- 1FG 16: Mycotic and Parasitic Agents and Mycobacteria, Robert Koch-Institute, Berlin, Germany
- 2Department of Veterinary Medicine, Institute of Immunology, Freie Universität Berlin, Berlin, Germany
Toxoplasma gondii is a zoonotic intracellular parasite, able to infect any warm-blooded animal via ingestion of infective stages, either contained in tissue cysts or oocysts released into the environment. While immune responses during infection are well-studied, there is still limited knowledge about the very early infection events in the gut tissue after infection via the oral route. Here we briefly discuss differences in host-specific responses following infection with oocyst-derived sporozoites vs. tissue cyst-derived bradyzoites. A focus is given to innate intestinal defense mechanisms and early immune cell events that precede T. gondii's dissemination in the host. We propose stem cell-derived intestinal organoids as a model to study early events of natural host-pathogen interaction. These offer several advantages such as live cell imaging and transcriptomic profiling of the earliest invasion processes. We additionally highlight the necessity of an appropriate large animal model reflecting human infection more closely than conventional infection models, to study the roles of dendritic cells and macrophages during early infection.
Introduction
Infection by the intracellular apicomplexan parasite Toxoplasma gondii affects an estimated 25–30% of humans worldwide (Montoya and Liesenfeld, 2004), making this zoonotic parasite one of the most widespread human pathogens in the world. Infected felids excrete up to several hundred million environmentally resistant oocysts with their feces, which can infect any warm-blooded animal upon ingestion. There, T. gondii reproduces asexually via two distinct life cycle stages, the fast growing tachyzoite and the slower reproducing bradyzoite stage. The latter forms cysts in various host tissues, which may be consumed by carnivores or omnivores. Following ingestion, bradyzoites are released from cysts, reverting to the tachyzoite stage, replicating, and invading surrounding tissues before eventually disseminating throughout the body to other organs (Blader et al., 2015).
Different Outcomes Are Observed Following Experimental Infection With Different Parasite Stages and in Different Host Species
While T. gondii can be transmitted via any of the above-mentioned paths, it is known that infections with different forms of the parasite have different effects in different hosts. Sporozoites differ biochemically and cell biologically from tachyzoites and bradyzoites (Speer et al., 1995; Dubey et al., 1998; Jerome et al., 1998; Fritz et al., 2012). Understanding innate immune mechanisms will therefore require comparisons of infections with oocyst-derived sporozoites and bradyzoites, as well as consideration of naturally occurring species-specific transmission pathways.
The natural predator-prey interaction of cats and rodents serves as a convincing argument for studying rats and mice as natural hosts for T. gondii. Experimental data (Dubey, 2001, 2006) support the hypothesis that T. gondii has primarily evolved for transmission by carnivory in cats and via the fecal-oral route in herbivores (Dubey, 2006). Mus musculus is almost entirely an herbivorous organism, with occasional insectivorism (Butet and Delettre, 2011). Maternal cannibalism, as seen under lab conditions, is rather a stress-related behavior (Weber and Olsson, 2008; Weber et al., 2013) and is presumably much less often observed in nature. Consequently, the common use of oral T. gondii infection with bradyzoites in mice as a research model is problematic, particularly if we wish to gain insights relevant to human infection (Ehret et al., 2017; Sher et al., 2017). Notably, islands free of felids exhibited a low seroprevalence of T. gondii in wild pigs and humans, likely resulting from a lack of oocysts in the environment (Dubey et al., 1997a; de Wit et al., 2019). This underlines the important role that oocysts play in parasite dissemination, even for omnivorous species such as pigs or humans.
The Early Events of Intestinal Entry of T. gondii
Very little is known in any organism about the very early phase of infection with either bradyzoites or sporozoites regarding mechanisms employed by the parasite to pass through the intestinal epithelial barrier (IEB). Early in vivo studies reported that excysted sporozoites were observed in enterocytes 30 min post-infection, with few cytopathological lesions such as villi enlargement detected at the ultrastructural level (Dubey et al., 1997b; Speer and Dubey, 1998). Sporozoites could pass through enterocytes and goblet cells of the ileal epithelium 2 h post-infection and enter the lamina propria where parasites differentiated. However, recent reports have concluded that parasites are only reliably detectable by in vivo imaging 3–5 days post-infection (Coombes et al., 2013; Gregg et al., 2013).
There is therefore a clear need for cellular systems which mimic the in vivo situation and allow live cell imaging and transcriptomic profiling of the earliest invasion processes. Great advances in generation, cultivation and cell-type characterization of intestinal organoids (IOs) offer unique opportunities to observe these early events in different hosts with different T. gondii stages (Klotz et al., 2012; Derricott et al., 2019).
Intestinal Organoid Models to Study T. gondii Infections
IOs can serve as an unlimited source for primary intestinal epithelial tissues. They reflect the cellular content and functionality of the in vivo organ (Figure 1A) including the unique properties of the IEB, like composition of tight junctions (Chiba et al., 2008; Kozuka et al., 2017). By manipulating culture conditions IOs can display the different cell populations that vary throughout both small and large intestine. A major advantage of IOs is their long-term survival in vitro (in contrast to ex vivo organ cultures) and the savings on animal experiments once IOs have been established. They are also genetically tractable (Schwank et al., 2013). However, IOs also have drawbacks, like issues with reproducibility and comparability of results between labs due to non-standardized culture conditions and/or different donors for organoid preparations (Bartfeld, 2016; Yu et al., 2017). Moreover, although IOs provide a good representation of the complexity of the intestinal tissue, terminally differentiated IOs with all cell populations, in particular the less frequent Tuft- and M-cells, are difficult to obtain. Other aspects that are missing in IOs compared to whole animals or ex vivo short-term organ cultures are immune cells and the microbiota. However, recent technological advances allow these “missing” components to be gradually incorporated into the system to consecutively reproduce the in vivo situation (Bartfeld, 2016; Hill et al., 2017; Noel et al., 2017; Williamson et al., 2018). Another hurdle, in particular for infection studies, is the inverted topology of the IOs, i.e., the apical side of the epithelial cells faces the lumen of the organoid (Figures 1C,D). This requires the pathogens to be introduced via microinjection (Bartfeld and Clevers, 2015; Hill et al., 2017; Heo et al., 2018; Williamson et al., 2018). However, in the case of T. gondii infection of IOs is efficiently occurring (Figures 1B–D) when its lumen becomes accessible by physically breaking it open via simple pipetting. From this short discussion it is evident that for studying T. gondii biology, depending on the research question, three-dimensional IOs are superior to 2D- or even 3D-cultures of cell lines (Barrila et al., 2018; Danielson et al., 2018) but that they cannot yet fully replace mouse experiments.
