
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Cardiovasc. Med. , 11 December 2018
Sec. Atherosclerosis and Vascular Medicine
Volume 5 - 2018 | https://doi.org/10.3389/fcvm.2018.00177
This article is part of the Research Topic Cardioimmunology: Inflammation and Immunity in Cardiovascular Disease View all 22 articles
 Elena Bartoloni
Elena Bartoloni Alessia Alunno
Alessia Alunno Valentina Valentini
Valentina Valentini Filippo Luccioli
Filippo Luccioli Eleonora Valentini
Eleonora Valentini Giuliana Maria Concetta La Paglia
Giuliana Maria Concetta La Paglia Maria Comasia Leone
Maria Comasia Leone Giacomo Cafaro
Giacomo Cafaro Elisa Marcucci
Elisa Marcucci Roberto Gerli*
Roberto Gerli*Evidence for increased risk of cardiovascular morbidity and mortality in chronic inflammatory rheumatic diseases has accumulated during the last years. Traditional cardiovascular risk factors contribute in part to the excess of cardiovascular risk in these patients and several mechanisms, including precocious acceleration of subclinical atherosclerotic damage, inflammation, and immune system deregulation factors, have been demonstrated to strictly interplay in the induction and progression of atherosclerosis. In this setting, chronic inflammation is a cornerstone of rheumatic disease pathogenesis and exerts also a pivotal role in all stages of atherosclerotic damage. The strict link between inflammation and atherosclerosis suggests that cardiovascular risk may be reduced by rheumatic disease activity control. There are data to suggest that biologic therapies, in particular TNFα antagonists, may improve surrogate markers of cardiovascular disease and reduce CV adverse outcome. Thus, abrogation of inflammation is considered an important outcome for achieving not only control of rheumatic disease, but also reduction of cardiovascular risk. However, the actual effect of anti-rheumatic therapies on atherosclerosis progression and CV outcome in these patients is rather uncertain due to great literature inconsistency. In this paper, we will summarize some of the main mechanisms linking the inflammatory pathogenic background underlying rheumatic diseases and the vascular damage observed in these patients, with a particular emphasis on the pathways targeted by currently available therapies. Moreover, we will analyze current evidence on the potential atheroprotective effects of these treatments on cardiovascular outcome pointing out still unresolved questions.
The long-term prognosis of chronic inflammatory rheumatic diseases (RDs), such as rheumatoid arthritis (RA), psoriatic arthritis (PsA), and ankylosing spondylitis (AS), is significantly influenced by increased risk of cardiovascular (CV) morbidity and mortality. In a large population-based, observational study, CV events resulted the third most frequent comorbidity in RA patients after depression and asthma (1). However, the evidence that screening and management of CV comorbidities in these patients is far from optimal deserves attention considering that high prevalence of atherosclerosis seems to occur yet in the earliest stages of the disease and also in young subjects free from CV risk factors, as demonstrated in particular in RA patients (2).
Chronic RDs and atherosclerotic endothelial damage share a similar inflammatory pathogenic background and multiple mechanisms contribute to subclinical atherosclerosis in these patients (3). It is demonstrated that disease-related inflammatory and immune mechanisms have a pivotal role in the pathogenesis of atherosclerosis and CV risk and that the contribution of traditional CV risk factors is at least as important as disease-specific factors (4). Indeed, prevalence of classic CV risk factors is higher in these patients in comparison to general population (5–8). In particular, hypertension, and diabetes mellitus represent two major factors to monitor in RD patients, both being associated with other CV comorbidities, disease activity and increased risk of CV events (5–9).
As inflammation is a cornerstone of the pathogenesis of systemic RDs and considering its pivotal role in driving all stages of atherosclerosis, it is compelling to hypothesize that controlling the pathways that induce synovial and systemic inflammation may provide benefit on CV risk in these patients (10). Although inconsistency in results between studies mainly due to different study design and different outcome measures, there are data suggesting that biologic therapies, in particular tumor necrosis factor-α inhibitors (TNFα-i), improve surrogate markers of subclinical atherosclerosis. Moreover, better control of RA activity has recently been associated with fewer CV events (11, 12). In a recent prospective study, failure in achieving disease activity control increased from 4- to 8-fold the risk to develop subclinical atherosclerosis and CV events at 1 year of follow-up (13). Although it is quite difficult to provide an actual long-term estimation of CV risk due to the lack of validated scores, tight, and sustained control of RD activity is necessary to effectively prevent CV disease development. Treat-to-target and abrogation of inflammation are now considered two main outcomes for achieving RD control. In addition, effective pharmacological treatment could favor physical activity, with consequent decrease of risk of obesity, diabetes, hypertension, and at least, CV disease. It is to note, however, that introduction of biologic agents is less frequent in RA patients with multiple concomitant comorbidities, although with active disease, and that some medications commonly used in these patients, such as corticosteroids (CS) and non-steroidal anti-inflammatory drugs, are known to enhance CV risk (14). In particular, some drugs may exert a dual effect. Indeed, if short-term CS treatment may lead to initial beneficial effect due to rapid suppression of inflammatory burden, it is well-known that long-term side effects of CS therapy may have a net adverse association with CV disease risk (15). Of consequence, the real effect of non-biologic and biologic therapy on CV risk and outcome in these patients is still uncertain.
