- 1Pure North S'Energy Foundation, Calgary, AB, Canada
- 2Faculty of Nursing, University of Calgary, Calgary, AB, Canada
- 3St. Mary's University, Calgary, AB, Canada
Background: Cardiovascular disease (CVD) risk factors are associated with low serum 25 hydroxyvitamin D (25(OH)D) concentrations in observational studies; however, clinical trial findings are inconsistent.
Objective: We assessed the effect of vitamin D supplementation and increased serum 25(OH)D concentrations on CVD risk factors in a systemic review and meta-analysis of randomized controlled trials (RCTs).
Design: MEDLINE, CINAHL, EMBASE, and Google Scholar were searched for RCTs that evaluated vitamin D supplementation and cardiovascular outcomes [blood pressure, parathyroid hormone (PTH), serum high-sensitivity C-reactive protein (hs-CRP), total cholesterol, high and low density lipoprotein (HDL and LDL, respectively), triglycerides, peak wave velocity (PWV) and Augmentation Index (AI)] from 1992 through 2017. Meta-analysis was based on a random-effects model and inverse variance method to calculate standardized mean difference (SMD) as effect sizes, followed by a leave-one-out method for sensitivity analysis. Risk of publication bias was assessed using Cochrane checklist and Begg funnel plots. The systematic review is registered as CRD42015025346.
Results: We identified 2341 studies from which 81 met inclusion criteria. The meta-analysis indicated a significant reduction in systolic blood pressure (SMD = −0.102 ± 0.04 mmHg, 95% confidence interval (CI), −0.20 to −0.03), diastolic blood pressure (SMD = −0.07 ± 0.03 mmHg, 95% CI, −0.14 to −0.006), serum PTH (SMD = −0.66 ± 0.08 ng/L, 95% CI, −0.82 to −0.49), hs-CRP (SMD = −0.20 ± 0.07 mg/L, 95% CI, −0.34 to −0.06), total cholesterol (SMD = −0.15 ± 0.06 mmol/L, 95% CI, −0.25 to −0.04), LDL (SMD = −0.10 ± 0.05 mmol/L, 95% CI, −0.20 to −0.003), triglycerides (SMD = −0.12 ± 0.06 mmol/L, 95% CI, −0.23 to −0.003) and a significant increase in HDL (SMD = 0.09 ± 0.04 mmol/L, 95% CI, 0.00 to 0.17) with vitamin D supplementation. These findings remained significant in sensitivity analyses for blood pressure, lipid profile, serum PTH, and serum hs-CRP. There was no significant effect of vitamin D supplementation on PWV (SMD = −0.20 ± 0.13 m/s, 95% CI, −0.46 to 0.06, p = 0.14) and AI (SMD = −0.09 ± 0.14%, 95% CI, −0.37 to 0.19, p = 0.52) for vitamin D supplemented groups.
Conclusion: These findings suggest that vitamin D supplementation may act to protect against CVD through improving risk factors, including high blood pressure, elevated PTH, dyslipidemia, and inflammation.
Introduction
The main physiological role of vitamin D has long been regarded as regulation of calcium and phosphorous homeostasis and proper bone mineralization. In more recent years, however, inadequate vitamin D status has been linked to a number of non-skeletal chronic health conditions such as diabetes, cancer, and cardiovascular disease (CVD) (1–3). The prevalence of vitamin D deficiency is high in populations across the globe and an additional 30–50% are at risk of being vitamin D deficient (4, 5). Aging is also associated with decreased vitamin D synthesis in the body, putting individuals already vulnerable at an increased risk of these conditions (6).
Observational studies have consistently found an association between low serum 25-hydroxyvitamin D (25(OH)D) concentrations and presence of CVD risk factors, including blood pressure, dyslipidemia, and inflammation (7–11). A review of prospective studies found that serum 25(OH)D concentrations <25 or 37 nmol/L (10 or 15 ng/mL) were associated with an increased risk of CVD disease or mortality (12). This is supported by a recent meta-analysis that revealed a significant association between low 25(OH)D concentration and increased cardiovascular mortality, a consistent finding across countries, sexes, age groups, and season of blood testing (13).
Current evidence suggests a role for several different vitamins in the protection of proper heart function, especially those with antioxidant potency, and thus multiple vitamin deficiencies may contribute to development of CVD. Antioxidant vitamins such as vitamin C and vitamin E might diminish the rate of oxidative stress which is a crucial component in the pathogenesis of atherosclerosis and CVD. B vitamins, which play a role in ATP energy production, and vitamin D all induce cardioprotective effects and maintain cardiovascular health (14). B vitamins might inhibit homocysteine mediated superoxide production and attenuate the atherogenicity of homocysteine (15), and improve endothelial function through decreasing homocysteine levels leading to increased flow-mediated vasodilation (16). The presence of vitamin D receptor expression in endothelial cells, vascular smooth muscle cells, and cardiac myocytes provides biological support for these observations (17); vitamin D has also been associated with the improvement of endothelial function and glucose homeostasis, reduction of oxidative stress, inflammatory response, and thrombogenesis, as well as the modulation of calcium and lipoprotein metabolism (18). Secondary hyperparathyroidism, excess parathyroid hormone, resulting from chronic vitamin D deficiency has been associated with CVD potentially through several different pathological pathways, including: (1) increased insulin resistance and pancreatic β cell dysfunction, leading to metabolic syndrome and diabetes, (2) activation of renin-angiotensin-aldosterone system (RAAS), increasing blood pressure, leading to apoptosis and fibrosis, and (3) stimulation of systemic and vascular inflammation leading to atherogenesis (4, 19). Current evidence suggests vitamin D deficiency is an important new cardiovascular risk factor that may play a causal role in the development of cardiovascular disease (20).
Several published meta-analyses and systematic reviews have found no beneficial effect of vitamin D supplementation on CVD risk factors (21–26). Ford (24), for example, suggested that there is insufficient evidence to support vitamin D supplementation for the reduction of cardiovascular events, although these authors did raise the possibility that vitamin D supplementation might have an effect on heart failure. Several meta-analyses and systematic reviews have similarly failed to find an association. In their systematic review, Wang et al. (26) showed a statistically nonsignificant reduction in cardiovascular disease with moderate doses of vitamin D (approximately 1,000 IU/d). Mao et al. (25) showed that neither vitamin D supplementation nor calcium supplementation had an effect on major cardiovascular events, myocardial infarction, or stroke. However, a meta-analysis is only as good as the quality of studies included.
The quality of the RCTs included in these meta-analyses has been criticized (27). Many RCTs do not have the ability to detect any effect due to an effect size that is simply too narrow (28). Several RCTs provided vitamin D doses that are far too low to measure a detectable increase in serum 25(OH)D concentration (29–31) and/or are too short in duration (e.g., weeks rather than months or years) to expect a change in health outcomes (32, 33). Further, most of the RCTs were grossly underpowered to detect changes in secondary outcomes (28). Several of the studies do not report baseline and/or follow-up serum 25(OH)D concentrations making it impossible to determine whether a change in vitamin D status occurred and thus whether it can be implicated in observed outcomes.
Given these uncertainties, the question of whether vitamin D supplementation improves cardiovascular risk factors and reduces subsequent disease remains without a convincing answer. The current meta-analysis investigates the role of vitamin D supplementation on cardiovascular outcomes by imposing a stringent set of inclusion criteria for studies by aggregating trials that properly take into account the biology of vitamin supplementation and by understanding the implications of different study designs.
Methods
Review Design
We conducted a systematic review based on a predefined protocol registered with PROSPERO, International Prospective Register of Systematic Reviews (http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42015025346). We included randomized controlled trials that reported blood pressure, total cholesterol, triglyceride, high and low density lipoproteins (HDL and LDL, respectively), as well as parathyroid hormone (PTH) and high sensitivity C-reactive protein (hs-CRP), peak wave velocity (PWV), and augmentation index (AI).
Search Strategy
We searched Medline, the Cochrane Central Register of Controlled Trials, Cumulative Index of Nursing and Allied Health Literature (CINAHL), Excerpta Medica database (EMBASE) and gray literature (i.e., material not published in scientific, peer-reviewed journals) using Google and Google Scholar. We also searched the references of previously published systematic reviews and meta-analyses in this area. The search interval spanned January 1, 1992 through December 31, 2017. Search terms included vitamin D, vitamin D3, and cholecalciferol combined with blood pressure, hypertension, cardiovascular, heart disease, coronary disease, lipids, cholesterol, triglycerides, HDL, LDL, hs-CRP, inflammation, PTH, arterial stiffness, PWV, AI and randomized controlled trial. Studies were limited to those published in English.
Study Selection
Inclusion Criteria
Only studies that met the following criteria were considered for this systematic review and meta-analysis: (1) studies included participants with any baseline 25(OH)D level; (2) studies recorded changes in blood pressure, PTH, hs-CRP, lipid profile, peak wave velocity, and/or augmentation index; (3) a minimum of 3 months of supplementation/therapy to ensure that the intervention had sufficient time to produce an effect on serum 25(OH)D concentrations; (4) studies with daily, weekly, or monthly frequency of dosage; (5) studies reported pre- and post-serum 25(OH)D levels (or when it was supplied by authors following request); (6) studies with control groups using a placebo and those receiving placebo plus a co-intervention (if both arms of the study received the co-intervention); and (7) studies using vitamin D3 or cholecalciferol.
Exclusion Criteria
Studies were excluded if: (1) they were nonclinical studies, observational studies, case-control, or cross-sectional studies; (2) they were methodological reports, editorials, narrative reviews, comments, and letters; (3) participants were younger than 18 years old; (4) intervention periods were less than 3 months; (5) dosage was less frequent than monthly or if a bolus dose was used; and (6) studies provided inadequate information on outcomes or serum 25(OH)D levels; (7) studies showed on improvement in vitamin D status (serum 25(OH)D change over time ≤ 0).
Two authors (NM, JR) independently reviewed each reference title and abstract to determine whether the studies met the inclusion criteria. Any disagreements with study selection were resolved through the discussion with the third author (SMK). Full-text articles were retrieved for the selected abstracts. Full articles were again assessed by the two independent authors (NM, JR) to ensure that they were eligible to be included in meta-analysis and any disagreements were finalized by the third author (SMK).
Data Extraction
Two independent authors (NM, JR) extracted the following data from the included trials: first author and year of publication; number, age, and sex of participants; study population characteristics; latitude of residence, dosage details of vitamin D including frequency, duration and IU; any co-intervention; pre- and post-serum 25(OH)D levels; pre- and post-measures for blood pressure, PTH, hs-CRP, total cholesterol, LDL, HDL, triglycerides, PWV, and AI. NM or JR also contacted several authors to provide missing data or to clarify data within the primary report. All data was then reviewed by the third author (SMK).
Risk for Bias Assessment
We assessed each included study for risk of bias by using fields from the Cochrane checklist (34) to determine the following variables: quality of random allocation concealment, blinding of outcomes assessors, treatment and control group comparability, clear definition of inclusion and exclusion criteria, participant blinding to allocation, selective reporting, if intention-to-treat analysis applied, and description of withdrawals and dropouts. Each criterion was marked as (+) with adequate information, (−) with inadequate information and (?) with unclear information (Table S1).
We generated Begg funnel plots to visually examine possible publication bias. These plots were supplemented by formal statistical testing using the Egger weighted regression tests (35). The analysis for the effects of publication bias was adjusted using the Duval and Tweedie trim-and-fill method (36).
Strategy for Data Synthesis and Statistical Analysis
We performed the meta-analysis at the trial level using Comprehensive Meta-Analysis V3 (Biostat 2014, Englewood, NJ) (37). To calculate the effect size, the mean change in concentrations, calculated as measure at the end of intervention minus measure at baseline, and the standard deviation of the outcomes were used for both treatment groups (38) and the effect size was expressed as standardized mean difference between vitamin D intervention and placebo groups, with a 95% confidence interval. For all treatment effects, a negative value denoted a reduction in the outcomes within the vitamin D group compared with placebo. We used the I2 index to evaluate heterogeneity among the included studies and with a value ≥50%, random-effects model (using the DerSimonian–Laird and generic inverse variance method) was applied (39, 40).
We conducted a sensitivity analysis using the leave-one-out method to assess the effect of each study on the overall effect size (41). For studies with more than one vitamin D supplemented group (e.g., different daily doses given), the trial with the higher dose was selected and compared with the placebo group.
Subgroup Analysis
To further assess interactions among subgroup treatments and also to address heterogeneity among included studies, we defined a priori subgroups as followed: participant's age (<55 vs. ≥55 years old), which was the median of study population's age and the central value of data providing an equal distribution of information for comparison; vitamin D supplementation dose (<4,000 vs. ≥4,000 IU/day), which is the average dose required to provide optimal serum physiological levels of vitamin D (100–130 nmol/L) (30, 42); serum 25(OH)D concentration at the end of the intervention (<86 vs. ≥86 nmol/L), which was selected as the median value of serum 25(OH)D levels; duration of intervention (<6 months vs. ≥6 months), which was selected based on the half-life of serum 25(OH)D and the time required to maintain a steady serum levels and potentially influence other biomarkers (43–45); obesity (BMI < 30 vs. BMI ≥ 30 kg/m2) as defined by WHO (46) and based on and the fact that obese individuals need 2–3 times the amount of vitamin D to achieve the same serum 25(OH)D as normal weight individuals (47); vitamin D deficiency at the beginning of the intervention [serum 25(OH)D < 50 vs. ≥50 nmol/L] as defined by IOM (48) and based on evidence demonstrating a strong association between vitamin D deficiency and higher incidence of CVD risk factors (12); and, calcium co-intervention.
Results
Study Selection
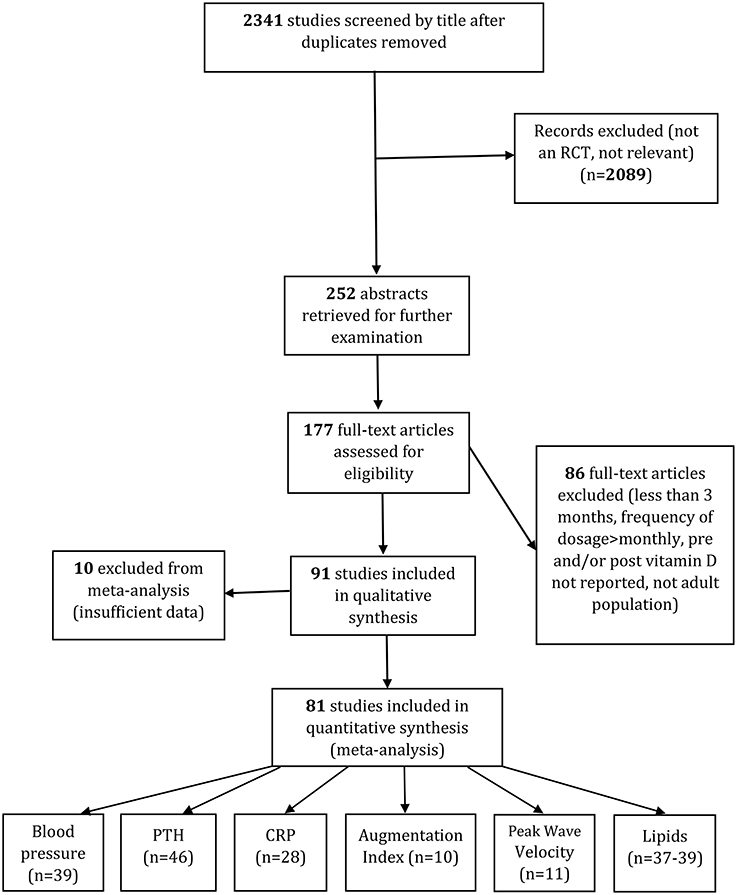
We screened the titles of 2,341 studies after duplicates were removed. After excluding any irrelevant studies, 252 abstracts were retrieved for further examination. Based on the abstracts, 177 full-text articles were assessed to determine whether they satisfied the inclusion criteria. Of these, 86 were excluded from analysis because the intervention was less than 3 months intervention, the frequency of dosage was more than monthly or bolus doses were given, pre- or post-serum 25(OH)D levels were not reported, or the trials were centered on children/ adolescents.
