- 1Department of Cardiothoracic Surgery, Erasmus University Medical Center, Rotterdam, Netherlands
- 2Imperial College London, National Heart and Lung Institute, London, United Kingdom
- 3Department of Cardiothoracic Surgery, Amsterdam University Medical Center, Amsterdam, Netherlands
In cardiovascular surgery, reconstruction and replacement of cardiac and vascular structures are routinely performed. Prosthetic or biological materials traditionally used for this purpose cannot be considered ideal substitutes as they have limited durability and no growth or regeneration potential. Tissue engineering aims to create materials having normal tissue function including capacity for growth and self-repair. These advanced materials can potentially overcome the shortcomings of conventionally used materials, and, if successfully passing all phases of product development, they might provide a better option for both the pediatric and adult patient population requiring cardiovascular interventions. This short review article overviews the most important cardiovascular pathologies where tissue engineered materials could be used, briefly summarizes the main directions of development of these materials, and discusses the hurdles in their clinical translation. At its beginnings in the 1980s, tissue engineering (TE) was defined as “an interdisciplinary field that applies the principles of engineering and the life sciences toward the development of biological substitutes that restore, maintain, or improve tissue function” (1). Currently, the utility of TE products and materials are being investigated in several fields of human medicine, ranging from orthopedics to cardiovascular surgery (2–5). In cardiovascular surgery, reconstruction and replacement of cardiac and vascular structures are routinely performed. Considering the shortcomings of traditionally used materials, the need for advanced materials that can “restore, maintain or improve tissue function” are evident. Tissue engineered substitutes, having growth and regenerative capacity, could fundamentally change the specialty (6). This article overviews the most important cardiovascular pathologies where TE materials could be used, briefly summarizes the main directions of development of TE materials along with their advantages and shortcomings, and discusses the hurdles in their clinical translation.
Clinical Need For Advanced Materials In Cardiovascular Surgery
Congenital Heart Disease
Congenital heart defects affect ~9 of 1,000 newborns (7) and often require corrective surgery at an early age. Invasive treatment of congenital cardiac defects results in an increased life expectancy and can significantly improve quality-of-life (8).
Repair with a prosthetic patch is the cornerstone of reconstruction of diseased or defective cardiac and vascular structures in pediatric cardiac surgery. Patches are used for the closure of atrial or ventricular septal defects, for complex reconstructions in atrioventricular canal defects; in right ventricular outflow tract reconstruction in Tetralogy of Fallot; for aortic reconstruction in interrupted aortic arch or hypoplastic left heart syndrome; or when establishing cavo-pulmonary connection is required (9–13). As most of these operations are performed at very young or even neonatal age, repair must stay effective and durable in a rapidly changing physiological environment. Traditionally used materials—autologous or xenogeneic pericardium, or prosthetic materials like Dacron (DuPont, Wilmington, DE) or PTFE (polytetrafluoroethylene)—are suboptimal in this respect as they tend to calcify over time and cannot grow with the child (14–16).
As children will inevitably outgrow a prosthetic heart valve (PHV) implanted at young age, reconstruction of native valves is always preferred over replacement whenever feasible. However, if reconstruction fails or appears not possible, replacement of the dysfunctional valve with a PHV becomes necessary. In childhood, most commonly the pulmonary valve requires replacement, often using a right ventricle-to-pulmonary artery conduit (17). Unfortunately, none of the currently available allogenic or xenogeneic conduits can be considered ideal, as they cannot grow and degenerate on the long term (18–21). Besides pathologies of the pulmonary valve and right ventricular outflow tract, congenital aortic stenosis (AS) often necessitates valve replacement in the pediatric population. Although balloon palliation can buy some time until valve repair or replacement (22), patients with congenital AS often require a sequence of re-operations until they reach adulthood (23, 24), largely due to the absence of an optimal valve substitute.
Acquired Valvular Heart Disease
Parallel to aging of the adult population, degenerative valvular heart diseases are becoming increasingly prevalent in the western world (25). Additionally, in developing countries, rheumatic valvular heart disease causes a substantial and often underestimated burden (26). Although valve replacement with a PHV improves symptom status and long-term survival, currently available heart valve prostheses are also associated with certain complications (27, 28). Mechanical PHVs necessitate lifelong anticoagulation, which increases the risk of bleeding and thromboembolic events and is suboptimal for women in childbearing age (29, 30), while the limited long-term durability and inherent structural degeneration of bioprosthetic valves remain a major issue for the younger patient population (31, 32).
Vascular Grafts
In coronary artery bypass grafting, peripheral vascular reconstructions or when creating arteriovenous shunts for renal dialysis, small caliber vascular grafts are required. Although autologous vessels harvested from other parts of body are potentially ideal for this purpose, they are not always available or eligible for use. Traditionally used prosthetic grafts have numerous limitations due to their limited patency (33–35) and increased susceptibility for infections (36). This, together with the magnitude of the affected population necessitates intensive research for alternative solutions (37).
