- 1Institute for Infectiology, University of Münster, Münster, Germany
- 2German Center for Infection Research (DZIF), Associated Site University of Münster, Münster, Germany
Infections with Shiga toxin-producing Escherichia coli (STEC) cause outbreaks of severe diarrheal disease in children and the elderly around the world. The severe complications associated with toxin production and release range from bloody diarrhea and hemorrhagic colitis to hemolytic-uremic syndrome, kidney failure, and neurological issues. As the use of antibiotics for treatment of the infection has long been controversial due to reports that antibiotics may increase the production of Shiga toxin, the recommended therapy today is mainly supportive. In recent years, a variety of alternative treatment approaches such as monoclonal antibodies or antisera directed against Shiga toxin, toxin receptor analogs, and several vaccination strategies have been developed and evaluated in vitro and in animal models. A few strategies have progressed to the clinical trial phase. Here, we review the current understanding of and the progress made in the development of treatment options against STEC infections and discuss their potential.
Introduction
Shiga-toxin producing (enterohemorrhagic) Escherichia coli (STEC/EHEC) are a major cause of severe gastrointestinal disease in industrialized countries and a major public health problem with most frequent and severe infections linked to serotype O157:H7 (Kaper and O'Brien, 2014). The bacteria are commonly transmitted through ingestion of contaminated food such as undercooked meat, particularly beef products, cross-contaminated raw vegetables, sprouts, and seeds (Caprioli et al., 2014).
The resulting disease ranges in intensity from watery diarrhea or hemorrhagic colitis to the life-threatening hemolytic uremic syndrome (HUS) leading to kidney failure and neurological episodes (Nataro and Kaper, 1998). Upon ingestion, EHEC resides in the intestinal tract and adheres to the gut epithelium of the distal ileum and colon. Initial binding is promoted by fimbriae, which, in EHEC infections (e.g., by EHEC O157:H7, O126, O103, O45, O111, O121, O145), is followed by the injection of effector proteins (Esp proteins) via a filamentous type III secretion system (T3SS) (Donnenberg and Kaper, 1992; Garmendia et al., 2005; Gaytan et al., 2016). Injection of the translocated intimin receptor (Tir), which integrates into the host cell plasma membrane and interacts with the bacterial outer membrane protein intimin, initiates bacterial attachment to the host cell and effacement of the brush border microvilli. The interaction between intimin and Tir leads to intimate attachment of the bacteria and initiates actin polymerization and subsequent formation of attaching and effacing (A/E) lesions (Kenny et al., 1997). The genes encoding Tir, intimin, and the T3SS are localized on the chromosomal “locus of enterocyte effacement” (LEE) pathogenicity island. Notably, this island is missing from LEE-negative STEC and from the unusual HUS-inducing E. coli strain EAHEC of serotype O104:H4, which is responsible for the major outbreak in Germany and parts of Europe in 2011. This latter strain is similar to enteroaggregative E. coli (EAEC) (Bielaszewska et al., 2011; Mellmann et al., 2011).
While the HUS-inducing strains belong to a variety of E. coli pathovars, their main discerning trait is the production of at least one of two genetically distinct Shiga toxins, named Stx1 and Stx2. Four subtypes of Stx1 (Stx1a, Stx1c, Stx1d, Stx1e) and seven subtypes of Stx2 (Stx2a-g) have been identified, of which especially the Stx2 variants Stx2a and Stx2c are commonly associated with HUS development in humans (Melton-Celsa, 2014). Both types of Shiga toxins are AB5 toxins that bind to the glycosphingolipids globotriaosylceramide (Gb3, CD77) and, to a lesser extent, globotetraosylceramide (Gb4) (Legros et al., 2018), which are found on a variety of human cells, such as glomerular and brain endothelial cells. The Stx toxins result in the arrest of protein translation and, ultimately, cell death (Melton-Celsa, 2014). The systemic consequences of intoxication are vascular dysfunction and thrombus formation, which lead to HUS. The genes encoding for Stx are located in the late region of a lambdoid phage, which adds additional complications to treatment options. As several antibiotics, especially those belonging to the quinolone family were shown to be potent inducers of the bacterial SOS response, which initiates the production and release of phages from the bacteria, treatment of STEC infections with antibiotics is generally not advised (Kakoullis et al., 2019). To date, there are no protective measures or therapies against STEC infections. Current treatment of STEC infections is solely supportive and includes rehydration therapy, and, where necessary, dialysis. However, over the past years, new therapeutic approaches and novel, promising strategies to manage the infection and the ensuing disease have been developed. These are outlined in this review.
Antibody Therapy
Stx-Targeted Antibodies
Antibodies are valuable therapeutics. As Stx-specific antibodies can completely neutralize the cytotoxicity of the toxin in cell culture and protect animals from developing Stx-induced symptoms when administered shortly after infection (Cheng et al., 2013), effective Stx-targeting antibodies are a suitable option for human therapy (Figure 1A).
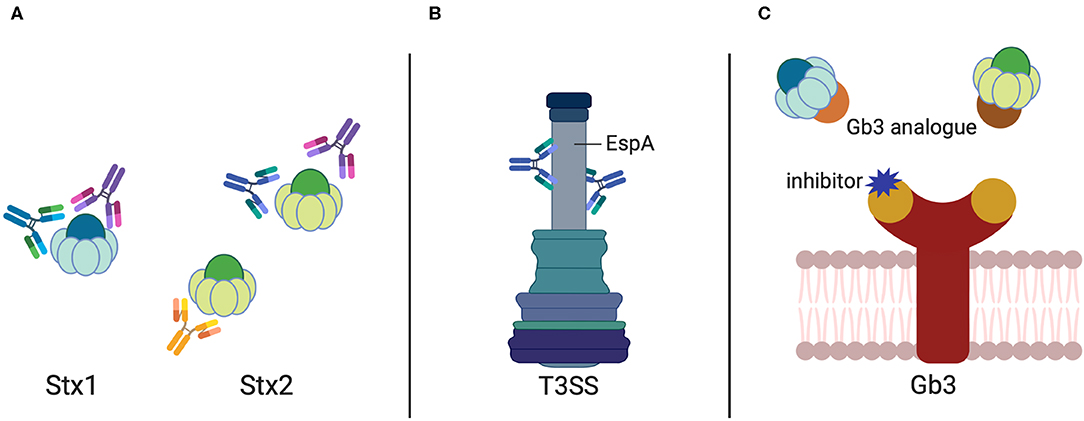
Figure 1. Antibodies and Gb3 analogs against STEC-induced diseases. Antibody targets in STEC treatment include (A) the Shiga toxins (Stx1 and Stx2) and (B) the sheath component EspA of the E. coli Type-3-Secretion System. (C) Analogs to the Stx receptor Gb3 harboring the Stx binding domains (given in brown and orange) sequestering Stx1 and Stx2. Inhibitors blocking the Stx binding site of the Gb3 receptor are illustrated in purple.
Mohawk et al. (2010) investigated the ability of polyclonal Stx2-neutralizing antibodies from rabbits to protect mice from lethal infection with the Stx2a-producing E. coli O157:H7 strain 86-24. The administration of the rabbit serum did not reduce the initial colonization of mice with EHEC 86-24, but it decreased the bacterial burden after 3–5 days and increased animal survival.
Bovine colostrum preparations harboring high titers of Stx1 and Stx2 antibodies were used to treat mice infected with E. coli O157:H7. This efficiently inhibited bacterial attachment, colonization, and growth (Funatogawa et al., 2002). Although the frequency of stool excretion was reduced, the presence of the bacterial toxin was not notably affected (Kuribayashi et al., 2006; Seita et al., 2013). Furthermore, colostral IgG against Shiga toxin and bovine lactoferrin completely prevented lethality of E. coli O157:H7 in a weaned mouse model (Albanese et al., 2018). In addition, an early study in children showed that bovine colostrum is well-tolerated, reduced the frequency of loose stools, and eliminated bacterial infection (Huppertz et al., 1999).
Another interesting concept for this strategy is the production of recombinant antibodies or even secretory antibodies specific for Stx by transgenic plants (e.g., thale grass—Arabidopsis thaliana) (Nakanishi et al., 2013, 2017), a concept that may be transferred to other plants, providing the possibility of an edible therapy.
Promising tools are also Stx-targeting humanized antibodies. Humanized antibodies were established by an exchange of the regions within the antigen-binding variable domains, which react with the antigen of the human IgG molecule with equivalent regions of the murine Stx-specific Mab (Nakao et al., 1999) to produce TMA-15 (Urtoxazumab) (Yamagami et al., 2001; Kimura et al., 2002). TMA-15 was shown to protect mice from a lethal challenge with STEC if given within 24 h of infection (Yamagami et al., 2001), while it was also able to reduce brain lesions and death in a gnotobiotic piglet model (Moxley et al., 2017). When tested in healthy adults or pediatric patients with a confirmed STEC infection, intravenous application of TMA-15 (Urtoxazumab) was found to be well-tolerated and safe (Lopez et al., 2010).
Chimeric murine-human MAbs (cαStx1/cαStx2) comprising the variable regions of the murine Stx1 (B-subunit) or Stx2 (A-subunit)-neutralizing antibodies 13C4 and 11E10 (Strockbine et al., 1985; Perera et al., 1988) fused to the light chain of human IgG1 were found to neutralize Stxs in mice (Bitzan et al., 2009). In addition, they were well–tolerated in healthy human volunteers when given as a single dose either separate or in combination (Dowling et al., 2005; Bitzan et al., 2009). Unfortunately, the efficacies of hybrid antibodies were often found to be lower compared to the murine parent antibodies (Tzipori et al., 2004). Another concern of chimeric MAbs is that they still retain murine IgG elements that could trigger antibody formation by treated patients. With this in mind, HuMAbs have been engineered, which encode the human heavy- and light-chain IgG genes, leading to the formation of human antibodies in response to immunization with an antigen, e.g., Stx1 or Stx2 [e.g., 5A4 (Stx1) and 5C12 (Stx2)] (Mukherjee et al., 2002a,b; Sheoran et al., 2005). These resulted in prolonged survival of mice in a Stx1 toxicosis model (Mukherjee et al., 2002b) and higher survival of gnotobiotic piglets when treated 48 h after challenge with an Stx2a-producing STEC strain (Sheoran et al., 2005). Interestingly, when piglets were infected with an Stx1- and Stx2-producing strain, only administration of 5C12 (αStx2) was protective (Jeong et al., 2010).
Another promising approach used hetero-multimeric camelid toxin-neutralizing agents containing two linked heavy-chain-only antibody VH domains that neutralize Stx1 or Stx2 co-administered with an antitag MAb—an “effector Ab”—that indirectly decorates each toxin with four Ab molecules in cell-based and in vivo mouse models (Tremblay et al., 2013). The effector antibody binds to multiple epitope tags engineered into the VHH-based toxin-neutralizing agent. When the toxin-neutralizing agent interacts with separate sites of the Stx toxins, and each, in turn, binds to two or more effector Abs through the tags, the Stxs become decorated with sufficient Abs to prevent all symptoms of Stx1 and Stx2 intoxication and protect mice from Stx lethality (Tremblay et al., 2013). Moreover, camelid antibodies, which are special as they only contain heavy chains, have been produced that target the Stx2 B-subunit. These antibodies decreased Shiga toxicity when injected into mice and were proposed as an alternative treatment for HUS sequelae (Mejias et al., 2016). Moreover, Luz et al. have produced recombinant antibody fragments that specifically bind to and neutralize Stx2 in vitro (Luz et al., 2015). They further showed that mice were protected from challenge with a lethal dose of Stx2 after pre-incubation of the toxin with the antibody fragment FabC11:Stx2 (Luz et al., 2018).
A combined antibody–antibiotic (e.g., tigecycline) treatment scheme that was found to eliminate the toxicity from STEC (Skinner et al., 2015) may help to eliminate bacteria in addition to inhibiting Shiga-toxin mediated disease, decreasing the probability of transmission to others due to continued bacterial carriage and excretion.
Evaluation of the different antibody therapies against HUS, mostly in piglets and mice, showed that they mainly differ in their protective efficacy and/or their specificity to Stx variants, whereby the A-subunit specific antibodies were better neutralizers than their B-subunit specific counterparts (Tzipori et al., 2004). A very critical point for passive immunization with Stx antibodies that has to be considered for successful therapy is the time point and dosage of antibody administration. Studies using piglets or mice demonstrated that administration of the Stx2-specific HuAbs 5C12 or TMA-15 protected the animals 48 or 24 h after infection, respectively. However, when TMA-15 was used as treatment 48 h after infection, no protection was observed (Yamagami et al., 2001). This indicated that infected patients might be protected against the development of HUS when the antibodies are given shortly after the onset of diarrhea (Orth et al., 2008). However, as mice and piglets do not develop either bloody diarrhea or HUS, results describing a protective effect of Stx-specific antibodies cannot easily be transferred to humans. Moreover, knowledge about the time when the Stxs enter the bloodstream and the Stx levels in the blood and infected tissues is scarce.
Effector- or Intimin-Targeted Antibodies
The E. coli secreted effector protein EspA forms the filamentous sheath of the T3SS, which aids in the transportation of the bacterial effectors into the host cells and elicits a protective immune response. A MAb (1H10) was identified to recognize the linear, conserved, and protective epitope Lys100-Val120 on the surface of EspA (Yu et al., 2011) (Figure 1B). This EspA-specific MAb was shown to inhibit EHEC-induced actin polymerization in vitro and conferred protection against EHEC, e.g., reduced their colonization efficiency in mice (Yu et al., 2011), which could be exploited for the development of epitope-based vaccine and MAb-based therapy. In addition, a camelid single-domain antibody (nanobody), TD4, which specifically recognized the Tir domain overlapping with the binding site of the adhesin intimin, was able to inhibit EHEC attachment and intimin-induced clustering of Tir, and reduced the colonization of EHEC on the human colonic mucosa (Ruano-Gallego et al., 2019).
HuMAb Against Complement Component 5 (C5): Eculizumab
Eculizumab is a recombinantly produced HuMAb against the complement component 5 (C5). Binding of the antibody to C5 results in the inhibition of complement activation. Originally not devised for the treatment of STEC-induced HUS, Eculizumab was initially trialed in patients with severe STEC-HUS during the outbreak in northern Europe in 2011, as Shiga toxin had been shown to mediate complement activation (Orth et al., 2009; Morigi et al., 2011; Noris et al., 2012; Karpman and Tati, 2016) reviewed in Buelli et al. (2019), which, in turn, negatively affects renal health. Unfortunately, the results obtained for the use of Eculizumab in STEC-HUS were inconsistent. While most studies reported no benefit on renal and extrarenal outcomes (Kielstein et al., 2012; Loos et al., 2012, 2017; Menne et al., 2012), other publications reported a beneficial effect of Eculizumab treatment in pediatric cases (Lapeyraque et al., 2011) or fewer severely infected patients (Delmas et al., 2014). This indicated that the early use of Eculizumab in children with HUS may be beneficial. However, a more recent study evaluating the short and intermediate outcome of Eculizumab treatment, including 18 children with STEC-HUS in a single-center matched cohort study did not reveal a benefit of Eculizumab on renal and extrarenal outcomes (Monet-Didailler et al., 2019). It has been discussed that the delay between HUS diagnosis and Eculizumab administration could affect patient recovery (Keenswijk et al., 2017). It was also suggested that Eculizumab might improve potential neurological outcomes (Pape et al., 2015; Monet-Didailler et al., 2019).
