- 1School of Sport, Exercise, and Rehabilitation Sciences, College of Life and Environmental Sciences, University of Birmingham, Edgbaston, United Kingdom
- 2Institute of Systems and Metabolism Research, College of Medical and Dental Sciences, University of Birmingham, Edgbaston, United Kingdom
- 3Department of Health and Human Performance, College of Idaho, Caldwell, ID, United States
Physical inactivity and excessive postprandial hyperglycemia are two major independent risk factors for type 2 diabetes and cardiovascular-related mortality. Current health policy guidelines recommend at least 150 min of physical activity per week coupled with reduced daily sedentary behavior by interrupting prolonged sitting with bouts of light activity every 30-min. This evidence-based strategy promotes health and quality of life. Since modern lifestyle enforces physical inactivity through motorized transportation and seated office working environments, this review examines the practical strategies (standing, walking, stair climbing, and strength-based circuit exercises) for reducing sitting time and increasing activity during the workday. Furthermore, since postprandial hyperglycemia poses the greatest relative risk for developing type 2 diabetes and its cardiovascular complications, this review examines a novel hypothesis that interrupting sitting time would be best focused on the postprandial period in order to optimize blood glucose control and maximize cardiometabolic health. In doing so, we aim to identify the science gaps which urgently need filling if we are to optimize healthcare policy in this critical area.
Introduction
Since Plato wrote, “And is not the bodily habit spoiled by rest and idleness, but preserved for a long time by motion and exercise?” (1), it is now an age-old message that physical inactivity is a cause of several chronic conditions. In 2012, the Global Burden of Diseases, Injuries and Risk Factors Study found low physical activity to be the fourth leading cause of global mortality, ahead of being overweight/obese; physical inactivity was estimated to contribute to ~1 in 10 premature deaths from coronary heart disease (2). For this reason, the UK Department of Health currently recommends all adults to undertake at least 150 min of moderate intensity exercise each week (or 75 min of vigorous intensity), combined with muscle-strengthening activities on at least 2 days per week, and to minimize the amount of time spent being sedentary (sitting) for extended periods. However, despite advances in scientific knowledge and large-scale media attention, in 2016 the Health Survey for England found that adults in the UK spend ~5 h per day sitting in their spare time (not including hours at paid work) and that only 26% of adults over 19 meet the above-described activity recommendations (3). Such questionnaire-based epidemiological assessments of activity levels are vast overestimates. The Health Survey for England in 2008 objectively measured physical activity levels using accelerometers, finding that only ~5% (vs. ~35% from self-report questionnaires) of the UK achieved physical activity guidelines (4). Similar data has emerged from the U.S. where the 2005–2006 National Health and Nutrition Examination Survey (NHANES) found that only ~3.2% of U.S. adults achieved physical activity guidelines, which are similar to the UK (5). In 2012, Ng and Popkin published a comprehensive historical analysis of physical activity levels and sedentary behavior (e.g., sitting time) in five populous nations: the US (1965 to 2009, the UK (1961 to 2005), China (1991 to 2009), Brazil (2002 to 2007), and India (2000 to 2005). The picture was clear: physical activity levels are declining and sedentary behavior is increasing (6). Because the majority of physical inactivity is derived from daily sitting time, this review will discuss appropriate and feasible physical activities that may be used to interrupt and prevent prolonged sitting. This review will also discuss the optimal timing between meals and interruption of sitting time (through different physical activity approaches) in the context of preventing poor postprandial blood glucose control, the hyperglycemic phenotype of diabetes that is a major contributor to cardiovascular-related mortality (7).
Type 2 Diabetes and Cardiometabolic Health—how is Inactivity Implicated and why does Physical Activity Matter?
Diabetes is a major health problem because it causes blindness, kidney failure, and lower limb amputations, and between 1980 and 2014 global prevalence doubled to 8.5%, equivalent to 422 million people (8). People with diabetes are also 2 to 3-times more likely to die of a cardiovascular event (7). Ultimately, diabetes drastically reduces quality of life and longevity, and places a huge economic burden on health care systems. Type 2 diabetes is characterized by persistent hyperglycemia caused by insufficient insulin secretion to compensate for poor insulin sensitivity. The disease is indicated when poor blood glucose control is detected with clinical tests for glycated hemoglobin (HbA1c), fasting glucose, or 2-h glucose during an oral glucose tolerance test (OGTT) (9). Prevention and treatment of type 2 diabetes involves a multifaceted approach including lifestyle modification, nutritional counseling, and pharmacological therapy (10). At the core of the lifestyle modification, are physical activity guidelines recommending that adults with diabetes should engage in ≥150-min of moderate-to-vigorous intensity aerobic exercise (or ≥75-min of vigorous or interval training) and 2–3 resistance exercise sessions per week, with no more than two consecutive days without activity (11). An additional recommendation is that sedentary behavior should be decreased by interrupting prolonged sitting every 30-min (11). Although these standards of care are almost identical to recommendations made for all adults, they are informed by published evidence. For example, the Nurses' Health Study examined type 2 diabetes incidence by measuring fasting and/or 2-h glucose during OGTT in a 6-year follow-up in ~70,000 individuals. Each 2-h/day increment in time spent watching TV or time spent sitting at work was associated with a 14 and 7% increased risk of diabetes, respectively (12). The same study also found that 2-h/day of standing or walking at home was associated with a 12% reduction in diabetes whereas brisk walking for 1-h/day was associated with a 34% reduction in diabetes (12). Experimental evidence from randomized controlled trials also demonstrate that the standard of care activity guidelines help maintain good blood glucose control in nondiabetic individuals (13) and help prevent deterioration of blood glucose control in patients with diabetes (14, 15). Since diabetes is a major risk for cardiovascular disease and mortality (7), the physical activity guidelines are also useful for optimizing cardiometabolic health. Similarly, sedentary behavior (sitting time) and low physical activity levels are strongly associated with increased cardiovascular disease risk and cardiovascular-related mortality in major studies like the Nurses' Health Study, the Lancet Sedentary Behavior Working Group, and the 45 and Up Study (12, 16–23). Therefore, as of 2018, these aforementioned epidemiological and experimental studies provide clear evidence to support the use of physical activity as a therapeutic modality for reducing diabetes and cardiometabolic risk. Given that ~3% of people are meeting activity guidelines [based on accelerometer data from the UK and US (4,5)], it is not surprising that type 2 diabetes continues to affect nearly 10% of the global population (8). With such a low attainment of physical activity guidelines, we are far from implementing the full potential of the beneficial health stimulus physical activity can provide. A more reasonable goal, therefore, might be first to interrupt sedentary time with physical activity.
What is the Impact of Interrupting Sitting Time on Type 2 Diabetes Risk and Cardiometabolic Health?
Although physical activity guidelines have long been available, the addition of inactivity guidelines to manage sedentary behavior by reducing sitting time is relatively new. For example, the recommendation to interrupt prolonged sitting was added to the American Diabetes Association's standards of care for diabetes in 2017 (24). More recently, experimental studies during single days have investigated the direct effects of interruption to inactivity (sitting time) on glucose control. The first of these by Dunstan and colleagues in 2012 showed that interrupting a 5-h period of sitting with 2-min of light or moderate walking every 20-min significantly reduced the blood glucose response to a mixed-nutrient liquid meal, in 19 overweight middle-aged individuals (25). Similarly, it was also found that regular treadmill walking breaks (1 min 40 s every 30 min) during 9-h of sitting significantly reduced the blood glucose response to mixed-nutrient meals during the day, in 70 young, lean, healthy individuals. In this work, the interruption protocol had a greater effect than a single 30-min walk (26). Such findings have been confirmed by Bailey and colleagues who found that interrupting sitting for 4-h following a mixed-nutrient breakfast with a 2-min treadmill walk every 20 min attenuated the postprandial increase in blood glucose in 14 young adults (27). These single-day observations have been extended to over 4-days by Duvivier and colleagues who studied 18 young, lean, healthy individuals in whom 14-h of sitting was interrupted with either 1-h of vigorous cycling or 4-h of walking and 2-h of standing (28). After the 4th day, neither fasting glucose nor AUC glucose during OGTT were different between groups while insulin sensitivity increased in the walking/standing group but not in the vigorous exercise group. This suggests that reducing inactivity with more time spent walking/standing is more effective than 1-h of vigorous exercise (28). In 2017, the same authors repeated this study in 19 older patients with type 2 diabetes using continuous glucose monitoring to assess 24-h glucose control during the 4-day interventions. Their findings showed that 24-h glucose AUC and time spent hyperglycemic (>10 mM) were significantly reduced by sitting less with 2-h of walking plus 3-h of standing, but not by ~1-h of cycling (29), again highlighting the effectiveness of replacing sedentary behavior with light physical activity, such as walking, in maintaining glucose control.
