- 1Faculdade de Ciências Farmacêuticas de Ribeirão Preto, Universidade de São Paulo, São Paulo, Brazil
- 2Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil
- 3Laboratory of Clinical Bacteriology and Mycology, Department of Microbiology and Immunology, KU Leuven, Leuven, Belgium
There are only few drugs available to treat fungal infections, and the lack of new antifungals, along with the emergence of drug-resistant strains, results in millions of deaths/year. An unconventional approach to fight microbial infection is to exploit nutritional vulnerabilities of microorganism metabolism. The metal gallium can disrupt iron metabolism in bacteria and cancer cells, but it has not been tested against fungal pathogens such as Aspergillus and Candida. Here, we investigate in vitro activity of gallium nitrate III [Ga(NO3)3] against these human pathogens, to reveal the gallium mechanism of action and understand the interaction between gallium and clinical antifungal drugs. Ga(NO3)3 presented a fungistatic effect against azole-sensitive and -resistant A. fumigatus strains (MIC50/90 = 32.0 mg/L) and also had a synergistic effect with caspofungin, but not with azoles and amphotericin B. Its antifungal activity seems to be reliant on iron-limiting conditions, as the presence of iron increases its MIC value and because we observed a synergistic interaction between gallium and iron chelators against A. fumigatus. We also show that an A. fumigatus mutant (ΔhapX) unable to grow in the absence of iron is more susceptible to gallium, reinforcing that gallium could act by disrupting iron homeostasis. Furthermore, we demonstrate that gallium has a fungistatic effect against different species of Candida ranging from 16.0 to 256.0 mg/L, including multidrug-resistant Candida auris, C. haemulonii, C. duobushaemulonii, and C. glabrata. Our findings indicate that gallium can inhibit fungal pathogens in vitro under iron-limiting conditions, showing that Ga(NO3)3 could be a potential therapy not only against bacteria but also as an antifungal drug.
Introduction
Fungal infection is an underestimated threat that affects over one billion people with a mortality rate higher than 1.6 million of people per year, similar to that estimated for tuberculosis and several fold greater than malaria (Brown et al., 2012a; Almeida et al., 2019). The four most fatal fungi isolated during clinical practices are species of Candida, Cryptococcus, Aspergillus, and Pneumocystis (Brown et al., 2012b). These microorganisms can cause severe and often fatal systemic infections without appropriate therapy (Perfect, 2017).
Currently, there are few available drugs to treat severe fungal infections that belong to classes of azole, pyrimidine analogs, polyene, or echinocandin antifungals (Perfect, 2017). However, the disadvantages of current antifungals include high toxicity, limited capability to inhibit multiple fungal cell targets, rapid development of drug resistance when used in single therapy, and low half-life (Perfect and Bicanic, 2015; Nett and Andes, 2016; Perfect, 2017). These factors, combined with the emergence of new multidrug-resistant species such as Candida auris, may explain why therapies against severe mycoses remain unsatisfactory. In this context, it is essential to search and develop new antifungal drugs (Perfect, 2017).
Gallium is a group IIIA metal in the periodic table of elements with several medical applications including its use as a diagnostic and therapeutic agent in cancer and disorders of calcium and bone metabolism (Chitambar, 2010). Furthermore, it has been demonstrated that gallium compounds, such as Ga(NO3)3 (gallium nitrate III), have antibacterial effects against Mycobaterium (Olakanmi et al., 2000), Francisella tulerens (Olakanmi et al., 2009), Acinetobacter baumannii (Antunes et al., 2012), Klebsiella pneumoniae (Thompson et al., 2015), Staphylococcus aureus (Richter et al., 2017), and Pseudomonas aeruginosa (Goss et al., 2018).
Gallium has a nearly identical ionic radius to iron and can be taken up by cellular iron transport systems and can replace it in iron-containing proteins (Chitambar and Narasimhan, 1991; Goss et al., 2018). Unlike iron, gallium cannot be reduced in physiological conditions (Apseloff, 1999), which inhibits the functionality of gallium-complexed proteins and arrests cell growth (Goss et al., 2018). Thus, this element acts as a “Trojan horse” by disrupting the structure of proteins that incorporate iron in both bacteria and cancer cells (Chitambar, 2010; Goss et al., 2018).
Despite its known antibacterial and antitumoral activity, to the best of our knowledge, the antifungal effect of gallium has been poorly reported (Bastos et al., 2010). Here, we tested in vitro gallium activity against A. fumigatus and Candida, performed experiments aiming to understand gallium mechanism of action, and studied the interaction between gallium and systemic antifungal drugs.
Materials and Methods
Microorganisms, Media, and Drugs
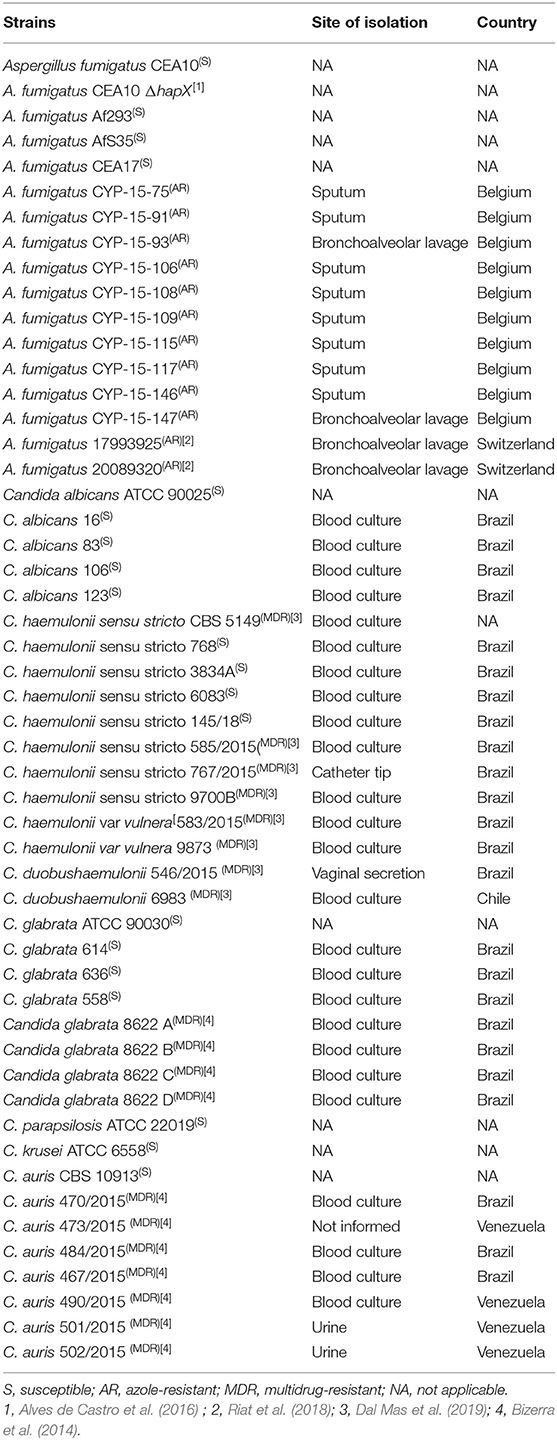
All of the fungal strains used in this work are described in Table 1. Azole-resistant Aspergillus fumigatus strains were isolated from different sources in Belgium or Switzerland. Susceptible Candida strains are reference lineages or isolates from patients from São Paulo Hospital (Brazil) and belonging to the collection of the Special Mycology Laboratory, São Paulo Federal University. Multidrug-resistant Candida isolates were previously characterized by our group as resistant to at least two classes of antifungal drugs (Bizerra et al., 2014; Dal Mas et al., 2019). All strains were maintained in glycerol 10% at −80°C until they were used. Strains were grown either in YPD (1% w/v yeast extract and 2% peptone and dextrose) (yeast strains) or glucose minimal medium (GMM) (1% w/v glucose, 50 ml/L of a 20× salt solution, 1 ml/L of 5× trace elements, pH 6.5) (A. fumigatus) (Alves de Castro et al., 2016). Iron-depleted MM (adapted minimal medium, AMM) was prepared in the same manner as GMM except that all iron-containing compounds were taken out from the trace elements (Alves de Castro et al., 2016). If required, agar was added to a final concentration of 1.7% w/v or 2% w/v to the GMM or YPD, respectively. We also used RPMI-1640 test medium (Gibco) buffered with morpholinepropanesulfonic acid (Sigma-Aldrich). All growth was carried out at 37°C for 24–48 h.
