- Skane University Hospital, Lund University, Malmö, Sweden
With increasing age, the cardiovascular risk increases, as does frailty, with negative health consequences such as coronary disease, stroke, and vascular dementia. However, this aging process seems to take a more rapid course in some individuals, as reflected in the Early Vascular Aging (EVA) syndrome that over the recent 10 years has attracted increased attention. The core of the EVA syndrome is arterial stiffness in the media layer of large elastic arteries, a process that can be measured by pulse wave velocity, for example, along the aorta. Hypertension is a well-known cardiovascular risk factor in its own right, but also linked to the EVA process. However, several studies have shown that non-hemodynamic factors also contribute to arterial stiffness and EVA, such as impaired glucose metabolism, chronic inflammation, and oxidative stress. New perspectives have been introduced for linking early life programming affecting new-born babies and birth weight, with a later risk of hypertension, arterial stiffness and EVA. New drugs are being developed to treat EVA when lifestyle intervention and conventional risk factor controlling drugs are not enough. Finally, the opposite phenotype of EVA is Healthy Vascular Aging (HVA) or even Super Normal Vascular Aging (SUPERNOVA). If protective mechanisms can be found and mapped in these fortunate subjects with a slower than expected aging process, there could exist a potential to find new drug targets for preventive therapy.
Patients with essential hypertension have an increased risk of cardiovascular disease (CVD), not only because of the hemodynamic burden inflicted by elevated blood pressure, but also due to the fact that hypertension often clusters with a number of cardiovascular risk factors in the same individual as described by the Lancet Commission on Hypertension 2016 (1). This was previously often referred to as the Metabolic syndrome, based on its most recent definition in 2009 with its components of abdominal obesity, dyslipidaemia, hyperglycemia, and elevated blood pressure (2). As the concept of the Metabolic syndrome has been subject to criticism ever since 2005, mostly because of the fact that the syndrome is not more that its components for risk prediction (3), there is a need to find new concepts.
The so called Early Vascular Aging (EVA) syndrome was first described in 2008 (4, 5) and this has been followed by a variety of studies that explored cardiovascular aging as a fruitful concept to look for new mechanisms and treatment targets. The core component of EVA is supposed to be arterial stiffness as measured by elevated carotid-femoral pulse wave velocity (c-f PWV) along the aorta, which can now be measured directly with some modern technical devices (6). Aortic distensibility can also be determined by use of ultrasound and magnetic resonance imaging (MRI). Other indirect methods try to provide an estimate of PWV, but inherent technical shortcomings may preclude researchers from making a correct assessment. In a recent study in patients undergoing coronary angiography, who thereby had their central hemodynamics measured at the same time, it was shown that most devices providing a direct measurement of central hemodynamic and aortic c-f PWV are reliable, but not all devices provided indirect measurements; for example, using age and systolic blood pressure in their algorithm for indirect estimation of aortic PWV (7).
Determinants of Arterial Stiffness
Population-based studies have shown that close correlations exist between the level of blood pressure and the degree of arterial stiffness (aortic PWV); the higher the brachial or central blood pressure, the higher the aortic PWV (Table 1). As hemodynamic factors play an important role for the morphology and functionality of the arterial wall, there is no doubt that hypertension is a crucial factor for determining the level of arterial stiffness c-f PWV) (8). However, some studies have also documented that a number of non-hemodynamic factors, linked to glucose metabolism and chronic inflammation, are also of importance for PWV (9, 10). Prospective studies have indicated that not only can increased blood pressure predict future arterial stiffness (PWV), but that arterial stiffness can also predict incident hypertension (11, 12), as well as incident type 2 diabetes (13). This shows the close association between these entities. In fact, genetic studies have documented that a genetic risk score for hyperglycemia, as a phenotypic trait in the normal, non-diabetic, elderly population, is independently associated with arterial stiffness (c-f PWV) (14). If a true causal mechanism exists, this could imply that more focused treatment directed toward this mechanism could also alleviate arterial stiffness beyond the effect of blood pressure lowering per se.
