- 1Department of Haematology Research, Murdoch Children's Research Institute, Melbourne, VIC, Australia
- 2Department of Paediatrics, The University of Melbourne, Melbourne, VIC, Australia
- 3Paediatric Intensive Care Unit, The Royal Children's Hospital, Melbourne, VIC, Australia
- 4Cardiothoracic Intensive Care Unit, National University Health System, Singapore, Singapore
- 5School of Biomedical Sciences, The University of Western Australia, Perth, WA, Australia
- 6Department of Clinical Haematology, The Royal Children's Hospital, Melbourne, VIC, Australia
Background: Despite increasing technical improvement and extracorporeal membrane oxygenation (ECMO)-related knowledge over the past three decades, morbidity and mortality associated with bleeding and clotting complications remain high in pediatric patients undergoing ECMO. Platelets, a key element of the coagulation system, have been proposed to be the main cause of coagulopathy in the setting of ECMO. This systematic review aims to summarize and discuss the existing knowledge of platelet phenotype and function in the pediatric ECMO population.
Methods: A systematic review was conducted for the Embase, Medline, and PubMed databases following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.
Results: The detailed study selection process yielded a total of 765 studies and only 3 studies that fulfilled the selection criteria were included in this review. Techniques used to assess platelet function in the three existing studies included platelet aggregometry, flow cytometry, and thromboelastography-platelet mapping. The finding that is common to the three studies is reduced platelet function in pediatric patients during ECMO either compared to before the initiation of ECMO or in non-survivors compared to survivors. Two studies demonstrated reduced platelet aggregation that are irreversible by platelet transfusion during ECMO. Two studies reported bleeding events and mortality in children on ECMO and none of the studies investigated thrombotic events.
Conclusions: This systematic review demonstrates the extremely limited information available for platelet phenotype and function in the pediatric ECMO population. Evidence from the existing literature suggests reduced platelet aggregation and increased platelet activation in children during ECMO. However, this needs to be interpreted with care due to the limitations associated with the techniques used for platelet function testing. Furthermore, the association between platelet dysfunction and clinical outcomes in the pediatric ECMO population remains elusive. Multiple research gaps have been identified when it comes to the knowledge of platelet phenotype and function of children on ECMO, highlighting the need for robust, well-designed studies in this setting.
Introduction
Extracorporeal membrane oxygenation (ECMO) is a modified form of cardiopulmonary bypass that aims to provide short to medium-length cardiac and/or respiratory support to patients. Despite the improvement in technologies and clinical practice over the years, bleeding, and/or thrombosis complications remain a major cause of morbidity and mortality in children on ECMO, with an overall survival rate of 54.9% (1). Using definitions of bleeding and thrombosis from the Extracorporeal Life Support Organization (ELSO), a study by Dalton et al. on 514 patients showed that bleeding is common in children on ECMO, with a rate of 70.2%, including intracranial hemorrhage in 16% of cases (1). Thrombotic events occurred in 37.5% of the patients, with 31.1% of patients requiring circuit component changes due to thrombosis (1).
The artificial surface and shear stress originating from the ECMO system have been proposed to be the main cause of coagulopathy seen in ECMO patients, via mechanisms such as activation of the hemostatic system. Modification of platelet function, a key element of the clotting system, has been suggested to be the main determinant of ECMO-induced bleeding and/or thrombosis in both the adult and pediatric ECMO population. Particularly, shear stress-induced platelet activation and loss of important platelet receptors have been reported in multiple studies for adults on ECMO and the resultant platelet dysfunction has been associated with increased thrombotic propensity and reduced hemostatic capacity (2–5).
Differences in platelet function and proteome between healthy adults and healthy children have been evaluated in multiple studies (6–8). Existing research in the ECMO population focuses on adults. However, the results may not be applicable to children due to the physiological differences between adults and children and the complex nature of ECMO. In addition, differences in response toward unfractionated heparin (UFH) (the main anticoagulant used in the setting of ECMO) between adults and children have also been documented (9, 10) and may be associated with the increased risk of anticoagulation-related complications in the pediatric ECMO cohort. Furthermore, differences in the clinical outcome between the adult and children ECMO patients (11) may also indicate differences in the pathophysiological development of coagulopathy between these two patient cohorts. Currently, there is very limited knowledge specific to platelet phenotype and function in children on ECMO (12–14) and there is no systematic review that summarizes the existing evidence for this population. On the other hand, existing studies relevant to platelet function and acquired von Willebrand syndrome were discussed previously for the adult ECMO population (15, 16). Hence, this systematic review aims to assemble and elaborate on existing studies focusing on platelet phenotype and function in children on ECMO.