For the other 10 trials we did not include data, they either shared similar designs and outcomes (49, 50), had no post serum 25(OH)D data available (51) or, even after contacting the corresponding author, had insufficient information (52–58). We only included papers where an increase in vitamin D status followed supplementation, so the Cooper et al. (59) study was excluded. Finally, 81 studies were included in systematic review and meta-analysis. Details of the complete search process and for each outcome are given in Figure 1.
Risk of Bias Assessment
Ten of the included studies lacked information on the blinding of participants and personnel, and one study did not provide information on allocation concealment. Less than half of the studies (n = 38) used an intent-to-treat analysis. However, the vast majority of the included studies had a low risk of bias. Details of the quality of bias assessment are provided in Table S1.
Study Characteristics
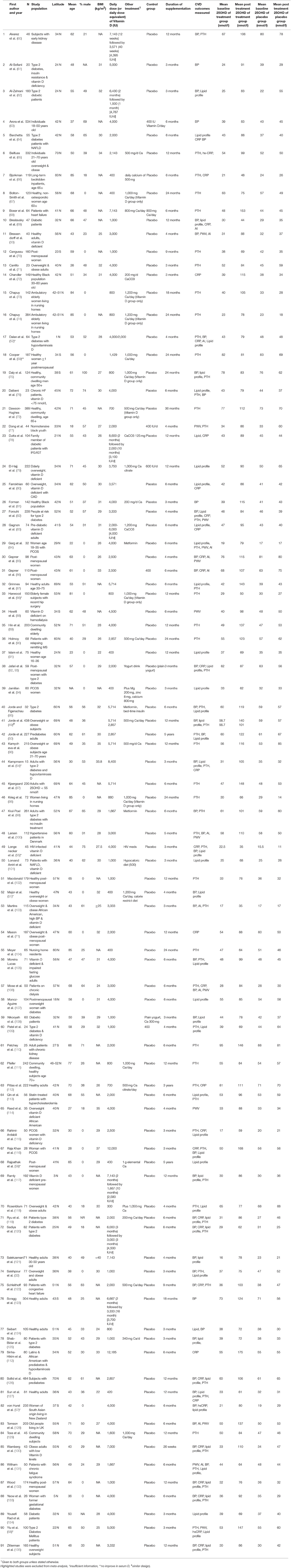
The characteristics of the included trials are given in Table 1 and studies that were excluded from the meta-analysis are highlighted. The included studies were published between 1992 and 2017. The average latitude of the studies conducted in the northern hemisphere was 43.2 ± 14.7°N (73 studies), with the maximum latitude of 70°N, and the average latitude of the studies from the southern hemisphere was 34.6 ± 6.8°S (eight studies), with the maximum latitude of 43S. Sample sizes varied from 20 (61) to 484 (126); a total of 9,993 participants are included in the meta-analysis, with 5,042 in a vitamin D-supplemented group and 4,951in a placebo group. Twenty of the studies only assessed females (Table 1), but the overall study population included 67% females and 33% males. The mean age of participants ranged from 18 (77) to 85 years (66, 74, 98, 104), with an overall average age of 55 ± 15 years.
Participants received treatment through capsules, pills, tablets, oil drops, or as a specially fortified milk or yogurt drink. Calcium was co-administered with vitamin D and placebo in 24 of the 81 studies (Table 1). The duration of intervention lasted 3 months to 5 years, with an average duration of 9.6 ± 9.2 months (median = 6 months). The daily dose of supplemental vitamin D ranged from 400 (66, 67, 91, 94, 104) to 12,000 IU (116), with an average of 2,967 ± 2,271 IU/day. Baseline serum 25(OH)D concentration varied widely from 16 (121) to 95 nmol/l (110), with the average of 45 ± 16 nmol/L in both vitamin D and placebo groups. The diversity of participants was considerable in these studies. Some were healthy and community dwelling populations, whereas others were institutionalized and/or had specific health conditions such as diabetes, kidney disease, women with polycystic ovary syndrome (PCOS), or included patients on hemodialysis. Forty-six of the studies reported serum PTH concentrations, 39 reported blood pressure and lipid profiles, 28 studies recorded hs-CRP concentrations, and 10/11 studies PWV and AI as their outcomes.
Effect of Vitamin D Supplementation on Serum 25(OH)D Level
Following vitamin D supplementation (average dose of ~3,000 IU/day), there was a significant increase in serum 25(OH)D levels in vitamin D group (48 ± 23 nmol/L) after an average of 9.6 months intervention, while it remained unchanged in placebo group (1 ± 9 nmol/L). Each of the studies reported an overall improvement in vitamin D status, with 27 recording serum 25(OH)D level greater than 100 nmol/L (Table 1). There is a significant dose-response effect between vitamin D supplementation dose and serum 25(OH)D concentration at the end of the intervention (R2 = 0.37, p < 0.001). Achieved serum 25(OH)D concentrations ≥100 nmol/L were observed in trials prescribing vitamin D at doses between 4,000 and 12,000 IU/day (50, 60, 68, 72, 74, 81, 85–87, 89, 90, 95–97, 99, 100, 110, 112, 116, 122, 123, 126, 128, 130, 136, 137).
Pooled Estimate of the Effect of Vitamin D on Cardiometabolic Parameters
Vitamin D and Blood Pressure
A total of 39 studies reported on outcomes of systolic and diastolic blood pressure (Figures 2, 3). The pooled effect size (standardized mean difference) of the effect of vitamin D supplementation on systolic blood pressure was −0.102 ± 0.04 mmHg, (95% CI −0.20 to −0.01, p = 0.02, I2 = 51%) across all studies. The pooled effect size for diastolic blood pressure was −0.072 ± 0.03 mmHg (95% CI −0.14 to −0.006, p = 0.03, I2 = 18%) across all studies. Overall results indicated that vitamin D supplementation was significantly associated with lower blood pressure.
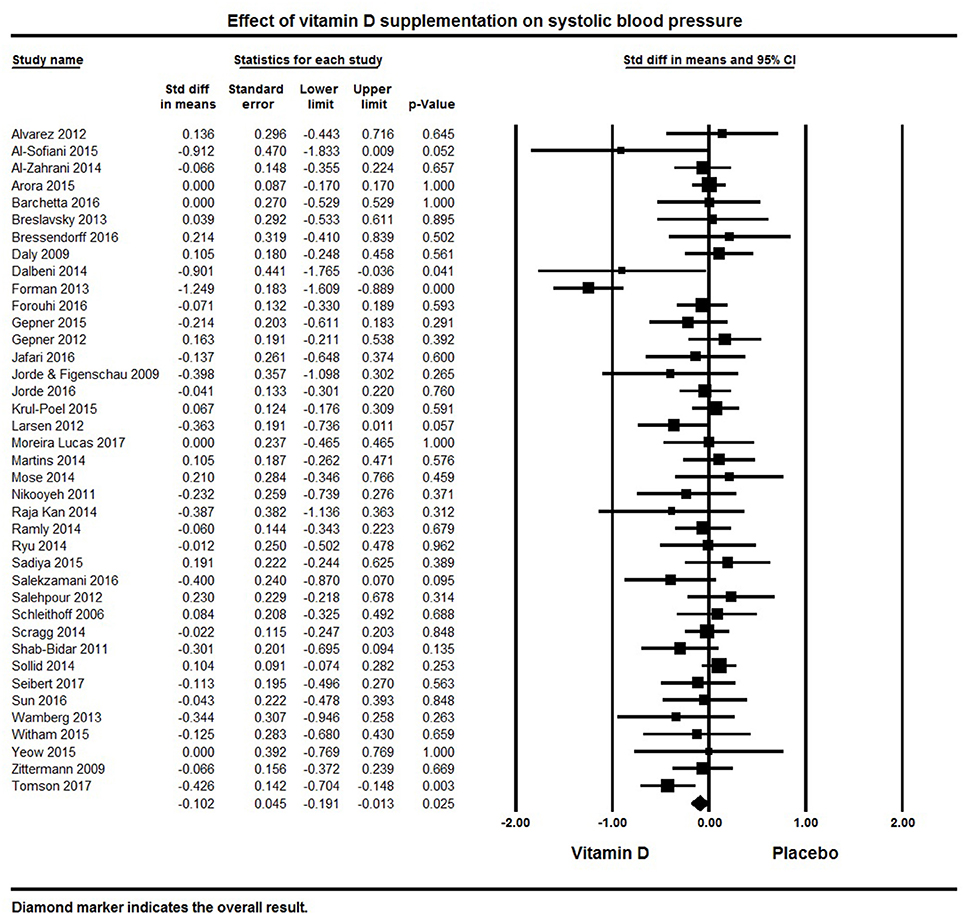
Figure 2. Forest plot detailing standardized mean difference for the impact of vitamin D on systolic blood pressure.
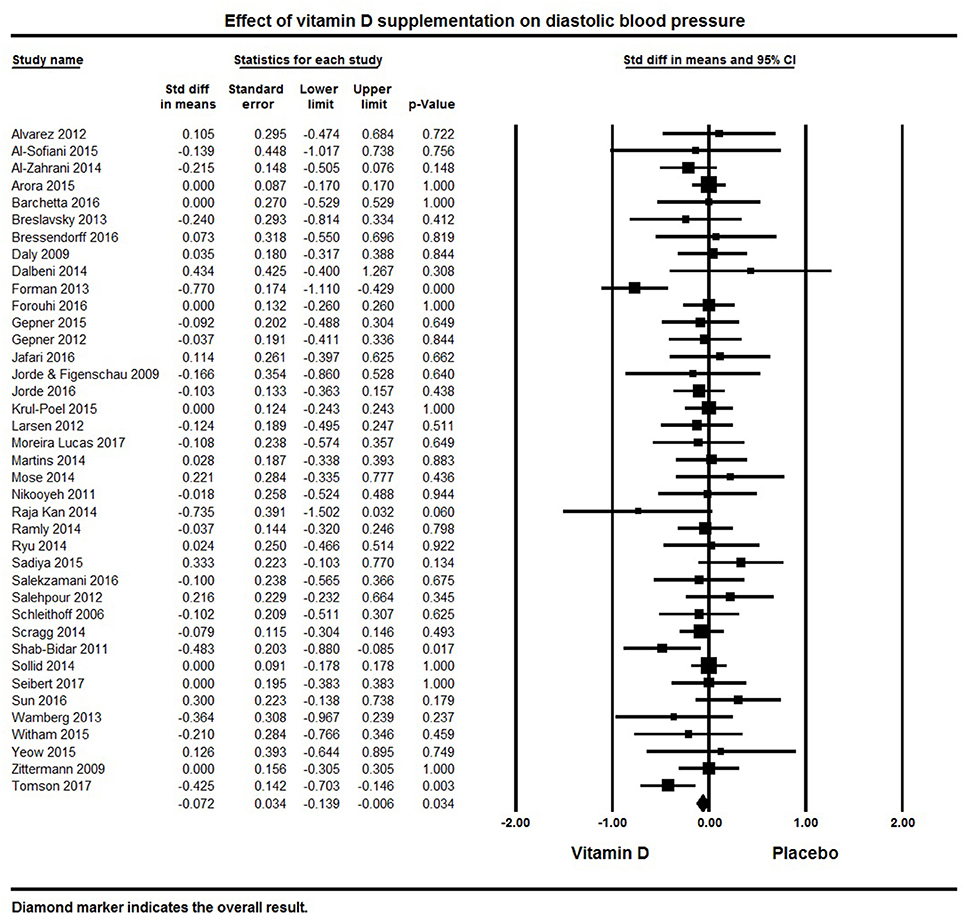
Figure 3. Forest plot detailing standardized mean difference for the impact of vitamin D on diastolic blood pressure.
Six studies showed a significant reduction in systolic blood pressure (61, 76, 81, 100, 121, 128) and four studies revealed significant reductions in diastolic blood pressure (81, 116, 125, 128). Seventeen studies demonstrated a decreasing trend in systolic and/or diastolic blood pressure following vitamin D supplementation, though these changes were not statistically significant (50, 62, 82, 86, 92, 95, 108, 116, 117, 119, 123–125, 127, 130, 131, 135). The remaining 16 studies with information on systolic blood pressure (32, 33, 60, 63, 64, 69, 75, 85, 99, 103, 105, 106, 120, 122, 126, 133) and 19 on diastolic blood pressure (32, 33, 60, 63, 64, 75, 76, 82, 92, 99, 103, 106, 119, 120, 124, 126, 127, 133, 135) showed either a null effect or an increase in blood pressure. In the majority of these studies, blood pressure was a secondary endpoint and the studies were not designed or powered for detecting the effects of vitamin D supplementation on blood pressure. Some studies also included patients with comorbid condition like kidney (60, 106) or heart failure (122), and others had all or a majority of their participants with normal blood pressure at baseline.
Only one of the included studies centered on hypertensive patients (100). After a five month intervention, this study found a significant reduction in blood pressure following vitamin D supplementation (3,000 IU/day) and improved serum 25(OH)D levels (50 nmol/L increase) compared to placebo.
Vitamin D and Lipid Profiles
Thirty-eight papers reported on the lipid profiles of participants (Table 1). Across all studies, vitamin D supplementation significantly decreased TG (pooled effect size −0.12 ± 0.06 mmol/L, 95% CI −0.24 to −0.003, p = 0.04, I2 = 64%) (Figure 4). Ten individual studies reported significant reductions in serum triglycerides with vitamin D supplementation (50, 84, 92, 94, 113, 121, 125, 127, 134, 135) and 11 studies indicated a decreasing trend with vitamin D supplementation (69, 78, 80, 101, 108, 109, 115, 116, 118, 120, 124). Seventeen of the 38 studies reported null findings or increased serum TG levels (Figure 4).
