
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Cardiovasc. Med. , 19 November 2019
Sec. Structural Interventional Cardiology
Volume 6 - 2019 | https://doi.org/10.3389/fcvm.2019.00168
This article is part of the Research Topic Structural Valve Degeneration and Failure in Transcatheter and Surgical Bioprosthesis View all 6 articles
Transcatheter aortic valve-in-valve replacement has been recently reported as a less-invasive alternative to re-do surgery in patients with bioprosthetic valve failure. Although procedural success is achieved in the great majority of patients, this therapy is associated with several potential complications, and coronary occlusion is one of the most feared. This is a rare event, but it is associated with an extremely poor prognosis. In this review, the mechanisms, the identification of patients at high risk, the primary prevention strategies, and treatment of coronary occlusion will be discussed.
In the last two decades, there has been a trend toward a greater prevalence of bioprosthetic heart valves use even in younger patients (1–3). Recently, transcatheter aortic valve replacement (TAVR) has emerged as a non-inferior or superior alternative to medical treatment or surgical aortic valve replacement (SAVR) in high, intermediate and low-risk patients when transfemoral approach is available (4). All surgical bioprostheses have limited durability and usually fail within 10–15 years (5, 6). Similarly, transcatheter heart valve (THV) durability up to 6 years is already a well-established reality, with low rates of structural valve dysfunction (SVD) demonstrated in large and methodologically rigorous studies (2). However, data on clinical outcomes and THV integrity after 6 years remain very scarce. Increased life expectancy and the use of the bioprosthetic valve in younger patients led to an increased incidence of bioprosthetic valve failure (7).
In this context, implantation of a THV inside the failed aortic bioprosthetic valve (valve-in-valve, ViV) has emerged as an effective and less invasive treatment for degenerated aortic bioprostheses (8). Although procedural success is achieved in the great majority of patients, this procedure is associated with several potential complications (9), being coronary occlusion one of the most impactful on acute patients' prognosis (10). In this review, the mechanisms, the identification of patients at high risk, the primary prevention strategies used during pre-TAVR ViV screening and during the procedure itself to avoid coronary occlusion will be discussed.
Coronary occlusion is a rare complication after TAVR-ViV procedures, but it is associated with a very high mortality rate (9). It is four- to six-fold more common in ViV procedures than TAVR in native valves, and a higher risk of delayed coronary occlusion has been also reported with self-expanding TAVR devices (11).To date, the incidence of coronary occlusion during TAVR is 0.5% in the Transcatheter Valve Therapy Registry data and 2.3% in the Valve-in-Valve International Data registry, with related in-hospital mortality of ~50% (12, 13). However, this phenomenon could be even underestimated because it can be incomplete and not well-recognized.
When coronary occlusion does occur, the clinical presentation is usually characterized by ST-segment changes, severe hypotension and procedural ventricular arrhythmias that may require temporary cardiopulmonary support and revascularization (14, 15). Similar to TAVR in native aortic stenosis, meticulous pre-procedural planning is necessary to avoiding peri and post-procedural complications. Assessing the risk of coronary occlusion requires a deep understanding of the involved mechanisms. In TAVR-ViV procedures, coronary occlusion usually occurs when a bioprosthetic leaflet comes in contact with the coronary ostia or when the bioprosthetic valve contacts the aortic wall above a coronary ostium at the level of the aortic root as the positioned THV creates a covered cylinder in the root or the aorta (16). This is common to all THV and is correlated with the different characteristics of surgical heart valve (SHV) and the relationship of its bioprosthetic leaflets with the coronary ostia.
A recent study evaluating the safety and efficacy of TAVR-ViV for stentless bioprosthetic aortic valves showed that subcoronary implant technique, short simulated radial valve-to-coronary distance and low coronary height are predictors of coronary occlusion (17).
