- 1Nuffield Department of Women's and Reproductive Health, University of Oxford, Oxford, United Kingdom
- 2The George Institute for Global Health, Oxford, United Kingdom
- 3The George Institute for Global Health, University of New South Wales, Sydney, NSW, Australia
- 4The George Institute for Global Health, New Delhi, India
- 5Manipal Academy of Higher Education, Manipal, India
- 6The Nuffield Department of Primary Care Health Sciences, University of Oxford, Oxford, United Kingdom
- 7THIS Institute (The Healthcare Improvement Studies Institute), University of Cambridge, Cambridge, United Kingdom
Cardiometabolic disorders (CMDs), including ischemic heart disease, stroke and type 2 diabetes are the leading causes of mortality and morbidity in women worldwide. The burden of CMDs falls disproportionately on low and middle-income countries (LMICs), placing substantial demands on already pressured health systems. Cardiometabolic disorders may present up to a decade earlier in some LMIC settings, and are associated with high-case fatality rates. Early identification and ongoing postpartum follow-up of women with pregnancy complications such as hypertensive disorders of pregnancy (HDPs), and gestational diabetes mellitus (GDM) may offer opportunities for prevention, or help delay onset of CMDs. This mini-review paper presents an overview of the key challenges faced in the early identification, referral and management of pregnant women at increased risk of CMDs, in low-resource settings worldwide. Evidence-based strategies, including novel diagnostics, technology and innovations for early detection, screening and management for pregnant women at high-risk of CMDs are presented. The review highlights the key research priorities for addressing cardiometabolic risk in pregnancy in low-resource settings.
Introduction
Significant physiological changes occur during pregnancy. These may affect a woman's cardiovascular, immune and metabolic functions and unmask susceptibility for developing cardiometabolic disorders (CMDs) (1, 2). Women with a history of Hypertensive Disorders of Pregnancy (HDPs, including preeclampsia), Gestational Diabetes Mellitus (GDM), spontaneous preterm birth, and delivery of a small for gestational age (SGA) baby, display increased risk of future CMDs: including cardiovascular disease (CVD), stroke, Type 2 Diabetes (T2DM) and chronic kidney disease (CKD) (3–7). Pregnancy complications are no longer seen as isolated conditions affecting pregnancy, but independent risk factors for future CVD (5, 8).
Women are more likely to die from CVD because of late presentation and differences in symptomatology (9). The burden of premature deaths from complications of pregnancy such as preeclampsia, preterm birth and SGA babies and CVD later in life, fall disproportionately upon Low-and Middle-Income Countries (LMICs) (10, 11). Women in rural areas of LMICs are further disadvantaged due to limitations in healthcare access and infrastructure, issues of poverty, and educational and socio-cultural barriers in accessing timely care as well as engaging with ongoing treatment (12, 13).
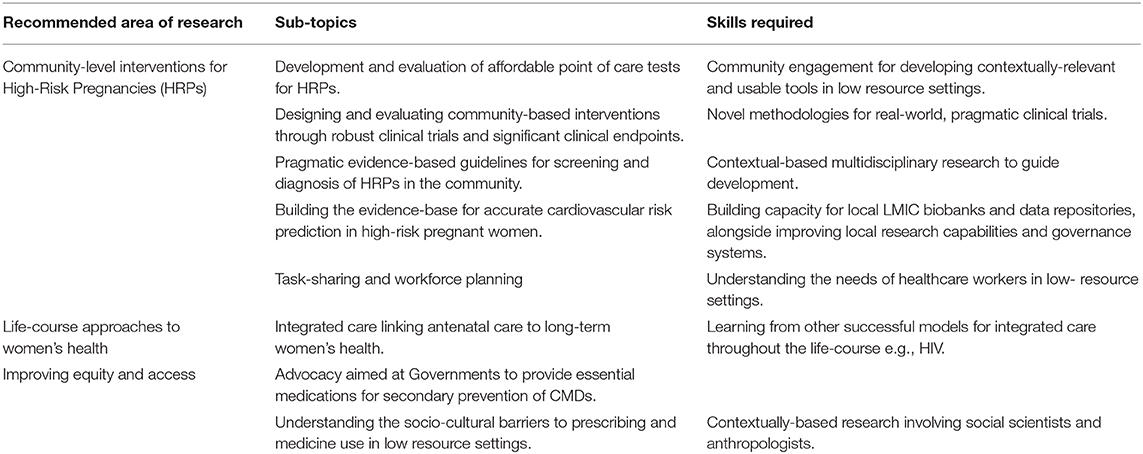
This review discusses the research priorities for improving women's cardiometabolic health in low-resource settings in LMICs following a high-risk pregnancy. We focus on three main areas related to the provision of universal health coverage: (1) Community-level interventions for high-risk pregnancies; (2) The need for life-course based approaches to women's health, and (3) Improving equity and access to affordable treatments for CMDs.
Community-Level Interventions for High-Risk Pregnancies
Preventative efforts to avert CMDs often start too late to be effective (3). Early detection and management of women with pregnancy complications associated with a high-risk of future CMDs requires active community engagement to encourage early, regular antenatal care (ANC) attendance (14).
In low-resource settings, where multiple clinic visits may not be feasible, point-of-care tests (POCT) have been used by Community Health Workers (CHWs), nurses and doctors to screen for HIV/AIDS, malaria and anemia during pregnancy (14). Potential biomarkers for first trimester preeclampsia screening include serum placental growth factor (PlGF), serum pregnancy-associated plasma protein A (PAPP-A), mean arterial pressure (MAP) and uterine artery pulsatility index (UTPI) (15). Point of care tests for these emerging biomarkers merit further country-specific, clinical, and economic evaluation (16). The implementation of novel diagnostics in LMICs, must be coupled with upgrading of laboratory facilities in primary care settings, which are insufficiently prioritized by governments worldwide (17).