Toxin Receptor Analogs
The Stxs, once released from the bacterial cell, spread through the body and target cells (Lingwood et al., 1987), which express the Gb3 receptor on their cell surface, such as renal glomerular and brain endothelial cells. Binding of Stxs to the Gb3 receptors is based on the multivalent interaction of the five B-subunits with the trisaccharide moiety of Gb3. Therefore, interfering with receptor binding by using receptor analogs to probe for free toxin in the gut or the circulation is a promising approach to reduce Stx-mediated disease. Over the last decade or so, a variety of strategies have been employed to produce (i) inhibitors of the Stx receptor Gb3 to prevent Stx binding and uptake, and (ii) Stx-neutralizing Gb3 analogs (Macconnachie and Todd, 2004; Serna and Boedeker, 2008; Rahal et al., 2015; Kavaliauskiene et al., 2017) (Figure 1C).
SYNSORB Pk
Synsorb Pk was one of the first and certainly a promising Stx receptor analog. It consists of silicon dioxide particles (diatomaceous earth) with covalently linked trisaccharides that functioned as an orally administered Stx adsorbent. SYNSORB Pk was tested in a large multicenter trial including 145 children diagnosed with STEC-induced HUS (Trachtman et al., 2003). Unfortunately, however, no treatment benefit could be observed. There were no significant differences in the number of deaths or incidence of extrarenal complications and no reduction in the need for dialysis was observed. Likely reasons for the observed failure are that (i) the agent was administered too late, (ii) the Stx toxins are mostly cell-associated and not free-floating, and (iii) the Stx binding capacity of monomeric SYNSORB Pk is substantially lower than that of Gb3 polymers.
Starfish and Daisy
Starfish is an oligovalent, water-soluble carbohydrate ligand with a sub-nanomolar inhibitory activity designed based on the crystal structure of the Stx1 B-subunit (Kitov et al., 2000). The in vitro inhibitory activity is very high as the two trisaccharide receptors at the tip of a 5-spacer arm engage all five Stx1 B-subunits. Daisy is a Gb3 analog (αGal(1,4)βGal) from the same group that developed Starfish, which was shown to protect mice from Stx1- and Stx2-mediated disease by subcutaneous administration (Mulvey et al., 2003).
SUPER TWIG (1) and (2)
Nishikawa and colleagues (Nishikawa et al., 2002, 2005) designed a series of carbosilane dendrimers carrying various numbers of terminal Gb3 moieties to bind Shiga toxin in the bloodstream before it reaches target cells expressing the receptor. The SUPER TWIG Gb3 analogs bound Stx1 and Stx2 with high affinity, prevented Stx uptake into host cells, induced phagocytosis of Stx by macrophages, and protected mice from a fatal challenge with EHEC.
Acrylamide Polymers With Gb3 Trisaccharides
Watanabe et al. (2004) constructed acrylamide polymers of Gb3 as toxin absorbent in the gut that bound both Stx1 and Stx2 with a very high affinity [e.g., it interacted with a higher affinity to the Stx B subunit than SUPER TWIG (1)]. They further showed that the oral administration of these polymers was able to protect mice that had been orally challenged with a fatal dose of STEC, whereby the toxin content in serum samples in the treated infected mice was significantly reduced. This protection was observed even if the polymers were administered after colonization.
Phage-Display Generated Stx-Neutralizing Peptides
Three peptides that bind to Gb3 receptor have been developed using phage-display (PC7-2, P12-26, and PC7-30). They efficiently competed with the Stx for binding and inhibited Stx-triggered cell toxicity. Peptide PC7-30 further inhibited Stx1-induced lethality in EHEC-infected animals, indicating that this peptide might be useful to prevent STEC-triggered diseases such as HUS (Bernedo-Navarro et al., 2014).
Bacteria Expressing Gb3 Analogs
In addition to previous attempts, E. coli strains and probiotics can be engineered to express Gb3 receptor mimics on their surface (Paton et al., 2000; Asahara et al., 2004; Hostetter et al., 2014). They absorb and neutralize Stx1, Stx2, Stx2c, and Stx2d in vitro and oral administration of Gb3 analog-expressing bacteria protected mice from fatal challenge with different highly virulent STEC strains.
Nanoparticles Displaying Stx Ligands
Kulkarni et al. (2010) established glycan-encapsulated gold nanoparticles that allowed multivalent display of glycans, e.g., the glycan Pk trisaccharide, which preferentially interacts with Stx. The coated nanoparticles neutralized Stx1 and Stx2, but not all variants in a Vero cell toxicity assay.
Gb3 Inhibitors
Inhibitors that interfere with the synthesis of the Stx receptor Gb3 are also attractive targets. The agent C-9 is a specific inhibitor of glucosylceramide synthase that downregulates the expression of Gb3, limiting the amount of receptor displayed on the surface of cells (Silberstein et al., 2011). C-9 addition to human kidney cells in a tissue culture model decreased Stx2-mediated tissue damage. Furthermore, administration of C-9 in a rat model decreased mortality by about 50% and significantly diminished toxin-mediated tubular necrosis and damage to goblet cells. In addition, PDMP, a ceramide analog shown to inhibit the synthesis of GlcCer and affect the composition of glycosphingolipids, reduced Stx binding and uptake and blocked initial transport of the toxin into the Golgi apparatus (Raa et al., 2009). It was also discovered that the glucose analog 2-fluoro-2-deoxy-D-glucose (FDG) reduced cellular uptake of Gb3 levels by 50%, likely by inhibiting precursor formation of Gb3 synthesis, and resulted in a decrease in Stx binding (Kavaliauskiene et al., 2015, 2016).
Intracellular Interference With Shiga Toxins
Not only Stx binding and uptake, but also its intracellular targeting from early endosomes to the Golgi apparatus and the endoplasmic reticulum (ER) can be inhibited by certain cell-permeable agents. One such substance is chloroquine, a weak base that can diffuse across membranes and accumulate in acidic compartments. Chloroquine treatment protected HEp-2 cells from Stx-mediated cytotoxicity, most likely by interfering with the translocation of the StxA subunit into the cytosol (Dyve Lingelem et al., 2012; Kavaliauskiene et al., 2017). The Retro-1 and−2 substances were shown to inhibit the retrograde transport of Stx1B from the endosomes to the Golgi apparatus, and it has been assumed that this could be mediated by the relocalization of the SNARE proteins syntaxin 5 and 6 (Noel et al., 2013; Kavaliauskiene et al., 2017). Later, it has been shown by Secher et al. (2015) that Retro-2 was able to protect mice against the toxic effects of Stx. Recently, a drug delivery system using Retro-2-loaded nanoglobules has been developed, which increased the solubility of the inhibitor and might enable a more successful therapeutic approach inhibiting the transport of Stx (Gandhi et al., 2019). Moreover, two other substances, Ac-PPP-tet and TVP, which also interfere with intracellular Stx trafficking, have been tested in animal models and were found to successfully prevent Stx2 intoxication (Watanabe-Takahashi et al., 2010; Stearns-Kurosawa et al., 2011).
Vaccination Strategies
Toxin-Based Vaccines
Stx is the main virulence factor associated with the potency of STEC-mediated disease pathology. It is released from the bacterial cell and exposed to the host immune system and has, therefore, long been considered one of the most prudent targets for vaccine strategy. Once released from the bacterial cell, Stx can be detected in the intestinal lumen and its target cells in the kidney and brain (Clements et al., 2012). Hence, immune cells primed to recognize Stx by prior vaccination can interfere and respond to the toxin as it travels from the site of release to the distal organs and eliminate it before it reaches its targets (Figure 2A). Of Stx1, Stx2, and the different varieties of each of these subtypes, Stx2 has been at the center of a greater number of vaccination approaches as it is commonly associated with more severe disease outcomes in humans, such as the development of HUS. Several vaccination strategies have been developed and tested that are solely toxin-based. With the active site of Stx known, inactive derivatives of the toxin are easy to make, and present safe alternatives for application. Several different studies showed that vaccination of animals with purified inactive Stx derivatives induced the production of neutralizing antibodies against the respective toxin and protected the animals from toxemia or limited shedding or disease after challenge (Gordon et al., 1992; Acheson et al., 1996; Konadu et al., 1999; Marcato et al., 2001, 2005; Ishikawa et al., 2003; Kerner et al., 2015; Schmidt et al., 2018). In addition to vaccines based on the inactive toxin, hybrid subunit vaccine approaches have been tested as the development of a vaccine, which may induce neutralizing antibodies against not only one but both types of Stx, would be ideal.
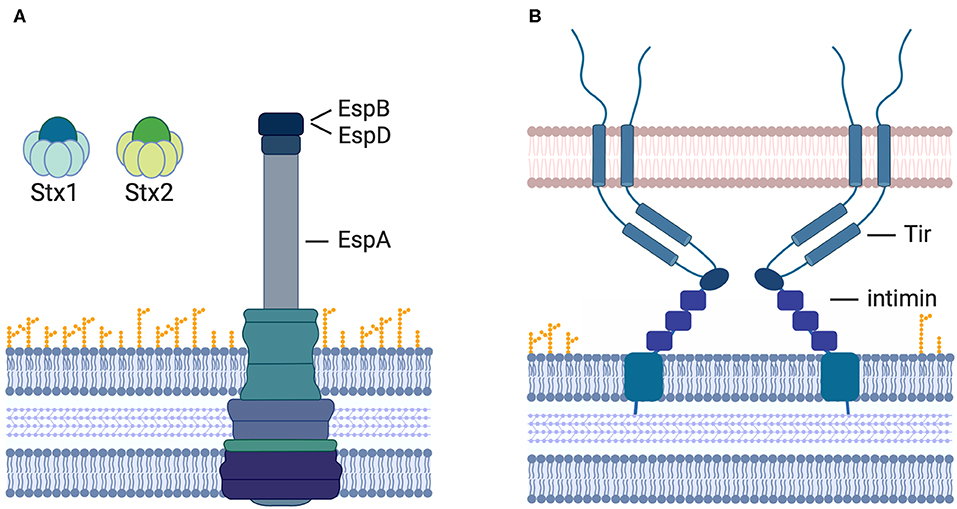
Figure 2. Vaccination strategies to prevent STEC-induced diseases. Currently assessed vaccine targets include (A) the A-and B-subunits of Stx1 and Stx2, the T3SS components EspA (sheath) and EspB (pore complex), as well as (B) the bacterial outer membrane protein intimin and its T3-translocated receptor Tir.
Vaccination with a hybrid toxin consisting of an inactive Stx2 A-subunit fused to the native Stx1 B-subunit was able to produce neutralizing antibodies against both Stx1 and Stx2. Mice immunized with this toxin were protected from subsequent lethal challenge with either Stx1 or Stx2, or both toxins (Smith et al., 2006).
Injection with a purified fusion protein consisting of the B-subunits of Stx subtypes 1 and 2 (Stx2B-Stx1B; short 2S) generated neutralizing antibodies against both Stxs and increased survival of mice after a challenge with E. coli O157:H7 lysates (Gao et al., 2009). Interestingly, the protective effects of the vaccine were stronger with the Stx2B-Stx1B-subunit fusion than when separate B-subunits were used for immunization (Gao et al., 2009).
Moreover, a fusion protein comprising the B-subunit of Stx1 and the inactive A-subunit of Stx2 (Stx2Am-Stx1) was constructed. Immunization with this protein resulted in a strong induction of neutralizing antibodies against both types of Shiga toxin and increased the survival rate of mice after challenge with E. coli O157:H7 lysates (Cai et al., 2011). A vector-based DNA vaccine encoding the C-terminal 32 amino acids of the Stx2 A-subunit and the complete B subunit (pStx2ΔAB) also produced neutralizing antibodies against both Stx2 subunits. It further decreased the mortality of mice after lethal challenge with Stx2 (Bentancor et al., 2009).
Intranasal immunization with the Stx2 B-subunit in combination with a mutant of heat-labile toxin induced neutralizing antibodies against both Stx1 and Stx2 in vivo and protected mice against fatal disease. Interestingly, immunization of mice with the B-subunit of Stx1 only protected against subsequent challenge with Stx1 (Tsuji et al., 2008). A fusion of the Stx B-subunit to the B-subunit of heat-labile toxin has also been assessed for toxin subtype 2e. Here, the ability of the fusion protein to induce neutralizing antibody production was much higher than for the Stx2 B-subunit alone. Furthermore, mice that had been immunized with the Stx2eB-LTB fusion protein were protected against challenge with a lethal dose of toxin (Ran et al., 2008).
In another study, the B subunit of Stx2 was fused with Brucella lumazine synthase, a protein that forms a dimer of pentamers thereby creating a scaffold for the presentation of the Stx. The resulting fusion protein induced a lasting immune response in mice after three vaccinations and vaccinated mice were protected from intravenous challenge with Stx2. Furthermore, antibodies isolated from vaccinated mice neutralized Stx2 as well as its variants. In addition, weaned mice inoculated with the immune sera were protected against oral infection with EHEC (Mejias et al., 2013).
Vaccine Approaches Based on LEE-Encoded Proteins
While Stx is produced by all strains that are classed as STEC, only some STEC strains encode the locus of enterocyte effacement pathogenicity island (LEE), which encodes for the T3SS. Therefore, the use of T3-secreted proteins in vaccine approaches is valid but limits the specificity of the vaccines to LEE-positive STEC strains. As the most common EHEC strains including those of the O157:H7 serotype are LEE-positive, the T3-S protein-based approaches will target many of the most common serotypes. However, they do not affect other pathotypes such as the O104:H4 strain, which caused the outbreak in Germany in 2011.
Only a few of the T3-secreted proteins can be used as targets for vaccination strategies, however, as most are translocated from the bacterial cell directly into the host cell cytoplasm and are never exposed to the surrounding environment. A few proteins including EspA, EspB (Figure 2A), Tir, and intimin (Figure 2B) are exposed on the outside of the cell at times as antibodies to these proteins are detectable in humans after an EHEC infection (Li et al., 2000; Asper et al., 2011). These proteins have been assessed as targets for vaccination strategies. The translocated intimin receptor (Tir) inserts into the host cell membrane upon translocation. Its surface-exposed receptor domain then interacts with the bacterial outer membrane protein intimin to induce intimate attachment of the bacteria to the host cell surface.
Furthermore, protein components of the T3 secretion system such as the sheath protein EspA, as well as EspB and EspD, the proteins required for forming a pore in the host cell membrane, have also been assessed for their immunogenicity (Loureiro et al., 1998; Martinez et al., 1999; Asper et al., 2011; Guirro et al., 2013). Most T3-secreted protein-based vaccines have been assessed using the intranasal immunization route. This vaccination approach promises a needle-free application and has, so far, yielded promising results.
Subcutaneous and intranasal immunization of mice with T3-secreted proteins showed that while subcutaneous injection was unable to raise an immune response, intranasal vaccination induced the production of anti-Tir and EspA antibodies. This reduced E. coli O157:H7 shedding after infection (Babiuk et al., 2008). Subcutaneous and intranasal immunization with purified Tir showed similar results. Mice immunized intranasally produced neutralizing antibodies, which resulted in reduced fecal shedding of E. coli O157:H7 after infection and increased animal survival (Fan et al., 2012). Intranasal immunization of mice with a purified fusion protein consisting of EspB and the C-terminus of intimin induced neutralizing antibodies against both EspB and intimin and antisera of immunized mice had promising anti-hemolytic effects in vitro (Cataldi et al., 2008).
