- 1The School of Pharmacy and Pharmaceutical Sciences, Trinity Biomedical Sciences Institute, Trinity College Dublin, The University of Dublin, Dublin, Ireland
- 2College of Pharmacy, University of Kufa, Najaf, Iraq
- 3School of Medicine, Trinity College Dublin, The University of Dublin, Dublin, Ireland
Nanomaterials have been recently introduced as potential diagnostic and therapeutic tools in the medical field. One of the main concerns in relation to the use of nanomaterials in humans is their potential toxicity profile and blood compatibility. In fact, and due to their small size, NPs can translocate into the systemic circulation even after dermal contact, inhalation, or oral ingestion. Once in the blood stream, nanoparticles become in contact with the different components of the blood and can potentially interfere with normal platelet function leading to bleeding or thrombosis. Metallic NPs have been already used for diagnosis and treatment purposes due to their unique characteristics. However, the potential interactions between metallic NPs and platelets has not been widely studied and reported. This review focuses on the factors that can affect platelet activation and aggregation by metal NPs and the nature of such interactions, providing a summary of the effect of various metal NPs on platelet function available in the literature.
Introduction
Nanoparticles (NPs) are defined as particles which range from 1 to 100 nm in size in at least one dimension (length, width, or depth) (1, 2). Although, the development of nanotechnology is a modern science, NPs have always existed in the natural environment and throughout the ages, humans have been continually exposed to airborne NPs (3). However, with the onset of the industrial revolution and the more recent explosion of interest in NPs for scientific development, the exposure to different types of NPs is on the rise. In fact, the use of engineered nanomaterials from a wide range of compositions and sizes together with the study of their potential hazards is continuously increasing.
This review will focus on the interactions of metallic NPs developed for medical applications with blood platelets. The search has been conducted using PubMed, Scopus, and Google Scholar and relevant full text original and review articles published in English up to April 2019 have been included.
Factors Associated to Nanoparticle's Toxicity
Since the introduction of nanomedicine to describe the application of nanotechnology to benefit patients by The Royal Society and Royal Academy Engineering in 2004 (4), engineered NPs have become promising tools for the monitoring, diagnosis and treatment of human diseases. Despite the ability of humans to avoid, tolerate, and adapt to naturally occurring NPs, the exposure and accumulation of engineered NPs in our body can lead to plausible side effects (4). Multiple studies have already demonstrated that NPs interactions with biological systems depend on size, shape, charge, and the constituent material of the nanomaterial. In fact, those parameters can have a profound effect on cellular uptake and toxicity (5) and therefore on platelet's function (6–8).
Nanoparticles Size
Alveolar clearance by macrophages in the lungs constitutes an important barrier for inhaled NPs. However, the efficacy of this mechanism decreases with NPs size leading to a local increased deposition, greater access of NPs to the circulatory system and to potential cardiovascular side effects (9). It has been previously demonstrated that the degree of cytotoxicity and platelet activation and aggregation is inversely correlated with the NPs size (6, 10–12). Therefore, to dampen their potential toxic effect, while improving their stability, various types of surface coatings have been used (7, 13, 14). Surface modification using polyethylene glycol (PEG) for example, has been successfully employed to improve the platelet compatibility of gold NPs (15).
Nanoparticles Shape
The physical dimensions of nanomaterials are also strongly correlated with their potential toxicity profiles when compared with the bulk materials of the same composition (16, 17). For example, although carbon black is non-toxic, inhaled carbon nanotubes can result highly toxic (18). One of the factors that strongly influences NPs toxicity is the NPs shape. However, for a given geometric shape, NPs size largely determines the cellular uptake (19–22). Gratton et al. have demonstrated that nanorods larger than 100 nm exhibit the highest rate of cellular uptake followed by spheres, cylinders, and cubes of similar size (23). In contrast, studies using particles smaller than 100 nm have found that spheres show a greater cellular uptake than nanorods (19, 24). However, little is known about the influence of NPs shape on platelet function. Holzer et al. have shown that carbon-based nanomaterials could induce thrombus formation in rodents independently of their shape (25). In contrast, He et al. have recently demonstrated that cuboidal cyclodextrin frameworks enhanced platelet aggregation when compared with their spherical counterpart (26).
Protein Binding
When NPs become in contact with biological fluids, electrostatic, dispersive, and covalent interactions will regulate the adsorption of proteins onto NPs, leading to the formation of a dynamic “protein corona” (27, 28). Binding of plasma proteins induce changes in the biophysical properties of the NPs modifying their biocompatibility. In fact, the nature and the concentration of the proteins adsorbed on the NPs can affect their bio uptake, biodistribution, and potential side effects (29–34). The adsorption of some types of plasma proteins may result in an inflammatory response and platelet activation and aggregation. For instance, carbon nanotubes have been reported to bind complement proteins leading to complement activation via both classical and alternative pathways. In contrast, adsorption of complement proteins on the surface of gold colloids has not been associated with complement activation (35–37). The ability of PEG-coatings to protect against platelet aggregation corroborates the hypothesis that physical barriers around NPs may also contribute to the loss of their pro-aggregatory effect (15, 38). However, although the adsorption of fibrinogen onto NPs can contribute to platelet adhesion and initiate thrombogenesis, it has been also demonstrated that the composition of the protein corona on PEGylated NPs doesn't predict their hemocompatibility (39).
Nanoparticle Charge
NPs charge plays also an important role in NP-plasma protein interactions (29, 40) and therefore in the blood compatibility of NPs. Positively charge NPs can potentially interact with the negatively charged platelet surface and induce platelet aggregation (41, 42). However, Love et al. found that gold NPs with the same size and opposite charge did not induced platelet aggregation (43). Marginal variation in charge may contribute to a significant difference in protein binding to the NP surface and that may be the case with albumin binding to colloidal gold NPs (37).
Metallic Nanoparticles and Their Applications in Medicine
Metallic NPs can be easily synthesized and modified with various chemical functional groups which allow them to be conjugated with antibodies, ligands, and drugs. Therefore, the potential applications of metallic NPs in a variety of health-related applications over conventional pharmaceuticals, including targeted drug delivery systems, vehicles for gene and drug delivery, and imaging has dramatically increased (44).
Medical diagnostic applications of metal NPs are plentiful, owing to their ability to interact with external stimuli, including infra-red radiation, ultrasonic waves, and magnetic fields (45–48). Paramagnetic and superparamagnetic NPs have shown a great potential for cancer detection, as evidenced, for example, by the superior ability of iron oxide NPs to detect liver metastases and metastatic lymph nodes (49, 50). Metallic NPs can be also used as therapeutics agents. The antibacterial nature of silver has been already well-established and gold NPs have been found to induce cell toxicity when delivered orally. In fact, silver and gold NPs have been demonstrated to be ideal candidates for the treatment of both, multi drug resistant infections and cancer (51–55). In addition, gold NPs have also shown to exert some anti-angiogenic effect by inhibiting the VEGF activity in collagen-induced arthritis in rats and in human umbilical vein endothelial cells (56–58). The use of multifunctional NPs that integrate diagnostic and therapeutic functions in the same system (theranostic) has been gaining increasing momentum in the nanomedical research and development (R&D). For the management of patients suffering from cancer, for example, nanotheranostic platforms could comprise advanced diagnostics, hyperthermia treatment, and targeted delivery of anticancer drugs. Actually, magnetic iron oxide NPs constitute a good example of such “multitasking” platforms (59).