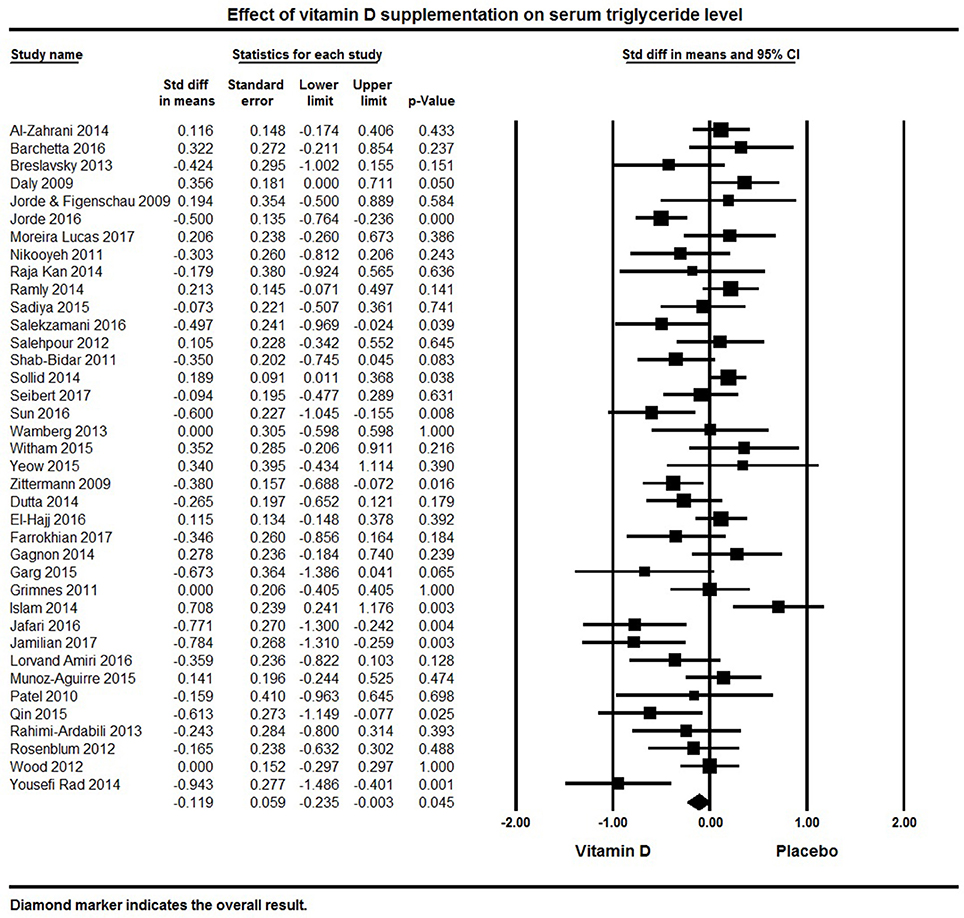
Figure 4. Forest plot detailing standardized mean difference for the impact of vitamin D on serum triglyceride level.
Thirty-eight studies included in the meta-analysis examined TC levels. The pooled effect size of vitamin D supplementation on TC was −0.15 ± 0.06 mmol/L (95% CI −0.26 to −0.04, p = 0.009, I2 = 57%; Figure 5). Vitamin D supplemented groups had lower TC levels at follow-up in seven individual studies (82, 94, 107, 113, 118, 125, 134), 18 studies found a non-significant trend for lower TC (50, 79, 80, 92, 95, 101, 105, 108, 109, 115, 119–121, 126, 127, 130–132) and 13 studies reported null effect or increased TC levels (32, 62, 64, 69, 75, 76, 83, 84, 87, 91, 116, 124, 133).
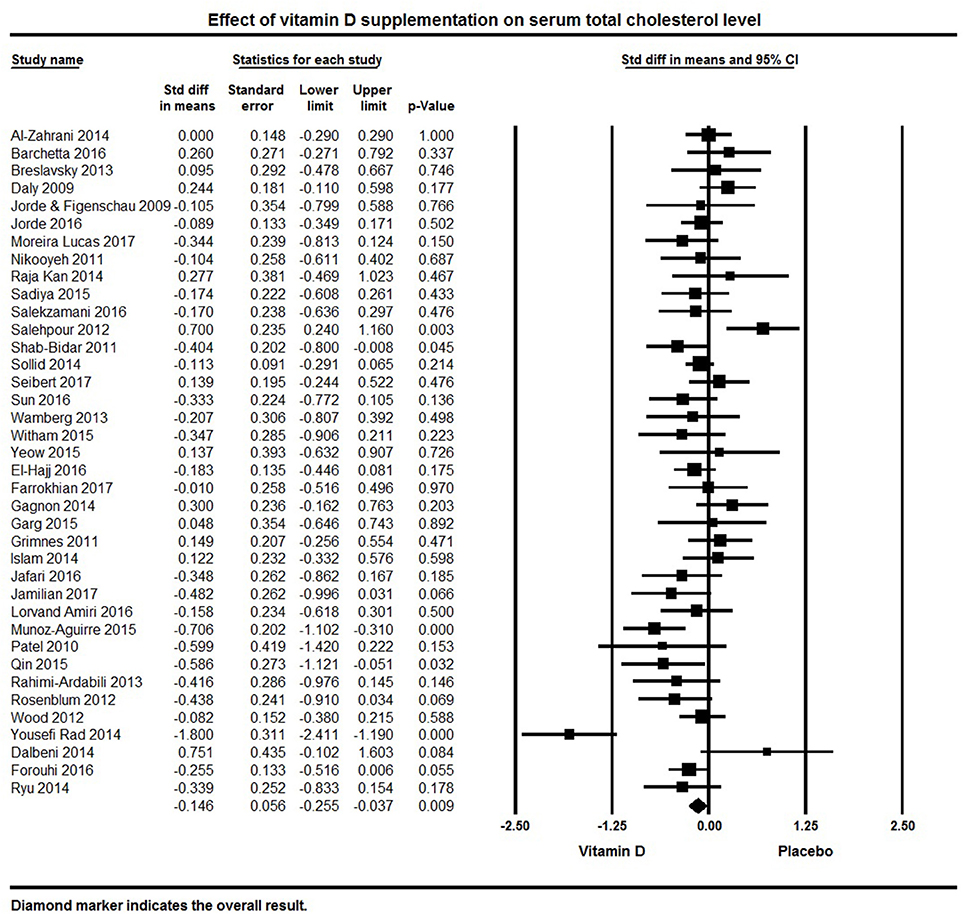
Figure 5. Forest plot detailing standardized mean difference for the impact of vitamin D on serum total cholesterol level.
Thirty-seven studies were included that reported LDL levels. The pooled effect size of vitamin D supplementation on LDL was −0.10 ± 0.05 mmol/L (95% CI −0.20 to −0.003, p = 0.04, I2 = 49%; Figure 6). Vitamin D supplementation was associated with reduced LDL levels in five individual trials (92, 113, 126, 127, 132), 17 studies reported a non-significant trend for decreased serum LDL (50, 78–80, 94, 95, 105, 107–109, 115, 120, 121, 124, 125, 131, 134), and 15 trials did not find any effect on LDL levels (32, 62, 64, 69, 75, 83, 84, 87, 91, 101, 116, 117, 119, 133, 135).
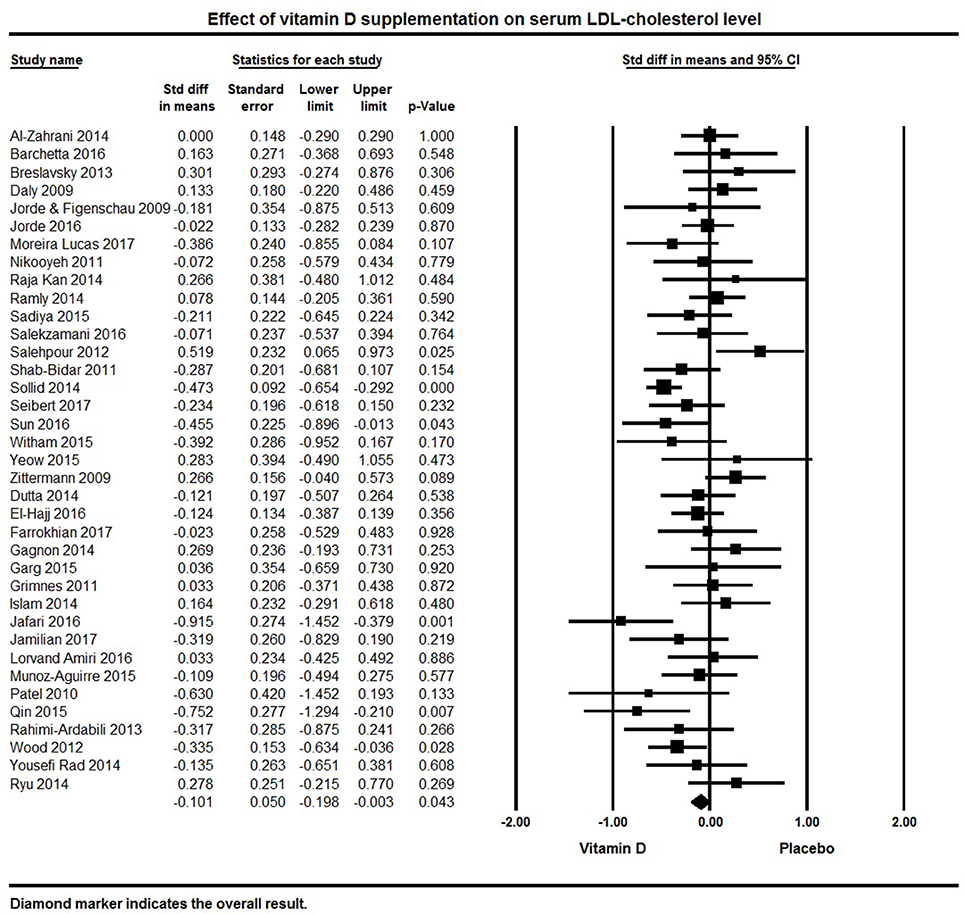
Figure 6. Forest plot detailing standardized mean difference for the impact of vitamin D on serum LDL-cholesterol level.
Serum HDL was a reported outcome for 39 studies. A significant effect of vitamin D supplementation on increased serum HDL was found with a pooled effect size of 0.09 ± 0.04 mmol/L [95% CI 0.00 to 0.17, p = 0.05, I2 = 37%; Figure 7). Vitamin D supplementation significantly increased serum HDL in 6 individual studies (32, 80, 92, 107, 113, 125). Serum HDL cholesterol remained unchanged following vitamin D supplementation in 17 studies (75, 95, 115, 119–121) and an increase in serum HDL levels in 16 studies (62, 64, 69, 78, 82–84, 91, 105, 108, 118, 126, 127, 130, 132, 134) (Figure 7).
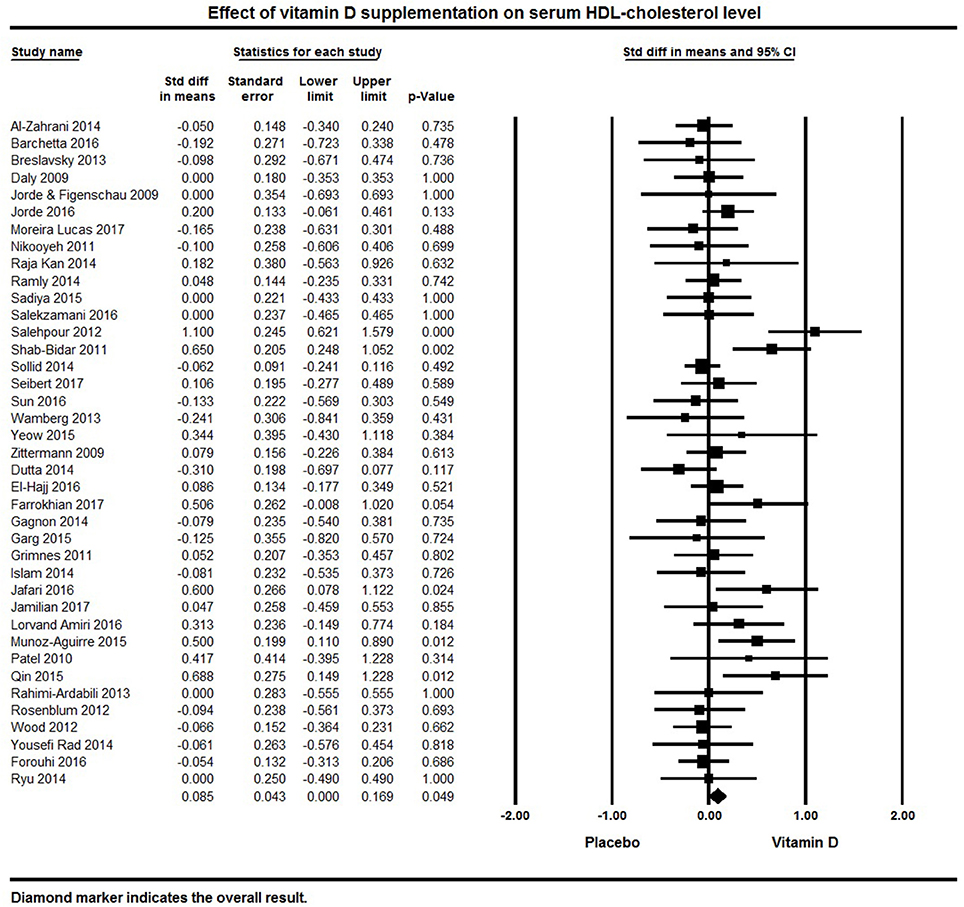
Figure 7. Forest plot detailing standardized mean difference for the impact of vitamin D on serum HDL-cholesterol level.
Vitamin D and PTH
Forty-five papers reported serum PTH levels as a primary or secondary endpoint. The pooled effect size of vitamin D on serum PTH levels was −0.66 ± 0.08 ng/L (95% CI −0.82 to −0.50, p < 0.001, I2 = 87%) across all studies (Figure 8). Twenty eight individual studies reported a significant reduction in PTH levels with vitamin D supplementation (32, 50, 65, 68, 70, 73–76, 82, 84, 87, 89, 92, 96–100, 102, 105, 117, 120, 125, 127, 131, 133, 136), 15 reported a non-significant reduction in PTH (60, 66, 67, 77, 90, 95, 104, 109, 116, 118, 119, 122, 129, 135, 138) and two studies found no change or an increase in PTH levels (110, 139).
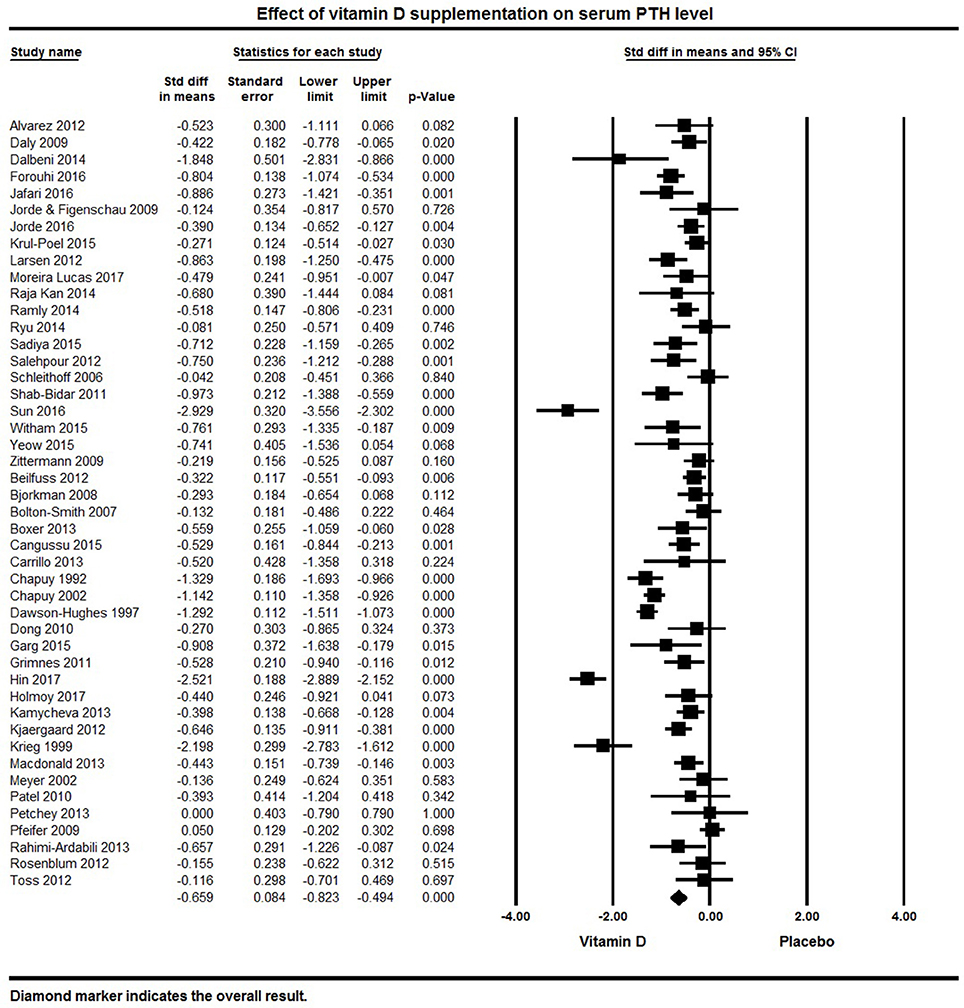
Figure 8. Forest plot detailing standardized mean difference for the impact of vitamin D on serum PTH level.