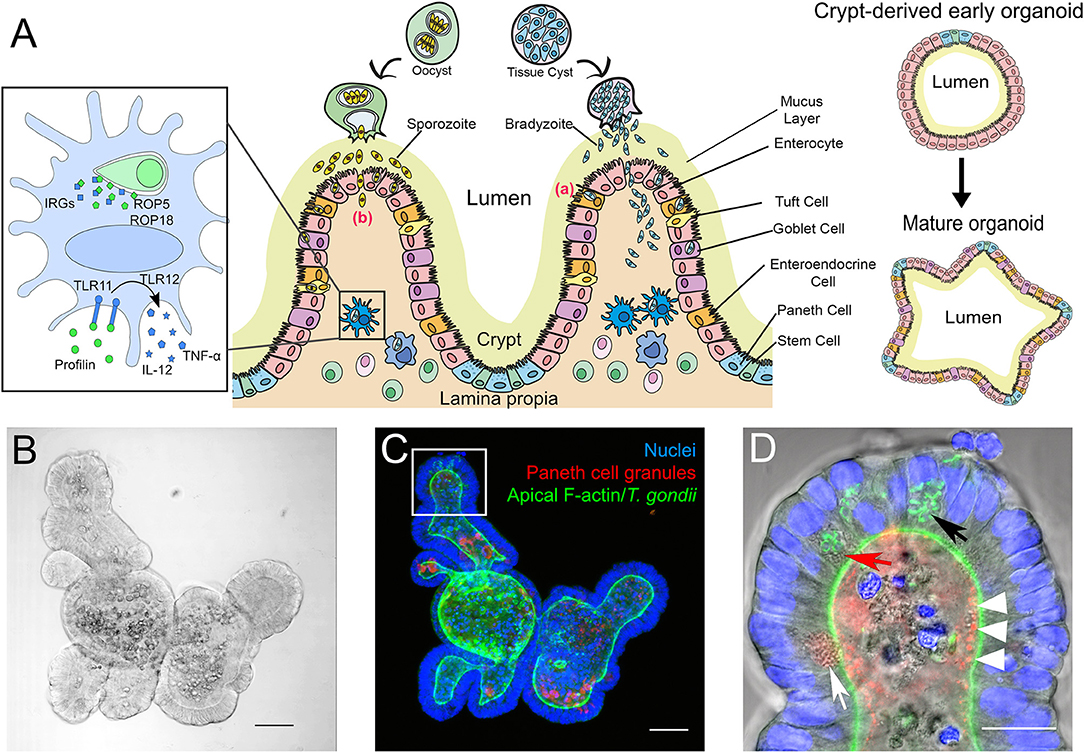
Figure 1. (A) Simplified scheme of the intestinal mucosa with its different cell populations and its derived organoids, and its infection with T. gondii bradyzoites or sporozoites. Center: After oral uptake of oocysts or tissue cysts into the small intestine sporozoites or bradyzoites leave the cyst and enter the intestinal epithelium to reach the lamina propria. The principle strategies used by the parasite to cross this barrier are either via invasion of the intestinal epithelial cells (a) or by transepithelial migration (b). Left: Infected murine DC. T. gondii ROP5 and ROP18 phosphorylate host IRGs, thus protecting the PV from degradation. Extracellular profilin is recognized by the cell via TLRs 11 and 12, inducing the release of IL-12 and TNF-α. Right: Extracellular matrix-embedded crypt (generated via physical disruption of the intestine) leads under appropriate culture conditions to the initial formation of a crypt-derived organoid which then can differentiate into a more complex “mature” intestinal organoid. (B–D) Microscopic images of a mouse small intestine-derived organoid infected with T. gondii RH strain tachyzoites. (B) Representative bright-field confocal image of a mouse IO after 7 days in culture. Scale bar 50 μm. (C) Projection of a confocal z-stack of the IO shown in C, stained with FITC-phalloidin for apical F-actin (green), TRITC-labeled UEA-1 lectin for Paneth cell granules (red) and DAPI for nuclei (blue). Note that due to the projection of the stack fluorescent signals might appear mis-localized within the organoid compared to the single plane shown in (D). Scale bar 50 μm. (D) Enlarged view of an organoid villus-like structure (white square in C) of a single plane. Parasites (identifiable by their GFP-tagged green tubular mitochondrion (Thomsen-Zieger et al., 2003; black arrow) had replicated in IECs for 48 h. Paneth cells, identifiable by their multiple granules (white arrow) can be detected in the villus-like structure. Due to its granular appearance the red arrow indicates a possible Paneth cell containing replicating parasites. The IO's lumen is filled with cell debris from apoptotic cells, constantly shed as part of the high turnover rate of IECs. The red structures in the lumen marked with white arrow heads might indicate Paneth cell degranulation, as previously described by Farin et al. (2014). Scale bar 20 μm.
Intestinal epithelial cells (IECs) are constantly renewed every 3–4 days (Clevers and Bevins, 2013). The main absorptive cell type, enterocytes, are characterized by a columnar architecture and microvilli at the apical surface. Goblet cells secrete mucins, which form a thick mucus layer along the epithelial surface, limiting access and promoting removal of potential invading pathogens (Johansson and Hansson, 2016). Enteroendocrine cells release hormones at the basolateral site, mediating paracrine effects to neighboring cells (Allaire et al., 2018). Tuft cells are thought to serve as luminal chemosensors, but their main function is largely unknown (Gerbe and Jay, 2016). Paneth cells are located in the crypt base and play a pivotal role in innate immune defense in the intestine by secreting antimicrobial molecules, such as defensins/cryptidins, lysozyme and phospholipases (Cheng and Leblond, 1974; Clevers and Bevins, 2013). The release of these granules is highly dependent on several stimuli such as cholinergic agonists (Satoh et al., 1995; Clevers and Bevins, 2013). Paneth cell-specific autophagy has been shown recently as essential for protection against interferon-γ (IFN-γ) dependent intestinal inflammation and crypt integrity, also in the context of a T. gondii infection (Raetz et al., 2013; Burger et al., 2018). Another important role of Paneth cells is the maintenance of the stem cell niche in the small intestine (Sato et al., 2011; Clevers and Bevins, 2013).
IOs allow the real-time study of early infection event dynamics in specific gut epithelial cell types upon T. gondii infection. This includes the ability to study IOs derived from a range of host species, including rodents, pigs and humans (Klotz et al., 2012; Derricott et al., 2019). They can therefore serve to highlight differences in these processes in different species under comparable experimental conditions, due to the absence of the immune system and microbiota. In the past, infection studies were performed using either the murine in vivo model, or small intestinal cell lines derived from immortalized or cancer cells. It will therefore be crucial to compare results to T. gondii-infected IOs.
Innate Defense Mechanisms of Intestinal Epithelial Cells to T. gondii Infection
Studies in mice have provided a comprehensive picture of innate immune responses in the lamina propria and beyond (Yarovinsky, 2014; Cohen and Denkers, 2015; Dunay and Diefenbach, 2018) upon T. gondii infection, as well as the role of the microbiota (Cohen and Denkers, 2014; Leung et al., 2018). However, much less is known about how parasites are able to overcome the IEB or their fate in the individual cells of it (Jones et al., 2017).
The intestinal mucosa is protected by physical barriers that include the mucus layer, the glycocalyx of enterocytes and the tight junctions between intestinal epithelial cells (Pelaseyed et al., 2014; Okumura and Takeda, 2017). It shields the mucosa from invasion by the microbiota. Although T. gondii is apparently able to penetrate this barrier the efficiency of this process and how the hurdles are overcome is unknown.
Few studies have addressed the role of glycocalyx and mucus during T. gondii infection. However, one described an increase in mucus-producing goblet cells in rats upon infection with oocysts (Trevizan et al., 2016). Conflicting results were reported for the role that trefoil factor family (TFF) peptides, major constituents of intestinal mucus, play in a T. gondii infection. While one study in mice showed a protective effect of TFF2 against immunopathology (McBerry et al., 2012), another reported the opposite effect for TFF3 (Fu et al., 2015).
Different pathways have been proposed to be used by T. gondii for transmigration into the intestinal epithelial tissue (Jones et al., 2017) (Figure 1A). The first one is paracellular transmigration, in which the parasites, aided by their gliding motility, move through the intercellular junctions without altering the barrier integrity. In vitro studies using intestinal polarized monolayers showed that tachyzoites of type I strains exhibited a higher migratory capacity compared to type II and type III strains. This might contribute to the higher virulence of type I strains seen in lab mice (Barragan and Sibley, 2002). It was later shown that parasites rapidly cluster between the cellular junctions upon entry (Weight and Carding, 2012; Briceño et al., 2016; Jones et al., 2017), and the tight junction protein occludin was identified as a specific target of tachyzoites during passage through the paracellular pathway (Weight et al., 2015). T. gondii might use it to efficiently cross the monolayers by the interaction of intracellular adhesion molecule-1 with the parasite adhesion molecule MIC2, without affecting barrier permeability (Barragan et al., 2005). However, contradictory results were reported in an experimental set-up with Caco-2 cells (Briceño et al., 2016) where it was shown that intestinal barrier function was disturbed. These examples highlight the need to evaluate these crucial events in a cellular system like IOs that closely resemble the in vivo IEB.