Further studies have examined the effects of simply standing to interrupt prolonged sitting. Epidemiological observations indicate that increased standing to break up sitting is protective against cardiovascular (30) and all-cause (31) mortality, but some detailed intervention studies have also been conducted. Henson and colleagues objectively compared standing vs. walking at ~3 km per h as means of interrupting 7.5-h of sitting for 5-min every 30-min, during a single-day in 22 overweight, middle-aged women. They found that standing or walking elicited the same improvement in blood glucose AUC following mixed-nutrient meal ingestion (32). Similarly, daylong postprandial glucose responses are reduced by interrupting daylong sitting with either standing, walking, or cycling during the day, in 9 overweight/obese adults (33). Buckley and colleagues also found lower AUC glucose (via continuous glucose monitoring) during 185-min of standing vs. sitting while individuals worked in an office environment in a non-crossover design of 10 people (34). Furthermore, direct comparisons between interstitial glucose derived from CGM in this study with plasma glucose measurements made in other studies should be made with caution. On the contrary, in a pooled analysis of three trials in 9 overweight, middle to older aged adults, another study found that regular standing breaks (2-min every 20-min) during a 5-h sit was insufficient to improve postprandial glucose control (35). In contrast, the same study found that light- and moderate-intensity walking caused progressively larger improvements in blood glucose AUC following mixed-nutrient meal ingestion (35). These findings are important since they indicate that activities that increase energy expenditure more so than just standing still may be required to optimize glucose control. Indeed similar observations have also been documented during a single day in younger and healthier weight adults (36–38). A contrary hypothesis is that longer-term standing interventions are required to improve blood glucose control. For example, in 2014 it was found that 5-days of alternating between sitting and standing every 30 min during the work day significantly reduced postprandial area under the glucose curve in overweight/obese sedentary office workers, compared to 5-days of prolonged sitting (39).
From the evidence presented above, it is clear that interruption of prolonged sitting with walking or cycling or even just standing up may be effective for improving postprandial blood glucose control, an independent cardiometabolic risk factor. However, as of 2018, no long-term large-scale experimental study has determined the effect of interrupting sitting time on diabetes risk and/or hard endpoints like subsequent cardiovascular complications and mortality.
When is the Right Time to use Physical Activity to Interrupt Sitting in order to Maximize Blood Glucose Control and Cardiometabolic Health?
Epidemiological and experimental studies show that the degree to which blood glucose is elevated 1–2-h following a meal is associated with increased cardiovascular disease risk (40). Furthermore, evidence also shows that postprandial hyperglycemia in well-controlled diabetes patients is the predominant contributor to elevated levels of glycated hemoglobin (HbA1c), the gold standard biomarker of mean glucose control over the prior 6–8 weeks (41). Accordingly, management of postprandial hyperglycemia is highly prudent particularly given that people spend a large proportion of the day in a postprandial state, and given that diabetes patients are hyperglycemic (>10 mmol/L) for up to 24% of their day (42).
The studies described in the previous section provide evidence that interruption of prolonged sitting with physical activity may improve postprandial blood glucose control. However, such studies did not examine the timing between physical activity and meals and, as of 2018, no long-term randomized, controlled physical activity or exercise intervention study with a primary focus on blood glucose control has reported activity-meal timing. Whether activity/exercise is completed in the fed or fasted state is seldom reported in training studies and as authors of prior studies in this field, we too are guilty. Sometimes this information is not known since activity interventions are self-administered, but activity-meal timing information is even lacking in studies where bouts of activity have been fully-supervised.
Postprandial blood glucose levels are determined by several factors, such as the total caloric value of a meal, macronutrient composition, and carbohydrate quality (e.g., glycemic index/load), all of which may be monitored and controlled. However, multiple other factors are more complex because they cannot be controlled and are variable between individuals. These include gastric emptying rate, intestinal absorption rate, enteroendocrine incretin secretion, incretin sensitivity, pancreatic beta-cell insulin secretory function, hepatic insulin extraction, hepatic glucose production, glucose effectiveness, glucose uptake in all tissues (especially brain, adipose, liver, muscle), insulin sensitivity, and renal glucose reabsorption. An abundance of studies (far too many to cite) also show that the above factors are also altered by a single bout of exercise and/or changed following a prolonged period of increased physical activity or structured exercise training. That said, large inter-individual variability exists in the hyperglycemia-lowering effect of physical activity in individuals with prediabetes or type 2 diabetes (13, 43, 44). For example, HbA1c improved in only two-thirds of patients enrolled in a 3–4-month training intervention (43) while 42% of participants in the HERITAGE study showed no improvement or a deterioration in insulin sensitivity (13). One plausible source of such variability is activity-meal timing.
In 2014, Elsamma Chacko published a letter stating that mid-postprandial moderate-intensity activity (commencing 30-min post-ingestion and lasting up to an hour) is the best time for lowering postprandial hyperglycemia (45). This suggestion was derived from the author's own anecdotal experiences as a medical doctor living with type 2 diabetes in combination with evidence from the very few published experimental studies. Fed vs. fasted exercise has been examined in the context of VO2max and/or fat oxidation for optimizing athletes' performance in hundreds of publications. A large number of studies have also studied the interactions between exercise-timing, insulin dosing, and carbohydrate intake for managing blood glucose and preventing hypoglycemia in patients with type 1 diabetes. However, there is a relative paucity of data comparing fed vs. fasted physical activity in relation to type 2 diabetes and cardiometabolic risk. One example from 2001 found that in 10 middle-aged men with type 2 diabetes blood glucose levels were significantly lower when 60-min of moderate-intensity bicycle ergometry were completed 2-h after breakfast, rather than before breakfast (46). While a non-activity control intervention was not included, postprandial plasma free fatty acids were reported but not different between trials. Further work by Colberg and colleagues found that when 12 older-aged, obese, men and women with type 2 diabetes completed 20-min of self-paced treadmill walking starting 15–20 min after eating dinner, blood glucose was significantly lowered from baseline when compared to the pre-dinner walking or no walking (47). However, daylong blood glucose levels show a different pattern: Borer and colleagues found that daylong blood glucose levels were significantly lower when two 2-h low-intensity treadmill walks were completed pre-meal compared to post-meal walking, in 9 overweight, middle to older aged women (48). That said, subjects had noticeable hypoglycemia following the first meal, while no differences in postprandial plasma free fatty acid levels were noted between the pre- or post-meal exercise groups. Another experimental design compared pre- vs. post-breakfast treadmill walking (60-min of continuous moderate intensity or intervals of 1-min hard/3 min easy) to a non-walking control group in 10 older aged, overweight/obese, men and women with type 2 diabetes (49). Similarly, pre-breakfast exercise was more effective at lowering total postprandial hyperglycemia during the day than post-breakfast exercise (49). Moreover, the reduction in total postprandial hyperglycemia during the day was equal between interval- and continuous-walking groups. However, interval walking significantly lowered post-breakfast AUC glucose compared to no exercise and was more effective than continuous walking (49). In addition to aerobic exercise, other work also examined resistance exercise-meal timing in 13 middle-aged, obese, men and women with type 2 diabetes (50). Pre- and post-meal resistance exercise equally improved blood glucose AUC following dinner regardless of timing. However, postprandial triglycerides were significantly lower in the post-meal exercise group, suggesting that post-dinner resistance exercise may more effectively improve cardiometabolic health in patients with diabetes since it lowers both postprandial hyperglycemia and dyslipidemia.