Overview Of Tissue Engineered Solutions
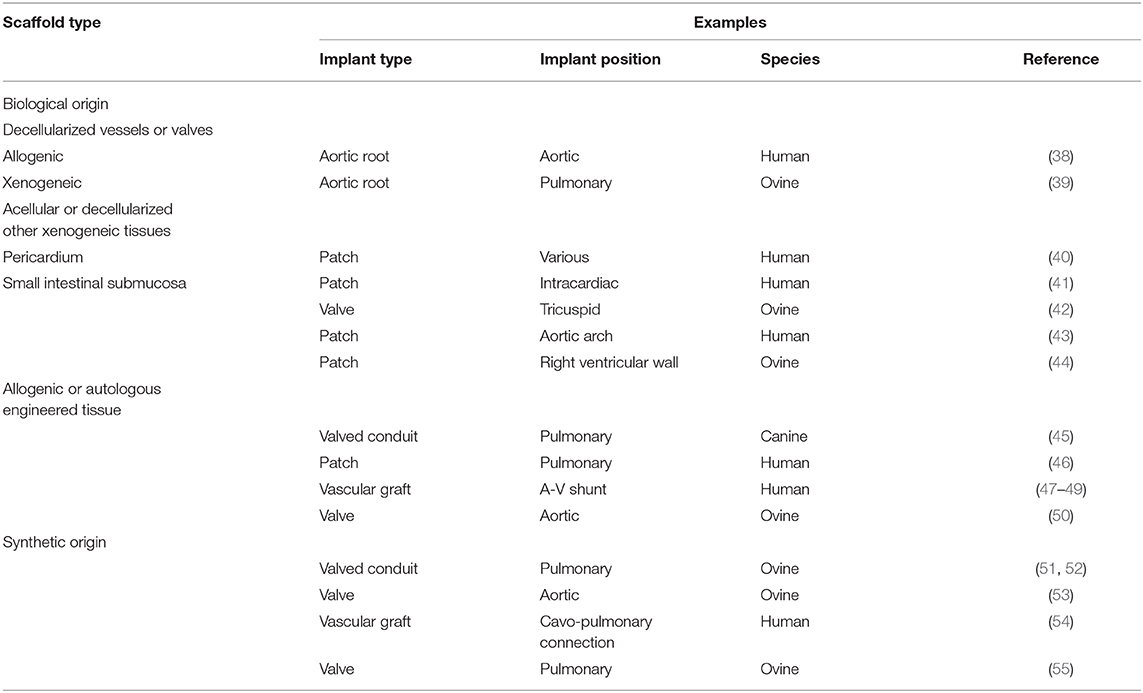
Tissue engineering, by providing advanced materials with physiological function and ability for growth and regeneration, could potentially fulfill these clinical needs. To create a TE product, the following are required: (i) a (biodegradable) scaffold to guide tissue formation; (ii) cells able to populate the scaffold; and (iii) (in the classical way of TE) a bioreactor, which simulates a physiological environment to augment tissue formation. Scaffolds used in cardiovascular TE can be from various origin. The most commonly used scaffold materials are summarized in (Table 1). Similarly, multiple cell-types might be used: among others, mesenchymal stem cells, endothelial progenitor cells or induced pluripotent stem cells can be utilized during the TE process (56). Bioreactors are specifically designed containers intended to provide an optimized, controlled environment where cell-scaffold interaction can take place (57). During “classical” TE, scaffolds are seeded with progenitor cells and incubated in vitro in a bioreactor. Following a period of maturation under controlled conditions, the TE product is implanted to the patient. Besides this “conventional” approach, various other, “incomplete” methods exist, where one or more steps of the “conventional” TE process are bypassed (58).
Some “TE” products are already cleared for clinical use: decellularized valves, porcine small intestinal submucosa and decellularized bovine pericardium (SynerGraft®, CryoLife Inc, Kennesaw, GA, United States; CorMatrix®, CorMatrix Cardiovascular, Roswell, GA, United States; CardioCel® Bioscaffold, LeMaitre Vascular, Burlington, MA, United States) are already parts of cardiovascular surgeon's armamentarium. Apart from decellularized allografts and porcine small intestinal submucosa, all other products are treated with glutaraldehyde (59) which can have a negative impact on cellular ingrowth following implantation (60). Furthermore, it is unclear if the above mentioned decellularized scaffolds degrade at all and whether they can truly be considered as TE products. Besides, all these products have inherent shortcomings. Allogenic tissues are generally cumbersome to procure and might still fail in the long term (61). Xenogeneic tissues can provoke inflammatory response leading to early degeneration and calcification. The first commercially available decellularized porcine valve dramatically failed due to a strong inflammatory response resulting in rapid degeneration and early structural failure in pediatric patients (62). Although a promising concept, acellular porcine small intestinal submucosa patches can also provoke an inflammatory response and can demonstrate early degeneration or calcifications leading to valve insufficiency when used for aortic valve repair (63, 64), or aneurysm formation when used for aortic reconstruction in pediatric patients (65). Decellularized bovine pericardium, though treated with low concentrations of glutaraldehyde (66), was found to be safe and effective in the mid-term when used for patch repair of complex congenital cardiac anomalies (40) and exhibited greater strain resistance compared to porcine small intestinal submucosa (67). Nevertheless, the possibility of calcification of the decellularized bovine pericardium has also been raised recently (68) and the quest for the “ideal” tissue engineered material continues.