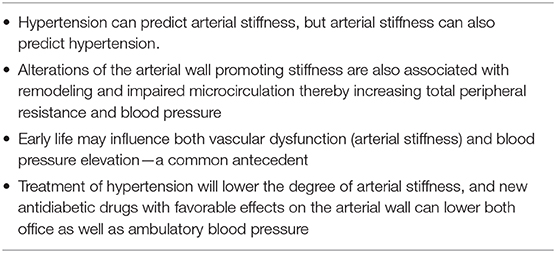
Table 1. The links between hypertension and Early Vascular Aging (EVA) with arterial stiffness as core feature.
How to Define EVA?
The definition of EVA has recently been discussed (15), but no established definition is yet available. However, as increased c-f PWV is the core feature of EVA one can try to define EVA as the upper 10, 20, or 25% of the c-f PWV distribution in relation to a background population. For example, a reference population in Europe with PWV exists for comparison (16). Several studies and meta-analyses have documented that PWV is predictive of fatal and non-fatal CVD events, but also of total mortality (17–19). It seems that the predictive power of PWV is more pronounced in middle-aged subjects, compared to the elderly, where selective survival bias may influence the observational findings (18). Differential aging in general, and of the vasculature in particular, may be more visible in middle-aged subjects and this is linked to risk. European guidelines therefore mention the usefulness of determination of arterial stiffness (PWV), although with a lower level of evidence than, for example, the measurement of blood pressure—an established risk factor with intervention studies showing clear benefits (20). As impaired glucose metabolism is closely associated with vascular aging when measured by PWV (9, 10) it makes sense to offer the measurement of fasting glucose or even an oral glucose tolerance test (OGTT, 75 g glucose) to risk subjects, for example, following a myocardial infarction. Even if chronic inflammation seems to be of importance for EVA there is no consensus today whether inflammatory biomarkers should be measured in the clinic or not.
Mechanisms of Importance to Modify Arterial Stiffness
Several studies have documented the association of chronic inflammation with increased arterial stiffness and PWV; for example, rheumatoid arthritis and inflammatory bowel disease, i.e., ulcerous colitis and morbus Crohn (21). It is suggested that chronic inflammation and increased oxidative stress will have a negative impact the proteins and structure of the arterial wall, but also on impaired vasodilation. A recent review has documented the importance of vascular smooth muscle cell (VSMC) changes in relation to arterial stiffness (22). These authors state that the first components that contribute to arterial stiffening are extracellular matrix (ECM) proteins that support the mechanical load, while the second important components are VSMCs, which not only regulate actomyosin interactions for contraction but mediate also so-called mechanotransduction in cell-ECM homeostasis. It seems that VSMC plasticity and signaling in both conductance and resistance arteries are highly relevant to the physiology of normal and EVA (22). This process also involves the architecture of cytoskeletal proteins and focal adhesion, the large/small arteries cross-talk that gives rise to target organ damage, and inflammatory pathways leading to calcification or atherosclerosis (22).
Factors in Early Life Influencing Arterial Stiffness and EVA
A new aspect of EVA is the hypothesis that early life factors such as fetal growth, birth weight adjusted for gestational age, prematurity, and post-natal growth patterns, could influence both arterial stiffness and blood pressure regulation, as measured by PWV (23) or Augmentation index (Aix), another but more complex marker of stiffness and central hemodynamics, as well as total peripheral resistance (24). In one recent study from Austria these early life factors were found to be predictive of EVA in adolescents (mean age 16 years) (25). There is an ongoing debate trying to clarify whether genetic factors form the basis of the link between parental hypertension and the same trait's presence in their offspring—when low birth weight is just a marker of the trait (26)—or whether environmental factors play the most important role, i.e., maternal diet (27), calorie intake, and lifestyle (smoking, alcohol). Probably genetic factors form a background structure, whereas environmental factors can play a modifying role (epigenetics) for the phenotypic outcome.