A recombinant fusion protein of EspA, intimin, and Tir (EIT) was created and used for immunization of mice. Subcutaneous or oral immunization of mice with the EIT protein resulted in a significant decrease of bacterial colonization and shedding after challenge with EHEC O157:H7 and an increase in anti-EIT IgG and IgA (Amani et al., 2010). In a follow-up study, rEIT was linked to chitosan and used for either intranasal electrospray (Doavi et al., 2016) or oral (Khanifar et al., 2019a) immunization of mice. Intranasal and oral administration both induced specific immune responses and reduced bacterial shedding after challenge with E. coli O157:H7 (Doavi et al., 2016). Oral administration additionally helped protect mice against E. coli O157 challenge and reduced damage (Khanifar et al., 2019a). An additional approach was made by encapsulating EIT together with the B-subunit of Stx2 (Khanifar et al., 2019b). Mice were subcutaneously or orally immunized and either infected with E. coli O157:H7 or challenged with a fatal dose of Stx2. While the former mice showed reduced colonization and bacterial shedding, the latter showed increased survival (Khanifar et al., 2019b). A shortened variant of the EIT fusion protein consisting of only EspA and intimin (EI) was recombinantly expressed and its immunogenicity was assessed after subcutaneous injection with two subcutaneous boosters and a third booster that was administered i.p. (Rad et al., 2013). This fusion protein, too, induced an immune response and decreased bacterial shedding and histopathological changes in the intestine after challenge (Rad et al., 2013).
In addition, the immunogenicity and protective efficacy of a DNA vaccine against a truncated version of the EHEC factor for adherence-1 (Efa-1'; the homolog of LifA in EPEC and C. rodentium) was evaluated in mice. Intranasal immunization with plasmid DNA induced efa-1-specific immune responses and protected mice from subsequent challenge with E. coli O157:H7 (Riquelme-Neira et al., 2015).
Peptide-based approaches to vaccination include the KT-12 peptide, which is based on a predicted B-cell epitope of intimin conjugated to adjuvant (Wan et al., 2011) and the synthetic peptides CoilA and CoilB, which interact with EspA (Larzabal et al., 2013). KT-12, when used for intranasal immunization, induced the production of neutralizing antibodies and protected mice from challenge with E. coli O157:H7 (Wan et al., 2011). Immunization of mice with CoilA and CoilB was shown to block intestinal damage in mice infected with C. rodentium (Larzabal et al., 2013).
A fusion of EspA, the C-terminus of intimin and the B-subunit of Stx2 (EIS), was constructed and assessed for its ability to induce the production of neutralizing antibodies. Indeed, antibodies against all three components of the fusion protein were detected, and immunized mice were protected from challenge with E. coli O157:H7 or lysates thereof (Gu et al., 2009). Fusion of the processed, active form of the Stx2A-subunit (Stx2A1) to the N-terminus of EspA induced the production of neutralizing antibodies in immunized mice (Cheng et al., 2009) and a fusion of the B-subunits of Stx1, Stx2, to a truncated version of intimin resulted in increased immune responses and protection of mice after a fatal challenge with E. coli O157:H7 (Gao et al., 2011).
Intranasal immunization with a novel EspA-Tir fusion protein (EspA-Tir-M; designating that the middle domain of Tir was used) showed high levels of neutralizing antibodies while subcutaneous vaccination had little effect. Additionally, intranasal immunization increased the survival of mice from subsequent challenge with E. coli O157:H7 and reduced organ damage (Lin et al., 2017b).
Bacteria-Based Vaccines
Attenuated or Vaccine Strains
Several vaccination approaches use non-pathogenic bacteria or bacterial vaccine strains as delivery vehicles and to increase immunogenicity. These approaches include genetically modified EHEC, EPEC, and Salmonella strains as well as probiotic strains such as Lactococcus lactis and Lactobacillus acidophilus.
A non-pathogenic variant of the EHEC O157:H7 86-24 strain was created by deletion of both the gene encoding the transcriptional regulator of the LEE (ler) and the stx gene. These deletions completely abolished cytotoxicity in vitro when compared to EHEC EDL933. A derivative of this strain that expresses the inactive forms of Stx1 and Stx2 from a plasmid also showed highly diminished cytotoxicity in vitro. Injection with either stx/ler deletion mutant or the respective Stx1/Stx2-expressing strain reduced the colonization of E. coli O157:H7 after infection of mice. Furthermore, if mice were immunized when pregnant, they passed the immunity on to their offspring, which were protected against E. coli O157:H7 infection (Liu et al., 2009).
Enteropathogenic E. coli (EPEC) also presents a vaccine alternative for EHEC as it is less pathogenic and shares the LEE pathogenicity island-encoded virulence genes. Immunization with EPEC raised neutralizing antibodies against EspB and intimin and conferred some protection against an EHEC infection. Vaccinated mice showed only mild disease phenotypes such as slight intestinal damage, while no kidney pathology could be detected (Calderon Toledo et al., 2011).
Immunization of mice with a Salmonella Typhimurium vaccine strain expressing an inactive Stx2 variant consisting of the A2- and B-subunit of Stx2 (Stx2ΔAB) resulted in efficient colonization of the Peyer's patches and production of neutralizing antibodies. Serum collected from immunized mice was able to neutralize Stx2-mediated toxicity in vitro. However, there was only minimal protection observed when mice were challenged with a lethal dose of Stx2, and no protective effect was seen for kidney health (Rojas et al., 2010).
Additionally, another group constructed a Salmonella Typhimurium strain expressing intimin, which was used to immunize mice orally (Oliveira et al., 2012). This immunization resulted in a significant increase in the levels of serum IgG and fecal IgA and reduced fecal shedding after an E. coli O157:H7 infection (Oliveira et al., 2012). A boost vaccination 2 weeks after the initial immunization led to continuously high colonization levels of the vaccine strain and dissemination into the underlying tissues such as Peyer's patches and spleen (Oliveira et al., 2012). Oral immunization of mice with attenuated Salmonella expressing a hybrid protein consisting of EspA in combination with the C-terminus of intimin and the Stx2 B-subunit (EIS, also see above) raised neutralizing antibodies against the respective proteins and protected mice from a lethal challenge with EHEC for more than 70 days. This period could be extended by a subcutaneous boost with purified EIS (Gu et al., 2011).
Inoculation of mice with a recombinant Mycobacterium bovis BCG (rBCG) vaccine, which was modified to express the Stx2 B-subunit, induced the production of neutralizing antibodies against Stx2. Two high-dose intraperitoneal immunizations resulted in decreased colonization and increased survival after fatal challenge with a STEC strain (Fujii et al., 2012).
The probiotic lactic acid bacterium Lactococcus lactis is considered a safe vaccine vehicle. Use of a L. lactis strain expressing the Stx2 A1-subunit (the A-subunit missing the 15 C-terminal amino acids) for the immunization of mice resulted in increased levels of fecal and serum IgA. Immunized animals had significantly reduced intestinal and kidney damage. Furthermore, immunized mice showed increased survival after challenge with a lethal dose of Shiga toxin isolated from either E. coli O157:H7 or Shigella dysenteriae (Sreerohini et al., 2019).
L. lactis expressing the T3-secreted protein EspB did not yield neutralizing antibodies when used to infect mice. After an i.p. boost with recombinant EspB, however, specific IgG and IgA levels increased (Ahmed et al., 2013). In a follow-up study, the L. lactis was modified to secrete EspB after expression, which resulted in an increased production of neutralizing antibodies. Also, mice immunized with this version of the EspB-expressing L. lactis were protected against E. coli O157:H7 colonization (Ahmed et al., 2014). An L. lactis strain expressing the EspA protein has also been designed. However, this strain has so far only been used for the production of recombinant EspA, as described above. Here, too, a system to either display the protein at the cell surface or secrete it from the cell will probably be needed but may be worthwhile (Luan et al., 2010). A recombinant L. lactis strain that displays the Stx1 B-subunit via albumin binding domains (single-domain non-immunoglobulin scaffolds) on the bacterial cell surface was recently designed by Zadravec et al. (2016). ELISA and FACS analysis confirmed the ability of this strain to bind Stx1. The immunogenicity and safety of this strain and its ability to protect against challenge were, however, not yet tested in animals.
Lastly, a recombinant Lactobacillus acidophilus variant expressing EspA and the Tir central domain (EspA-Tir-M) inhibited A/E lesions formation by EHEC O157:H7 after pre-incubation in vitro. Oral immunization of mice induced the production of specific and systemic neutralizing antibodies and reduced EHEC O157:H7 colonization. It also inhibited intestinal A/E lesions and toxin-mediated organ damage (Lin et al., 2017a).
Bacterial Ghosts
Bacterial ghosts (BGs) remain when bacteria are treated with viral E protein. This protein forms tubes across the bacterial cell membrane releasing the cytoplasm of the bacteria into the surroundings. What remains are bacterial ghosts, empty membranes with intact bacterial morphology and cell surface structures. Because of this, bacterial ghosts are highly immunogenic. Moreover, due to the ubiquitous process used to create them, bacterial ghosts can be produced from whichever strain is desirable (Lubitz et al., 2009; Hajam et al., 2017).
E. coli O157:H7 N°CIP 105282 encode Stx1 and Stx2. BGs were produced from this strain by combining treatment with viral E protein to remove the cytoplasm and addition of staphylococcal nuclease A to degrade pathogenic DNA. Oral immunization of mice induced a specific immune response and mice were protected from a subsequent challenge with an EHEC strain (Mayr et al., 2005). When oral immunization was followed by an oral boost on day 28, antibody production increased, resulting in even better survival (Mayr et al., 2005). A later study showed that a single rectal inoculation of mice with these BGs led to the production of neutralizing antibodies that completely protected mice from infection with a lethal dose of bacteria without requiring a boost (Mayr et al., 2012).
The use of E. coli O157:H7 (EDL933) bacterial ghosts yielded similar results. Here, too, an increase in neutralizing antibodies and protection was observed after a boost (Cai et al., 2010). When the BGs were modified to display an inactive Stx2A–Stx1B fusion (Stx2Am–Stx1B) on the cell surface, a stronger induction of neutralizing antibodies was observed, which correlated with better survival and reduced organ damage upon challenge with EHEC. Furthermore, bacterial ghosts displaying the Stx2Am–Stx1B on the surface performed better than those that did not, suggesting that the combination of surface antigens such as intimin in combination with the toxin resulted in even better immune responses (Cai et al., 2015).
Outer Membrane Vesicles
Outer membrane vesicles (OMVs) are nanoparticles that are released by many Gram-negative bacteria including E. coli. Their major component is bacterial LPS, making them highly immunogenic. Modified OMVs from Shiga toxin A-subunit-deficient E. coli O157:H7 were prepared and tested by eyedrop application for their activity against HUS development. Mice received a boost after 2 weeks, and subsequent intraperitoneal challenge with wild-type OMVs was carried out 4 weeks after the initial immunization. The vaccinated mice were shown to be protected from the lethality usually observed upon challenge with wildtype OMVs (Choi et al., 2014). Immunization with chemically inactivated OMVs obtained from a virulent E. coli O157 strain was also effective in the murine infection model. Here, mice were immunized subcutaneously on days 0 and 21 and received an intraperitoneal challenge with concentrated cell supernatants 2 weeks after application of second immunization. While 90% of control mice died by day seven post-challenge, all immunized mice survived (Fingermann et al., 2018).
Plant-Based Vaccines
Plant-based vaccines have been trialed with the idea that they can easily be used for oral vaccination.
Toxin-Targeted Plant-Based Vaccines
The inactive A-subunit of Stx2 was produced by expression in a tobacco plant cell line (NT-1). Subsequent immunization of mice by feeding or by parenteral immunization with an oral boost resulted in increased Stx2 IgA and IgG levels. It was able to protect mice from a lethal challenge with an EHEC strain. Sera of immunized mice further neutralized toxicity in vitro (Wen et al., 2006). For the treatment of porcine edema disease, which is a severe and often fatal disease in pigs that is also mediated by Stx (in this case subtype Stx2e), Hamabata et al. have recently developed stx2eB-transgenic lettuce for immunization. Infection of piglets with STEC after oral vaccination by feeding the lettuce showed decreased levels of pathogenesis in lettuce-fed piglets (Hamabata et al., 2019).
Virulence Protein-Targeted Vaccines
The immunization capacity of plant-codon optimized intimin expressed in the NT-1 tobacco cell line was also assessed by either i.p. injection of purified protein, feeding of transgenic plant cells, or a combination thereof. Here, mice immunized by injection and boosted by feeding developed neutralizing antibodies against intimin and reduced the time of bacterial shedding after challenge with E. coli O157:H7 (Judge et al., 2004). In another approach, chimeric protein composed of the LEE-encoded proteins EspA, intimin, and Tir (named EIT and further described above) was codon-optimized for expression in tobacco and canola plants. Using this plant-based expression strategy, recombinant EIT was prepared, and immune responses in mice were assessed after parenteral and oral immunization as well as after a combination. Here, a combination of subcutaneous injection and oral gavage yielded the highest immune responses and resulted in significantly reduced fecal shedding of E. coli O157:H7 after challenge (Amani et al., 2011).
Inhibitors
Pyocins
R(rod)-type pyocins are bacterial structures that have developed from phages and show a high similarity to bacteriophage tails. R-type pyocins bind to the core polysaccharides in the outer membrane of bacteria via specific receptor binding proteins, resulting in selective targeting of certain bacteria. The LPS recognition induces contraction of the phage-like tail and puncturing of the bacterial cell envelope, leading to loss of bacterial membrane potential and subsequent cell death. This mechanism is best described for Pseudomonas aeruginosa, which use pyocins to target competitors. However, by designing hybrid proteins that possess the receptor binding proteins or tail fibers of known bacteriophages, the specificity of the pyocins can be changed (Kim et al., 2019). An EHEC O157:H7 specific pyocin (AvR2-V10) developed by Scholl et al. was able to specifically recognize and degrade O157 LPS and kill E. coli O157:H7 without inducing Stx expression (Scholl et al., 2009). A new version of this pyocin (AvR2-10.3) was tested in vivo in a rabbit EHEC infection model. Here, reduced diarrhea colonization and bacterial shedding, as well as less intestinal damage, were observed (Ritchie et al., 2011). Furthermore, Scholl et al. identified an O104-specific bacteriophage tail protein and showed that fusion of this protein to the pyocin tail fiber resulted in specific targeting and killing of O104 strains by the pyocin (AvR2-104.1) (Scholl et al., 2012), showing that this approach is promising for a variety of different serotypes.
Alternative Approaches Using Antimicrobial Agents
Probiotics
Management of STEC-mediated diseases including HUS onset (curative or preventive) by probiotics has also recently been addressed in many studies. The administration of certain probiotics to humans or reservoir animals may reduce colonization and carriage of STEC, which will prevent and/or reduce the risk of infection and transmission of the pathogenic bacteria (Sargeant et al., 2007; Corr et al., 2009). Protective and beneficial capabilities of probiotics have been described in several studies in which probiotics have been applied prior to an STEC infection of cultured cells or in mice (recently reviewed in detail by (Eaton et al., 2011; Mogna et al., 2012; Chen et al., 2013; Kakisu et al., 2013; Rund et al., 2013; Cordonnier et al., 2017; Giordano et al., 2019)). Significant inhibitory effects against the growth of STEC have been demonstrated for several Lactobacillus strains (L. rhamnosus LR04, LR06, L. delbrueckii, L. pentosus, L. fermentum, L. crispatus, L. plantarum, L. lactis, L. kefir, L. gasseri CCDM215, L. accidophila CCDM149, L. helveticus KLDS, L. casei, L. paracasei NZU101), E. coli Nissle, Enterococcus faecium YF5, Enterococcus faecalis (Symbioflor), Bifidobacterium longum, and others. Moreover, use of direct-fed microbiota was found to reduce shedding of E. coli O157:H7 in cattle (Peterson et al., 2007; Callaway et al., 2009; Rahal et al., 2015; Wisener et al., 2015; Giordano et al., 2019). The success seems to depend on the probiotic strain(s), immunomodulation of the host, and their metabolism and ability to modify the local milieu, e.g., by the production of short chain acids, such as lactate, butyrate, and acetate (Ogawa et al., 2001; Takahashi et al., 2004; Carey et al., 2008; Fukuda et al., 2011), or/and occupy similar niches in the intestinal tract in which they compete for adhesion to the gut epithelium and nutrients. In fact, recent studies demonstrated that the efficiency of colonization of the different gut section by intestinal pathogens is dependent on the composition of the microbiota (Baumler and Sperandio, 2016; Litvak et al., 2019).