In Situ Tissue Engineering With Polymer Scaffolds
During in situ TE, an unseeded biodegradable scaffold is implanted to the recipient. After implantation, the scaffold will be populated in vivo by cells scrambled from the circulation, with the recipient's own body acting as a bioreactor. This simplified approach saves substantial costs and prevents potential complications associated with incubating the scaffold in a bioreactor. Neo-tissue formation begins only after scaffold implantation and can occur under completely physiological shear and pressure conditions (69).
Compared to biological materials, polymers materials are relatively easy to produce, handle, sterilize or store, and they can be manufactured in virtually any size or form. This creates the possibility of manufacturing directly off-the-shelf available TE cardiovascular implants and increases the interest in in situ TE using polymer scaffolds.
However, this technique also has certain shortcomings which have to be considered. During in situ TE, neo-tissue formation occurs in a less-controlled environment and cells repopulating the scaffold must be gathered from high blood flows which might not be ideal (70). Furthermore, a delicate balance between the pace of scaffold degeneration and neo-tissue formation is required to achieve an optimal result. Fortunately, in contrast to TE products from biological origin, the design of the prosthesis and the characteristics of the polymer material such as scaffold composition, scaffold or fiber thickness or fiber orientation can be relatively easily modified, if necessary (71–75).
Heart valve constructs from polymer scaffolds are currently under investigation in preclinical experiments. In sheep, these scaffolds have demonstrated satisfactory durability and function on mid- term when implanted in the pulmonary position as a surgical prosthesis (55) or as a valved conduit (76), or when used as a transcatheter aortic valve (53). Based on these encouraging results, the first-in-man investigations of the polymer scaffolds has recently been started (54).
Challenges In Clinical Translation
As any new technology awaiting clinical introduction (77), TE products in cardiovascular surgery must find a therapeutic gap, a “niche,” where no ideal treatment option exists, and where the advantages of the new technology can be proven. Although the shortcomings of currently used materials are evident and some “TE” products are already approved and used in the clinical setting, there are many hurdles to overcome before TE materials can be routinely used in cardiovascular surgery (78).
Cardiovascular implants have to fulfill strict safety and performance criteria during their regulatory assessment before clinical introduction (79–81). During regulatory assessment, standards of the International Organization for Standardization (ISO) are widely used. The ISO 5840 standard on heart valve prostheses provides guidance on in vitro and in vivo hemodynamic and durability testing, as well as Objective Performance Criteria (OPC) for the assessment of complications after implantation (82). However, regulatory assessment of TE implants is complicated. During manufacturing, achieving consistence in the biological properties of cell-based products between batches is difficult, rendering TE products less reproducible than conventional cardiovascular implants. Furthermore, difficulties in sterilization, packaging and storage of cell-based TE products further limit their regulatory approval and widespread clinical use. On the other hand, in situ TE cardiovascular implants cannot be considered as “final products,” as their properties are expected to change after implantation while they transform into normally functioning living tissue. Of note, this transformation might not be the same in all subjects receiving the same implant and tissue formation might occur differently in animals used for pre-clinical in vivo testing than in human recipients (83). These issues make the interpretation of the results derived from in vitro and in vivo testing cumbersome, and together with the plethora of approaches and techniques used for manufacturing, makes the regulatory assessment of TE cardiovascular implants difficult. To date, no specific ISO standard on TE heart valves exist and it is not clear how these products should be classified or assessed.
Besides, considering these unique properties of TE cardiovascular implants, important ethical issues might arise when it comes to in human testing or clinical introduction of these products (84). Irrespective of the local circumstances or the clinical need, the risk of implanting a prosthesis that might fail must always be carefully weighed against the perceived benefits (26, 85, 86).
Another important aspect of successful clinical introduction is the cost-effectiveness of the novel device or technique, compared to standard treatment (87). Although development of TE materials are expensive, in-situ TE valves constructed from biodegradable polymer scaffolds could be potentially cost effective, according to a recent early health technology assessment study (88).
Perspectives
Materials used in cardiovascular surgery must fulfill a few essential requirements: they must be hemostatic, hold sutures, be resistant to pressure and stress while being tissue-friendly and resistant to thrombosis. Additionally, an ideal material has normal tissue function and capability for growth and self-repair.
TE materials can potentially fulfill all of these essential requirements and in situ TE using polymer materials can offer a simplified and potentially cost-effective method to produce off-the-shelf available, TE cardiovascular implants. Although initial results are promising (55), future research is necessitated and there are still many obstacles to overcome before the use of these materials can become a part of the everyday practice of cardiovascular surgery (89). The use of novel cell free techniques to enhance the process of regeneration in TE include adding exosomes (90), hydrogels (91), direct (92, 93), or indirect induced pluripotent stem cell reprogramming using gene editing (94, 95) as well as stimulating myocardial cell division (96). Adding such strategies holds great promise in the future.
Author Contributions
AD: drafting the first manuscript. MY and JK: critically revising the work for important intellectual content.