In this perspective, a literature search was performed to identify articles investigating medium- and long-term effect of non-biologic and biologic therapies on subclinical atherosclerosis measures and CV outcome in patients with RA, PsA, and AS. Articles were identified in PubMed by using Mesh terms and keywords. Search was restricted to English language.
The definite demonstration that atherosclerosis is a dynamic process greatly driven by inflammatory factors has highlighted interesting pathogenic links between atherosclerotic arterial wall damage and inflammatory mechanisms underlying the pathogenesis of systemic RDs (16) (Figure 1). Systemic inflammation contributes to all stages of atherosclerosis starting from activation of endothelial layer and recruitment of inflammatory cells within arterial layer to monocyte differentiation and foam cell formation, with subsequent plaque development. Moreover, these molecules promote apoptosis of arterial smooth muscle cells, matrix degradation, and fibrosis with subsequent destabilization and rupture of atherosclerotic plaques. Immune dysregulation, through the involvement of T lymphocytes, contributes to amplification of inflammatory response driving atherosclerotic damage. T helper (Th)1 cells, in particular, secrete several cytokines, such as interferon (IFN)γ, interleukin (IL)-2, IL-12, IL-18, and TNFα, which contribute to vascular endothelial damage and plaque progression (17). Interestingly, these cytokines, in particular TNFα, IL-6, and IL-18, have been associated with endothelial dysfunction, carotid atherosclerosis, CV morbidity, and risk of CV events and mortality in patients with systemic RDs (18). Among inflammatory biomarkers, C-reactive protein (CRP), IL-6, IL-1, and TNFα have been extensively studied and employed as predictive tools of CV risk and future CV events (16, 17, 19). Strong evidence supports the direct role of these molecules in contributing to atherogenesis by favoring endothelial dysfunction, vascular oxidative stress, foam cell formation, and atherosclerotic plaque destabilization (16, 17, 19). In addition, pro-inflammatory cytokines may induce atherosclerosis causing an alteration of lipid profile. In particular, TNFα and IL-6 have been shown to induce a pro-atherogenic profile and insulin resistance in patients with RDs (18).
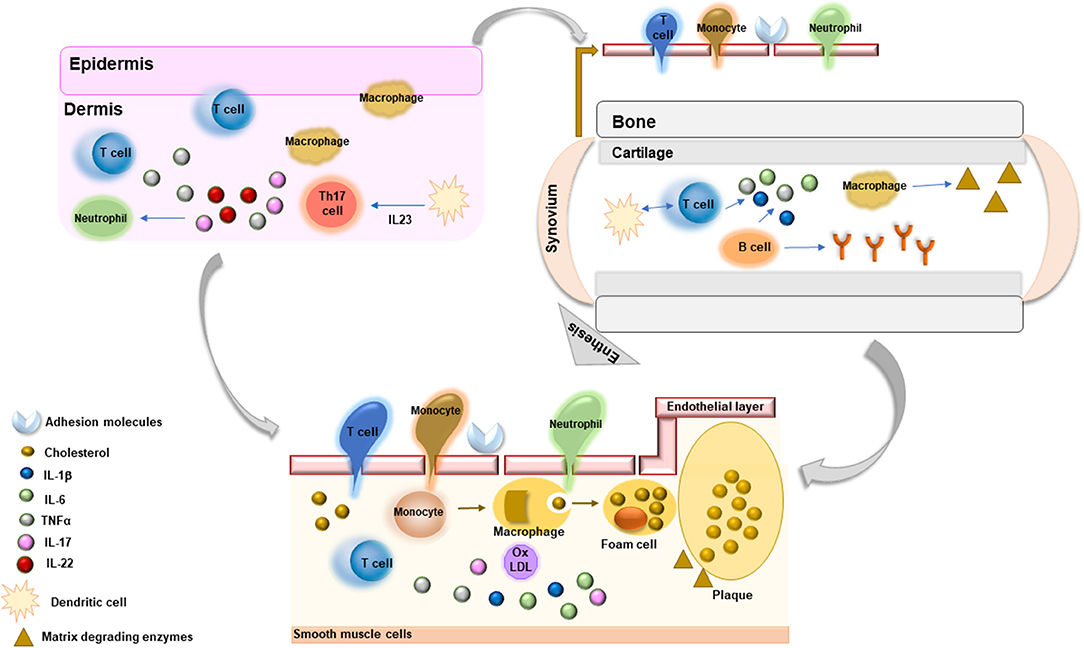
Figure 1. Common inflammatory mechanisms driving the pathogenesis of chronic rheumatic diseases and athrosclerosis.
Given the importance of pro-inflammatory cytokines in atherosclerosis and CV disease risk, effective modulation of inflammatory response in systemic RDs is expected to reduce risk and incidence of CV events and multiple pathways have been identified as potential therapeutic targets for the prevention and treatment of CV disease. In this setting, canakinumab, an inhibitor of IL-1β, was associated with significant reduction of recurrent CV events in patients with previous myocardial infarction and persistently elevated CRP levels (20). Three doses of canakinumab were tested and only the 150 mg dose reduced the relative risk of composite CV endpoint by 15%, mainly driven by a 24% reduction of relative risk of myocardial infarction. No significant reduction in CV death was observed and canakinumab was associated with an increased risk of fatal infection and sepsis. Surely, given the modest absolute clinical benefit, routine use of canakinumab in patients with previous myocardial infarction is not justified until more data are available.