Vitamin D and hs-CRP
Twenty eight studies reported hs-CRP concentration as an outcome. The pooled effect size (standardized mean difference) of vitamin D supplementation on serum hs-CRP was −0.20 ± 0.07 mg/L (95% CI −0.34 to −0.06, p = 0.006, I2 = 73%) across all studies (Figure 9). Eight individual studies reported a significant reduction in serum hs-CRP following vitamin D supplementation (64, 66, 69, 83, 92, 112, 130, 133), 13 indicated a non-significant reduction in hs-CRP (65, 71, 72, 78, 80, 82, 86, 87, 116, 120, 126, 131, 137), and seven found either a null effect (85) or an increase in hs-CRP in the vitamin D supplemented group (106, 115, 119, 122, 127, 135).
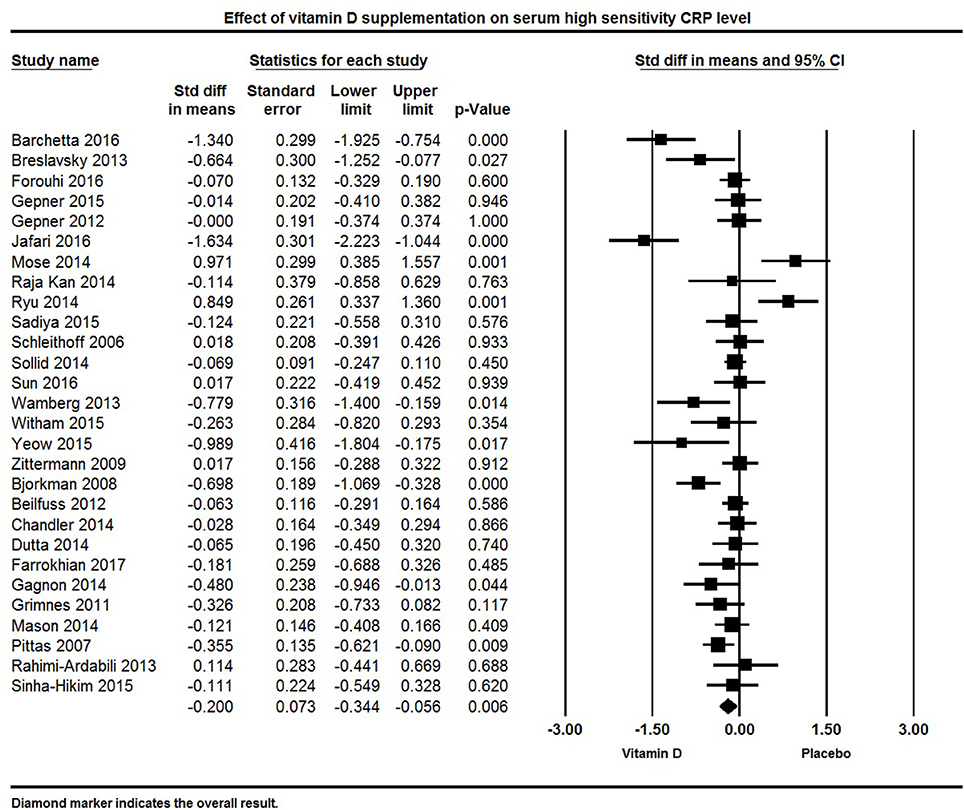
Figure 9. Forest plot detailing standardized mean difference for the impact of vitamin D on serum high sensitivity CRP level.
Vitamin D and Peak Wave Velocity
Eleven papers reported PWV as a primary or secondary outcome. Overall, there was no significant effect of vitamin D supplementation on PWV. The pooled effect size of vitamin D on PWV was −0.20 ± 0.13 m/s [95% CI −0.46 to 0.06, p = 0.13, I2 = 72%) across all studies (Figure 10). Four individual studies reported a significant reduction in PWV in the group supplemented with vitamin D (77, 82, 114, 128), two studies found a non-significant trend for reduction in PWV (88, 131), and five trials did not find any significant effect of vitamin D on PWV (33, 84, 85, 100, 106).
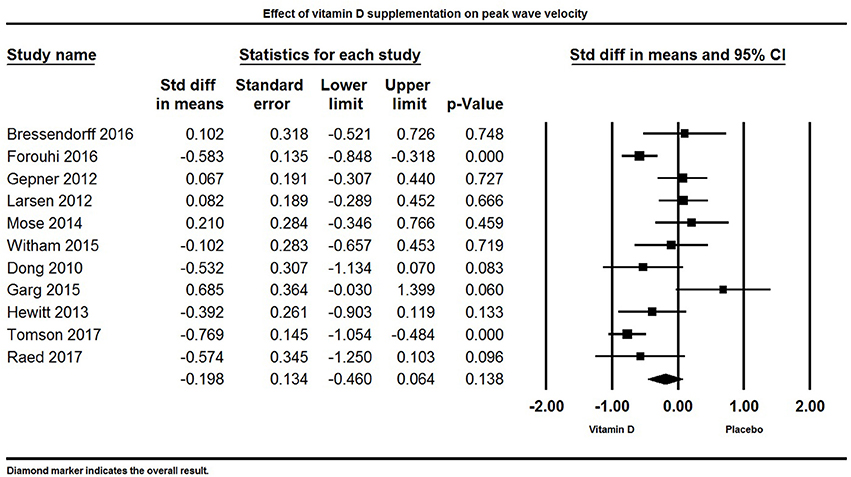
Figure 10. Forest plot detailing standardized mean difference for the impact of vitamin D on peak wave velocity.
Vitamin D and Augmentation Index
Ten studies reported AI as an outcome (Figure 11). Overall, vitamin D supplementation did not have an effect on AI. The pooled estimate (standardized mean difference) of the effect of vitamin D administration on AI was −0.09 ± 0.14% (95% CI −0.37 to 0.20%, p = 0.52, I2 = 74%).
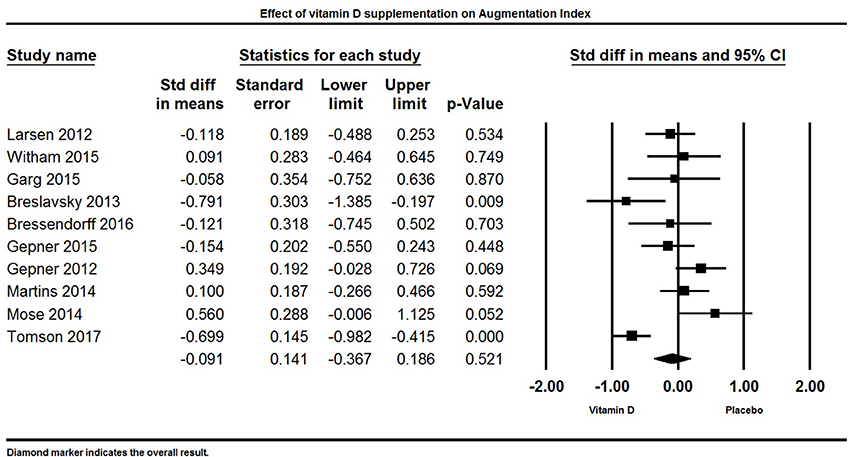
Figure 11. Forest plot detailing standardized mean difference for the impact of vitamin D on augmentation index.
Two studies reported a significant decrease in AI (69, 128), two studies reported a significant increase in AI (85, 106), and six trials did not find any influence of vitamin D supplementation on AI (33, 84, 86, 100, 103, 131).
Sensitivity Analysis
Using leave-one-out sensitivity analysis, the effect size of vitamin D remained significant for blood pressure, lipid profile, serum PTH, and serum hs-CRP, confirming that difference between treatment groups is not due to the effect of any single study.
We then removed more than one study based on a number of possible outliers and repeated meta-analysis. For serum PTH, three studies were removed (89, 98, 127) and vitamin D supplementation still significantly lowered serum PTH (−0.53 ± 0.06 ng/L, 95% CI −0.65 to −0.41, p < 0.001). For serum hs-CRP, two studies were removed (64, 92) and we found significant reduction in serum hs-CRP following vitamin D supplementation (−0.12 ± 0.06 mg/L, 95% CI −0.24 to −0.003, p = 0.04). Three studies were removed as outliers for serum TC (32, 76, 134) and there was still a significant lowering effect of vitamin D supplementation on serum TC (−0.14 ± 0.04 mmol/L, 95% CI −0.22 to −0.06, p < 0.001). There was one outlier for serum LDL (92); after removing this study, serum LDL decreased following vitamin D supplementation by −0.08 ± 0.05 mmol/L (95% CI −0.18 to 0.01, p = 0.07). Salehpour (32) study was removed as an outlier for serum HDL and the overall vitamin D effect size was 0.06 ± 0.04 mmol/L (95% CI −0.01 to 0.12, p = 0.12). For systolic BP, three studies were removed (61, 76, 81) and there was a non-significant decreased trend in systolic BP following vitamin D supplementation (−0.04 ± 0.03 mmHg, 95% CI −0.09 to 0.02, p = 0.2). We did not identify any outliers for diastolic BP, PWV, AI, and serum TG.
Publication Bias
Visual inspection of funnel plot symmetry suggested potential publication bias for the comparison of systolic and diastolic BP, PTH, PWV, TC, HDL, and hs-CRP between vitamin D-administered and placebo groups, though funnel plots for AI, TG, and LDL looked symmetric (Figures S1–S10). Egger's linear regressions did not indicate publication bias. After adjusting the effect size using the Trim and Fill method for potential publication bias, except for PWV, the effect size of vitamin D on lowering CVD risk parameters increased (BP, hs-CRP, PTH, AI, TC, LDL) or remained unchanged (TG, HDL) (Table S2).
Sub-Group Analysis
We investigated the effect of dose, achieved mean serum 25(OH)D, length of intervention, obesity, vitamin D deficiency, co-administration of calcium supplementation and age.
Achieved Serum 25(OH)D Concentrations at the End of the Trial
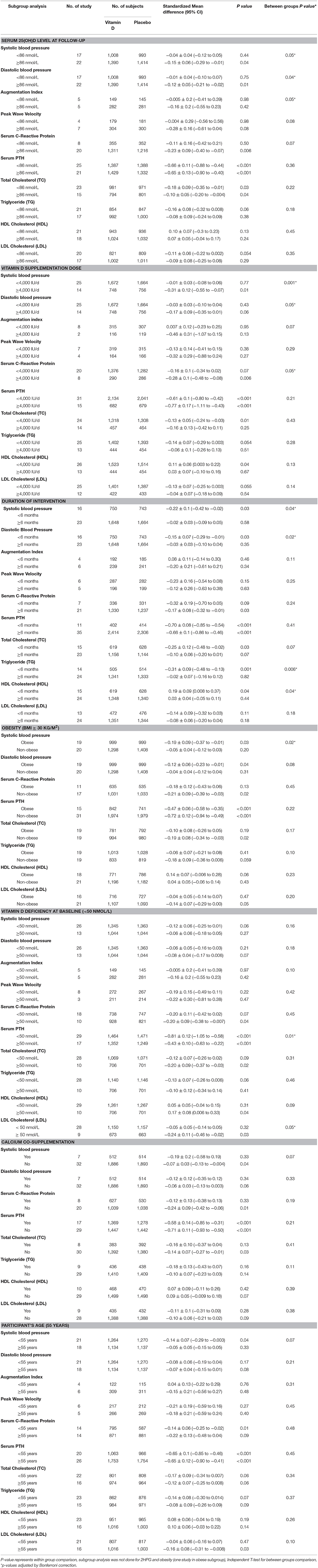
We investigated the effect of achieved serum 25(OH)D as high vs. low based on median levels (Table 2). Serum 25(OH)D concentrations ≥86 nmol/L resulted in a significantly higher reduction in systolic (−0.15 ± 0.06 vs. −0.04 ± 0.04 mmHg, p = 0.05), diastolic BP (−0.12 ± 0.05 vs. −0.01 ± 0.04 mmHg, p = 0.04), PWV (−0.28 ± 0.16 vs. −0.004 ± 0.29 m/s, p = 0.08), and hs-CRP (−0.23 ± 0.09 vs. −0.11 ± 0.16 md/L, p = 0.07). AI, for which there was no significant effect overall, was found to be significantly lower among participants with serum 25(OH)D concentrations ≥86 nmol/L (−0.16 ± 0.2% vs. −0.005 ± 0.2%, p = 0.05). PTH and lipid changes did not significantly differ based on achieved serum 25(OH)D concentration.
Vitamin D Supplementation Dose
We investigated dose effect on outcomes by comparing trials in which subjects received ≥4,000 IU/d of vitamin D to those in which subjects received <4,000 IU/d (Table 2). Trials with vitamin D doses ≥4,000 IU/d had significantly greater reduction in systolic (−0.31 ± 0.12 vs. −0.01 ± 0.03 mmHg, p = 0.001), diastolic BP (−0.17 ± 0.09 vs. −0.03 ± 0.03 mmHg, p = 0.05), and hs-CRP (−0.28 ± 0.1 vs. −0.16 ± 0.1 mg/L, p = 0.05). AI was significantly lowered in trials with vitamin D doses ≥4,000 IU/d (−0.46 ± 0.31% vs. 0.007 ± 0.12%, p = 0.07).
Duration of Intervention
To investigate whether the length of the trial influenced the effect of vitamin D supplementation on outcomes we investigated trials that supplemented for ≥6 months in comparison with those <6 months in duration (Table 2). In trials that assessed vitamin D supplementation for <6 months there was a significantly greater decrease in systolic (−0.22 ± 0.1 vs. −0.02 ±0.03 mmHg, p = 0.04), diastolic BP (−0.15 ± 0.07 vs. −0.03 ± 0.03 mmHg, p = 0.02), and TG (−0.31 ± 0.09 vs. −0.02 ± 0.07 mmol/L), and a greater increase in HDL (0.19 ± 0.09 vs. 0.03 ± 0.04 mmol/L, p = 0.04).