Methods
Search Strategy
This systematic review was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (17). The Embase, Medline and PubMed databases were searched systematically on August 1, 2019 to identify studies in human and limited to the English language only. The following search terms were used for each of the database:
Embase: (*extracorporeal oxygenation OR *membrane oxygenator OR extracorporeal-membrane-oxygenation OR extra-corporeal-membrane-oxygenation OR extracorporeal-life-support OR extra-corporeal-life-support OR extracorporeal-cardiopulmonary-resuscitation OR extra-corporeal-cardiopulmonary-resuscitation OR ecmo OR ecls OR ecpr) AND (thrombocyte OR thrombocyte activation OR antithrombocytic agent OR platelet count OR blood clotting parameters OR thrombocytopheresis OR thrombocytopenia OR thrombocytosis OR thrombocyte transfusion OR fluoroimmunoassay OR *flow cytometry OR flow-cytometry OR aggregometry or platelet* OR thrombocyt* OR *thrombocyte function OR *thrombocyte dysfunction OR thrombocyte-count) AND (newborn* OR baby OR babies OR neonat* OR infan* OR toddler* OR pre-schooler* OR preschooler* OR kindergarten OR boy OR boys OR girl OR girls OR child OR children OR childhood OR adolescen* OR pediatric* OR paediatric* OR youth* OR teen OR teens OR teenage* OR school-aged* OR school-child* OR school-girl* OR school-boy* OR schoolgirl* OR schoolboy*).
Medline: (extracorporeal membrane oxygenation OR extracorporeal circulation OR oxygenators, membrane) AND (blood platelets OR platelet activation OR platelet aggregation inhibitors OR platelet count OR platelet function tests OR plateletpheresis OR thrombocytopenia OR thrombocytopenia OR thrombocytosis OR platelet transfusion OR fluoroimmunonoassay OR flow cytometry OR flow-cytometry or aggregometry or platelet* or thrombocyt*) AND (newborn* OR baby OR babies OR neonat* OR infan* OR toddler* OR pre-schooler* OR preschooler* OR kindergarten OR boy OR boys OR girl OR girls OR child OR children OR childhood OR adolescen* OR pediatric* OR paediatric* OR youth* OR teen OR teens OR teenage* OR school-aged* OR school-child* OR school-girl* OR school-boy* OR schoolgirl* OR schoolboy*).
PubMed: #1 (Extracorporeal-oxygenation OR Extra-corporeal-oxygenation OR Extracorporeal-membrane-oxygenation OR Extra-corporeal-membrane-oxygenation OR extracorporeal-life-support OR extra-corporeal-life-support OR extracorporeal-cardiopulmonary-resuscitation OR extra-corporeal-cardiopulmonary-resuscitation OR ECMO OR ECLS OR ECPR OR Extracorporeal-circulation OR Extra-corporeal-circulation OR membrane-oxygenator*) AND (Platelet* OR thrombocyt* OR Platelet-count OR Platelet-activation* OR thrombocyte-activation* OR Platelet-function* OR blood-clotting-parameter* OR Thrombocytopaenia* OR Thrombocytopenia* OR Thrombo-cytopaenia* OR Thrombo-cytopenia* OR Platelet-transfusion* OR thrombocyte-transfusion* OR Flow-cytometry OR fluoroimmunoassay* OR fluoro -immunoassay* OR Platelet-aggregation OR antithrombocytic-agent* OR anti-thrombocytic-agent* OR Plateletpheresis OR Thrombopheresis OR thrombocytopheresis OR aggregometry) AND (NOTNLM OR publisher[sb] OR inprocess[sb] OR pubmednotmedline[sb] OR indatareview[sb] OR pubstatusaheadofprint)) NOT ((animal OR animals OR rat OR rats OR mouse OR mice OR rodent* OR murine OR sheep) NOT (human OR humans OR newborn* OR baby OR babies OR neonat* OR infan* OR toddler* OR pre-schooler* OR preschooler* OR kindergarten OR boy OR boys OR girl OR girls OR child OR children OR childhood OR adolescen* OR pediatric* OR paediatric* OR youth* OR teen OR teens OR teenage* OR school-aged* OR school-child* OR school-girl* OR school-boy* OR schoolgirl* OR schoolboy*).