On the other hand, in systemic RDs, randomized controlled trials (RCT)s of disease-modifying anti-rheumatic drugs (DMARDs), and biologic anti-cytokine therapies have not been powered to detect the impact of these agents on the modification of subclinical atherosclerosis and CV disease risk. Of consequence, data on the effect of these therapies on CV outcome in patients with RDs have been mainly driven by observational and pharmaco-epidemiological studies which suggest that close control of inflammation and disease activity in RDs may favorably affect some CV disease risk factors, reducing the rate of progression of subclinical atherosclerosis and the incidence of CV events (12) (Table 1).
Endothelial dysfunction, a potentially reversible step in atherosclerosis development, and structural vascular wall damage, assessed either as intima-media thickness (IMT) and carotid plaque, are considered important predictors of subsequent CV events in the general population as well as in patients with RA (21, 22). Multiple mechanisms, including systemic inflammatory burden, have been implicated in the pathophysiology of micro and macro-vascular endothelial dysfunction in patients with RDs and different methods are employed to detect precocious atherosclerosis in these patients (23). Thus, therapies reducing inflammation and disease activity are expected to improve vascular function and, possibly, arterial wall organic damage. In this setting, however, no definite conclusions can be driven on the effect of anti-rheumatic therapies on vascular endothelial function in inflammatory RDs. Multiple variables, including differences in study design, population enrolled, disease duration, instrumental technique employed in the assessment of subclinical atherosclerosis, length of follow-up, class of biologic drug used and concomitant therapies, hamper data interpretation and explain the high variability of study results. However, analysis of data derived by meta-analysis and systematic reviews, observational studies, and few RCTs allows to highlight some observations.
• Hydroxychloroquine (HCQ) has been associated with lower risk of diabetes mellitus (24), a better lipid profile characterized by reduced low-density lipoprotein and trygliceride levels (25) and antithrombotic effect on platelet aggregation. Moreover, in vitro studies demonstrated a potential vasoprotective effect by reduction of vascular endothelial adhesion molecule expression (26). Despite this beneficial evidence on lipid and glucose homeostasis, no studies explored the effects of HCQ on surrogate markers of atherosclerosis. Interestingly, a recent meta-analysis demonstrated that patients with RA and systemic connective diseases assuming HCQ are characterized by a significant reduction of CV events in comparison to non-HCQ users (27).
• Methotrexate (MTX) has several favorable effects on markers of CV damage. In particular, MTX therapy has been associated with improvement in reverse cholesterol transport (28), reduction of foam cell formation (29), down-regulation of adhesion molecule expression on endothelial surface (30), and reduced risk of metabolic syndrome (31). Moreover, response to MTX therapy is associated with reduction of circulating cytokines, including TNFα, IL-6, and IL-1, which exert atherogenic activity. Effects of MTX on measures of subclinical atherosclerosis has been explored in few studies showing a favorable response in atherosclerosis progression (32–34). In a recent observational study, 6-month MTX monotherapy was associated with a more pronounced favorable effect on endothelial function in comparison to TNFα-i ± MTX in a cohort of RD patients (35). The effect was independent of disease activity improvement. However, the small number of patients enrolled and the method used to detect atherosclerosis progression (change in Reactive Hyperemic Index) suggest caution in data interpretation.
• Short and medium-term studies demonstrated that TNFα-i are effective in improving arterial stiffness, evaluated as reduction of pulse wave velocity (PWV), and endothelial dysfunction, expressed as improvement in flow-mediated vasodilation (FMD), in RA patients, thus suggesting a link between chronic inflammation and endothelial dysfunction and arterial stiffness (11, 36, 37).
• TNFα-i therapy is associated with prevention or reversion of IMT progression in RD patients responding to treatment in studies with up to 5-year follow-up (36). The effect on IMT seems more relevant in RA patients with early disease (38).
• A beneficial effect on measures of microvascular endothelial dysfunction has been depicted in a small cohort of AS patients following 1 month of etanercept therapy, thus suggesting that suppression of inflammation is associated with rapid reversal of microvascular dysfunction in these patients (39). On the other hand, no effect of TNFα-i treatment has been detected on arterial stiffness and augmentation index (AIx) in wider cohorts of AS patients, suggesting that different disease-specific mechanims may contribute to endothelial impairment (40).
• AIx, a composite measure of arterial stiffness and speed of reflected wave from peripheral vascular resistances, usually does not change following TNFα-i therapy (36, 37). Intriguingly, this may suggest that arterial stiffness, a surrogate measure of macrovacular function, is more sensitive to inflammatory burden in RDs in comparison to other vascular functional parameters.