Obesity
We investigated whether outcomes differed between trials that enrolled an obese (BMI ≥ 30 kg/m2) population vs. non-obese (Table 2). In trials with obese subjects there was a significantly greater reduction in systolic (−0.19 ± 0.09 vs. −0.05 ± 0.04 mmHg, p = 0.02) and diastolic BP (−0.12 ± 0.06 vs. −0.04 ± 0.04 mmHg, p = 0.08). There was no significant difference in other outcomes based on obesity.
Vitamin D Deficiency at Baseline
We investigated whether the effect of vitamin D on outcomes was dependent on vitamin D deficiency at baseline by comparing trials vitamin D deficient subjects at baseline [serum 25(OH)D <50 nmol/L] vs. vitamin D sufficient subjects (Table 2). Vitamin D supplementation in trials with participants who were vitamin D deficient had a significantly greater reduction in PTH (−0.81 ± 0.12 vs. −0.43 ± 0.1 ng/L, p = 0.01), LDL (−0.24 ± 0.11 vs. −0.05 ± 0.05 mmol/L, p = 0.05) and AI (−0.16 ± 0.2% vs. −0.005 ± 0.2%, p = 0.10), and a greater increase in HDL (0.17 ± 0.08 vs. 0.05 ± 0.05 mmol/L, p = 0.09) in comparison with vitamin D sufficient participants.
Calcium Co-administration
We investigated whether calcium co-administration influenced outcomes by comparing those trials with those that supplemented with vitamin D alone (Table 2). Participants supplemented with both vitamin D and calcium had a significantly greater reduction in systolic BP (−0.19 ± 0.2 vs. −0.07 ±0.03 mmHg, p = 0.07) and TG levels (−0.18 ± 0.13 vs. −0.10 ±0.07 mmol/L, p = 0.11), compared with those who received vitamin D alone. There was no difference among the remaining parameters.
Effect of Participants' Age
Age itself is a risk factor for CVD and thus we compared trials that enrolled populations ≥55 y vs. <55 y. Vitamin D supplementation in trials with populations <55 y had significantly greater reduction in systolic BP (−0.14 ± 0.07 vs.−0.05 ± 0.05 mmHg, p = 0.07). There was no significant difference in other outcomes based on participant age grouping.
Discussion
As the leading cause of death and disability worldwide, cardiovascular disease (CVD) is a major public health burden (140). Much effort has been devoted to identifying modifiable risk factors to prevent CVD. Vitamins may have a role in the prevention and treatment of CVD. Antioxidant vitamins such as vitamin C, vitamin E, folic acid and vitamin B6 might decrease the rate of oxidative stress, a key component of atherosclerosis and CVD (14). Vitamin D and folic acid can inhibit inflammation with their anti-atherogenic effects. Vitamin E can inhibit platelet aggregation and B vitamins might have anti-thrombotic activity by lowering serum homocysteine levels (14, 141, 142). Among these, vitamin D, with its deficiency highly prevalent worldwide and having many pleiotropic effects, has been associated with CVD prevention in different community settings. Vitamin D deficiency impairs vascular function and is strongly associated with the heightened risks of various cardiovascular diseases such as hypertension, metabolic syndrome, heart failure, and stroke (3, 24).
Evidence suggests that vitamin D exerts beneficial cardiovascular effects through many pathways. Improved vitamin D status reduces RAAS activity and lowers blood pressure, it has anti-inflammatory, anti-proliferative, anti-hypertrophic, anti-fibrotic and anti-thrombotic impacts as well (111). Following vitamin D supplementation, suppression of renin production and downregulation of RAAS directly impacts myocardium and vasculature through modulating hypertrophic stimuli (143). Vitamin D inhibits the proliferation of vascular smooth muscle cells through influx of calcium into the cells, thus preserving endothelial function (144). Antihypertensive benefits of vitamin D include suppression of RAAS, an anti-proteinuric effect, a direct effect on endothelial cells and calcium metabolism as well as preventing secondary hyperparathyroidism (145, 146). Vitamin D may have both direct and indirect impacts on modifying lipid profiles. Vitamin D supplementation might decrease serum levels of triglyceride via increasing the activity of lipoprotein lipase in adipose tissue (147). Also, through improving calcium absorption, vitamin D might reduce fatty acid absorption via the formation of insoluble calcium-fatty acid complexes in the gut leading to decreased LDL cholesterol levels (148). Yet, despite these observations, evidence linking corrections to vitamin D status with improved cardiometabolic parameters is somewhat inconclusive (24, 43, 149).
Considering the alternate postulation, vitamin D deficiency might be a consequence of chronic conditions such as inflammation. There is a bacterial pathogenesis theory explains that intracellular bacteria commonly seen in chronic inflammation might invade different nucleated cells and affect vitamin D metabolism and its endocrine function resulting in low vitamin D status. This occurs concurrent to increased production of 1,25(OH)2D which is required for upregulating vitamin D receptors to transcribes more adenosine monophosphate. And more 25(OH)D should be metabolized in this process leading to low vitamin D status (150, 151). In another study conducted on the patients recovering from knee arthroplasty, there was a significant reduction in serum 25(OH)D levels during the process of systemic inflammatory response in these patients after surgery (152). Sattar et al. (153) also mentioned that vitamin D is an acute phase reactant and declines with the increase in inflammatory cytokine in different chronic conditions. Several mechanisms including decreased vitamin D carrier proteins, increased conversion of 25(OH)D to 1,25(OH)2D and hemodilution could be responsible for this reduction (154, 155). However for CVD, using Hill's criteria for causality, Weyland found that all relevant Hill criteria are satisfied suggesting low 25(OH)D level is an independent risk factor for CVD (156).
To better understand this incongruence, we analyzed 81 studies that evaluated the effect of vitamin D supplementation on various cardiometabolic risk parameters, including blood pressure, serum PTH, hs-CRP, lipid profile, and arterial PWV and AI. Unlike many previous studies, we imposed several strict inclusion criteria to select only well-designed trials. Overall, vitamin D supplementation was found to improve cardiovascular risk factors. Specifically, vitamin D supplementation, with doses above 4,000 IU/d and increased serum 25(OH)D concentrations ≥86 nmol/L decreased systolic and diastolic blood pressure, serum PTH, serum hs-CRP and improved lipid profiles (total cholesterol, triglyceride, HDL and LDL). Markers of arterial stiffness (PWV and AI) may also improve with vitamin D supplementation.
Subgroup analyses revealed that the co-administration of calcium with vitamin D led to greater reductions in blood pressure. The combination of calcium with vitamin D has been suggested to improve blood pressure by facilitating calcium absorption into the blood stream and optimizing serum calcium and PTH levels (157, 158). Although the greatest benefits of vitamin D supplementation can be achieved in vitamin D deficient populations, such that we observed as the lowering impact of vitamin D on serum PTH. Remarkably, we also found notable improvements in lipid profile in participants considered vitamin D sufficient prior to intervention. Further, individuals who were obese at baseline had a greater reduction in blood pressure, likely due to the higher percentage of obese individuals that are pre-hypertensive or hypertensive (159) and the higher daily doses of vitamin D provided to obese participants (61, 76, 81).
The results of our study compare closely with those from a number of recent meta-analyses. Jafari et al. (93), for instance, found significant reductions in the serum total cholesterol, triglyceride, and LDL levels of type 2 diabetics following vitamin D supplementation. Studying heterogeneous populations that consisted of healthy individuals, pregnant women, bedridden elderly people and those with different diseases (e.g., diabetes, heart failure, PCOS, and insulin resistant condition), both Chen et al. (160) and Rodriguez et al. (161) found that vitamin D supplementation significantly decreased inflammatory markers (i.e., hs-CRP). Chen et al. (160) additionally concluded that vitamin D supplementation led to a significantly greater reduction among those with baseline hs-CRP levels ≥5 mg/l. This is in line with increased hs-CRP levels in diabetic patients (64, 69, 92). Diabetes usually results in higher levels of hs-CRP and lower levels of 25(OH)D concentrations suggesting a larger effect size in subjects with this condition. Moreover, Wu et al. (162) and Witham et al. (163) found a significant modest reduction in blood pressure following vitamin D supplementation. Vitamin D supplementation may affect arterial stiffness and vascular aging through decreased synthesis of angiotensin II, following inhibition of RAAS, to increase vascular tone and arterial stiffness (164). However, limited data to assess the impact of vitamin D supplementation on the markers of arterial stiffness (PWV and AI) were inconclusive (43, 165), and may be due to inappropriate study design including insufficient duration of supplementation and insufficient power (119).
In contrast, Beveridge et al. (21) found no significant reduction in blood pressure after participants whose mean SBP was ≥140 mm Hg at baseline were supplemented with vitamin D. Of note, there are significant methodological differences in our approach. Beveridge et al. (21) included trials that combined vitamin D with antihypertensive drugs, administered large bolus doses to elderly populations, included subjects with resistant HTN, and/or supplemented with very low doses of vitamin D (i.e., 600 IU). These issues could mask any effects of vitamin D supplementation or simply not lead to any observable benefits. Our strict inclusion criteria resulted in the exclusion of 14 of the 27 studies that were analyzed by Beveridge et al. We had similar concerns with the meta-analysis conducted by Wang et al. (26) who reported that vitamin D supplementation led to a statistically significant increase in LDL and included RCTs that provided very low doses of vitamin D (i.e., 300 IU) or supplemented for durations considered too short (i.e., 42 days). Of the 12 studies included, and as mentioned by the authors, none were sufficiently powered to detect changes in CVD outcomes. The current meta-analysis revealed significant impact of vitamin D supplementation on lipid profiles with increased HDL and reduced LDL and TG.
Many observational studies support an association between cardiovascular risk factors and low vitamin D status. Perhaps most importantly, what constitutes vitamin D deficiency and repletion is somewhat debateable and, at times, contentious. The Institute of Medicine issues dietary recommendations, such as the Recommended Dietary Allowance (RDA), at the request of the U.S. and Canadian governments. In 2010, the Institute of Medicine set the RDA for vitamin D at 600 IU per day for individuals between the age of 1 and 70 (48). This RDA is assumed to achieve serum 25(OH)D levels of ≥50 nmol/l in 97.5% of the population. The methodology used to calculate this RDA, however, has been deemed erroneous (166) and estimates of much higher magnitude have been calculated by others – 3,875 IU/day (167) to 8,895 IU/day (168).
Similarly, the definition of what is an “optimal” serum 25(OH)D concentration is also controversial. Serum 25(OH)D concentrations >75 nmol/l (12) and >80 nmol/l (28) have been suggested as necessary for lipid and cardiovascular health. Serum 25(OH)D concentrations of 100–150 are defined as optimal by the U.S. Endocrine Society with values below 75 nmol/l deemed insufficient (169, 170). The results of the present meta-analysis suggest that serum 25(OH)D concentrations ≥86 nmol/L are optimal for reductions in blood pressure, markers of arterial stiffness, and reductions in hs-CRP. It is important to note that these serum 25(OH)D concentrations were achieved with vitamin D supplement doses ≥4,000 IU/d—the current tolerable upper level of intake. Using the standards of the U.S. Endocrine Society, 27 of the 81 included studies in our meta-analyses reached optimal 25(OH)D levels post-supplementation, and only 16 had post-supplement 25(OH)D levels that were insufficient.
The duration of supplementation is an important factor in assessments of vitamin D. With a half-life of 2 months, to achieve and maintain a steady serum 25(OH)D concentration requires a follow-up period of at least 3 months. Here, we included trials that ranged from 3 months to 5 years of intervention in the meta-analysis. Somewhat surprisingly, we found better improvement in some outcomes (blood pressure and lipids) in trials that were less than 6 months, although this is likely related to higher compliance in short-term interventions (171). Improvements in blood pressure and lipid profile were also witnessed in a short, 3 month vitamin D intervention in obese PCOS patients (116). The women received 12,000 IU/d of vitamin D for an increase in their serum 25(OH)D of 50–168 nmol/L. In contrast, a 5 year trial of obese and vitamin D-insufficient prediabetics provided 2,800 IU/d of vitamin D found no change in blood pressure (50). It is known that overweight and obese individuals require two to three times the amount of vitamin D to increase serum 25(OH)D concentrations to the same extent as those with a normal BMI (31, 47).
The present study has several strengths and limitations. Even after enforcing a strict inclusion criteria, the included studies varied with regard to participant age, serum 25(OH)D concentrations at baseline, concurrent use of other nutrients or medication, and overall health status. We used a random-effect model and performed sensitivity analyses to mitigate these limitations. For some of the studies, cardiovascular outcomes of interest were secondary outcomes or the trial was not of sufficient power to detect a change in these outcomes. Many of the studies also did not describe dietary intakes, season of treatment, or sun exposure. Further, some included trials assessed relatively small populations (10–13 participants per intervention group), but taken together offer support to the larger trials. Strengths include the large sample and the consideration of a wide variety of CVD risk parameters from at least 28 clinical trials for each CVD outcome (with the exception of arterial PWV and AI).
Conclusion
Vitamin D deficiency is a highly prevalent condition and is independently associated with most CVD risk factors. The present meta-analysis demonstrated that vitamin D supplementation improved serum 25(OH)D concentrations significantly lowered blood pressure, serum PTH, hs-CRP, TC, LDL, and TG and increased HDL. Vitamin D supplementation also appears to improve arterial stiffness (PWV), but large and well-designed RCTs are required to confirm these findings. The present analysis suggests that for improvements in CVD risk factors vitamin D supplementation ≥4,000 IU/d and achieved serum 25(OH)D concentrations ≥86 nmol/L are required.