The second reported entry pathway is by penetration of the apical cell membrane and passing through the basolateral side in order to reach the underlying lamina propria where leukocytes reside (Barragan et al., 2005; Lambert and Barragan, 2010). Several authors have proposed a third pathway, which involves a Trojan-horse-like model (Gregg et al., 2013; Jones et al., 2017). Upon infection of IECs neutrophils are rapidly recruited to the site of infection and subsequently infected by the parasite. These are then capable of migrating through the epithelial cell layer and crossing the lumen, thereby facilitating parasite spread not only in the intestine but also to other tissues (Coombes et al., 2013). However, most of these data were generated in the above-mentioned traditional model systems. Therefore, it will be interesting to see how IOs compare to these models and which reflect best the in vivo situation.
Besides the physical barrier discussed above an independent biochemical barrier exists, composed mainly of antimicrobial peptides and proteins (e.g., cryptidins, defensins, lysozyme). The vulnerability of T. gondii bradyzoites or sporozoites toward these molecules is largely unknown. A differential effect of oocysts of different genetic backgrounds on Paneth cell-derived lysozyme expression following infection of BALB/c mice was reported (Lu et al., 2018). Likewise, only a single study provided some indirect evidence for an effect of the antimicrobial activity of cryptidins on bradyzoites in the lumen of mice prior to invasion of the epithelial layer (Foureau et al., 2010). Evidently, there is still more to learn in this area.
Upon parasite exposure IECs (and immune cells, see below) recognize the parasite through pattern recognition receptors on the cell surface, such as Toll-like receptors (TLRs), which activate secretion of pro-inflammatory cytokines that induce a subsequent Th1 response (Gopal et al., 2008). Among these, Paneth cell-resident TLR9 has been shown to modulate recognition of external pathogens and to induce the immune response through mechanisms such as defensin release (Rumio et al., 2004; Buzoni-Gatel et al., 2006; Foureau et al., 2010). Surprisingly, a recent transcriptomic study with rat intestinal IEC-18 cells did not find evidence of pathogen-associated molecular patterns being induced upon infection with oocyst-derived T. gondii sporozoites (Guiton et al., 2017).
Early Interaction of T. gondii With Host Immune Cells
The lamina propria and Peyer's patches are rich in dendritic cells (DCs) and macrophages (MΦs). Once the intestinal barrier is overcome by T. gondii, these are the first immune cells to recognize parasite infection and to initiate the mounting of the host immune response. This recognition can occur via at least three distinct routes. (1) DCs and MΦs directly phagocytose free, opsonized parasites upon crossing the epithelial barrier. (2) Both cell types also phagocytose infected apoptotic IECs (Buzoni-Gatel and Werts, 2006). (3) DCs are able to elongate through the tight junctions of the epithelium, and in mice recognize the soluble T. gondii antigen profilin in the lumen via TLR 11 and 12 (Yarovinsky et al., 2005; Koblansky et al., 2013). DCs and MΦs exhibit directly toxoplasmacidal effects to phagocytosed parasites. However, once stimulated they also begin to secrete interleukin (IL) 12 and tumor necrosis factor α (TNF-α) (Buzoni-Gatel and Werts, 2006). IL-12 and TNF-α induce the differentiation of CD4+ T cells into Th1 cells, which secrete IFN-γ. In parallel, IL-12, along with IL-15 secreted by infected IECs, stimulate natural killer (NK) cells and CD8+ T cells to begin secreting IFN-γ, the primary mediator of resistance to T. gondii (Suzuki et al., 1988). This leads to containment of the parasite and its conversion into the bradyzoite form, thereby hiding from the immune system (Hunter and Sibley, 2012; Ahmed et al., 2017).
Role of T. gondii Rhoptry Proteins in Early Immune Cell Modification
One mechanism by which IFN-γ mediates parasite destruction in mice is through upregulation of immunity-related GTPases (IRGs) (Gazzinelli et al., 2014; Müller and Howard, 2016). IRGs are intracellular host proteins, some of which localize to the PV membrane in an infected cell causing membrane rupture, parasite release into the host cell cytosol and its subsequent degradation. In order to avoid destruction, T. gondii has evolved a means to subvert this host defense mechanism (Buzoni-Gatel and Werts, 2006; Gazzinelli et al., 2014). It is dependent on several parasite proteins which are derived from unique secretory organelles (rhoptries and dense granules) and transported into the infected cell. ROP18 is able to phosphorylate host IRGs such as Irga6 while ROP5 modulates this activity, all eventually resulting in PV membrane destruction (Reese et al., 2011; Behnke et al., 2012; Fleckenstein et al., 2012; Niedelman et al., 2012; Etheridge et al., 2014). However, depending on the genetic background of the mouse, this virulence mechanism of the parasite can be overcome by a highly polymorphic IRG protein (Irgb2-b1). Some of its variants can act as decoys for ROP5/18 binding, enabling other IRGs to degrade the PV (Lilue et al., 2013). Surprisingly, it is unknown if or to what extent this IRG response plays a role in the intestine. Mouse IOs could be very useful to shed light on this immediate obstacle the parasite has to overcome in order to proliferate and disseminate.
Phenotypic Changes of DCs Utilized as “Trojan Horse” Vehicles for Dissemination of T. gondii
After infection T. gondii is able to rapidly disseminate throughout the body. Within hours it is found in the spleen and it is also able to cross the blood-brain barrier, the placental barrier in pregnant hosts, and enter immune privileged sites such as the eyes (Lambert and Barragan, 2010; Harker et al., 2015). This is achieved through invasion and utilization of migratory leukocytes as “Trojan horses.” There is evidence suggesting that several cell types may be used for this purpose, including DCs, MΦs, neutrophils, NK cells, and T cells (Courret et al., 2006). However, extracellular tachyzoites released from infected endothelial cells in the brain vasculature have also recently been implicated in overcoming the blood-brain barrier (Konradt et al., 2016).
Mostly DCs, inherently able to become migratory, have been implicated in early dissemination (Weidner and Barragan, 2014; Kanatani et al., 2015; Brasil et al., 2017; Ólafsson et al., 2018). Following activation by an antigen they undergo a series of phenotypic changes required for its efficient presentation. This includes comprehensive remodeling of the actin cytoskeleton and the loss of actin-rich structures called podosomes. These changes are essential for switching from a strongly adhesive to a migratory phenotype, allowing cells to reach the lymph nodes. Using lipopolysaccharide to activate DCs indicated that remodeling was dependent on TLR4 signaling and prostaglandin E2 (PGE2) secretion (van Helden et al., 2010; Weidner et al., 2013). In contrast, following infection with T. gondii tachyzoites, phenotypic changes occurred < 10 min post-invasion and were not reliant on TLR4 or PGE2. This was shown experimentally to require active manipulation by live T. gondii (Weidner et al., 2013). Recent studies indicated that T. gondii infection results in a marked reduction in pericellular proteolytic activity by DCs, mediated via the release of tissue inhibitor of metalloproteinase 1. This suggests a compensatory mechanism for an upregulation of matrix metalloproteinases, which have been demonstrated to perform diverse catalytic and non-catalytic functions in amoeboid migration (Orgaz et al., 2014; Ólafsson et al., 2018).