#2 (Case Reports[ptyp]) OR (Review[ptyp]).
#3 #1 NOT #2.
Study Selection
Inclusion criteria: (I) pediatric patients (0–18 years), (II) primary research, (III) platelet function assessed, (IV) English language, and (V) human study.
Exclusion criteria: (I) adult patients (>18 years), (II) review, case report, guideline, editorial correspondence, or conference abstract, (III) only platelet count measured, and (IV) animal study.
Results
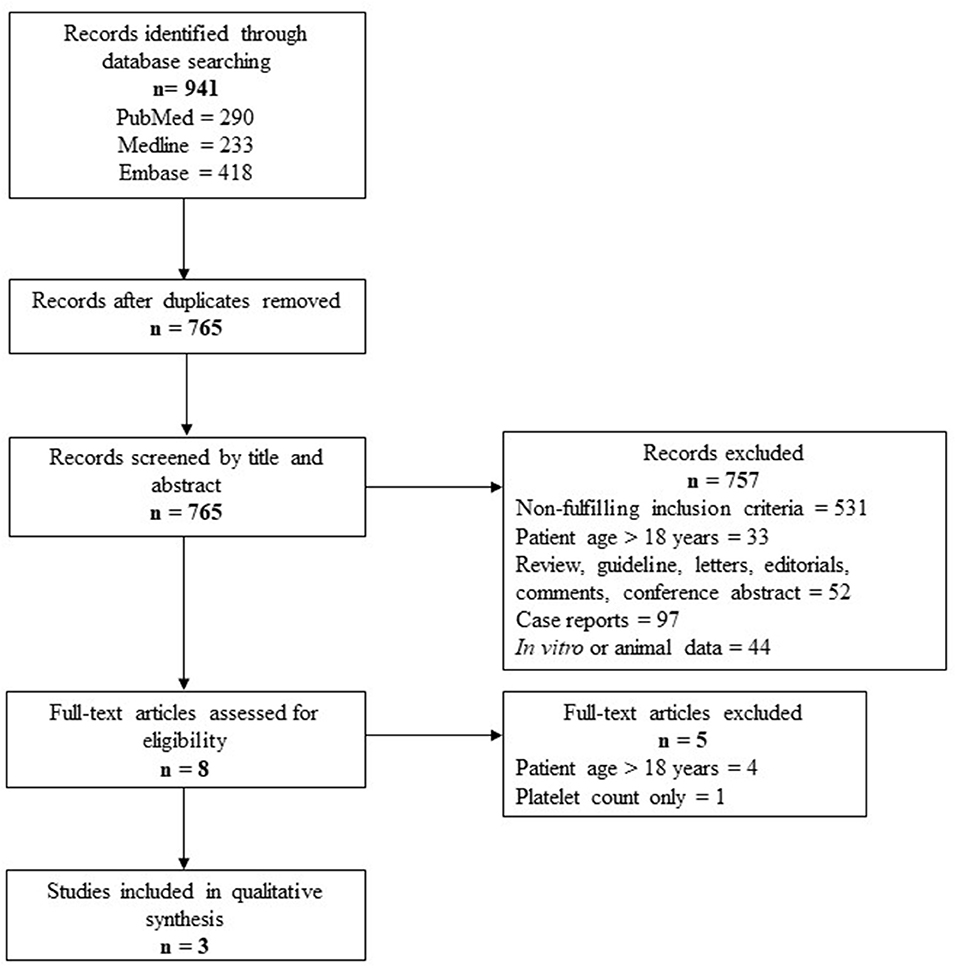
A total of 765 studies were identified with this systematic review search strategy. The majority of the studies (n = 757) that did not fulfill the inclusion criteria were excluded, including case reports that did not focus on assessing platelet phenotype and function. Eight studies were included for full-text screening and five of them were excluded for either patients aged >18 years or had assessed only platelet count in the study. A total of three studies matched the inclusion criteria and were summarized in this review. The steps used following the PRISMA guidelines are outlined in Figure 1. The summary for each of the included studies is included in Table 1.