Vascular Aging and the Brain
Hypertension is a well-documented risk factor for stroke and other cerebrovascular disease manifestations such as microangiopathy and white matter lesions (WML), often affecting the elderly. Also, arterial stiffness can contribute to these pathologies in different ways (28, 29). One result is the impaired cognition and increased risk of dementia in these patients. Thus, hypertension and EVA may cause more damage in the population at large than visible from hospital statistics of stroke alone. If impaired cognition and dementia occurs several years earlier than in people without these risk conditions, this will substantially impact actual daily living (ADL) capacities and independence of care in aging populations.
Treatment of EVA and Hypertension
The treatment of EVA and hypertension is based on an improved healthy lifestyle and treatment of conventional risk factors, based on current best evidence as shown in both European (20) and US guidelines (30). Several observational studies have indicated that antihypertensive treatment may reduce arterial stiffness (PWV) beyond the blood pressure reduction itself, when blockers of the renin-angiotensin-aldosterone system (RAS) in particular seem to be of great value (31). Recently, in the SPRINT study an estimated PWV (ePWV) was shown to be lowered by relatively more than the intensive blood pressure control implicated in the intervention arm (32). These results suggest that, in this trial, ePWV predicted outcomes independent of the Framingham Risk Score (where blood pressure is included), indicating an incremental role of markers of aortic stiffness on cardiovascular risk. The authors concluded that better survival of individuals whose ePWV responded to antihypertensive treatment independently of systolic blood pressure reduction suggests a role of markers of aortic stiffness as effective treatment targets in individuals with hypertension (32).
The ultimate proof of the usefulness of PWV for risk stratification and as a target for therapy will come with the SPARTE study in France where risk individuals have been randomized to either a treatment strategy aimed to lower PWV or to conventional treatment aiming for multiple risk factor intervention and control based on guidelines (33). SPARTE has been underway for a few years and the results should be reported during 2020.
Interestingly enough, it has been shown that newer anti-diabetes drugs such as the SGLT-2 inhibitor empagliflozin may lower both office and ambulatory blood pressure (34), and in addition show beneficial effects on Aix—a marker of aortic dysfunction—in patients with type 2 diabetes (35).
Look for Protection, Not Only Risk!
A very novel aspect of EVA is to turn it around and look for factors that protect from EVA and are associated with Healthy Vascular Aging (HVA) (36–38), or even Super Normal Vascular Aging (SUPERNOVA) (39). If such protection from vascular aging could be better defined and understood, based on advanced phenotyping using genetics and omics, there is a potential to find new drug targets of protection. Also, other models of protection exist, but are poorly understood; for example the astonishing lack of major complications in a few patients with type 1 diabetes with more than 40–50 years of diabetes (40–42). It would be interesting to further examine vascular function and the escape from hemodynamic aging in these fortunate subjects (43). Another example includes obese subjects who were not hospitalized for decades in mid-life in spite of risk factors and drug treatment (44, 45). Some of them seem to be “fat and fit” as a way to cope with obesity and its risks. Even if obesity is a strong risk factor for the development of type 2 diabetes, many of these subjects with “Metabolically Healthy Obesity” (HMO) escape diabetes. Even if they are not thought to be protected from complications in the long run, such HMO subjects may benefit from a postponement of complications for a substantial time.