Differences in the Macrophage/DC Responses of Mice and Humans to T. gondii: the Pig as Human-Relevant Model
As discussed, murine DCs are able to undergo maturation in response to the detection of the soluble T. gondii antigen profilin via TLR11 and TLR12. However, in humans TLR12 is entirely absent, and TLR11 is apparently a non-functional pseudogene (Zhang et al., 2004; Roach et al., 2005; Ishii et al., 2008). Consequently, profilin does not elicit an immune response in humans; instead it relies on phagocytosis of tachyzoites (Tosh et al., 2016; Sher et al., 2017). Although the pattern recognition receptors responsible for the recognition of T. gondii in humans have not been definitively identified, human PBMCs produce pro-inflammatory cytokines following stimulation with T. gondii RNA or DNA. This implicates the involvement of TLRs 7, 8, and 9 which are responsible for the recognition of nucleic acids from pathogens (Forsbach et al., 2008; Andrade et al., 2013; Jennes et al., 2017). The specific subsets of monocytes and DCs secreting IL-12 in response to T. gondii also differ between mice and humans. In mice, inflammatory monocytes and CD8α+ DCs respond, whereas the human analogs—classical monocytes and the cDC2 subset—do not. In contrast, human non-classical and intermediate monocytes and the cDC1 subset produce IL-12, which are analogous to murine patrolling monocytes and CD8α− DCs (Tosh et al., 2016; Sher et al., 2017).
There is a clear need for an immunologically more human-like large animal model to understand the mechanisms underlying T. gondii infection and immunity in humans (Ahmed et al., 2017; Sher et al., 2017). The pig is one such candidate that could be utilized for this purpose. Genomic studies have indicated that 80% of porcine immune response genes resemble human equivalents, whereas for mice <10% are similar (Meurens et al., 2012; Mair et al., 2014) (Table 1). Of particular note is that like humans, pigs lack TLRs 11 and 12 (Uenishi and Shinkai, 2009; Mair et al., 2014) and so are presumably also unable to respond to profilin. They do however exhibit TLRs 7, 8, and 9, and so are likely able to recognize T. gondii via the same mechanism as humans (Uenishi et al., 2012; Jennes et al., 2017). Thus, the initial porcine DC and MΦ responses to T. gondii deserve further examination regarding their similarity to the human response.
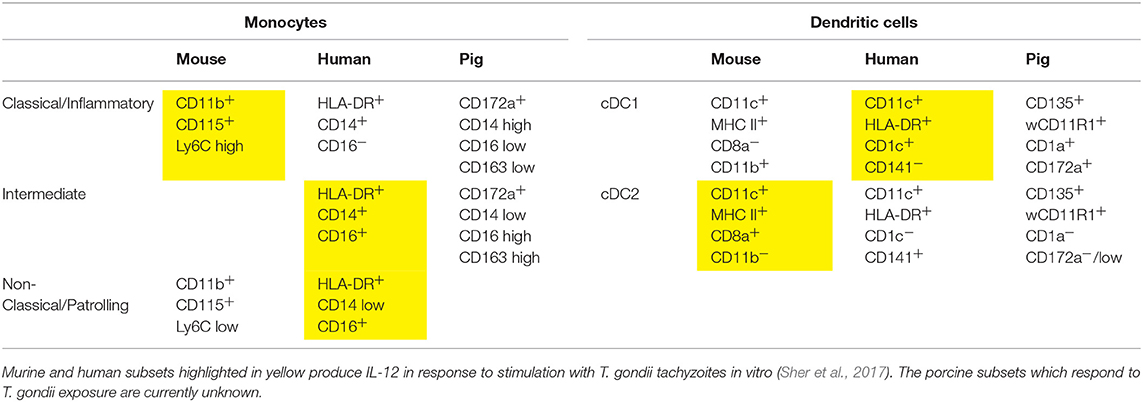
Table 1. Markers of monocyte and DC subsets in mice, humans, and pigs (Fairbairn et al., 2013; Summerfield et al., 2015; Sher et al., 2017).
There are also clinical similarities between the human and porcine responses to T. gondii, which further suggest the pig may be an appropriate model for human infection. For example, postnatal infection with T. gondii is usually asymptomatic or mild in humans and pigs, whereas infections with some parasite strains can be fatal in mice (Dubey, 1986; Nau et al., 2017). During pregnancy in humans and pigs parasites can often cross the placental barrier and result in abortion or congenital toxoplasmosis (Jungersen et al., 2001), whereas fetal infections are rare in immunocompetent mice (Shiono et al., 2007; Nau et al., 2017). Furthermore, as omnivorous mammals pigs, like humans, are naturally at risk of exposure to both T. gondii tissue cysts and oocysts in their diet (Meurens et al., 2012). This makes them a more natural host for research into the early stages of infection with both bradyzoites and sporozoites.
Although fewer immunological reagents are currently available for swine in comparison to mice and humans, this is an area undergoing rapid progress, not the least because of the increased interest in pig organs for xenotransplantation (Meier et al., 2018). After mice and primates, the porcine immune system is perhaps the next most thoroughly characterized, with pigs being firmly established as a model organism for infection research. This includes their use as a model for infection with other human-relevant, orally-acquired pathogens such as Helicobacter pylori and human rotavirus, as well as the protozoan parasite Cryptosporidium parvum (Meurens et al., 2012). In recent years the body of literature on the porcine cellular immune response specifically to T. gondii also increased (e.g., Miranda et al., 2015; Jennes et al., 2017; Nau et al., 2017). Notably, porcine IOs have also been described recently (Derricott et al., 2019).
Concluding Remarks
IOs closely resemble the in vivo intestinal barrier and represent a source of species-specific IECs. To mechanistically study interactions of pathogens with such a complex organ it is advantageous to examine the contribution of individual epithelial cells in the absence of immune cells and microbiota. However, several reports have illustrated that IOs can be co-cultured with DCs, MΦs, IELS (Nozaki et al., 2016; Noel et al., 2017; Ihara et al., 2018; Nakamura, 2018) and also with bacteria (Hill et al., 2017; Williamson et al., 2018), thereby complementing this system as required.
The pig allows for tissue-specific translational research since the immune parameters depicted so far closely resemble humans. Future studies will show whether porcine intestinal innate and adaptive parameters better reflect human early infection events in comparison to mice.
Ethics Statement
The use of animal material was approved by the responsible local authorities of the German Federal State Berlin (permit T0173/14).
Author Contributions
ED provided parts of Figure 1A and Figures 1B–D. BH provided parts of Figure 1A. All authors contributed to the text and approved its final version.