• Different TNFα-i may exert different effects on subclinical atherosclerosis. In this setting, adalimumab and etanercept have been associated with significant reduction of arterial stiffness in RA patients, while no change in the same measure was detected following infliximab administration (40). However, the limited number of studies does not allow to demonstrate a clear class-specific effect of TNFα-i on endothelial function in these patients (11).
Very few data are available on the effect of other non-TNFα-i targeted therapies on subclinical vascular endothelial damage. Inhibition of IL-6, a potent inflammatory cytokine inducing hepatic acute phase reactant production, has been associated with improvement of endothelial function, expressed as FMD, arterial stiffness and AIx, in open-label RCTs (40, 41). Interestingly, no changes in carotid IMT were reported (42), suggesting that rapid suppression of inflammation exerts more pronounced effect on endothelial function and that longer follow-up may be needed to detect significant changes of structural arterial wall damage. Conversely, B-cell blockade with rituximab was associated with improvement of carotid IMT in a pilot study without exerting significant effect on arterial stiffness and AIx in open label studies (43–45). Despite studies on atherosclerosis-prone mice demonstrated a favorable effect of abatacept in atherogenesis reduction, treatment with abatacept in humans was not associated with an improvement of surrogate measures of subclinical atherosclerosis, including aortic stiffness, AIx, carotid IMT, and plaques (40, 46).
• Although no RCT explored the independent effect of MTX on major CV outcomes, robust evidence supports that patients treated with MTX are characterized by a significant lower risk of all CV events, myocardial infarction and stroke in comparison to RD patients not receiving MTX (12, 47, 48). The effect was more evident in responders to therapy and the pooled relative reduction resulted 28% for all CV events and 19% for myocardial infarction (12). Moreover, the evidence was stronger for overall reduction of CV morbidity and mortality and weaker for stroke risk reduction (12).
• As observed with MTX, no randomized studies evaluated the risk of CV diseases in RD patients treated with non-MTX non-biologic therapies. Observational data suggest that long exposure to leflunomide and sulfasalazine may be associated with a reduced risk of all CV events and myocardial infarction (38, 46).
• Meta-analysis of cohort studies demonstrated that use of TNFα-i in RA patients is associated with a 30% relative reduction in all CV events and a 41% reduction of myocardial infarction in comparison to other non-biologic therapies (12). Subsequent systematic literature review of different studies confirmed the safety of biologic therapies in RDs patients with respect to CV outcome (40). However, the high variability in study design, CV outcome definition, populations enrolled and disease activity hamper data interpretation and makes it difficult to compare results among studies (40).
• As observed for MTX, the favorable effect on CV outcome may depend on clinical response since a lower incidence of myocardial infarction has been observed in responders to therapy (49).
• A recent prospective study with a longer follow-up (median 5 years) demonstrated that TNFα-i therapy in RA patients is associated with a significant reduction of 39% in the risk of myocardial infarction in comparison to DMARD therapy (50). This is the first demonstration that duration of TNFα-i exposure may be associated with reduction of CV risk in these patients and suggests that stable suppression of inflammation and disease activity control are mandatory targets in the prevention of CV disease risk.
• A prospective analysis of the same cohort depicted that, compared to DMARDs, ever-exposure to TNFα-i therapy is not associated to a significant effect on the risk of first ischemic stroke over a median period of 5 years. Although not statistically significant, there was a trend toward a reduction in mortality at 30 days and at 1 year following the event among patients treated with TNFα-i at stroke occurrence compared to the other group (51). This may suggest different and still unexplored pathogenic mechanims underlying ischemic cerebrovascular events in RA patients.
• Unfavorable lipid profile has been observed following TCZ therapy. However, pooled analysis of clinical trials and post-marketing safety data suggest that the CV disease risk in TCZ users is comparable to the risk associated with other biologic therapies (52–54). Indeed, a clear inverse relationship, known as the “lipid paradox,” has been demonstrated between lipid levels and CV risk in RA patients with an increased risk of CV disease also in patients with low total cholesterol and low-density lipoprotein (LDL) levels in the setting of active inflammation (55). Despite the global increase in LDL, total cholesterol, and triglyceride levels following the reduction of inflammatory burden, a favorable anti-inflammatory change of high-density lipoprotein composition and function has been demonstrated following tocilizumab administration, thus suggesting its positive net effect on CV risk (56).
• Abatacept may be associated with lower risk of myocardial infarction in comparison with TNFα-i. A retrospective study enrolling RA patients initiating biologic therapies, patients treated with abatacept were characterized by a lower risk of myocardial infarction in comparison to patients on TNFα-i therapy (57). Interestingly, these data have been recently confirmed in a large population-based cohort of RA patients demonstrating that abatacept was associated with a significant 29% reduced risk of a CV composite endopoint (myocardial infarction, stroke/transient ischemic attack, and coronary revascularization) when compared with TNFα-i therapy, in particular in patients with diabetes mellitus (58).
• Data on CV outcome in patients treated with rituximab are scarce. Observational studies did not observe significant differences in CV event rates in patients treated with rituximab in comparison to TNFα-i therapy or abatacept (40, 46).
• The period passed from the introduction of anti IL-12/23 targeted therapies is too short to draw hypothesis on their effect on CV outcome (40, 59).