The detection of HDPs mainly involves blood pressure (BP) measurement and urinalysis. Whilst non-invasive, these skills can be intimidating to rural healthcare workers. To improve community-level detection of HDPs in LMICs, low-cost instruments have been developed and validated for use by frontline healthcare workers in low-resource settings. The CRADLE Vital Signs Alert device is a novel, semi-automated BP device, validated for use in pregnancy and to stratify risk in pregnant women with both high and very low BP, costing ~$20 USD (18). Feasibility studies have shown high levels of acceptability by Community Health Workers (CHWs) in Nigeria, Mozambique, Zimbabwe, Ethiopia and India (18, 19). A large stepped-wedge cluster-randomized controlled trial across 10 LMICs was; however, unable to demonstrate impact on the primary composite outcomes of maternal mortality and morbidity (20). This may be due, in part, to insufficient power and sample size, and significant variations between clusters (20). Low cost urinalysis devices for proteinuria detection have also been developed and piloted (21–23), although evidence of impact on clinical endpoints is lacking.
Community-based screening for GDM is complicated by a lack of attendance, and lack of consolidated criteria for testing and diagnosis of GDM (24). Routine application of the gold standard fasting oral glucose tolerance test (OGTT), followed by venous blood being drawn at 0, 1, and 2 hours post-glucose load is not feasible in rural settings where mothers have to travel long distances and wait substantial amounts of time to receive antenatal services, and healthcare staff skilled in drawing blood are not available at specified times (24). Some rural centers perform OGTTs irrespective of fasting status. A study from India, testing women irrespective of their fasting state, did not reveal statistically different results compared to the WHO-recommended fasting 75-g OGTT (25). Subsequent studies have, however, found the sensitivity of non-fasting tests to be low (26). In response to the growing burden of GDM in India, pragmatic guidelines for low-resource settings have been developed (24, 27); however, the operability of such guidelines relies heavily on the presence of good laboratory and primary care infrastructure.
The Role of Mobile Technologies
Mobile health (mHealth) technologies have the potential to increase equity, quality and efficiency of service delivery in LMICs (28). mHealth technologies have contributed to reductions in delays in accessing maternal health in LMICs (29), and can be useful in the diagnosis, monitoring, providing clinical decision support, education and health promotion (30, 31).
A large-scale cluster randomized trial of a multi-faceted smartphone-based mHealth intervention (ImTECHO) used by CHWs to deliver care to pregnant women in their homes, involving a population of almost half a million in rural Gujarat in India, demonstrated improved engagement and delivery of antenatal and postnatal care by CHWs (32). The platform facilitated longitudinal tracking, scheduling of health services, screening for complications, counseling and behavior change communication, and real-time mentoring and supportive supervision of CHWs. This study highlighted the feasibility and effectiveness of mobile phone technologies as job aids to frontline healthcare workers to strengthen the local health system, but did not demonstrate a positive impact on maternal or neonatal mortality (32).
Similar interventions have potential to extend beyond the immediate postpartum period for long-term follow up of women at high risk of future CMDs. Future research should focus on rigorous evaluation of mHealth interventions beyond pilot studies (33), and include process evaluation and cost-effectiveness analyses, with a focus on local ownership and integration within existing health systems.
Risk stratification tools for pregnant women with preeclampsia have been developed for predicting risk of adverse maternal outcomes (34, 35). The full-Pre-eclampsia Integrated Estimate of RiSk model (fullPIERS) is a prediction model based on clinical history, signs and symptoms and laboratory tests. Developed in a High-Income Country (HIC) context, it has also been validated for use in low-resource settings (36, 37); however, is reliant on full laboratory-based support (35). A succinct version, based on symptoms and signs alone (miniPIERS), has also been developed for community-based risk assessment (34). These tools provide clinical decision support to frontline healthcare workers and may be integrated into mHealth platforms, such as the PIERS-on-the-move (POM) mHealth platform (38). A study of the POM mHealth platform, demonstrated good levels of acceptability, feasibility, and moderate utility for the prediction of adverse maternal outcomes in women with HDPs (39).
While these tools may be used for identification and risk-stratification of high-risk women during pregnancy, little evidence is currently available to calculate or predict long-term cardiovascular risk in this population (40). Robust data collection systems are needed for the long-term follow-up of women in LMICs to study the true prevalence and impact of high-risk conditions in pregnancy on future CMDs, and enable accurate risk stratification of high-risk women. It is unclear if existing cardiovascular risk prediction models could be improved through the addition of history of pregnancy complications (41, 42). There is a need for prognostic models using sample populations reflecting the diversity of target populations, and involving both nulliparous and multiparous women to better identify women at high-risk of CMD during and after pregnancy (40). Women who develop T2DM following GDM in some LMIC settings are more likely to exhibit certain characteristics such as increased body mass index postpartum, family history of T2DM, and certain ethnicities (43, 44). It is unclear, however, if these clinical features may be used to guide risk stratification of women with GDM and their progression to T2DM across other LMIC settings, as they are based on small-scale studies. A systematic review on the progression of GDM to T2DM concluded that a markedly raised fasting glucose level during pregnancy was most highly predictive of progression to T2DM, and did not support the use of features such as ethnicity, BMI, and family history of T2DM for risk stratification of progression to T2DM in pregnant women (45).
Task-Sharing in the Community
Task-sharing has potential to empower and engage community members, improve efficiency, and “expand the reach of delegated medical acts” (46). In areas with a shortage of doctors and nurses in LMICs, CHWs have been deployed to deliver interventions for the early detection of high-risk pregnant women (18, 47–49) and enable community-based data collection (50). CHWs have high levels of trust and respect within their communities, and motivate women to engage with antenatal care (51). Task-sharing relies upon continuous training and supervision, as CHWs may have limited literacy in low-resource settings (14).
The community-based management of hypertension in Nepal (COBIN) cluster randomized controlled trial in the general adult population of Nepal, established the effectiveness of a CHW-led home-based health education and screening for the reduction of Systolic BP (of almost 5 mmHg), in adults with hypertension; and amelioration of age-related increases in BP in adults without hypertension (52). Further examples of community-based programmes with potential to reduce cardiovascular risk in LMIC settings exist (53–56), however as the COBIN trial team concluded; long-term trials with hard clinical outcomes, such as myocardial infarction and stroke as primary endpoints are needed to confirm the effect of CHW-led interventions on cardiovascular mortality and morbidity (52). Important areas for future research would be to conduct adequately sized, robustly designed trials, demonstrating tangible impact upon mortality across the life-course, including cost effectiveness analyses, and exploration of the impact of climate change and seasonal variations on BP-related endpoints (57, 58).