Funding
The work was supported by the German Research Council: GRK 2046 to ED, BH, CK, SH, and FS. Work by CK and FS cited is supported by the Robert Koch-Institute. BF is supported by the Robert Koch-Institute as part of the German One Health Initiative (GOHI).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Ahmed, N., French, T., Rausch, S., Kühl, A., Hemminger, K., Dunay, I. R., et al. (2017). Toxoplasma co-infection prevents Th2 differentiation and leads to a Helminth-specific Th1 response. Front. Cell. Infect. Microbiol. 7:341. doi: 10.3389/fcimb.2017.00341
Allaire, J. M., Crowley, S. M., Law, H. T., Chang, S. Y., Ko, H. J., and Vallance, B. A. (2018). The intestinal epithelium: central coordinator of mucosal immunity. Trends Immunol. 39, 677–696. doi: 10.1016/j.it.2018.04.002
Andrade, W. A., Souza Mdo, C., Ramos-Martinez, E., Nagpal, K., Dutra, M. S., Melo, M. B., et al. (2013). Combined action of nucleic acid-sensing Toll-like receptors and TLR11/TLR12 heterodimers imparts resistance to Toxoplasma gondii in mice. Cell Host Microbe 13, 42–53. doi: 10.1016/j.chom.2012.12.003
Barragan, A., Brossier, F., and Sibley, L. D. (2005). Transepithelial migration of Toxoplasma gondii involves an interaction of intercellular adhesion molecule 1 (ICAM-1) with the parasite adhesin MIC2. Cell. Microbiol. 7, 561–568. doi: 10.1111/j.1462-5822.2005.00486.x
Barragan, A., and Sibley, L. D. (2002). Transepithelial migration of Toxoplasma gondii is linked to parasite motility and virulence. J. Exp. Med. 195, 1625–1633. doi: 10.1084/jem.20020258
Barrila, J., Crabbé, A., Yang, J., Franco, K., Nydam, S. D., Forsyth, R. J., et al. (2018). Modeling host-pathogen interactions in the context of the microenvironment: three-dimensional cell culture comes of age. Infect. Immun. 86:e00282–18. doi: 10.1128/IAI.00282-18
Bartfeld, S. (2016). Modeling infectious diseases and host-microbe interactions in gastrointestinal organoids. Dev. Biol. 420, 262–270. doi: 10.1016/j.ydbio.2016.09.014
Bartfeld, S., and Clevers, H. (2015). Organoids as model for infectious diseases: culture of human and murine stomach organoids and microinjection of Helicobacter pylori. J. Vis. Exp. 105. doi: 10.3791/53359
Behnke, M. S., Fentress, S. J., Mashayekhi, M., Li, L. X., Taylor, G. A., and Sibley, L. D. (2012). The polymorphic pseudokinase ROP5 controls virulence in Toxoplasma gondii by regulating the active kinase ROP18. PLoS Pathog. 8:e1002992. doi: 10.1371/journal.ppat.1002992
Blader, I. J., Coleman, B. I., Chen, C. T., and Gubbels, M. J. (2015). Lytic cycle of Toxoplasma gondii: 15 years later. Annu. Rev. Microbiol. 69, 463–485. doi: 10.1146/annurev-micro-091014-104100
Brasil, T. R., Freire-de-Lima, C. G., Morrot, A., and Vetö Arnholdt, A. C. (2017). Host-Toxoplasma gondii coadaptation leads to fine tuning of the immune response. Front. Immunol. 8:1080. doi: 10.3389/fimmu.2017.01080
Briceño, M. P., Nascimento, L. A., Nogueira, N. P., Barenco, P. V., Ferro, E. A., Rezende-Oliveira, K., et al. (2016). Toxoplasma gondii infection promotes epithelial barrier dysfunction of Caco-2 Cells. J. Histochem. Cytochem. 64, 459–469. doi: 10.1369/0022155416656349
Burger, E., Araujo, A., López-Yglesias, A., Rajala, M. W., Geng, L., Levine, B., et al. (2018). Loss of paneth cell autophagy causes acute susceptibility to Toxoplasma gondii-mediated inflammation. Cell Host Microbe 23, 177–190.e174. doi: 10.1016/j.chom.2018.01.001
Butet, A., and Delettre, Y. R. (2011). Diet differentiation between European arvicoline and murine rodents. Acta Theriol. 56, 297–304. doi: 10.1007/s13364-011-0049-6
Buzoni-Gatel, D., Schulthess, J., Menard, L. C., and Kasper, L. H. (2006). Mucosal defences against orally acquired protozoan parasites, emphasis on Toxoplasma gondii infections. Cell. Microbiol. 8, 535–544. doi: 10.1111/j.1462-5822.2006.00692.x
Buzoni-Gatel, D., and Werts, C. (2006). Toxoplasma gondii and subversion of the immune system. Trends Parasitol. 22, 448–452. doi: 10.1016/j.pt.2006.08.002
Cheng, H., and Leblond, C. P. (1974). Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. I. Columnar cell. Am. J. Anat. 141, 461–479. doi: 10.1002/aja.1001410403
Chiba, H., Osanai, M., Murata, M., Kojima, T., and Sawada, N. (2008). Transmembrane proteins of tight junctions. Biochim. Biophys. Acta 1778, 588–600. doi: 10.1016/j.bbamem.2007.08.017
Clevers, H. C., and Bevins, C. L. (2013). Paneth cells: maestros of the small intestinal crypts. Annu. Rev. Physiol. 75, 289–311. doi: 10.1146/annurev-physiol-030212-183744
Cohen, S. B., and Denkers, E. Y. (2014). Border maneuvers: deployment of mucosal immune defences against Toxoplasma gondii. Mucosal Immunol. 7, 744–752. doi: 10.1038/mi.2014.25
Cohen, S. B., and Denkers, E. Y. (2015). The gut mucosal immune response to Toxoplasma gondii. Parasite Immunol. 37, 108–117. doi: 10.1111/pim.12164
Coombes, J. L., Charsar, B. A., Han, S.-J., Halkias, J., Chan, S. W., Koshy, A. A., et al. (2013). Motile invaded neutrophils in the small intestine of Toxoplasma gondii-infected mice reveal a potential mechanism for parasite spread. Proc. Natl. Acad. Sci. U.S.A. 110, E1913–1922. doi: 10.1073/pnas.1220272110
Courret, N., Darche, S., Sonigo, P., Milon, G., Buzoni-Gâtel, D., and Tardieux, I. (2006). CD11c- and CD11b-expressing mouse leukocytes transport single Toxoplasma gondii tachyzoites to the brain. Blood 107, 309–316. doi: 10.1182/blood-2005-02-0666
Danielson, J. J., Perez, N., Romano, J. D., and Coppens, I. (2018). Modelling Toxoplasma gondii infection in a 3D cell culture system in vitro: comparison with infection in 2D cell monolayers. PLoS ONE 13:e0208558. doi: 10.1371/journal.pone.0208558
de Wit, L. A., Croll, D. A., Tershy, B., Correa, M. D, Luna-Pasten, H., Quadri, P., et al. (2019). Potential public health benefits from cat eradications on islands. PLoS Negl. Trop. Dis. 13:e0007040. doi: 10.1371/journal.pntd.0007040
Derricott, H., Luu, L., Fong, W. Y., Hartley, C. S., Johnston, L. J., Armstrong, S. D., et al. (2019). Developing a 3D intestinal epithelium model for livestock species. Cell Tissue Res. 375, 409–424. doi: 10.1007/s00441-018-2924-9
Dubey, J. P. (1986). A review of toxoplasmosis in pigs. Vet. Parasitol. 19, 181–223. doi: 10.1016/0304-4017(86)90070-1
Dubey, J. P. (2001). Oocyst shedding by cats fed isolated bradyzoites and comparison of infectivity of bradyzoites of the VEG strain Toxoplasma gondii to cats and mice. J. Parasitol. 87, 215–219. doi: 10.1645/0022-3395(2001)087[0215:OSBCFI]2.0.CO;2