Conclusions
In conclusion, the EVA concept has enriched translational research activities to find new mechanisms of CVD risk (46) and also inspired researchers to find new treatment targets to further lower the CVD risk beyond what can be achieved by conventional risk factor control. Screening of EVA has been tried based on measurements at pharmacies offered to the public (47) and seems to be feasible. The most recent development is focused on the opposite of EVA, namely, Healthy Vascular Aging (HVA) or even Super Normal Vascular Aging (SUPERNOVA) in order to understand vascular protection and find new treatment targets.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Funding
This review was supported by grants from the Swedish Research Council (Grant no. 521-2013-2756) and Heart- and Lung Foundation (Grant no. 2015-0427) to PN.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Olsen MH, Angell SY, Asma S, Boutouyrie P, Burger D, Chirinos JA, et al. A call to action and a lifecourse strategy to address the global burden of raised blood pressure on current and future generations: the Lancet Commission on hypertension. Lancet. (2016) 388:2665–712. doi: 10.1016/S0140-6736(16)31134-5
2. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. (2009) 120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644
3. Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal. Joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. (2005) 48:1684–99. doi: 10.1007/s00125-005-1876-2
4. Nilsson PM. Early vascular aging (EVA): consequences and prevention. Vasc Health Risk Manag. (2008) 4:547–52. doi: 10.2147/VHRM.S1094
5. Nilsson PM, Lurbe E, Laurent S. The early life origins of vascular ageing and cardiovascular risk: the EVA syndrome. J Hypertens. (2008) 26:1049–57. doi: 10.1097/HJH.0b013e3282f82c3e
6. Vlachopoulos C, Xaplanteris P, Aboyans V, Brodmann M, Cífková R, Cosentino F, et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: Endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis. (2015) 241:507–32. doi: 10.1016/j.atherosclerosis.2015.05.007
7. Salvi P, Scalise F, Rovina M, Moretti F, Salvi L, Grillo A, et al. Noninvasive estimation of aortic stiffness through different approaches. Hypertension. (2019) 74:117–29. doi: 10.1161/HYPERTENSIONAHA.119.12853
8. Nilsson PM, Boutouyrie P, Laurent S. Vascular aging: a tale of EVA and ADAM in cardiovascular risk assessment and prevention. Hypertension. (2009) 54:3–10. doi: 10.1161/HYPERTENSIONAHA.109.129114
9. Gottsäter M, Östling G, Persson M, Engström G, Melander O, Nilsson PM. Non-hemodynamic predictors of arterial stiffness after 17 years of follow-up: the Malmö Diet and Cancer study. J Hypertens. (2015) 33:957–65. doi: 10.1097/HJH.0000000000000520
10. Guzik TJ, Touyz RM. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension. (2017) 70:660–7. doi: 10.1161/HYPERTENSIONAHA.117.07802
11. Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, et al. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. (2012) 308:875–81. doi: 10.1001/2012.jama.10503
12. AlGhatrif M, Strait JB, Morrell CH, Canepa M, Wright J, Elango P, et al. Longitudinal trajectories of arterial stiffness and the role of blood pressure: the Baltimore Longitudinal Study of Aging. Hypertension. (2013) 62:934–41. doi: 10.1161/HYPERTENSIONAHA.113.01445
13. Muhammad IF, Borné Y, Östling G, Kennbäck C, Gottsäter M, Persson M, et al. Arterial stiffness and incidence of diabetes: a population-based cohort study. Diabetes Care. (2017) 40:1739–45. doi: 10.2337/dc17-1071
14. Gottsäter M, Hindy G, Orho-Melander M, Nilsson PM, Melander O. A genetic risk score for fasting plasma glucose is independently associated with arterial stiffness: a Mendelian randomization study. J Hypertens. (2018) 36:809–14. doi: 10.1097/HJH.0000000000001646
15. Cunha PG, Boutouyrie P, Nilsson PM, Laurent S. Early vascular ageing (EVA): definitions and clinical applicability. Curr Hypertens Rev. (2017) 13:8–15. doi: 10.2174/1573402113666170413094319
16. Reference Values for Arterial Stiffness' Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: 'establishing normal and reference values'. Eur Heart J. (2010) 31:2338–50. doi: 10.1093/eurheartj/ehq165
17. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. (2010) 55:1318–27. doi: 10.1016/j.jacc.2009.10.061
18. Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. (2014) 63:636–46. doi: 10.1016/j.jacc.2013.09.063
19. Zhong Q, Hu MJ, Cui YJ, Liang L, Zhou MM, Yang YW, et al. Carotid-femoral pulse wave velocity in the prediction of cardiovascular events and mortality: an updated systematic review and meta-analysis. Angiology. (2018) 69:617–29. doi: 10.1177/0003319717742544
20. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. (2018) 36:1953–2041. doi: 10.1097/HJH.0000000000001940
21. Zanoli L, Rastelli S, Granata A, Inserra G, Empana JP, Boutouyrie P, et al. Arterial stiffness in inflammatory bowel disease: a systematic review and meta-analysis. J Hypertens. (2016) 34:822–9. doi: 10.1097/HJH.0000000000000867
22. Lacolley P, Regnault V, Segers P, Laurent S. Vascular smooth muscle cells and arterial stiffening: relevance in development, aging, and disease. Physiol Rev. (2017) 97:1555–617. doi: 10.1152/physrev.00003.2017
23. Visentin S, Grumolato F, Nardelli GB, Di Camillo B, Grisan E, Cosmi E. Early origins of adult disease: low birth weight and vascular remodeling. Atherosclerosis. (2014) 237:391–9. doi: 10.1016/j.atherosclerosis.2014.09.027
24. Sperling J, Nilsson PM. Does early life programming influence arterial stiffness and central hemodynamics in adulthood? J Hypertens. (2019). doi: 10.1097/HJH.0000000000002292. [Epub ahead of print].