LMIC-Based Data Repositories and Biobanks
Research associating pregnancy complications with CVD risk have, to date, been derived from linkage of large national data sets from high income countries (HIC) (41, 59, 60). Unlike HICs, the majority of CVD deaths in sub-Saharan Africa are due to stroke rather than ischemic heart disease (61), which may reflect differences in etiology. Currently, there are insufficient data on the life-long health of women living in LMIC settings. Encouraging collaboration across LMICs to form consortia for uniform women's health related data collection such as the COLLECT database for collaborative pregnancy and placental research (62), started by the Global Pregnancy Collaboration (CoLab) (63), might facilitate the use of big data analytics to enable risk stratification of women with pregnancy-related risk factors for CMDs in LMICs, and identify key timings for interventions.
With the fast-developing world of genomics, proteomics and metabolomics, LMICs might benefit from establishing biobanks. This would encourage locally-driven -omics research, based on the needs and priorities of LMICs, with local data ownership. South-south as well as north-south collaborations have potential to improve research into biomarkers for risk factors for CMD in women, including pregnancy-related risk factors such as preterm birth, pre-eclampsia, and GDM. The significant genetic variations in South Asian and African populations are important to furthering our knowledge of disease etiology and drug development. Currently, the majority of DNA used for research studies come from participants of European descent, with only 2% of data contributed from African data sets (64). In response, a new pan-Africa biobank start-up, 54-gene (64) and additionally, the first pan-Asia biobank have launched (65), both with the aim of solving the problem of lack of global representation. Similar initiatives are found in Brazil (66). Due to the heterogeneity of the samples and different collection strategies of existing biobanks, adequate skills training, capacity-building of LMIC-based researchers, and regulatory environments would need to be in place to support standardization of biobanks globally (63), as well as the infrastructure (such as 24-h electricity) for sample storage.
The Need for Life-Course Based Approaches to Women's Health
Health systems in low-resource settings are often designed to provide emergency services only. Preventative services, however, are fundamental to ensuring a healthy population. A recent study showed that each dollar spent on a package of essential preventive services leads to a net health gain of 1.8 dollars in India (67). Provision of integrated care for women throughout their life-course is one way in which women may be engaged within the health system at key intervals in their life. By using entry points (e.g., antenatal care) into the health system as opportunities to engage women, opportunistic screening for cardiometabolic risk factors might be feasible at critical points during the life-course.
There are few integrated care models that link antenatal care and non-communicable disease (NCD) prevention (68–71), although those demonstrating effectiveness for communicable diseases such as HIV and life-long health, exist (72, 73). Future research into how existing successful models of integrated care (such as the HIV programmes in sub-Saharan Africa) could be adapted for NCD prevention will be valuable in designing health systems responsive to the needs of women throughout their life-course.
There is a 2-fold increased risk of developing CVD, and a 3-5-fold risk of chronic hypertension in the decades following a pregnancy complicated by HDP (59, 74–77). The cumulative incidence of T2DM following GDM increases markedly within the first 5 years postpartum and plateaus after 10 years (45, 78). The American Heart Association (79) and the American Diabetes Association (80) have recommended incorporating pregnancy-related risk factors as part of screening for adult cardiovascular disease (81). Postpartum screening for ongoing problems with BP (79, 82) and glucose control through the use of an OGTT at 6–12 weeks postpartum and 1–3 yearly thereafter are advised (80). Despite these recommendations, most women with a complicated pregnancy do not routinely receive postpartum follow-up (83, 84) and certainly not for 5 years following the index pregnancy, when the long-term sequelae are likely to manifest.
Although challenges to postpartum screening of women are faced worldwide, there are specific contextual challenges in LMICs. Postnatal follow-up is lower in LMICs than in high income settings (85, 86). In low-resource settings, the burden of CMDs on daily life, household expenditure and economic stability have considerable implications for women and their entire household. Cultural practices after birth, workforce shortages, particularly in rural areas, and a lack of health system infrastructure are additional barriers to providing life-long care. Many women with hypertension and T2DM remain undiagnosed, although population-based screening for GDM shows high rates of conversion from GDM to T2DM in both urban and rural areas of LMICs (87). Further education and training of women and healthcare staff are needed to encourage postpartum follow-up and repeat testing of women at high risk of CMDs (84). Postpartum interventions targeting high-risk women might learn from adult NCD prevention programmes that have shown evidence of clinical benefit (88, 89). Successful lifestyle interventions are characterized by addressing more than one area of prevention and taking a holistic approach to change (90).
Improving Equity and Access to Affordable Treatments for CMDs
A study of 596 urban and rural communities in 18 countries concluded that improving the availability and affordability of medicines for CMDs is essential for increasing their uptake and use (91). This is of great importance for women identified at high-risk of CMDs early in their life-course. Although common medications for cardiovascular disease and diabetes are widely available in some LMICs, the out-of-pocket expenditure for households already struggling to meet their daily needs, is a significant barrier to their continued life-long use for women diagnosed with CMDs earlier in their life-course. The situation is even more pronounced in rural areas (92). Even with improved access to affordable medicines, there are significant socio-cultural barriers affecting compliance to lifelong treatment (93).
The WHO has committed to achieving the goal of 80% availability of affordable, essential medicines for NCDs by 2025 in their Global Action Plan (94). Current rates of medicine use for the secondary prevention of CVD are, however, substantially lower (91, 92, 95). The proportion of patients with coronary heart disease receiving medications for secondary prevention of CVD in 10 countries (including several LMICs) in the Prevention of REcurrences of Myocardial Infarction and StrokE (WHO-PREMISE) PREMISE study, was lower than 50% for all major classes of CVD prevention medicines, including beta blockers (48%), ACE inhibitors (40%), and statins (30%) (96).