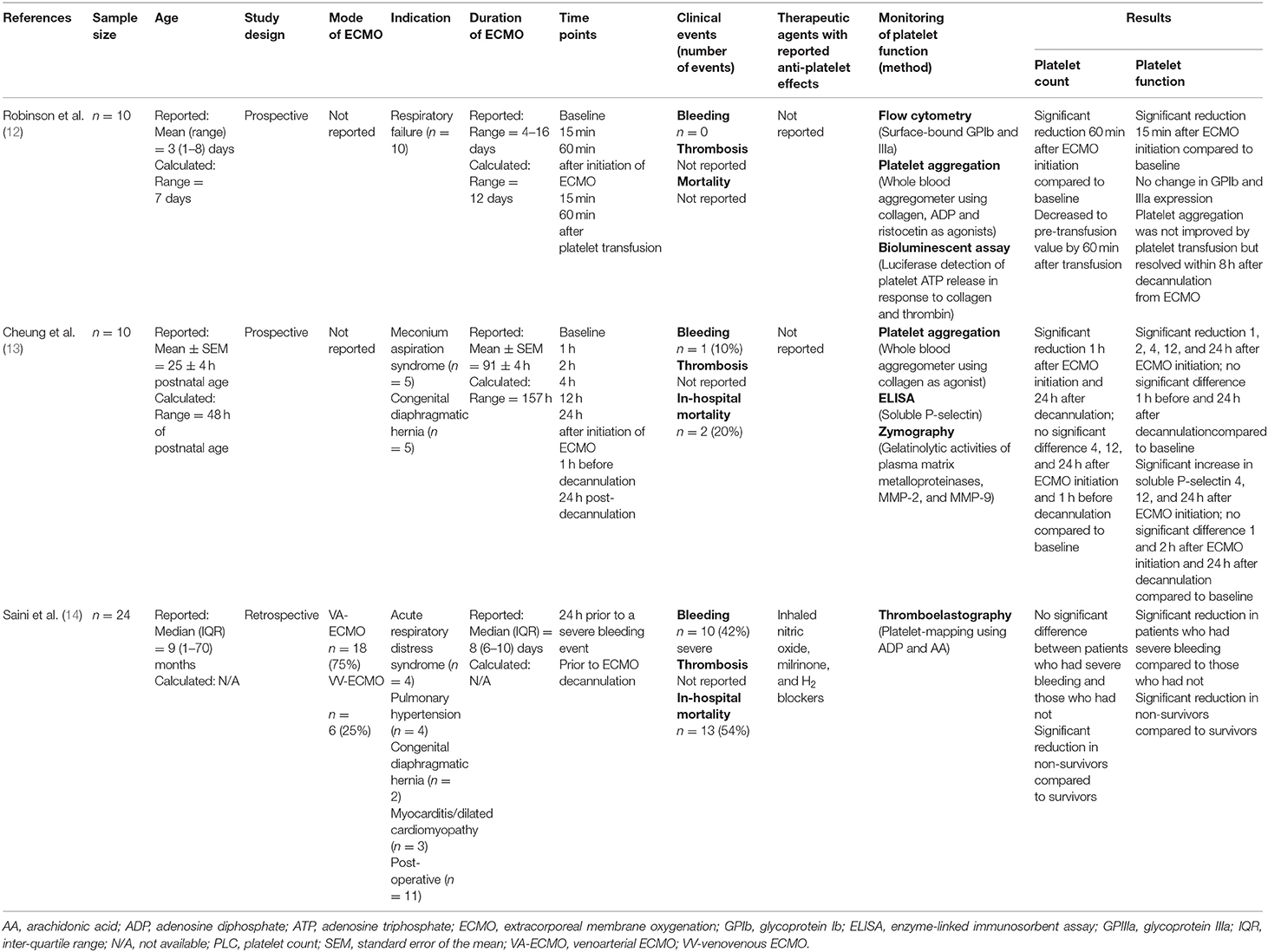
Table 1. The summary of the studies included in the systematic review for platelet phenotype and function in pediatric patients on ECMO.
