- 1State Key Laboratory for Infectious Disease Prevention and Control, Chinese Center for Disease Control and Prevention, National Institute for Communicable Disease Control and Prevention, Beijing, China
- 2Key Laboratory of Laboratory Medicine, Ministry of Education, School of Laboratory Medicine and Life Sciences, Wenzhou Medical University, Wenzhou, China
The P61 protein is an immunodominant antigen of Nocardia brasiliensis that is observed in the sera from patients infected with the bacterium. However, the B-cell epitopes of N. brasiliensis are still unresolved. To identify the antigenic determinants of P61, we screened seven monoclonal antibodies (mAbs) against P61 protein that was expressed in the Escherichia coli system. A series of truncated peptides of P61 were then generated and the mAbs were used to screen these peptides by Western blot analyses. Three B-cell epitopes were recognized by the P61 specific mAbs: 461-FEYWTKVDPEIGKRIEEG-478, 427-LVREVFNDAQRDRLVSNVVGGVQEPV. LSRVFEYWTKVDPEIGKRIEEGVRAG-482, and 447-HVLGGVQEPVLSRVFEY WTKVDPEI GKRIEEGVRAGLD-484. The latter two epitopes were further identified by N. brasiliensis-infected mouse serum. These results facilitate future investigations of serodiagnostic methods to identify Nocardia infections.
Introduction
Nocardia brasiliensis is a facultative, intracellular, filamentous, gram-positive, and partially acid-fast bacterium that infects skin via traumatic inoculation. Infection produces symptoms of chronic inflammation including fistulas, abscesses, cellulitis, ulcers, and mycetoma (Smego and Gallis, 1984; Salinas-Carmona, 2000; Salinas-Carmona et al., 2009). Infections can later spread to muscles, bones, and adjacent organs. Infections can also be transmitted via cutaneous or respiratory inhalation resulting in CNS disease (Smego and Gallis, 1984; Beaman and Beaman, 1994; Chen et al., 2016). Primary cutaneous nocardiosis is an infectious disease caused by bacteria in the genus Nocardia, but is most often caused by N. brasiliensis infection (Smego and Gallis, 1984; Wilson, 2012; Chen et al., 2016). N. brasiliensis is the primary etiologic agent of human mycetoma in Mexico, and about 86% of the mycetoma cases there are caused by the bacterium (Lopez Martinez et al., 1992; Salinas-Carmona et al., 1992; Licón-Trillo et al., 2003; Castro-Matteotti et al., 2008). The numbers of human nocardiosis cases are increasing in developed countries, and especially in immunocompromised patients (Salinas-Carmona et al., 1992; Brown-Elliott et al., 2006). The proper diagnosis of this disease is therefore important to promote rapid and efficient clinical treatment of infected patients. The diagnosis of mycetoma caused by N. brasiliensis is currently based on isolation and cultivation techniques. However, the confirmation of its presence using conventional microbiological techniques usually takes quite a long time, and it is also difficult to clinically differentiate N. brasiliensis infections from cutaneous infection by Staphylococcus aureus and Streptococcus pyogenes (Chen et al., 2016). Further, N. brasiliensis is closely related to Mycobacterium tuberculosis and shares many of its morphological, antigenic, and physiological characteristics (Castro-Matteotti et al., 2008). Due to a lack of purified antigens, the cross-reactivity of N. brasiliensis antigens with sera from leprosy and tuberculosis patients remains an important, unresolved problem in disease diagnosis (Humphreys et al., 1975; Salinas-Carmona et al., 1992).
Two proteins from a culture filtrate of N. asteroides with molecular weights of 55,000 and 31,000 Da have been demonstrated as highly specific markers to identify patients infected with N. asteroides, and can thus be used as diagnostic tools for nocardiosis (Sugar et al., 1985). In addition, Vera-Cabrera et al. purified two proteins from a crude extract of N. brasiliensis, termed P61 and P24. These two proteins are recognized by sera from nocardial mycetoma patients, but not sera from mycobacterial-infected or healthy controls, and therefore could have great value in the serological diagnosis of N. brasiliensis-infected patients (Vera-Cabrera et al., 1992). The P61 protein is encoded by the gene O3I_001640 and is the target of the humoral immune response in patients suffering from nocardial mycetoma (Gordon et al., 2013). This immunodominant protein is highly conserved in the Nocardia genus (Vera-Cabrera et al., 1999), furthering its potential as a tool for clinical diagnosis of nocardiosis.