Dubey, J. P. (2006). Comparative infectivity of oocysts and bradyzoites of Toxoplasma gondii for intermediate (mice) and definitive (cats) hosts. Vet. Parasitol. 140, 69–75. doi: 10.1016/j.vetpar.2006.03.018
Dubey, J. P., Lindsay, D. S, and Speer, C. A. (1998). Structures of Toxoplasma gondii tachyzoites, bradyzoites and sporozoites and biology and development of tissue cysts. Clin. Microbiol. Rev. 11:267. doi: 10.1128/CMR.11.2.267
Dubey, J. P., Rollor, E. A., Smith, K., Kwok, O. C., and Thulliez, P. (1997a). Low seroprevalence of Toxoplasma gondii in feral pigs from a remote island lacking cats. J. Parasitol. 83, 839–841. doi: 10.2307/3284277
Dubey, J. P., Speer, C. A., Shen, S. K., Kwok, O. C., and Blixt, J. A. (1997b). Oocyst-induced murine toxoplasmosis: life cycle, pathogenicity, and stage conversion in mice fed Toxoplasma gondii oocysts. J. Parasitol. 83, 870–882. doi: 10.2307/3284282
Dunay, I. R., and Diefenbach, A. (2018). Group 1 innate lymphoid cells in Toxoplasma gondii infection. Parasite Immunol. 40:e12516. doi: 10.1111/pim.12516
Ehret, T., Torelli, F., Klotz, C., Pedersen, A. B., and Seeber, F. (2017). Translational rodent models for research on parasitic protozoa-a review of confounders and possibilities. Front. Cell. Infect. Microbiol. 7:238. doi: 10.3389/fcimb.2017.00238
Etheridge, R. D., Alaganan, A., Tang, K., Lou, H. J., Turk, B. E., and Sibley, L. D. (2014). The Toxoplasma pseudokinase ROP5 forms complexes with ROP18 and ROP17 kinases that synergize to control acute virulence in mice. Cell Host Microbe 15, 537–550. doi: 10.1016/j.chom.2014.04.002
Fairbairn, L., Kapetanovic, R., Beraldi, D., Sester, D. P., Tuggle, C. K., Archibald, A. L., et al. (2013). Comparative analysis of monocyte subsets in the pig. J. Immunol. 190, 6389–6396. doi: 10.4049/jimmunol.1300365
Farin, H. F., Karthaus, W. R., Kujala, P., Rakhshandehroo, M., Schwank, G., Vries, R. G., et al. (2014). Paneth cell extrusion and release of antimicrobial products is directly controlled by immune cell-derived IFN-gamma. J. Exp. Med. 211, 1393–1405. doi: 10.1084/jem.20130753
Fleckenstein, M. C., Reese, M. L., Könen-Waisman, S., Boothroyd, J. C., Howard, J. C., and Steinfeldt, T. (2012). A Toxoplasma gondii pseudokinase inhibits host IRG resistance proteins. PLoS Biol. 10:e1001358. doi: 10.1371/journal.pbio.1001358
Forsbach, A., Nemorin, J. G., Montino, C., Müller, C., Samulowitz, U., Vicari, A. P., et al. (2008). Identification of RNA sequence motifs stimulating sequence-specific TLR8-dependent immune responses. J. Immunol. 180, 3729–3738. doi: 10.4049/jimmunol.180.6.3729
Foureau, D. M., Mielcarz, D. W., Menard, L. C., Schulthess, J., Werts, C., Vasseur, V., et al. (2010). TLR9-dependent induction of intestinal alpha-defensins by Toxoplasma gondii. J. Immunol. 184, 7022–7029. doi: 10.4049/jimmunol.0901642
Fritz, H. M., Bowyer, P. W., Bogyo, M., Conrad, P. A., and Boothroyd, J. C. (2012). Proteomic analysis of fractionated Toxoplasma oocysts reveals clues to their environmental resistance. PLoS ONE 7:e29955. doi: 10.1371/journal.pone.0029955
Fu, T., Znalesniak, E. B., Kalinski, T., Mohle, L., Biswas, A., Salm, F., et al. (2015). Tff peptides play a role in the immune response following oral infection of mice with Toxoplasma gondii. Eur. J. Microbiol. Immunol. 5, 221–231. doi: 10.1556/1886.2015.00028
Gazzinelli, R. T., Mendonça-Neto, R., Lilue, J., Howard, J., and Sher, A. (2014). Innate resistance against Toxoplasma gondii: an evolutionary tale of mice, cats, and men. Cell Host Microbe 15, 132–138. doi: 10.1016/j.chom.2014.01.004
Gerbe, F., and Jay, P. (2016). Intestinal tuft cells: epithelial sentinels linking luminal cues to the immune system. Mucosal Immunol. 9, 1353–1359. doi: 10.1038/mi.2016.68
Gopal, R., Birdsell, D., and Monroy, F. P. (2008). Regulation of toll-like receptors in intestinal epithelial cells by stress and Toxoplasma gondii infection. Parasite Immunol. 30, 563–576. doi: 10.1111/j.1365-3024.2008.01055.x
Gregg, B., Taylor, B. C., John, B., Tait-Wojno, E. D., Girgis, N. M., Miller, N., et al. (2013). Replication and distribution of Toxoplasma gondii in the small intestine after oral infection with tissue cysts. Infect. Immun. 81, 1635–1643. doi: 10.1128/IAI.01126-12
Guiton, P. S., Sagawa, J. M., Fritz, H. M., and Boothroyd, J. C. (2017). An in vitro model of intestinal infection reveals a developmentally regulated transcriptome of Toxoplasma sporozoites and a NF-κB-like signature in infected host cells. PLoS ONE 12:e0173018. doi: 10.1371/journal.pone.0173018
Harker, K. S., Ueno, N., and Lodoen, M. B. (2015). Toxoplasma gondii dissemination: a parasite's journey through the infected host. Parasite Immunol. 37, 141–149. doi: 10.1111/pim.12163
Heo, I., Dutta, D., Schaefer, D. A., Iakobachvili, N., Artegiani, B., Sachs, N., et al. (2018). Modelling Cryptosporidium infection in human small intestinal and lung organoids. Nat. Microbiol. 3, 814–823. doi: 10.1038/s41564-018-0177-8
Hill, D. R., Huang, S., Nagy, M. S., Yadagiri, V. K., Fields, C., Mukherjee, D., et al. (2017). Bacterial colonization stimulates a complex physiological response in the immature human intestinal epithelium. Elife 6:e29132. doi: 10.7554/eLife.29132
Hunter, C. A., and Sibley, L. D. (2012). Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nat. Rev. Microbiol. 10, 766–778. doi: 10.1038/nrmicro2858
Ihara, S., Hirata, Y., Hikiba, Y., Yamashita, A., Tsuboi, M., Hata, M., et al. (2018). Adhesive interactions between mononuclear phagocytes and intestinal epithelium perturb normal epithelial differentiation and serve as a therapeutic target in inflammatory bowel disease. J. Crohns Colitis 12, 1219–1231. doi: 10.1093/ecco-jcc/jjy088
Ishii, K. J., Koyama, S., Nakagawa, A., Coban, C., and Akira, S. (2008). Host innate immune receptors and beyond: making sense of microbial infections. Cell Host Microbe 3, 352–363. doi: 10.1016/j.chom.2008.05.003
Jennes, M., De Craeye, S., Devriendt, B., Dierick, K., Dorny, P., and Cox, E. (2017). Strain- and dose-dependent reduction of Toxoplasma gondii burden in pigs is associated with interferon-gamma production by CD8(+) lymphocytes in a heterologous challenge model. Front. Cell. Infect. Microbiol. 7:232. doi: 10.3389/fcimb.2017.00232
Jerome, M. E., Radke, J. R., Bohne, W., Roos, D. S., and White, M. W. (1998). Toxoplasma gondii bradyzoites form spontaneously during sporozoite-initiated development. Infect. Immun. 66, 4838–4844.