Discussion
This systematic review demonstrates the absolute lack of studies that have focused on platelet phenotype and function in pediatric patients on ECMO. Such a lack of information in this population could reflect challenges in ethical approval and limited availability of blood sample volume for pediatric research. The main techniques used in the three existing studies included aggregometry, flow cytometry, and thromboelastography-platelet mapping. The common findings are reduced platelet function during ECMO. Particularly, evidence showed that the acquired platelet dysfunction during ECMO is not resolved by platelet transfusion. The relationship of plasma problems such as acquired von Willebrand's disease to the platelet dysfunction is unknown. In addition, the association between platelet dysfunction and bleeding and/or thrombosis commonly seen in children on ECMO has not been investigated to date.
Two out of the three studies utilized platelet aggregometry to investigate platelet function using agonists such as collagen, ADP and ristocetin (12, 13). Both studies reported reduced platelet aggregation in pediatric patients during ECMO despite the platelet transfusions given to the patients during ECMO. Robinson et al. reported reduced platelet aggregation in response to ADP and ristocetin but not for collagen within 15 min upon initiation of ECMO (12) whereas Cheung et al. have shown a decrease in platelet aggregation in response to collagen in a time-dependent manner starting from within 24 h upon cannulation for ECMO (13). The difference in collagen-induced platelet aggregation between the two studies may be related to the difference in concentration of collagen (5 vs. 10 μg/mL) used.
Despite being the gold standard in platelet function testing for many years, the applicability of aggregometry to the pediatric patients is often limited by the requirement of a large volume of blood. Specifically, platelet aggregometry usually requires 0.5–2 mL of blood which is a significant volume of blood to be withdrawn on multiple occasions from children who are critically ill and are sampled very frequently for a variety of clinical assays. Dalton et al. showed in children on ECMO that laboratory blood sampling is the primary cause of significant blood loss and is the main reason of transfusion in 42.2% of the studied population (1). Furthermore, multiple studies have suggested a strong association between platelet aggregometry and platelet count (18–20), which is problematic given that thrombocytopaenia is commonly found in children on ECMO. A study by Fernia et al. showed that light transmission aggregometry can be affected by platelet counts of <150 × 109 /L (20). None of the existing studies have reported whether any cut-off value was set for the platelet count for patients to be included in the analysis of platelet function using platelet aggregometry. Hence, results from the existing studies need to be interpreted with care.
In recent years, whole blood flow cytometry analysis of platelet function has gained attention in the pediatric setting for the requirement of only a minimal amount of blood and enabling analysis of platelets in their physiological environment, in the presence of the other blood cells with minimal manipulation. However, multiple technical challenges such as the requirement for trained-personnel and expensive reagents have limited its usage in a wider population. Only one study utilized flow cytometry to evaluate the expression of GPIb and IIIa, platelet markers important for adhesion and aggregation (12). There was no difference in the expression of these two receptors during ECMO compared to before initiation of ECMO. However, this study is now over 25 years old and flow cytometry techniques have improved considerably. In addition, these results must be interpreted with care as platelets are highly subjected to pre-analytical activation and have a limited processing window. Optimization of the flow cytometry methods for evaluation of platelet phenotype and function is important and this was not indicated as part of the study by Robinson et al. (12). Flow cytometric analysis of surface receptors on circulating platelets in adults on mechanical circulatory support (MCS) showed that ECMO patients had significantly lower expression of GPIbα and GPVI (platelet adhesion receptors) compared to healthy individuals (21). Whether the same changes occur in children of different ages remains to be determined.
In addition to platelet aggregometry and flow cytometry, thromboelastography-platelet mapping (TEG®-PM) has gained popularity as a platelet function assay in recent years and few centers have incorporated this system for hemostasis monitoring in children on ECMO (22). By using TEG®-PM, Saini et al. reported severe platelet dysfunction in more than 75% of the patient cohort (14). Using Multiplate® analyser with ADP and ristocetin, Nair et al. demonstrated 50–72% incidence of platelet dysfunction in the adult ECMO population (23). Furthermore, platelet count and TEG®-PM were shown to be a better predictor of severe bleeding and mortality compared to the activated clotting time in children on ECMO (14). A study by Weitzel et al. on the effects of cardiopulmonary bypass on cardiac surgery patients [mean (standard deviation) age: 58 (17)] also found reduced platelet aggregation in response to collagen, ADP and AA in the patient cohort and reported 83% sensitivity and 68% specificity for TEG®-PM to predict post-operative bleeding (24).