Funding
We acknowledged the financial support from the Netherlands Cardio Vascular Research Initiative: the Dutch Heart Foundation, Dutch Federation of University Medical Centres, the Netherlands Organisation for Health Research and Development, and the Royal Netherlands Academy of Sciences.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Langer R, Vacanti JP. Tissue engineering. Science. (1993) 260:920–6. doi: 10.1126/science.8493529
2. Langer R, Vacanti J. Advances in tissue engineering. J Pediatr Surg. (2016) 51:8–12. doi: 10.1016/j.jpedsurg.2015.10.022
3. Vijayavenkataraman S, Lu WF, Fuh JY. 3D bioprinting of skin: a state-of-the-art review on modelling, materials, and processes. Biofabrication. (2016) 8:032001. doi: 10.1088/1758-5090/8/3/032001
4. Nie X, Wang DA. Decellularized orthopaedic tissue-engineered grafts: biomaterial scaffolds synthesised by therapeutic cells. Biomater Sci. (2018) 6:2798–811. doi: 10.1039/C8BM00772A
5. Bowles RD, Setton LA. Biomaterials for intervertebral disc regeneration and repair. Biomaterials. (2017) 129:54–67. doi: 10.1016/j.biomaterials.2017.03.013
6. Yacoub MH, Takkenberg JJ. Will heart valve tissue engineering change the world? Nat Clin Pract Cardiovasc Med. (2005) 2:60–1. doi: 10.1038/ncpcardio0112
7. van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. (2011) 58:2241–7. doi: 10.1016/j.jacc.2011.08.025
8. Loup O, von Weissenfluh C, Gahl B, Schwerzmann M, Carrel T, Kadner A. Quality of life of grown-up congenital heart disease patients after congenital cardiac surgery. Eur J Cardiothorac Surg. (2009) 36:105–11; discussion 11. doi: 10.1016/j.ejcts.2009.03.023
9. Backer CL, Stewart RD, Bailliard F, Kelle AM, Webb CL, Mavroudis C. Complete atrioventricular canal: comparison of modified single-patch technique with two-patch technique. Ann Thorac Surg. (2007) 84:2038–46; discussion −46. doi: 10.1016/j.athoracsur.2007.04.129
10. Kaza AK, Lim HG, Dibardino DJ, Bautista-Hernandez V, Robinson J, Allan C, et al. Long-term results of right ventricular outflow tract reconstruction in neonatal cardiac surgery: options and outcomes. J Thorac Cardiovasc Surg. (2009) 138:911–6. doi: 10.1016/j.jtcvs.2008.10.058
11. Backer CL, Paape K, Zales VR, Weigel TJ, Mavroudis C. Coarctation of the aorta. Repair with polytetrafluoroethylene patch aortoplasty. Circulation. (1995) 92:II132–6. doi: 10.1161/01.CIR.92.9.132
12. Vitanova K, Cleuziou J, Pabst von Ohain J, Burri M, Eicken A, Lange R. Recoarctation after norwood i Procedure for hypoplastic left heart syndrome: impact of patch material. Ann Thorac Surg. (2017) 103:617–21. doi: 10.1016/j.athoracsur.2016.10.030
13. Stamm C, Friehs I, Mayer JE Jr., Zurakowski D, Triedman JK, et al. Long-term results of the lateral tunnel fontan operation. J Thorac Cardiovasc Surg. (2001) 121:28–41. doi: 10.1067/mtc.2001.111422
14. Lee C, Lee CH, Hwang SW, Lim HG, Kim SJ, Lee JY, et al. Midterm follow-up of the status of gore-Tex graft after extracardiac conduit fontan procedure. Eur J Cardiothorac Surg. (2007) 31:1008–12. doi: 10.1016/j.ejcts.2007.03.013
15. Kadowaki MH, Levett JM, Manjoney DL, Grina NM, Glagov S. Comparison of prosthetic graft materials as intracardiac right atrial patches. J Surg Res. (1986) 41:65–74. doi: 10.1016/0022-4804(86)90010-7
16. Giannico S, Hammad F, Amodeo A, Michielon G, Drago F, Turchetta A, et al. Clinical outcome of 193 extracardiac fontan patients: the first 15 years. J Am Coll Cardiol. (2006) 47:2065–73. doi: 10.1016/j.jacc.2005.12.065
17. The Society of Thoracic Surgeons. Congenital Heart Surgery Database. The Society of Thoracic Surgeons. (2019). Available online at: https://www.sts.org/registries-research-center/sts-national-database/congenital-heart-surgery-database.