Governments need to set policy objectives to ensure that essential medicines to stem the tide of the rising CMD epidemic are affordable and available to their populations, including those in rural areas, by ensuring continuity of supply chains, i.e., the manufacturing, transport and distribution of medicines. Multidisciplinary research is needed to explore socio-cultural barriers to prescribing and taking medications, if we are to ensure that women with, or at risk of CMDs in LMICs, receive essential care.
Discussion: The Research Agenda for LMICs
Only a multi-dimensional research strategy can help improve women's health in LMICs. Our review highlights the need for further well-designed experimental studies of novel technologies and biomarkers, embedded within the real-world context. Such studies would need to be adequately powered to demonstrate tangible benefit to clinical outcomes, such as maternal and neonatal mortality in the short-term, and cardiovascular endpoints in the longer term, and include cost-effectiveness analyses for future scalability. Data collection and monitoring are important strategies for improving healthcare provider practices (90). Future research should prioritize high-quality community-based data collection and linkage to existing hospital level health information systems, through prospective cohort studies with appropriate representation of women living in low-resource settings. Exploration of the complex social factors that impact the health of women both during pregnancy and beyond, with reference to CMDs has also been highlighted as a key area for future research (see Table 1).
The Academy of Medical Sciences have emphasized the need to develop locally driven solutions and diagnostics for NCDs, including disruptive technologies (17). Significant challenges include the commercially unattractive nature of research into novel low-cost diagnostics for low-resource settings, affecting the development and scalability of new diagnostic tests (17). Nevertheless, given that pregnancy complications associated with future CMDs still result in significant maternal mortality worldwide (97), there is a moral imperative to give women in LMICs the same access as those living in HICs to screening tests that predict life-threatening conditions in pregnancy, and beyond.
Author Contributions
SN was responsible for the conceptualisation of the mini-review and for writing the first draft. All authors contributed to the subsequent editing and review of the draft paper for publication.
Funding
SN is supported by an Medical Research Council Clinical Research Training Fellowship (MR/R017182/1). LH is supported by the NIHR Oxford Biomedical Research Centre (BRC), grant BRC-1215-20008 to the Oxford University Hospitals NHS Foundation Trust and the University of Oxford.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Sattar N, Greer IA. Pregnancy complications and maternal cardiovascular risk: opportunities for intervention and screening. BMJ. (2002) 325:157–60. doi: 10.1136/bmj.325.7356.157
2. Thilaganathan B, Kalafat E. Cardiovascular system in preeclampsia and beyond. Hypertension. (2019) 73:522–31. doi: 10.1161/HYPERTENSIONAHA.118.11191
3. Rich-Edwards JW, Fraser A, Lawlor DA, Catov JM. Pregnancy characteristics and women's future cardiovascular health: An underused opportunity to improve women's health? Epidemiol Rev. (2014) 36:57–70. doi: 10.1093/epirev/mxt006
4. Hauspurg A, Ying W, Hubel CA, Michos ED, Ouyang P. Adverse pregnancy outcomes and future maternal cardiovascular disease. Clin Cardiol. (2018) 41:239–46. doi: 10.1002/clc.22887
5. Heida KY, Bots ML, De Groot CJ, Van Dunné FM, Hammoud NM, Hoek A, et al. Cardiovascular risk management after reproductive and pregnancy-related disorders: A Dutch multidisciplinary evidence-based guideline. Eur J Prev Cardiol. (2016) 23:1863–79. doi: 10.1177/2047487316659573
6. Morken NH, Halland F, DeRoo LA, Wilcox AJ, Skjærven R. Offspring birthweight by gestational age and parental cardiovascular mortality: a population-based cohort study. BJOG. (2018) 125:336–41. doi: 10.1111/1471-0528.14522
7. Kristensen JH, Basit S, Wohlfahrt J, Damholt MB, Boyd HA. Pre-eclampsia and risk of later kidney disease: nationwide cohort study. BMJ. (2019) 365:1516. doi: 10.1136/bmj.l1516
8. Nahum Sacks K, Friger M, Shoham-Vardi I, Spiegel E, Sergienko R, Landau D, et al. Prenatal exposure to preeclampsia as an independent risk factor for long-term cardiovascular morbidity of the offspring. Pregnancy Hyper. (2018) 13:181–6. doi: 10.1016/j.preghy.2018.06.013
9. GenMed Cardiovascular Clinical Study Group, Regitz-Zagrosek V, Oertelt-Prigione S, Prescott E, Franconi F, et al. Gender in cardiovascular diseases: impact on clinical manifestations, management, and outcomes. Eur Heart J. (2015) 37:24–34. doi: 10.1093/eurheartj/ehv598
10. Miranda JJ, Barrientos-Gutiérrez T, Corvalan C, Hyder AA, Lazo-Porras M, Oni T, et al. Understanding the rise of cardiometabolic diseases in low-and middle-income countries. Nat Med. (2019) 25. doi: 10.1038/s41591-019-0644-7
11. World Health Organization. Trends in Maternal Mortality 2000 to 2017: Estimates by WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division. Available online at: https://apps.who.int/iris/handle/10665/327595 (Last accessed January 15, 2020).
12. Ralston J, Nugent R. Toward a broader response to cardiometabolic disease. Nat Med. (2019) 25:1644–6. doi: 10.1038/s41591-019-0642-9
13. Gupta R, Yusuf S. Challenges in management and prevention of ischemic heart disease in low socioeconomic status people in LLMICs. BMC Med. (2019) 17:209. doi: 10.1186/s12916-019-1454-y
14. Young N, Taegtmeyer M, Aol G, Bigogo GM, Phillips-Howard PA, Hill J, et al. Integrated point-of-care testing (POCT) of HIV, syphilis, malaria and anaemia in antenatal clinics in western Kenya: a longitudinal implementation study. PLoS ONE. (2018) 13:e0198784. doi: 10.1371/journal.pone.0198784
15. Poon LC, Shennan A, Hyett JA, Kapur A, Hadar E, Divakar H, et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: a pragmatic guide for first-trimester screening and prevention. Int J Gynecol Obstet. (2019) 145:1–33. doi: 10.1002/ijgo.12802
16. Katoba J, Kuupiel D, Mashamba-Thompson TP. Toward improving accessibility of point-of-care diagnostic services for maternal and child health in low-and middle-income countries. Point Care. (2019) 18:17–25. doi: 10.1097/POC.0000000000000180
17. The Academy of Medical Sciences. Improving the Development and Deployment of Rapid Diagnostic Tests in LMICs. Workshop report. (2016). p. 1–30. Available online at: https://acmedsci.ac.uk/file-download/18597310 (Last accessed January 15, 2020).