Metallic Nanoparticles-Platelets Interactions
In 1977, Berry et al. described for the first time the translocation of nano-sized particles across the alveolar epithelium following intratracheal instillations of 30 nm gold particles in rats. They found large amounts of these particles in platelets of pulmonary capillaries and assumed that there might be a pre-disposing factor for platelet aggregation and microthrombi formation even when those NPs are coated with a biocompatible material (60). In fact, NPs which are not intended for systemic use can, due to their ability to cross epithelial barriers, reach the systemic circulation and interfere with physiological platelet function increasing the risk of cardiovascular disease and vascular thrombosis (61–63). Although, some NPs have been developed for therapeutic purposes aimed to target the injured vascular site mimicking platelet function (64) or to enhance blood clotting (65), the potential unwanted, pro- and/or anti-aggregating, properties of NPs are of significant concern in the field of nanomedicine and may impede the progression of promising engineered NPs to the clinical setting.
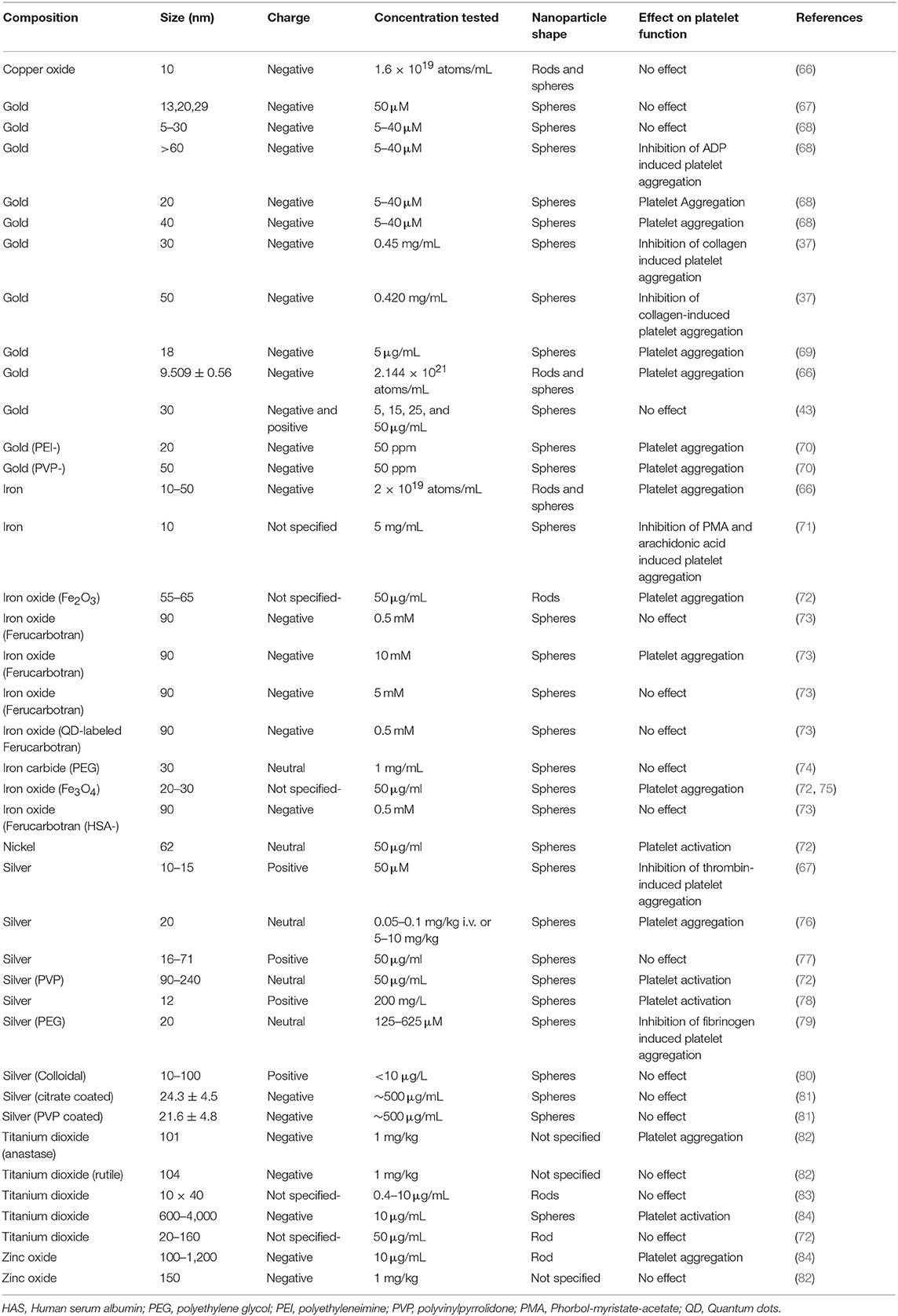
Induction of platelet aggregation by metallic NPs has been found by multiple research groups. However, some studies also indicate that metal NPs can inhibit or not affect platelet function (Table 1). The extent to which NPs induce platelet aggregation may depend on multiple NPs factors, as explained in the previous sections, but also on the physiological state of platelets prior exposure to NPs. When platelets are in resting state most metallic NPs seem to be inert (7) becoming more sensitive to NPs in the presence of a threshold shear force or “pre-activation” by critical concentrations of ADP (10, 66, 85). However, non-metallic NPs such as carbon nano-tubes or polymer-based NPs seem to induce platelet aggregation in the absence of any “pre-activating factor” (42, 62).
The exact molecular mechanisms by which metallic NPs influence platelet function is not well-understood. However, their effect on platelet granules and integrin receptors seems to be crucial during the process. Metallic NP-induced platelet aggregation is inhibited when ADP pathway is blocked by an appropriate concentration of clopidogrel or apyrase inhibiting therefore the secondary wave of platelet aggregation which depends mainly on granule release. GPIIbIIIa seems to play also an important role in metallic NP-induced platelet aggregation as in the presence of Arg-Gly-Asp-Ser (RGDS), a tetra-peptide which binds and inhibits activated but not resting GpIIbIIIa, platelet aggregation is attenuated (86).
Silver NPs
The broad-spectrum antimicrobial properties of silver NPs are well-documented. Their widespread use in both commercial and biomedical applications, due to their large surface-area-to-volume ratio which increases their efficacy against bacteria in comparison to common antibiotics, has raised them to the status of the most commercialized NPs.