18. Dearani JA, Danielson GK, Puga FJ, Schaff HV, Warnes CW, Driscoll DJ, et al. Late follow-up of 1095 patients undergoing operation for complex congenital heart disease utilizing pulmonary ventricle to pulmonary artery conduits. Ann Thorac Surg. (2003) 75:399–410; discussion −1. doi: 10.1016/S0003-4975(02)04547-2
19. Boethig D, Goerler H, Westhoff-Bleck M, Ono M, Daiber A, Haverich A, et al. Evaluation of 188 consecutive homografts implanted in pulmonary position after 20 years. Eur J Cardiothorac Surg. (2007) 32:133–42. doi: 10.1016/j.ejcts.2007.02.025
20. Homann M, Haehnel JC, Mendler N, Paek SU, Holper K, Meisner H, et al. Reconstruction of the RVOT with valved biological conduits: 25 years experience with allografts and xenografts. Eur J Cardiothorac Surg. (2000) 17:624–30. doi: 10.1016/S1010-7940(00)00414-0
21. Yong MS, Yim D, d'Udekem Y, Brizard CP, Robertson T, Galati JC, et al. Medium-term outcomes of bovine jugular vein graft and homograft conduits in children. ANZ J Surg. (2015) 85:381–5. doi: 10.1111/ans.13018
22. McCrindle BW, Blackstone EH, Williams WG, Sittiwangkul R, Spray TL, Azakie A, et al. Are outcomes of surgical versus transcatheter balloon valvotomy equivalent in neonatal critical aortic stenosis? Circulation. (2001) 104(12 Suppl. 1):I152–8. doi: 10.1161/hc37t1.094837
23. Siddiqui J, Brizard CP, Galati JC, Iyengar AJ, Hutchinson D, Konstantinov IE, et al. Surgical valvotomy and repair for neonatal and infant congenital aortic stenosis achieves better results than interventional catheterization. J Am Coll Cardiol. (2013) 62:2134–40. doi: 10.1016/j.jacc.2013.07.052
24. d'Udekem Y, Siddiqui J, Seaman CS, Konstantinov IE, Galati JC, Cheung MM, et al. Long-term results of a strategy of aortic valve repair in the pediatric population. J Thorac Cardiovasc Surg. (2013) 145:461–7; discussion 7–9. doi: 10.1016/j.jtcvs.2012.11.033
25. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. (2006) 368:1005–11. doi: 10.1016/S0140-6736(06)69208-8
26. Zilla P, Yacoub M, Zuhlke L, Beyersdorf F, Sliwa K, Khubulava G, et al. Global unmet needs in cardiac surgery. Glob Heart. (2018) 13:293–303. doi: 10.1016/j.gheart.2018.08.002
27. Kapadia SR, Leon MB, Makkar RR, Tuzcu EM, Svensson LG, Kodali S, et al. 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. (2015) 385:2485–91. doi: 10.1016/S0140-6736(15)60290-2
28. Brennan JM, Edwards FH, Zhao Y, O'Brien S, Booth ME, Dokholyan RS, et al. Long-term safety and effectiveness of mechanical versus biologic aortic valve prostheses in older patients: results from the society of thoracic surgeons adult cardiac surgery national database. Circulation. (2013) 127:1647–55. doi: 10.1161/CIRCULATIONAHA.113.002003
29. Hammermeister K, Sethi GK, Henderson WG, Grover FL, Oprian C, Rahimtoola SH. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the veterans affairs randomized trial. J Am Coll Cardiol. (2000) 36:1152–8. doi: 10.1016/S0735-1097(00)00834-2
30. Steinberg ZL, Dominguez-Islas CP, Otto CM, Stout KK, Krieger EV. Maternal and fetal outcomes of anticoagulation in pregnant women with mechanical heart valves. J Am Coll Cardiol. (2017) 69:2681–91. doi: 10.1016/j.jacc.2017.03.605
31. Fatima B, Mohananey D, Khan FW, Jobanputra Y, Tummala R, Banerjee K, et al. Durability data for bioprosthetic surgical aortic valve: a systematic review. JAMA Cardiol. (2018) 4:71–80. doi: 10.1001/jamacardio.2018.4045
32. Saleeb SF, Newburger JW, Geva T, Baird CW, Gauvreau K, Padera RF, et al. Accelerated degeneration of a bovine pericardial bioprosthetic aortic valve in children and young adults. Circulation. (2014) 130:51–60. doi: 10.1161/CIRCULATIONAHA.114.009835
33. Desai M, Seifalian AM, Hamilton G. Role of prosthetic conduits in coronary artery bypass grafting. Eur J Cardiothorac Surg. (2011) 40:394–8. doi: 10.1016/j.ejcts.2010.11.050
34. Kashyap VS, Ahn SS, Quinones-Baldrich WJ, Choi BU, Dorey F, Reil TD, et al. Infrapopliteal-lower extremity revascularization with prosthetic conduit: a 20-year experience. Vasc Endovascular Surg. (2002) 36:255–62. doi: 10.1177/153857440203600402