18. Nathan HL, Boene H, Munguambe K, Sevene E, Akeju D, Adetoro OO, et al. The CRADLE vital signs alert: qualitative evaluation of a novel device designed for use in pregnancy by healthcare workers in low-resource settings. Reprod Health. (2018) 15:5. doi: 10.1186/s12978-017-0450-y
19. Vousden N, Lawley E, Nathan HL, Seed PT, Brown A, Muchengwa T, et al. Evaluation of a novel vital sign device to reduce maternal mortality and morbidity in low-resource settings: a mixed method feasibility study for the CRADLE-3 trial. BMC Preg Childbirth. (2018) 18:115. doi: 10.1186/s12884-018-1737-x
20. Vousden N, Lawley E, Nathan HL, Seed PT, Gidiri MF, Goudar S, et al. Effect of a novel vital sign device on maternal mortality and morbidity in low-resource settings: a pragmatic, stepped-wedge, cluster-randomised controlled trial. Lancet Global Health. (2019) 7:e347–56. doi: 10.1016/S2214-109X(18)30526-6
21. Wirth M, Biswas N, Ahmad S, Nayak HS, Pugh A, Gupta T, et al. A prospective observational pilot study to test the feasibility of a smartphone enabled uChek© urinalysis device to detect biomarkers in urine indicative of preeclampsia/eclampsia. Health Technol. (2019) 9:31–6. doi: 10.1007/s12553-018-0248-0
22. Rood KM, Buhimschi CS, Dible T, Webster S, Zhao G, Samuels P, et al. Congo Red Dot Paper Test for Antenatal Triage and Rapid Identification of Preeclampsia. EClin Med. (2019) 8:47–56. doi: 10.1016/j.eclinm.2019.02.004
23. von Dadelszen P, Ansermino JM, Dumont G, Hofmeyr GJ, Magee LA, Mathai M, et al. Improving maternal and perinatal outcomes in the hypertensive disorders of pregnancy: a vision of a community-focused approach. Int J Gynecol Obstetr. (2012). 119:S30-4. doi: 10.1016/j.ijgo.2012.03.012
24. Bhavadharini B, Uma R, Saravanan P, Mohan V. Screening and diagnosis of gestational diabetes mellitus–relevance to low and middle income countries. Clin Diabet Endocrinol. (2016) 2:13. doi: 10.1186/s40842-016-0031-y
25. Anjalakshi C, Balaji V, Balaji MS, Ashalata S, Suganthi S, Arthi T, et al. A single test procedure to diagnose gestational diabetes mellitus. Acta Diabetol. (2009) 46:51–4. doi: 10.1007/s00592-008-0060-9
26. Mohan V, Mahalakshmi MM, Bhavadharini B, Maheswari K, Kalaiyarasi G, Anjana RM, et al. Comparison of screening for gestational diabetes mellitus by oral glucose tolerance tests done in the non-fasting (random) and fasting states. Acta Diabetol. (2014) 51:1007–13. doi: 10.1007/s00592-014-0660-5
27. Mishra S, Bhadoria A, Kishore S, Kumar R. Gestational diabetes mellitus 2018 guidelines: An update. J Fam Med Primary Care. (2018) 7:1169. doi: 10.4103/jfmpc.jfmpc_178_18
28. Balakrishnan R, Gopichandran V, Chaturvedi S, Chatterjee R, Mahapatra T, Chaudhuri I. Continuum of Care Services for Maternal and Child Health using mobile technology–a health system strengthening strategy in low and middle income countries. BMC Med Inform Deci Making. (2016) 16:84. doi: 10.1186/s12911-016-0326-z
29. Oyeyemi SO, Wynn R. Giving cell phones to pregnant women and improving services may increase primary health facility utilization: a case–control study of a Nigerian project. Reprod Health. (2014) 11:8. doi: 10.1186/1742-4755-11-8
30. Rivera-Romero O, Olmo A, Muñoz R, Stiefel P, Miranda ML, Beltrán LM. Mobile health solutions for hypertensive disorders in pregnancy: scoping literature review. JMIR mHealth and uHealth. (2018) 6:e130. doi: 10.2196/mhealth.9671
31. Carter J, Sandall J, Shennan AH, Tribe RM. Mobile phone apps for clinical decision support in pregnancy: a scoping review. BMC Med Inform Decis Making. (2019) 19:219. doi: 10.1186/s12911-019-0954-1
32. Modi D, Dholakia N, Gopalan R, Venkatraman S, Dave K, Shah S, et al. mHealth intervention “ImTeCHO” to improve delivery of maternal, neonatal, and child care services—A cluster-randomized trial in tribal areas of Gujarat, India. PLoS Med. (2019) 16:e1002939. doi: 10.1371/journal.pmed.1002939
33. Hall CS, Fottrell E, Wilkinson S, Byass P. Assessing the impact of mHealth interventions in low- and middle-income countries - what has been shown to work? Global Health Action. (2014) 7:1–12. doi: 10.3402/gha.v7.25606
34. Payne BA, Hutcheon JA, Ansermino JM, Hall DR, Bhutta ZA, Bhutta SZ, et al. A risk prediction model for the assessment and triage of women with hypertensive disorders of pregnancy in low-resourced settings: the miniPIERS (Pre-eclampsia Integrated Estimate of RiSk) multi-country prospective cohort study. PLoS Med. (2014) 11:e1001589. doi: 10.1371/journal.pmed.1001589
35. von Dadelszen P, Payne B, Li J, Ansermino JM, Pipkin FB, Côté AM, et al. Prediction of adverse maternal outcomes in pre-eclampsia: development and validation of the fullPIERS model. Lancet. (2011) 377:219–27. doi: 10.1016/S0140-6736(10)61351-7
36. Ukah UV, Payne B, Lee T, Magee LA, von Dadelszen P. External validation of the fullPIERS model for predicting adverse maternal outcomes in pregnancy hypertension in low-and middle-income countries. Hypertension. (2017) 69:705–11. doi: 10.1161/HYPERTENSIONAHA.116.08706