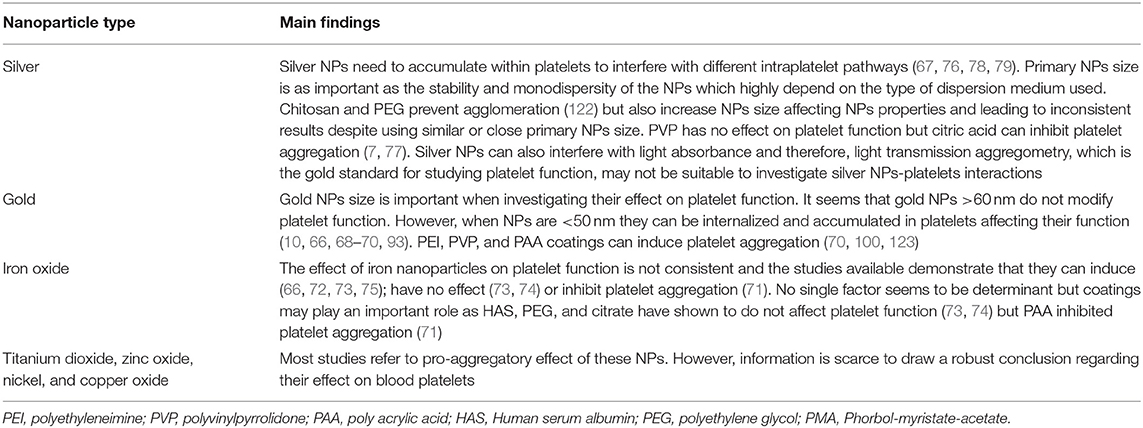
The blood compatibility of silver NPs, remains controversial, with several studies that report contradictory results. Spherical silver NPs (10–100 nm) have been found to induce platelet aggregation by increasing intraplatelet Ca2+ levels, upregulating GPIIb/IIIa and P-selectin and serotonin secretion (76, 78). Laloy et al. reported that silver NPs increased platelet adhesion but did not exert any further effect on platelet aggregation (77). Coating with PEG inhibits platelet aggregation induced by collagen, ADP, thrombin, and arachidonic acid in a concentration dependent manner (79). Silver NPs spherical in shape, 10–15 nm in diameter and monodispersed, have also been shown to have antiplatelet properties (67). Some variability between studies can be due to the use of different dispersing media (87–89) and Deb et al. have argued that the inhibition of platelet function by silver NPs may be due to the presence of the citric acid used for coating their surface (7). In addition, most of the studies use light transmission aggregometry (LTA) for measuring platelet aggregation and LTA has some limitations: (1) it may not be sensitive enough for studying NP-induced platelet aggregation (90). (2) As silver NPs have light absorbance properties, which are significant from 10 μg/mL, the use of concentrations over this threshold can have a profound effect on the results obtained (77). On the other hand, Smock et al. performed a prospective, placebo controlled in healthy human volunteers to assess the effect of commercially available oral colloidal silver NPs on platelet aggregation ex vivo using LTA. They found that platelet activation was not enhanced at peak silver serum concentrations (<10 μg/mL) (80).
Gold NPs
Colloidal gold was first described as a novel NP vector for tumor directed drug delivery back to 2004 (91). Gold NPs are considered to be one of the safest and most attractive drug-delivery agents due of their inert, non-toxic, and highly permeable properties (92). Still, there are some studies that document toxicity of gold NPs depending on the physical dimensions, surface chemistry, and shape of the NPs studied (19, 20, 93–97).
The compatibility of gold NPs with blood components and their effect on platelet function is not well-established. Although, gold NPs >60 nm in size have no effect on platelet function, smaller NPs (20 nm) can activate platelets. Those NPs could trigger platelet aggregation by a molecular mechanism which involves the platelet canalicular system and the activation of intracellular signaling mechanisms that comprise tyrosine phosphorylation, alpha granule release and P-selectin translocation (10, 98). On the other hand, it has been shown that spherical gold NPs of ~30 nm do not affect platelet aggregation, probably due to the total surface contact and/or the formation of the protein corona (37, 43).
Santos-Martinez et al. have investigated the effect that different NPs may exert on platelet function under flow conditions using a Quartz Crystal Microbalance with Dissipation (QCM-D). The group has demonstrated that QCM-D is more sensitive than the traditional LTA when investigating NP-platelet interactions (69, 99) and found that PEGylation of gold NPs improves their platelet compatibility (15). However, the use of polyethyleneimine (PEI) and polyvinylpyrrolidone (PVP) conjugated NPs can induce platelet activation as revealed by the formation of numerous filopodia and degranulation in equine platelets (70). This effect may be related to the adsorption of fibrinogen onto the NPs surface (5) and could be even more obvious if polyacrylic acid (PAA)-conjugated gold nanoparticles are exposed to platelets as PAA binds to and induce conformational changes of fibrinogen (100) that could potentially have a greater impact on the hemocompatibility of those NPs.
Iron Oxide NPs
Iron oxide NPs have been extensively used as contrast agents. With the introduction of theranostic systems their use has become more attractive as a novel approach for cancer therapy. The use of those NPs loaded with cytotoxic drugs and functionalized to detect and specifically attack malignant cells could potentially reduce significantly both, side effects of cytotoxic drugs and healthy cells damage.
The effect of iron-based NPs on platelet function in the literature is somehow inconsistent. Some iron-based NPs have been found to induce platelet aggregation as demonstrated by morphological changes using scanning electron microscopy (72). Bircher et al. found that carbon-coated iron carbide magnetic NPs incubated with whole blood induced upregulation of GPIIb-IIIa and P-Selectin but this effect was reversed when NPs were PEGylated (74). In another study, the use of dextran-stabilized iron oxide NPs developed for hyperthermia did not affect either platelet function (101). Deb et al. show in their work that starch-stabilized iron oxide NPs do not exert any effect of platelets while citric acid-stabilized iron oxide NPs inhibited platelet aggregation (7).
Platelet labeling can be of great interest for evaluating the influence of different methods on platelet survival when preparing platelet concentrates or when there is a need to distinguishing between donor and recipient platelets. Iron oxide NPs conjugated with quantum dots have been used previously by Aurich et al. as a non-radioactive alternative for platelet labeling. NPs were successfully internalized in platelets but impaired platelet function at the concentrations needed for labeling. However, this effect was abolished when the NPs where functionalized further with human serum albumin (73).
Nickel NPs
Compared to its elemental state, nickel NPs exhibit exceptional electrochemical properties and show unusual superparamagnetic properties and stability that make them very attractive in the nanotechnology field (102, 103). In addition, nickel NPs have been used in medicine as catalysts in the production of hydrogen nanoparticles (104). Some studies have demonstrated that nickel NPs induce cytotoxic effects in vitro (105, 106) and changes in platelet shape (72). However, despite their wide use in industry, their potential toxic effect to humans has not been extensively investigated.
Zinc Oxide NPs
Zinc oxide NPs are commonly used in nanomedicine. Spherical NPs have been used as anti-cancer and anti-bacterial agents due to their ability to produce reactive oxygen species (ROS) and induce apoptosis (107, 108). In fact, tetrapod NPs have been recently studied for their antibacterial activity (109), antiviral activity (110, 111), and as a vaccine adjuvant (112). Composite types of nanostructures are also synthesized in various forms including zinc oxide quantum dots and zinc oxide nanoclusters as anti-cancer and anti-bacterial agents (113–115).
Zinc oxide NPs prepared in dispersion medium, citrate or glucose solution have been shown to induce human and canine platelet activation (84). On the other hand, systemic administration of zinc oxide NPs to mice did not induce thrombus formation (82). However, studies looking at the blood compatibility of those NPs are once again scarce.
Copper NPs
Deb et al. have shown that copper NPs have a pro-aggregatory effect activating purinergic receptors (P2Y12) in the presence of ADP at suboptimal concentrations (66). However, Major et al. using a nitric oxide generating polymeric material (polyurethane containing copper NPs) combined with the intravenous infusion of a nitric oxide donor (S-nitroso-N-acetylpenicillamine-SNAP) observed that platelet function was preserved in rabbit blood exposed to extracorporeal circulation (116).