Retrospective analyses of patient food and activity logs from a 12-week training study including three supervised 60-min sessions/week of moderate intensity bicycle ergometry, in 19 middle-aged, overweight men with type 2 diabetes, provided insight into the optimal activity-meal timing (51). Again, blood glucose levels were significantly decreased when cycling was initiated following ingestion of meals but not when cycling was completed in the fasting state. The amount of time after a meal may also be important. Another retrospective study where 15 older aged, obese, men and women with type 2 diabetes completed five 30- to 60-min supervised exercise sessions/week for 12-weeks, found significantly greater reductions in blood glucose when meals were ingested less than 2-h prior to exercise, rather than more than 2-h (52). However, neither a control group nor post-meal exercise data were included.
The above-described literature addressing activity-meal timing is indeed scant with small sample sizes and dichotomous outcomes (Table 1). Some studies are retrospective and some do not include a non-activity control group. Despite current efforts to elucidate the optimal exercise-meal timing, a prospective randomized controlled trial that thoroughly assesses the time course of activity-meal timing on postprandial hyperglycemia and other cardiometabolic risk factors (such as postprandial lipemia) is urgently required. Such an intervention should also separately examine nondiabetic individuals and people with type 2 diabetes, in order to inform guidelines for diabetes prevention as well as diabetes treatment. Since carbohydrate quality influences postprandial glycemia and has been shown to influence exercise adaptations for some (53) but not all (54) variables, examination of the timing between exercise and meals of differing glycemic index/load is also prudent. Besides walking, other practical means to interrupt sitting time, such as standing, stair climbing, or body-weight circuit exercises, also remain to be investigated in the context of an activity-meal time course. Given the different effects of pre- vs. post-meal activity on blood glucose control, the lack of exercise-induced improvement in blood glucose control documented in some studies (55–58) may have been influenced by activity-meal timing. To enhance knowledge, it is prudent for future training studies to consider and report activity-meal timing.
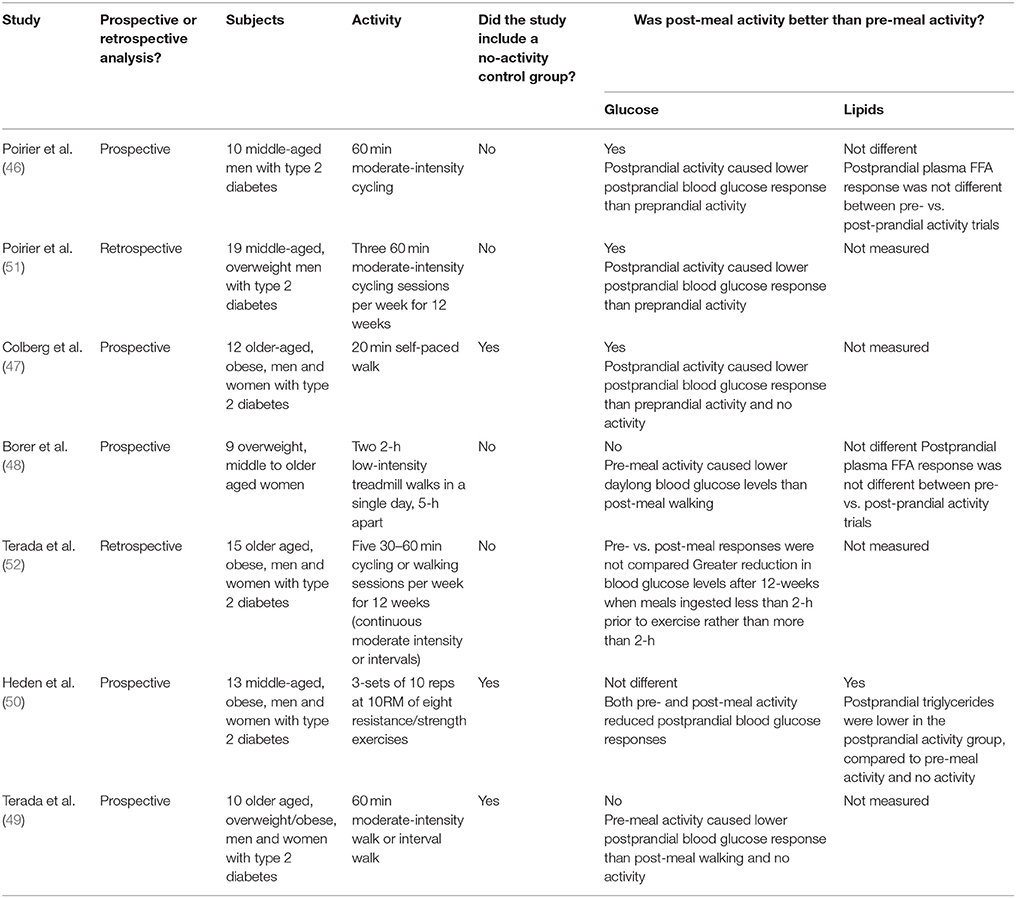
Table 1. A summary of published studies that have examined the effect of pre-meal vs. post-meal physical activity on postprandial glycemia and lipemia.
How can Sitting Time be Interrupted?
Recent data shows that physical inactivity accounts for more CVD-related deaths (37%) than smoking (19%), and hypertension (13%) combined, and that 15–17% of all premature deaths is attributable to low fitness (59, 60). Therefore, it is now critical that strategies to reduce inactivity are developed. Although guidelines often provide clear examples for physical activities (61), many of them are impractical. For instance, only brisk walking or using a skipping rope would be feasible activities for use as voluntary substitutes for sitting when voluntary habits like TV viewing, reading, or computer/tablet/smartphone use occur. Muscle strengthening exercises or yoga are also possible. However, sitting is often involuntary and enforced during a commute (driving cars or taking public transport) or while at school, university, or work (including office workers and delivery/public transport drivers). Accordingly, alternative approaches to reducing sitting time and inactivity during a commute and/or at the workplace are necessary.
Previous works by Levine extensively examine the ability of different types of simple physical activities to increase daily energy expenditure above resting levels (62, 63). Not surprisingly, standing up increases energy expenditure above levels induced by sitting, while walking at incremental speeds further increases energy expenditure in a dose-dependent fashion (62). However, the magnitude of the increase in energy expenditure above basal levels that is induced by motionless activity (i.e., standing) is minor in comparison to activities that require ambulation (62, 63). For instance, Levine found that stair climbing increases energy expenditure above resting levels and expends more energy than standing in an elevator (64). Such observations have led to small-scale stair climb interventions which show some benefits to postprandial glucose control in healthy and diabetic individuals (65–69). Therefore, from an energy expenditure perspective, some form of movement to interrupt sitting would be preferable to simply standing up. This point is highlighted in Figure 1 using metabolic equivalent data extracted from the compendium of physical activities (70). That said, some evidence described above supports the use of standing alone as a means for interrupting sitting time and optimizing blood glucose control and cardiometabolic health (30–34). Further research is necessary to determine how much, when, and for whom standing is sufficient to improve blood glucose control and cardiometabolic health. Regardless, if standing is the sole activity permitted or available to interrupt sitting time, it is indeed a useful starting point.