We purchased gallium nitrate III [Ga(NO3)3] and systemic antifungal drugs, posaconazole, voriconazole, caspofungin, and amphotericin B from Sigma-Aldrich. Gallium nitrate and caspofungin were dissolved in water, and the other drugs were dissolved in dimethyl sulfoxide (DMSO) (Sigma-Aldrich).
Antifungal Activity Assays
Minimum inhibitory concentration (MIC) of gallium nitrate was determined using RPMI-1640 based on M38-A2 (molds) (Clinical Laboratory Standards Institute, 2017a) or M27-A3 (Clinical Laboratory Standards Institute, 2017b) (yeasts) protocols of the Clinical and Laboratory Standards Institute (CLSI) broth microdilution for antifungal susceptibility assays. GMM and AMM also were used when stated. MIC was determined as the lowest concentration of gallium or other drugs that visually inhibited 100% (except when stated) fungal growth.
After performing the MIC assay, we checked if gallium nitrate has a fungistatic or fungicidal effect. Aliquots of 100 μl were removed from the wells where there was no visible growth and from the original inoculum and then subcultured in GMM or YPD and incubated at 37°C for 2 days. Samples were seeded in Petri dishes in duplicate. The compound was classified as fungicide if it was able to reduce 99% of fungal load comparing it with the initial inoculum; otherwise, it was considered fungistatic.
Killing Assay
Killing curves were obtained by following a procedure described previously with some modifications (Öz et al., 2016). Briefly, microplates containing 1-, 4-, and 16-fold higher than the MIC of gallium, diluted in RPMI, were prepared. Then, 100 μl of an inoculum suspension at the concentration of 2 × 106 was added to each well. The plates were incubated at 37°C for 2, 8, 16, 24, or 48 h before measurements of cellular viability with XTT-menadione. Each plate was taken from the incubator 2 h prior to the end of the incubation time, and 50 μl of XTT-menadione (1 mg/ml XTT with 125 μM menadione in saline) solution was added to each well. After 2 h of incubation with XTT-menadione, the plates were centrifuged (3,000 × g, 10 min), the supernatant was replaced in a new microplate, and the plates were read at 492 nm with a microplate reader. The experiment was performed with four replicates, and the result was evaluated by comparing the absorbance in the growth in the presence of gallium with the negative control conditions (without gallium).
Antifungal Drug Combination Activity Assay
We tested combination between gallium nitrate and antifungal drugs against A. fumigatus conidia using a checkerboard microdilution method (Santos et al., 2006), which provides a matrix of all possible drug combinations in the required concentration range. The concentrations ranged from 0.125 to 64.0 mg/L for gallium, 0.125–8.0 mg/L for posaconazole and voriconazole, 4.0–256.0 mg/L for caspofungin, and 0.5–32.0 mg/L for amphotericin B. Briefly, 50 μl of each dilution of clinical antifungals and gallium was added to 96-well plates in the horizontal and vertical orientation, respectively. Then, 100 μl of the inoculum (1 × 104 conidia/ml) was added to the plate containing various combinations of drug and gallium concentrations. The plates were incubated at 37°C during 48 h and one plate was used to test each strain. The MIC endpoint was 100% of growth inhibition. The interaction was quantitatively evaluated by determining the fractional inhibitory concentration index (FICI): FICI = [MIC gallium in combination/MIC gallium] + [MIC clinical drug in combination/MIC clinical drug]. The FICI was calculated for all of the possible combinations of different concentrations for the same isolate and the final result was expressed as the mean of the FICIs (Gomez-lopez et al., 2003). Also, interaction curves were constructed. The interaction between these drugs was classified as synergism if FICI ≤ 0.5, indifferent if 0.5 < FICI ≤ 4.0, and antagonism for FICI > 4.0 (Odds, 2003). Wells of the checkerboard plates with the combination between gallium and caspofungin were also recorded using Tucsen (ISH500) camera coupled to a Nikon (eclipse E100) microscope.
The interaction between iron and gallium was evaluated using the checkerboard methodology using RPMI-1640 and AMM media. First, the FICI for the interaction of gallium (0.125–64.0 mg/L) with the iron chelator Bathophenanthrolinedisulfonic acid (4,7-diphenyl-1,10-phenanthrolinedisulfonic acid [BPS] (Sigma-Aldrich) (7.8–500 μM) was calculated. Further, we combined different concentrations of gallium and FeSO4 (0.007–0.5% w/v) in microplates. After 48 h at 37°C, we analyzed the MIC of gallium in presence of iron and imaged the wells (plates with RPMI) as described before.
Radial Growth and Caspofungin Paradoxical Effect (CPE) Test
To determine radial growth in the presence of gallium, wild-type and ΔhapX strains from A. fumigatus were grown from 105 spores for 5 days on plates containing AMM supplemented with different concentrations of gallium (16.0–512.0 mg/L). Growth results were expressed as ratios, dividing colony radial diameter (cm) of growth in the gallium condition by colony radial diameter in the control (no gallium) (Ries et al., 2017).
In order to study if Ga(NO3)3 can interfere in CPE, we determined the radial growth of wild-type strain in AMM supplemented with caspofungin at a lower (1 mg/L) and higher (8 mg/L) concentration combined or not with gallium at sub-inhibitory concentration.