25. Stock K, Schmid A, Griesmaier E, Gande N, Hochmayr C, Knoflach M, et al. The impact of being born preterm or small for gestational age on early vascular aging in adolescents. J Pediatr. (2018) 201:49–54. doi: 10.1016/j.jpeds.2018.05.056
26. Warrington NM, Beaumont RN, Horikoshi M, Day FR, Helgeland Ø, Laurin C, et al. Maternal and fetal genetic effects on birth weight and their relevance to cardio-metabolic risk factors. Nat Genet. (2019) 51:804–14. doi: 10.1038/s41588-019-0403-1
27. Symonds ME, Stephenson T, Budge H. Early determinants of cardiovascular disease: the role of early diet in later blood pressure control. Am J Clin Nutr. (2009) 89:1518S−22S. doi: 10.3945/ajcn.2009.27113F
28. Henskens LH, Kroon AA, van Oostenbrugge RJ, Gronenschild EH, Fuss-Lejeune MM, Hofman PA, et al. Increased aortic pulse wave velocity is associated with silent cerebral small-vessel disease in hypertensive patients. Hypertension. (2008) 52:1120–6. doi: 10.1161/HYPERTENSIONAHA.108.119024
29. Savoia C, Battistoni A, Calvez V, Cesario V, Montefusco G, Filippini A. Microvascular alterations in hypertension and vascular aging. Curr Hypertens Rev. (2017) 13:16–23. doi: 10.2174/1573402113666170505115010
30. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. (2019) 140:e563–95. doi: 10.1161/CIR.0000000000000677
31. Ong KT, Delerme S, Pannier B, Safar ME, Benetos A, Laurent S, et al. Aortic stiffness is reduced beyond blood pressure lowering by short-term and long-term antihypertensive treatment: a meta-analysis of individual data in 294 patients. J Hypertens. (2011) 29:1034–42. doi: 10.1097/HJH.0b013e328346a583
32. Vlachopoulos C, Terentes-Printzios D, Laurent S, Nilsson PM, Protogerou AD, Aznaouridis K, et al. Association of estimated pulse wave velocity with survival: a secondary analysis of SPRINT. JAMA Netw Open. (2019) 2:e1912831. doi: 10.1001/jamanetworkopen.2019.12831
33. Laurent S, Schlaich M, Esler M. New drugs, procedures, and devices for hypertension. Lancet. (2012) 380:591–600. doi: 10.1016/S0140-6736(12)60825-3
34. Mancia G, Cannon CP, Tikkanen I, Zeller C, Ley L, Woerle HJ, et al. Impact of empagliflozin on blood pressure in patients with type 2 diabetes mellitus and hypertension by background antihypertensive medication. Hypertension. (2016) 68:1355–64. doi: 10.1161/HYPERTENSIONAHA.116.07703
35. Chilton R, Tikkanen I, Cannon CP, Crowe S, Woerle HJ, Broedl UC, et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab. (2015) 17:1180–93. doi: 10.1111/dom.12572
36. Niiranen TJ, Lyass A, Larson MG, Hamburg NM, Benjamin EJ, Mitchell GF, et al. Prevalence, correlates, and prognosis of healthy vascular aging in a western community-dwelling cohort: the Framingham Heart Study. Hypertension. (2017) 70:267–74. doi: 10.1161/HYPERTENSIONAHA.117.09026
37. Nilsson PM, Laurent S, Cunha PG, Olsen MH, Rietzschel E, Franco OH, et al. Characteristics of healthy vascular ageing in pooled population-based cohort studies: the global Metabolic syndrome and Artery Research Consortium. J Hypertens. (2018) 36:2340–9. doi: 10.1097/HJH.0000000000001824