Titanium Dioxide NPs
Titanium dioxide NPs are widely used in cosmetic products and sunscreens (117) and they are very well-known photocatalysts able to generate different reactive species that could be potentially toxic to microorganisms. In fact, in vitro studies have shown deleterious effect of those NPs on different cell lines (118, 119). Titanium dioxide NPs have shown to activate dog and, to a lesser degree, human platelets in vitro (84). When administered systemically to rats, titanium dioxide anatase but not titanium dioxide rutile NPs of similar size (around 100 nm) were found to increase murine platelet aggregation (82). In another study, systemic administration of titanium dioxide rutile NPs did not activate platelets or exert prothrombotic effects in mice either (120). However, the intratracheal administration of rutile titanium oxide nanorods has resulted in platelet aggregation in rats (83).
Conclusions
Medicine is envisaged to be one of the primary beneficiaries of the nanotechnological development. The growth of nanotechnology has fired the “technological boom” in diagnostics, imaging, and drug delivery (121). The scientific community has shown significant interest in further developing the potential medical applications of metal NPs. However, nanomaterial-blood interactions are inevitable regardless of the use NPs are intended to.
The compatibility of NPs with blood elements remains as a controversial topic. Several studies utilizing similar experimental protocols and assessing similar end points report contradictory results and Table 2 summarizes the main effects of metallic NPs on platelets found in the literature. Although there are multiple “NP dependent” factors that can play an important role on NPs-platelets interaction, other circumstances should be kept on mind when carrying out those experiments as they may potentially contribute to the conflicting results: (a) platelet preparation/platelet handling; (b) use of different solutions/dispersing media when preparing the NPs/performing the experiments; (c) presence of impurities or additional substances in metallic NPs or their suspensions; (d) interference of the NPs tested with the method used; (e) use of different concentrations of NPs and or platelet agonists in different experimental settings; and (f) lack of sensibility for detecting platelet microaggregates with the method used.
Unwanted side effects of NPs is of significant concern in the field of nanomedicine and often hampers the progression of promising nanomaterials to the clinical setting. Knowledge regarding NPs toxic potential is still limited along with an absence of appropriated regulatory policies for their use and testing (124, 125). In fact, not all commercially available nanomaterials have a Safety Data Sheet and novel nanomaterials are sometimes manipulated without full assessment of their potential health risk (124).
There is no doubt that the development of nanomaterials intended for human use should be always carried out in tandem with an extensive toxicological evaluation. Those studies must include the investigation of the effect of nanomaterials on platelet function as they can lead to secondary effects (bleeding or thrombosis) that can affect individuals involved in their production, manipulation or use during medical applications.
Author Contributions
NH, MS-M, and CM designed the structure of the different sections of the manuscript. NH did the search and wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
NH was funded from Ministry of Higher Education and Scientific Research in Iraq (MoHER).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
NPs, Nanoparticles; VEGF, Vascular endothelial growth factor; PEG, polyethylene glycol; LTA, light transmission Aggregometry; QCM-D, Quartz Crystal Microbalance with Dissipation, PEI, polyethyleneimine; PVP, polyvinylpyrrolidone; PMA, Phorbol-myristate-acetate; PAA, polyacrylic acid; ROS, reactive oxygen species; SNAP, S-nitroso-N-acetylpenicillamine; HAS, human serum albumin.
References
1. Morimoto Y, Kobayashi N, Shinohara N, Myojo T, Tanaka I, Nakanishi J. Hazard assessments of manufactured nanomaterials. J Occup Health. (2010) 52:325–34. doi: 10.1539/joh.R10003
2. Scuri M, Chen BT, Castranova V, Reynolds JS, Johnson VJ, Samsell L, et al. Effects of titanium dioxide nanoparticle exposure on neuroimmune responses in rat airways. J Toxicol Environ Health. (2010) 73:1353–69. doi: 10.1080/15287394.2010.497436
3. Buffle J. The key role of environmental colloids/nanoparticles for the sustainability of life. Environ Chem. (2006) 3:155–8. doi: 10.1071/ENv3n3_ES
4. Magaye R, Zhao J, Bowman L, Ding M. Genotoxicity and carcinogenicity of cobalt-, nickel- and copper-based nanoparticles. Exp Ther Med. (2012) 4:551–61. doi: 10.3892/etm.2012.656
5. Albanese A, Tang PS, Chan WC. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu Rev Biomed Eng. (2012) 14:1–16. doi: 10.1146/annurev-bioeng-071811-150124
6. Mayer A, Vadon M, Rinner B, Novak A, Wintersteiger R, Frohlich E. The role of nanoparticle size in hemocompatibility. Toxicology. (2009) 258:139–47. doi: 10.1016/j.tox.2009.01.015
7. Deb S, Raja SO, Dasgupta AK, Sarkar R, Chattopadhyay AP, Chaudhuri U, et al. Surface tunability of nanoparticles in modulating platelet functions. Blood Cells Mol Dis. (2012) 48:36–44. doi: 10.1016/j.bcmd.2011.09.011
8. Tomaszewski KA, Radomski MW, Santos-Martinez MJ. Nanodiagnostics, nanopharmacology and nanotoxicology of platelet-vessel wall interactions. Nanomedicine. (2015) 10:1451–75. doi: 10.2217/nnm.14.232
9. Yamawaki H, Iwai N. Mechanisms underlying nano-sized air-pollution-mediated progression of atherosclerosis: carbon black causes cytotoxic injury/inflammation and inhibits cell growth in vascular endothelial cells. Circ J. (2006) 70:129–40. doi: 10.1253/circj.70.129
10. Deb S, Patra HK, Lahiri P, Dasgupta AK, Chakrabarti K, Chaudhuri U. Multistability in platelets and their response to gold nanoparticles. Nanomedicine. (2011) 7:376–84. doi: 10.1016/j.nano.2011.01.007
11. Park MV, Neigh AM, Vermeulen JP, de la Fonteyne LJ, Verharen HW, Briede JJ, et al. The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. Biomaterials. (2011) 32:9810–7. doi: 10.1016/j.biomaterials.2011.08.085