Clinical diagnosis assays relying on synthesized peptides are considered to have more advantages than those using recombinant or native protein antigens (Goyal et al., 2014). Consequently, it is important to analyze specific epitopes for the development of epitope peptide-based diagnostic tools. B-cell epitopes are regions on the surface of the native antigen that are recognized by binding to B-cell receptors or specific antibodies (Viudes et al., 2001; Zhang et al., 2015). To date, there has been no validation of a peptide-based serodiagnostic assay of the P61 protein. In this study, we generated seven monoclonal antibodies (mAbs) against recombinant P61 protein and used them to screen for B-cell epitopes using Western blot analyses. Two epitopes were additionally recognized by N. brasiliensis-infected mouse serum. These results represent a promising first step in the development of specific serological tools for nocardiosis diagnosis.
Materials and Methods
Ethics Statement
Laboratory animal care and experimentation were conducted in accordance with animal ethics guidelines and protocols approved by the Ethics Review Committee of the National Institute for Communicable Disease Control and Prevention at the Chinese Center for Disease Control and Prevention.
Bacterial Strains, Plasmids, and Animals
The standard strain of N. brasiliensis (ATCC700358) was purchased from the German Resource Centre for Biological Materials and grown in brain-heart-infusion (BHI) medium (Difco Laboratories, Detroit, MI), as previously described (Vera-Cabrera et al., 1992; Salinas-Carmona et al., 1999). pET30a and pMAL-c5x plasmids were used as expression vectors (New England Biolabs, Beijing) and E. Coli strain BL21 (DE3) was used as the vector host. E. coli (TransGen Biotech, China) was grown in Luria-Bertani (LB) medium. Female BALB/c mice that were 9–12 weeks of age were maintained under pathogen-free conditions and used for serological testing.
Preparation of P61 Protein
P61 protein that is used as an antigen in the generation of mAbs was expressed in BL21 (DE3) E. coli cells. Briefly, the katN gene codon was optimized and synthesized by Sangon Biotech (the restriction endonuclease sites are NdeI and HindIII), cloned into the expression vector pET-30a(+), and transformed into BL21 (DE3) competent cells. Recombinant BL21 cells were cultured at 37°C in LB medium containing 50 μg/ml kanamycin with agitation until their optical density (measured at 600 nm) reached 0.8. Expression was then induced with 0.2 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) at 30°C for 6 h. Cells were sonicated and centrifuged at 12,000 rpm for 20 min at 4°C. The supernatant was then carefully collected. Production of recombinant P61 protein was evaluated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The expressed P61 proteins were purified with a Ni-NTA kit (Novagen) according to the manufacturer's instructions and stored at −80°C.
The purified, recombinant P61 protein was confirmed by Western blot analysis. Briefly, purified recombinant P61 and N. brasiliensis whole-cell protein were electro-blotted onto a polyvinylidene fluoride (PVDF) membrane at 100 mA for 1 h, then blocked overnight at 4°C in blocking buffer (5% skim milk in PBS, pH 7.4, with 0.05% Tween 20). Membranes were incubated at room temperature with anti-N. brasiliensis mouse serum for 2 h, and then incubated with HRP-conjugated goat anti-mouse IgG (TransGen Biotech, China) for 1 h. Protein detection was performed using chemiluminescent luminol reagents (Takara, China).
Preparation and Identification of mAbs Against P61 Protein
We used standard hybridoma techniques to screen for specific anti-p61 MAbs (Chaithirayanon et al., 2002). Briefly, purified His-P61 protein was emulsified with equal volumes of complete/incomplete Freund's adjuvant (Sigma–Aldrich) at a final concentration of 0.25 mg/ml. Female BALB/c mice (6–8 weeks old) were subcutaneously immunized with purified His-P61 protein in 2 week intervals over 6 weeks to generate hybridoma lines that secreted antibodies. The spleen cells of immunized BALB/c mice were fused with mouse myeloma cells (SP2/0) 3 days after the last injection. The fused hybridoma clones were then screened by indirect enzyme-linked immunosorbent assays (ELISA) for mAbs exhibiting strong reactivity to the P61 protein. Selected clones that produced mAbs against the P61 protein were subcloned three times by limiting dilution. Ascites was induced in pristine-primed BALB/c mice. Finally, the mAbs were identified by Western blot and indirect ELISA analyses. Additionally, mAb subtypes were identified using a SBA Clonotyping System/HRP Kit (Southern Biotech, USA).