Despite broad evidence suggests that non-biologic and biologic therapies may be associated with a reduced risk of CV events and more favorable CV outcome in RD patients, several points should be considered in data interpretation, suggesting caution in their feasibility.
• The high variability in study designs and inclusion/exclusion criteria, in disease characteristics (grade of activity, seropositivity, duration, concomitant CV risk factors, concomitant therapies as non-steroidal anti-inflammatory drugs), in CV event definition, and in cohort enrolled represent a major limit to consider.
• The median follow-up of almost all studies was too short to effectively detect a significant reduction of long-term CV events. Similarly, the variable follow-up across studies makes it difficult to verify the durability of therapy effect on subclinical atherosclerosis measure improvement.
• The application in many studies of surrogate markers of atherosclerosis to estimate CV disease risk due to the low number of CV events, which limited statistical significance detection, remains an important limit in the interpretation of study results.
• Further studies are needed to investigate if the reduction of CV risk is a direct effect of these targeted therapies on atherosclerotic process or an indirect manifestation of the general reduction of systemic inflammation and disease activity.
• Research should focus on evaluation of drug-specific class effects on CV disease risk in order to enable better and personalized use of targeted therapies according to patient CV risk phenotype and disease characteristics.
• Further studies are needed to more deeply elucidate the contribution of inflammation to the pathophysiology of atherosclerosis in RDs and to identify specific non-invasive biomarkers to be employed as tool to identify patients with higher CV risk and guide therapy selection.
• The effect of targeted therapies on CV risk as well as pathogenic mechanisms leading to atherosclerotic damage in patients with SA and PsA should be further investigated.
• Larger, prospective studies with longer follow-up and RCTs with hard CV end-points are urgently needed to better characterize the CV outcome in these patients.
• Specific CV disease screening by validated CV risk score in RD patients should be implemented in order to quantify the CV long-term outcome and guide the better primary and secondary CV preventive therapeutic strategy.
• Despite advances in the treatment of these chronic RDs and better control of disease activity, CV-related mortality remains elevated in these patients. Under-recognition and suboptimally treatment of CV risk factors in association with the unavailability of validated treatment recommendations represent major causes for the lack of proper CV risk management in usual clinical care.
EB wrote the whole manuscript. RG revised and approved the final manuscript draft. All Authors revised and approved the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Dougados M, Soubrier M, Antunez A, Balint P, Balsa A, Buch MH, et al. Prevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: results of an international, cross-sectional study (COMORA). Ann Rheum Dis. (2014) 73:62–8. doi: 10.1136/annrheumdis-2013-204223
2. Bartoloni E, Alunno A, Bistoni O, Gerli R. How early is the atherosclerotic risk in rheumatoid arthritis? Autoimmun Rev. (2010) 9:701–7. doi: 10.1016/j.autrev.2010.06.001
3. Bartoloni E, Shoenfeld Y, Gerli R. Inflammatory and autoimmune mechanisms in the induction of atherosclerotic damage in systemic rheumatic diseases: two faces of the same coin. Arthritis Care Res. (2011) 63:178–83. doi: 10.1002/acr.20322
4. Crowson CS, Rollefstad S, Ikdahl E, Kitas G, van Riel PL, Gabriel S, et al. Impact of risk factors associated with cardiovascular outcomes in patients with rheumatoid arthritis. Ann Rheum Dis. (2018) 77:48–54. doi: 10.1136/annrheumdis-2017-211735
5. Bartoloni E, Alunno A, Valentini V, Luccioli F, Valentini E, La Paglia G, et al. Role of inflammatory diseases in hypertension. High Blood Press Cardiovasc Prev. (2017) 24:353–61. doi: 10.1007/s40292-017-0214-3
6. Bartoloni E, Alunno A, Valentini V, Valentini E, La Paglia GCM, Leone MC, et al. The prevalence and relevance of traditional cardiovascular risk factors in primary Sjögren's syndrome. Clin Exp Rheumatol. (2018) 36(Suppl. 112):113–120.