35. Johnson WC, Lee KK. A comparative evaluation of polytetrafluoroethylene, umbilical vein, and saphenous vein bypass grafts for femoral-popliteal above-knee revascularization: a prospective randomized department of veterans affairs cooperative study. J Vasc Surg. (2000) 32:268–77. doi: 10.1067/mva.2000.106944
36. Kirkton RD, Prichard HL, Santiago-Maysonet M, Niklason LE, Lawson JH, Dahl SLM. Susceptibility of ePTFE vascular grafts and bioengineered human acellular vessels to infection. J Surg Res. (2018) 221:143–51. doi: 10.1016/j.jss.2017.08.035
37. Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. The Lancet. (2015) 385:1975–82. doi: 10.1016/S0140-6736(14)61601-9
38. Zehr KJ, Yagubyan M, Connolly HM, Nelson SM, Schaff HV. Aortic root replacement with a novel decellularized cryopreserved aortic homograft: postoperative immunoreactivity and early results. J Thorac Cardiovasc Surg. (2005) 130:1010–5. doi: 10.1016/j.jtcvs.2005.03.044
39. Paniagua Gutierrez JR, Berry H, Korossis S, Mirsadraee S, Lopes SV, da Costa F, et al. Regenerative potential of low-concentration sDS-decellularized porcine aortic valved conduits in vivo. Tissue Engineer Part A. (2015) 21:332–42. doi: 10.1089/ten.tea.2014.0003
40. Neethling WM, Strange G, Firth L, Smit FE. Evaluation of a tissue-engineered bovine pericardial patch in paediatric patients with congenital cardiac anomalies: initial experience with the aDAPT-treated cardioCel(R) patch. Interact Cardiovasc Thorac Surg. (2013) 17:698–702. doi: 10.1093/icvts/ivt268
41. Al Haddad E, LaPar DJ, Dayton J, Stephens EH, Bacha E. Complete atrioventricular canal repair with a decellularized porcine small intestinal submucosa patch. Congenit Heart Dis. (2018) doi: 10.1111/chd.12666
42. Zafar F, Hinton RB, Moore RA, Baker RS, Bryant R 3rd, Narmoneva DA, et al. Physiological growth, remodeling potential, and preserved function of a novel bioprosthetic tricuspid valve: tubular bioprosthesis made of small intestinal submucosa-Derived extracellular matrix. J Am Coll Cardiol. (2015) 66:877–88. doi: 10.1016/j.jacc.2015.06.1091
43. Jacobsen RM, Mitchell ME, Woods RK, Loomba RS, Tweddell JS. Porcine small intestinal submucosa may be a suitable material for norwood arch reconstruction. Ann Thorac Surg. (2018) 106:1847–52. doi: 10.1016/j.athoracsur.2018.06.033
44. Baker RS, Zafar F, Kimura N, Knilans T, Osinska H, Robbins J, et al. In vivo remodeling of an extracellular matrix cardiac patch in an ovine model. Asaio J. (2018) doi: 10.1097/MAT.0000000000000864
45. Yamanami M, Yahata Y, Uechi M, Fujiwara M, Ishibashi-Ueda H, Kanda K, et al. Development of a completely autologous valved conduit with the sinus of valsalva using in-body tissue architecture technology: a pilot study in pulmonary valve replacement in a beagle model. Circulation. (2010) 122(11 Suppl.):S100–6. doi: 10.1161/CIRCULATIONAHA.109.922211
46. Kato N, Yamagishi M, Kanda K, Miyazaki T, Maeda Y, Yamanami M, et al. First successful clinical application of the in vivo tissue-Engineered autologous vascular graft. Ann Thorac Surg. (2016) 102:1387–90. doi: 10.1016/j.athoracsur.2016.06.095
47. McAllister TN, Maruszewski M, Garrido SA, Wystrychowski W, Dusserre N, Marini A, et al. Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: a multicentre cohort study. Lancet. (2009) 373:1440–6. doi: 10.1016/S0140-6736(09)60248-8
48. Lawson JH, Glickman MH, Ilzecki M, Jakimowicz T, Jaroszynski A, Peden EK, et al. Bioengineered human acellular vessels for dialysis access in patients with end-stage renal disease: two phase 2 single-arm trials. Lancet. (2016) 387:2026–34. doi: 10.1016/S0140-6736(16)00557-2
49. Wystrychowski W, McAllister TN, Zagalski K, Dusserre N, Cierpka L, L'Heureux N. First human use of an allogeneic tissue-engineered vascular graft for hemodialysis access. J Vasc Surg. (2014) 60:1353–7. doi: 10.1016/j.jvs.2013.08.018
50. Syedain Z, Reimer J, Schmidt J, Lahti M, Berry J, Bianco R, et al. 6-month aortic valve implantation of an off-the-shelf tissue-engineered valve in sheep. Biomaterials. (2015) 73:175–84. doi: 10.1016/j.biomaterials.2015.09.016
51. Bennink G, Torii S, Brugmans M, Cox M, Svanidze O, Ladich E, et al. A novel restorative pulmonary valved conduit in a chronic sheep model: mid-term hemodynamic function and histologic assessment. J Thorac Cardiovasc Surg. (2018) 155:2591–601 e3. doi: 10.1016/j.jtcvs.2017.12.046