Expression of P61-Derived Polypeptides and Screening by Western Blot Analysis
To identify the approximate locations of the epitopes on the P61 protein, consecutively truncated P61 protein fragments were expressed in E. Coli. Primers were then designed that were specific for the P2–P19 fragments (Table 1). The P2, P3, and P4 fragment genes were cloned into pET30a vectors and the rest of the fragments were cloned into pMAL-c5x vectors. Recombinant BL21 E. coli cells were cultured at 37°C in LB medium containing 50 μg/ml kanamycin with agitation until their optical density (measured at 600 nm) reached 0.8. The cells were subsequently induced with 1 mM IPTG at 16°C for 6 h. Fragments that reacted with the mAbs were then screened by Western blot analysis. Briefly, the expressed polypeptides were subjected to SDS-PAGE and electro-blotted onto PVDF membranes. The membranes were then incubated with mAbs followed by a secondary HRP-conjugated goat anti-mouse antibody.
Identification of the Precise Location of Epitopes
In order to identify the minimal antigenic epitopes that were recognized by our P61 mAbs, consecutive primers (Table 1) or genes (Table 2) were synthesized and cloned into the pMAL-c5x expression vector. Maltose-binding protein (MBP)-fused recombinant proteins were expressed in BL21 E. coli cells and then identified by SDS-PAGE. These proteins were then used to determine the epitopes that were recognized by mAbs using the Western blot protocol described above.
Reactivity of the Identified B-Cell Epitopes With N. brasiliensis-Positive Mouse Serum
To assess whether the screened B-cell epitopes of P61 could be identified by N. brasiliensis-positive mouse serum, the MBP-fused proteins containing the peptides A3, A7, B, B1, B6, and B7 were tested with N. brasiliensis-positive mouse serum using Western blot analysis, as described above. N. brasiliensis-positive mouse serum was used as the primary antibody and HRP-conjugated goat anti-mouse IgG was used as the secondary antibody.
Location of the B-Cell Epitopes in the P61 Protein
The three-dimensional (3D) structure of the P61 protein was unavailable in the Protein Data Bank (PDB). Consequently, we predicted its structure by 3D-modeling the P61 protein with the SWISSMODEL online sever. We then investigated the location of the epitopes on the protein using the Pymol software package, and presented them diagrammatically in the predicted protein model.
Results
Expression, Purification, and Identification of Recombinant P61 Protein
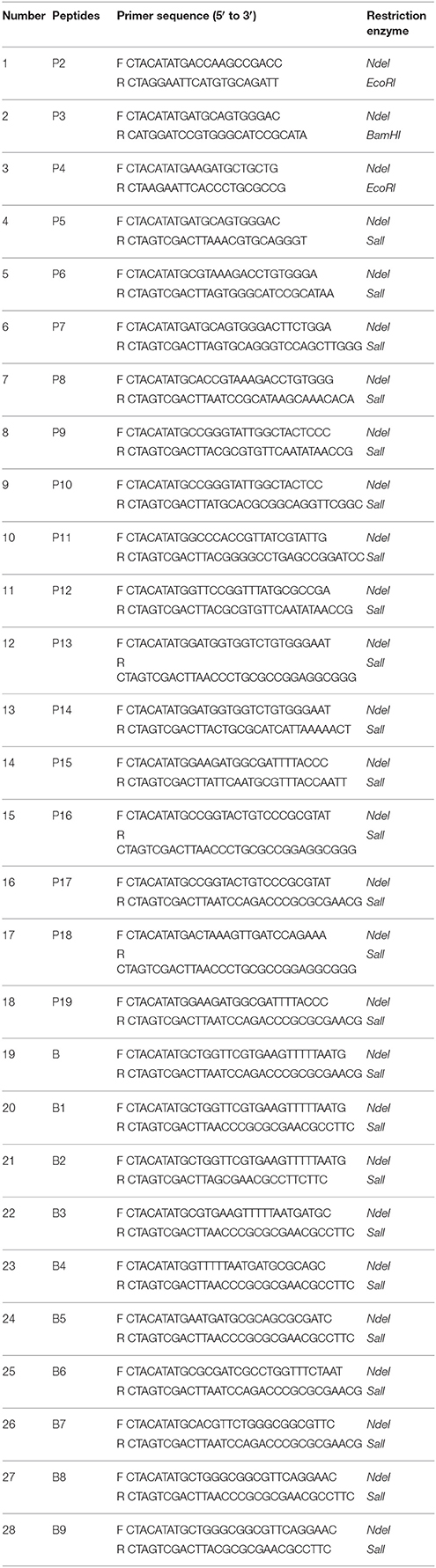
Recombinant P61 fused with a six-histidine tag (His-P61) was successfully expressed in BL21 (DE3) E. coli cells and a Ni–NTA affinity kit was used to produce purified P61 protein. Subsequent SDS-PAGE indicated that the P61 protein was soluble at 30°C, was present in its intact form, and had a molecular weight of 61 kDa (Figure 1A). Purified P61 protein exhibited a single band in Western blot analysis (Figure 1B). Western blot analysis further revealed that the P61 protein can be specifically identified by the endogenous antibodies present in serum from N. brasiliensis-infected mice (Figure 1C).