37. Agrawal S, Maitra N. Prediction of adverse maternal outcomes in preeclampsia using a risk prediction model. J Obstet Gynecol India. (2016) 66:104–11. doi: 10.1007/s13224-015-0779-5
38. Dunsmuir DT, Payne BA, Cloete G, Petersen CL, Görges M, Lim J, et al. Development of mHealth applications for pre-eclampsia triage. IEEE J Biomed Health Inform. (2014) 18:1857–64. doi: 10.1109/JBHI.2014.2301156
39. Lim J, Cloete G, Dunsmuir DT, Payne BA, Scheffer C, von Dadelszen P, et al. Usability and feasibility of PIERS on the move: an mHealth app for pre-eclampsia triage. JMIR mHealth. (2015) 3:e37. doi: 10.2196/mhealth.3942
40. Grandi SM, Smith GN, Platt RW. The relative contribution of pregnancy complications to cardiovascular risk prediction: are we getting it wrong? Circulation. (2019) 140:1965–7. doi: 10.1161/CIRCULATIONAHA.119.040917
41. Markovitz AR, Stuart JJ, Horn J, Williams PL, Rimm EB, Missmer SA, et al. Does pregnancy complication history improve cardiovascular disease risk prediction? Findings from the HUNT study in Norway. Eur Heart J. (2018) 40:1113–20. doi: 10.1093/eurheartj/ehy863
42. Stuart JJ, Tanz LJ, Cook NR, Spiegelman D, Missmer SA, Rimm EB, et al. Hypertensive disorders of pregnancy and 10-year cardiovascular risk prediction. J Am Coll Cardiol. (2018) 72:1252–63. doi: 10.1016/j.jacc.2018.05.077
43. Chivese T, Norris SA, Levitt NS. Progression to type 2 diabetes mellitus and associated risk factors after hyperglycemia first detected in pregnancy: A cross-sectional study in Cape Town, South Africa. PLoS Med. (2019) 16:e1002865. doi: 10.1371/journal.pmed.1002865
44. Herath H, Herath R, Wickremasinghe R. Gestational diabetes mellitus and risk of type 2 diabetes 10 years after the index pregnancy in Sri Lankan women—A community based retrospective cohort study. PLoS ONE. (2017) 12:e0179647. doi: 10.1371/journal.pone.0179647
45. Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. (2002) 25:1862–8. doi: 10.2337/diacare.25.10.1862
46. Vedanthan R, Bernabe-Ortiz A, Herasme OI, et al. Innovative approaches to hypertension control in low- and middle-income countries. Cardiol Clin. (2017) 35:99–115. doi: 10.1016/j.ccl.2016.08.010
47. Ramadurg U, Vidler M, Charanthimath U, Katageri G, Bellad M, Mallapur A, et al. Community health worker knowledge and management of pre-eclampsia in rural Karnataka State, India. Reprod Health. (2016) 13:113. doi: 10.1186/s12978-016-0219-8
48. Charanthimath U, Vidler M, Katageri G, Ramadurg U, Karadiguddi C, Kavi A, et al. The feasibility of task-sharing the identification, emergency treatment, and referral for women with pre-eclampsia by community health workers in India. Reprod Health. (2018) 15:101. doi: 10.1186/s12978-018-0532-5
49. Bellad MB, Vidler M, Honnungar Nv, Mallapur A, Ramadurg U, Charanthimath U, et al. Maternal and newborn health in Karnataka State, India: The community level interventions for pre-eclampsia (CLIP) trial's baseline study results. PLoS ONE. (2017) 12:e0166623. doi: 10.1371/journal.pone.0166623
50. Lewin S, Munabi-Babigumira S, Glenton C, Daniels K, Bosch-Capblanch X, van Wyk BE, et al. Lay health workers in primary and community health care for maternal and child health and the management of infectious diseases. Coch Database Syst Rev. (2010) 17: CD004015. doi: 10.1002/14651858.CD004015.pub3
51. Namazzi G, Okuga M, Tetui M, Muhumuza Kananura R, Kakaire A, Namutamba S, et al. Working with community health workers to improve maternal and newborn health outcomes: implementation and scale-up lessons from eastern Uganda. Global Health Action. (2017) 10:1345495. doi: 10.1080/16549716.2017.1345495
52. Neupane D, McLachlan CS, Mishra SR, Olsen MH, Perry HB, Karki A, et al. Effectiveness of a lifestyle intervention led by female community health volunteers versus usual care in blood pressure reduction (COBIN): an open-label, cluster-randomised trial. Lancet Global Health. (2018) 6:e66–73. doi: 10.1016/S2214-109X(17)30411-4
53. Cappuccio FP, Kerry SM, Micah FB, Plange-Rhule J, Eastwood JB. A community programme to reduce salt intake and blood pressure in Ghana [ISRCTN88789643]. BMC Public Health. (2006) 6:13. doi: 10.1186/1471-2458-6-13
54. Xavier D, Gupta R, Kamath D, Sigamani A, Devereaux PJ, George N, et al. Community health worker-based intervention for adherence to drugs and lifestyle change after acute coronary syndrome: a multicentre, open, randomised controlled trial. Lancet Diab Endocrinol. (2016) 4:244–53. doi: 10.1016/S2213-8587(15)00480-5
55. van de Vijver S, Oti S, Addo J, de Graft-Aikins A, Agyemang C. Review of community-based interventions for prevention of cardiovascular diseases in low- and middle-income countries. Ethnicity Health. (2012) 17:651–76. doi: 10.1080/13557858.2012.754409
56. Patel A, Praveen D, Maharani A, Oceandy D, Pilard Q, Kohli MPS, et al. Association of multifaceted mobile technology-enabled primary care intervention with cardiovascular disease risk management in rural indonesia. JAMA Cardiol. (2019) 4:978–86. doi: 10.1001/jamacardio.2019.2974
57. Lewington S, Li L, Sherliker P, Guo Y, Millwood I, Bian Z, et al. Seasonal variation in blood pressure and its relationship with outdoor temperature in 10 diverse regions of China: the China Kadoorie Biobank. J Hyper. (2012) 30:1383–91. doi: 10.1097/HJH.0b013e32835465b5