All radial growth experiment was done in triplicate.
Statistical Analysis
All experiments were repeated at least twice. All statistical analyses were performed using GraphPad Prism, version 8.00, for Windows (GraphPad Software, San Diego, CA, USA) with P < 0.05 considered significant. CPE interference was analyzed by Student's t-test and radial growth in the presence of gallium by two-way ANOVA followed by Bonferroni test.
Results
Gallium Has a Fungistatic Effect Against Azole-Sensitive and -Resistant Aspergillus fumigatus Strains
We tested if gallium nitrate III [Ga(NO3)3] could inhibit A. fumigatus growth by determining the MIC in microdilution plates. We used azole-sensitive A. fumigatus strains common in laboratory practice (CEA10, CEA17, Af293, and AfS35) and also azole-resistant isolates, with different resistance mechanisms, cultured from different sample sites from patients from Belgium and Switzerland (Table 1) (MIC for azole drugs in Table S1). The most common azole-resistance mechanisms include amino acid substitutions in the target Cyp51A protein and tandem repeat sequence insertions at the cyp51A promoter (Hagiwara et al., 2016). The cyp51A gene is not mutated in the Belgian strains (except CYP-15-91 in which cyp51A was not sequenced), suggesting different mechanisms of azole resistance. In contrast, strains 1799392 and 20089320 isolated from Switzerland have TR34 tandem repeats at the cyp51 promoter region and L98H amino acid replacement at the Cyp51A (Riat et al., 2018).
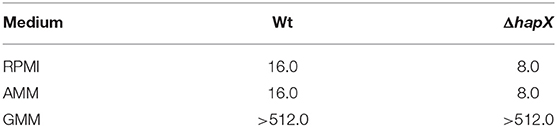
Initially, the antifungal effect of gallium was tested in two media, GMM and RPMI. Growth inhibition was observed only in RPMI, with both MIC50 and MIC90 = 32.0 mg/L (MIC values that inhibited 50 and 90% of the strains, respectively) (Table 2). Furthermore, azole-resistant strains had MIC one dilution higher than susceptible isolated when RPMI was used (Table 2). Subsequently, we plated the content of those wells where the fungi had not visibly grown after MIC assay in RPMI to determine whether gallium has a fungistatic or fungicidal effect. Ga(NO3)3 was classified as fungistatic as it did not reduce 99% of fungal load compared to initial inoculum for all of the strains.
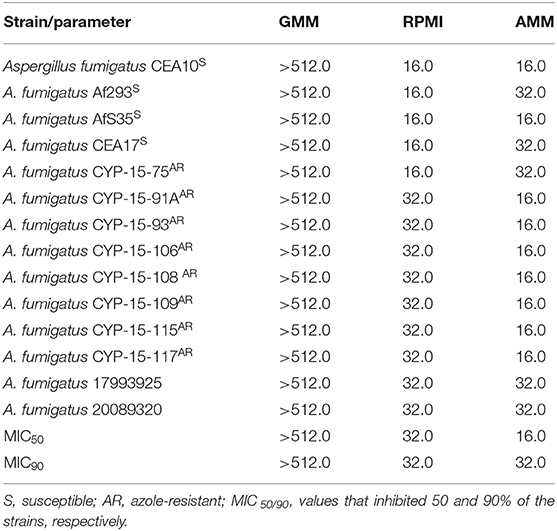
Table 2. Minimum inhibitory concentration (MIC, mg/L) of gallium nitrate for A. fumigatus strains in glucose minimal medium (GMM), iron-depleted minimal medium (AMM), and RPMI.
Kinetics of the Action of Gallium
To evaluate the kinetics of the action of gallium, A. fumigatus CEA10 was selected to test cell viability using XTT (Figure 1). We observed that increasing the gallium concentration has significant influence (P < 0.05) on the time to reduce the fungal viability. The best inhibitory effect of gallium was achieved at 24 h for all concentrations tested. At the highest concentration (MIC 16 ×), gallium was able to reduce 80% of the metabolic activity at 24 h, which was maintained until 48 h. However, at the other concentrations, part of metabolic activity was recovered after 24 h.
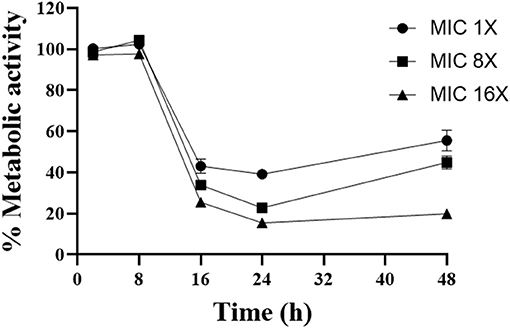
Figure 1. Time kill curve performed with different concentrations of gallium nitrate (1-, 4-, 16-fold MIC) against A. fumigatus CEA10.
Synergistic Effect Between Gallium and Caspofungin Against A. fumigatus CEA10
Combination of drugs is a potent alternative treatment used as a therapy against fungal infection (Perfect, 2017). In this context, drugs can have three types of interactions: antagonistic, indifferent, and synergistic (Odds, 2003). In order to study the effect of combining gallium with antifungal drugs used to treat aspergillosis (voriconazole, posaconazole, amphotericin B, and caspofungin), we determined the FICI.
Gallium presented indifferent interactions with voriconazole, posaconazole, and amphotericin B (0.5 < FICI ≥ 4.0) (Figures 2A–C), but a synergistic interaction (FICI ≤ 0.5) with caspofungin (32.0 mg/L of gallium and 8 mg/L of caspofungin) (Figure 2D). This synergistic interaction became more evident when we imaged the wells from the checkerboard assay (Figure 3). When the conidia were challenged with both drugs (from column 2 and row B), hyphal growth decreased compared to the treatment with single drugs and the growth control (Figure 3).