Despite the observed beneficial effects in animals, it is very difficult to extrapolate the data to humans, considering that the beneficial ratio of probiotic to pathogen varied from 1:1 to 1:105 colony forming units (CFU). Furthermore, it remains unclear how and when the probiotic should be administered.
Phages
Another upcoming potential measure to prevent infectious bacterial diseases is phage therapy, i.e., the application of lytic phages to kill and decrease the number of pathogens in food, animal reservoirs, or patients. One of the first attempts to eliminate STEC from animals (e.g., mice, sheep, and cattle) and food was performed with bacteriophages (e.g., the T-even bacteriophage CEV1, rV5, WV8, WV7, wV11, e11/2, and e4/1c), which have the potential to lyse E. coli O:157:H7 (Raya et al., 2006; Sheng et al., 2006; Abuladze et al., 2008; Rivas et al., 2010; Stanford et al., 2010). To date, a large variety of phages have been isolated and shown to be highly effective in the killing of STEC strains in vitro, but the application of individual bacteriophages in vivo seemed less promising, likely due to the fact that bacterial access of the phages in the gut is reduced, or the intestinal environment is disadvantageous for phage survival and/or replication (Niu et al., 2009; Dini et al., 2016; Sabouri et al., 2017; Wang et al., 2017; Safwat-Mohamed et al., 2018). However, more recently, use of certain phages and phage cocktails including multiple STEC-specific phages for oral or rectal administration to ruminants or for spraying on fruits and vegetables has shown the potential of phage therapy to reduce STEC carriage in domestic animals (Niu et al., 2009; Dini et al., 2016; Sabouri et al., 2017; Wang et al., 2017; Safwat-Mohamed et al., 2018).
Antibiotics
Early studies investigating the effect of antibiotics in the treatment of STEC infections have suggested that antibiotics induce the bacterial SOS response, resulting in an increase of Shiga toxin production and release (O'Brien et al., 1984; Muhldorfer et al., 1996; Kimmitt et al., 2000). This has raised concerns that antibiotics may increase the risk of HUS development (Zimmerhackl, 2000; Panos et al., 2006; Kakoullis et al., 2019), and therefore, their use during STEC infections has been contraindicated. However, not all studies were able to confirm Stx induction or an increase in the amount of HUS incidences in response to antibiotics. Furthermore, the effects of antibiotics on stx expression vary greatly and are dependent on the antibiotic class, the antibiotic concentration, the respective STEC strain, as well as the Stx subtype (Walterspiel et al., 1992; Grif et al., 1998; Kimmitt et al., 2000; Ochoa et al., 2007; Pedersen et al., 2008; Zhang et al., 2009; McGannon et al., 2010; Bielaszewska et al., 2012; Nassar et al., 2013). While the results obtained from some antibiotic classes, such as β-lactams, are conflicting (Yoh et al., 1997, 1999; Grif et al., 1998; Yoshimura et al., 1999; McGannon et al., 2010; Muhlen et al., 2020), ansamycins and chloramphenicol consistently yielded promising results in in vitro studies (Kimmitt et al., 2000; Ochoa et al., 2007; McGannon et al., 2010; Kakoullis et al., 2019; Muhlen et al., 2020) while fluoroquinolones were regularly associated with toxin induction (Zhang et al., 2000; Hiramatsu et al., 2003; Bielaszewska et al., 2012; Berger et al., 2019; Muhlen et al., 2020).
Some animal studies used to mimic STEC- or Stx-mediated disease in response to antibiotic treatment confirmed previous in vitro results, while others were contradicting the in vitro data (Zhang et al., 2000, 2009; Rahal et al., 2011a; Zangari et al., 2014; Kakoullis et al., 2019). Interestingly, the in vivo studies confirmed a detrimental effect of fluoroquinolone antibiotics (Zhang et al., 2000; Hiramatsu et al., 2003; Muhlen et al., 2020) and a reduction of disease pathology when animals were treated with rifamycin antibiotics (Rahal et al., 2011a,b; Muhlen et al., 2020).
As studies using antibiotics that inhibit transcription or translation, such as rifamycins, have regularly been shown to inhibit Stx production in vitro and in vivo (Rahal et al., 2011a,b; Corogeanu et al., 2012; Fadlallah et al., 2015; Berger et al., 2019; Muhlen et al., 2020) even after pre-treatment with fluoroquinolone ciprofloxacin (Berger et al., 2019), this opens up the possibility of a potential treatment regimen for STEC infection using antibiotics combination therapy consisting of a transcriptional inhibitor supplied in advance of or simultaneously with an antibiotic such as ciprofloxacin, which efficiently clears the bacterial infection.
Natural Products
Several studies have tested natural products for their ability to reduce or prevent Stx-induced cell or tissue damage in cell cultures in vitro or in animal infection models. An inhibition of cytotoxicity of Stx of E. coli O:157:H7 was found for white carob tree (Prosopsis alba) and Ziziphus mistol extracts (Pellarin et al., 2013) and for Ellagitannin from the Aleppo oak (Quercus infectoria) (Voravuthikunchai et al., 2012). Moreover, bacterial products such as lactic acid, linoleic acid, (Pittman et al., 2012), green tea extracts (Isogai et al., 1998, 2001), fruit juices (Nogueira et al., 2003), and other plant products (Takemasa et al., 2009; Lacombe et al., 2010; Lee and Stein, 2011; Sheng et al., 2016; Sewlikar and D'Souza, 2017; Patel et al., 2018) have been shown to have a beneficial effect on STEC-infected cells and animals alone or in combination of other agents, e.g., antibiotics.
Conclusions
Although almost four decades have passed since the first clinical human HUS case (Centers for Disease Control, 1982) and the incidence of HUS increases, a generally accepted and successful therapy for STEC-induced HUS in patients is still missing. Nevertheless, a number of promising approaches and clinical studies employing different strategies have been performed. Here, we have reviewed therapeutic strategies including Stx toxin receptor analogs, Stx-specific antibodies, and alternative antimicrobial agents. For some, a beneficial effect has been reported, although the outcome seems to depend on multiple factors, e.g., the dose of the agent, the STEC isolate/strain, the Stx variants, the time point of administration, the route of application, the severity of the infection/symptoms, and the type of agent. Another important problem is that our information about the concentration, activity, and the precise localization of the toxins during the course of the infection is still scarce. Further studies examining these issues and testing the efficacy of Stx-inhibiting agents according to gained knowledge from these experiments are required to select the most successful regimens that could then be assessed in clinical trials. As none of the current animal models mimic EHEC infections and HUS development in humans, clinical trials and cohort studies are urgently needed to evaluate whether newly developed treatment strategies are effective. In this context, it should also be mentioned that early detection of an EHEC infection is crucial for the success of most newly developed therapeutics. Thus, the development of fast and reliable diagnostic tools to screen for Stx is also of utter importance. Moreover, a vaccination strategy for humans, but also for cattle limiting STEC carriage in their reservoirs would be of great benefit for public health.
What is the current situation of possible therapies and vaccines? While many different treatment strategies have been employed in order to develop a therapeutic or prophylactic treatment against STEC-induced disease, few have, to date, progressed past Phase II trials. Several approaches including monoclonal antibodies, receptor analogs such as Synsorb Pk, or the use of Eculizumab looked promising, but when evaluated systematically or in Phase III trials showed little evidence of success. The disadvantage of monomeric antibodies may likely be that the Stx receptor forms polymers, thus leaving Stx docking spaces available. Newly developed antibodies and neutralizing peptides therefore aim at providing multimeric recognition sites to capture as many receptor-binding sites as possible. These have, however, yet to be tested in clinical trials.
Vaccine approaches face other difficulties. While STEC infections are an important cause for diarrhea disease and especially dangerous as they can lead to HUS and systemic complications, the total amount of STEC infections is rather low. Therefore, a prophylactic vaccine may only be of interest for countries in which these infections are endemic. In general, while phase II clinical trials can be carried out, the availability of patients with STEC infections for phase III trials is limited. Furthermore, these infections will vary in the infection-causing strain and Stx subtype, both of which need to be controlled in a clinical trial. Also, the time of presentation at the physician or in hospital will most likely be after the onset of bloody diarrhea or late stages of watery diarrhea, making an early intervention difficult.
Author Contributions
All authors listed have made an equal, substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
The work on potential novel therapeutic strategies of STEC infections by the authors is supported by the German Center of Infection Research (DZIF).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank M. Fenner and U. Dobrindt for helpful discussion.
References
Abuladze, T., Li, M., Menetrez, M. Y., Dean, T., Senecal, A., and Sulakvelidze, A. (2008). Bacteriophages reduce experimental contamination of hard surfaces, tomato, spinach, broccoli, and ground beef by Escherichia coli O157:H7. Appl. Environ. Microbiol. 74, 6230–6238. doi: 10.1128/AEM.01465-08
Acheson, D. W., Levine, M. M., Kaper, J. B., and Keusch, G. T. (1996). Protective immunity to shiga-like toxin I following oral immunization with shiga-like toxin I B-subunit-producing Vibrio cholerae CVD 103-HgR. Infect. Immun. 64, 355–357. doi: 10.1128/IAI.64.1.355-357.1996
Ahmed, B., Loos, M., Vanrompay, D., and Cox, E. (2013). Mucosal priming of the murine immune system against enterohemorrhagic Escherichia coli O157:H7 using Lactococcus lactis expressing the type III secretion system protein EspB. Vet. Immunol. Immunopathol. 152, 141–145. doi: 10.1016/j.vetimm.2012.09.019
Ahmed, B., Loos, M., Vanrompay, D., and Cox, E. (2014). Oral immunization with Lactococcus lactis-expressing EspB induces protective immune responses against Escherichia coli O157:H7 in a murine model of colonization. Vaccine 32, 3909–3916. doi: 10.1016/j.vaccine.2014.05.054
Albanese, A., Sacerdoti, F., Seyahian, E. A., Amaral, M. M., Fiorentino, G., Fernandez Brando, R., et al. (2018). Immunization of pregnant cows with shiga toxin-2 induces high levels of specific colostral antibodies and lactoferrin able to neutralize E. coli O157:H7 pathogenicity. Vaccine 36, 1728–1735. doi: 10.1016/j.vaccine.2018.02.060
Amani, J., Mousavi, S. L., Rafati, S., and Salmanian, A. H. (2011). Immunogenicity of a plant-derived edible chimeric EspA, intimin and tir of Escherichia coli O157:H7 in mice. Plant Sci. 180, 620–627. doi: 10.1016/j.plantsci.2011.01.004
Amani, J., Salmanian, A. H., Rafati, S., and Mousavi, S. L. (2010). Immunogenic properties of chimeric protein from espA, eae and tir genes of Escherichia coli O157:H7. Vaccine 28, 6923–6929. doi: 10.1016/j.vaccine.2010.07.061
Asahara, T., Shimizu, K., Nomoto, K., Hamabata, T., Ozawa, A., and Takeda, Y. (2004). Probiotic bifidobacteria protect mice from lethal infection with shiga toxin-producing Escherichia coli O157:H7. Infect. Immun. 72, 2240–2247. doi: 10.1128/IAI.72.4.2240-2247.2004
Asper, D. J., Karmali, M. A., Townsend, H., Rogan, D., and Potter, A. A. (2011). Serological response of shiga toxin-producing Escherichia coli type III secreted proteins in sera from vaccinated rabbits, naturally infected cattle, and humans. Clin. Vaccine Immunol. 18, 1052–1057. doi: 10.1128/CVI.00068-11
Babiuk, S., Asper, D. J., Rogan, D., Mutwiri, G. K., and Potter, A. A. (2008). Subcutaneous and intranasal immunization with type III secreted proteins can prevent colonization and shedding of Escherichia coli O157:H7 in mice. Microb. Pathog. 45, 7–11. doi: 10.1016/j.micpath.2008.01.005
Baumler, A. J., and Sperandio, V. (2016). Interactions between the microbiota and pathogenic bacteria in the gut. Nature 535, 85–93. doi: 10.1038/nature18849
Bentancor, L. V., Bilen, M., Brando, R. J., Ramos, M. V., Ferreira, L. C., Ghiringhelli, P. D., et al. (2009). A DNA vaccine encoding the enterohemorragic Escherichia coli shiga-like toxin 2 A2 and B subunits confers protective immunity to shiga toxin challenge in the murine model. Clin. Vaccine Immunol. 16, 712–718. doi: 10.1128/CVI.00328-08
Berger, M., Aijaz, I., Berger, P., Dobrindt, U., and Koudelka, G. (2019). Transcriptional and translational inhibitors block SOS response and shiga toxin expression in enterohemorrhagic Escherichia coli. Sci. Rep. 9:18777. doi: 10.1038/s41598-019-55332-2
Bernedo-Navarro, R. A., Miyachiro, M. M., Da Silva, M. J., Reis, C. F., Conceicao, R. A., Gatti, M. S., et al. (2014). Peptides derived from phage display libraries as potential neutralizers of shiga toxin-induced cytotoxicity in vitro and in vivo. J. Appl. Microbiol. 116, 1322–1333. doi: 10.1111/jam.12451
Bielaszewska, M., Idelevich, E. A., Zhang, W., Bauwens, A., Schaumburg, F., Mellmann, A., et al. (2012). Effects of antibiotics on shiga toxin 2 production and bacteriophage induction by epidemic Escherichia coli O104:H4 strain. Antimicrob. Agents Chemother. 56, 3277–3282. doi: 10.1128/AAC.06315-11
Bielaszewska, M., Mellmann, A., Zhang, W., Kock, R., Fruth, A., Bauwens, A., et al. (2011). Characterisation of the Escherichia coli strain associated with an outbreak of haemolytic uraemic syndrome in Germany, 2011: a microbiological study. Lancet Infect. Dis. 11, 671–676. doi: 10.1016/S1473-3099(11)70165-7
Bitzan, M., Poole, R., Mehran, M., Sicard, E., Brockus, C., Thuning-Roberson, C., et al. (2009). Safety and pharmacokinetics of chimeric anti-shiga toxin 1 and anti-shiga toxin 2 monoclonal antibodies in healthy volunteers. Antimicrob. Agents Chemother. 53, 3081–3087. doi: 10.1128/AAC.01661-08
Buelli, S., Zoja, C., Remuzzi, G., and Morigi, M. (2019). Complement activation contributes to the pathophysiology of shiga toxin-associated hemolytic uremic syndrome. Microorganisms 7:15. doi: 10.3390/microorganisms7010015
Cai, K., Gao, X., Li, T., Hou, X., Wang, Q., Liu, H., et al. (2010). Intragastric immunization of mice with enterohemorrhagic Escherichia coli O157:H7 bacterial ghosts reduces mortality and shedding and induces a Th2-type dominated mixed immune response. Can. J. Microbiol. 56, 389–398. doi: 10.1139/W10-025
Cai, K., Gao, X., Li, T., Wang, Q., Hou, X., Tu, W., et al. (2011). Enhanced immunogenicity of a novel Stx2Am-Stx1B fusion protein in a mice model of enterohemorrhagic Escherichia coli O157:H7 infection. Vaccine 29, 946–952. doi: 10.1016/j.vaccine.2010.11.035
Cai, K., Tu, W., Liu, Y., Li, T., and Wang, H. (2015). Novel fusion antigen displayed-bacterial ghosts vaccine candidate against infection of Escherichia coli O157:H7. Sci. Rep. 5:17479. doi: 10.1038/srep17479
Calderon Toledo, C., Arvidsson, I., and Karpman, D. (2011). Cross-reactive protection against enterohemorrhagic Escherichia coli infection by enteropathogenic E. coli in a mouse model. Infect. Immun. 79, 2224–2233. doi: 10.1128/IAI.01024-10
Callaway, T. R., Carr, M. A., Edrington, T. S., Anderson, R. C., and Nisbet, D. J. (2009). Diet, Escherichia coli O157:H7, and cattle: a review after 10 years. Curr. Issues Mol. Biol. 11, 67–79.