58. Peiris D, Praveen D, Mogulluru K, Ameer MA, Raghu A, Li Q, et al. SMARThealth India: a stepped-wedge, cluster randomised controlled trial of a community health worker managed mobile health intervention for people assessed at high cardiovascular disease risk in rural India. PLoS ONE. (2019) 14:e0213708. doi: 10.1371/journal.pone.0213708
59. Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. (2007) 335:974. doi: 10.1136/bmj.39335.385301.BE
60. Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. (2009) 373:1773–9. doi: 10.1016/S0140-6736(09)60731-5
61. Mensah GA, Roth GA, Sampson UKA, Moran AE, Feigin VL, Forouzanfar MH, et al. Mortality from cardiovascular diseases in sub-Saharan Africa, 1990-2013: a systematic analysis of data from the Global Burden of Disease Study (2013). Cardiovasc J Africa. (2015) 26:S6–10. doi: 10.5830/CVJA-2015-036
62. Myers J, Myatt L, Roberts J, Redman C. COLLECT, a collaborative database for pregnancy and placental research studies worldwide. BJOG. (2019) 126:8–10. doi: 10.1111/1471-0528.15393
63. Roberts JM, Mascalzoni D, Ness RB, Poston L. Collaboration to understand complex diseases: Preeclampsia and adverse pregnancy outcomes. Hypertension. (2016) 67:681–7. doi: 10.1161/HYPERTENSIONAHA.115.06133
64. Adepoju P. Africa's first biobank start-up receives seed funding. Lancet. (2019) 394:108. doi: 10.1016/S0140-6736(19)31614-9
65. Consortium G. The GenomeAsia 100K Project enables genetic discoveries across Asia. Nature. (2019). 576:106–11. doi: 10.1038/s41586-019-1793-z
66. de Oliveira L, Dias MAB, Jeyabalan A, Payne B, Redman CW, Mageee L, et al. Creating biobanks in low and middle-income countries to improve knowledge – The PREPARE initiative. Pregnancy Hyper. (2018) 13:62–4. doi: 10.1016/j.preghy.2018.05.007
67. Wu DC, Banzon EP, Gelband H, Chin B, Malhotra V, Khetrapal S, et al. Health-care investments for the urban populations, Bangladesh and India. Bull World Health Organ. (2020) 98:19–29. doi: 10.2471/BLT.19.234252
68. Rahman A, Abid M, Siham S, Roberts C, Creed F, Malik A, et al. Cognitive behaviour therapy-based intervention by community health workers for mothers with depression and their infants in rural Pakistan: a cluster-randomised controlled trial. Lancet. (2008) 372:902–9. doi: 10.1016/S0140-6736(08)61400-2
69. Sorsdahl K, Petersen Williams P, Everett-Murphy K, Vythilingum B, de Villiers P, Myers B, et al. Feasibility and preliminary responses to a screening and brief intervention program for maternal mental disorders within the context of primary care. Comm Mental Health J. (2015) 51:962–9. doi: 10.1007/s10597-015-9853-9
70. Honikman S, Heyningen T, van Field S, Baron E, Tomlinson M, van Heyningen T, et al. Stepped care for maternal mental health: a case study of the perinatal mental health project in South Africa. PLoS Med. (2012) 9:e1001222. doi: 10.1371/journal.pmed.1001222
71. Anyanwu L-JJC, Anyanwu OM, Yakubu AA. Missed opportunities for breast awareness information among women attending the maternal and child health services of an urban tertiary hospital in Northern Nigeria. J Cancer Res Ther. (2016) 12:765–9. doi: 10.4103/0973-1482.163791
72. Suthar AB, Hoos D, Beqiri A, Lorenz-Dehne K, McClure C, Duncomb C. Integrating antiretroviral therapy into antenatal care and maternal and child health settings: a systematic review and meta-analysis. Bull World Health Organ. (2013) 91:46–56. doi: 10.2471/BLT.12.107003
73. Tudor Car L, van Velthoven MHMMT, Brusamento S, Elmoniry H, Car J, Majeed A, et al. Integrating prevention of mother-to-child HIV transmission programs to improve uptake: a systematic review. PLoS ONE. (2012) 7:e35268. doi: 10.1371/journal.pone.0035268
74. Behrens I, Basit S, Melbye M, Lykke JA, Wohlfahrt J, Bundgaard H, et al. Risk of post-pregnancy hypertension in women with a history of hypertensive disorders of pregnancy: nationwide cohort study. BMJ. (2017) 2017:358. doi: 10.1136/bmj.j3078
75. Timpka S, Stuart JJ, Tanz LJ, Rimm EB, Franks PW, Rich-Edwards JW. Lifestyle in progression from hypertensive disorders of pregnancy to chronic hypertension in Nurses' Health Study II: observational cohort study. BMJ. (2017) 2017:358. doi: 10.1136/bmj.j3024
76. Grandi SM, Vallée-Pouliot K, Reynier P, Eberg M, Platt RW, Arel R, et al. Hypertensive disorders in pregnancy and the risk of subsequent cardiovascular disease. Paediatr Perinatal Epidemiol. (2017) 31:412–21. doi: 10.1111/ppe.12388
77. Brown MC, Best KE, Pearce MS, Waugh J, Robson SC, Bell R. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol. (2013) 28:1–19. doi: 10.1007/s10654-013-9762-6
78. Mahalakshmi M, Bhavadharini B, Kumar M, Anjana R, Shah S, Bridgette A, et al. Clinical profile, outcomes, and progression to type 2 diabetes among Indian women with gestational diabetes mellitus seen at a diabetes center in south India. Indian J Endocrinol Metab. (2014) 18:400–6. doi: 10.4103/2230-8210.131205
79. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults. J Am Coll Cardiol. (2018) 71:e127–248. doi: 10.1016/j.jacc.2017.11.006