7. Bartoloni E, Alunno A, Gerli R. Hypertension as a cardiovascular risk factor in autoimmune rheumatic diseases. Nat Rev Cardiol. (2018) 15:33–44. doi: 10.1038/nrcardio.2017.118
8. Ruscitti P, Ursini F, Cipriani P, Ciccia F, Liakouli V, Carubbi F, et al. Prevalence of type 2 diabetes and impaired fasting glucose in patients affected by rheumatoid arthritis: results from a cross-sectional study. Medicine (2017) 96:e7896. doi: 10.1097/MD.0000000000007896
9. Crepaldi G, Scirè CA, Carrara G, Sakellariou G, Caporali R, Hmamouchi I, et al. Cardiovascular comorbidities relate more than others with disease activity in rheumatoid arthritis. PLoS ONE (2016) 11:e0146991. doi: 10.1371/journal.pone.0146991
10. Geovanini GR, Libby P. Atherosclerosis and inflammation: overview and updates. Clin Sci. (2018) 132:1243–52. doi: 10.1042/CS20180306
11. Ursini F, Leporini C, Bene F, D'Angelo S, Mauro D, Russo E, et al. Anti-TNF-alpha agents and endothelial function in rheumatoid arthritis: a systematic review and meta-analysis. Sci Rep. (2017) 7:5346. doi: 10.1038/s41598-017-05759-2
12. Roubille C, Richer V, Starnino T, McCourt C, McFarlane A, Fleming P, et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis. (2015) 74:480–9. doi: 10.1136/annrheumdis-2014-206624
13. Ruscitti P, Cipriani P, Masedu F, Romano S, Berardicurti O, Liakouli V, et al. Increased cardiovascular events and subclinical atherosclerosis in rheumatoid arthritis patients: 1 Year prospective single centre study. PLoS ONE (2017) 12:e0170108. doi: 10.1371/journal.pone.0170108
14. Radner H, Yoshida K, Hmamouchi I, Dougados M, Smolen JS, Solomon DH. Treatment patterns of multimorbid patients with rheumatoid arthritis: results from an international cross-sectional study. J Rheumatol. (2015) 42:1099–104. doi: 10.3899/jrheum.141534
15. Bartoloni E, Alunno A, Santoboni G, Gerli R. Beneficial cardiovascular effects of low-dose glucocorticoid therapy in inflammatory rheumatic diseases. J Rheumatol. (2012) 39:1758–60. doi: 10.3899/jrheum.120192
16. Raggi P, Genest J, Giles JT, Rayner KJ, Dwivedi G, Beanlands RS, et al. Role of inflammation in the pathogenesis of atherosclerosis and therapeutic interventions. Atherosclerosis (2018) 276:98–108. doi: 10.1016/j.atherosclerosis.2018.07.014
17. Moriya J. Critical roles of inflammation in atherosclerosis. J Cardiol. (2018) 73:22–7. doi: 10.1016/j.jjcc.2018.05.010
18. Arida A, Protogerou AD, Kitas GD, Sfikakis PP. Systemic inflammatory response and atherosclerosis: the paradigm of chronic inflammatory rheumatic diseases. Int J Mol Sci. (2018) 19:1890. doi: 10.3390/ijms19071890
19. Avan A, Tavakoly Sany SB, Ghayour-Mobarhan M, Rahimi HR, Tajfard M, Ferns G. Serum C-reactive protein in the prediction of cardiovascular diseases: overview of the latest clinical studies and public health practice. J Cell Physiol. (2018) 233:8508–25. doi: 10.1002/jcp.26791
20. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. (2017) 377:1119–31. doi: 10.1056/NEJMoa1707914
21. Gutterman DD, Chabowski DS, Kadlec AO, Durand MJ, Freed JK, Ait-Aissa K, et al. The human microcirculation: regulation of flow and beyond. Circ. Res. (2016) 118:157–72. doi: 10.1161/CIRCRESAHA.115.305364
22. Evans MR, Escalante A, Battafarano DF, Freeman GL, O'Leary DH, del Rincón I. Carotid atherosclerosis predicts incident acute coronary syndromes in rheumatoid arthritis. Arthritis Rheum. (2011) 63:1211–20. doi: 10.1002/art.30265
23. Bordy R, Totoson P, Prati C, Marie C, Wendling D, Demougeot C. Microvascular endothelial dysfunction in rheumatoid arthritis. Nat Rev Rheumatol. (2018) 14:404–20. doi: 10.1038/s41584-018-0022-8
24. Solomon DH, Massarotti E, Garg R, Liu J, Canning C, Schneeweiss S. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA (2011) 305:2525–31. doi: 10.1001/jama.2011.878
25. Morris SJ, Wasko MC, Antohe JL, Sartorius JA, Kirchner HL, Dancea S, et al. Hydroxychloroquine use associated with improvement in lipid profiles in rheumatoid arthritis patients. Arthritis Care Res. (2011) 63:530–4. doi: 10.1002/acr.20393
26. Le NT, Takei Y, Izawa-Ishizawa Y, Heo KS, Lee H, Smrcka AV, et al. Identification of activators of ERK5 transcriptional activity by high-throughput screening and the role of endothelial ERK5 in vasoprotective effects induced by statins and antimalarial agents. J Immunol. (2014) 193:3803–15. doi: 10.4049/jimmunol.1400571
27. Mathieu S, Pereira B, Tournadre A, Soubrier M. Cardiovascular effects of hydroxychloroquine: a systematic review and meta-analysis. Ann Rheum Dis. (2018) 77:e65. doi: 10.1136/annrheumdis-2017-212668
28. Reiss AB, Carsons SE, Anwar K, Rao S, Edelman SD, Zhang H, et al. Atheroprotective effects of methotrexate on reverse cholesterol transport proteins and foam cell transformation in human THP-1 monocyte/macrophages. Arthritis Rheum. (2008) 58:3675–83. doi: 10.1002/art.24040
29. Ronda N, Greco D, Adorni MP, Zimetti F, Favari E, Hjeltnes G, et al. Newly identified antiatherosclerotic activity of methotrexate and adalimumab: complementary effects on lipoprotein function and macrophage cholesterol metabolism. Arthritis Rheumatol. (2015) 67:1155–64. doi: 10.1002/art.39039
30. Hjeltnes G, Hollan I, Forre O, Wiik A, Lyberg T, Mikkelsen K, et al. Serum levels of lipoprotein(a) and E-selectin are reduced in rheumatoid arthritis patients treated with methotrexate or methotrexate in combination with TNF-alpha-inhibitor. Clin Exp Rheumatol. (2013) 31:415–21.