Considering all these aspects, it is crucial to know the patient's anatomy before a ViV. The main predisposing factor in ViV procedures is the proximity of a coronary ostium to the anticipated final position of the displaced bioprosthetic leaflets after the new THV implantation (18), calculated by the virtual THV to coronary distance (VTC). This is a CT-obtained predictor of the proximity of the coronary ostia to the anticipated final position of the displaced bioprosthetic leaflets after THV implantation. It added the THV size to the classical risk factors of coronary ostia height and sinus width, taking into account also the THV tilting into the aortic annulus (16). To calculate the VTC, it is first necessary to identify the basal ring plane and the geometric center of the SHV. Then, a virtual cylinder that represents the THV is placed and aligned in the middle of the basal ring. At the end, the horizontal distance between the ostia of the coronary arteries and the edge of this cylinder is measured with a tool of the CT imaging software (16). If it is <4 mm the risk is maximum, between 4 and 6 mm is borderline and >6 mm is a low risk for coronary occlusion (Figure 1) (16). Furthermore, others possible risk factors for coronary occlusion after a ViV implantation may be determined by anatomic factors such as narrow sinuses of Valsalva and sinotubular junction, bioprosthetic valve factors such as supra-annular position, high leaflet profile, THV factors such as extended sealing cuff of high THV implantation, and finally internal stent frame (e.g., Mitroflow, Trifecta) or no stent frame (homograft, stentless valve) and bulky leaflets (19).
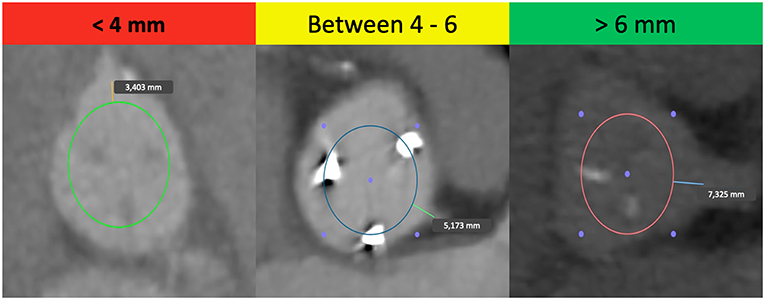
Figure 1. Examples of the “virtual THV to coronary artery” distance (VTC) in high-risk, borderline and low-risk patients for coronary occlusion in TAVR-ViV procedure.
A meticulous understanding of the differences of SHVs design is important to allow the optimal selection of THV and to prevent coronary occlusion.
Coronary occlusion may be more common in stentless bioprosthetic valves or those that are internally stented (e.g., Mitroflow or Trifecta) because the leaflets of these bioprostheses may extend outward in tubular fashion after a ViV procedure beyond the surgical device frame (10). Furthermore, it is crucial to know the nature of surgical implantation. In the subcoronary technique, the risk of coronary obstruction is greater than in full root replacement, because the suture line between the stentless prosthesis and the aorta is closer to the native coronary ostia (Figure 2) (20). In the case of stented SHVs, it is important to consider the “true internal diameter” for the choice of THV size and type.
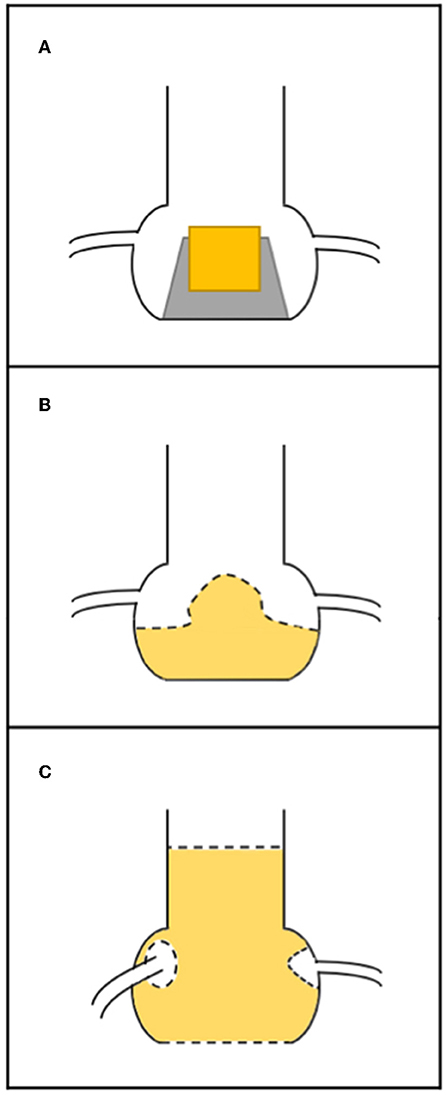
Figure 2. Relationship between surgical bioprosthesis and the coronary ostia. (A) Stented valve; (B) stentless valve replaced as subcoronary implantation (the dashed line represents the suture); (C) stentless valve replaced as full root, with suture line (dashed line) at the level of annulus and the coronary ostia have been reimplanted higher up.