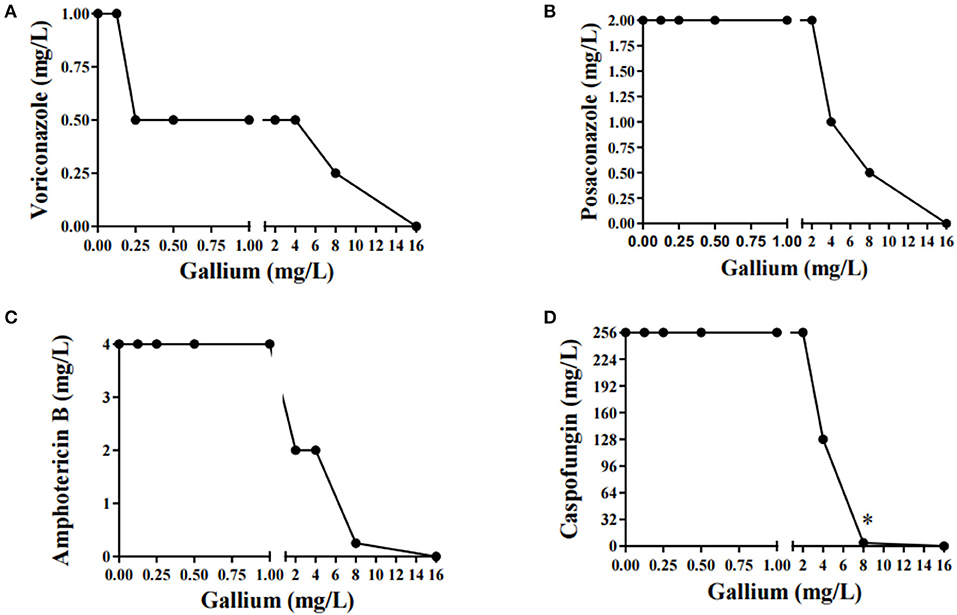
Figure 2. Combination curve of gallium and voriconazole (A), posaconazole (B), amphotericin B (C), and caspofungin (D) against A. fumigatus CEA10. *synergism.
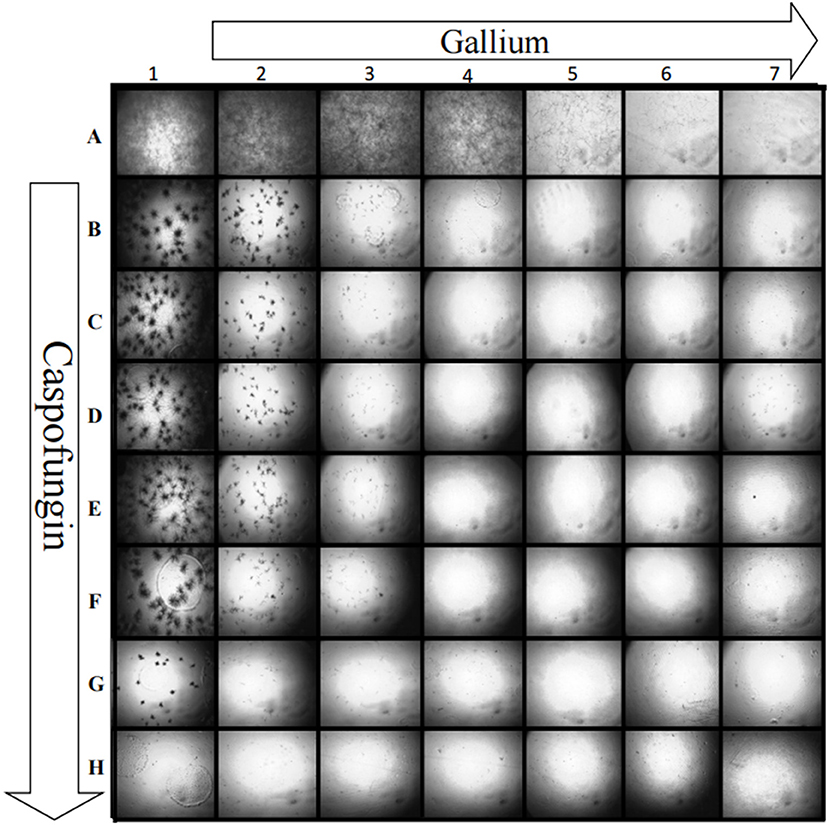
Figure 3. Growth of A. fumigatus CEA10 in checkerboard assay showing that when gallium (row) is combined with caspofungin (column), there is a visible decrease in the hyphae growth compared to the single drug challenge (column A and row 1). Image A1 shows the growth control, without drugs. Increase = 50×.
CPE Is Affected by Gallium
Since gallium had a synergistic interaction with caspofungin in A. fumigatus CEA10, we evaluated whether it would affect the CPE, which is characterized by reduced activity of the drug at higher concentrations (Ries et al., 2017). In order to test that, first we determined the MIC of gallium in solid AMM. Then, we quantified the radial growth of the spores grown in AMM plus caspofungin, at lower (1 mg/L) and higher (8 mg/L) concentrations, and gallium at MIC/2 (64 mg/L) concentration. Figure 4 shows that A. fumigatus CEA10, independently of the presence of gallium, grew better at the highest concentration of caspofungin than at the lowest. However, the difference between the growth at lower and higher concentration of caspofungin is decreased by gallium (Figure 4), indicating that [Ga(NO3)3] interferes in CPE in A. fumigatus CEA10.
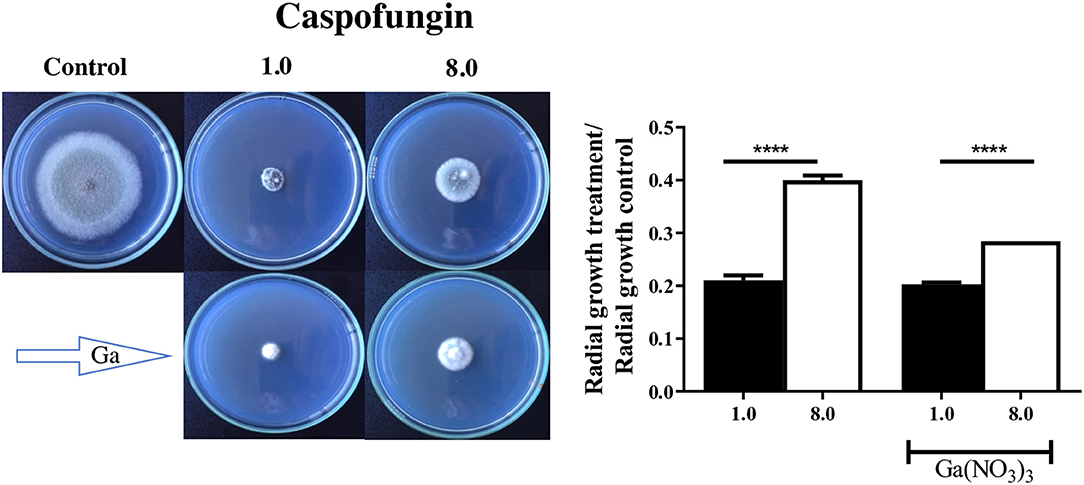
Figure 4. Gallium was combined with caspofungin at lower (1 mg/L) and higher (8 mg/L) concentrations. Radial growth indicates that gallium decreased caspofungin paradoxical effect in A. fumigatus CEA10. ****P < 0.0001.