31. Kerekes G, Nurmohamed MT, Gonzalez-Gay MA, Seres I, Paragh G, Kardos Z, et al. Rheumatoid arthritis andmetabolic syndrome. Nat Rev Rheumatol. (2014) 10:691–6. doi: 10.1038/nrrheum.2014.121
32. Vandhuick T, Allanore Y, Borderie D, Louvel JP, Fardellone P, Dieude P, et al. Early phase clinical and biological markers associated with subclinical atherosclerosis measured at 7 years of evolution in an early inflammatory arthritis cohort. Clin Exp Rheumatol. (2016) 34:58–67.
33. Kim HJ, Kim MJ, Lee CK, Hong YH. Effects of methotrexate on carotid intima-media thickness in patients with rheumatoid arthritis. J Korean Med Sci. (2015) 30:1589–96. doi: 10.3346/jkms.2015.30.11.1589
34. Guin A, Chatterjee Adhikari M, Chakraborty S, Sinhamahapatra P, Ghosh A. Effects of disease modifying anti-rheumatic drugs on subclinical atherosclerosis and endothelial dysfunction which has been detected in early rheumatoid arthritis: 1-year follow-up study. Semin Arthritis Rheum. (2013) 43:48–54. doi: 10.1016/j.semarthrit.2012.12.027
35. Deyab G, Hokstad I, Whist JE, Smastuen MC, Agewall S, Lyberg T, et al. Methotrexate and anti-tumor necrosis factor treatment improves endothelial function in patients with inflammatory arthritis. Arthritis Res Ther. (2017) 19:232. doi: 10.1186/s13075-017-1439-1
36. Tam LS, Kitas GD, González-Gay MA. Can suppression of inflammation by anti-TNF prevent progression of subclinical atherosclerosis in inflammatory arthritis? Rheumatology (2014) 53:1108–19. doi: 10.1093/rheumatology/ket454
37. Dulai R, Perry M, Twycross-Lewis R, Morrissey D, Atzeni F, Greenwald S. The effect of tumor necrosis factor-α antagonists on arterial stiffness in rheumatoid arthritis: a literature review. Semin Arthritis Rheum. (2012) 42:1–8. doi: 10.1016/j.semarthrit.2012.02.002
38. Zekanecz Z, Kerekes G, Végh E, Kardos Z, Baráth Z, Tamási L, et al. Autoimmune atherosclerosis in 3D: how it develops, how to diagnose and what to do. Autoimmun Rev. (2016) 15:756–69. doi: 10.1016/j.autrev.2016.03.014
39. van Eijk IC, Peters MJ, Serné EH, van der Horst-Bruinsma IE, Dijkmans BA, Smulders YM, et al. Microvascular function is impaired in ankylosing spondylitis and improves after tumour necrosis factor alpha blockade. Ann Rheum Dis. (2009) 68:362–6. doi: 10.1136/ard.2007.086777
40. Nurmohamed M, Choy E, Lula S, Kola B, DeMasi R, Accossato P. The impact of biologics and tofacitinib on cardiovascular risk factors and outcomes in patients with rheumatic disease: a systematic literature review. Drug Saf. (2018) 41:473–88. doi: 10.1007/s40264-017-0628-9
41. Protogerou AD, Zampeli E, Fragiadaki K, Stamatelopoulos K, Papamichael C, Sfikakis PP. A pilot study of endothelial dysfunction and aortic stiffness after interleukin-6 receptor inhibition in rheumatoid arthritis. Atherosclerosis (2011) 219:734–6. doi: 10.1016/j.atherosclerosis.2011.09.015
42. Kume K, Amano K, Yamada S, Hatta K, Ohta H, Kuwaba N. Tocilizumab monotherapy reduces arterial stiffness as effectivelyvas etanercept or adalimumab monotherapy in rheumatoid arthritis: an open-label randomized controlled trial. J Rheumatol. (2011) 38:2169–71. doi: 10.3899/jrheum.110340
43. Kerekes G, Soltesz P, Der H, Veres K, Szabo Z, Vegvari A, et al. Effects of rituximab treatment on endothelial dysfunction, carotid atherosclerosis, and lipid profile in rheumatoid arthritis. Clin Rheumatol. (2009) 28:705–10. doi: 10.1007/s10067-009-1095-1
44. Provan SA, Berg IJ, Hammer HB, Mathiessen A, Kvien TK, Semb AG. The impact of newer biological disease modifying anti-rheumatic drugs on cardiovascular risk factors: a 12-month longitudinal study in rheumatoid arthritis patients treated with rituximab, abatacept and tociliziumab. PLoS ONE (2015) 10:e0130709. doi: 10.1371/journal.pone.0130709
45. Mathieu S, Pereira B, Dubost JJ, Lusson JR, Soubrier M. No significant change in arterial stiffness in RA after 6 months and 1 year of rituximab treatment. Rheumatology (2012) 51:1107–11. doi: 10.1093/rheumatology/kes006
46. Giles J. Rheumatoid arthritis pharmacotherapies: do they have anti-atherosclerotic activity? Curr Rheumatol Rep. (2016) 18:27. doi: 10.1007/s11926-016-0578-8