12. Shannahan JH, Lai X, Ke PC, Podila R, Brown JM, Witzmann FA. Silver nanoparticle protein corona composition in cell culture media. PLoS ONE. (2013) 8:e74001. doi: 10.1371/journal.pone.0074001
13. Gilbert B, Huang F, Zhang H, Waychunas GA, Banfield JF. Nanoparticles: strained and stiff. Science. (2004) 305:651–4. doi: 10.1126/science.1098454
14. Fröhlich E. Action of nanoparticles on platelet activation and plasmatic coagulation. Curr Med Chem. (2016) 23:408–30. doi: 10.2174/0929867323666160106151428
15. Santos-Martinez M, Rahme K, Corbalan J, Faulkner C, Holmes J, Tajber L, et al. Pegylation increases platelet biocompatibility of gold nanoparticles. J Biomed Nanotechnol. (2014) 10:1004–15. doi: 10.1166/jbn.2014.1813
16. Borm PJ, Muller-Schulte D. Nanoparticles in drug delivery and environmental exposure: same size, same risks? Nanomedicine. (2006) 1:235–49. doi: 10.2217/17435889.1.2.235
17. Dunphy Guzmán KA, Taylor MR, Banfield JF. Environmental risks of nanotechnology: national nanotechnology initiative funding, 2000–2004. Environ Sci Technol. (2006) 40:1401–7. doi: 10.1021/es0515708
18. Carrero-Sanchez JC, Elias AL, Mancilla R, Arrellin G, Terrones H, Laclette JP, et al. Biocompatibility and toxicological studies of carbon nanotubes doped with nitrogen. Nano Lett. (2006) 6:1609–16. doi: 10.1021/nl060548p
19. Chithrani BD, Ghazani AA, Chan WC. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. (2006) 6:662–8. doi: 10.1021/nl052396o
20. Chithrani BD, Chan WC. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. (2007) 7:1542–50. doi: 10.1021/nl070363y
21. Jin H, Heller DA, Sharma R, Strano MS. Size-dependent cellular uptake and expulsion of single-walled carbon nanotubes: single particle tracking and a generic uptake model for nanoparticles. ACS Nano. (2009) 3:149–58. doi: 10.1021/nn800532m
22. Lu F, Wu SH, Hung Y, Mou CY. Size effect on cell uptake in well-suspended, uniform mesoporous silica nanoparticles. Small. (2009) 5:1408–13. doi: 10.1002/smll.200900005
23. Gratton SE, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, et al. The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci USA. (2008) 105:11613–8. doi: 10.1073/pnas.0801763105
24. Qiu Y, Liu Y, Wang L, Xu L, Bai R, Ji Y, et al. Surface chemistry and aspect ratio mediated cellular uptake of Au nanorods. Biomaterials. (2010) 31:7606–19. doi: 10.1016/j.biomaterials.2010.06.051
25. Holzer M, Bihari P, Praetner M, Uhl B, Reichel C, Fent J, et al. Carbon-based nanomaterials accelerate arteriolar thrombus formation in the murine microcirculation independently of their shape. J Appl Toxicol. (2014) 34:1167–76. doi: 10.1002/jat.2996
26. He Y, Xu J, Sun X, Ren X, Maharjan A, York P, et al. Cuboidal tethered cyclodextrin frameworks tailored for hemostasis and injured vessel targeting. Theranostics. (2019) 9:2489–504. doi: 10.7150/thno.31159
27. Gebauer JS, Malissek M, Simon S, Knauer SK, Maskos M, Stauber RH, et al. Impact of the nanoparticle-protein corona on colloidal stability and protein structure. Langmuir. (2012) 28:9673–9. doi: 10.1021/la301104a
28. Treuel L, Nienhaus GU. Toward a molecular understanding of nanoparticle-protein interactions. Biophys Rev. (2012) 4:137–47. doi: 10.1007/s12551-012-0072-0
29. Lundqvist M, Stigler J, Elia G, Lynch I, Cedervall T, Dawson KA. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc Natl Acad Sci USA. (2008) 105:14265–70. doi: 10.1073/pnas.0805135105
30. Lynch I, Salvati A, Dawson KA. Protein-nanoparticle interactions: what does the cell see? Nat Nanotechnol. (2009) 4:546–7. doi: 10.1038/nnano.2009.248
31. Monopoli MP, Walczyk D, Campbell A, Elia G, Lynch I, Bombelli FB, et al. Physical-chemical aspects of protein corona: relevance to in vitro and in vivo biological impacts of nanoparticles. J Am Chem Soc. (2011) 133:2525–34. doi: 10.1021/ja107583h
32. Lesniak A, Salvati A, Santos-Martinez MJ, Radomski MW, Dawson KA, Aberg C. Nanoparticle adhesion to the cell membrane and its effect on nanoparticle uptake efficiency. J Am Chem Soc. (2013) 135:1438–44. doi: 10.1021/ja309812z
33. Allemann E, Gravel P, Leroux JC, Balant L, Gurny R. Kinetics of blood component adsorption on poly(D,L-lactic acid) nanoparticles: evidence of complement C3 component involvement. J Biomed Mater Res. (1997) 37:229–34.
34. Monopoli MP, Aberg C, Salvati A, Dawson KA. Biomolecular coronas provide the biological identity of nanosized materials. Nat Nanotechnol. (2012) 7:779–86. doi: 10.1038/nnano.2012.207
35. Salvador-Morales C, Flahaut E, Sim E, Sloan J, Green ML, Sim RB. Complement activation and protein adsorption by carbon nanotubes. Mol Immunol. (2006) 43:193–201. doi: 10.1016/j.molimm.2005.02.006
36. Ilinskaya AN, Shah A, Enciso AE, Chan KC, Kaczmarczyk JA, Blonder J, et al. Nanoparticle physicochemical properties determine the activation of intracellular complement. Nanomedicine. (2019) 17:266–75. doi: 10.1016/j.nano.2019.02.002
37. Dobrovolskaia MA, Patri AK, Zheng J, Clogston JD, Ayub N, Aggarwal P, et al. Interaction of colloidal gold nanoparticles with human blood: effects on particle size and analysis of plasma protein binding profiles. Nanomedicine. (2009) 5:106–17. doi: 10.1016/j.nano.2008.08.001
38. You J, Zhou J, Zhou M, Liu Y, Robertson JD, Liang D, et al. Pharmacokinetics, clearance, and biosafety of polyethylene glycol-coated hollow gold nanospheres. Particle Fibre Toxicol. (2014) 11:26. doi: 10.1186/1743-8977-11-26
39. Dobrovolskaia MA, Neun BW, Man S, Ye X, Hansen M, Patri AK, et al. Protein corona composition does not accurately predict hematocompatibility of colloidal gold nanoparticles. Nanomedicine. (2014) 10:1453–63. doi: 10.1016/j.nano.2014.01.009
40. Cedervall T, Lynch I, Lindman S, Berggard T, Thulin E, Nilsson H, et al. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc Natl Acad Sci USA. (2007) 104:2050–5. doi: 10.1073/pnas.0608582104
41. Enciso AE, Neun B, Rodriguez J, Ranjan AP, Dobrovolskaia MA, Simanek EE. Nanoparticle effects on human platelets in vitro: a comparison between PAMAM and triazine dendrimers. Molecules. (2016) 21:428. doi: 10.3390/molecules21040428
42. McGuinnes C, Duffin R, Brown S, Mills LN, Megson IL, Macnee W, et al. Surface derivatization state of polystyrene latex nanoparticles determines both their potency and their mechanism of causing human platelet aggregation in vitro. Toxicol Sci. (2011) 119:359–68. doi: 10.1093/toxsci/kfq349