38. Ji H, Teliewubai J, Lu Y, Xiong J, Yu S, Chi C, et al. Vascular aging and preclinical target organ damage in community-dwelling elderly: the Northern Shanghai Study. J Hypertens. (2018) 36:1391–8. doi: 10.1097/HJH.0000000000001692
39. Laurent S, Boutouyrie P, Cunha PG, Lacolley P, Nilsson PM. Concept of extremes in vascular aging. Hypertension. (2019) 74:218–28. doi: 10.1161/HYPERTENSIONAHA.119.12655
40. Bain SC, Gill GV, Dyer PH, Jones AF, Murphy M, Jones KE, et al. Characteristics of Type 1 diabetes of over 50 year's duration (the Golden Years Cohort). Diabet Med. (2003) 20:808–11. doi: 10.1046/j.1464-5491.2003.01029.x
41. Sun JK, Keenan HA, Cavallerano JD, Asztalos BF, Schaefer EJ, Sell DR, et al. Protection from retinopathy and other complications in patients with type 1 diabetes of extreme duration: the Joslin 50-year medalist study. Diabetes Care. (2011) 34:968–974. doi: 10.2337/dc10-1675
42. Adamsson Eryd S, Svensson AM, Franzén S, Eliasson B, Nilsson PM, Gudbjörnsdottir S. Risk of future microvascular and macrovascular disease in people with Type 1 diabetes of very long duration: a national study with 10-year follow-up. Diabet Med. (2017) 34:411–8. doi: 10.1111/dme.13266
43. Nilsson PM. Hemodynamic aging as the consequence of structural changes associated with early vascular aging (EVA). Aging Dis. (2014) 5:109–13. doi: 10.14336/AD.2014.0500109
44. Tremmel M, Lyssenko V, Zöller B, Engström G, Magnusson M, Melander O, et al. Characteristics and prognosis of healthy severe obesity (HSO) subjects – The Malmo Preventive Project. Obesity Med. (2018) 11:6–12. doi: 10.1016/j.obmed.2018.06.005
45. Korduner J, Bachus E, Jujic A, Magnusson M, Nilsson PM. Metabolically Healthy Obesity (MHO) in the Malmö Diet Cancer Study – epidemiology and prospective risks. Obes Res Clin Pract. (2019) 13:548–54. doi: 10.1093/eurheartj/ehz748.0079
46. Nilsson PM, Boutouyrie P, Cunha P, Kotsis V, Narkiewicz K, Parati G, et al. Early vascular ageing in translation: from laboratory investigations to clinical applications in cardiovascular prevention. J Hypertens. (2013) 31:1517–26. doi: 10.1097/HJH.0b013e328361e4bd
Keywords: aging, artery, glucose, hypertension, inflammation, oxidative stress
Citation: Nilsson PM (2020) Early Vascular Aging in Hypertension. Front. Cardiovasc. Med. 7:6. doi: 10.3389/fcvm.2020.00006
Received: 19 October 2019; Accepted: 14 January 2020;
Published: 04 February 2020.
Edited by:
Michel Burnier, Lausanne University Hospital (CHUV), SwitzerlandReviewed by:
Carmine Savoia, Sapienza University of Rome, ItalyStefano Omboni, Istituto Italiano di Telemedicina, Italy
Copyright © 2020 Nilsson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter M. Nilsson, cGV0ZXIubmlsc3NvbiYjeDAwMDQwO21lZC5sdS5zZQ==
 Peter M. Nilsson
Peter M. Nilsson