Sizing, position and type of implanted THV may influence the risk of coronary occlusion after ViV. The selection of a small diameter THVs or intentionally under-expanded balloon-expandable THVs may result in less lateral displacement of surgical valve leaflets and posts with a minor risk of coronary occlusion after ViV (21).
Accurate positioning of the THV is essential for achieving good procedural results. The recently developed Valve in Valve Aortic App provides a very useful guide for THV positioning in available bioprostheses (22). In patient with high risk of coronary occlusion, a lower positioning of THV within the bioprostheses may cause less outward displacement of the surgical valve leaflets and posts than a THV implanted high.
The choice of the THV type for ViV-TAVR procedures should be individualized for each patient. The assessment of the risk for coronary occlusion may indeed influence THV selection. THV device that could be immediately retrieved after partial device implantation is advantageous (e.g., Lotus, Portico, Evolut-R, etc.). Furthermore, new-generation, fully retrievable THV devices or those with aortic leaflet clipping (JenaValve; JenaValve Technology GmbH, Munich, Germany) may be preferable if the risk of coronary occlusion is estimated to be high (23). The benefit of using devices with clipping mechanism in high-risk cases for coronary occlusion should be studied further.
The management in patients with a coronary occlusion by a displaced bioprosthetic valve leaflet after ViV represents a challenge because they are commonly unstable and delivery of wire and successively a stent toward the coronary vasculature may be very challenging. In patients at high risk of coronary occlusion, it is essential to implement invasive primary prevention strategies, such as coronary protection with a supportive coronary guidewire (BHW, Abbott, Vascular) and an undeployed balloon or stent in the periphery of the left anterior descending in order to be ready to inflate the balloon or to place the stent at the coronary ostium to maintain coronary flow in case of its impairment (Figure 3) (19, 24). If the patency of the coronary cannot be restored and the hemodynamic is poor, the valve should instantly be snared (MCV), or removed from its anatomical position by using an oversized balloon (i.e., ESV prosthesis) and pulled up out into the ascending aorta to maintain coronary flow (18). However, ostial stenting after a leaflet obstruction is related with delayed coronary occlusion, high restenosis risk, difficulty re-engaging the coronary ostia, and difficulty retrieving undeployed stents (19, 25).
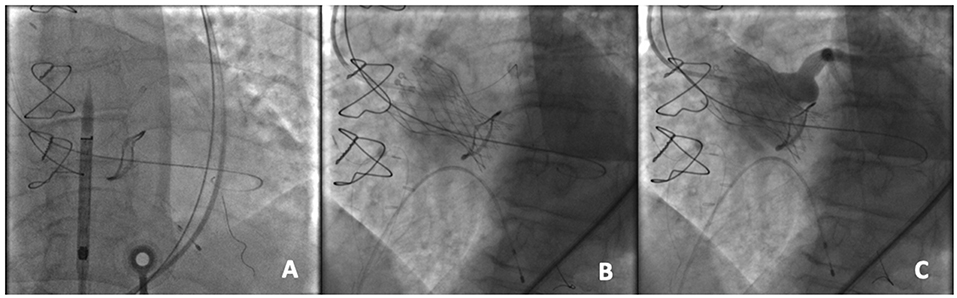
Figure 3. An example of coronary protection during a TAVR-ViV procedure. (A) Placement of the coronary guidewire in the left anterior descending (LAD) and A 5.5 × 15 mm undeployed balloon in the proximal LAD prior to the transcatheter aortic ViV implantation; (B) Deployment of the CoreValve Evolut PRO 23 mm device within a Mitroflow 21 mm; (C) Aortography to demonstrate the patency of the coronary ostium.