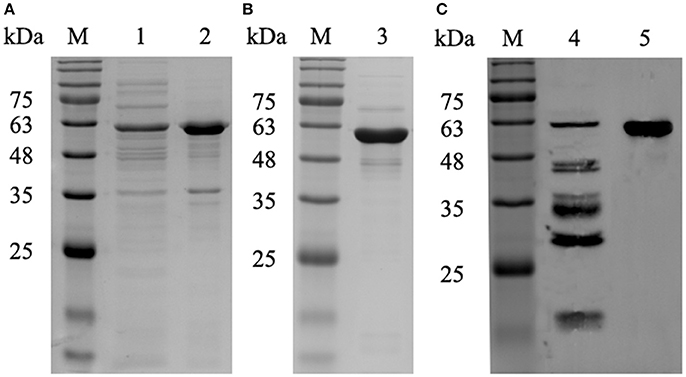
Figure 1. SDS-PAGE and Western blot analysis of the P61 protein. Lane M: protein marker. (A) Lanes 1 and 2: supernatant and precipitate fractions, respectively, of recombinant BL21 cells expressing P61 protein that were cultured for 6 h with 0.2 mM IPTG at 30°C. (B) Lane 3: purified P61 protein. (C) Lane 4: whole-cell protein of N. brasiliensis immunoblotted with the sera of mice infected with N. brasiliensis; Lane 5: purified P61 immunoblotted with the sera of mice infected with N. brasiliensis.
Production and Characterization of mAbs Against P61 Protein
After screening of hybridoma cells by indirect ELISA, we selected hybridoma cells that secreted antibodies to the P61 protein. Seven mAbs were thus generated and designated as 2A7, 4A10, 4E9, 8E15, 9A8, 9H9, and 10C6. The isotypes of the mAbs are shown in Table 3. The titers of the mAbs in cell cultures were greater than 1:2430 and the titers of the mAbs in the ascites fluid were 1:1 × 105-1 × 106. The mAbs reacted with recombinant P61 protein, but could not recognize another recombinant Mce1C protein that also contained the His fusion tag and that is also expressed by Nocardia spp. (Figure 2). These results indicate the specificity of the mAbs for the P61 protein of N. brasiliensis.
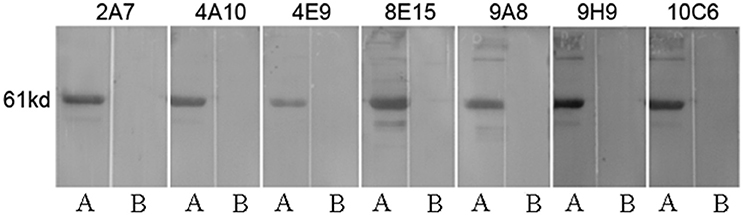
Figure 2. Specificity of the mAbs to recombinant His-P61 protein demonstrated by Western blot. Lane A: purified recombinant P61 protein; Lane B: recombinant His-Mce1C protein as the negative control.
Identification of the Truncated P61 Protein
To identify the approximate location of the P61 epitope, a series of overlapping P61 peptides (designated P1–P19) were generated, expressed, and analyzed (Figure 3). Of these, mAb 8E15 recognized the P17 peptide of the P61 protein and mAbs 2A7, 4A10, 4E9, 9A8, 9H9, and 10C6 recognized the P19 peptide.
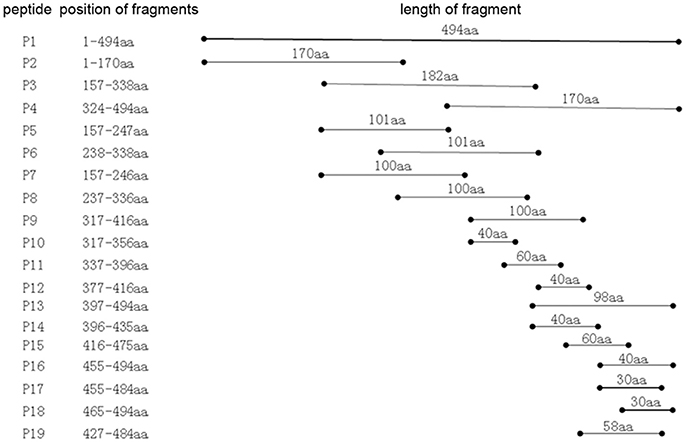
Figure 3. Schematic diagram of the P61 protein. Truncated peptides of the P61 protein were generated and used to identify P61 regions of reactivity with mAbs by Western blot analysis.