43. Love SA, Thompson JW, Haynes CL. Development of screening assays for nanoparticle toxicity assessment in human blood: preliminary studies with charged Au nanoparticles. Nanomedicine. (2012) 7:1355–64. doi: 10.2217/nnm.12.17
44. Mody VV, Siwale R, Singh A, Mody HR. Introduction to metallic nanoparticles. J Pharm Bioallied Sci. (2010) 2:282–9. doi: 10.4103/0975-7406.72127
45. Chen J, Saeki F, Wiley BJ, Cang H, Cobb MJ, Li ZY, et al. Gold nanocages: bioconjugation and their potential use as optical imaging contrast agents. Nano Lett. (2005) 5:473–7. doi: 10.1021/nl047950t
46. Fu A, Gu W, Boussert B, Koski K, Gerion D, Manna L, et al. Semiconductor quantum rods as single molecule fluorescent biological labels. Nano Lett. (2007) 7:179–82. doi: 10.1021/nl0626434
47. Gobin AM, Lee MH, Halas NJ, James WD, Drezek RA, West JL. Near-infrared resonant nanoshells for combined optical imaging and photothermal cancer therapy. Nano Lett. (2007) 7:1929–34. doi: 10.1021/nl070610y
48. Lee S, Chon H, Lee M, Choo J, Shin SY, Lee YH, et al. Surface-enhanced Raman scattering imaging of HER2 cancer markers overexpressed in single MCF7 cells using antibody conjugated hollow gold nanospheres. Biosens Bioelectron. (2009) 24:2260–3. doi: 10.1016/j.bios.2008.10.018
49. Pan D, Schmieder AH, Wickline SA, Lanza GM. Manganese-based MRI contrast agents: past, present and future. Tetrahedron. (2011) 67:8431–44. doi: 10.1016/j.tet.2011.07.076
50. Zhu L, Zhou Z, Mao H, Yang L. Magnetic nanoparticles for precision oncology: theranostic magnetic iron oxide nanoparticles for image-guided and targeted cancer therapy. Nanomedicine. (2016) 12:73–87. doi: 10.2217/nnm-2016-0316
51. Bowman M-C, Ballard TE, Ackerson CJ, Feldheim DL, Margolis DM, Melander C. Inhibition of HIV fusion with multivalent gold nanoparticles. J Am Chem Soc. (2008) 130:6896–7. doi: 10.1021/ja710321g
52. Sun L, Singh A, Vig K, Pillai SR, Singh SR. Silver nanoparticles inhibit replication of respiratory syncytial virus. J Biomed Nanotechnol. (2008) 4:149–58. doi: 10.1166/jbn.2008.012
53. Baram-Pinto D, Shukla S, Perkas N, Gedanken A, Sarid R. Inhibition of herpes simplex virus type 1 infection by silver nanoparticles capped with mercaptoethane sulfonate. Bioconjug Chem. (2009) 20:1497–502. doi: 10.1021/bc900215b
54. Lara HH, Ayala-Nuñez NV, Ixtepan-Turrent L, Rodriguez-Padilla C. Mode of antiviral action of silver nanoparticles against HIV-1. J Nanobiotechnol. (2010) 8:1. doi: 10.1186/1477-3155-8-1
55. Mallipeddi R, Rohan LC. Nanoparticle-based vaginal drug delivery systems for HIV prevention. Expert Opin Drug Deliv. (2010) 7:37–48. doi: 10.1517/17425240903338055
56. Bhattacharya R, Mukherjee P, Xiong Z, Atala A, Soker S, Mukhopadhyay D. Gold nanoparticles inhibit VEGF165-induced proliferation of HUVEC cells. Nano Lett. (2004) 4:2479–81. doi: 10.1021/nl0483789
57. Tsai CY, Shiau AL, Chen SY, Chen YH, Cheng PC, Chang MY, et al. Amelioration of collagen-induced arthritis in rats by nanogold. Arthritis Rheum. (2007) 56:544–54. doi: 10.1002/art.22401
58. Arvizo RR, Rana S, Miranda OR, Bhattacharya R, Rotello VM, Mukherjee P. Mechanism of anti-angiogenic property of gold nanoparticles: role of nanoparticle size and surface charge. Nanomedicine. (2011) 7:580–7. doi: 10.1016/j.nano.2011.01.011
59. Gobbo OL, Sjaastad K, Radomski MW, Volkov Y, Prina-Mello A. Magnetic nanoparticles in cancer theranostics. Theranostics. (2015) 5:1249–63. doi: 10.7150/thno.11544
60. Berry JP, Arnoux B, Stanislas G, Galle P, Chretien J. A microanalytic study of particles transport across the alveoli: role of blood platelets. Biomedicine. (1977) 27:354–7.
61. Pope CA III, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. (2002) 287:1132–41. doi: 10.1001/jama.287.9.1132
62. Radomski A, Jurasz P, Alonso-Escolano D, Drews M, Morandi M, Malinski T, et al. Nanoparticle-induced platelet aggregation and vascular thrombosis. Br J Pharmacol. (2005) 146:882–93. doi: 10.1038/sj.bjp.0706386
63. Gaffney AM, Santos-Martinez MJ, Satti A, Major TC, Wynne KJ, Gun'ko YK, et al. Blood biocompatibility of surface-bound multi-walled carbon nanotubes. Nanomedicine. (2015) 11:39–46. doi: 10.1016/j.nano.2014.07.005
64. Anselmo AC, Modery-Pawlowski CL, Menegatti S, Kumar S, Vogus DR, Tian LL, et al. Platelet-like nanoparticles: mimicking shape, flexibility, and surface biology of platelets to target vascular injuries. ACS Nano. (2014) 8:11243–53. doi: 10.1021/nn503732m
65. Roy SC, Paulose M, Grimes CA. The effect of TiO2 nanotubes in the enhancement of blood clotting for the control of hemorrhage. Biomaterials. (2007) 28:4667–72. doi: 10.1016/j.biomaterials.2007.07.045
66. Deb S, Chatterjee M, Bhattacharya J, Lahiri P, Chaudhuri U, Choudhuri SP, et al. Role of purinergic receptors in platelet-nanoparticle interactions. Nanotoxicology. (2007) 1:93–103. doi: 10.1080/17435390600772978
67. Shrivastava S, Bera T, Singh SK, Singh G, Ramachandrarao P, Dash D. Characterization of antiplatelet properties of silver nanoparticles. ACS Nano. (2009) 3:1357–64. doi: 10.1021/nn900277t
68. Aseychev AV, Azizova OA, Beckman EM, Dudnik LB, Sergienko VI. Effect of gold nanoparticles coated with plasma components on ADP-induced platelet aggregation. Bull Exp Biol Med. (2013) 155:685–8. doi: 10.1007/s10517-013-2226-x
69. Santos-Martinez MJ, Inkielewicz-Stepniak I, Medina C, Rahme K, D'Arcy DM, Fox D, et al. The use of quartz crystal microbalance with dissipation (QCM-D) for studying nanoparticle-induced platelet aggregation. Int J Nanomed. (2012) 7:243–55. doi: 10.2147/IJN.S26679
70. Hecold M, Buczkowska R, Mucha A, Grzesiak J, Rac-Rumijowska O, Teterycz H, et al. The effect of PEI and PVP-stabilized gold nanoparticles on equine platelets activation: potential application in equine regenerative medicine. J Nanomater. (2017) 2017:11. doi: 10.1155/2017/8706921
71. Villegas MG, Ceballos MT, Urquijo J, Torres EY, Ortiz-Reyes BL, Arnache-Olmos OL, et al. Poly(acrylic acid)-coated iron oxide nanoparticles interact with mononuclear phagocytes and decrease platelet aggregation. Cell Immunol. (2019) 338:51–62. doi: 10.1016/j.cellimm.2019.03.005
72. Guildford AL, Poletti T, Osbourne LH, Di Cerbo A, Gatti AM, Santin M. Nanoparticles of a different source induce different patterns of activation in key biochemical and cellular components of the host response. J R Soc Interf . (2009) 6:1213–21. doi: 10.1098/rsif.2009.0021