Caprioli, A., Scavia, G., and Morabito, S. (2014). Public health microbiology of shiga toxin-producing Escherichia coli. Microbiol. Spectr. 2. doi: 10.1128/microbiolspec.EHEC-0014-2013
Carey, C. M., Kostrzynska, M., Ojha, S., and Thompson, S. (2008). The effect of probiotics and organic acids on shiga-toxin 2 gene expression in enterohemorrhagic Escherichia coli O157:H7. J. Microbiol Methods 73, 125–132. doi: 10.1016/j.mimet.2008.01.014
Cataldi, A., Yevsa, T., Vilte, D. A., Schulze, K., Castro-Parodi, M., Larzabal, M., et al. (2008). Efficient immune responses against Intimin and EspB of enterohaemorragic Escherichia coli after intranasal vaccination using the TLR2/6 agonist MALP-2 as adjuvant. Vaccine 26, 5662–5667. doi: 10.1016/j.vaccine.2008.07.027
Chen, Y. P., Lee, T. Y., Hong, W. S., Hsieh, H. H., and Chen, M. J. (2013). Effects of Lactobacillus kefiranofaciens M1 isolated from kefir grains on enterohemorrhagic Escherichia coli infection using mouse and intestinal cell models. J. Dairy Sci. 96, 7467–7477. doi: 10.3168/jds.2013-7015
Cheng, L. W., Henderson, T. D., Patfield, S., Stanker, L. H., and He, X. (2013). Mouse in vivo neutralization of Escherichia coli shiga toxin 2 with monoclonal antibodies. Toxins 5, 1845–1858. doi: 10.3390/toxins5101845
Cheng, Y., Feng, Y., Luo, P., Gu, J., Yu, S., Zhang, W. J., et al. (2009). Fusion expression and immunogenicity of EHEC EspA-Stx2Al protein: implications for the vaccine development. J. Microbiol. 47, 498–505. doi: 10.1007/s12275-009-0116-8
Choi, K. S., Kim, S. H., Kim, E. D., Lee, S. H., Han, S. J., Yoon, S., et al. (2014). Protection from hemolytic uremic syndrome by eyedrop vaccination with modified enterohemorrhagic E. coli outer membrane vesicles. PLoS ONE 9:e100229. doi: 10.1371/journal.pone.0100229
Clements, A., Young, J. C., Constantinou, N., and Frankel, G. (2012). Infection strategies of enteric pathogenic Escherichia coli. Gut Microbes 3, 71–87. doi: 10.4161/gmic.19182
Control, C. F. D. (1982). Isolation of E. coli O157:H7 from sporadic cases of hemorrhagic colitis - United States. MMWR 31, 580–585.
Cordonnier, C., Thevenot, J., Etienne-Mesmin, L., Alric, M., Livrelli, V., and Blanquet-Diot, S. (2017). Probiotic and enterohemorrhagic Escherichia coli: an effective strategy against a deadly enemy? Crit. Rev. Microbiol. 43, 116–132. doi: 10.1080/1040841X.2016.1185602
Corogeanu, D., Willmes, R., Wolke, M., Plum, G., Utermohlen, O., and Kronke, M. (2012). Therapeutic concentrations of antibiotics inhibit shiga toxin release from enterohemorrhagic E. coli O104:H4 from the 2011 German outbreak. BMC Microbiol. 12:160. doi: 10.1186/1471-2180-12-160
Corr, S. C., Hill, C., and Gahan, C. G. (2009). Understanding the mechanisms by which probiotics inhibit gastrointestinal pathogens. Adv. Food Nutr. Res. 56, 1–15. doi: 10.1016/S1043-4526(08)00601-3
Delmas, Y., Vendrely, B., Clouzeau, B., Bachir, H., Bui, H. N., Lacraz, A., et al. (2014). Outbreak of Escherichia coli O104:H4 haemolytic uraemic syndrome in France: outcome with eculizumab. Nephrol. Dial Transplant. 29, 565–572. doi: 10.1093/ndt/gft470
Dini, C., Bolla, P. A., and De Urraza, P. J. (2016). Treatment of in vitro enterohemorrhagic Escherichia coli infection using phage and probiotics. J. Appl. Microbiol. 121, 78–88. doi: 10.1111/jam.13124
Doavi, T., Mousavi, S. L., Kamali, M., Amani, J., and Fasihi Ramandi, M. (2016). Chitosan-based intranasal vaccine against Escherichia coli O157:H7. Iran Biomed. J. 20, 97–108. doi: 10.7508/ibj.2016.02.005
Donnenberg, M. S., and Kaper, J. B. (1992). Enteropathogenic Escherichia coli. Infect. Immun. 60, 3953–3961. doi: 10.1128/IAI.60.10.3953-3961.1992
Dowling, T. C., Chavaillaz, P. A., Young, D. G., Melton-Celsa, A., O'Brien, A., Thuning-Roberson, C., et al. (2005). Phase 1 safety and pharmacokinetic study of chimeric murine-human monoclonal antibody c alpha Stx2 administered intravenously to healthy adult volunteers. Antimicrob. Agents Chemother. 49, 1808–1812. doi: 10.1128/AAC.49.5.1808-1812.2005
Dyve Lingelem, A. B., Bergan, J., and Sandvig, K. (2012). Inhibitors of intravesicular acidification protect against shiga toxin in a pH-independent manner. Traffic 13, 443–454. doi: 10.1111/j.1600-0854.2011.01319.x
Eaton, K. A., Honkala, A., Auchtung, T. A., and Britton, R. A. (2011). Probiotic Lactobacillus reuteri ameliorates disease due to enterohemorrhagic Escherichia coli in germfree mice. Infect. Immun. 79, 185–191. doi: 10.1128/IAI.00880-10
Fadlallah, S. M., Rahal, E. A., Sabra, A., Kissoyan, K. A., and Matar, G. M. (2015). Effect of rifampicin and gentamicin on shiga toxin 2 expression level and the SOS response in Escherichia coli O104:H4. Foodborne Pathog. Dis. 12, 47–55. doi: 10.1089/fpd.2014.1824
Fan, H. Y., Wang, L., Luo, J., and Long, B. G. (2012). Protection against Escherichia coli O157:H7 challenge by immunization of mice with purified tir proteins. Mol. Biol. Rep. 39, 989–997. doi: 10.1007/s11033-011-0824-0
Fingermann, M., Avila, L., De Marco, M. B., Vazquez, L., Di Biase, D. N., Muller, A. V., et al. (2018). OMV-based vaccine formulations against shiga toxin producing Escherichia coli strains are both protective in mice and immunogenic in calves. Hum. Vaccine Immunother. 14, 2208–2213. doi: 10.1080/21645515.2018.1490381
Fujii, J., Naito, M., Yutsudo, T., Matsumoto, S., Heatherly, D. P., Yamada, T., et al. (2012). Protection by a recombinant mycobacterium bovis bacillus calmette-guerin vaccine expressing shiga toxin 2 B subunit against shiga toxin-producing Escherichia coli in mice. Clin. Vaccine Immunol. 19, 1932–1937. doi: 10.1128/CVI.00473-12
Fukuda, S., Toh, H., Hase, K., Oshima, K., Nakanishi, Y., Yoshimura, K., et al. (2011). Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469, 543–547. doi: 10.1038/nature09646
Funatogawa, K., Ide, T., Kirikae, F., Saruta, K., Nakano, M., and Kirikae, T. (2002). Use of immunoglobulin enriched bovine colostrum against oral challenge with enterohaemorrhagic Escherichia coli O157:H7 in mice. Microbiol. Immunol. 46, 761–766. doi: 10.1111/j.1348-0421.2002.tb02761.x
Gandhi, T., Patki, M., Kong, J., Koya, J., Yoganathan, S., Reznik, S., et al. (2019). Development of an arginine anchored nanoglobule with retrograde trafficking inhibitor (Retro-2) for the treatment of an enterohemorrhagic Escherichia coli outbreak. Mol. Pharm. 16, 4405–4415. doi: 10.1021/acs.molpharmaceut.9b00727
Gao, X., Cai, K., Li, T., Wang, Q., Hou, X., Tian, R., et al. (2011). Novel fusion protein protects against adherence and toxicity of enterohemorrhagic Escherichia coli O157:H7 in mice. Vaccine 29, 6656–6663. doi: 10.1016/j.vaccine.2011.06.106
Gao, X., Cai, K., Shi, J., Liu, H., Hou, X., Tu, W., et al. (2009). Immunogenicity of a novel Stx2B-Stx1B fusion protein in a mice model of Enterohemorrhagic Escherichia coli O157:H7 infection. Vaccine 27, 2070–2076. doi: 10.1016/j.vaccine.2009.01.115
Garmendia, J., Frankel, G., and Crepin, V. F. (2005). Enteropathogenic and enterohemorrhagic Escherichia coli infections: translocation, translocation, translocation. Infect. Immun. 73, 2573–2585. doi: 10.1128/IAI.73.5.2573-2585.2005
Gaytan, M. O., Martinez-Santos, V. I., Soto, E., and Gonzalez-Pedrajo, B. (2016). Type three secretion system in attaching and effacing pathogens. Front. Cell Infect. Microbiol. 6:129. doi: 10.3389/fcimb.2016.00129
Giordano, M., Baldassarre, M. E., Palmieri, V., Torres, D. D., Carbone, V., Santangelo, L., et al. (2019). Management of STEC Gastroenteritis: is there a role for probiotics? Int. J. Environ. Res. Public Health 16:1649. doi: 10.3390/ijerph16091649
Gordon, V. M., Whipp, S. C., Moon, H. W., O'Brien, A. D., and Samuel, J. E. (1992). An enzymatic mutant of shiga-like toxin II variant is a vaccine candidate for edema disease of swine. Infect. Immun. 60, 485–490. doi: 10.1128/IAI.60.2.485-490.1992
Grif, K., Dierich, M. P., Karch, H., and Allerberger, F. (1998). Strain-specific differences in the amount of shiga toxin released from enterohemorrhagic Escherichia coli O157 following exposure to subinhibitory concentrations of antimicrobial agents. Eur. J. Clin. Microbiol. Infect. Dis. 17, 761–766. doi: 10.1007/s100960050181
Gu, J., Liu, Y., Yu, S., Wang, H., Wang, Q., Yi, Y., et al. (2009). Enterohemorrhagic Escherichia coli trivalent recombinant vaccine containing EspA, intimin and Stx2 induces strong humoral immune response and confers protection in mice. Microbes Infect. 11, 835–841. doi: 10.1016/j.micinf.2009.04.024
Gu, J., Ning, Y., Wang, H., Xiao, D., Tang, B., Luo, P., et al. (2011). Vaccination of attenuated EIS-producing Salmonella induces protective immunity against enterohemorrhagic Escherichia coli in mice. Vaccine 29, 7395–7403. doi: 10.1016/j.vaccine.2011.07.069
Guirro, M., De Souza, R. L., Piazza, R. M., and Guth, B. E. (2013). Antibodies to intimin and Escherichia coli-secreted proteins EspA and EspB in sera of Brazilian children with hemolytic uremic syndrome and healthy controls. Vet Immunol. Immunopathol. 152, 121–125. doi: 10.1016/j.vetimm.2012.09.016
Hajam, I. A., Dar, P. A., Won, G., and Lee, J. H. (2017). Bacterial ghosts as adjuvants: mechanisms and potential. Vet. Res. 48:37. doi: 10.1186/s13567-017-0442-5
Hamabata, T., Sato, T., Takita, E., Matsui, T., Imaoka, T., Nakanishi, N., et al. (2019). shiga toxin 2eB-transgenic lettuce vaccine is effective in protecting weaned piglets from edema disease caused by shiga toxin-producing Escherichia coli infection. Anim. Sci. J. 90, 1460–1467. doi: 10.1111/asj.13292
Hiramatsu, K., Murakami, J., Kishi, K., Hirata, N., Yamasaki, T., Kadota, J., et al. (2003). Treatment with rokitamycin suppresses the lethality in a murine model of Escherichia coli O157:H7 infection. Int. J. Antimicrob. Agents 21, 471–477. doi: 10.1016/S0924-8579(03)00007-4
Hostetter, S. J., Helgerson, A. F., Paton, J. C., Paton, A. W., and Cornick, N. A. (2014). Therapeutic use of a receptor mimic probiotic reduces intestinal shiga toxin levels in a piglet model of hemolytic uremic syndrome. BMC Res. Notes 7:331. doi: 10.1186/1756-0500-7-331
Huppertz, H. I., Rutkowski, S., Busch, D. H., Eisebit, R., Lissner, R., and Karch, H. (1999). Bovine colostrum ameliorates diarrhea in infection with diarrheagenic Escherichia coli, shiga toxin-producing E. coli, and E. coli expressing intimin and hemolysin. J. Pediatr. Gastroenterol. Nutr. 29, 452–456. doi: 10.1097/00005176-199910000-00015
Ishikawa, S., Kawahara, K., Kagami, Y., Isshiki, Y., Kaneko, A., Matsui, H., et al. (2003). Protection against shiga toxin 1 challenge by immunization of mice with purified mutant shiga toxin 1. Infect. Immun. 71, 3235–3239. doi: 10.1128/IAI.71.6.3235-3239.2003
Isogai, E., Isogai, H., Hirose, K., Hayashi, S., and Oguma, K. (2001). In vivo synergy between green tea extract and levofloxacin against enterohemorrhagic Escherichia coli O157 infection. Curr. Microbiol. 42, 248–251. doi: 10.1007/s0028403357
Isogai, E., Isogai, H., Takeshi, K., and Nishikawa, T. (1998). Protective effect of Japanese green tea extract on gnotobiotic mice infected with an Escherichia coli O157:H7 strain. Microbiol. Immunol. 42, 125–128. doi: 10.1111/j.1348-0421.1998.tb02260.x
Jeong, K. I., Tzipori, S., and Sheoran, A. S. (2010). Shiga toxin 2-specific but not shiga toxin 1-specific human monoclonal antibody protects piglets challenged with enterohemorrhagic Escherichia coli producing shiga toxin 1 and shiga toxin 2. J. Infect. Dis. 201, 1081–1083. doi: 10.1086/651198
Judge, N. A., Mason, H. S., and O'Brien, A. D. (2004). Plant cell-based intimin vaccine given orally to mice primed with intimin reduces time of Escherichia coli O157:H7 shedding in feces. Infect. Iimmun. 72, 168–175. doi: 10.1128/IAI.72.1.168-175.2004