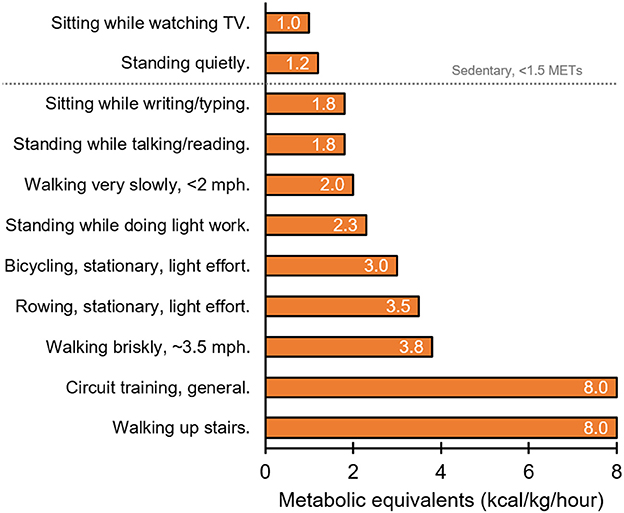
Figure 1. Representation of relative energy expenditure (kcal/kg/hour) during different behaviors as indicated by metabolic equivalents (METs; the ratio of the work metabolic rate to the resting metabolic rate) (70).
A number of additional practical approaches to reducing sedentary time exist. Levine's work showed that greater levels of energy expenditure are induced by walk-commuting vs. drive-commuting (64). This work also found that favoring stair climbing over elevators/escalators induces greater energy expenditure (64) (Figure 1). While replacing a whole commute with a physical activity like walking is not feasible for all people, parking further from work, taking the stairs rather than an elevator, or getting off the train/bus early to integrate a brisk walk into the remainder of the commute is achievable for many. Furthermore, if taking public transport, one may consider standing up rather than sitting on the bus or train. If interrupting sitting time is not possible during a commute, physical activity may be integrated into the workday during break times or during work itself. This is important for office workers and school/university students. For instance, sitting breaks in the form of resistance exercise or body-weight circuit exercises such as squats, lunges, calf raises, press-ups, and sit-ups, can effectively improve blood glucose control and cardiometabolic risk factors (50). Such circuits, along with standing, may be favorable options since they could be undertaken at an office workstation. However, interrupting sitting time during the workday may cause distraction and reduction in work productivity/performance because ambulatory tasks like walking or cycling require information processing. Tudor-Locke (63) and MacEwen (71) have carefully evaluated the paucity of experimental studies in this area. They highlight the small samples sizes, the lack of thorough comparisons between different activities, and the heterogeneous outcomes between studies with respect to mouse use, typing speed, error rate, transcription speed, reading, and cognitive skills. It is clear, therefore, replacing workstation sedentary behavior with a physical activity must be individualized so it does not distract the worker from their work tasks. Additional strategies are also necessary and important to reduce sitting time for individuals who are unable to stand up (i.e., wheelchair users) (72).
Delivery drivers and public transportation drivers should also develop strategies to take regular breaks from sitting. Comparisons between seated workers (bus drivers) and active workers (bus conductors, postmen) in the 1950s showed increased cardiovascular morbidity in the former (73, 74), and recent data has confirmed such observations in delivery drivers (75). Furthermore, attention to people's leisure time is also essential. Epidemiological evidence shows that when people are not at work on average they sit for approximately 5 h per day watching TV, reading, or using a computer (3). Prolonged sitting time (leisure or otherwise) should be interrupted every 30-min as per diabetes prevention and treatment guidelines (11). Some data shows that home exercise can be an effective means of reducing sedentary behavior, although reduction in adherence over time is documented to necessitate additional strategies (76).
There is a clear disconnect between the knowledge of the detriments of inactivity and actual implementation of physical activity at multiple levels. Health care services have a responsibility to formulate and disseminate policy. The media have a responsibility to facilitate this dissemination to the public, with accuracy. The public also have a responsibility to empower themselves to maximize their own health. Despite these responsibilities, overwhelming evidence that scientific knowledge fails to inform public implementation emerges from objective epidemiological assessments of activity levels, where people on average are far from meeting public health activity guidelines (4, 5). However, education was an effective means to increase physical activity levels of children according to a recent meta-analysis (77). Furthermore, several groups have assessed the feasibility of workplace interventions that employers may use to keep their workforce healthy. A promising recent example is Stand Up Victoria, a multi-component intervention in Australia consisting of organizational, environmental (sit-stand workstations), and individual behavioral (i.e., face-to-face and telephone health coaching) support. In this intervention, Healy and colleagues objectively measured posture and ambulation in 231 healthy office workers (78). After 3-months intervention, overall daily sitting time and workplace sitting time were reduced, −78 min/16 h and −99 min/8 h, respectively. After 12-months, reductions in overall daily sitting time (−36 min/16 h) and workplace (−45 min/8 h) sitting time persisted, corresponding to reductions in fasting glucose and cardiometabolic risk score. The office workers primarily replaced sitting with more standing but not more ambulatory activity (78). The same group found that the reduction in workplace sitting was more effective following multi-component intervention when compared to the provision of standing desks alone (−89 vs. −33 min/8 h, respectively) over a 3-months period (79). Education alone is not always effective as the 2015 Project STAND program failed to find a significant reduction in sitting time, blood glucose or cardiometabolic risk factors in young overweight/obese adults after a 12-month education-only intervention where participants were encouraged to self-monitor and self-regulate their behavior (80). While, Aadahl and colleagues also found no reduction in sitting time after 6-months of motivational counseling, standing time was significantly increased along with improvements in cardiometabolic risk factors (waist circumference and fasting insulin) (81). From these very limited numbers of studies addressing workplace sitting time, the current meta-analysis data indicate that existing workplace interventions are not highly effective at reducing sitting time and have mixed effects at reducing cardiometabolic risk factors (82). However, interventions differ in effectiveness and the Cochrane group found that sit-stand desks were more likely to lead to less sitting than other behavioral interventions such as mindfulness training, education, or other organizational changes (83). Technology may also prove effective as randomized clinical trials have reduced sedentary time using education combined with smartphone technology to alert people when they have been inactive for a period of time (84) or by making personalized activity recommendations (85). Other studies have increased step count in the work place through cash incentives (86). However, merely wearing an activity monitor does not lead to increased physical activity in multiple studies (87, 88). Consequently, to win the war on inactivity such behavioral strategies and interventions must be optimized.
Barriers Against Interrupting Sitting Time
To win the war on physical inactivity it is necessary to understand the health psychology of physical activity in addition to the physiology of the optimal timing between meals and physical activity. Health psychology is critical to create the optimal societal environment in which the physical activity guidelines can be achieved. As such, a huge societal shift in physical environment is required to implement physical activity into daily routines. For instance, incredible architectural foresight in major cities in Scandinavia and the Netherlands has had great impact on daily physical activity by making a commute via bicycle simple, affordable, safe, and enjoyable. In the 1970s, the Danish Government set out to develop the infrastructure needed to increase cycling as a means to reduce traffic accidents, reduce pollution, an improve health. In 2002, this culminated with a long-term “cycling policy” being established in the city of Copenhagen to increase commuting via bicycle (89). The restructuring of the physical environment to increase active transport and/or provision of sit-stand desks have also shown to be promising interventions that reduce sedentary behavior (90). The built environment interacts with health psychology to create barriers that reduce physical activity and prevent the interruption of sitting time. Regrettably, given the variability in individuals' environmental barriers against physical activity, there is no one-size-fits-all approach for interrupting sedentary time.
Besides the built environment, psychological barriers to energy expenditure also exist. Minimization of energy costs is biologically advantageous and is a strategy that is evolutionarily conserved (91). For example, when walking for transport, humans adopt a stride frequency, length, and width that minimizes the energy cost of the behavior (92). This minimization is learnt, linked to changes in visual perception associated with walking (93–95). Standing costs less energy than walking and sitting costs less energy than standing (Figure 1) thereby creating an incentive to avoid energy expenditure by sitting when possible. Objective data from NHANES suggest that adults sit for ≥60% of their waking hours (96). Awareness of sitting is also an issue as individuals are unware that they are sitting, and instead report the task associated with sitting rather than sitting itself (97). In support if this, when asked to categorize the behavior depicted in photographs, individuals were less likely to use posture, i.e. “sitting” vs. “standing,” to describe the task (98). Typical tasks during which individuals sit are TV viewing, computer use and/or electronic games and transportation in cars (99). Although sitting is the default behavior to minimize energy cost, tasks like TV viewing etc. dictate the behavioral choice to sit down. Therefore, it is prudent to intervene with physical activity during such tasks.