Antifungal Effect of Gallium Is Dependent on Iron Concentration in the Medium
Our results have shown that gallium cannot inhibit A. fumigatus in GMM (MIC > 512.0 mg/L) (Table 2), suggesting that some components in the medium may interfere with gallium antifungal effect. In addition, it has been reported that gallium exerts its antibacterial and anticancer effect by disrupting iron metabolism (Chitambar, 2010; Goss et al., 2018). Thus, we investigated if the absence of inhibition in GMM would be related to the presence of iron in the medium by performing MIC tests in GMM without iron. In iron-depleted GMM, gallium inhibited A. fumigatus growth with MIC50 and MIC90 = 16.0 and 32.0 mg/L, respectively, similar values observed when the test was done in RPMI (Table 2).
To confirm that iron interferes with the anti-Aspergillus effect of gallium, conidia from A. fumigatus CEA10 were inoculated in microplates containing AMM and RPMI with combined concentrations of gallium and FeSO4. Figure 5A shows that the gallium concentration able to inhibit fungal growth in the absence of iron was 16.0 mg/L in both media. However, with iron addition, the gallium MIC increased, especially when RPMI was used (Figure 5A). Images from the wells (plates with RPMI) also confirmed that increased concentrations of iron negatively affect the antifungal activity of gallium against A. fumigatus CEA10 (Figure 5B).
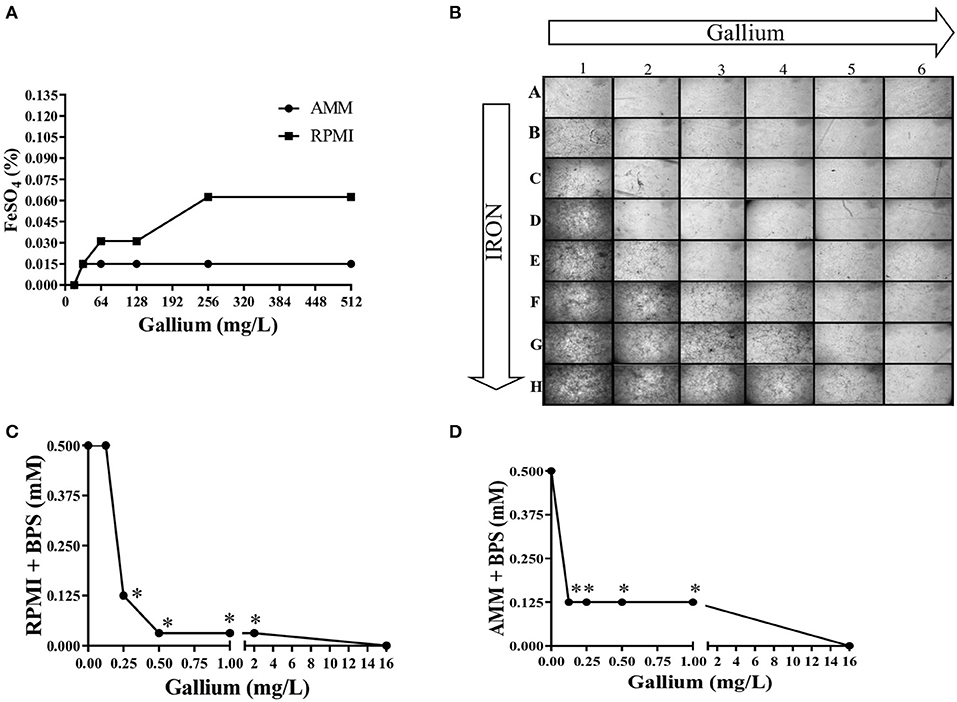
Figure 5. Iron presence interferes in gallium anti-Aspergillus effect. The MIC of gallium for against A. fumigatus CEA10 increases adding FeSO4 (A,B). Combination between gallium and BPS (iron chelator) has a strong synergism in RPMI (C) and iron-depleted medium (D). Increase = 50×. *synergism.
Combination Between Gallium and Iron-Chelator Is Synergistic in A. fumigatus
We tested the combination of BPS, an iron chelator, and gallium in RPMI and AMM and calculated the FICI. The FICI mean was 0.33 and 0.28 for the combination in RPMI and AMM, respectively, indicating a strong synergism between gallium and the iron chelator (BPS) (Figures 5C,D). Our results indicate that iron-limiting conditions enhance the gallium antifungal activity. To investigate whether the fungistatic effect of gallium is related to iron metabolism, we used an A. fumigatus ΔhapX mutant. HapX is an essential transcription factor for iron assimilation under iron starvation conditions (Haas, 2014). We observed that the MIC of gallium for ΔhapX was slightly lower than for the wild-type strain (8.0 vs. 16.0 mg/L) in RPMI and AMM (Table 3), but there was no inhibition in iron-containing medium (GMM) (Table 3). As the MIC between the strains were not substantially different and could be due to impaired growth of the mutant in the media, we also calculated the normalized radial growth in AMM supplemented with gallium. The growth of ΔhapX was significantly lower than the wild-type strain at all concentrations of gallium tested (Figure 6). Together, these data show that the gallium anti-Aspergillus effect is more pronounced in an iron-deficient strain.
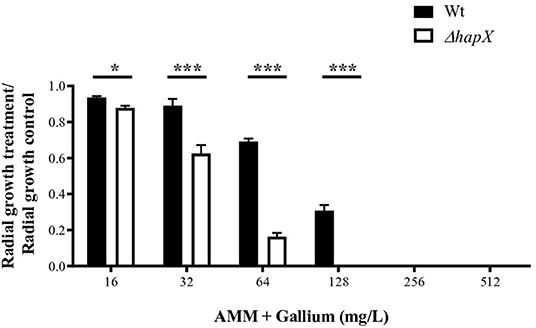
Figure 6. Radial growth of wt and ΔhapX in AMM with different concentrations of gallium showing that the mutant is more susceptible to gallium. *P < 0.05; ***P < 0.001.
We also tested the combination between gallium and systemic antifungals against ΔhapX. FICI showed that gallium interacts indifferently with voriconazole, posaconazole, and amphotericin B in ΔhapX as well (Table 3). The interaction with caspofungin, however, was synergistic and more evident in ΔhapX than in the wild-type strain (FICI means = 0.47 vs. 0.78, respectively) (Table 4).
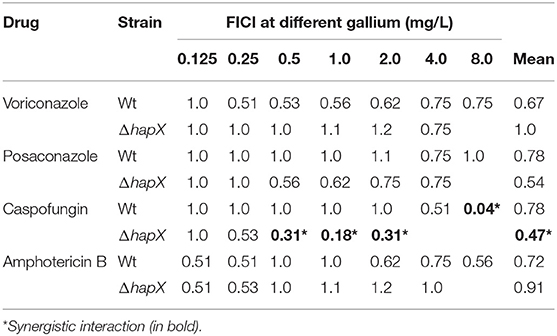
Table 4. Fractional inhibitory concentration index (FICI) of gallium nitrate and azoles, caspofungin, and amphotericin B against A. fumigatus wild-type and ΔhapX.