73. Aurich K, Wesche J, Palankar R, Schluter R, Bakchoul T, Greinacher A. Magnetic nanoparticle labeling of human platelets from platelet concentrates for recovery and survival studies. ACS Appl Mater Interf . (2017) 9:34666–73. doi: 10.1021/acsami.7b10113
74. Bircher L, Theusinger OM, Locher S, Eugster P, Roth-Z'graggen B, Schumacher CM, et al. Characterization of carbon-coated magnetic nanoparticles using clinical blood coagulation assays: effect of PEG-functionalization and comparison to silica nanoparticles. J Mater Chem B. (2014) 2:3753–8. doi: 10.1039/C4TB00208C
75. Aurich K, Spoerl M-C, Fürll B, Sietmann R, Greinacher A, Hosten N, et al. Development of a method for magnetic labeling of platelets. Nanomedicine. (2012) 8:537–44. doi: 10.1016/j.nano.2011.09.008
76. Jun EA, Lim KM, Kim K, Bae ON, Noh JY, Chung KH, et al. Silver nanoparticles enhance thrombus formation through increased platelet aggregation and procoagulant activity. Nanotoxicology. (2011) 5:157–67. doi: 10.3109/17435390.2010.506250
77. Laloy J, Minet V, Alpan L, Mullier F, Beken S, Toussaint O, et al. Impact of silver nanoparticles on haemolysis, platelet function and coagulation. Nanobiomedicine. (2014) 1:4. doi: 10.5772/59346
78. Krajewski S, Prucek R, Panacek A, Avci-Adali M, Nolte A, Straub A, et al. Hemocompatibility evaluation of different silver nanoparticle concentrations employing a modified Chandler-loop in vitro assay on human blood. Acta Biomater. (2013) 9:7460–8. doi: 10.1016/j.actbio.2013.03.016
79. Ragaseema VM, Unnikrishnan S, Kalliyana Krishnan V, Krishnan LK. The antithrombotic and antimicrobial properties of PEG-protected silver nanoparticle coated surfaces. Biomaterials. (2012) 33:3083–92. doi: 10.1016/j.biomaterials.2012.01.005
80. Smock KJ, Schmidt RL, Hadlock G, Stoddard G, Grainger DW, Munger MA. Assessment of orally dosed commercial silver nanoparticles on human ex vivo platelet aggregation. Nanotoxicology. (2014) 8:328–33. doi: 10.3109/17435390.2013.788749
81. Huang H, Lai W, Cui M, Liang L, Lin Y, Fang Q, et al. An evaluation of blood compatibility of silver nanoparticles. Sci Rep. (2016) 6:25518. doi: 10.1038/srep25518
82. Haberl N, Hirn S, Holzer M, Zuchtriegel G, Rehberg M, Krombach F. Effects of acute systemic administration of TiO2, ZnO, SiO2, and Ag nanoparticles on hemodynamics, hemostasis and leukocyte recruitment. Nanotoxicology. (2015) 9:963–71. doi: 10.3109/17435390.2014.992815
83. Nemmar A, Melghit K, Ali BH. The acute proinflammatory and prothrombotic effects of pulmonary exposure to rutile TiO2 nanorods in rats. Exp Biol Med. (2008) 233:610–9. doi: 10.3181/0706-RM-165
84. Šimundić M, Drašler B, Šuštar V, Zupanc J, Štukelj R, Makovec D, et al. Effect of engineered TiO2 and ZnO nanoparticles on erythrocytes, platelet-rich plasma and giant unilamelar phospholipid vesicles. BMC Vet Res. (2013) 9:7. doi: 10.1186/1746-6148-9-7
85. Geys J, Nemmar A, Verbeken E, Smolders E, Ratoi M, Hoylaerts MF, et al. Acute toxicity and prothrombotic effects of quantum dots: impact of surface charge. Environ Health Perspect. (2008) 116:1607–13. doi: 10.1289/ehp.11566
86. Deb S, Dasgupta AK. Thrombotic Inception at Nano-Scale Acute Coronary Syndromes. Open access peer-reviewed chapter (2012). Available online at: https://www.intechopen.com/books/acute-coronary-syndromes/thrombotic-inception-at-nano-scale
87. Bouwmeester H, Lynch I, Marvin HJ, Dawson KA, Berges M, Braguer D, et al. Minimal analytical characterization of engineered nanomaterials needed for hazard assessment in biological matrices. Nanotoxicology. (2011) 5:1–11. doi: 10.3109/17435391003775266
88. Taurozzi JS, Hackley VA, Wiesner MR. Ultrasonic dispersion of nanoparticles for environmental, health and safety assessment–issues and recommendations. Nanotoxicology. (2011) 5:711–29. doi: 10.3109/17435390.2010.528846
89. Strojan K, Leonardi A, Bregar VB, KriŽaj I, Svete J, Pavlin M. Dispersion of nanoparticles in different media importantly determines the composition of their protein corona. PLoS ONE. (2017) 12:e0169552. doi: 10.1371/journal.pone.0169552
90. Santos-Martínez MJ, Prina-Mello A, Medina C, Radomski MW. Analysis of platelet function: role of microfluidics and nanodevices. Analyst. (2011) 136:5120–6. doi: 10.1039/c1an15445a
91. Paciotti GF, Myer L, Weinreich D, Goia D, Pavel N, McLaughlin RE, et al. Colloidal gold: a novel nanoparticle vector for tumor directed drug delivery. Drug Deliv. (2004) 11:169–83. doi: 10.1080/10717540490433895
92. Huff TB, Tong L, Zhao Y, Hansen MN, Cheng JX, Wei A. Hyperthermic effects of gold nanorods on tumor cells. Nanomedicine. (2007) 2:125–32. doi: 10.2217/17435889.2.1.125
93. Goodman CM, McCusker CD, Yilmaz T, Rotello VM. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjug Chem. (2004) 15:897–900. doi: 10.1021/bc049951i
94. Pernodet N, Fang X, Sun Y, Bakhtina A, Ramakrishnan A, Sokolov J, et al. Adverse effects of citrate/gold nanoparticles on human dermal fibroblasts. Small. (2006) 2:766–73. doi: 10.1002/smll.200500492
95. Pan Y, Neuss S, Leifert A, Fischler M, Wen F, Simon U, et al. Size-dependent cytotoxicity of gold nanoparticles. Small. (2007) 3:1941–9. doi: 10.1002/smll.200700378
96. Alkilany AM, Murphy CJ. Toxicity and cellular uptake of gold nanoparticles: what we have learned so far? J Nanopart Res. (2010) 12:2313–33. doi: 10.1007/s11051-010-9911-8
97. Malugin A, Ghandehari H. Cellular uptake and toxicity of gold nanoparticles in prostate cancer cells: a comparative study of rods and spheres. J Appl Toxicol. (2010) 30:212–7. doi: 10.1002/jat.1486
98. White JG. Platelets are covercytes, not phagocytes: uptake of bacteria involves channels of the open canalicular system. Platelets. (2005) 16:121–31. doi: 10.1080/09537100400007390
99. Santos-Martinez MJ, Tomaszewski KA, Medina C, Bazou D, Gilmer JF, Radomski MW. Pharmacological characterization of nanoparticle-induced platelet microaggregation using quartz crystal microbalance with dissipation: comparison with light aggregometry. Int J Nanomed. (2015) 10:5107–19. doi: 10.2147/IJN.S84305
100. Deng ZJ, Liang M, Monteiro M, Toth I, Minchin RF. Nanoparticle-induced unfolding of fibrinogen promotes Mac-1 receptor activation and inflammation. Nat Nanotechnol. (2011) 6:39–44. doi: 10.1038/nnano.2010.250