Kakisu, E., Abraham, A. G., Farinati, C. T., Ibarra, C., and De Antoni, G. L. (2013). Lactobacillus plantarum isolated from kefir protects vero cells from cytotoxicity by type-II shiga toxin from Escherichia coli O157:H7. J. Dairy Res. 80, 64–71. doi: 10.1017/S0022029912000659
Kakoullis, L., Papachristodoulou, E., Chra, P., and Panos, G. (2019). shiga toxin-induced haemolytic uraemic syndrome and the role of antibiotics: a global overview. J. Infect. 79, 75–94. doi: 10.1016/j.jinf.2019.05.018
Kaper, J. B., and O'Brien, A. D. (2014). Overview and historical perspectives. Microbiol. Spectr 2. doi: 10.1128/microbiolspec.EHEC-0028-2014
Karpman, D., and Tati, R. (2016). Complement contributes to the pathogenesis of shiga toxin-associated hemolytic uremic syndrome. Kidney Int. 90, 726–729. doi: 10.1016/j.kint.2016.07.002
Kavaliauskiene, S., Dyve Lingelem, A. B., Skotland, T., and Sandvig, K. (2017). Protection against shiga Toxins. Toxins 9:44. doi: 10.3390/toxins9020044
Kavaliauskiene, S., Skotland, T., Sylvanne, T., Simolin, H., Klokk, T. I., Torgersen, M. L., et al. (2015). Novel actions of 2-deoxy-D-glucose: protection against shiga toxins and changes in cellular lipids. Biochem J. 470, 23–37. doi: 10.1042/BJ20141562
Kavaliauskiene, S., Torgersen, M. L., Lingelem, A. B., Klokk, T. I., Lintonen, T., Simolin, H., et al. (2016). Cellular effects of fluorodeoxyglucose: global changes in the lipidome and alteration in intracellular transport. Oncotarget 7, 79885–79900. doi: 10.18632/oncotarget.13089
Keenswijk, W., Vanmassenhove, J., Raes, A., Dhont, E., and Vande Walle, J. (2017). Blood urea nitrogen to serum creatinine ratio is an accurate predictor of outcome in diarrhea-associated hemolytic uremic syndrome, a preliminary study. Eur. J. Pediatr. 176, 355–360. doi: 10.1007/s00431-016-2846-z
Kenny, B., Devinney, R., Stein, M., Reinscheid, D. J., Frey, E. A., and Finlay, B. B. (1997). Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91, 511–520. doi: 10.1016/S0092-8674(00)80437-7
Kerner, K., Bridger, P. S., Kopf, G., Frohlich, J., Barth, S., Willems, H., et al. (2015). Evaluation of biological safety in vitro and immunogenicity in vivo of recombinant Escherichia coli shiga toxoids as candidate vaccines in cattle. Vet. Res. 46:38. doi: 10.1186/s13567-015-0175-2
Khanifar, J., Hosseini, R. H., Kazemi, R., Ramandi, M. F., Amani, J., and Salmanian, A. H. (2019a). Prevention of EHEC infection by chitosan nano-structure coupled with synthetic recombinant antigen. J. Microbiol. Methods 157, 100–107. doi: 10.1016/j.mimet.2019.01.002
Khanifar, J., Salmanian, A. H., Haji Hosseini, R., Amani, J., and Kazemi, R. (2019b). Chitosan nano-structure loaded with recombinant E. coli O157:H7 antigens as a vaccine candidate can effectively increase immunization capacity. Artif. Cells Nanomed. Biotechnol. 47, 2593–2604. doi: 10.1080/21691401.2019.1629947
Kielstein, J. T., Beutel, G., Fleig, S., Steinhoff, J., Meyer, T. N., Hafer, C., et al. (2012). Best supportive care and therapeutic plasma exchange with or without eculizumab in shiga-toxin-producing E. coli O104:H4 induced haemolytic-uraemic syndrome: an analysis of the German STEC-HUS registry. Nephrol. Dial Transplant. 27, 3807–3815. doi: 10.1093/ndt/gfs394
Kim, B. O., Kim, E. S., Yoo, Y. J., Bae, H. W., Chung, I. Y., and Cho, Y. H. (2019). Phage-derived antibacterials: harnessing the simplicity, plasticity, and diversity of phages. Viruses 11, 268. doi: 10.3390/v11030268
Kimmitt, P. T., Harwood, C. R., and Barer, M. R. (2000). Toxin gene expression by shiga toxin-producing Escherichia coli: the role of antibiotics and the bacterial SOS response. Emerg. Infect. Dis. 6, 458–465. doi: 10.3201/eid0605.000503
Kimura, T., Co, M. S., Vasquez, M., Wei, S., Xu, H., Tani, S., et al. (2002). Development of humanized monoclonal antibody TMA-15 which neutralizes shiga toxin 2. Hybrid Hybridomics 21, 161–168. doi: 10.1089/153685902760173872
Kitov, P. I., Sadowska, J. M., Mulvey, G., Armstrong, G. D., Ling, H., Pannu, N. S., et al. (2000). shiga-like toxins are neutralized by tailored multivalent carbohydrate ligands. Nature 403, 669–672. doi: 10.1038/35001095
Konadu, E., Donohue-Rolfe, A., Calderwood, S. B., Pozsgay, V., Shiloach, J., Robbins, J. B., et al. (1999). Syntheses and immunologic properties of Escherichia coli O157 O-specific polysaccharide and shiga toxin 1 B subunit conjugates in mice. Infect. Immun. 67, 6191–6193. doi: 10.1128/IAI.67.11.6191-6193.1999
Kulkarni, A. A., Weiss, A. A., and Iyer, S. S. (2010). Glycan-based high-affinity ligands for toxins and pathogen receptors. Med. Res. Rev. 30, 327–393. doi: 10.1002/med.20196
Kuribayashi, T., Seita, T., Fukuyama, M., Furuhata, K., Honda, M., Matsumoto, M., et al. (2006). Neutralizing activity of bovine colostral antibody against verotoxin derived from enterohemorrhagic Escherichia coli O157:H7 in mice. J. Infect. Chemother. 12, 251–256. doi: 10.1007/s10156-006-0470-Y
Lacombe, A., Wu, V. C., Tyler, S., and Edwards, K. (2010). Antimicrobial action of the American cranberry constituents; phenolics, anthocyanins, and organic acids, against Escherichia coli O157:H7. Int J. Food Microbiol. 139, 102–107. doi: 10.1016/j.ijfoodmicro.2010.01.035
Lapeyraque, A. L., Malina, M., Fremeaux-Bacchi, V., Boppel, T., Kirschfink, M., Oualha, M., et al. (2011). Eculizumab in severe shiga-toxin-associated HUS. New Engl. J. Med. 364, 2561–2563. doi: 10.1056/NEJMc1100859
Larzabal, M., Zotta, E., Ibarra, C., Rabinovitz, B. C., Vilte, D. A., Mercado, E. C., et al. (2013). Effect of coiled-coil peptides on the function of the type III secretion system-dependent activity of enterohemorragic Escherichia coli O157:H7 and citrobacter rodentium. Int. J. Med. Microbiol. 303, 9–15. doi: 10.1016/j.ijmm.2012.12.001
Lee, J. H., and Stein, B. D. (2011). Antimicrobial activity of a combination of mume fructus, schizandrae fructus, and coptidis rhizoma on enterohemorrhagic Escherichia coli O26, O111, and O157 and its effect on shiga toxin releases. Foodborne Pathog. Dis. 8, 643–646. doi: 10.1089/fpd.2010.0710
Legros, N., Pohlentz, G., Steil, D., and Muthing, J. (2018). shiga toxin-glycosphingolipid interaction: status quo of research with focus on primary human brain and kidney endothelial cells. Int. J. Med. Microbiol. 308, 1073–1084. doi: 10.1016/j.ijmm.2018.09.003
Li, Y., Frey, E., Mackenzie, A. M., and Finlay, B. B. (2000). Human response to Escherichia coli O157:H7 infection: antibodies to secreted virulence factors. Infect. Immun. 68, 5090–5095. doi: 10.1128/IAI.68.9.5090-5095.2000
Lin, R., Zhang, Y., Long, B., Li, Y., Wu, Y., Duan, S., et al. (2017a). Oral immunization with recombinant Lactobacillus acidophilus expressing espA-Tir-M confers protection against enterohemorrhagic Escherichia coli O157:H7 challenge in mice. Front. Microbiol. 8:417. doi: 10.3389/fmicb.2017.00417
Lin, R., Zhu, B., Zhang, Y., Bai, Y., Zhi, F., Long, B., et al. (2017b). Intranasal immunization with novel EspA-Tir-M fusion protein induces protective immunity against enterohemorrhagic Escherichia coli O157:H7 challenge in mice. Microb. Pathog. 105, 19–24. doi: 10.1016/j.micpath.2017.01.062
Lingwood, C. A., Law, H., Richardson, S., Petric, M., Brunton, J. L., De Grandis, S., et al. (1987). Glycolipid binding of purified and recombinant Escherichia coli produced verotoxin in vitro. J. Biol. Chem. 262, 8834–8839.
Litvak, Y., Mon, K. K. Z., Nguyen, H., Chanthavixay, G., Liou, M., Velazquez, E. M., et al. (2019). Commensal enterobacteriaceae protect against salmonella colonization through oxygen competition. Cell Host. Microbe 25, 128–139 e125. doi: 10.1016/j.chom.2018.12.003
Liu, J., Sun, Y., Feng, S., Zhu, L., Guo, X., and Qi, C. (2009). Towards an attenuated enterohemorrhagic Escherichia coli O157:H7 vaccine characterized by a deleted ler gene and containing apathogenic shiga toxins. Vaccine 27, 5929–5935. doi: 10.1016/j.vaccine.2009.07.097
Loos, S., Ahlenstiel, T., Kranz, B., Staude, H., Pape, L., Hartel, C., et al. (2012). An outbreak of shiga toxin-producing Escherichia coli O104:H4 hemolytic uremic syndrome in Germany: presentation and short-term outcome in children. Clin. Infect. Dis. 55, 753–759. doi: 10.1093/cid/cis531
Loos, S., Aulbert, W., Hoppe, B., Ahlenstiel-Grunow, T., Kranz, B., Wahl, C., et al. (2017). Intermediate follow-up of pediatric patients with hemolytic uremic syndrome during the 2011 outbreak caused by E. coli O104:H4. Clin. Infect. Dis. 64, 1637–1643. doi: 10.1093/cid/cix218
Lopez, E. L., Contrini, M. M., Glatstein, E., Gonzalez Ayala, S., Santoro, R., Allende, D., et al. (2010). Safety and pharmacokinetics of urtoxazumab, a humanized monoclonal antibody, against shiga-like toxin 2 in healthy adults and in pediatric patients infected with shiga-like toxin-producing Escherichia coli. Antimicrob. Agents Chemother. 54, 239–243. doi: 10.1128/AAC.00343-09
Loureiro, I., Frankel, G., Adu-Bobie, J., Dougan, G., Trabulsi, L. R., and Carneiro-Sampaio, M. M. (1998). Human colostrum contains IgA antibodies reactive to enteropathogenic Escherichia coli virulence-associated proteins: intimin, BfpA, EspA, and EspB. J. Pediatr. Gastroenterol. Nutr. 27, 166–171. doi: 10.1097/00005176-199808000-00007
Luan, J., Zhuang, Z., Liu, Y., Yun, K., Chen, M., and Wang, P. G. (2010). Expression of EspA in Lactococcus lactis NZ9000 and the detection of its immune effect in vivo and vitro. Immunopharmacol. Immunotoxicol. 32, 133–140. doi: 10.3109/08923970903207083
Lubitz, P., Mayr, U. B., and Lubitz, W. (2009). Applications of bacterial ghosts in biomedicine. Adv. Exp. Med. Biol. 655, 159–170. doi: 10.1007/978-1-4419-1132-2_12
Luz, D., Amaral, M. M., Sacerdoti, F., Bernal, A. M., Quintilio, W., Moro, A. M., et al. (2018). Human recombinant fab fragment neutralizes shiga toxin type 2 cytotoxic effects in vitro and in vivo. Toxins 10:508. doi: 10.3390/toxins10120508
Luz, D., Chen, G., Maranhao, A. Q., Rocha, L. B., Sidhu, S., and Piazza, R. M. (2015). Development and characterization of recombinant antibody fragments that recognize and neutralize in vitro Stx2 toxin from shiga toxin-producing Escherichia coli. PLoS ONE 10:e0120481. doi: 10.1371/journal.pone.0120481
Macconnachie, A. A., and Todd, W. T. (2004). Potential therapeutic agents for the prevention and treatment of haemolytic uraemic syndrome in shiga toxin producing Escherichia coli infection. Curr. Opin. Infect. Dis. 17, 479–482. doi: 10.1097/00001432-200410000-00013
Marcato, P., Griener, T. P., Mulvey, G. L., and Armstrong, G. D. (2005). Recombinant shiga toxin B-subunit-keyhole limpet hemocyanin conjugate vaccine protects mice from shigatoxemia. Infect. Immun. 73, 6523–6529. doi: 10.1128/IAI.73.10.6523-6529.2005
Marcato, P., Mulvey, G., Read, R. J., Vander Helm, K., Nation, P. N., and Armstrong, G. D. (2001). Immunoprophylactic potential of cloned shiga toxin 2 B subunit. J. Infect. Dis. 183, 435–443. doi: 10.1086/318080
Martinez, M. B., Taddei, C. R., Ruiz-Tagle, A., Trabulsi, L. R., and Giron, J. A. (1999). Antibody response of children with enteropathogenic Escherichia coli infection to the bundle-forming pilus and locus of enterocyte effacement-encoded virulence determinants. J. Infect. Dis. 179, 269–274. doi: 10.1086/314549
Mayr, U. B., Haller, C., Haidinger, W., Atrasheuskaya, A., Bukin, E., Lubitz, W., et al. (2005). Bacterial ghosts as an oral vaccine: a single dose of Escherichia coli O157:H7 bacterial ghosts protects mice against lethal challenge. Infect. Immun. 73, 4810–4817. doi: 10.1128/IAI.73.8.4810-4817.2005
Mayr, U. B., Kudela, P., Atrasheuskaya, A., Bukin, E., Ignatyev, G., and Lubitz, W. (2012). Rectal single dose immunization of mice with Escherichia coli O157:H7 bacterial ghosts induces efficient humoral and cellular immune responses and protects against the lethal heterologous challenge. Microb. Biotechnol. 5, 283–294. doi: 10.1111/j.1751-7915.2011.00316.x