Mapping of the Epitopes to the P61 Protein
To map the minimal sequences of the epitopes recognized by the mAbs on the P61 protein, we generated a series of further truncated peptides from the P17 and P19 fragments (labeled A and B in Figure 4). These truncated peptides (A1–14 and B1–9) were reacted with the mAbs using Western blot analyses (Figure 5). Of these, mAb 8E15 reacted with fragments A1–A6, but not A7–A14; mAbs 4A10 and 4E9 reacted with fragments B5, B6, and B7, but not B8 and B9; and lastly, mAbs 2A7, 9A8, 9H9, and 10C6 reacted with fragments B and B1, but not B2–B5. Specifically, mAb 8E15 recognized the epitope comprising amino
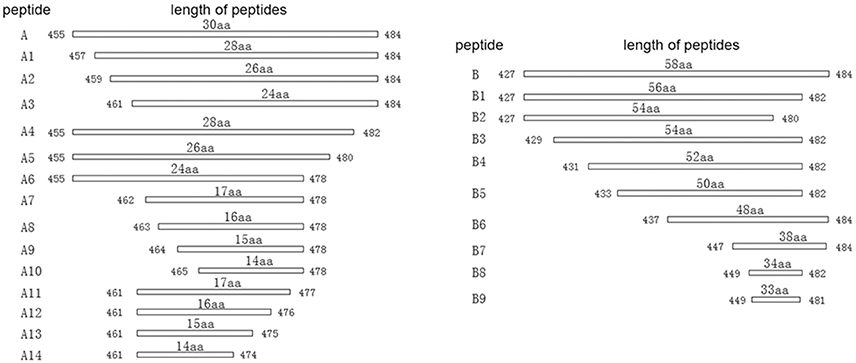
Figure 4. Schematic diagram of P17 (A) and P19 (B) fragments. The P17 (A) fragment was truncated into 14 different peptides for subsequent reaction with mAb 8E15 by Western blot analysis (left). The P19 (B) fragment was truncated into nine different peptides for subsequent reaction with mAbs 2A7, 4A10, 4E9, 9A8, 9H9, and 10C6 via Western blot analysis.
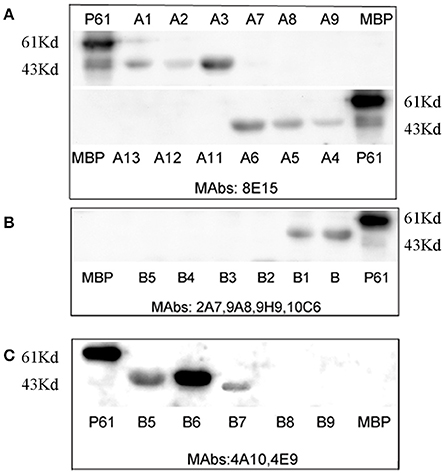
Figure 5. Minimal sequences of the epitopes were mapped using Western blot analysis. The full-length P61 protein was used as a positive control and MBP alone was the negative control in each blot. (A) The epitopes that were detected using mAb 8E15. (B) The epitopes detected using mAbs 2A7, 9A8, 9H9, and 10C6. (C) The epitopes detected using mAbs 4A10 and 4E9.
acid (aa) positions 461–478 aa of the P61 protein, mAbs 4A10, and 4E9 recognized the epitope at 447–484 aa, and mAbs 2A7, 9A8, 9H9, and 10C6 recognized the epitope at 427–482 aa. All of the epitopes that were recognized by the antibodies were in overlapping regions of the P61 protein between amino acids 427 and 484.
Reactivity of the Identified B-Cell Epitopes With N. brasiliensis-Positive Mouse Serum
The antigenicities of the screened epitopes were determined with N. brasiliensis-positive mouse serum. Peptides of P61 encompassing the regions 427–484, 427–482, 437–484, 447–484, and 461–484 aa reacted with N. brasiliensis-positive mouse serum (Figure 6). The peptide within the amino acid sequence 461–478 aa did not react at all with the infected serum. These results suggest that the screened epitopes at positions 427–482 and 447–484 aa are robustly identified by N. brasiliensis-positive mouse serum, and can potentially be used as markers for the diagnosis of nocardiosis caused by N. brasiliensis infection.