In the majority of the cases of coronary occlusion after VIV procedure published in the past, the only solution was immediate cardiac surgery to perform an aortocoronary bypass. In case of coronary occlusion a less invasive option to solve it could be PCI with a stent to get enough radial force to maintain coronary flow. Chimney technique has recently described for coronary stenting in patients at high risk of coronary occlusion during ViV (26, 27). Originally, this technique was described for renal and mesenteric preservation after endovascular aortic repair with an excellent outcome for the patient (28). It involves deployment of the stent into the coronary ostia, with the proximal parts placed between the aortic wall and the bioprosthetic leaflets, and extended beyond coronary ostia to ensure coronary flow.
It has been also previously reported in limited case reports in prophylactic setting (29, 30). The prophylactic Chimney technique offers a potential predictable stepwise method of coronary protection that may be employed in the highest coronary occlusion risk patients. Long-term outcome data from this technique are expected to give a perspective of the long-term durability of this technique.
The bioprosthetic aortic scallop intentional laceration to prevent iatrogenic coronary artery occlusion (BASILICA) procedure has recently emerged as a method for disrupting bioprosthetic leaflets in patients at high risk of coronary occlusion. This procedure is based on the LAMPOON procedure, which uses catheters to split the mitral valve leaflet to prevent the obstruction of the left ventricular outflow tract (LVOT) after transcatheter mitral valve replacement (31, 32). The aim is to create a triangular space that allows blood flow into the coronary artery. The BASILICA procedure has three steps: First, under fluoroscopic and/or echocardiographic guidance, a multipurpose guiding catheter with a combination of 300 cm 0.014″ guidewire and microcatheter is advanced to the coronary cusp targeted for laceration, and a snare catheter is positioned in the left ventricular outflow; Next, the cusp is penetrated with an electrified wire and it is snare-retrieved and externalized; Then, the aortic valve leaflet is lacerated with electrosurgery energy (typically 70 W) in short bursts, until it is complete and the guidewire is free. In this way, a triangular space that allows blood flow is created (Figure 4). Finally, a conventional TAVR is performed (33, 34). Prophylactically coronary artery stent systems and cracking of failed bioprosthetic heart valve frame, with a high-pressure balloon, may be used at operator discretion (34). Currently, BASILICA is being prospectively evaluated in a clinical trial (NCT03381989) and further data are awaited on this promising technique.
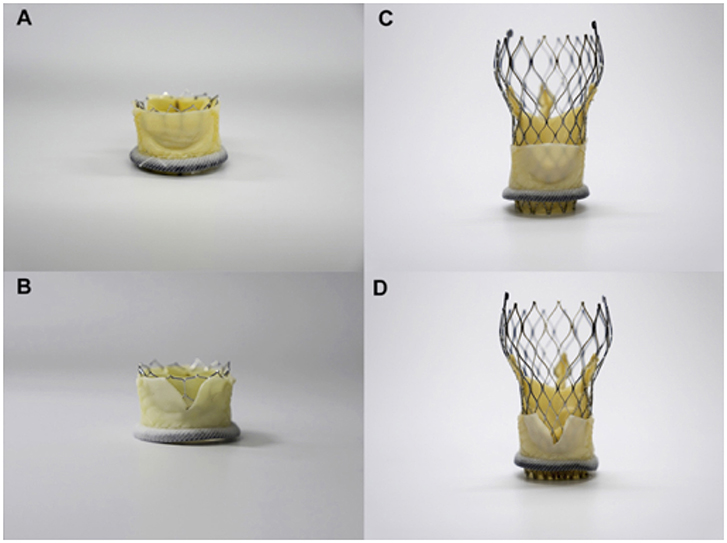
Figure 4. Simulation of BASILICA: Two different transcatheter heart valves [23-mm Sapien 3, (A,B); 26-mm Evolut Pro (C,D)] implanted in a 25-mm Mitroflow; (A,B) Without BASILICA, (C,D) With BASILICA.