To change behavior, health education informing individuals about the risks of prolonged sitting is key (83, 90). To facilitate this, health education combined with self-monitoring of one's own behavior is a potent technique because it maintains focus on any motivation to change behavior that can result from health education (100). Self-monitoring also helps highlight to an individual a sitting behavior that they may not have been aware was occurring (97). Combing self-monitoring with goal setting has also shown promise for reducing sedentary behavior (90). Nonetheless, evidence shows that translation of motivations like self-monitoring into actual behavior change often requires volitional processes, such as reminders about motivation (101, 102) or planning about how to change a particular behavior (103–105). For example, “point-of-choice prompts” (signage at the time a healthy choice can be made) are a volitional tool that remind people about the health benefits they can accrue from increased physically active behaviors. Studies show that such prompts delivered during the day on an individual's computer can remind individuals about interruptions to sitting (106). Similarly, signs next to the office clock have encouraged individuals to break up their sedentary time when they look at the time (107).
Going Forward
It is not a new message that physical activity helps optimize blood glucose control, improve cardiovascular health, and reduce cardiovascular-related mortality, but increased awareness of the dangers of inactivity and refinement of physical activity advice is essential. Experimental evidence demonstrates that interrupting inactivity (sitting time) with physical activity breaks is a useful approach for managing blood glucose levels but recommendations concerning the optimal time to interrupt sitting do not yet exist. Since postprandial hyperglycemia is an independent cardiovascular risk factor and since we spend many hours each day in a postprandial state, timing the interruption of sitting with physical activity to minimize postprandial fluctuations in blood glucose is a sensible approach to maximize cardiometabolic health. However, randomized controlled trials determining the optimal timing between meals and activity are required and studies of this nature must examine hard endpoints like cardiovascular-related mortality. It would also be prudent for such studies to examine other cardiovascular risk factors, such as postprandial lipemia. As scientists acquire such knowledge, health psychology interventions exploring the behavioral and environmental barriers that prevent people interrupting their sedentary time must be developed. This would include consideration of the environmental barriers that influence the practicality of different activities and thus minimize the distraction from work tasks (e.g., typing, thinking, reading) or hobbies (reading, TV viewing). Furthermore, greater focus is required for increasing employers' awareness of the long-term benefits to their work force by allowing activity breaks and creating a work environment that facilitates and encourages an active workday. Such approaches will help curb the ever-increasing incidence of diabetes and thereby improve cardiovascular health, longevity, and quality of life for the increasingly inactive global population.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
At the time of writing, TS was funded by a Marie Skłodowska-Curie Individual Fellowship awarded by the European Commission and was in receipt of research grants from the European Foundation for the Study of Diabetes/Astra Zeneca, and the Physiological Society.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Lisa Tindle for her critique of the manuscript prior to submission.
References
1. Plato. Theaetetus. (369BC). Available online at: http://classics.mit.edu//Plato/theatu.html
2. Lee I-M, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT, et al. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet (2012) 380:219–29. doi: 10.1016/S0140-6736(12)61031-9
3. NatCen Social Research and UCL. Summary of Key Findings. Health Survey England 2016 (2017) Available online at: https://digital.nhs.uk/data-and-information/publications/statistical/health-survey-for-england/health-survey-for-england-2016
4. Aresu M, Bécares L, Brage S, Chaudhury M, Doyle-Francis M, Esliger D, et al. Health survey for England: physical activity and fitness. Heal Surv Engl 2008 (2009) 1:1–395. Available online at: http://webarchive.nationalarchives.gov.uk/20100423112915/http://www.ic.nhs.uk/statistics-and-data-collections/health-and-lifestyles-related-surveys/health-survey-for-england/health-survey-for-england–2008-physical-activity-and-fitness
5. Tudor-Locke C, Brashear MM, Johnson WD, Katzmarzyk PT. Accelerometer profiles of physical activity and inactivity in normal weight, overweight, and obese U.S. men and women. Int J Behav Nutr Phys Act. (2010) 7:60. doi: 10.1186/1479-5868-7-60
6. Ng SW, Popkin BM. Time use and physical activity: a shift away from movement across the globe. Obes Rev. (2012) 13:659–80. doi: 10.1111/j.1467-789X.2011.00982.x
7. Sarwar N, Gao P, Kondapally Seshasai SR, Gobin R, Kaptoge S, di Angelantonio E, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet (2010) 375:2215–22. doi: 10.1016/S0140-6736(10)60484-9
9. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes−2018. Diabetes Care (2018) 41:S13–27. doi: 10.2337/dc18-S002
10. American Diabetes Association. 5. Prevention or delay of type 2 diabetes: standards of medical care in diabetes−2018. Diabetes Care (2018) 41:S51–4. doi: 10.2337/dc18-S005
11. American Diabetes Association. 4. Lifestyle management: standards of medical care in diabetes−2018. Diabetes Care (2018) 41:S38–50. doi: 10.2337/dc18-S004
12. Hu FB. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA (2003) 289:1785. doi: 10.1001/jama.289.14.1785
13. Boulé NG, Weisnagel SJ, Lakka TA, Tremblay A, Bergman RN, Rankinen T, et al. Effects of exercise training on glucose homeostasis: the HERITAGE family study. Diabetes Care (2005) 28:108–14. doi: 10.2337/diacare.28.1.108
14. Sigal RJ, Kenny GP, Boulé NG, Wells GA, Prud'homme D, Fortier M, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes. Ann Intern Med. (2007) 147:357. Available online at: http://www.annals.org/content/147/6/357.short
15. Church TS, Blair SN, Cocreham S, Johannsen N, Johnson W, Kramer K, et al. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. JAMA (2010) 304:2253–62. doi: 10.1001/jama.2010.1710
16. Patel AV, Bernstein L, Deka A, Feigelson HS, Campbell PT, Gapstur SM, et al. Leisure time spent sitting in relation to total mortality in a prospective cohort of US adults. Am J Epidemiol. (2010) 172:419–29. doi: 10.1093/aje/kwq155
17. Hu FB, Willett WC, Li T, Stampfer MJ, Colditz GA, Manson JE. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med. (2004) 351:2694–703. doi: 10.1056/NEJMoa042135