Author Contributions
NM, JR, and SK designed the study, NM and JR searched databases and performed the selection of studies. NM, JR, and SK wrote the manuscript. NM analyzed the data. SK and JR critically evaluated the review, commented on it, and approved the last version. All authors reviewed and approved the final manuscript. SK is the guarantor of this study.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Ken Fyie for preparing funnel plots and Brian Rankin for reviewing this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2018.00087/full#supplementary-material
References
1. Thomas GN, Hartaigh BO, Bosch JA, Pilz S, Loerbroks A, Kleber ME, et al. Vitamin D levels predict all-cause and cardiovascular disease mortality in subjects with the metabolic syndrome, the ludwigshafen risk and cardiovascular health (LURIC) study. Diabetes Care (2012) 35:1158–64. doi: 10.2337/dc11–1714
2. Wang J, Khaw KT, Bingham Sh, Wareham NJ, Forouhi NG. Dietary Energy Density Predicts the Risk of Incident Type 2 Diabetes, The European Prospective Investigation of Cancer (EPIC)-Norfolk Study. Diabetes Care (2008) 31:2120–5. doi: 10.2337/dc08–1085
3. Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. (2008) 168:1629–37. doi: 10.1001/archinte.168.15.1629
4. Lee JH, O'Keefe JH, Bell D, Hensrud DD, Holick MF. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? J Am Coll Cardiol. (2008) 24:1949–56. doi: 10.1016/j.jacc.2008.08.050
5. Tangpricha V, Pearce EN, Chen TC, Holick MF. Vitamin D insufficiency among free-living healthy young adults. Am J Med. (2002) 112:659–62. doi: 10.1016/S0002–9343(02)01091–4
6. Meehan M, Penckofer S. The Role of Vitamin D in the aging adult. J Aging Gerontol. (2014) 2:60–71. doi: 10.12974/2309–6128.2014.02.02.1
7. Fraser A, Williams D, Lawlor DA. Associations of serum 25-hydroxyvitamin D, parathyroid hormone and calcium with cardiovascular risk factors: analysis of 3 NHANES cycles (2001–2006). PLoS ONE (2010) 5:e13882. doi: 10.1371/journal.pone.0013882
8. Kunutsor SK, Burgess S, Munroe PB, Khan H. Vitamin D and high blood pressure: causal association or epiphenomenon? Eur J Epidemiol. (2014) 29:1–14. doi: 10.1007/s10654-013-9874-z
9. Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the Third National Health and Nutrition Examination Survey. Am J Hypertens (2007) 20:713–9. doi: 10.1016/j.amjhyper.2007.01.017
10. Kendrick J, Targher G, Smits G, Chonchol M. 25-Hydroxyvitamin D deficiency is independently associated with cardiovascular disease in the Third National Health and Nutrition Examination Survey. Atherosclerosis (2009) 205:255–60. doi: 10.1016/j.atherosclerosis.2008.10.033
11. Yin K, Agrawal DK. Vitamin D and inflammatory diseases. J Inflamm Res. (2014) 7:69–87. doi: 10.2147/JIR.S63898
12. Leu M, Giovannucci E. Vitamin D: epidemiology of cardiovascular risks and events. Best Pract Res Clin Endocrinol Metab. (2011) 25:633–46. doi: 10.1016/j.beem.2011.04.001
13. Schottker B, Jorde R, Peasey A, Thorand B, Jansen EH, Groot Ld, et al. Vitamin D and mortality: meta-analysis of individual participant data from a large consortium of cohort studies from Europe and the United States. BMJ (2014) 348:g3656. doi: 10.1136/bmj.g3656
14. Debreceni B, Debreceni L. Role of vitamins in cardiovascular health and disease. Res Rep Clin Cardiol. (2014) 5:283–95. doi: 10.2147/RRCC.S44465
15. Au-Yeung KK, Yip JC, Siow YL OK. Folic acid inhibits homocysteineinduced superoxide anion production and nuclear factor kappa B activation in macrophages. Can J Physiol Pharmacol. (2006) 84:141–7. doi: 10.1139/Y05-136
16. Bellamy MF, McDowell IF, Ramsey MW, Brownlee M, Newcombe RG, Lewis MJ. Oral folate enhances endothelial function in hyperhomocysteinaemic subjects. Eur J Clin Invest. (1999) 29:659–62. doi: 10.1046/j.1365-2362.1999.00527.x
17. Challoumas DS, Stavrou A, Pericleous A, Dimitrakakis G. Effects of combined vitamin D–calcium supplements on the cardiovascular system: should we be cautious? Atherosclerosis (2015) 238:388–98. doi: 10.1016/j.atherosclerosis.2014.12.050
18. Carvalho LSF, Sposito AC. Vitamin D for the prevention of cardiovascular disease: are we ready for that? Atherosclerosis (2015) 241:729–40. doi: 10.1016/j.atherosclerosis.2015.06.034
19. Wallis DE, Sizemore GW. The “sunshine deficit” and cardiovascular disease. Circulation (2008) 118:1476–85. doi: 10.1161/CIRCULATIONAHA.107.713339
20. Anderson JL, May HT, Horne BD, Bair TL, Hall NL, Carlquist JF, et al. Relation of Vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. Am J Cardiol. (2010) 106:963–8. doi: 10.1016/j.amjcard.2010.05.027
21. Beveridge LA, Struthers AD, Khan F, Jorde R, Scragg R, Macdonald HM, et al. Effect of vitamin D supplementation on blood pressure: a systematic review and meta-analysis incorporating individual patient data. JAMA Intern Med. (2015) 175:745. doi: 10.1001/jamainternmed.2015.0237
22. Bolland MJ, Grey A, Gamble GD, Reid IR. The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: a trial sequential meta-analysis. Lancet Diabetes Endocrinol. (2014) 2:307–20. doi: 10.1016/S2213-8587(13)70212-2
23. Elamin MB, Abu Elnour NO, Elamin KB, Fatourechi MM, Alkatib AA, Almandoz JP, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. (2011) 96:1931–42. doi: 10.1210/jc.2011-0398
24. Ford JAM, MacLennan GS, Avenell A, Bolland M, Grey A, Witham M, for the RECORD Trial Group. Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am J Clin Nutr. (2014) 100:746–55. doi: 10.3945/ajcn.113.082602
25. Mao PJZ, Zhang C, Tang L, Xian YQ, Li YS, Wang WD, et al. Effect of calcium or vitamin D supplementation on vascular outcomes: a meta-analysis of randomized controlled trials. Int J Cardiol. (2013) 169:106–11. doi: 10.1016/j.ijcard.2013.08.055
26. Wang L, Manson JE, Song Y, Sesso HD. Systematic review: vitamin D and calcium supplementation in prevention of cardiovascular events. Ann Intern Med. (2010) 152:315–23. doi: 10.7326/0003-4819-152-5-201003020-00010
27. Lappe JM, Heaney RP. Why randomized controlled trials of calcium and vitamin D sometimes fail. Dermatoendocrinolgy (2012) 4:95–100. doi: 10.4161/derm.19833
28. Jorde R, Grimnes G. Vitamin D and health: the need for more randomized controlled trials. J Steroid Biochem Mol Biol. (2015) 148:269–74. doi: 10.1016/j.jsbmb.2015.01.021
29. Heaney RP. Vitamin D—baseline status and effective dose. N Engl J Med. (2012) 367:77–8. doi: 10.1056/NEJMe1206858
30. Heaney RP. Toward a physiological referent for the vitamin D requirement. J Endocrinol Invest. (2014) 37:1127–30. doi: 10.1007/s40618-014-0190-6
31. Kimball SM, Mirhosseini N, Holick MF. Evaluation of vitamin D3 intakes up to 15,000 international units/day and serum 25-hydroxyvitamin D concentrations up to 300 nmol/L on calcium metabolism in a community setting. Dermatoendocrinology (2017) 9:e1300213. doi: 10.1080/19381980.2017.1300213
32. Salehpour A, Shidfar F, Hosseinpanah F, Vafa MR, Razaghi M, Hoshiarrad A, et al. Vitamin D3 and the risk of CVD in overweight and obese women: a randomised controlled trial. Br J Nutr. (2012) 108:1866–73. doi: 10.1017/S0007114512000098
33. Bressendorff I, Brandi L, Schou M, Nygaard B, Frandsen NE, Rasmussen K, et al. The effect of high dose cholecalciferol on arterial stiffness and peripheral and central blood pressure in healthy humans: a randomized controlled trial. PLOS ONE (2016) 11:e0160905. doi: 10.1371/journal.pone.0160905
34. Higgins JPT, Green SE, Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration (2009). Available online at: www.handbook.cochrane.org
35. Egger M, Davey Smith G, Schneider M, Minder C. Bias in metaanalysis detected by a simple, graphical test. BMJ (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
36. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in metaanalysis. Biometrics (2000) 56:455–63. doi: 10.1111/j.0006-341X.2000.00455.x
37. Borenstein MHL, Higgins J, Rothstein H. Comprehensive Meta-Analysis. Version2. Englewood Cliffs, NJ: Biostat (2005).
38. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration (2011). Available online at: www.handbook.cochrane.org
39. Sutton AJ, Abrams KR, Jones DR. Methods for Meta-Analysis in Medical Research. West Sussex: John Wiley & Sons (2000).
40. Mazidi M, Rezaie P, Ferns GA, Gao HK. Impact of different types of tree nut, peanut, and soy nut consumption on serum C-reactive protein (CRP): a systematic review and meta-analysis of randomized controlled clinical trials. Medicine (Baltimore) (2016) 95:e5165. doi: 10.1097/MD.0000000000005165
41. Mazidi M, Rezaie P, Ferns GA, Gao HK. Impact of probiotic administration on serum c-reactive protein concentrations: systematic review and meta-analysis of randomized control trials. Nutrients (2017) 9:20. doi: 10.3390/nu9010020
42. Heaney RP Armas LA. Quantifying the vitamin D economy. Nutr Rev. (2015) 73:51–67. doi: 10.1093/nutrit/nuu004
43. Rodriguez AJ, Srikanth V, Ebeling P. Effect of vitamin D supplementation on measures of arterial stiffness: a systematic review and meta-analysis of randomized controlled trials. Clin Endocrinol. (2016) 84:645–57. doi: 10.1111/cen.13031
44. Zheng Y, Zhou M, Cui L, Yao W, Liu Y. Meta-analysis of long-term vitamin D supplementation on overall mortality. PLoS ONE (2013) 8:e82109. doi: 10.1371/journal.pone.0082109
45. Vieth V. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr. (1999) 69:842–56.
46. World Health Organization. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation. WHO Technical Report Series 894. Geneva: World Health Organization (2000).
47. Ekwaru JP, Holick MF, Giovannucci E, Veugelers PJ. The importance of body weight for the dose response relationship of oral vitamin D supplementation and serum 25-hydroxyvitamin D in healthy volunteers. PLoS ONE (2014) 9:e111265. doi: 10.1371/journal.pone.0111265
48. Institute of Medicine IoM. Dietary reference intakes for calcium and vitamin D. Washington DC: The National Academic Press (2011).
49. Jorde R, Sneve M, Torjesen P, Figenschau Y. No improvement in cardiovascular risk factors in overweight and obese subjects after supplementation with vitamin D3 for 1 year. J Intern Med. (2010) 267:462–72. doi: 10.1111/j.1365-2796.2009.02181.x
50. Jorde R, Sollid ST, Svartberg J, Schirmer H, Joakimsen RM, Njolstad I, et al. Vitamin D 20,000 IU per week for five years does not prevent progression from prediabetes to diabetes. J Clin Endocrinol Metab. (2016) 101:1647–55. doi: 10.1210/jc.2015–4013
51. Major GC, Alarie F, Doré J, Phouttama S, Tremblay A. Supplementation with calcium + vitamin D enhances the beneficial effect of weight loss on plasma lipid and lipoprotein concentrations. Am J Clin Nutr. (2007) 85:54–9. doi: 10.1093/ajcn/85.1.54
52. Dalan R, Liew H, Assam PN, Chan ES, Siddiqui FJ, Tan AW, et al. A randomised controlled trial evaluating the impact of targeted vitamin D supplementation on endothelial function in type 2 diabetes mellitus: the DIMENSION trial. Diab Vasc Dis Res. (2016) 13:192–200. doi: 10.1177/1479164115621667
53. Harwood RH, Sahota O, Gaynor K, Masud T, Hosking DJ, Nottingham Neck of Femur (NONOF) Study. A randomised, controlled comparison of different calcium and vitamin D supplementation regimens in elderly women after hip fracture: The Nottingham Neck of Femur (NONOF) Study. Age and Aging. (2004) 33:45–51. doi: 10.1093/ageing/afh002
54. Kampmann U, Mosekilde L, Juhl C, Moller N, Christensen B, Rejnmark L, et al. Effects of 12 weeks high dose vitamin D3 treatment on insulin sensitivity, beta cell function, and metabolic markers in patients with type 2 diabetes and vitamin D insufficiency – a double-blind, randomized, placebo-controlled trial. Metab Clin Exp. (2014) 63:1115–24. doi: 10.1016/j.metabol.2014.06.008
55. Longenecker CT, Hileman CO, Carman TL, Ross AC, Seydafkan Sh, Brown TT, et al. Vitamin D supplementation and endothelial function in vitamin D deficient HIV-infected patients: a randomized placebo-controlled trial. Antivir Ther. (2012) 17:613–21. doi: 10.3851/IMP1983
56. Rajpathak SN, Xue X, Wassertheil-Smoller S, Van Horn L, Robinson JG, Liu S, et al. Effect of 5 y of calcium plus vitamin D supplementation on change in circulating lipids: results from the Women's Health Initiative. Am J Clin Nutr. (2010) 91:894–9. doi: 10.3945/ajcn.2009.28579
57. von Hurst PR S, Stonehouse W, Coad J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient – a randomised, placebo-controlled trial. Br J Nutr. (2010) 103:549–55. doi: 10.1017/S0007114509992017
58. Yiu YF, Yiu KH, Siu CW, Chan YH, Li SW, Wong LY, et al. Randomized controlled trial of vitamin D supplement on endothelial function in patients with type 2 diabetes. Atherosclerosis (2013) 227:140–6. doi: 10.1016/j.atherosclerosis.2012.12.013
59. Cooper L, Clifton-Bligh PB, Nery ML, Figtree G, Twigg S, Hibbert E, et al. Vitamin D supplementation and bone mineral density in early postmenopausal women. Am J Clin Nutr. (2003) 77:1324–9. doi: 10.1093/ajcn/77.5.1324
60. Alvarez JA, Law J, Coakley KE, Zughaier SM, Hao L, Shahid Salles KH, et al. High-dose cholecalciferol reduces parathyroid hormone in patients with early chronic kidney disease: a pilot, randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. (2012) 96:672–9. doi: 10.3945/ajcn.112.040642
61. Al-Sofiani ME, Jammah A, Racz M, Khawaja RA, Hasanato R, El-Fawal HAN, et al. Effect of Vitamin D supplementation on glucose control and inflammatory response in Type II diabetes: a double blind, randomized clinical trial. Int J Endocrinol Metab. (2015) 13:e22604. doi: 10.5812/ijem.22604
62. Al-Zahrani MK, Elnasieh AM, Alenezi FM, Almoushawah AA, Almansour M, Alshahrani F, et al. A 3-month oral vitamin D supplementation marginally improves diastolic blood pressure in Saudi patients with type 2 diabetes mellitus. Int J Clin Exp Med. (2014) 7:5421–8.