52. Brugmans M, Serrero A, Cox M, Svanidze O, Schoen FJ. Morphology and mechanisms of a novel absorbable polymeric conduit in the pulmonary circulation of sheep. Cardiovasc Pathol. (2018) 38:31–8. doi: 10.1016/j.carpath.2018.10.008
53. Miyazaki Y, Soliman OII, Abdelghani M, Katsikis A, Naz C, Lopes S, et al. Acute performance of a novel restorative transcatheter aortic valve: preclinical results. EuroIntervention. (2017) 13:e1410–e7. doi: 10.4244/EIJ-D-17-00554
54. Bockeria LA, Svanidze O, Kim A, Shatalov K, Makarenko V, Cox M, et al. Total cavopulmonary connection with a new bioabsorbable vascular graft: first clinical experience. J Thorac Cardiovasc Surg. (2017) 153:1542–50. doi: 10.1016/j.jtcvs.2016.11.071
55. Kluin J, Talacua H, Smits AI, Emmert MY, Brugmans MC, Fioretta ES, et al. In situ heart valve tissue engineering using a bioresorbable elastomeric implant - from material design to 12 months follow-up in sheep. Biomaterials. (2017) 125:101–17. doi: 10.1016/j.biomaterials.2017.02.007
56. Jover E, Fagnano M, Angelini G, Madeddu P. Cell sources for tissue engineering strategies to treat calcific valve disease. Front Cardiovasc Med. (2018) 5:155. doi: 10.3389/fcvm.2018.00155
57. Berry JL, Steen JA, Koudy Williams J, Jordan JE, Atala A, Yoo JJ. Bioreactors for development of tissue engineered heart valves. Ann Biomed Eng. (2010) 38:3272–9. doi: 10.1007/s10439-010-0148-6
58. Rabkin E, Schoen FJ. Cardiovascular tissue engineering. Cardiovasc Pathol. (2002) 11:305–17. doi: 10.1016/S1054-8807(02)00130-8
59. Pattar SS, Fatehi Hassanabad A, Fedak PWM. Acellular extracellular matrix bioscaffolds for cardiac repair and regeneration. Front Cell Dev Biol. (2019) 7:63. doi: 10.3389/fcell.2019.00063
60. Honge JL, Funder J, Hansen E, Dohmen PM, Konertz W, Hasenkam JM. Recellularization of aortic valves in pigs?. Eur J Cardio-Thor Surg. (2011) 39:829–34. doi: 10.1016/j.ejcts.2010.08.054
61. Horke A. Decellularization of aortic valves: only time will tell. Eur J Cardiothorac Surg. (2016) 49:707–8. doi: 10.1093/ejcts/ezv361
62. Simon P, Kasimir MT, Seebacher G, Weigel G, Ullrich R, Salzer-Muhar U, et al. Early failure of the tissue engineered porcine heart valve sYNERGRAFT in pediatric patients. Eur J Cardiothorac Surg. (2003) 23:1002–6; discussion 6. doi: 10.1016/S1010-7940(03)00094-0
63. Hofmann M, Schmiady MO, Burkhardt BE, Dave HH, Hubler M, Kretschmar O, et al. Congenital aortic valve repair using corMatrix((R)) : a histologic evaluation. Xenotransplantation. (2017) 24:12341. doi: 10.1111/xen.12341
64. Mosala Nezhad Z, Baldin P, Poncelet A, El Khoury G. Calcific degeneration of corMatrix 4 years after bicuspidization of unicuspid aortic valve. Ann Thorac Surg. (2017) 104:e431–e3. doi: 10.1016/j.athoracsur.2017.07.040
65. Erek E, Aydin S, Suzan D, Yildiz O, Demir IH, Odemis E. Early degeneration of extracellular matrix used for aortic reconstruction during the norwood operation. Ann Thorac Surg. (2016) 101:758–60. doi: 10.1016/j.athoracsur.2015.04.051
66. Strange G, Brizard C, Karl TR, Neethling L. An evaluation of admedus' tissue engineering process-treated (ADAPT) bovine pericardium patch (CardioCel) for the repair of cardiac and vascular defects. Expert Rev Med Devices. (2015) 12:135–41. doi: 10.1586/17434440.2015.985651
67. Neethling WML, Puls K, Rea A. Comparison of physical and biological properties of cardioCel(R) with commonly used bioscaffolds. Interact Cardiovasc Thorac Surg. (2018) 26:985–92. doi: 10.1093/icvts/ivx413
68. Salameh A, Greimann W, Vondrys D, Kostelka M. Calcification or not. This is the question. A 1-year study of bovine pericardial vascular patches (CardioCel) in minipigs. Semin Thorac Cardiovasc Surg. (2018) 30:54–9. doi: 10.1053/j.semtcvs.2017.09.013
69. Riem Vis PW, Kluin J, Sluijter JP, van Herwerden LA, Bouten CV. Environmental regulation of valvulogenesis: implications for tissue engineering. Eur J Cardiothorac Surg. (2011) 39:8–17. doi: 10.1016/j.ejcts.2010.05.032
70. Lichtenberg A, Cebotari S, Tudorache I, Sturz G, Winterhalter M, Hilfiker A, et al. Flow-dependent re-endothelialization of tissue-engineered heart valves. J Heart Valve Dis. (2006) 15:287–93; discussion 93–4.