Thrombocytopaenia is the main trigger of platelet administration in ECMO patients and has been shown to be an important predictor of hemorrhage (25–27). Nevertheless, a study by Reed and Rutledge found no correlation between platelet level and hemorrhage or thrombosis in a cohort of deceased pediatric ECMO patients (28). On average, children on ECMO received 1.3 platelet transfusions per day to maintain platelet level of >100 × 109/L (29). However, frequent platelet transfusions in ECMO patients with excessive bleeding who had normal platelet counts may indicate occurrence of platelet dysfunction. Hence, in addition to the platelet count, it will also be important to monitor platelet function, which has been proposed to be the main cause of coagulopathy in ECMO patients.
In recent years, interactions between platelets and leukocytes have gained increased attention for their important roles in the cross-talk between the hemostatic and inflammatory systems. Currently, there are no studies that have investigated platelet-leukocyte interactions in children or adults on ECMO. Initiation of ECMO is known to induce inflammation via different mechanisms (30, 31) and elevated monocyte-platelet levels have been found in various thromboinflammatory conditions such as cardiovascular diseases (32, 33). Hence, future research focusing on investigating platelet-leukocyte interactions will be important for better understanding of how the interaction between the hemostatic and inflammatory systems may be associated with the development of clinical events in the pediatric ECMO population.
Conclusions
This systematic review revealed the existing research gaps in platelet function for children on ECMO. Existing studies are limited by small sample sizes and the types of platelet function tests performed. Pediatric ECMO patients represent a complex patient cohort with diverse etiologies and complications, resulting in pathological bleeding, and clotting. Hence, it will be crucial for future assessment of platelet function to investigate platelet phenotype and function from multiple aspects tailored to the extensive roles of platelets that are not only limited to coagulation but also as immune and inflammatory cells. In particular, future research using the multi-advantageous whole blood flow cytometry should focus on optimized and robust experimental design to ensure standardization and validity of the method used. Furthermore, it will be important to take into account the multiple patient-related factors such as interventions and procedures leading up to commencement of ECMO, age, and duration on ECMO for analysis in relation to the complex nature of this patient cohort.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This study was funded by National Health and Medical Research Council (NHMRC), Grant Number: APP1129317.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Dalton HJ, Reeder R, Garcia-Fillon P, Holubkov R, Berg RA, Zuppa A, et al. Factors associated with bleeding and thrombosis in children receiving extracorporeal membrane oxygenation. Am J Respir Crit Care Med. (2017) 196:762–71. doi: 10.1164/rccm.2016091945OC
2. Yoshimoto Y, Hasebe T, Takahashi K, Amari M, Nagashima S, Kamijo A, et al. Ultrastructural characterization of surface-induced platelet activation on artificial materials by transmission electron microscopy. Microsc Res Tech. (2013) 76:342–9. doi: 10.1002/jemt.22172
3. Chen Z, Mondal NK, Ding J, Koenig SC, Slaughter MS, Wu ZJ. Paradoxical effect of non-physiological shear stress on platelets and von Willebrand factor. Artif Organs. (2016) 40:659–68. doi: 10.1111/aor.12606
4. Chen Z, Mondal NK, Zheng S, Koenig SC, Slaughter MS, Griffith BP, et al. High shear induces platelet dysfunction leading to enhanced thrombotic propensity and diminished hemostatic capacity. Platelets. (2019) 30:112–9. doi: 10.1080/09537104.2017.1384542
5. Heilmann C, Geisen U, Beyersdorf F, Nakamura L, Benk C, Trummer G, et al. Acquired von Willebrand syndrome in patients with extracorporeal life support (ECLS). Intensive Care Med. (2012) 38:62–8. doi: 10.1007/s00134-011-2370-6
6. Yip C, Ignjatovic V, Attard C, Monagle P, Linden MD. First report of elevated monocyte-platelet aggregates in healthy children. PLoS ONE. (2013) 8:e67416. doi: 10.1371/journal.pone.0067416