18. Ekelund U, Steene-Johannessen J, Brown WJ, Fagerland MW, Owen N, Powell KE, et al. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet (2016) 388:1302–10. doi: 10.1016/S0140-6736(16)30370-1
19. van der Ploeg HP, Chey T, Korda RJ, Banks E, Bauman A. Sitting time and all-cause mortality risk in 222 497 Australian adults. Arch Intern Med. (2012) 172:494–500. doi: 10.1001/archinternmed.2011.2174
20. Laaksonen D, Lindström J, Lakka T. Physical activity in the prevention of type 2 diabetes the Finnish Diabetes Prevention Study. Diabetes (2005) 54:158–65. doi: 10.2337/diabetes.54.1.158
21. Fretts AM, Howard B V, McKnight B, Duncan GE, Beresford SA, Calhoun D, et al. Modest levels of physical activity are associated with a lower incidence of diabetes in a population with a high rate of obesity: the strong heart family study. Diabetes Care (2012) 35:1743–5. doi: 10.2337/dc11-2321
22. van der Berg JD, Stehouwer CD, Bosma H, van der Velde JHPM, Willems PJB, Savelberg HHCM, et al. Associations of total amount and patterns of sedentary behaviour with type 2 diabetes and the metabolic syndrome: the maastricht study. Diabetologia (2016) 59:709–18. doi: 10.1007/s00125-015-3861-8
23. Balkau B, Mhamdi L, Oppert J, Nolan J, Golay A, Porcellati F, et al. Physical activity and insulin sensitivity. The RISC study. Diabetes (2008) 57:2613–8. doi: 10.2337/db07-1605
24. American Diabetes Association. Lifestyle management. Diabetes Care (2017) 40:S33–43. doi: 10.2337/dc17-S007
25. Dunstan D, Kingwell B, Larsen R, Healy GN, Cerin E, Hamilton MT, et al. Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care (2012) 35:976–83. doi: 10.2337/dc11-1931
26. Peddie MC, Bone JL, Rehrer NJ, Skeaff CM, Gray AR, Perry TL. Breaking prolonged sitting reduces postprandial glycemia in healthy, normal-weight adults: a randomized crossover trial. Am J Clin Nutr. (2013) 98:358–66. doi: 10.3945/ajcn.112.051763
27. Bailey DP, Maylor BD, Orton CJ, Zakrzewski-Fruer JK. Effects of breaking up prolonged sitting following low and high glycaemic index breakfast consumption on glucose and insulin concentrations. Eur J Appl Physiol. (2017) 117:1299–307. doi: 10.1007/s00421-017-3610-4
28. Duvivier BMFM, Schaper NC, Bremers MA, van Crombrugge G, Menheere PPCA, Kars M, Savelberg HHCM. Minimal intensity physical activity (standing and walking) of longer duration improves insulin action and plasma lipids more than shorter periods of moderate to vigorous exercise (cycling) in sedentary subjects when energy expenditure is comparable. PLoS ONE (2013) 8:e55542. doi: 10.1371/journal.pone.0055542
29. Duvivier BMFM, Schaper NC, Hesselink MKC, van Kan L, Stienen N, Winkens B, et al. Breaking sitting with light activities vs structured exercise: a randomised crossover study demonstrating benefits for glycaemic control and insulin sensitivity in type 2 diabetes. Diabetologia (2017) 60:490–98. doi: 10.1007/s00125-016-4161-7
30. Katzmarzyk PT. Standing and mortality in a prospective cohort of canadian Adults. Med Sci Sports Exerc. (2014) 46:940–6. doi: 10.1249/MSS.0000000000000198
31. Van der Ploeg HP, Chey T, Ding D, Chau JY, Stamatakis E, Bauman AE. Standing time and all-cause mortality in a large cohort of Australian adults. Prev Med. (2014) 69:187–91. doi: 10.1016/j.ypmed.2014.10.004
32. Henson J, Davies MJ, Bodicoat DH, Edwardson CL, Gill JMR, Stensel DJ, et al. Breaking up prolonged sitting with standing or walking attenuates the postprandial metabolic response in postmenopausal women: a randomized acute study. Diabetes Care (2016) 39:130–8. doi: 10.2337/dc15-1240
33. Crespo NC, Mullane SL, Zeigler ZS, Buman MP, Gaesser GA. Effects of standing and light-intensity walking and cycling on 24-h glucose. Med Sci Sports Exerc. (2016) 48:2503–11. doi: 10.1249/MSS.0000000000001062
34. Buckley JP, Mellor DD, Morris M, Joseph F. Standing-based office work shows encouraging signs of attenuating post-prandial glycaemic excursion. Occup Environ Med. (2014) 71:109–11. doi: 10.1136/oemed-2013-101823
35. Larsen RN, Dempsey PC, Dillon F, Grace M, Kingwell BA, Owen N, et al. Does the type of activity “break” from prolonged sitting differentially impact on postprandial blood glucose reductions? An exploratory analysis. Appl Physiol Nutr Metab. (2017) 4:1–4. doi: 10.1139/apnm-2016-0642
36. Hawari NSA, Al-Shayji I, Wilson J, Gill JMR. Frequency of breaks in sedentary time and postprandial metabolic responses. Med Sci Sports Exerc. (2016) 48:2495–502. doi: 10.1249/MSS.0000000000001034
37. Bailey DP, Locke CD. Breaking up prolonged sitting with light-intensity walking improves postprandial glycemia, but breaking up sitting with standing does not. J Sci Med Sport (2015) 18:294–8. doi: 10.1016/j.jsams.2014.03.008
38. Pulsford RM, Blackwell J, Hillsdon M, Kos K. Intermittent walking, but not standing, improves postprandial insulin and glucose relative to sustained sitting: a randomised cross-over study in inactive middle-aged men. J Sci Med Sport (2017) 20:278–83. doi: 10.1016/j.jsams.2016.08.012
39. Thorp AA, Kingwell BA, Sethi P, Hammond L, Owen N, Dunstan DW. Alternating bouts of sitting and standing attenuate postprandial glucose responses. Med Sci Sports Exerc. (2014) 46:2053–61. doi: 10.1249/MSS.0000000000000337
40. Ceriello A. Postprandial hyperglycemia and diabetes complications. Is it time to treat? Diabetes (2005) 54:1–7. doi: 10.2337/diabetes.54.1.1
41. Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycaemia of type 2 diabetic patients. Diabetes Care (2003) 26:881–5. doi: 10.2337/diacare.26.3.881
42. van Dijk JW, Manders RJF, Hartgens F, Stehouwer CD, Praet SFE, van Loon LJC. Postprandial hyperglycemia is highly prevalent throughout the day in type 2 diabetes patients. Diabetes Res Clin Pract. (2011) 93:31–7. doi: 10.1016/j.diabres.2011.03.021
43. Solomon TPJ, Malin SK, Karstoft K, Kashyap SR, Haus JM, Kirwan JP. Pancreatic β-cell function is a stronger predictor of changes in glycemic control after an aerobic exercise intervention than insulin sensitivity. J Clin Endocrinol Metab. (2013) 98:4176–86. doi: 10.1210/jc.2013-2232
44. Van dijk J, Manders R, Canfora E, Mechelen W, Hartgens F, Stehouwer C, et al. Exercise and 24-h glycemic control: equal effects for all type 2 diabetes patients? Med Sci Sport Exerc. (2013) 45:628–35. doi: 10.1249/MSS.0b013e31827ad8b4
45. Chacko E. Timing and intensity of exercise for glucose control. Diabetologia (2014) 57:2425–6. doi: 10.1007/s00125-014-3339-0
46. Poirier P, Mawhinney S, Grondin L, Tremblay A, Broderick T, Cléroux J, et al. Prior meal enhances the plasma glucose lowering effect of exercise in type 2 diabetes. Med Sci Sports Exerc. (2001) 33:1259–64. doi: 10.1097/00005768-200108000-00003
47. Colberg SR, Zarrabi L, Bennington L, Nakave A, Thomas Somma C, Swain DP, et al. Postprandial walking is better for lowering the glycemic effect of dinner than pre-dinner exercise in type 2 diabetic individuals. J Am Med Dir Assoc. (2009) 10:394–7. doi: 10.1016/j.jamda.2009.03.015
48. Borer KT, Wuorinen EC, Lukos JR, Denver JW, Porges SW, Burant CF. Two bouts of exercise before meals, but not after meals, lower fasting blood glucose. Med Sci Sport Exerc. (2009) 41:1606–14. doi: 10.1249/MSS.0b013e31819dfe14