Antifungal Effect of Gallium Against Other Fungal Pathogens
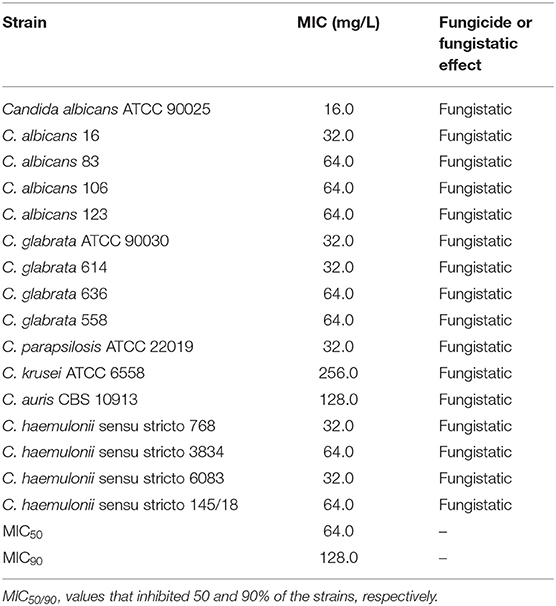
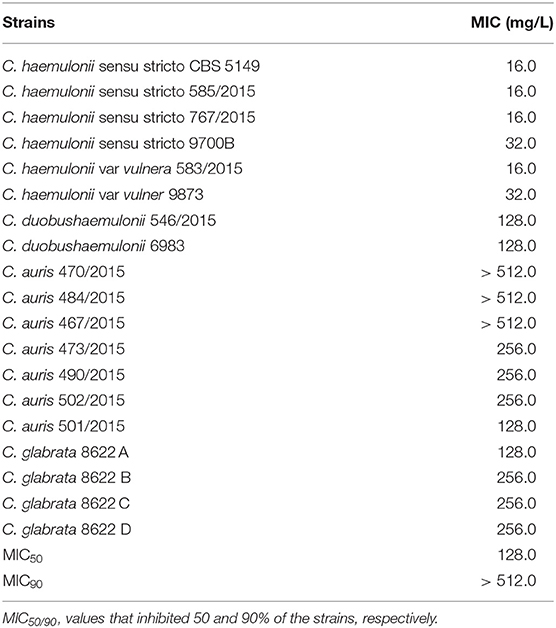
We investigated the inhibition of other important fungal pathogens. The MIC of gallium in RPMI for different species of drug-susceptible Candida (MIC for antifungal drugs in Table S2) ranged from 16.0 to 256.0 mg/L (MIC50 = 64.0 and MIC90 = 128.0 mg/ml), being more effective against C. albicans, C. haemulonii, C. glabrata, and C. parapsilosis (Table 5). The antifungal effect of gallium was also tested against multidrug resistant Candida. The lower MIC was obtained against multidrug-resistant C. haemulonii strains (range 16.0–32.0 mg/L) (Table 6). For C. duobushaemulonii, 128.0 mg/L of gallium was able to inhibit the fungal growth and for multidrug-resistant C. glabrata strains, the MIC range was 128–256 mg/L (Table 6). Regarding C. auris, the strains 473/2015, 490/2015, 501/2015, and 502/2015 were completely inhibited by gallium at concentration of 128–256 mg/L. However, the strains 467/2015, 470/2015, and 484/2015 survived the challenge at the higher concentration tested (Table 6). For all pathogens, gallium presented a fungistatic activity (Tables 5, 6). These results indicate that gallium has a potential antifungal activity against several important fungal pathogens.
Discussion
Fungal infections kill millions of people annually (Almeida et al., 2019). This high fatality rate can be explained by a combination of factors including host conditions, time to diagnose the infection, as well as the limited antifungal drugs available and the emergence of resistant strains (Colombo et al., 2017; Perfect, 2017). An unconventional approach to fight fungal infection is to exploit nutritional vulnerabilities of the fungal metabolism (Li et al., 2018), similar to what has been used in cancer and antibacterial therapies (Chitambar, 2010; Goss et al., 2018).
In this paper, we have tested the gallium nitrate III antifungal effect against azole-sensitive and -resistant A. fumigatus strains. Our results showed that gallium presents a fungistatic activity against A. fumigatus conidia and its inhibitory effect is drug-concentration-dependent as revealed by the time kill curve. Gallium anti-Aspergillus effect appears to be hampered by the presence of iron since it is unable to inhibit fungal growth in medium containing iron. This result is in agreement with other studies showing that gallium can inhibit bacterial growth only in iron-depleted medium (Chitambar, 2010; Goss et al., 2018). To verify the hypothesis that gallium can interfere in cell iron homeostasis in A. fumigatus, first we challenged the conidia with different concentrations of gallium at increasing levels of FeSO3 and we observed that iron presence negatively affected the antifungal activity. Then, we detected a strong synergism when an iron chelator (BPS) was combined with gallium, proving that gallium antifungal activity is iron-dependent.
The redox properties of iron are important in several cellular processes, such as electron transport chains, respiration, DNA synthesis, Krebs cycle, and oxygen transport/storage (Li et al., 2018). This importance shows why pathogens require adequate iron concentration inside the host to grow and express their virulence factors. On the other hand, hosts limit iron by compartmentalization or through iron complexation with proteins, such as hemoglobin, transferrin, and lactoferrin (Haas, 2014; Li et al., 2018). Furthermore, additional iron restriction mechanisms occur during infection in a process named “nutritional immunity” (Cassat and Skaar, 2013). The activity of these host defenses, together with the insolubility of iron in aerobic environments, explain the extremely low iron concentration found in vivo (~10−20 M) (Bullen et al., 2005).
To counterbalance the iron limitation inside the host, A. fumigatus acquired mechanisms for obtaining iron, which are controlled at the transcriptional level by two transcription factors, SreA (important for iron-replete conditions) and HapX (important for iron-depletion condition) (Haas, 2014). In our work, we used ΔhapX to investigate if a strain unable to grow in total absence of iron would be more susceptible to gallium. Our findings showed that this transcription factor is important to tolerate more gallium because the mutant presented lower MIC and radial growth than the wild-type strain.
Antifungal combination is a common approach aiming to (i) potentialize the antimicrobial effect; (ii) reduce the dose of single drug usage with increased drug efficacy, consequently decreasing the drug toxicity; and (iii) hinder the development of resistant strains (Spitzer et al., 2017; Simm and May, 2019). We tested the combination between gallium and antifungal agents against A. fumigatus and observed that gallium had a synergistic effect with caspofungin, a second-line therapy against invasive aspergillosis. In addition, gallium decreased the CPE. Other studies have also exploited and proved the synergistic effect between substances that affect iron homeostasis and azoles (Gautam et al., 2011; Simm and May, 2019), unlike gallium, which had the same effect only with caspofungin.