Coronary occlusion remains one of the major concerns of transcatheter aortic Valve-in-Valve implantation. Despite its low frequency, it is related to very poor prognosis. To avoid this complication, meticulous procedural planning is necessary to choose the correct prosthesis type and size, because this concern is universal to all THV designs. In this context, coronary protection should be used in high-risk cases to restore instantly coronary flow and improve clinical outcome. The Chimney technique has recently described for coronary stenting in patients at high risk of coronary occlusion during ViV. Furthermore, the bioprosthetic aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction (BASILICA) procedure is emerging as an effective method for preventing coronary occlusion. We can expect that new tools and techniques to treat failed bioprosthetic valves will continue to be designed.
RV and GC provided the first revision of the manuscript. MB made critical revisions of the text and gave final approval.
MB is consultant for Edwards Lifesciences, and an advisory board member for Biotronik.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Barbanti M, Costa G, Zappulla P, Todaro D, Picci A, Rapisarda G, et al. Incidence of long-term structural valve dysfunction and bioprosthetic valve failure after transcatheter aortic valve replacement. J Am Heart Assoc. (2018) 7:e008440. doi: 10.1161/JAHA.117.008440
2. Søndergaard L, Ihlemann N, Capodanno D, Jørgensen TH, Nissen H, Kjeldsen BJ, et al. Durability of transcatheter and surgical bioprosthetic aortic valves in patients at lower surgical risk. J Am Coll Cardiol. (2019) 73:546–53. doi: 10.1016/j.jacc.2018.10.083
3. Fatima B, Mohananey D, Khan FW, Jobanputra Y, Tummala R, Banerjee K, et al. Durability data for bioprosthetic surgical aortic valve: a systematic review. JAMA Cardiol. (2019) 4:71–80. doi: 10.1001/jamacardio.2018.4045
4. Baumgartner H, Falk V, Bax J, De Bonis M, Hamm C, Holm P. 2017 ESC/EACTS Guidelines on the management of valvular heart disease. Eur Heart J. (2017) 38:2739–91. doi: 10.1093/eurheartj/ehx391
5. Gao G, Wu YX, Grunkemeier GL, Furnary AP, Starr A. Durability of pericardial versus porcine aortic valves. J Am Coll Cardiol. (2004) 44:384–8. doi: 10.1016/j.jacc.2004.01.053
6. David TE, Ivanov J, Armstrong S, Feindel CM, Cohen G. Late results of heart valve replacement with the Hancock II bioprosthesis. J Thorac Cardiovasc Surg. (2001) 121:268–77. doi: 10.1067/mtc.2001.112208
7. Capodanno D, Petronio AS, Prendergast B, Eltchaninoff H, Vahanian A, Modine T, et al. Standardized definitions of structural deterioration and valve failure in assessing long-term durability of transcatheter and surgical aortic bioprosthetic valves: a consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) endorsed by the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. (2017) 38:3382–90. doi: 10.1093/eurheartj/ehx303
8. Milburn K, Bapat V, Thomas M. Valve-in-valve implantations: is this the new standard for degenerated bioprostheses? Review of the literature. Clin Res Cardiol. (2014) 103:417–29. doi: 10.1007/s00392-013-0653-3
9. Dvir D, Webb J, Brecker S, Bleiziffer S, Hildick-Smith D, Colombo A, et al. Transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: Results from the global valve-in-valve registry. Circulation. (2012) 126:2335–44. doi: 10.1161/CIRCULATIONAHA.112.104505