63. Arora P, Song Y, Dusek J, Plotnikoff G, Sabatine M, Cheng S, et al. Vitamin D Therapy in Individuals with Pre-Hypertension or Hypertension: the DAYLIGHT trial. Circulation (2015) 131:254–62. doi: 10.1161/CIRCULATIONAHA.114.011732
64. Barchetta I, Del Ben M, Angelico F, Di Martino M, Fraioli A, La Torre G, et al. No effects of oral vitamin D supplementation on non-alcoholic fatty liver disease in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. BMC Med. (2016) 14:92. doi: 10.1186/s12916-016-0638-y
65. Beilfuss J, Berg V, Sneve M, Jorde R, Kamycheva E. Effects of a 1-year supplementation with cholecalciferol on interleukin-6, tumor necrosis factor-alpha and insulin resistance in overweight and obese subjects. Cytokine (2012) 60:870–4. doi: 10.1016/j.cyto.2012.07.032
66. Bjorkman MP, Sorva AJ, Tilvis RS. C-reactive protein and fibrinogen of bedridden older patients in a six-month vitamin D supplementation trial. J Nutr Health Aging. (2009) 13:436–9. doi: 10.1007/s12603-009-0080-3
67. Bolton-Smith C, McMurdo MET, Paterson CR, Mole PA, Harvey JM, Fenton ST, et al. Two-Year Randomized Controlled Trial of Vitamin K1 (Phylloquinone) and Vitamin D3 Plus Calcium on the Bone Health of Older Women. J Bone Miner Res. (2007) 22:509–19. doi: 10.1359/jbmr.070116
68. Boxer RS, Kenny AM, Schmotzer BJ, Vest M, Fiutem JJ, Pina IL. A randomized controlled trial of high-dose vitamin D3 in patients with heart failure. JACC Heart Fail (2013) 1:84–90. doi: 10.1016/j.jchf.2012.11.003
69. Breslavsky A, Frand J, Matas Z, Boaz M, Barnea Z, Shargorodsky M. Effect of high doses of vitamin D on arterial properties, adiponectin, leptin and glucose homeostasis in type 2 diabetic patients. Clin Nutr. (2013) 32:970–5. doi: 10.1016/j.clnu.2013.01.020
70. Cangussu LM, Nahas-Neto J, Orsatti CL, Bueloni-Dias FN, Nahas EA. Effect of vitamin D supplementation alone on muscle function in postmenopausal women: a randomized, double-blind, placebo-controlled clinical trial. Osteoporos Int. (2015) 26:2413–21. doi: 10.1007/s00198-015-3151-9
71. Mason C, Xiao L, Imayama I, Duggan C, Wang CHY, Korde L, et al. Vitamin D3 supplementation during weight loss: a double-blind randomized controlled trial. Am J Clin Nutr. (2014) 99:1015–25. doi: 10.3945/ajcn.113.073734
72. Chandler PD, Scott JB, Drake BF, Ng K, Manson JE, Rifai N, et al. Impact of Vitamin D Supplementation on Inflammatory Markers in African-Americans: results of a Four-Arm, Randomized, Placebo-Controlled Trial. Cancer Prev Res. (2014) 7:218–25. doi: 10.1158/1940-6207.CAPR-13-0338-T
73. Chapuy MC, Arlot ME, Duboeuf F, Brun J, Crouzet B, Arnaud S, et al. Vitamin D3 and calcium to prevent hip fracture in elderly women. N Engl J Med. (1992) 327:1637–42. doi: 10.1056/NEJM199212033272305
74. Chapuy MC, Pamphile R, Paris E, Kempf C, Schlichting M, Arnaud S, et al. Combined calcium and vitamin D3 supplementation in elderly women: confirmation of reversal of secondary hyperparathyroidism and hip fracture risk: the Decalyos II study. Osteoporos Int. (2002) 13:257–64. doi: 10.1007/s001980200023
75. Daly RM, Nowson CA. Long-term effect of calcium-vitamin D3 fortified milk on blood pressure and serum lipid concentrations in healthy older men. Eur J Clin Nutr. (2009) 63:993–1000. doi: 10.1038/ejcn.2008.79
76. Dalbeni A, Scaturro G, Degan M, Minuz P, Delva P. Effects of six months of vitamin D supplementation in patients with heart failure: a randomized double-blind controlled trial. Nutr Metab Cardiovasc Dis. (2014) 24:861–8. doi: 10.1016/j.numecd.2014.02.015
77. Dong Y, Stallmann-Jorgensen IS, Pollock NK, Harris RA, Keeton D, Huang Y, et al. A 16-Week Randomized Clinical Trial of 2000 International Units Daily Vitamin D3 Supplementation in Black Youth: 25-Hydroxyvitamin D, Adiposity, and Arterial Stiffness J Clin Endocrinol Metab. (2010) 95:4584–91. doi: 10.1210/jc.2010-0606
78. Dutta D, Mondal SA, Choudhuri S, Maisnam I, Hasanoor Reza AH, Bhattacharya B, et al. Vitamin-D supplementation in prediabetes reduced progression to type 2 diabetes and was associated with decreased insulin resistance and systemic inflammation: an open label randomized prospective study from Eastern India. Diabetes Res Clin Pract (2014) 103:e18-e23. doi: 10.1016/j.diabres.2013.12.044
79. El-Hajj Fuleihan G, Baddoura R, Habib RH, Halaby G, Arabi A, Rahme M, et al. Effect of vitamin D replacement on indexes of insulin resistance in overweight elderly individuals: a randomized controlled trial. Am J Vlin Nutr. (2016) 104:315–23. doi: 10.3945/ajcn.116.132589
80. Farrokhian AR, Raygan F, Bahmani F, Talari HR, Esfandiari R, Esmaillzadeh A, et al. Long-Term vitamin D supplementation affects metabolic status in vitamin D–deficient type 2 diabetic patients with Coronary Artery disease. J Nutr. (2017) 147:384–9. doi: 10.3945/jn.116.242008
81. Forman JP, Scott JB, Ng K, Drake BF, Gonzalez Suarez E, Hayden DL, et al. Effect of Vitamin D Supplementation on Blood Pressure in African-Americans. Hypertension (2013) 61:779–85. doi: 10.1161/HYPERTENSIONAHA.111.00659
82. Forouhi NG, Menon RK, Sharp SJ, Mannan N, Timms PM, Martineau AR, et al. Effects of vitamin D2 or D3 supplementation on glycaemic control and cardiometabolic risk among people at risk of type 2 diabetes: results of a randomized double-blind placebo-controlled trial. Diabetes Obes Metab. (2016) 18:392–400. doi: 10.1111/dom.12625
83. Gagnon C, Daly RM, Carpentier A, Lu ZX, Shore-Lorenti C, Sikaris K, et al. Effects of combined calcium and vitamin D supplementation on insulin secretion, insulin sensitivity and b-cell function in multi-ethnic vitamin D-deficient adults at risk for type 2 diabetes: a pilot randomized, placebo-controlled trial. PLoS ONE (2014) 9:e109607. doi: 10.1371/journal.pone.0109607
84. Garg G, Kachhawa G, Ramot R, Khadgawat R, Tandon N, Sreenivas V, et al. Effect of vitamin D supplementation on insulin kinetics and cardiovascular risk factors in polycystic ovarian syndrome: a pilot study. Endocr Connect (2015) 4:108–16. doi: 10.1530/EC-15-0001
85. Gepner AD, Krueger DC, Korcarz CE, Binkley N, Stein JH. A prospective randomized controlled trial of the effects of vitamin D supplementation on cardiovascular disease risk. PLOS ONE (2012) 7:e36617. doi: 10.1371/journal.pone.0036617
86. Gepner AD, Haller IV, Krueger DC, Claudia E. Korcarz CE, Binkley N, et al. A randomized controlled trial of the effects of vitamin D supplementation on arterial stiffness and aortic blood pressure in native american women. Atherosclerosis (2015) 240:526–8. doi: 10.1016/j.atherosclerosis.2015.04.795
87. Grimnes G, Figenschau Y, Almas B, Jorde R. Vitamin D, insulin secretion, sensitivity, and lipids: results from a case-control study and a randomized controlled trial using hyperglycemic clamp technique. Diabetes (2011) 60:2748–57. doi: 10.2337/db11-0650
88. Hewitt NA, O'Connor AA, O'Shaughnessy DV, Elder GJ. Effects of Cholecalciferol on Functional, Biochemical, Vascular, and Quality of Life Outcomes in Hemodialysis Patients. Clin J Am Soc Nephrol (2013) 8:1143–9. doi: 10.2215/CJN.02840312
89. Hin H, Tomson J, Newman C, Kurien R, Lay M, Cox J, et al. Optimum dose of vitamin D for disease prevention in older people: BEST-D trial of vitamin D in primary care. Osteoporos Int. (2017) 28:841–51. doi: 10.1007/s00198-016-3833-y
90. Holmoy T, Lindstrom JCH, Eriksen EF, Steffensen LH, Kampman MT. High dose vitamin D supplementation does not affect biochemical bone markers in multiple sclerosis – a randomized controlled trial. BMC Neurol. (2017) 17:67. doi: 10.1186/s12883-017-0851-0
91. Islam MdZ, Shamim AA, Akhtaruzzaman M, Kärkkäinen M, Lamberg-Allardt Ch. Effect of vitamin D, calcium and multiple micronutrients supplementation on lipid profile in pre-menopausal bangladeshi garment factory workers with hypovitaminosis D. J Health Popul Nutr. (2014) 32:687–95.
92. Jafari T, Faghihimani E, Feizi A, Iraj B, Haghjooy Javanmard SH, Esmaillzadeh A, et al. Effects of vitamin D-fortified low fat yogurt on glycemic status, anthropometric indexes, inflammation, and bone turnover in diabetic postmenopausal women: A randomised controlled clinical trial. Clin Nutr. (2016) 35:67–76. doi: 10.1016/j.clnu.2015.02.014
93. Jafari T, Fallah AA, Barani A. Effects of vitamin D on serum lipid profile in patients with type 2 diabetes: a meta-analysis of randomized controlled trials. Clin Nutr (2016) 35:1259–68. doi: 10.1016/j.clnu.2016.03.001
94. Jamilian M, Maktabi M, Asemi Z. A Trial on the effects of magnesium-zinc-calcium-vitamin D co-supplementation on glycemic control and markers of cardio-metabolic risk in women with polycystic ovary syndrome. Arch Iran Med. (2017) 20:640–5.
95. Jorde RF, Figenschau Y. Supplementation with cholecalciferol does not improve glycaemic control in diabetic subjects with normal serum 25-hydroxyvitamin D levels. Eur J Nutr. (2009) 48:349–54. doi: 10.1007/s00394-009-0020-3
96. Kamycheva E, Berg V, Jorde R. Insulin-like growth factor I, growth hormone, and insulin sensitivity: the effects of a one-year cholecalciferol supplementation in middle-aged overweight and obese subjects. Endocrine (2013) 43:412–8. doi: 10.1007/s12020-012-9825-6
97. Kjærgaard M, Waterloo K, Wang CEA, Almas B, Figenschau Y, Hutchinson MS, et al. Effect of vitamin D supplement on depression scores in people with low levels of serum 25-hydroxyvitamin D: nested case–control study and randomised clinical trial. Br J Psychiatry (2012) 201:360–8. doi: 10.1192/bjp.bp.111.104349
98. Krieg MA, Jacquet AF, Bremgartner M, Cuttelod S, Thiebaud D, Burckhardt P. Effect of Supplementation with Vitamin D3 and calcium on quantitative ultrasound of bone in elderly institutionalized women: a longitudinal study. Osteoporos Int. (1999) 9:483–8.
99. Krul-Poel YH, Westra S, ten Boekel E, ter Wee MM, van Schoor NM, van Wijland H, et al. Effect of Vitamin D supplementation on glycemic control in patients with Type 2 Diabetes (SUNNY Trial): a randomized placebo-controlled trial. Diabetes Care (2015) 38:1420–6. doi: 10.2337/dc15-0323
100. Larsen T, Mose FH, Jesper N. Bech JN, Hansen AB, Pedersen EB. Effect of cholecalciferol supplementation during winter months in patients with hypertension: a randomized, placebo-controlled trial. Am J Hypertens (2012) 25:1215–22. doi: 10.1038/ajh.2012.111
101. Lorvand Amiri H, Agah SH, Mousavi SN, Hosseini AF, Shidfar F. Regression of non-alcoholic fatty liver by vitamin D supplement: a double-blind randomized controlled clinical trial. Arch Iran Med. (2016) 19:631–8.
102. Macdonald HM, Aucott LS, Black AJ, Fraser WD, Mavroeidi A, Reid DM, et al. Hip bone loss is attenuated with 1000 IU but not 400 IU daily vitamin D3: a 1-year double-blind RCT in postmenopausal women. J Bone Miner Res. (2013) 28:2202–13. doi: 10.1002/jbmr.1959
103. Martins D, Meng YX, Tareen N, Artaza J, Lee JE, Farodolu C, et al. The effect of short term vitamin D supplementation on the inflammatory and oxidative mediators of arterial stiffness. Health (Irvine Calif) (2014) 6:1503–11. doi: 10.4236/health.2014.612185
104. Meyer HE, Smedshaug GB, Kvaavik E, Falch JA, Tverdal A, Pedersen JI. Can vitamin D supplementation reduce the risk of fracture in the elderly? A randomized controlled trial. J Bone Miner Res. (2002) 17:709–15. doi: 10.1359/jbmr.2002.17.4.709
105. Moreira-Lucas TS, Duncan AM, Rabasa-Lhoret R, Vieth R, Gibbs AL, Badawi A, et al. Effect of vitamin D supplementation on oral glucose tolerance in individuals with low vitamin D status and increased risk for developing type 2 diabetes (EVIDENCE): A double-blind, randomized, placebo-controlled clinical trial. Diabetes Obes Metab. (2017) 19:133–41. doi: 10.1111/dom.12794
106. Mose FH, Vase H, Larsen T, Kancir ASP, Kosierkiewic R, Jonczy B, et al. Cardiovascular effects of cholecalciferol treatment in dialysis patients – a randomized controlled trial. BMC Nephrol. (2014) 15:50. doi: 10.1186/1471-2369-15-50
107. Munoz-Aguirre P, Flores M, Macias N, Quezada AD, Denova-Gutierrez E, Salmeron J. The effect of vitamin D supplementation on serum lipids in postmenopausal women with diabetes: a randomized controlled trial. Clin Nutr. (2015) 34:799–804. doi: 10.1016/j.clnu.2014.10.002
108. Nikooyeh B, Neyestani TR, Farvid M, Alavi-Majd H, Houshiarrad A, Kalayi A, et al. Daily consumption of vitamin D– or vitamin D + calcium–fortified yogurt drink improved glycemic control in patients with type 2 diabetes: a randomized clinical trial. Am J Clin Nutr. (2011) 93:764–71. doi: 10.3945/ajcn.110.007336
109. Patel P, Poretsky L, Liao E. Lack of effect of subtherapeutic vitamin D treatment on glycemic and lipid parameters in Type 2 diabetes: A pilot prospective randomized trial. J Diabetes (2010) 2:36–40. doi: 10.1111/j.1753-0407.2009.00057.x
110. Petchey WG, Hickman IJ, Prins JB, Hawley CM, Johnson DW, Isbel NM. Vitamin D does not improve the metabolic health of patients with chronic kidney disease stage 3–4: a randomized controlled trial. Nephrology (2013) 18:26–35. doi: 10.1111/j.1440-1797.2012.01662.x
111. Mozos I, Marginean O. Links between vitamin D deficiency and Cardiovascular diseases. Biomed Res Int. (2015) 2015:109275.