Figure 6. Reactivity of epitopes was analyzed with N. brasiliensis-positive mouse serum by Western blot. Lane 1–6: peptide 427–484 aa, peptide 427–482 aa, peptide 437–484 aa, peptide 447–484 aa, peptide 461–484 aa, and peptide 462–478 aa, respectively, immunoblotted with N. brasiliensis-infected mouse serum. Lane 7: MBP (negative control).
Location of the Screened Epitopes in the Fully Folded P61 Protein
The location of the screened, overlapping epitopes comprising the positions 427–482, 447–484, and 461–478 aa were determined within the three–dimensional structure of the P61 protein. The 3D structural modeling analyses indicated that these three peptides were located on the external surface of the P61 protein (Figure 7).
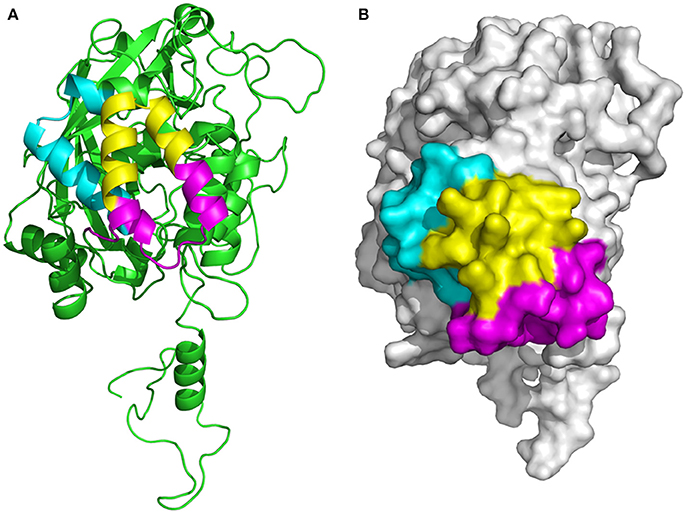
Figure 7. Determination of the location of epitopes on the P61 protein. (A) Ribbon diagram of the P61 protein. (B) Space-filling 3D model of P61. The screened epitopes (of P61 amino acid sequences) 427–446 aa is highlighted in cyan, 447–460 and 479–484 aa are highlighted in magenta, and 461–478 aa is highlighted in yellow.
Discussion
The P61 protein is a highly conserved, immunodominant protein in Nocardia bacteria, and is the target of the body's humoral response to Nocardia infection. Thus, the protein is a potentially valuable tool for nocardiosis disease diagnosis (Vera-Cabrera et al., 1999). Peptide-based tools play a critical role in clinical diagnoses and the use of a specific epitope to diagnose nocardiosis could avoid misdiagnoses that result from cross-reaction with bacteria similar to N. brasiliensis. However, no studies have been conducted to investigate epitopes of the N. brasiliensis P61 protein. In this study, mice were immunized with purified, recombinant His-P61 protein and seven mAbs were produced and were then used to screen for the B-cell epitope of P61. All of the mAbs reacted with the full-length P61 protein and truncated peptides of P61.
During initial screening, the P61 peptide comprising the amino acid sequence positions of 455–484 aa was screened by mAb 8E15 and the peptide comprising the positions 427–484 aa was screened by mAbs 2A7, 4A10, 4E9, 9A8, 9H9, and 10C6. These results indicated that the amino acid sequences recognized by the mAbs clearly overlap. As expected, mAb 8E15 recognized both of the peptides, while mAbs 2A7, 4A10, 4E9, 9A8, 9H9, and 10C6 only reacted with the peptide comprising positions 427–484 aa. To identify the minimal epitope (minimum amino acid positions of P61 required for antibody binding), a series of further truncated peptides were reacted with the same mAbs by Western blot analysis. Three minimal sequences were subsequently isolated: the epitope 461-FEYWTKVD PEIGKRIEEG-478 was recognized by mAb 8E15; the epitope 427-LVREVFNDAQRDRLVSNVVG GVQEPVLSRVFEYWTKV.