10. Gurvitch R, Cheung A, Bedogni F, Webb JG. Coronary obstruction following transcatheter aortic valve-in-valve implantation for failed surgical bioprostheses. Catheter Cardiovasc Interv. (2011) 77:439–44. doi: 10.1002/ccd.22861
11. Jabbour RJ, Tanaka A, Finkelstein A, Mack M, Tamburino C, Van Mieghem N, et al. Delayed coronary obstruction after transcatheter aortic valve replacement. J Am Coll Cardiol. (2018) 71:1513–24. doi: 10.1016/j.jacc.2018.01.066
12. Holmes DR Jr, Nishimura RA, Grover FL, Brindis RG, Carroll JD, Edwards FH, et al. Annual outcomes with transcatheter valve therapy from the STS/ACC TVT registry. J Am Coll Cardiol. (2015) 66:2813–23. doi: 10.1016/j.jacc.2015.10.021
13. Ribeiro HB, Rodés-Cabau J, Blanke P, Leipsic J, Kwan Park J, Bapat V, et al. Incidence, predictors, and clinical outcomes of coronary obstruction following transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: Insights from the VIVID registry. Eur Heart J. (2018) 39:687–95. doi: 10.1093/eurheartj/ehx455
14. Ribeiro HB, Nombela-Franco L, Urena M, Mok M, Pasian S, Doyle D, et al. Coronary obstruction following transcatheter aortic valve implantation: a systematic review. JACC Cardiovasc Interv. (2013) 6:452–61. doi: 10.1016/j.jcin.2012.11.014
15. Ribeiro HB, Webb JG, Makkar RR, Cohen MG, Kapadia SR, Kodali S, et al. Predictive factors, management, and clinical outcomes of coronary obstruction following transcatheter aortic valve implantation: insights from a large multicenter registry. J Am Coll Cardiol. (2013) 62:1552–62. doi: 10.1016/j.jacc.2013.07.040
16. Blanke P, Soon J, Dvir D, Park JK, Naoum C, Kueh SH, et al. Computed tomography assessment for transcatheter aortic valve in valve implantation: The vancouver approach to predict anatomical risk for coronary obstruction and other considerations. J Cardiovasc Comput Tomogr. (2016) 10:491–9. doi: 10.1016/j.jcct.2016.09.004
17. Miller M, Snyder M, Horne BD, Harkness JR, Doty JR, Miner EC, et al. Transcatheter aortic valve-in-valve replacement for degenerated stentless bioprosthetic aortic valves. JACC Cardiovasc Interv. (2019) 12:1217–26. doi: 10.1016/j.jcin.2019.05.022
18. Barbanti M. Avoiding coronary occlusion and root rupture in TAVI - The role of pre-procedural imaging and prosthesis selection. Interv Cardiol Rev. (2015) 10:94–7. doi: 10.15420/icr.2015.10.2.94
19. Dvir D, Leipsic J, Blanke P, Ribeiro HB, Kornowski R, Pichard A, et al. Coronary obstruction in transcatheter aortic valve-in-valve implantation preprocedural evaluation, device selection, protection, and treatment. Circ Cardiovasc Interv. (2015) 8:e002079. doi: 10.1161/CIRCINTERVENTIONS.114.002079
20. Bapat V, Davies W, Attia R, Hancock J, Bolter K, Young C, et al. Use of balloon expandable transcatheter valves for valve-in-valve implantation in patients with degenerative stentless aortic bioprostheses: technical considerations and results. J Thorac Cardiovasc Surg. (2014) 148:917–22; discussion: 922–4. doi: 10.1016/j.jtcvs.2014.05.029
21. Barbanti M, Leipsic J, Binder R, Dvir D, Tan J, Freeman M, et al. Underexpansion and ad hoc post-dilation in selected patients undergoing balloon-expandable transcatheter aortic valve replacement. J Am Coll Cardiol. (2014) 63:976–81. doi: 10.1016/j.jacc.2013.10.014
22. Bapat V. Valve-in-valve apps: why and how they were developed and how to use them. EuroIntervention. (2014) 10:U44–51. doi: 10.4244/EIJV10SUA7
23. Conradi L, Kloth B, Seiffert M, Schirmer J, Koschyk D, Blankenberg S, et al. The challenge of valve-in-valve procedures in degenerated Mitroflow bioprostheses and the advantage of using the JenaValve transcatheter heart valve. EuroIntervention. (2014) 10:990–4. doi: 10.4244/EIJV10I8A167