101. Easo SL, Mohanan PV. in vitro hematological and in vivo immunotoxicity assessment of dextran stabilized iron oxide nanoparticles. Colloids Surf B. (2015) 134:122–30. doi: 10.1016/j.colsurfb.2015.06.046
102. Barry L, Holmes JD, Otway DJ, Copley MP, Kazakova O, Morris MA. Unusual magnetism in templated NiS nanoparticles. J Phys. (2010) 22:076001. doi: 10.1088/0953-8984/22/7/076001
103. Sun C, Ma M, Yang J, Zhang Y, Chen P, Huang W, et al. Phase-controlled synthesis of α-NiS nanoparticles confined in carbon nanorods for high performance supercapacitors. Sci Rep. (2014) 4:7054. doi: 10.1038/srep07054
104. Alonso F, Riente P, Yus M. Nickel nanoparticles in hydrogen transfer reactions. Acc Chem Res. (2011) 44:379–91. doi: 10.1021/ar1001582
105. Ahamed M, Alhadlaq HA. Nickel nanoparticle-induced dose-dependent cyto-genotoxicity in human breast carcinoma MCF-7 cells. Onco Targets Ther. (2014) 7:269–80. doi: 10.2147/OTT.S58044
106. Di Bucchianico S, Gliga AR, Åkerlund E, Skoglund S, Wallinder IO, Fadeel B, et al. Calcium-dependent cyto- and genotoxicity of nickel metal and nickel oxide nanoparticles in human lung cells. Part Fibre Toxicol. (2018) 15:32. doi: 10.1186/s12989-018-0268-y
107. Wahab R, Kaushik NK, Kaushik N, Choi EH, Umar A, Dwivedi S, et al. ZnO nanoparticles induces cell death in malignant human T98G gliomas, KB and non-malignant HEK cells. J Biomed Nanotechnol. (2013) 9:1181–9. doi: 10.1166/jbn.2013.1652
108. Wahab R, Siddiqui MA, Saquib Q, Dwivedi S, Ahmad J, Musarrat J, et al. ZnO nanoparticles induced oxidative stress and apoptosis in HepG2 and MCF-7 cancer cells and their antibacterial activity. Colloids Surf B Biointerf . (2014) 117:267–76. doi: 10.1016/j.colsurfb.2014.02.038
109. Xu X, Chen D, Yi Z, Jiang M, Wang L, Zhou Z, et al. Antimicrobial mechanism based on H2O2 generation at oxygen vacancies in ZnO crystals. Langmuir. (2013) 29:5573–80. doi: 10.1021/la400378t
110. Mishra YK, Adelung R, Rohl C, Shukla D, Spors F, Tiwari V. Virostatic potential of micro-nano filopodia-like ZnO structures against herpes simplex virus-1. Antiviral Res. (2011) 92:305–12. doi: 10.1016/j.antiviral.2011.08.017
111. Antoine TE, Mishra YK, Trigilio J, Tiwari V, Adelung R, Shukla D. Prophylactic, therapeutic and neutralizing effects of zinc oxide tetrapod structures against herpes simplex virus type-2 infection. Antiviral Res. (2012) 96:363–75. doi: 10.1016/j.antiviral.2012.09.020
112. Antoine TE, Hadigal SR, Yakoub AM, Mishra YK, Bhattacharya P, Haddad C, et al. Intravaginal zinc oxide tetrapod nanoparticles as novel immunoprotective agents against genital herpes. J Immunol. (2016) 196:4566–75. doi: 10.4049/jimmunol.1502373
113. Ahmad J, Wahab R, Siddiqui MA, Musarrat J, Al-Khedhairy AA. Zinc oxide quantum dots: a potential candidate to detain liver cancer cells. Bioprocess Biosyst Eng. (2015) 38:155–63. doi: 10.1007/s00449-014-1254-x
114. Wahab R, Khan F, Mishra YK, Musarrat J, Al-Khedhairy AA. Antibacterial studies and statistical design set data of quasi zinc oxide nanostructures. RSC Adv. (2016) 6:32328–39. doi: 10.1039/C6RA05297E
115. Wahab R, Kaushik N, Khan F, Kaushik NK, Choi EH, Musarrat J, et al. Self-styled ZnO nanostructures promotes the cancer cell damage and supresses the epithelial phenotype of glioblastoma. Sci Rep. (2016) 6:19950. doi: 10.1038/srep19950
116. Major TC, Brant DO, Burney CP, Amoako KA, Annich GM, Meyerhoff ME, et al. The hemocompatibility of a nitric oxide generating polymer that catalyzes S-nitrosothiol decomposition in an extracorporeal circulation model. Biomaterials. (2011) 32:5957–69. doi: 10.1016/j.biomaterials.2011.03.036
117. Warheit DB, Sayes CM, Reed KL, Swain KA. Health effects related to nanoparticle exposures: environmental, health and safety considerations for assessing hazards and risks. Pharmacol Ther. (2008) 120:35–42. doi: 10.1016/j.pharmthera.2008.07.001
118. Hussain SM, Hess KL, Gearhart JM, Geiss KT, Schlager JJ. in vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol In Vitro. (2005) 19:975–83. doi: 10.1016/j.tiv.2005.06.034
119. Sayes CM, Wahi R, Kurian PA, Liu Y, West JL, Ausman KD, et al. Correlating nanoscale titania structure with toxicity: a cytotoxicity and inflammatory response study with human dermal fibroblasts and human lung epithelial cells. Toxicol Sci. (2006) 92:174–85. doi: 10.1093/toxsci/kfj197
120. Bihari P, Holzer M, Praetner M, Fent J, Lerchenberger M, Reichel CA, et al. Single-walled carbon nanotubes activate platelets and accelerate thrombus formation in the microcirculation. Toxicology. (2010) 269:148–54. doi: 10.1016/j.tox.2009.08.011
121. Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR. Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm Res. (2016) 33:2373–87. doi: 10.1007/s11095-016-1958-5
122. Tajdidzadeh M, Azmi BZ, Yunus WMM, Talib ZA, Sadrolhosseini AR, Karimzadeh K, et al. Synthesis of silver nanoparticles dispersed in various aqueous media using laser ablation. Sci World J. (2014) 2014:324921. doi: 10.1155/2014/324921
123. Albanese A, Tang PS, Chan CW The effect of nanoparticle size shape and surface chemistry on biological systems. Annu Rev Biomed Eng. (2012) 14:1–16. doi: 10.1146/annurev-bioeng-071811-150124
124. Hulla J, Sahu S, Hayes A. Nanotechnology: history and future. Hum Exp Toxicol. (2015) 34:1318–21. doi: 10.1177/0960327115603588
Keywords: platelets, metallic nanoparticles, nanoparticles, activation, aggregation, cytotoxicity, nanoparticles charge, nanoparticles shape
Citation: Hante NK, Medina C and Santos-Martinez MJ (2019) Effect on Platelet Function of Metal-Based Nanoparticles Developed for Medical Applications. Front. Cardiovasc. Med. 6:139. doi: 10.3389/fcvm.2019.00139
Received: 14 June 2019; Accepted: 03 September 2019;
Published: 18 September 2019.
Edited by:
Marie Lordkipanidzé, Université de Montréal, CanadaReviewed by:
Christoph Eugen Hagemeyer, Monash University, AustraliaMatthew Dean Linden, University of Western Australia, Australia
Copyright © 2019 Hante, Medina and Santos-Martinez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carlos Medina, Y2FybG9zLm1lZGluYSYjeDAwMDQwO3RjZC5pZQ==; Maria Jose Santos-Martinez, c2FudG9zbW0mI3gwMDA0MDt0Y2QuaWU=
 Nadhim Kamil Hante
Nadhim Kamil Hante Carlos Medina1*
Carlos Medina1* Maria Jose Santos-Martinez
Maria Jose Santos-Martinez