Johansson, M. E., and Hansson, G. C. (2016). Immunological aspects of intestinal mucus and mucins. Nat. Rev. Immunol. 16, 639–649. doi: 10.1038/nri.2016.88
Jones, E. J., Korcsmaros, T., and Carding, S. R. (2017). Mechanisms and pathways of Toxoplasma gondii transepithelial migration. Tissue Barriers 5:e1273865. doi: 10.1080/21688370.2016.1273865
Jungersen, G., Bille-Hansen, V., Jensen, L., and Lind, P. (2001). Transplacental transmission of Toxoplasma gondii in minipigs infected with strains of different virulence. J. Parasitol. 87, 108–113. doi: 10.1645/0022-3395(2001)087[0108:TTOTGI]2.0.CO;2
Kanatani, S., Uhlén, P., and Barragan, A. (2015). Infection by Toxoplasma gondii induces amoeboid-like migration of dendritic cells in a three-dimensional collagen matrix. PLoS ONE 10:e0139104. doi: 10.1371/journal.pone.0139104
Klotz, C., Aebischer, T., and Seeber, F. (2012). Stem cell-derived cell cultures and organoids for protozoan parasite propagation and studying host-parasite interaction. Int. J. Med. Microbiol. 302, 203–209. doi: 10.1016/j.ijmm.2012.07.010
Koblansky, A. A., Jankovic, D., Oh, H., Hieny, S., Sungnak, W., Mathur, R., et al. (2013). Recognition of profilin by Toll-like receptor 12 is critical for host resistance to Toxoplasma gondii. Immunity 38, 119–130. doi: 10.1016/j.immuni.2012.09.016
Konradt, C., Ueno, N., Christian, D. A., Delong, J. H., Pritchard, G. H., Herz, J., et al. (2016). Endothelial cells are a replicative niche for entry of Toxoplasma gondii to the central nervous system. Nat. Microbiol. 1:16001. doi: 10.1038/nmicrobiol.2016.1
Kozuka, K., He, Y., Koo-McCoy, S., Kumaraswamy, P., Nie, B., Shaw, K., et al. (2017). Development and characterization of a human and mouse intestinal epithelial cell monolayer platform. Stem Cell Rep. 9, 1976–1990. doi: 10.1016/j.stemcr.2017.10.013
Lambert, H., and Barragan, A. (2010). Modelling parasite dissemination: host cell subversion and immune evasion by Toxoplasma gondii. Cell. Microbiol. 12, 292–300. doi: 10.1111/j.1462-5822.2009.01417.x
Leung, J. M., Graham, A. L., and Knowles, S. C. L. (2018). Parasite-microbiota interactions with the vertebrate gut: synthesis through an ecological lens. Front. Microbiol. 9:843. doi: 10.3389/fmicb.2018.00843
Lilue, J., Müller, U. B., Steinfeldt, T., and Howard, J. C. (2013). Reciprocal virulence and resistance polymorphism in the relationship between Toxoplasma gondii and the house mouse. Elife 2:e01298. doi: 10.7554/eLife.01298
Lu, Y. Y., Dong, H., Feng, Y. J., Wang, K., Jiang, Y. B., Zhang, L. X., et al. (2018). Avirulence and lysozyme secretion in Paneth cells after infection of BALB/c mice with oocysts of Toxoplasma gondii strains TgCatCHn2 (ToxoDB#17) and TgCatCHn4 (ToxoDB#9). Vet. Parasitol. 252, 1–8. doi: 10.1016/j.vetpar.2018.01.016
Mair, K. H., Sedlak, C., Käser, T., Pasternak, A., Levast, B., Gerner, W., et al. (2014). The porcine innate immune system: an update. Dev. Comp. Immunol. 45, 321–343. doi: 10.1016/j.dci.2014.03.022
McBerry, C., Egan, C. E., Rani, R., Yang, Y., Wu, D., Boespflug, N., et al. (2012). Trefoil factor 2 negatively regulates type 1 immunity against Toxoplasma gondii. J. Immunol. 189, 3078–3084. doi: 10.4049/jimmunol.1103374
Meier, R. P. H., Muller, Y. D., Balaphas, A., Morel, P., Pascual, M., Seebach, J. D., et al. (2018). Xenotransplantation: back to the future? Transpl. Int. 31, 465–477. doi: 10.1111/tri.13104
Meurens, F., Summerfield, A., Nauwynck, H., Saif, L., and Gerdts, V. (2012). The pig: a model for human infectious diseases. Trends Microbiol. 20, 50–57. doi: 10.1016/j.tim.2011.11.002
Miranda, F. J., Souza, D. B., Frazão-Teixeira, E., Oliveira, F. C., Melo, J. C., Mariano, C. M., et al. (2015). Experimental infection with the Toxoplasma gondii ME-49 strain in the Brazilian BR-1 mini pig is a suitable animal model for human toxoplasmosis. Mem. Inst. Oswaldo Cruz 110, 95–100. doi: 10.1590/0074-02760140318
Montoya, J. G., and Liesenfeld, O. (2004). Toxoplasmosis. Lancet 363, 1965–1976. doi: 10.1016/S0140-6736(04)16412-X
Müller, U. B., and Howard, J. C. (2016). The impact of Toxoplasma gondii on the mammalian genome. Curr. Opin. Microbiol. 32, 19–25. doi: 10.1016/j.mib.2016.04.009
Nakamura, T. (2018). Recent progress in organoid culture to model intestinal epithelial barrier functions. Int. Immunol. 31, 13–21. doi: 10.1093/intimm/dxy065
Nau, J., Eller, S. K., Wenning, J., Spekker-Bosker, K. H., Schroten, H., Schwerk, C., et al. (2017). Experimental porcine Toxoplasma gondii infection as a representative model for human toxoplasmosis. Mediators Inflamm. 2017:3260289. doi: 10.1155/2017/3260289
Niedelman, W., Gold, D. A., Rosowski, E. E., Sprokholt, J. K., Lim, D., Farid Arenas, A., et al. (2012). The rhoptry proteins ROP18 and ROP5 mediate Toxoplasma gondii evasion of the murine, but not the human, interferon-gamma response. PLoS Pathog. 8:e1002784. doi: 10.1371/journal.ppat.1002784
Noel, G., Baetz, N. W., Staab, J. F., Donowitz, M., Kovbasnjuk, O., Pasetti, M. F., et al. (2017). A primary human macrophage-enteroid co-culture model to investigate mucosal gut physiology and host-pathogen interactions. Sci. Rep. 7:45270. doi: 10.1038/srep45270
Nozaki, K., Mochizuki, W., Matsumoto, Y., Matsumoto, T., Fukuda, M., Mizutani, T., et al. (2016). Co-culture with intestinal epithelial organoids allows efficient expansion and motility analysis of intraepithelial lymphocytes. J. Gastroenterol. 51, 206–213. doi: 10.1007/s00535-016-1170-8
Okumura, R., and Takeda, K. (2017). Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Nat. Exp. Mol. Med. 49:e338. doi: 10.1038/emm.2017.20
Ólafsson, E. B., Varas-Godoy, M., and Barragan, A. (2018). Toxoplasma gondii infection shifts dendritic cells into an amoeboid rapid migration mode encompassing podosome dissolution, secretion of TIMP-1, and reduced proteolysis of extracellular matrix. Cell. Microbiol. 20:e12808. doi: 10.1111/cmi.12808
Orgaz, J. L., Pandya, P., Dalmeida, R., Karagiannis, P., Sanchez-Laorden, B., Viros, A., et al. (2014). Diverse matrix metalloproteinase functions regulate cancer amoeboid migration. Nat. Commun. 5:4255. doi: 10.1038/ncomms5255
Pelaseyed, T., Bergström, J. H., Gustafsson, J. K., Ermund, A., Birchenough, G. M., Schütte, A., et al. (2014). The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol. Rev. 260, 8–20. doi: 10.1111/imr.12182
Raetz, M., Hwang, S.-H., Wilhelm, C. L., Kirkland, D., Benson, A., Sturge, C. R., et al. (2013). Parasite-induced TH1 cells and intestinal dysbiosis cooperate in IFN-γ-dependent elimination of Paneth cells. Nat. Immunol. 14, 136–142. doi: 10.1038/ni.2508
Reese, M. L., Zeiner, G. M., Saeij, J. P., Boothroyd, J. C., and Boyle, J. P. (2011). Polymorphic family of injected pseudokinases is paramount in Toxoplasma virulence. Proc. Natl. Acad. Sci. U.S.A. 108, 9625–9630. doi: 10.1073/pnas.1015980108
Roach, J. C., Glusman, G., Rowen, L., Kaur, A., Purcell, M. K., Smith, K. D., et al. (2005). The evolution of vertebrate Toll-like receptors. Proc. Natl. Acad. Sci. U.S.A. 102, 9577–9582. doi: 10.1073/pnas.0502272102