7. Yip C, Linden MD, Attard C, Monagle P, Ignjatovic V. Platelets from children are hyper-responsive to activation by thrombin receptor activator peptide and adenosine diphosphate compared to platelets from adults. Br J Haematol. (2015) 168:526–32. doi: 10.1111/bjh.13153
8. Cini C, Yip C, Karlaftis V, Monagle P, Linden M, Ignajatovic V. Differences in the resting platelet proteome and platelet releasate between healthy children and adults. J Proteomics. (2015) 123:78–88. doi: 10.1016/j.jprot.2015.04.003
9. Ignjatovic V, Straka E, Summerhayes R, Monagle P. Age-specific differences in binding of heparin to plasma proteins. J Throm Haemos. (2010) 8:1290–4. doi: 10.1111/j.1538-7836.2010.03847.x
10. Newall F, Ignjatovic V, Johnston L, Summerhayes R, Lane G, Cranswick N, et al. Clinical use of unfractionated heparin therapy in children: time for change? Br J Haematol. (2010) 150:674–8. doi: 10.1111/j.1365-2141.2010.08302.x.
12. Robinson TM, Kickler TS, Walker LK, Ness P, Bell W. Effect of extracorporeal membrane oxygenation on platelets in newborn. Crit Care Med. (1993) 21:1029–34.
13. Cheung PY, Sawicki G, Salas E, Etches PC, Schulz R, Radomski MW. The mechanisms of platelet dysfunction during extracorporeal membrane oxygenation in critically ill neonates. Crit Care Med. (2000) 28:2584–90. doi: 10.1097/00003246-200007000-00067
14. Saini A, Hartmen ME, Gage BF, Said A, Gazit AZ, Boston US, et al. Incidence of platelet dysfunction by thromboelastography-platelet mapping in children supported with ECMO: a pilot retrospective study. Front Pediatr. (2016) 3:116. doi: 10.3389/fped.2015.00116
15. Malfertheiner MV, Pimenta LP, Bahr VV, Millar JE, Obonyo NG, Suen JY, et al. Acquired von Willebrand syndrome in respiratory extracorporeal life support: a systematic review of the literature. Crit Care Resusc. (2017) 19:45–52.
16. Balle CM, Jeppesen AN, Christensen S, Hvas AM. Platelet function during extracorporeal membrane oxygenation in adult patients: a systematic review. Front Cardiovasc Med. (2018) 5:157. doi: 10.3389/fcvm.2018.00157
17. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4:1. doi: 10.1186/2046-4053-4-1
18. Wurtz M, Hvas AM, Kristensen SD, Grove EL. Platelet aggregation is dependent on platelet count in patients with coronary artery disease. Throm Res. (2012) 129:56–61. doi: 10.1016/j.thromres.2011.08.019
19. Stissing T, Dridi MP, Ostrowski SR, Bochsen L, Johansson PI. The influence of low platelet count on whole blood aggregometry assessed by Multiplate. Clin Appl Throm Hemost. (2011) 17:E211–7. doi: 10.1177/1076029610397183
20. Fernia EA, Scavone M, Lecchi A, Cattaneo M. Effect of platelet count on platelet aggregation measured with impedance aggregometry (Multiplate™ analyzer) and with light transmission aggregometry. J Throm Haemost. (2013) 11:2193–6. doi: 10.1111/jth.12432
21. Lukito P, Wong A, Jing J, Arthur JF, Marasco SF, Murphy DA, et al. Mechanical circulatory support is associated with loss of platelet receptors glycoprotein Ibalpha and glycoprotein VI. J Throm Haemost. (2016) 14:2253–60. doi: 10.1111/jth.13497
22. Bembea MM, Schwartz JM, Shah N, Colantuoni E, Lehmann CU, Kickler T, et al. Anticoagulation monitoring during pediatric extracorporeal membrane oxygenation. ASAIO J. (2013) 59:63–8. doi: 10.1097/MAT.0b013e318279854a
23. Nair P, Hoechter DJ, Buscher H, Venkatesh K, Whittam S, Joseph J, et al. Prospective observational study of hemostatic alterations during adult extracorporeal membrane oxygenation (ECMO) using point-of-care thromboelastometry and platelet aggregometry. J Cardiothorac Vasc Anesth. (2015) 29:288–96. doi: 10.1053/j.jvca.2014.06.006
24. Weitzel NS, Weitzel LB, Epperson LE, Karimpour-Ford A, Tran ZV, Seres T. Platelet mapping as part of modified thromboelastography (TEG®) in patients undergoing cardiac surgery and cardiopulmonary bypass. Anaesthesia. (2012) 67:1158–65. doi: 10.1111/j.1365-2044.2012.07231.x
25. Hirthler MA, Blackwell E, Abbe D, Doe-Chapman R, LeClair Smith C, Goldthorn J, et al. Coagulation parameter instability as an early predictor of intracranial hemorrhage during extracorporeal membrane oxygenation. J Pediatr Surg. (1992) 27:40–3.