47. Micha R, Imamura F, Wyler von Ballmoos M, Solomon DH, Hernàn M, Ridker P, et al. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol. (2011) 108:1362–70. doi: 10.1016/j.amjcard.2011.06.054
48. De Vecchis R, Baldi C, Palmisani L. Protective effects of methotrexate against ischemic cardiovascular disorders in patients treated for rheumatoid arthritis or psoriasis: novel therapeutic insights coming from a meta-analysis of the literature data. Anatol J Cardiol. (2016) 16:2–9. doi: 10.5152/akd.2015.6136
49. Dixon WG, Watson KD, Lunt M, Hyrich KL, British Society for Rheumatology Biologics Register Control Centre Consortium, Silman AJ, et al. Reduction in the incidence ofmyocardial infarction in patients with rheumatoid arthritis who respond to anti-tumor necrosis factor alpha therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. (2007) 56:2905–12. doi: 10.1002/art.22809
50. Low AS, Symmons DP, Lunt M, Mercer LK, Gale CP, Watson KD, et al. Relationship between exposure to tumour necrosis factor inhibitor therapy and incidence and severity of myocardial infarction in patients with rheumatoid arthritis. Ann Rheum Dis. (2017) 76:654–660. doi: 10.1136/annrheumdis-2016-209784
51. Low AS, Lunt M, Mercer LK, Watson KD, Dixon WG, Symmons DP, et al. Association between ischemic stroke and tumor necrosis factor inhibitor therapy in patients with rheumatoid arthritis. Arthritis Rheumatol. (2016) 68:1337–45. doi: 10.1002/art.39582
52. Xie F, Yun H, Levitan EB, Muntner P, Curtis JR. Tocilizumab and the risk of cardiovascular disease: a direct comparison among biologic disease-modifying antirheumatic drugs for rheumatoid arthritis patients. Arthritis Care Res. (2018). doi: 10.1002/acr.23737. [Epub ahead of print].
53. Kim SC, Solomon DH, Rogers JR, Gale S, Klearman M, Sarsour K, et al. No difference in cardiovascular risk of tocilizumab versus abatacept for rheumatoid arthritis: a multi-database cohort study. Semin Arthritis Rheum. (2018). doi: 10.1016/j.semarthrit.2018.03.012. [Epub ahead of print].
54. Generali E, Carrara G, Selmi C, Verstappen S, Zambon A, Bortoluzzi A, et al. Comparison of the risks of hospitalisation for cardiovascular events in patients with rheumatoid arthritis treated with tocilizumab and etanercept. Clin Exp Rheumatol. (2018) 36:310–13.
55. Myasoedova E. Lipids and lipid changes with synthetic and biologic disease-modifying antirheumatic drug therapy in rheumatoid arthritis: implications for cardiovascular risk. Curr Opin Rheumatol. (2017) 29:277–84. doi: 10.1097/BOR.0000000000000378
56. McInnes IB, Thompson L, Giles JT, Bathon JM, Salmon JE, Beaulieu AD, et al. Effect of interleukin-6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo-controlled study. Ann Rheum Dis. (2015) 74:694–702. doi: 10.1136/annrheumdis-2013-204345
57. Zhang J, Xie F, Yun H, Chen L, Muntner P, Levitan EB, et al. Comparative effects of biologics on cardiovascular risk among older patients with rheumatoid arthritis. Ann Rheum Dis. (2016) 75:1813–8. doi: 10.1136/annrheumdis-2015-207870
58. Kang EH, Jin Y, Brill G, Lewey J, Patorno E, Desai RJ, et al. Comparative cardiovascular risk of Abatacept and Tumor Necrosis Factor Inhibitors in patients with rheumatoid arthritis with and without diabetes mellitus: A multidatabase cohort study. J Am Heart Assoc. (2018) 7:e007393. doi: 10.1161/JAHA.117.007393
Keywords: rheumatic disease, inflammation, cardiovascular disease, atherosclerosis, biologic therapies, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis
Citation: Bartoloni E, Alunno A, Valentini V, Luccioli F, Valentini E, La Paglia GMC, Leone MC, Cafaro G, Marcucci E and Gerli R (2018) Targeting Inflammation to Prevent Cardiovascular Disease in Chronic Rheumatic Diseases: Myth or Reality? Front. Cardiovasc. Med. 5:177. doi: 10.3389/fcvm.2018.00177
Received: 17 September 2018; Accepted: 29 November 2018;
Published: 11 December 2018.
Edited by:
Pietro Enea Lazzerini, Università degli Studi di Siena, ItalyReviewed by:
Michael T. Nurmohamed, VU University Medical Center, NetherlandsCopyright © 2018 Bartoloni, Alunno, Valentini, Luccioli, Valentini, La Paglia, Leone, Cafaro, Marcucci and Gerli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roberto Gerli, cm9iZXJ0by5nZXJsaUB1bmlwZy5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.