49. Terada T, Wilson BJ, Myette-C?té E, Kuzik N, Bell GJ, McCargar LJ, et al. Targeting specific interstitial glycemic parameters with high-intensity interval exercise and fasted-state exercise in type 2 diabetes. Metabolism (2016) 65:599–608. doi: 10.1016/j.metabol.2016.01.003
50. Heden TD, Winn NC, Mari A, Booth FW, Rector RS, Thyfault JP, et al. Postdinner resistance exercise improves postprandial risk factors more effectively than predinner resistance exercise in patients with type 2 diabetes. J Appl Physiol. (2015) 118:624–34. doi: 10.1152/japplphysiol.00917.2014
51. Poirier P, Tremblay A, Catellier C, Tancrède G, Garneau C, Nadeau A. Impact of time interval from the last meal on glucose response to exercise in subjects with type 2 diabetes. J Clin Endocrinol Metab. (2000) 85:2860–4. doi: 10.1210/jc.85.8.2860
52. Terada T, Friesen A, Chahal BS, Bell GJ, Mccargar LJ, Boulé NG. Exploring the variability in acute glycemic responses to exercise in type 2 diabetes. J Diabetes Res. (2013) 2013:591574. doi: 10.1155/2013/591574
53. Solomon TPJ, Haus JM, Cook MA, Flask CA, Kirwan JP. A low-glycemic diet lifestyle intervention improves fat utilization during exercise in older obese humans. Obesity (2013) 21:2272–8. doi: 10.1002/oby.20411
54. Malin SK, Niemi N, Solomon TPJ, Haus JM, Kelly KR, Filion J, et al. Exercise training with weight loss and either a high- or low-glycemic index diet reduces metabolic syndrome severity in older adults. Ann Nutr Metab. (2012) 61:135–41. doi: 10.1159/000342084
55. Karstoft K, Winding K, Knudsen SH, Nielsen JS, Thomsen C, Pedersen BK, et al. The effects of free-living interval- walking training on glycemic control, body composition, and physical fitness in type 2 diabetic patients. Diabetes Care (2013) 36:228–36. doi: 10.2337/dc12-0658
56. Burns N, Finucane FM, Hatunic M, Gilman M, Murphy M, Gasparro D, et al. Early-onset type 2 diabetes in obese white subjects is characterised by a marked defect in beta cell insulin secretion, severe insulin resistance and a lack of response to aerobic exercise training. Diabetologia (2007) 50:1500–8. doi: 10.1007/s00125-007-0655-7
57. Dela F, von Linstow ME, Mikines KJ, Galbo H. Physical training may enhance beta-cell function in type 2 diabetes. Am J Physiol Endocrinol Metab. (2004) 287:E1024–31. doi: 10.1152/ajpendo.00056.2004
58. Terada T, Friesen A, Chahal BS, Bell GJ, McCargar LJ, Boulé NG. Feasibility and preliminary efficacy of high intensity interval training in type 2 diabetes. Diabetes Res Clin Pract. (2013) 99:120–9. doi: 10.1016/j.diabres.2012.10.019
59. Lee I-M, Bauman AE, Blair SN, Heath GW, Kohl HW, Pratt M, et al. Annual deaths attributable to physical inactivity: whither the missing 2 million? Lancet (2013) 381:992–3. doi: 10.1016/S0140-6736(13)60705-9
60. McPherson K., Britton A, National Heart Forum. Monitoring the Progress of the 2010 Target for Coronary Heart Disease Mortality: Estimated Consequences on Chd Incidence Mortality From Changing Prevalence of Risk Factors: A Report for the Chief Medical Officer. London: London School of Hygiene Tropical Medicine (2001). Available online at: http://researchonline.lshtm.ac.uk/17972/
61. NHS. Physical Activity Guidelines for Adults. NHS Choices (2015) Available online at: https://www.nhs.uk/Livewell/fitness/Pages/physical-activity-guidelines-for-adults.aspx
62. Levine JA, Schleusner SJ, Jensen MD. Energy expenditure of nonexercise activity. Am J Clin Nutr. (2000) 72:1451–4. doi: 10.1093/ajcn/72.6.1451
63. Tudor-Locke C, Schuna JM, Frensham LJ, Proenca M. Changing the way we work: elevating energy expenditure with workstation alternatives. Int J Obes. (2014) 38:755–65. doi: 10.1038/ijo.2013.223
64. Lanningham-Foster L, Nysse LJ, Levine JA. Labor saved, calories lost: the energetic impact of domestic labor-saving devices. Obes Res. (2003) 11:1178–81. doi: 10.1038/oby.2003.162
65. Allison MK, Baglole JH, Martin BJ, Macinnis MJ, Gurd BJ, Gibala MJ. Brief intense stair climbing improves cardiorespiratory fitness. Med Sci Sport Exerc. (2017) 49:298–307. doi: 10.1249/MSS.0000000000001188
66. Takaishi T, Hayashi T. Stair ascending–descending exercise accelerates the decrease in postprandial hyperglycemia more efficiently than bicycle exercise. BMJ Open Diab Res Care (2017) 5:e000428. doi: 10.1136/bmjdrc-2017-000428
67. Takaishi T, Imaeda K, Tanaka T, Moritani T, Hayashi T. A short bout of stair climbing-descending exercise attenuates postprandial hyperglycemia in middle-aged males with impaired glucose tolerance. Appl Physiol Nutr Metab. (2012) 37:193–6. doi: 10.1139/h11-140
68. Honda H, Igaki M, Hatanaka Y, Komatsu M, Tanaka SI, Miki T, et al. Stair climbing/descending exercise for a short time decreases blood glucose levels after a meal in participants with type 2 diabetes. BMJ Open Diabetes Res Care (2016) 4:e000232. doi: 10.1136/bmjdrc-2016-000232
69. Honda H, Igaki M, Hatanaka Y, Komatsu M, Tanaka S-I, Miki T, et al. Repeated 3-minute stair climbing-descending exercise after a meal over 2 weeks increases serum 1,5-anhydroglucitol levels in people with type 2 diabetes. J Phys Ther Sci. (2017) 29:75–8. doi: 10.1589/jpts.29.75
70. Ainsworth B, Haskell W, Leon A, Jacobs D, Montoye H, Sallis J, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sport Exerc. (1993) 25:71–80. doi: 10.1249/00005768-199301000-00011
71. MacEwen BT, MacDonald DJ, Burr JF. A systematic review of standing and treadmill desks in the workplace. Prev Med. (2015) 70:50–8. doi: 10.1016/j.ypmed.2014.11.011
72. Durstine JL, Painter P, Franklin BA, Morgan D, Pitetti KH, Roberts SO. Physical activity for the chronically ill and disabled. Sport Med. (2000) 30:207–19. doi: 10.2165/00007256-200030030-00005
73. Morris JN, Heady J, Raffle P, Roberts C, Parks J. Coronary heart-disease and physical activity of work. Lancet (1953) 265:1053–7. doi: 10.1016/S0140-6736(53)90665-5
74. Morris JN, Heady J, Raffle P, Roberts C, Parks J. Coronary heart-disease and physical activity of work. Lancet (1953) 262:1111–20. doi: 10.1016/S0140-6736(53)91495-0
75. Thiese MS, Hanowski RJ, Moffitt G, Kales SN, Porter RJ, Ronna B, et al. A retrospective analysis of cardiometabolic health in a large cohort of truck drivers compared to the American working population. Am J Ind Med. (2018) 61:103–10. doi: 10.1002/ajim.22795
76. Rhodes RE, Beauchamp MR, Blanchard CM, Bredin SSD, Warburton DER, Maddison R. Use of in-home stationary cycling equipment among parents in a family-based randomized trial intervention. J Sci Med Sport (2018) 18:30097–5. doi: 10.1016/j.jsams.2018.03.013
77. Brown HE, Atkin AJ, Panter J, Wong G, Chinapaw MJM, van Sluijs EMF. Family-based interventions to increase physical activity in children: a systematic review, meta-analysis and realist synthesis. Obes Rev. (2016) 17:345–60. doi: 10.1111/obr.12362
78. Healy GN, Winkler EAH, Eakin EG, Owen N, Lamontagne AD, Moodie M, et al. A cluster RCT to reduce workers' sitting time: impact on cardiometabolic biomarkers. Med Sci Sports Exerc. (2017) 49:2032–9. doi: 10.1249/MSS.0000000000001328