The most severe, common, and difficult-to-treat mycoses are those caused by three fungi: A. fumigatus, Candida spp., and Cryptococcus spp. (Brown et al., 2012a; Perfect, 2017). As gallium had antifungal activity against A. fumigatus, we tested it against Candida. Gallium inhibited the growth of all species tested, being more effective against C. albicans, C. haemulonii, C. glabrata, and C. parapsilosis. These differences may reflect distinct forms of how these species combat iron disruption inside the cell. Furthermore, gallium inhibited multidrug-resistant C. haemulonii, C. duobushaemulonii, C. glabrata, and C. auris strains, demonstrating that it may be a potential agent for treating infections caused by emergent pathogens.
We were able to demonstrate that gallium nitrate III exhibits in vitro antifungal inhibitory activity against human fungal pathogens, including azole-resistant A. fumigatus and multidrug-resistant Candida strains. More studies need to be done addressing whether the effect that we have reported also manifests in vivo. In addition, it could be interesting to investigate if gallium nitrate could act against bacteria and fungi in polymicrobial infection, as those that frequently happen in patients with cystic fibrosis (Zhao and Yu, 2018).
In this study, we have investigated the antifungal effect of gallium nitrate III, which is an FDA-approved drug (GaniteTM), and the first generation of gallium compounds. Additionally, there are several other gallium-based metallodrugs and novel gallium agents available and in development (Chitambar, 2010; Hijazi et al., 2018) that can exert distinct antimicrobial effects (Hijazi et al., 2018). In the future, studies should focus on testing if other gallium compounds are more effective and specific against fungal cells and the safety of those drugs for the host.
In conclusion, gallium has a fungistatic effect against mold and yeasts, like A. fumigatus and Candida spp. This effect seems to be due to iron disruption caused by gallium inside the cell. In addition, it has a synergistic interaction with caspofungin against A. fumigatus.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
RB and GG conceived and designed the experiments. RB, LR, and CV performed the experiments. RB, LR, CV, and GG analyzed the data. GG, KL, and AC contributed reagents, materials, and analysis tools. RB, AC, and GG contributed to the writing of the manuscript.
Funding
This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Grant Nos. 2016/07870-9, 2017-19821-5, 2017/19095-2, and 2017/02203-7) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), both from Brazil.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Taylor Schoen for the critical reading of the manuscript, Dr. Hubertus Haas for sending the A. fumigatus ΔhapX mutant and the two reviewers for their comments and suggestions.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2019.00414/full#supplementary-material
References
Almeida, F., Rodrigues, M. L., and Coelho, C. (2019). The still underestimated problem of fungal diseases worldwide. Front. Microbiol. 10:214. doi: 10.3389/fmicb.2019.00214
Alves de Castro, P., Dos Reis, T. F., Dolan, S. K., Oliveira Manfiolli, A, Brown, N. A., Jones, G. W., et al. (2016). The Aspergillus fumigatus SchASCH9 kinase modulates SakAHOG1 MAP kinase activity and it is essential for virulence. Mol. Microbiol. 102, 642–671. doi: 10.1111/mmi.13484
Antunes, L. C. S., Imperi, F., Minandri, F., and Visca, P. (2012). In vitro and in vivo antimicrobial activities of gallium nitrate against multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 56, 5961–5970. doi: 10.1128/AAC.01519-12
Apseloff, G. (1999). Therapeutic uses of gallium nitrate: past, present, and future. Am. J. Ther. 6, 327–339. doi: 10.1097/00045391-199911000-00008
Bastos, T. O., Soares, B. M., Cisalpino, P. S., Mendes, I. C., dos Santos, R. G., and Beraldo, H. (2010). Coordination to gallium (III) strongly enhances the potency of 2-pyridineformamide thiosemicarbazones against Cryptococcus opportunistic fungi. Microbiol. Res. 165, 573–577. doi: 10.1016/j.micres.2009.10.005
Bizerra, F. C., Jimenez-Ortigosa, C., Souza, A. C., Breda, G. L., Queiroz-Telles, F., Perlin, D. S., et al. (2014). Breakthrough candidemia due to multidrug-resistant Candida glabrata during prophylaxis with a low dose of micafungin. Antimicrob. Agents Chemother. 58, 2438–2440. doi: 10.1128/AAC.02189-13
Brown, G. D., Denning, D. W., Gow, N. A., Levitz, S. M., Netea, M. G., and White, T. C. (2012b). Hidden killers: human fungal infections. Sci. Transl. Med. 4:165rv13. doi: 10.1126/scitranslmed.3004404
Brown, G. D., Denning, D. W., and Levitz, S. M. (2012a). Tackling human fungal infections. Science. 336:647. doi: 10.1126/science.1222236
Bullen, J. J., Rogers, H. J., Spalding, P. B., and Ward, C. G. (2005). Iron and infection: the heart of the matter. FEMS Immunol. Med. Microbiol. 43, 325–330. doi: 10.1016/j.femsim.2004.11.010
Cassat, J. E., and Skaar, E. P. (2013). Iron in infection and immunity. Cell Host Microbe. 13, 509–519. doi: 10.1016/j.chom.2013.04.010
Chitambar, C. R. (2010). Medical applications and toxicities of gallium compounds. J. Environ. Res. Public Health. 7, 2337–2361. doi: 10.3390/ijerph7052337
Chitambar, C. R., and Narasimhan, J. (1991). Targeting iron-dependent DNA synthesis with gallium and transferrin-gallium. Pathobiology 59, 3–10. doi: 10.1159/000163609
Clinical and Laboratory Standards Institute (2017a). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi: Approved Standard M38-A2. Wayne, PA: CLSI.
Clinical and Laboratory Standards Institute (2017b). Reference Method for Broth Dilution Antifungal Susceptibility Testing Yeasts: Approved Standard M27-A3. Wayne, PA: CLSI.