McGannon, C. M., Fuller, C. A., and Weiss, A. A. (2010). Different classes of antibiotics differentially influence shiga toxin production. Antimicrob. Agents Chemother. 54, 3790–3798. doi: 10.1128/AAC.01783-09
Mejias, M. P., Ghersi, G., Craig, P. O., Panek, C. A., Bentancor, L. V., Baschkier, A., et al. (2013). Immunization with a chimera consisting of the B subunit of shiga toxin type 2 and brucella lumazine synthase confers total protection against shiga toxins in mice. J. Immunol. 191, 2403–2411. doi: 10.4049/jimmunol.1300999
Mejias, M. P., Hiriart, Y., Lauche, C., Fernandez-Brando, R. J., Pardo, R., Bruballa, A., et al. (2016). Development of camelid single chain antibodies against shiga toxin type 2 (Stx2) with therapeutic potential against Hemolytic Uremic Syndrome (HUS). Sci. Rep. 6:24913. doi: 10.1038/srep24913
Mellmann, A., Harmsen, D., Cummings, C. A., Zentz, E. B., Leopold, S. R., Rico, A., et al. (2011). Prospective genomic characterization of the German enterohemorrhagic Escherichia coli O104:H4 outbreak by rapid next generation sequencing technology. PLoS ONE 6:e22751. doi: 10.1371/journal.pone.0022751
Melton-Celsa, A. R. (2014). Shiga toxin (Stx) classification, structure, and function. Microbiol Spectr 2:EHEC-0024-2013. doi: 10.1128/microbiolspec.EHEC-0024-2013
Menne, J., Nitschke, M., Stingele, R., Abu-Tair, M., Beneke, J., Bramstedt, J., et al. (2012). Validation of treatment strategies for enterohaemorrhagic Escherichia coli O104:H4 induced haemolytic uraemic syndrome: case-control study. BMJ 345:e4565. doi: 10.1136/bmj.e4565
Mogna, L., Del Piano, M., Deidda, F., Nicola, S., Soattini, L., Debiaggi, R., et al. (2012). Assessment of the in vitro inhibitory activity of specific probiotic bacteria against different Escherichia coli strains. J. Clin. Gastroenterol. 46, S29–32. doi: 10.1097/MCG.0b013e31826852b7
Mohawk, K. L., Melton-Celsa, A. R., Robinson, C. M., and O'Brien, A. D. (2010). Neutralizing antibodies to shiga toxin type 2 (Stx2) reduce colonization of mice by Stx2-expressing Escherichia coli O157:H7. Vaccine 28, 4777–4785. doi: 10.1016/j.vaccine.2010.04.099
Monet-Didailler, C., Chevallier, A., Godron-Dubrasquet, A., Allard, L., Delmas, Y., Contin-Bordes, C., et al. (2019). Outcome of children with shiga toxin-associated haemolytic uraemic syndrome treated with eculizumab: a matched cohort study. Nephrol. Dial Transplant. 14:gfz158. doi: 10.1093/ndt/gfz158
Morigi, M., Galbusera, M., Gastoldi, S., Locatelli, M., Buelli, S., Pezzotta, A., et al. (2011). Alternative pathway activation of complement by shiga toxin promotes exuberant C3a formation that triggers microvascular thrombosis. J. Immunol. 187, 172–180. doi: 10.4049/jimmunol.1100491
Moxley, R. A., Francis, D. H., Tamura, M., Marx, D. B., Santiago-Mateo, K., and Zhao, M. (2017). Efficacy of urtoxazumab (TMA-15 Humanized monoclonal antibody specific for shiga toxin 2) against post-diarrheal neurological sequelae caused by Escherichia coli O157:H7 infection in the neonatal gnotobiotic piglet model. Toxins 9:49. doi: 10.3390/toxins9020049
Muhldorfer, I., Hacker, J., Keusch, G. T., Acheson, D. W., Tschape, H., Kane, A. V., et al. (1996). Regulation of the shiga-like toxin II operon in Escherichia coli. Infect. Immun. 64, 495–502. doi: 10.1128/IAI.64.2.495-502.1996
Muhlen, S., Ramming, I., Pils, M. C., Koeppel, M., Glaser, J., Leong, J., et al. (2020). Identification of antibiotics that diminish disease in a murine model of enterohemorrhagic Escherichia coli infection. Antimicrob. Agents Chemother. 64. doi: 10.1128/AAC.02159-19
Mukherjee, J., Chios, K., Fishwild, D., Hudson, D., O'donnell, S., Rich, S. M., et al. (2002a). Human Stx2-specific monoclonal antibodies prevent systemic complications of Escherichia coli O157:H7 infection. Infect. Immun. 70, 612–619. doi: 10.1128/IAI.70.2.612-619.2002
Mukherjee, J., Chios, K., Fishwild, D., Hudson, D., O'donnell, S., Rich, S. M., et al. (2002b). Production and characterization of protective human antibodies against shiga toxin 1. Infect. Immun. 70, 5896–5899. doi: 10.1128/IAI.70.10.5896-5899.2002
Mulvey, G. L., Marcato, P., Kitov, P. I., Sadowska, J., Bundle, D. R., and Armstrong, G. D. (2003). Assessment in mice of the therapeutic potential of tailored, multivalent shiga toxin carbohydrate ligands. J. Infect. Dis. 187, 640–649. doi: 10.1086/373996
Nakanishi, K., Morikane, S., Ichikawa, S., Kurohane, K., Niwa, Y., Akimoto, Y., et al. (2017). Protection of human colon cells from shiga toxin by plant-based recombinant secretory IgA. Sci. Rep. 7:45843. doi: 10.1038/srep45843
Nakanishi, K., Narimatsu, S., Ichikawa, S., Tobisawa, Y., Kurohane, K., Niwa, Y., et al. (2013). Production of hybrid-IgG/IgA plantibodies with neutralizing activity against shiga toxin 1. PLoS ONE 8:e80712. doi: 10.1371/journal.pone.0080712
Nakao, H., Kiyokawa, N., Fujimoto, J., Yamasaki, S., and Takeda, T. (1999). Monoclonal antibody to shiga toxin 2 which blocks receptor binding and neutralizes cytotoxicity. Infect. Immun. 67, 5717–5722. doi: 10.1128/IAI.67.11.5717-5722.1999
Nassar, F. J., Rahal, E. A., Sabra, A., and Matar, G. M. (2013). Effects of subinhibitory concentrations of antimicrobial agents on Escherichia coli O157:H7 shiga toxin release and role of the SOS response. Foodborne Pathog. Dis. 10, 805–812. doi: 10.1089/fpd.2013.1510
Nataro, J. P., and Kaper, J. B. (1998). Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11, 142–201. doi: 10.1128/CMR.11.1.142
Nishikawa, K., Matsuoka, K., Kita, E., Okabe, N., Mizuguchi, M., Hino, K., et al. (2002). A therapeutic agent with oriented carbohydrates for treatment of infections by shiga toxin-producing Escherichia coli O157:H7. Proc. Natl. Acad. Sci. U.S.A. 99, 7669–7674. doi: 10.1073/pnas.112058999
Nishikawa, K., Matsuoka, K., Watanabe, M., Igai, K., Hino, K., Hatano, K., et al. (2005). Identification of the optimal structure required for a shiga toxin neutralizer with oriented carbohydrates to function in the circulation. J. Infect. Dis. 191, 2097–2105. doi: 10.1086/430388
Niu, Y. D., Johnson, R. P., Xu, Y., Mcallister, T. A., Sharma, R., Louie, M., et al. (2009). Host range and lytic capability of four bacteriophages against bovine and clinical human isolates of shiga toxin-producing Escherichia coli O157:H7. J. Appl. Microbiol. 107, 646–656. doi: 10.1111/j.1365-2672.2009.04231.x
Noel, R., Gupta, N., Pons, V., Goudet, A., Garcia-Castillo, M. D., Michau, A., et al. (2013). N-methyldihydroquinazolinone derivatives of retro-2 with enhanced efficacy against shiga toxin. J. Med. Chem. 56, 3404–3413. doi: 10.1021/jm4002346
Nogueira, M. C., Oyarzabal, O. A., and Gombas, D. E. (2003). Inactivation of Escherichia coli O157:H7, Listeria monocytogenes, and Salmonella in cranberry, lemon, and lime juice concentrates. J. Food Prot. 66, 1637–1641. doi: 10.4315/0362-028X-66.9.1637
Noris, M., Mescia, F., and Remuzzi, G. (2012). STEC-HUS, atypical HUS and TTP are all diseases of complement activation. Nat. Rev. Nephrol. 8, 622–633. doi: 10.1038/nrneph.2012.195
O'Brien, A. D., Newland, J. W., Miller, S. F., Holmes, R. K., Smith, H. W., and Formal, S. B. (1984). Shiga-like toxin-converting phages from Escherichia coli strains that cause hemorrhagic colitis or infantile diarrhea. Science 226, 694–696. doi: 10.1126/science.6387911
Ochoa, T. J., Chen, J., Walker, C. M., Gonzales, E., and Cleary, T. G. (2007). Rifaximin does not induce toxin production or phage-mediated lysis of shiga toxin-producing Escherichia coli. Antimicrob. Agents Chemother. 51, 2837–2841. doi: 10.1128/AAC.01397-06
Ogawa, M., Shimizu, K., Nomoto, K., Tanaka, R., Hamabata, T., Yamasaki, S., et al. (2001). Inhibition of in vitro growth of shiga toxin-producing Escherichia coli O157:H7 by probiotic Lactobacillus strains due to production of lactic acid. Int. J. Food Microbiol. 68, 135–140. doi: 10.1016/S0168-1605(01)00465-2
Oliveira, A. F., Cardoso, S. A., Almeida, F. B., De Oliveira, L. L., Pitondo-Silva, A., Soares, S. G., et al. (2012). Oral immunization with attenuated salmonella vaccine expressing Escherichia coli O157:H7 intimin gamma triggers both systemic and mucosal humoral immunity in mice. Microbiol. Immunol. 56, 513–522. doi: 10.1111/j.1348-0421.2012.00477.x
Orth, D., Grif, K., Zimmerhackl, L. B., and Wurzner, R. (2008). Prevention and treatment of enterohemorrhagic Escherichia coli infections in humans. Expert. Rev. Anti. Infect. Ther. 6, 101–108. doi: 10.1586/14787210.6.1.101
Orth, D., Khan, A. B., Naim, A., Grif, K., Brockmeyer, J., Karch, H., et al. (2009). Shiga toxin activates complement and binds factor H: evidence for an active role of complement in hemolytic uremic syndrome. J. Immunol. 182, 6394–6400. doi: 10.4049/jimmunol.0900151
Panos, G. Z., Betsi, G. I., and Falagas, M. E. (2006). Systematic review: are antibiotics detrimental or beneficial for the treatment of patients with Escherichia coli O157:H7 infection? Aliment Pharmacol. Ther. 24, 731–742. doi: 10.1111/j.1365-2036.2006.03036.x
Pape, L., Hartmann, H., Bange, F. C., Suerbaum, S., Bueltmann, E., and Ahlenstiel-Grunow, T. (2015). Eculizumab in typical Hemolytic Uremic Syndrome (HUS) With neurological involvement. Medicine 94:e1000. doi: 10.1097/MD.0000000000001000
Patel, J., Keelara, S., and Green, J. (2018). Inactivation of Escherichia coli O157:H7 and salmonella on fresh herbs by plant essential oils. Foodborne Pathog. Dis. 15, 332–338. doi: 10.1089/fpd.2017.2377
Paton, A. W., Morona, R., and Paton, J. C. (2000). A new biological agent for treatment of shiga toxigenic Escherichia coli infections and dysentery in humans. Nat. Med. 6, 265–270. doi: 10.1038/73111
Pedersen, M. G., Hansen, C., Riise, E., Persson, S., and Olsen, K. E. (2008). Subtype-specific suppression of shiga toxin 2 released from Escherichia coli upon exposure to protein synthesis inhibitors. J. Clin. Microbiol. 46, 2987–2991. doi: 10.1128/JCM.00871-08
Pellarin, M. G., Albrecht, C., Rojas, M. J., Aguilar, J. J., Konigheim, B. S., Paraje, M. G., et al. (2013). Inhibition of cytotoxicity of shiga toxin of Escherichia coli O157:H7 on vero cells by prosopis alba griseb (fabaceae) and ziziphus mistol griseb (rhamnaceae) extracts. J. Food Prot. 76, 1733–1739. doi: 10.4315/0362-028X.JFP-13-087
Perera, L. P., Marques, L. R., and O'Brien, A. D. (1988). Isolation and characterization of monoclonal antibodies to shiga-like toxin II of enterohemorrhagic Escherichia coli and use of the monoclonal antibodies in a colony enzyme-linked immunosorbent assay. J. Clin. Microbiol. 26, 2127–2131. doi: 10.1128/JCM.26.10.2127-2131.1988
Peterson, R. E., Klopfenstein, T. J., Erickson, G. E., Folmer, J., Hinkley, S., Moxley, R. A., et al. (2007). Effect of Lactobacillus acidophilus strain NP51 on Escherichia coli O157:H7 fecal shedding and finishing performance in beef feedlot cattle. J. Food Prot 70, 287–291. doi: 10.4315/0362-028X-70.2.287
Pittman, C. I., Geornaras, I., Woerner, D. R., Nightingale, K. K., Sofos, J. N., Goodridge, L., et al. (2012). Evaluation of lactic acid as an initial and secondary subprimal intervention for Escherichia coli O157:H7, non-O157 shiga toxin-producing E. coli, and a nonpathogenic E. coli surrogate for E. coli O157:H7. J. Food. Prot. 75, 1701–1708. doi: 10.4315/0362-028X.JFP-11-520
Raa, H., Grimmer, S., Schwudke, D., Bergan, J., Walchli, S., Skotland, T., et al. (2009). Glycosphingolipid requirements for endosome-to-Golgi transport of shiga toxin. Traffic 10, 868–882. doi: 10.1111/j.1600-0854.2009.00919.x
Rad, H. S., Mousavi, S. L., Rasooli, I., Amani, J., and Nadooshan, M. R. (2013). EspA-Intimin chimeric protein, a candidate vaccine against Escherichia coli O157:H7. Iran J. Microbiol. 5, 244–251.