80. American Diabetes Association. Management of diabetes in pregnancy: standards of Medical Care in Diabetes−2018. Diabetes Care. (2018). 41(Supplement 1):S137–43. doi: 10.2337/dc18-S013
81. Bushnell C, McCullough LD, Awad IA, Chireau MV, Fedder WN, Furie KL, et al. Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2014) 45:1545–88. doi: 10.1161/01.str.0000442009.06663.48
82. Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, Blomström-Lundqvist C, Cífková R, De Bonis M, et al. ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. (2018). 39:3165–241. doi: 10.1093/eurheartj/ehy340
83. Keely E. An opportunity not to be missed–how do we improve postpartum screening rates for women with gestational diabetes? Diabetes Metab Res Rev. (2012) 28:312–6. doi: 10.1002/dmrr.2274
84. Sanderson H, Loveman E, Colquitt J, Royle P, Waugh N, Tan B. Improving uptake of postnatal checking of blood glucose in women who had gestational diabetes mellitus in universal healthcare settings: a systematic review. J Clin Med. (2019) 8:4. doi: 10.3390/jcm8010004
85. Langlois E, Miszkurka M, Zunzunegui MMV, Ghaffar A, Ziegler D, Karp I, et al. Inequities in postnatal care in low- and middle-income countries: a systematic review and meta-analysis. Bull World Health Organ. (2015) 93:259–270. doi: 10.2471/BLT.14.140996
86. Warren C, Daly P, Toure L, Mongi P. Postnatal Care. Opportunities for Africa' s Newborns. Cape Town, South Africa: Partnership for Maternal, Newborn and Child Health. Geneva, Switzerland: PMNCH. (2006). p. 79-90.
87. Gupta Y, Kapoor D, Desai A, Praveen D, Joshi R, Rozati R, et al. Conversion of gestational diabetes mellitus to future Type 2 diabetes mellitus and the predictive value of HbA 1c in an Indian cohort. Diabetic Med. (2017) 34:37–43. doi: 10.1111/dme.13102
88. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. (2002) 346:393–403. doi: 10.1056/NEJMoa012512
89. Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia. (2006) 49:289–97. doi: 10.1007/s00125-005-0097-z
90. Rowe AK, Rowe SY, Peters DH, Holloway KA, Chalker J, Ross-Degnan D. Effectiveness of strategies to improve health-care provider practices in low-income and middle-income countries: a systematic review. Lancet Global Health. (2018) 6:e1163–75. doi: 10.1016/S2214-109X(18)30398-X
91. Attaei MW, Khatib R, McKee M, Lear S, Dagenais G, Igumbor EU, et al. Availability and affordability of blood pressure-lowering medicines and the effect on blood pressure control in high-income, middle-income, and low-income countries: an analysis of the PURE study data. Lancet Public Health. (2017) 2:e411–9. doi: 10.1016/S2468-2667(17)30141-X
92. Yusuf S, Islam S, Chow CK, Rangarajan S, Dagenais G, Diaz R, et al. Use of secondary prevention drugs for cardiovascular disease in the community in high-income, middle-income, and low-income countries (the PURE Study): A prospective epidemiological survey. Lancet. (2011) 378:1231–43. doi: 10.1016/S0140-6736(11)61215-4
93. Khatib R, Schwalm JD, Yusuf S, Haynes RB, McKee M, Khan M, et al. Patient and healthcare provider barriers to hypertension awareness, treatment and follow up: a systematic review and meta-analysis of qualitative and quantitative studies. PLoS ONE. (2014) 9:e84238. doi: 10.1371/journal.pone.0084238
94. World Health Organization. Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020. (2013). Available online at: https://apps.who.int/iris/bitstream/handle/10665/94384/9789241506236_eng.pdf?sequence=1 (accessed March 11, 2020).
95. Wirtz VJ, Kaplan WA, Kwan GF, Laing RO. Access to medications for cardiovascular diseases in low- and middle-income countries. Circulation. (2016) 133:2076–85. doi: 10.1161/CIRCULATIONAHA.115.008722
96. Mendis S, Abegunde D, Yusuf S, Ebrahim S, Shaper G, Ghannem H, et al. WHO study on Prevention of REcurrences of Myocardial Infarction and StrokE (WHO-PREMISE). Bull World Health Organ. (2005) 83:820–9. Available online at: https://www.who.int/bulletin/volumes/83/11/820.pdf (accessed March 11, 2020).
Keywords: high-risk pregnancy, preeclampsia, gestational diabetes, cardiometabolic disorders, cardiovascular disease
Citation: Nagraj S, Kennedy SH, Norton R, Jha V, Praveen D, Hinton L and Hirst JE (2020) Cardiometabolic Risk Factors in Pregnancy and Implications for Long-Term Health: Identifying the Research Priorities for Low-Resource Settings. Front. Cardiovasc. Med. 7:40. doi: 10.3389/fcvm.2020.00040
Received: 15 January 2020; Accepted: 03 March 2020;
Published: 20 March 2020.
Edited by:
Elsayed Z. Soliman, Wake Forest School of Medicine, United StatesReviewed by:
Marcelo Arruda Nakazone, Faculty of Medicine of São José Do Rio Preto, BrazilChristoph Sinning, Universitäres Herzzentrum Hamburg GmbH (UHZ), Germany
Copyright © 2020 Nagraj, Kennedy, Norton, Jha, Praveen, Hinton and Hirst. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shobhana Nagraj, c2hvYmhhbmEubmFncmFqQHdyaC5veC5hYy51aw==
 Shobhana Nagraj
Shobhana Nagraj Stephen H. Kennedy
Stephen H. Kennedy Robyn Norton2,3
Robyn Norton2,3 Jane E. Hirst
Jane E. Hirst