DPEIGKRIEEGVRAG-482 was recognized by mAbs 2A7, 9A8, 9H9, and 10C6; and the epitope 447-HVLGGVQEP VLSRVFEYW TKVDPEI GKRIEE GVRAGLD −484 was recognized by mAbs 4A10 and 4E9. These three epitopes were then expressed in the pMAL-c5x plasmid vector and their reactivity with N. brasiliensis-positive mouse serum was determined. Results from these experiments indicated that epitopes comprising the positions 427–482 and 447–484 aa were identified by N. brasiliensis-positive serum, but that epitope 461–478 aa was not identified by the serum. Interestingly, the peptide comprising positions 461–484 aa, which overlapped with the 461–478 aa peptide, successfully reacted with infected mouse serum, suggesting that positions 461–484 on the P61 protein contains critical binding sites for the mAbs. However, it is possible that the MBP fusion tag was large enough to block the binding sites in peptide 461–478 aa, while leaving sufficient binding sites open on the larger peptide 461–484 aa. The SWISSMODEL online server was used to predict the 3D structure of the P61 protein and Pymol was used to map the locations of the screened epitopes on the 3D model of P61.
All three screened peptides were identified on the external surface of the P61 protein, indicating that they would be easily recognized by the infected host's immune cells. In future investigations, serum will be collected from patients infected with N. brasiliensis to verify the applicability of the screened peptides for clinical diagnosis of this bacterial infection. Peptides will then be synthesized and their reactivity will be verified using ELISA.
In conclusion, we produced seven mAbs for the P61 protein of N. brasiliensis and isolated three epitopes from the P61 protein (461–478, 427–482, and 447–484 aa) that can facilitate the future development of a serodiagnostic tool for nocardiosis diagnosis.
Author Contributions
XJ and JL conceived and designed the experiments. XJ wrote the manuscript. XJ and NS performed the experiments. XJ and XH analyzed the data. QL, SX, TQ, LT, and BW contributed reagents, materials, analysis tools. JL supported the research financially and administratively, and provided final approval of manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the National Key R&D Program of China (grant number 2017YFC1200303, 2016YFC1200701); the China Special Grant for the Prevention and Control of Infectious Diseases (grant number 2017ZX10303401).
References
Beaman, B. L., and Beaman, L. (1994). Nocardia species: host-parasite relationships. Clin. Microbiol. Rev. 7, 213–264. doi: 10.1128/CMR.7.2.213
Brown-Elliott, B. A., Brown, J. M., Conville, P. S., and Wallace, R. J. Jr. (2006). Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin. Microbiol. Rev. 19, 259–282. doi: 10.1128/CMR.19.2.259-282.2006
Castro-Matteotti, B., Vera-Cabrera, L., Ocampo-Candiani, J., Rendón, A., Salinas-Carmona, M. C., and Welsh, O. (2008). Immune response to Nocardia brasiliensis extracellular antigens in patients with mycetoma. Mycopathologia 165, 127–134. doi: 10.1007/s11046-008-9093-4
Chaithirayanon, K., Wanichanon, C., Vichasri-Grams, S., Ardseungneon, P., Grams, R., Viyanant, V., et al. (2002). Production and characterization of a monoclonal antibody against 28.5 kDa tegument antigen of Fasciola gigantica. Acta Trop. 84, 1–8. doi: 10.1016/S0001-706X(02)00138-9
Chen, B., Tang, J., Lu, Z., Wang, N., Gao, X., and Wang, F. (2016). Primary cutaneous nocardiosis in a patient with nephrotic syndrome: a case report and review of the literature. Medicine 95:e2490. doi: 10.1097/md.0000000000002490
Gordon, S. V., Vera-Cabrera, L., Ortiz-Lopez, R., Elizondo-Gonzalez, R., and Ocampo-Candiani, J. (2013). complete genome sequence analysis of nocardia brasiliensis HUJEG-1 reveals a saprobic lifestyle and the genes needed for human pathogenesis. PLoS ONE 8:e65425. doi: 10.1371/journal.pone.0065425
Goyal, B., Kumar, K., Gupta, D., Agarwal, R., Latawa, R., Sheikh, J. A., et al. (2014). Utility of B-cell epitopes based peptides of RD1 and RD2 antigens for immunodiagnosis of pulmonary tuberculosis. Diagn. Microbiol. Infect. Dis. 78, 391–397. doi: 10.1016/j.diagmicrobio.2013.12.018
Humphreys, D. W., Crowder, J. G., and White, A. (1975). Serological reactions to nocardia antigens. Am. J. Med. Sci. 269, 323–326. doi: 10.1097/00000441-197505000-00005
Licón-Trillo, A., Angeles Castro-Corona, M., and Salinas-Carmona, M. C. (2003). Immunogenicity and biophysical properties of a Nocardia brasiliensisprotease involved in pathogenesis of mycetoma. FEMS Immunol. Med. Microbiol. 37, 37–44. doi: 10.1016/s0928-8244(03)00102-0
López Martínez, R., Méndez Tovar, L. J., Lavalle, P., Welsh, O., Saúl, A., and Macotela Ruíz, E. (1992). Epidemiology of mycetoma in Mexico: study of 2105 cases. Gac. Med. Mex. 128, 477–481.