Rahal, E. A., Fadlallah, S. M., Nassar, F. J., Kazzi, N., and Matar, G. M. (2015). Approaches to treatment of emerging shiga toxin-producing Escherichia coli infections highlighting the O104:H4 serotype. Front. Cell Infect. Microbiol. 5:24. doi: 10.3389/fcimb.2015.00024
Rahal, E. A., Kazzi, N., Kanbar, A., Abdelnoor, A. M., and Matar, G. M. (2011a). Role of rifampicin in limiting Escherichia coli O157:H7 shiga-like toxin expression and enhancement of survival of infected BALB/c mice. Int J. Antimicrob. Agents 37, 135–139. doi: 10.1016/j.ijantimicag.2010.10.009
Rahal, E. A., Kazzi, N., Sabra, A., Abdelnoor, A. M., and Matar, G. M. (2011b). Decrease in shiga toxin expression using a minimal inhibitory concentration of rifampicin followed by bactericidal gentamicin treatment enhances survival of Escherichia coli O157:H7-infected BALB/c mice. Ann. Clin. Microbiol. Antimicrob. 10:34. doi: 10.1186/1476-0711-10-34
Ran, X. Q., Wang, H. Z., Liu, J. J., Li, S., and Wang, J. F. (2008). The immunogenicity of fusion protein linking the carboxyl terminus of the B subunit of shiga toxin 2 to the B subunit of E. coli heat-labile enterotoxin. Vet. Microbiol. 127, 209–215. doi: 10.1016/j.vetmic.2007.08.021
Raya, R. R., Varey, P., Oot, R. A., Dyen, M. R., Callaway, T. R., Edrington, T. S., et al. (2006). Isolation and characterization of a new T-even bacteriophage, CEV1, and determination of its potential to reduce Escherichia coli O157:H7 levels in sheep. Appl. Environ. Microbiol. 72, 6405–6410. doi: 10.1128/AEM.03011-05
Riquelme-Neira, R., Rivera, A., Saez, D., Fernandez, P., Osorio, G., Del Canto, F., et al. (2015). Vaccination with DNA Encoding truncated enterohemorrhagic Escherichia coli (EHEC) Factor for adherence-1 Gene (efa-1') Confers protective immunity to mice infected with E. coli O157:H7. Front. Cell Infect. Microbiol. 5:104. doi: 10.3389/fcimb.2015.00104
Ritchie, J. M., Greenwich, J. L., Davis, B. M., Bronson, R. T., Gebhart, D., Williams, S. R., et al. (2011). An Escherichia coli O157-specific engineered pyocin prevents and ameliorates infection by E. coli O157:H7 in an animal model of diarrheal disease. Antimicrob. Agents Chemother. 55, 5469–5474. doi: 10.1128/AAC.05031-11
Rivas, L., Coffey, B., Mcauliffe, O., Mcdonnell, M. J., Burgess, C. M., Coffey, A., et al. (2010). In vivo and ex vivo evaluations of bacteriophages e11/2 and e4/1c for use in the control of Escherichia coli O157:H7. Appl. Environ. Microbiol. 76, 7210–7216. doi: 10.1128/AEM.01530-10
Rojas, R. L., Gomes, P. A., Bentancor, L. V., Sbrogio-Almeida, M. E., Costa, S. O., Massis, L. M., et al. (2010). Salmonella enterica serovar typhimurium vaccine strains expressing a nontoxic shiga-like toxin 2 derivative induce partial protective immunity to the toxin expressed by enterohemorrhagic Escherichia coli. Clin. Vaccine Immunol. 17, 529–536. doi: 10.1128/CVI.00495-09
Ruano-Gallego, D., Yara, D. A., Di Ianni, L., Frankel, G., Schuller, S., and Fernandez, L. A. (2019). A nanobody targeting the translocated intimin receptor inhibits the attachment of enterohemorrhagic E. coli to human colonic mucosa. PLoS Pathog. 15:e1008031. doi: 10.1371/journal.ppat.1008031
Rund, S. A., Rohde, H., Sonnenborn, U., and Oelschlaeger, T. A. (2013). Antagonistic effects of probiotic Escherichia coli nissle 1917 on EHEC strains of serotype O104:H4 and O157:H7. Int. J. Med. Microbiol. 303, 1–8. doi: 10.1016/j.ijmm.2012.11.006
Sabouri, S., Sepehrizadeh, Z., Amirpour-Rostami, S., and Skurnik, M. (2017). A minireview on the in vitro and in vivo experiments with anti-Escherichia coli O157:H7 phages as potential biocontrol and phage therapy agents. Int. J. Food Microbiol. 243, 52–57. doi: 10.1016/j.ijfoodmicro.2016.12.004
Safwat-Mohamed, D., Farouk-Ahmed, E., Mohamed-Mahmoud, A., Abd El-Baky, R. M., and John, J. (2018). Isolation and evaluation of cocktail phages for the control of multidrug-resistant Escherichia coli serotype O104: H4 and E. coli O157: H7 isolates causing diarrhea. FEMS Microbiol. Lett. 365:fnx275. doi: 10.1093/femsle/fnx275
Sargeant, J. M., Amezcua, M. R., Rajic, A., and Waddell, L. (2007). Pre-harvest interventions to reduce the shedding of E. coli O157 in the faeces of weaned domestic ruminants: a systematic review. Zoonoses Public Health 54, 260–277. doi: 10.1111/j.1863-2378.2007.01059.x
Schmidt, N., Barth, S. A., Frahm, J., Meyer, U., Danicke, S., Geue, L., et al. (2018). Decreased STEC shedding by cattle following passive and active vaccination based on recombinant Escherichia coli shiga toxoids. Vet. Res. 49:28. doi: 10.1186/s13567-018-0523-0
Scholl, D., Cooley, M., Williams, S. R., Gebhart, D., Martin, D., Bates, A., and Mandrell, R. (2009). An engineered R-type pyocin is a highly specific and sensitive bactericidal agent for the food-borne pathogen Escherichia coli O157:H7. Antimicrob. Agents Chemother. 53, 3074–3080. doi: 10.1128/AAC.01660-08
Scholl, D., Gebhart, D., Williams, S. R., Bates, A., and Mandrell, R. (2012). Genome sequence of E. coli O104:H4 leads to rapid development of a targeted antimicrobial agent against this emerging pathogen. PLoS ONE 7:e33637. doi: 10.1371/journal.pone.0033637
Secher, T., Shima, A., Hinsinger, K., Cintrat, J. C., Johannes, L., Barbier, J., et al. (2015). Retrograde trafficking inhibitor of shiga toxins reduces morbidity and mortality of mice infected with enterohemorrhagic Escherichia coli. Antimicrob. Agents Chemother. 59, 5010–5013. doi: 10.1128/AAC.00455-15
Seita, T., Kuribayashi, T., Honjo, T., and Yamamoto, S. (2013). Comparison of efficacies of bovine immune colostral antibody and each immunoglobulin class against verotoxin 2, flagellum and somatic cells of Escherichia coli O157:H7 in mice. J. Microbiol. Immunol. Infect. 46, 73–79. doi: 10.1016/j.jmii.2012.01.002
Serna, A. T., and Boedeker, E. C. (2008). Pathogenesis and treatment of shiga toxin-producing Escherichia coli infections. Curr. Opin. Gastroenterol. 24, 38–47. doi: 10.1097/MOG.0b013e3282f2dfb8
Sewlikar, S., and D'Souza, D. H. (2017). Antimicrobial effects of quillaja saponaria extract against Escherichia coli O157:H7 and the emerging non-o157 shiga toxin-producing E. coli. J. Food Sci. 82, 1171–1177. doi: 10.1111/1750-3841.13697
Sheng, H., Knecht, H. J., Kudva, I. T., and Hovde, C. J. (2006). Application of bacteriophages to control intestinal Escherichia coli O157:H7 levels in ruminants. Appl. Environ. Microbiol. 72, 5359–5366. doi: 10.1128/AEM.00099-06
Sheng, L., Olsen, S. A., Hu, J., Yue, W., Means, W. J., and Zhu, M. J. (2016). Inhibitory effects of grape seed extract on growth, quorum sensing, and virulence factors of CDC “top-six” non-O157 shiga toxin producing E. coli. Int. J. Food Microbiol. 229, 24–32. doi: 10.1016/j.ijfoodmicro.2016.04.001
Sheoran, A. S., Chapman-Bonofiglio, S., Harvey, B. R., Mukherjee, J., Georgiou, G., Donohue-Rolfe, A., et al. (2005). Human antibody against shiga toxin 2 administered to piglets after the onset of diarrhea due to Escherichia coli O157:H7 prevents fatal systemic complications. Infect. Immun. 73, 4607–4613. doi: 10.1128/IAI.73.8.4607-4613.2005
Silberstein, C., Lucero, M. S., Zotta, E., Copeland, D. P., Lingyun, L., Repetto, H. A., et al. (2011). A glucosylceramide synthase inhibitor protects rats against the cytotoxic effects of shiga toxin 2. Pediatr. Res. 69, 390–394. doi: 10.1203/PDR.0b013e318211dd57
Skinner, C., Zhang, G., Patfield, S., and He, X. (2015). An in vitro combined antibiotic-antibody treatment eliminates toxicity from shiga toxin-producing Escherichia coli. Antimicrob. Agents Chemother. 59, 5435–5444. doi: 10.1128/AAC.00763-15
Smith, M. J., Teel, L. D., Carvalho, H. M., Melton-Celsa, A. R., and O'Brien, A. D. (2006). Development of a hybrid shiga holotoxoid vaccine to elicit heterologous protection against shiga toxins types 1 and 2. Vaccine 24, 4122–4129. doi: 10.1016/j.vaccine.2006.02.035
Sreerohini, S., Balakrishna, K., and Parida, M. (2019). Oral immunization of mice with lactococcus lactis expressing shiga toxin truncate confers enhanced protection against shiga toxins of Escherichia coli O157:H7 and Shigella dysenteriae. APMIS 127, 671–680. doi: 10.1111/apm.12983
Stanford, K., Mcallister, T. A., Niu, Y. D., Stephens, T. P., Mazzocco, A., Waddell, T. E., et al. (2010). Oral delivery systems for encapsulated bacteriophages targeted at Escherichia coli O157:H7 in feedlot cattle. J. Food Prot. 73, 1304–1312. doi: 10.4315/0362-028X-73.7.1304
Stearns-Kurosawa, D. J., Collins, V., Freeman, S., Debord, D., Nishikawa, K., Oh, S. Y., et al. (2011). Rescue from lethal shiga toxin 2-induced renal failure with a cell-permeable peptide. Pediatr. Nephrol. 26, 2031–2039. doi: 10.1007/s00467-011-1913-y
Strockbine, N. A., Marques, L. R., Holmes, R. K., and O'Brien, A. D. (1985). Characterization of monoclonal antibodies against shiga-like toxin from Escherichia coli. Infect. Immun. 50, 695–700. doi: 10.1128/IAI.50.3.695-700.1985
Takahashi, M., Taguchi, H., Yamaguchi, H., Osaki, T., Komatsu, A., and Kamiya, S. (2004). The effect of probiotic treatment with clostridium butyricum on enterohemorrhagic Escherichia coli O157:H7 infection in mice. FEMS Immunol. Med. Microbiol. 41, 219–226. doi: 10.1016/j.femsim.2004.03.010
Takemasa, N., Ohnishi, S., Tsuji, M., Shikata, T., and Yokoigawa, K. (2009). Screening and analysis of spices with ability to suppress verocytotoxin production by Escherichia coli O157. J. Food Sci. 74, M461–466. doi: 10.1111/j.1750-3841.2009.01326.x
Trachtman, H., Cnaan, A., Christen, E., Gibbs, K., Zhao, S., Acheson, D. W., et al. (2003). Effect of an oral shiga toxin-binding agent on diarrhea-associated hemolytic uremic syndrome in children: a randomized controlled trial. JAMA 290, 1337–1344. doi: 10.1001/jama.290.10.1337
Tremblay, J. M., Mukherjee, J., Leysath, C. E., Debatis, M., Ofori, K., Baldwin, K., et al. (2013). A single VHH-based toxin-neutralizing agent and an effector antibody protect mice against challenge with shiga toxins 1 and 2. Infect. Immun. 81, 4592–4603. doi: 10.1128/IAI.01033-13
Tsuji, T., Shimizu, T., Sasaki, K., Tsukamoto, K., Arimitsu, H., Ochi, S., et al. (2008). A nasal vaccine comprising B-subunit derivative of shiga toxin 2 for cross-protection against shiga toxin types 1 and 2. Vaccine 26, 2092–2099. doi: 10.1016/j.vaccine.2008.02.034
Tzipori, S., Sheoran, A., Akiyoshi, D., Donohue-Rolfe, A., and Trachtman, H. (2004). Antibody therapy in the management of shiga toxin-induced hemolytic uremic syndrome. Clin. Microbiol. Rev. 17, 926–941, table of contents. doi: 10.1128/CMR.17.4.926-941.2004
Voravuthikunchai, S. P., Suwalak, S., and Mitranan, W. (2012). Ellagitannin from quercus infectoria eradicates intestinal colonization and prevents renal injuries in mice infected with Escherichia coli O157 : H7. J. Med. Microbiol. 61, 1366–1372. doi: 10.1099/jmm.0.044495-0
Walterspiel, J. N., Ashkenazi, S., Morrow, A. L., and Cleary, T. G. (1992). Effect of subinhibitory concentrations of antibiotics on extracellular shiga-like toxin I. Infection 20, 25–29. doi: 10.1007/BF01704889
Wan, C. S., Zhou, Y., Yu, Y., Peng, L. J., Zhao, W., and Zheng, X. L. (2011). B-cell epitope KT-12 of enterohemorrhagic Escherichia coli O157:H7: a novel peptide vaccine candidate. Microbiol. Immunol. 55, 247–253. doi: 10.1111/j.1348-0421.2011.00316.x
Wang, L., Qu, K., Li, X., Cao, Z., Wang, X., Li, Z., et al. (2017). Use of bacteriophages to control Escherichia coli O157:H7 in domestic ruminants, meat products, and fruits and vegetables. Foodborne Pathog. Dis. 14, 483–493. doi: 10.1089/fpd.2016.2266
Watanabe, M., Matsuoka, K., Kita, E., Igai, K., Higashi, N., Miyagawa, A., et al. (2004). Oral therapeutic agents with highly clustered globotriose for treatment of shiga toxigenic Escherichia coli infections. J. Infect. Dis. 189, 360–368. doi: 10.1086/381124
Watanabe-Takahashi, M., Sato, T., Dohi, T., Noguchi, N., Kano, F., Murata, M., et al. (2010). An orally applicable shiga toxin neutralizer functions in the intestine to inhibit the intracellular transport of the toxin. Infect. Immun. 78, 177–183. doi: 10.1128/IAI.01022-09
Wen, S. X., Teel, L. D., Judge, N. A., and O'Brien, A. D. (2006). A plant-based oral vaccine to protect against systemic intoxication by shiga toxin type 2. Proc. Natl. Acad. Sci. U.S.A. 103, 7082–7087. doi: 10.1073/pnas.0510843103
Wisener, L. V., Sargeant, J. M., O'connor, A. M., Faires, M. C., and Glass-Kaastra, S. K. (2015). The use of direct-fed microbials to reduce shedding of Escherichia coli O157 in beef cattle: a systematic review and meta-analysis. Zoonoses Public Health 62, 75–89. doi: 10.1111/zph.12112
Yamagami, S., Motoki, M., Kimura, T., Izumi, H., Takeda, T., Katsuura, Y., and Matsumoto, Y. (2001). Efficacy of postinfection treatment with anti-shiga toxin (Stx) 2 humanized monoclonal antibody TMA-15 in mice lethally challenged with Stx-producing Escherichia coli. J. Infect. Dis. 184, 738–742. doi: 10.1086/323082
Yoh, M., Frimpong, E. K., and Honda, T. (1997). Effect of antimicrobial agents, especially fosfomycin, on the production and release of Vero toxin by enterohaemorrhagic Escherichia coli O157:H7. FEMS Immunol. Med. Microbiol. 19, 57–64. doi: 10.1111/j.1574-695X.1997.tb01072.x
Yoh, M., Frimpong, E. K., Voravuthikunchai, S. P., and Honda, T. (1999). Effect of subinhibitory concentrations of antimicrobial agents (quinolones and macrolide) on the production of verotoxin by enterohemorrhagic Escherichia coli O157:H7. Can J. Microbiol. 45, 732–739. doi: 10.1139/w99-069
Yoshimura, K., Fujii, J., Taniguchi, H., and Yoshida, S. (1999). Chemotherapy for enterohemorrhagic Escherichia coli O157:H infection in a mouse model. FEMS Immunol. Med. Microbiol. 26, 101–108. doi: 10.1111/j.1574-695X.1999.tb01376.x
Yu, S., Gu, J., Wang, H. G., Wang, Q. X., Luo, P., Wu, C., et al. (2011). Identification of a novel linear epitope on EspA from enterohemorrhagic E. coli using a neutralizing and protective monoclonal antibody. Clin. Immunol. 138, 77–84. doi: 10.1016/j.clim.2010.09.009
Zadravec, P., Mareckova, L., Petrokova, H., Hodnik, V., Perisic Nanut, M., Anderluh, G., et al. (2016). Development of recombinant lactococcus lactis displaying albumin-binding domain variants against shiga toxin 1 B Subunit. PLoS ONE 11:e0162625. doi: 10.1371/journal.pone.0162625
Zangari, T., Melton-Celsa, A. R., Panda, A., Smith, M. A., Tatarov, I., De Tolla, L., et al. (2014). Enhanced virulence of the Escherichia coli O157:H7 spinach-associated outbreak strain in two animal models is associated with higher levels of Stx2 production after induction with ciprofloxacin. Infect. Immun. 82, 4968–4977. doi: 10.1128/IAI.02361-14
Zhang, Q., Donohue-Rolfe, A., Krautz-Peterson, G., Sevo, M., Parry, N., Abeijon, C., et al. (2009). Gnotobiotic piglet infection model for evaluating the safe use of antibiotics against Escherichia coli O157:H7 infection. J. Infect. Dis. 199, 486–493. doi: 10.1086/596509
Zhang, X., Mcdaniel, A. D., Wolf, L. E., Keusch, G. T., Waldor, M. K., and Acheson, D. W. (2000). Quinolone antibiotics induce shiga toxin-encoding bacteriophages, toxin production, and death in mice. J. Infect. Dis. 181, 664–670. doi: 10.1086/315239
Keywords: STEC, Shiga toxin, antibiotics, antibodies, vaccines
Citation: Mühlen S and Dersch P (2020) Treatment Strategies for Infections With Shiga Toxin-Producing Escherichia coli. Front. Cell. Infect. Microbiol. 10:169. doi: 10.3389/fcimb.2020.00169
Received: 10 February 2020; Accepted: 31 March 2020;
Published: 06 May 2020.
Edited by:
Tânia Aparecida Tardelli Gomes, Federal University of São Paulo, BrazilReviewed by:
Marina Sandra Palermo, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), ArgentinaVernon L. Tesh, Texas A&M University, United States
Copyright © 2020 Mühlen and Dersch. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Petra Dersch, cGV0cmEuZGVyc2NoJiN4MDAwNDA7dW5pLW11ZW5zdGVyLmRl
 Sabrina Mühlen
Sabrina Mühlen Petra Dersch
Petra Dersch