Colombo, A. L., de Almeida Júnior, J. N., Slavin, M. A., Chen, S. C., and Sorrell, T. C. (2017). Candida and invasive mould diseases in non-neutropenic critically ill patients patients with haematological cancer. Lancet Infect. Dis. 17, e344–e356. doi: 10.1016/S1473-3099(17)30304-3
Dal Mas, C., Rossato, L., Shimizu, T., Oliveira, E. B., da Silva Junior, P. I., Meis, J. F., et al. (2019). Effects of the natural peptide crotamine from a South American rattlesnake on Candida auris, an emergent multidrug antifungal resistant human pathogen. Biomolecules 9:E205. doi: 10.3390/biom9060205
Gautam, P., Upadhyay, S. K., Hassan, W., Madan, T., Sirdeshmukh, R., and Sundaram, C. S. (2011). Transcriptomic and proteomic profile of Aspergillus fumigatus on exposure to artemisinin. Mycopathologia 172:331. doi: 10.1007/s11046-011-9445-3
Gomez-lopez, A., Cuenca-estrella, M., Mellado, E., and Rodriguez-Tudela, J. L. (2003).In vitro evaluation of combination of terbinafine with itraconazole or amphotericin B against Zygomycota. Diagn. Microbiol. Infect. Dis. 45, 199–202. doi: 10.1016/S0732-8893(02)00509-6
Goss, C. H., Kaneko, Y., Khuu, L., Anderson, G. D., Ravishankar, S., Aitken, M. L., et al. (2018). Gallium disrupts bacterial iron metabolism and has therapeutic effects in mice and humans with lung infections. Sci. Transl. Med. 10:eaat7520. doi: 10.1126/scitranslmed.aat7520
Haas, H. (2014). Fungal siderophore metabolism with a focus on Aspergillus fumigatus. Nat. Prod. Rep. 31, 1266–1276. doi: 10.1039/C4NP00071D
Hagiwara, D., Watanabe, A., Kamei, K., and Goldman, G. H. (2016). Epidemiological and genomic landscape of azole resistance mechanisms in Aspergillus fungi. Front. Microbiol. 7:1382. doi: 10.3389/fmicb.2016.01382
Hijazi, S., Visaggio, D., Pirolo, M., Frangipani, E., Bernstein, L., and Visca, P. (2018). Antimicrobial activity of gallium compounds on ESKAPE pathogens. Front. Cell. Infect. Microbiol. 8:316. doi: 10.3389/fcimb.2018.00316
Li, Y., Sun, L., Lu, C., Gong, Y., Li, M, and Sun, S. (2018). Promising antifungal targets against Candida albicans based on ion homeostasis. Front. Cell. Infect. Microbiol. 8:286. doi: 10.3389/fcimb.2018.00286
Nett, J. E., and Andes, D. R. (2016). Antifungal agents: spectrum of activity, pharmacology, and clinical indications. Infect. Dis. Clin. North. Am. 30, 51–83. doi: 10.1016/j.idc.2015.10.012
Odds, F. C. (2003). Synergy, antagonism and what the chequerboard puts between them. J. Antimicrob. Chemot. 52:1. doi: 10.1093/jac/dkg301
Olakanmi, O., Britigan, B. E., and Schlesinger, L. S. (2000). Gallium disrupts iron metabolism of mycobacteria residing within human macrophages. Infect. Immun. 68, 5619–5627. doi: 10.1128/IAI.68.10.5619-5627.2000
Olakanmi, O., Gunn, J. S., Su, S., Soni, S., Hassett, D. J., and Britigan, B. E. (2009). Gallium disrupts iron uptake by intracellular and extracellular Francisella strains and exhibits therapeutic efficacy in a murine pulmonary infection model. Antimicrob. Agents Chemother. 54, 244–253. doi: 10.1128/AAC.00655-09
Öz, Y., Özdemir, H. G., Gökbolat, E., Kiraz, N., Ilkit, M., and Seyedmousavi, S (2016). Time-kill kinetics and in vitro antifungal susceptibility of non-fumigatus Aspergillus species isolated from patients with ocular mycoses. Mycopathologia 181, 225–233. doi: 10.1007/s11046-015-9969-z
Perfect, J. R. (2017). The antifungal pipeline: a reality check. Nat. Rev. Drug Discov. 16, 603–616. doi: 10.1038/nrd.2017.46
Perfect, J. R., and Bicanic, T. (2015). Cryptococcosis diagnosis and treatment: what do we know now. Fungal Genet. Biol. 78, 49–54. doi: 10.1016/j.fgb.2014.10.003
Riat, A., Plojoux, J., Gindro, K., Schrenzel, J., and Sanglard, D. (2018). Azole resistance of environmental and clinical aspergillus fumigatus isolates from Switzerland. Antimicrob Agents Chemother. 62:e02088-17. doi: 10.1128/AAC.02088-17
Richter, K., Thomas, N., Zhang, G., Prestidge, C. A., Coenye, T., and Wormald, P. J. (2017). Deferiprone and gallium-protoporphyrin have the capacity to potentiate the activity of antibiotics in Staphylococcus aureus small colony variants. Front. Cell Infect. Microbiol. 7:280. doi: 10.3389/fcimb.2017.00280
Ries, L. N. A., Rocha, M. C., de Castro, P. A., Silva-Rocha, R., Silva, R. N., Freitas, F. Z., et al. (2017). The Aspergillus fumigatus CrzA transcription factor activates chitin synthase gene expression during the caspofungin paradoxical effect. mBio. 8, e00705–e00717. doi: 10.1128/mBio.00705-17
Santos, D. A., Barros, M. E. S., and Hamdan, J. S. (2006). Establishing a method of inoculum preparation for susceptibility testing of Trichophyton rubrum and Trichophyton mentagrophytes. J. Clin. Microbiol. 44, 98–101. doi: 10.1128/JCM.44.1.98-101.2006
Simm, C., and May, R. C. (2019). Zinc and iron homeostasis: target-based drug screening as new route for antifungal drug development. Front. Cell Infect. Microbiol. 9:181. doi: 10.3389/fcimb.2019.00181
Spitzer, M., Robbins, N., and Wright, G. D. (2017). Combinatorial strategies for combating invasive fungal infections. Virulence 8, 169–185. doi: 10.1080/21505594.2016.1196300
Thompson, M. G., Truong-Le, V., Alamneh, Y. A., Black, C. C., Anderl, J., Honnold, C. L., et al. (2015). Evaluation of gallium citrate formulations against a multidrug-resistant strain of Klebsiella pneumoniae in a murine wound model of infection. Antimicrob. Agents Chemother. 59, 6484–6493. doi: 10.1128/AAC.00882-15
Keywords: Aspergillus fumigatus, Candida, gallium nitrate, multidrug resistant, iron
Citation: Bastos RW, Rossato L, Valero C, Lagrou K, Colombo AL and Goldman GH (2019) Potential of Gallium as an Antifungal Agent. Front. Cell. Infect. Microbiol. 9:414. doi: 10.3389/fcimb.2019.00414
Received: 07 August 2019; Accepted: 20 November 2019;
Published: 11 December 2019.
Edited by:
Yanan Zhao, Center for Discovery and Innovation, Hackensack Meridian Health, United StatesReviewed by:
Alexandre Alanio, Paris Diderot University, FranceCheshta Sharma, University of Tennessee Health Science Center (UTHSC), United States
Copyright © 2019 Bastos, Rossato, Valero, Lagrou, Colombo and Goldman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gustavo H. Goldman, Z2dvbGRtYW5AdXNwLmJy
 Rafael Wesley Bastos
Rafael Wesley Bastos Luana Rossato
Luana Rossato Clara Valero
Clara Valero Katrien Lagrou
Katrien Lagrou Arnaldo Lopes Colombo
Arnaldo Lopes Colombo Gustavo H. Goldman
Gustavo H. Goldman