Rumio, C., Besusso, D., Palazzo, M., Selleri, S., Sfondrini, L., Dubini, F., et al. (2004). Degranulation of paneth cells via toll-like receptor 9. Am. J. Pathol. 165, 373–381. doi: 10.1016/S0002-9440(10)63304-4
Sato, T., van Es, J. H., Snippert, H. J., Stange, D. E., Vries, R. G., van den Born, M., et al. (2011). Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469, 415–418. doi: 10.1038/nature09637
Satoh, Y., Habara, Y., Ono, K., and Kanno, T. (1995). Carbamylcholine- and catecholamine-induced intracellular calcium dynamics of epithelial cells in mouse ileal crypts. Gastroenterology 108, 1345–1356. doi: 10.1016/0016-5085(95)90681-9
Schwank, G., Koo, B. K., Sasselli, V., Dekkers, J. F., Heo, I., Demircan, T., et al. (2013). Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell 13, 653–658. doi: 10.1016/j.stem.2013.11.002
Sher, A., Tosh, K., and Jankovic, D. (2017). Innate recognition of Toxoplasma gondii in humans involves a mechanism distinct from that utilized by rodents. Cell. Mol. Immunol. 14, 36–42. doi: 10.1038/cmi.2016.12
Shiono, Y., Mun, H. S., He, N., Nakazaki, Y., Fang, H., Furuya, M., et al. (2007). Maternal-fetal transmission of Toxoplasma gondii in interferon-gamma deficient pregnant mice. Parasitol. Int. 56, 141–148. doi: 10.1016/j.parint.2007.01.008
Speer, C. A., and Dubey, J. P. (1998). Ultrastructure of early stages of infections in mice fed Toxoplasma gondii oocysts. Parasitology 116(Pt 1), 35–42. doi: 10.1017/S0031182097001959
Speer, C. A., Tilley, M., Temple, M. E., Blixt, J. A., Dubey, J. P., and White, M. W. (1995). Sporozoites of Toxoplasma gondii lack dense-granule protein GRA3 and form a unique parasitophorous vacuole. Mol. Biochem. Parasitol. 75, 75–86. doi: 10.1016/0166-6851(95)02515-4
Summerfield, A., Auray, G., and Ricklin, M. (2015). Comparative dendritic cell biology of veterinary mammals. Annu. Rev. Anim. Biosci. 3, 533–557. doi: 10.1146/annurev-animal-022114-111009
Suzuki, Y., Orellana, M. A., Schreiber, R. D., and Remington, J. S. (1988). Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science 240, 516–518. doi: 10.1126/science.3128869
Thomsen-Zieger, N., Schachtner, J., and Seeber, F. (2003). Apicomplexan parasites contain a single lipoic acid synthase located in the plastid. FEBS Lett. 547, 80–86. doi: 10.1016/S0014-5793(03)00673-2
Tosh, K. W., Mittereder, L., Bonne-Annee, S., Hieny, S., Nutman, T. B., Singer, S. M., et al. (2016). The IL-12 response of primary human dendritic cells and monocytes to Toxoplasma gondii is stimulated by phagocytosis of live parasites rather than host cell invasion. J. Immunol. 196, 345–356. doi: 10.4049/jimmunol.1501558
Trevizan, A. R., Vicentino-Vieira, S. L., da Silva Watanabe, P., Góis, M. B., de Melo Gde, A., Garcia, J. L., et al. (2016). Kinetics of acute infection with Toxoplasma gondii and histopathological changes in the duodenum of rats. Exp. Parasitol. 165, 22–29. doi: 10.1016/j.exppara.2016.03.015
Uenishi, H., and Shinkai, H. (2009). Porcine toll-like receptors: the front line of pathogen monitoring and possible implications for disease resistance. Dev. Comp. Immunol. 33, 353–361. doi: 10.1016/j.dci.2008.06.001
Uenishi, H., Shinkai, H., Morozumi, T., and Muneta, Y. (2012). Genomic survey of polymorphisms in pattern recognition receptors and their possible relationship to infections in pigs. Vet. Immunol. Immunopathol. 148, 69–73. doi: 10.1016/j.vetimm.2011.07.019
van Helden, S. F., van den Dries, K., Oud, M. M., Raymakers, R. A., Netea, M. G., van Leeuwen, F. N., et al. (2010). TLR4-mediated podosome loss discriminates gram-negative from gram-positive bacteria in their capacity to induce dendritic cell migration and maturation. J. Immunol. 184, 1280–1291. doi: 10.4049/jimmunol.0900764
Weber, E. M., Algers, B., Hultgren, J., and Olsson, I. A. (2013). Pup mortality in laboratory mice–infanticide or not? Acta Vet. Scand. 55:83. doi: 10.1186/1751-0147-55-83
Weber, E. M., and Olsson, I. A. S. (2008). Maternal behaviour in Mus musculus sp.: an ethological review. Appl. Anim. Behav. Sci. 114, 1–22. doi: 10.1016/j.applanim.2008.06.006
Weidner, J. M., and Barragan, A. (2014). Tightly regulated migratory subversion of immune cells promotes the dissemination of Toxoplasma gondii. Int. J. Parasitol. 44, 85–90. doi: 10.1016/j.ijpara.2013.09.006
Weidner, J. M., Kanatani, S., Hernández-Castañeda, M. A., Fuks, J. M., Rethi, B., Wallin, R. P., et al. (2013). Rapid cytoskeleton remodelling in dendritic cells following invasion by Toxoplasma gondii coincides with the onset of a hypermigratory phenotype. Cell. Microbiol. 15, 1735–1752. doi: 10.1111/cmi.12145
Weight, C. M., and Carding, S. R. (2012). The protozoan pathogen Toxoplasma gondii targets the paracellular pathway to invade the intestinal epithelium. Ann. N.Y. Acad. Sci. 1258, 135–142. doi: 10.1111/j.1749-6632.2012.06534.x
Weight, C. M., Jones, E. J., Horn, N., Wellner, N., and Carding, S. R. (2015). Elucidating pathways of Toxoplasma gondii invasion in the gastrointestinal tract: involvement of the tight junction protein occludin. Microb. Infect. 17, 698–709. doi: 10.1016/j.micinf.2015.07.001
Williamson, I. A., Arnold, J. W., Samsa, L. A., Gaynor, L., DiSalvo, M., Cocchiaro, J. L., et al. (2018). A high-throughput organoid microinjection platform to study gastrointestinal microbiota and luminal physiology. Cell. Mol. Gastroenterol. Hepatol. 6, 301–319. doi: 10.1016/j.jcmgh.2018.05.004
Yarovinsky, F. (2014). Innate immunity to Toxoplasma gondii infection. Nat. Rev. Immunol. 14, 109–121. doi: 10.1038/nri3598
Yarovinsky, F., Zhang, D., Andersen, J. F., Bannenberg, G. L., Serhan, C. N., Hayden, M. S., et al. (2005). TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science 308, 1626–1629. doi: 10.1126/science.1109893
Yu, H., Hasan, N. M., In, J. G., Estes, M. K., Kovbasnjuk, O., Zachos, N. C., et al. (2017). The contributions of human mini-intestines to the study of intestinal physiology and pathophysiology. Annu. Rev. Physiol. 79, 291–312. doi: 10.1146/annurev-physiol-021115-105211
Keywords: intestinal organoids, Apicomplexa, intestinal epithelial barrier, innate response, Toxoplasma gondii, Paneth cells
Citation: Delgado Betancourt E, Hamid B, Fabian BT, Klotz C, Hartmann S and Seeber F (2019) From Entry to Early Dissemination—Toxoplasma gondii's Initial Encounter With Its Host. Front. Cell. Infect. Microbiol. 9:46. doi: 10.3389/fcimb.2019.00046
Received: 15 December 2018; Accepted: 13 February 2019;
Published: 05 March 2019.
Edited by:
Jeroen P. J. Saeij, University of California, Davis, United StatesReviewed by:
Antonio Barragan, Stockholm University, SwedenMelissa Lodoen, University of California, Irvine, United States
Copyright © 2019 Delgado Betancourt, Hamid, Fabian, Klotz, Hartmann and Seeber. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Frank Seeber, c2VlYmVyZkBya2kuZGU=
†These authors have contributed equally to this work
 Estefania Delgado Betancourt
Estefania Delgado Betancourt Benjamin Hamid
Benjamin Hamid Benedikt T. Fabian
Benedikt T. Fabian Christian Klotz
Christian Klotz Susanne Hartmann
Susanne Hartmann Frank Seeber
Frank Seeber