26. Sell LL, Cullen ML, Whittlesey GC, Yedlin ST, Philippart AI, Bedard MP, et al. Hemorrhagic complications during extracorporeal membrane oxygenation: prevention and treatment. J Pediatr Surg. (1986) 21:1087–91.
27. Dela Cruz TV, Stewart DL, Winston SJ, Weatherman KS, Phelps JL, Mendoza JC. Risk factors for intracranial hemorrhage in the extracorporeal membrane oxygenation patients. J Perinatol. (1997) 17:18–23.
28. Reed RC, Rutledge JC. Laboratory and clinical predictors of thrombosis and hemorrhage in 29 pediatric extracorporeal membrane oxygenation nonsurvivors. Pediatr Dev Pathol. (2010) 13:385–92. doi: 10.2350/09-09-0704-OA.1
29. Chevuru SC, Sola MC, Theriaque DW, Hutson AD, Leung WC, Rerez JA, et al. Multiple analysis of platelet transfusion usage among neonates on extracorporeal membrane oxygenation. Pediatrics. (2002) 109:e89. doi: 10.1542/peds.109.6.e89
30. Plotz FB, van Oeveren W, Bartlett RH, Wildevuur CR. Blood activation during neonatal extracorporeal life support. J Thorac Cardiovasc Surg. (1993) 105:823–32.
31. Millar JE, Fanning JP, McDonald CI, McAuley DF, Fraser JF. The inflammatory response to extracorporeal membrane oxygenation (ECMO): a review of the pathophysiology. Crit Care. (2016) 20:387. doi: 10.1186/s13054-016-1570-4
32. Furman MI, Barnard MR, Krueger LA, Fox ML, Shilale EA, et al. Circulating monocyte-platelet aggregates are an early marker of acute myocardial infarction. J Am Coll Cardiol. (2001) 38:1002–6. doi: 10.1016/s0735-1097(01)01485-1
Keywords: platelet, extracorporeal membrane oxygenation (ECMO), pediatric, bleeding, clotting, phenotype and function
Citation: Yaw HP, Van Den Helm S, MacLaren G, Linden M, Monagle P and Ignjatovic V (2019) Platelet Phenotype and Function in the Setting of Pediatric Extracorporeal Membrane Oxygenation (ECMO): A Systematic Review. Front. Cardiovasc. Med. 6:137. doi: 10.3389/fcvm.2019.00137
Received: 20 June 2019; Accepted: 29 August 2019;
Published: 18 September 2019.
Edited by:
Rory R. Koenen, Maastricht University, NetherlandsReviewed by:
Patricia B. Maguire, University College Dublin, IrelandPhilipp Diehl, University Heart Center Freiburg, Germany
Heidi Dalton, Inova Health System, United States
Copyright © 2019 Yaw, Van Den Helm, MacLaren, Linden, Monagle and Ignjatovic. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Ping Yaw, aHB5YXcxMDA2QGdtYWlsLmNvbQ==
 Hui Ping Yaw
Hui Ping Yaw Suelyn Van Den Helm
Suelyn Van Den Helm Graeme MacLaren1,2,3,4
Graeme MacLaren1,2,3,4 Matthew Linden
Matthew Linden Vera Ignjatovic
Vera Ignjatovic