Salinas-Carmona, M. C. (2000). Nocardia brasiliensis: from microbe to human and experimental infections. Microbes Infect. 2, 1373–1381. doi: 10.1016/S1286-4579(00)01291-0
Salinas-Carmona, M. C., Torres-Lopez, E., Ramos, A. I., Licon-Trillo, A., and Gonzalez-Spencer, D. (1999). Immune response to nocardia brasiliensis antigens in an experimental model of actinomycetoma in BALB/c mice. Infect. Immun. 67, 2428–2432.
Salinas-Carmona, M. C., Vera, L., Welsh, O., and Rodríguez, M. (1992). Antibody response to Nocardia brasiliensis antigens in man. Z. Bakteriol. 276, 390–397. doi: 10.1016/s0934-8840(11)80546-3
Salinas-Carmona, M. C., Zúñiga, J. M., Pérez-Rivera, L. I., Segoviano-Ramírez, J. C., and Vázquez-Marmolejo, A. V. (2009). Nocardia brasiliensis modulates IFN-gamma, IL-10, and IL-12 cytokine production by macrophages from BALB/c Mice. J. Interferon Cytokine Res. 29, 263–271. doi: 10.1089/jir.2008.0059
Smego, R. A. Jr., and Gallis, H. A. (1984). The clinical spectrum of Nocardia brasiliensis infection in the United States. Rev. Infect. Dis. 6, 164–180. doi: 10.1093/clinids/6.2.164
Sugar, A. M., Schoolnik, G. K., and Stevens, D. A. (1985). Antibody response in human nocardiosis: identification of two immunodominant culture-filtrate antigens derived from Nocardia asteroides. J. Infect. Dis. 151, 895–901. doi: 10.1093/infdis/151.5.895
Vera-Cabrera, L., Johnson, W. M., Welsh, O., Resendiz-Uresti, F. L., and Salinas-Carmona, M. C. (1999). Distribution of a nocardia brasiliensiscatalase gene fragment in members of the generanocardia, gordona, andrhodococcus. J. Clin. Microbiol. 37, 1971–1976.
Vera-Cabrera, L., Salinas-Carmona, M., Welsh, O., and Rodriguez, M. A. (1992). Isolation and purification of two immunodominant antigens from Nocardia brasiliensis. J. Clin. Microbiol. 30, 1183–1188.
Viudes, A., Perea, S., and Lopez-Ribot, J. L. (2001). Identification of continuous B-cell epitopes on the protein moiety of the 58-kiloDalton cell wall mannoprotein of Candida albicans belonging to a family of immunodominant fungal antigens. Infect. Immun. 69, 2909–2919. doi: 10.1128/iai.69.5.2909-2919.2001
Wilson, J. W. (2012). Nocardiosis: updates and clinical overview. Mayo Clin. Proc. 87, 403–407. doi: 10.1016/j.mayocp.2011.11.016
Keywords: Nocardia brasiliensis, P61, monoclonal antibodies, B-cell epitope, serodiagnostic
Citation: Ji X, Sun NL, Hou XX, Xu S, Qiu TX, Tang L, Li QH, Wang BX and Li JZ (2018) Screening and Identification of B-Cell Epitopes in the P61 Protein of Nocardia brasiliensis. Front. Cell. Infect. Microbiol. 8:224. doi: 10.3389/fcimb.2018.00224
Received: 09 April 2018; Accepted: 12 June 2018;
Published: 02 July 2018.
Edited by:
Wenjun Liu, Institute of Microbiology (CAS), ChinaReviewed by:
José Ascención Martínez-Álvarez, Universidad de Guanajuato, MexicoDapeng Wang, Shanghai Jiao Tong University, China
Copyright © 2018 Ji, Sun, Hou, Xu, Qiu, Tang, Li, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Zhen Li, bGl6aGVuanVuQGljZGMuY24=
 Xingzhao Ji
Xingzhao Ji Na Li Sun1
Na Li Sun1 Shuai Xu
Shuai Xu