71. Emmert MY, Schmitt BA, Loerakker S, Sanders B, Spriestersbach H, Fioretta ES, et al. Computational modeling guides tissue-engineered heart valve design for long-term in vivo performance in a translational sheep model. Sci Transl Med. (2018) 10:4587. doi: 10.1126/scitranslmed.aan4587
72. Van Lieshout M, Peters G, Rutten M, Baaijens F. A knitted, fibrin-covered polycaprolactone scaffold for tissue engineering of the aortic valve. Tissue Eng. (2006) 12:481–7. doi: 10.1089/ten.2006.12.481
73. Vaz CM, van Tuijl S, Bouten CV, Baaijens FP. Design of scaffolds for blood vessel tissue engineering using a multi-layering electrospinning technique. Acta Biomater. (2005) 1:575–82. doi: 10.1016/j.actbio.2005.06.006
74. Fioretta ES, Simonet M, Smits AI, Baaijens FP, Bouten CV. Differential response of endothelial and endothelial colony forming cells on electrospun scaffolds with distinct microfiber diameters. Biomacromolecules. (2014) 15:821–9. doi: 10.1021/bm4016418
75. Sohier J, Carubelli I, Sarathchandra P, Latif N, Chester AH, Yacoub MH. The potential of anisotropic matrices as substrate for heart valve engineering. Biomaterials. (2014) 35:1833–44. doi: 10.1016/j.biomaterials.2013.10.061
76. Soliman OI, Miyazaki Y, Abdelghani M, Brugmans M, Witsenburg M, Onuma Y, et al. Midterm performance of a novel restorative pulmonary valved conduit: preclinical results. EuroIntervention. (2017) 13:e1418–e27. doi: 10.4244/EIJ-D-17-00553
77. Dearani JA, Rosengart TK, Marshall MB, Mack MJ, Jones DR, Prager RL, et al. Incorporating innovation and new technology into cardiothoracic surgery. Ann Thorac Surg. (2018) 107:1267–74. doi: 10.1016/j.athoracsur.2018.10.022
78. Emmert MY, Fioretta ES, Hoerstrup SP. Translational challenges in cardiovascular tissue engineering. J Cardiovasc Transl Res. (2017) 10:139–49. doi: 10.1007/s12265-017-9728-2
81. US Food And Drug Administration. Premarket Approval (PMA): US Department of Health and Human Services. (2018). Available online at: https://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/HowtoMarketYourDevice/PremarketSubmissions/PremarketApprovalPMA/ucm2007514.htm#data.
82. ISO. International Standard, ISO 5840:2015, Cardiovascular Implants - Cardiac Valve Prostheses. International Organization for Standardization (ISO). (2015).
83. Klopfleisch R, Jung F. The pathology of the foreign body reaction against biomaterials. J Biomed Mater Res A. (2017) 105:927–40. doi: 10.1002/jbm.a.35958
84. Taylor DA, Caplan AL, Macchiarini P. Ethics of bioengineering organs and tissues. Expert opinion on biological therapy. (2014) 14:879–82. doi: 10.1517/14712598.2014.915308
85. Zilla P, Bolman RM, Yacoub MH, Beyersdorf F, Sliwa K, Zuhlke L, et al. The cape town declaration on access to cardiac surgery in the developing world. Eur J Cardiothorac Surg. (2018) 54:407–10. doi: 10.1093/ejcts/ezy272
86. Angell M. The ethics of clinical research in the third world. N Engl J Med. (1997) 337:847–9. doi: 10.1056/NEJM199709183371209
87. Antonides CFJ, Cohen DJ, Osnabrugge RLJ. Statistical primer: a cost-effectiveness analysis†. Eur J Cardio-Thor Surg. (2018) 54:209–13. doi: 10.1093/ejcts/ezy187
88. Huygens SA, Rutten-van Molken M, Noruzi A, Etnel JRG, Ramos IC, Bouten CVC, et al. What is the potential of tissue-engineered pulmonary valves in children? Ann Thorac Surg. (2018) doi: 10.1016/j.athoracsur.2018.11.066
89. Yacoub MH. In search of living valve substitutes. J Am Coll Cardiol. (2015) 66:889–91. doi: 10.1016/j.jacc.2015.07.007
90. Jing H, He X, Zheng J. Exosomes and regenerative medicine: state of the art and perspectives. Transl Res. (2018) 196:1–16. doi: 10.1016/j.trsl.2018.01.005
91. El-Sherbiny IM, Yacoub MH. Hydrogel scaffolds for tissue engineering: progress and challenges. Glob Cardiol Sci Pract. (2013) 2013:316–42. doi: 10.5339/gcsp.2013.38
92. Grath A, Dai G. Direct cell reprogramming for tissue engineering and regenerative medicine. J Biol Eng. (2019) 13:14. doi: 10.1186/s13036-019-0144-9
93. Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. (2010) 142:375–86. doi: 10.1016/j.cell.2010.07.002
94. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. (2007) 131:861–72. doi: 10.1016/j.cell.2007.11.019
95. Armstrong JPK, Stevens MM. Emerging technologies for tissue engineering: from gene editing to personalized medicine. Tissue Eng Part A. (2019) 25:688–92. doi: 10.1089/ten.tea.2019.0026
Keywords: tissue-engineering, bioengineering, cardiac surgery, heart surgery, in-situ tissue engineered, TEHV
Citation: Durko AP, Yacoub MH and Kluin J (2020) Tissue Engineered Materials in Cardiovascular Surgery: The Surgeon's Perspective. Front. Cardiovasc. Med. 7:55. doi: 10.3389/fcvm.2020.00055
Received: 05 September 2019; Accepted: 20 March 2020;
Published: 15 April 2020.
Edited by:
Jesper Hjortnaes, University Medical Center Utrecht, NetherlandsReviewed by:
Sharan Ramaswamy, Florida International University, United StatesElisa Avolio, University of Bristol, United Kingdom
Copyright © 2020 Durko, Yacoub and Kluin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jolanda Kluin, ai5rbHVpbkBhbXN0ZXJkYW11bWMubmw=
 Andras P. Durko
Andras P. Durko Magdi H. Yacoub
Magdi H. Yacoub Jolanda Kluin3*
Jolanda Kluin3*