24. Yamamoto M, Shimura T, Kano S, Kagase A, Kodama A, Koyama Y, et al. Impact of preparatory coronary protection in patients at high anatomical risk of acute coronary obstruction during transcatheter aortic valve implantation. Int J Cardiol. (2016) 217:58–63. doi: 10.1016/j.ijcard.2016.04.185
25. Nelson BC, Chadderdon S, Song H, Zahr FE. Bioprosthetic aortic valve leaflet disruption with high energy electrocautery to prevent coronary artery obstruction during valve-in-valve transcatheter aortic valve replacement. Catheter Cardiovasc Interv. (2019) 93:164–8. doi: 10.1002/ccd.27721
26. Hidalgo F, Ojeda S, Romero M. Chimney stent technique in a valve-in-valve procedure. Rev Esp Cardiol. (2018) 71:972. doi: 10.1016/j.rec.2017.10.049
27. Romano V, Buzzatti N, Latib A, Colombo A, Montorfano M. Chimney technique for coronary obstruction after aortic valve in valve: Pros and cons. Eur Heart J Cardiovasc Imaging. (2018) 19:1194. doi: 10.1093/ehjci/jey092
28. Liu B, Pan H, Song X, Liu C, Wu W, Chen Y, et al. Chimney stents for endovascular repair of juxtarenal aortic aneurysms with unfavourable anatomy. Int Angiol. (2013) 32:307–11.
29. Fetahovic T, Hayman S, Cox S, Cole C, Rafter T, Camuglia A. The prophylactic chimney snorkel technique for the prevention of acute coronary occlusion in high risk for coronary obstruction transcatheter aortic valve replacement/implantation cases. Hear Lung Circ. (2019) 28:e126–30. doi: 10.1016/j.hlc.2019.04.009
30. Chakravarty T, Jilaihawi H, Nakamura M, Kashif M, Kar S, Cheng W, et al. Pre-emptive positioning of a coronary stent in the left anterior descending artery for left main protection: a prerequisite for transcatheter aortic valve-in-valve implantation for failing stentless bioprostheses? Catheter Cardiovasc Interv. (2013) 82:E630–6. doi: 10.1002/ccd.25037
31. Khan JM, Rogers T, Schenke WH, Mazal JR, Faranesh AZ, Greenbaum AB, et al. Intentional laceration of the anterior mitral valve leaflet to prevent left ventricular outflow tract obstruction during transcatheter mitral valve replacement: pre-clinical findings. JACC Cardiovasc Interv. (2016) 9:1835–43. doi: 10.1016/j.jcin.2016.06.020
32. Babaliaros VC, Greenbaum AB, Khan JM, Rogers T, Wang DD, Eng MH, et al. Intentional percutaneous laceration of the anterior mitral leaflet to prevent outflow obstruction during transcatheter mitral valve replacement: first-in-human experience. JACC Cardiovasc Interv. (2017) 10:798–809. doi: 10.1016/j.jcin.2017.01.035
33. Dvir D, Khan J, Kornowski R, Komatsu I, Chatriwalla A, Mackenson GB, et al. Novel strategies in aortic valve-in-valve therapy including bioprosthetic valve fracture and BASILICA. EuroIntervention. (2018) 14:AB74–82. doi: 10.4244/EIJ-D-18-00667
Keywords: TAVR, valve-in-valve, coronary occlusion, coronary protection, BASILICA
Citation: Valvo R, Costa G and Barbanti M (2019) How to Avoid Coronary Occlusion During TAVR Valve-in-Valve Procedures. Front. Cardiovasc. Med. 6:168. doi: 10.3389/fcvm.2019.00168
Received: 16 May 2019; Accepted: 04 November 2019;
Published: 19 November 2019.
Edited by:
Darren Mylotte, National University of Ireland Galway, IrelandReviewed by:
Augusto D'Onofrio, University of Padova, ItalyCopyright © 2019 Valvo, Costa and Barbanti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marco Barbanti, bWJhcmJhbnRpODNAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.