112. Pittas AG, Harris SS, Stark PC, Dawson-Hughes B. The effects of calcium and vitamin d supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care (2007) 30:980–6. doi: 10.2337/dc06-1994
113. Qin XF, Zhao LS, Chen WR, Yin DW, Wang H. Effects of vitamin D on plasma lipid profiles in statin-treated patients with hypercholesterolemia: A randomized placebo-controlled trial. Clin Nutr. (2015) 34:201–6. doi: 10.1016/j.clnu.2014.04.017
114. Raed A, Bhagatwala J, Zhu H, Pollock NK, Parikh SJ, Huang Y, et al. Dose responses of vitamin D3 supplementation on arterial stiffness in overweight African Americans with vitamin D deficiency: a placebo controlled randomized trial. PLoS ONE (2017) 12:e0188424. doi: 10.1371/journal.pone.0188424
115. Rahimi-Ardabili B, Gargaro P, Farzadi L. Effects of vitamin D on cardiovascular disease risk factors in polycystic ovary syndrome women with vitamin D deficiency. J Endocrinol Invest. (2013) 36:28–32. doi: 10.3275/8303
116. Raja-Khan N, Shah J, Stetter ChM, Lott MEJ, Kunselman AR, Dodson WC, et al. High-dose vitamin D supplementation and measures of insulin sensitivity in polycystic ovary syndrome: a randomized, controlled pilot trial. Fertil Steril. (2014) 101:1740–6. doi: 10.1016/j.fertnstert.2014.02.021
117. Ramly M, Ming MF, Chinna K, Suboh S, Pendek R. Effect of vitamin D supplementation on cardiometabolic risks and health-related quality of life among urban premenopausal women in a tropical country – a randomized controlled trial. PLoS ONE (2014) 9:e110476. doi: 10.1371/journal.pone.0110476
118. Rosenblum JL, Castro VM, Moore CE, Kaplan LM. Calcium and vitamin D supplementation is associated with decreased abdominal visceral adipose tissue in overweight and obese adults. Am J Clin Nutr. (2012) 95:101–8. doi: 10.3945/ajcn.111.019489
119. Ryu OH, Chung W, Lee S, Hong KS, Choi MG, Yoo HJ. The effect of high-dose vitamin D supplementation on insulin resistance and arterial stiffness in patients with type 2 diabetes. Korean. J Intern Med. (2014) 29:620–9. doi: 10.3904/kjim.2014.29.5.620
120. Sadiya A, Ahmed SM, Carlsson M, Tesfa Y, George M, Ali SH, et al. Vitamin D supplementation in obese type 2 diabetes subjects in Ajman, UAE: a randomized controlled double-blinded clinical trial. Eur J Clin Nutr. (2015) 69:707–11. doi: 10.1038/ejcn.2014.251
121. Salekzamani S, Mehralizadeh H, Ghezel A, Salekzamani Y, Jafarabadi MA, Bavil AS, et al. Effect of high-dose vitamin D supplementation on cardiometabolic risk factors in subjects with metabolic syndrome: a randomized controlled double-blind clinical trial. J Endocrinol Invest. (2016) 39:1303–13. doi: 10.1007/s40618-016-0507-8
122. Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. (2006) 83:754–9. doi: 10.1093/ajcn/83.4.754
123. Scragg R, Slow S, Stewart AW, Jennings LC, Chambers ST, Priest PC, et al. Long-term high-dose vitamin D3 supplementation and blood pressure in healthy adults a randomized controlled trial. Hypertension (2014) 64:725–30. doi: 10.1161/HYPERTENSIONAHA.114.03466
124. Seibert E, Lehmann U, Riedel A, Ulrich C, Hirche F, Brandsch C, et al. Vitamin D3 supplementation does not modify cardiovascular risk profile of adults with inadequate vitamin D status. Eur J Nutr. (2017) 56:621–34. doi: 10.1007/s00394-015-1106-8
125. Shab-Bidar S, Neyestani TR, Djazayery A, Eshraghian MR, Houshiarrad A, Gharavi A. Regular consumption of vitamin D-fortified yogurt drink (Doogh) improved endothelial biomarkers in subjects with type 2 diabetes: a randomized double-blind clinical trial. BMC Med. (2011) 9:125. doi: 10.1186/1741-7015-9-125
126. Sollid ST, Hutchinson MYS, Fuskevag OM, Figenschau Y, Joakimsen RM, Schirmer H, et al. No Effect of High-Dose Vitamin D Supplementation on Glycemic Status or Cardiovascular Risk Factors in Subjects With Prediabetes. Diabetes Care (2014) 37:2123–31. doi: 10.2337/dc14-0218
127. Sun X, Cao ZB, Tanisawa K, Ito T, Oshima S, Higuchi M. Vitamin D supplementation reduces insulin resistance in Japanese adults: a secondary analysis of a double-blind, randomized, placebo-controlled trial. Nutr Res. (2016) 36:1121–9. doi: 10.1016/j.nutres.2016.07.006
128. Tomson J, Hin H, Emberson J, Kurien R, Lay M, Cox J, et al. Effects of vitamin D on blood pressure, arterial stiffness, and cardiac function in older people after 1 year: BEST-D (Biochemical Efficacy and Safety Trial of Vitamin D). J Am Heart Assoc. (2017) 6:e005707. doi: 10.1161/JAHA.117.005707
129. Toss G, Magnusson P. Is a daily supplementation with 40 microgram vitamin D3 sufficient? A randomised controlled trial. Eur J Nutr. (2012) 51:939–45. doi: 10.1007/s00394-011-0271-7
130. Wamberg L, Kampmann U, Stodkilde-Jorgensen H, Rejnmark L, Pedersen SB, Richelsen B. Effects of vitamin D supplementation on body fat accumulation, inflammation, and metabolic risk factors in obese adults with low vitamin D levels — Results from a randomized trial. Eur J Int Med. (2013) 24:644–9. doi: 10.1016/j.ejim.2013.03.005
131. Witham MD, Adams F, McSwiggan S, Kennedy G, Kabir G, Belch JJF, et al. Effect of intermittent vitamin D3 on vascular function and symptoms in chronic fatigue syndrome e A randomised controlled trial. Nutr Metab Cardiovasc Dis. (2015) 25:287–94. doi: 10.1016/j.numecd.2014.10.007
132. Wood AD, Secombes KR, Thies F, Aucott L, Black AJ, Mavroeidi A, et al. Vitamin D3 supplementation has no effect on conventional cardiovascular risk factors: a parallel-group, double-blind, placebo-controlled RCT. J Clin Endocrinol Metab. (2012) 97:3557–68. doi: 10.1210/jc.2012-2126
133. Yeow TP, Lim SL, Hor CP, Khir AS, Wan Mohamud WN, Pacini G. Impact of Vitamin D Replacement on markers of glucose metabolism and cardio-metabolic risk in women with former gestational diabetes—a double-blind, randomized controlled trial. PLOS ONE (2015) 10:e0129017. doi: 10.1371/journal.pone.0129017
134. Yousefi Rad E, Djalali M, Koohdani F, Saboor-Yaraghi AA, Eshraghian MR, Javanbakht MH, et al. The effects of vitamin D supplementation on glucose control and insulin resistance in patients with diabetes type 2: a randomized clinical trial study. Iran J Public Health (2014) 43:1651–6. Available online at: http://ijph.tums.ac.ir/index.php/ijph
135. Zittermann A, Frisch S, Berthold HK, Gotting Ch, Kuhn J, Kleesiek K, et al. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am J Clin Nutr. (2009) 89:1321–7. doi: 10.3945/ajcn.2008.27004
136. Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. (1997) 337:670–6. doi: 10.1056/NEJM199709043371003
137. Sinha-Hikim I, Duran P, Shen R, Lee M, Friedman THC, Davidson MB. Effect of long term vitamin D supplementation on biomarkers of inflammation in latino and african-american subjects with pre-diabetes and hypovitaminosis D. Horm Metab Res. (2015) 47:280–3. doi: 10.1055/s-0034-1383652
138. Carrillo AE, Flynn MG, Pinkston C, Markofski MM, Jiang Y, Donkin SHS, et al. Impact of vitamin D supplementation during a resistance training intervention on body composition, muscle function, and glucose tolerance in overweight and obese adults. Clin Nutr. (2013) 32:375–81. doi: 10.1016/j.clnu.2012.08.014
139. Pfeifer M, Begerow B, Minne HW, Suppan K, Fahrleitner-Pammer A, Dobnig H. Effects of a long-term vitamin D and calcium supplementation on falls and parameters of muscle function in community-dwelling older individuals. Osteoporos Int. (2009) 20:315–22. doi: 10.1007/s00198-008-0662-7
140. World Health Organozation. Global Status Report on Noncommunicable Diseases 2010. Geneva (2011).
141. Freedman JE, Farhat JH, Loscalzo J, Keaney JF Jr. Alpha-tocopherol inhibits aggregation of human platelets by a protein kinase C-dependent mechanism. Circulation (1996) 94:2434–40. doi: 10.1161/01.CIR.94.10.2434
142. Ogawa M, Abe S, Saigo M, Biro S, Toda H, Matsuoka T, et al. Homocysteine and hemostatic disorder as a risk factor for myocardial infarction at a young age. Thromb Res. (2003) 109:253–8.
143. Patel R, Rizvi AA. Vitamin D deficiency in patients with congestive heart failure: mechanisms, manifestations, and management. South Med J. (2011) 104:325–30. doi: 10.1097/SMJ.0b013e318213cf6b
144. Davies MR Hruska KA. Pathophysiological mechanisms of vascular calcification in end-stage renal disease. Kidney Int. (2001) 60:472–9. doi: 10.1046/j.1523-1755.2001.060002472.x
145. Aggarwal N, Michos ED. Vitamin D deficiency and its implications on cardiovascular disease. Curr Cardiovasc Risk Reports (2010) 4:68–75. doi: 10.1007/s12170-009-0072-1
146. Pliz S, Tomaschitz A, Ritz E, Pieber TR. Vitamin D status and arterial hypertension: a systematic review. Nat Rev Cardiol. (2009) 6:621-30. doi: 10.1038/nrcardio.2009.135
147. Wang JH, Keisala T, Solakivi T, Minasyan A, Kalueff AV, Tuohimaa P. Serum cholesterol and expression of ApoAI, LXRbeta and SREBP2 in vitamin D receptor knock-out mice. J Steroid Biochem Mol Biol. (2009) 113:222–6. doi: 10.1016/j.jsbmb.2009.01.003
148. Christensen R, Lorenzen JK, Svith CR, Bartels EM, Melanson EL, Saris WH, et al. Effect of calcium from dairy and dietary supplements on faecal fat excretion: a meta-analysis of randomized controlled trials. Obes Rev. (2009) 10:475–86. doi: 10.1111/j.1467-789X.2009.00599.x
149. Manousopoulou A, Al-Daghri NM, Spiros D. Garbis SD, Chrousos GP. Vitamin D and cardiovascular risk among adults with obesity: a systematic review and meta-analysis. Eur J Clin Invest. (2015) 45:1113–26. doi: 10.1111/eci.12510
150. Rolhion N, Darfeuille-Michaud A. Adherent-invasive Escherichia coli in inflammatory bowel disease. Inflamm Bowel Dis. (2007) 13:1277–83. doi: 10.1002/ibd.20176
151. Mangin M, Sinha R, Fincher K. Inflammation and vitamin D: the infection connection. Inflamm Res. (2014) 63:803–19. doi: 10.1007/s00011-014-0755-z
152. Reid D, Toole BJ, Knox S, Talwar D, Harten J, O'Reilly DS, et al. The relation between acute changes in the systemic inflammatory response and plasma 25-hydroxyvitamin D concentrations after elective knee arthroplasty. Am J Clin Nutr. (2011) 93:1006–11. doi: 10.3945/ajcn.110.008490
153. Sattar N, Welsh P, Panarelli M, Forouhi NG. Increasing requests for vitamin D measurement: costly, confusing, and without credibility. Lancet (2012) 379:95–6. doi: 10.1016/S0140-6736(11)61816-3
154. Dahl B, Schiødt FV, Kiaer T, Ott P, Bondesen S, Tygstrup N. Serum Gc-globulin in the early course of multiple trauma. Crit Care Med. (1998) 26:285–9. doi: 10.1097/00003246-199802000-00027
155. Krishnan A, Ochola J, Mundy J, Jones M, Kruger P, Duncan E, et al. Acute fluid shifts influence the assessment of serum vitamin D status in critically ill patients. Crit Care (2010) 14:R216. doi: 10.1186/cc9341
156. Weyland PGGW, Howie-Esquivel J. Does sufficient evidence exist to support a causal association between vitamin D status and Cardiovascular disease risk? an assessment using hill's criteria for causality. Nutrients (2014) 6:3403–30. doi: 10.3390/nu6093403
157. Jorde R, Bonaa KH. Calcium from dairy products, vitamin D intake, and blood pressure: the Tromsø study. Am J Clin Nutr. (2000) 71:1530–5. doi: 10.1093/ajcn/71.6.1530
158. Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C. Effects of a short-term vitamin D3 and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab. (2001) 86:1633–7. doi: 10.1210/jc.86.4.1633
159. Wang Y, Wang WQ. The prevalence of prehypertension and hypertension among US adults according to the new joint national committee guidelines, new challenges of the old problem. Arch Intern Med. (2004) 164:2126–34. doi: 10.1001/archinte.164.19.2126
160. Chen N, Wang Z, Han SF, Li BY, Zhang ZL, Qin LQ. Effect of vitamin D supplementation on the level of circulating high-sensitivity c-reactive protein: a meta-analysis of randomized controlled trials. Nutrients (2014) 6:2206–16. doi: 10.3390/nu6062206
161. Rodriguez AJ, Mousa A, Ebeling PR, Scott D, de Courten B. Effects of vitamin D supplementation on inflammatory markers in heart failure: a systematic review and meta-analysis of randomized controlled trials. Sci Rep. (2018) 8:1169. doi: 10.1038/s41598-018-19708-0
162. Wu SH, Ho S, Zhong L. Effects of vitamin D supplementation on blood pressure. South Med J. (2010) 103:729–37. doi: 10.1097/SMJ.0b013e3181e6d389
163. Witham MD, Nadir M, Struthers AD. Effect of vitamin D on blood pressure: a systematic review and meta-analysis. J Hypertens (2009) 27:1948–54. doi: 10.1097/HJH.0b013e32832f075b
164. Al Mheid I, Patel RS, Tangpricha V, Quyyumi AA. Vitamin D and cardiovascular disease: is the evidence solid? Eur Heart J. (2013) 34:3691–8. doi: 10.1093/eurheartj/eht166
165. Veloudi P, Jones G, Sharman JE. Effectiveness of vitamin D supplementation for cardiovascular health outcomes. Pulse (2017) 4:193–207. doi: 10.1159/000452742
166. Allison DB, Murphy SP. Purported Mathematical Errors in the 2011. IOM Report, Dietary Reference Intakes: Calcium and Vitamin D (2017).
167. Heaney R, Garland C, Baggerly C, French C, Gorham E. Letter to Veugelers, P.J. and Ekwaru, J.P., A Statistical error in the estimation of the recommended dietary allowance for vitamin D. Nutrients 2014:6, 4472–4475; doi: 10.3390/nu6104472. Nutrients (2015) 7:1688–90. doi: 10.3390/nu7031688
168. Veugelers PJ, Ekwaru JP. A statistical error in the estimation of the recommended dietary allowance for vitamin D. Nutrients (2014) 6:4472–5. doi: 10.3390/nu6104472
169. Holick MF, Binkley N, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. (2011) 96:1911–30. doi: 10.1210/jc.2011-0385
170. Pramyothin P, Holick M. Vitamin D supplementation: guidelines and evidence for subclinical deficiency. Curr Opin Gastroenterol. (2012) 28:139–50. doi: 10.1097/MOG.0b013e32835004dc
Keywords: vitamin D, cardiovascular, blood pressure, lipids, inflammation, parathyroid hormone, arterial stiffness, meta-analysis
Citation: Mirhosseini N, Rainsbury J and Kimball SM (2018) Vitamin D Supplementation, Serum 25(OH)D Concentrations and Cardiovascular Disease Risk Factors: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 5:87. doi: 10.3389/fcvm.2018.00087
Received: 02 April 2018; Accepted: 18 June 2018;
Published: 12 July 2018.
Edited by:
Sang-Hyun Kim, Seoul Boramae Hospital, South KoreaReviewed by:
Hack-Lyoung Kim, SMG-SNU Boramae Medical Center, South KoreaDietmar Fuchs, Innsbruck Medical University, Austria
Copyright © 2018 Mirhosseini, Rainsbury and Kimball. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samantha M. Kimball, c2FtYW50aGEua2ltYmFsbEBwdXJlbm9ydGguY2E=
 Naghmeh Mirhosseini1
Naghmeh Mirhosseini1 Samantha M. Kimball
Samantha M. Kimball