79. Neuhaus M, Healy GN, Dunstan DW, Owen N, Eakin EG. Workplace sitting and height-adjustable workstations: a randomized controlled trial. Am J Prev Med. (2014) 46:30–40. doi: 10.1016/j.amepre.2013.09.009
80. Biddle SJH, Edwardson CL, Wilmot EG, Yates T, Gorely T, Bodicoat DH, et al. A randomised controlled trial to reduce sedentary time in young adults at risk of type 2 diabetes Mellitus: project STAND (Sedentary Time ANd Diabetes). PLoS ONE (2015) 10:e0143398. doi: 10.1371/journal.pone.0143398
81. Aadahl M, Linneberg A, Møller TC, Rosenørn S, Dunstan DW, Witte DR, et al. Motivational counseling to reduce sitting time: a community-based randomized controlled trial in adults. Am J Prev Med. (2014) 47:576–86. doi: 10.1016/j.amepre.2014.06.020
82. Chau JY, van der Ploeg HP, van Uffelen JGZ, Wong J, Riphagen I, Healy GN, et al. Are workplace interventions to reduce sitting effective? A systematic review. Prev Med. (2010) 51:352–6. doi: 10.1016/j.ypmed.2010.08.012
83. Shrestha N, Ijaz S, Kt K, Kumar S, Cp N. Workplace interventions for reducing sitting at work. Cochrane Database Syst Rev. (2016) CD010912. doi: 10.1002/14651858.CD010912.pub3
84. Rosenberg DE, Lee AK, Anderson M, Renz A, Matson TE, Kerr J, et al. Reducing sedentary time for obese older adults: protocol for a randomized controlled trial. JMIR Res Protoc. (2018) 7:e23. doi: 10.2196/resprot.8883
85. Recio-Rodriguez JI, Gomez-Marcos MA, Agudo-Conde C, Ramirez I, Gonzalez-Viejo N, Gomez-Arranz A, et al. EVIDENT 3 study: a randomized, controlled clinical trial to reduce inactivity and caloric intake in sedentary and overweight or obese people using a smartphone application: study protocol. Medicine (2018) 97:e9633. doi: 10.1097/MD.0000000000009633
86. Finkelstein EA, Haaland BA, Bilger M, Sahasranaman A, Sloan RA, Nang EEK, et al. Effectiveness of activity trackers with and without incentives to increase physical activity (TRIPPA): a randomised controlled trial. Lancet Diabetes Endocrinol. (2016) 4:983–95. doi: 10.1016/S2213-8587(16)30284-4
87. Sullivan AN, Lachman ME. Behavior change with fitness technology in sedentary adults: a review of the evidence for increasing physical activity. Front Public Health (2017) 4: 289. doi: 10.3389/fpubh.2016.00289
88. Sloan RA, Kim Y, Sahasranaman A, Müller-Riemenschneider F, Biddle SJH, Finkelstein EA. The influence of a consumer-wearable activity tracker on sedentary time and prolonged sedentary bouts: secondary analysis of a randomized controlled trial. BMC Res Notes (2018) 11:189. doi: 10.1186/s13104-018-3306-9
89. City of Copenhagen. Cykelpolitik 2002–2012. (2002) 1–40. Available online at: http://divritenis.lv/box/files/webpage.pdf
90. Gardner B, Smith L, Lorencatto F, Hamer M, Biddle SJH. How to reduce sitting time? A review of behaviour change strategies used in sedentary behaviour reduction interventions among adults. Health Psychol Rev. (2016) 10:89–112. doi: 10.1080/17437199.2015.1082146
91. Nolet B. Efficiency as a foraging currency in animals attaining a gain below the energetic ceiling. Behav Ecol. (2002) 13:571–4. doi: 10.1093/beheco/13.4.571
92. Srinivasan M. Optimal speeds for walking and running, and walking on a moving walkway. Chaos (2009) 19:26112. doi: 10.1063/1.3141428
93. Prokop T, Schubert M, Berger W. Visual influence on human locomotion. Modulation to changes in optic flow. Exp Brain Res. (1997) 114:63–70. doi: 10.1007/PL00005624
94. White E, Shockley K, Riley MA. Multimodally specified energy expenditure and action-based distance judgments. Psychon Bull Rev. (2013) 20:1371–7. doi: 10.3758/s13423-013-0462-8
95. Zadra JR, Proffitt DR. Optic flow is calibrated to walking effort. Psychon Bull Rev. (2016) 23:1491–6. doi: 10.3758/s13423-016-1017-6
96. Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RR, et al. Amount of time spent in sedentary behaviors in the United States, 2003-2004. Am J Epidemiol. (2008) 167:875–81. doi: 10.1093/aje/kwm390
97. Martinez-Ramos E, Martin-Borras C, Trujillo J-M, Gine-Garriga M, Martin-Cantera C, Sola-Gonfaus M, et al. Prolonged sitting time: barriers, facilitators and views on change among primary healthcare patients who are overweight or moderately obese. PLoS ONE (2015) 10:e0125739. doi: 10.1371/journal.pone.0125739
98. Gardner B, Dewitt S, Smith L. The invisibility of sitting: mental representations of sedentary behaviour. Eur Heal Psychol. (2017) 19 (Suppl.). Available online at: http://ehps.net/ehp/index.php/contents/article/view/2698
99. Owen N, Healy GN, Matthews CE, Dunstan DW. Too much sitting: the population health science of sedentary behavior. Exerc Sport Sci Rev. (2010) 38:105–13. doi: 10.1097/JES.0b013e3181e373a2
100. Michie S, Abraham C, Whittington C, McAteer J, Gupta S. Effective techniques in healthy eating and physical activity interventions: a meta-regression. Health Psychol. (2009) 28:690–701. doi: 10.1037/a0016136
101. Lewis AL, Eves FF. Testing the theory underlying the success of point-of-choice prompts. Psychol Sport Exerc. (2012) 13:126–32. doi: 10.1016/j.psychsport.2011.10.001
102. Lewis AL, Eves FF. Specific effects of a calorie-based intervention on stair climbing in overweight commuters. Ann Behav Med. (2011) 42:257–61. doi: 10.1007/s12160-011-9283-z
103. Sniehotta FF. Towards a theory of intentional behaviour change: plans, planning, and self-regulation. Br J Health Psychol. (2009) 14:261–73. doi: 10.1348/135910708X389042
104. Sniehotta FF, Schwarzer R, Scholz U, Schüz B. Action planning and coping planning for long-term lifestyle change: theory and assessment. Eur J Soc Psychol. (2005) 35:565–76. doi: 10.1002/ejsp.258
105. Sniehotta FF, Scholz U, Schwarzer R. Bridging the intention–behaviour gap: planning, self-efficacy, and action control in the adoption and maintenance of physical exercise. Psychol Health (2005) 20:143–60. doi: 10.1080/08870440512331317670
106. Evans RE, Fawole HO, Sheriff SA, Dall PM, Grant PM, Ryan CG. Point-of-choice prompts to reduce sitting time at work: a randomized trial. Am J Prev Med. (2012) 43:293–7. doi: 10.1016/j.amepre.2012.05.010
Keywords: exercise, inactivity, sedentary behavior, sitting time, standing, walking, Type 2 diabetes, motivation
Citation: Solomon TPJ, Eves FF and Laye MJ (2018) Targeting Postprandial Hyperglycemia With Physical Activity May Reduce Cardiovascular Disease Risk. But What Should We Do, and When Is the Right Time to Move? Front. Cardiovasc. Med. 5:99. doi: 10.3389/fcvm.2018.00099
Received: 15 May 2018; Accepted: 02 July 2018;
Published: 18 July 2018.
Edited by:
Bradford G. Hill, University of Louisville, United StatesReviewed by:
Nicholas Kruse, University of Iowa Health Care, United StatesJill Kanaley, University of Missouri, United States
Copyright © 2018 Solomon, Eves and Laye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thomas P. J. Solomon, dC5zb2xvbW9uQGJoYW0uYWMudWs=
 Thomas P. J. Solomon
Thomas P. J. Solomon Frank F. Eves
Frank F. Eves Matthew J. Laye
Matthew J. Laye