- 1Laboratory Breeding Base of Green Pesticide and Agricultural Bioengineering, Key Laboratory of Green Pesticide and Agricultural Bioengineering Ministry of Education, Guizhou University, Guiyang, China
- 2School of Pharmacy, Guizhou University of Traditional Chinese Medicine, Guiyang, China
- 3R&D Center, Shenzhen AmTech Bioengineering Ltd., Inc., Shenzhen, China
A chemo- and diastereo-selective (3 + 2) cycloaddition reacition between Donor-Acceptor (D-A) cyclopropanes and α,β-unsaturated enamides is developed for efficient access to spiro(cyclopentane-1,3'-indoline) derivatives. Simple, inexpensive and readily available NaOH is used as the sole catalyst for this process. A broad range of D-A cyclopropanes could be used as the C-3 synthons to react with oxindole-derived α,β-unsaturated enamides. The structurally sophisticated spiro(cyclopentane-1,3'-indoline) derivatives bearing up to 3 adjacent chiral centers are afforded in excellent yields as single diastereomers.
Introduction
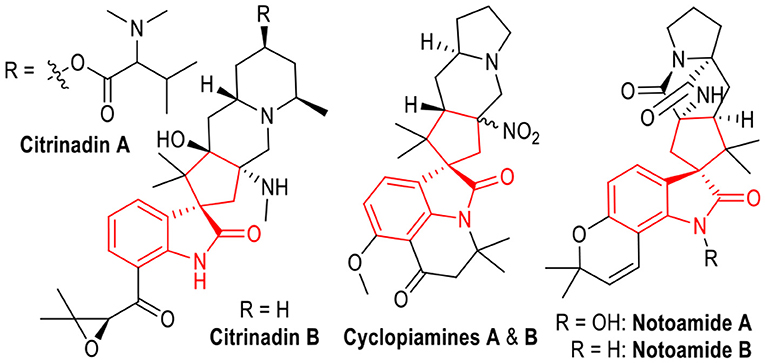
Spirocyclopentanes are interesting structural units with broad applications in organic synthesis and medicinal chemistry. They have existed as core structures in various bioactive molecules (Boeyens et al., 1979; Tsuda et al., 2004; Mugishima et al., 2005; Zhang et al., 2019). Specifically, spiro(cyclopentane-1,3'-indoline) derivatives are frequently found in natural products with proven biological activities (Figure 1). For example, Citrinadin A and B are active molecules against murine leukemia L1210 and human epidermoid carcinoma KB cells, which have been isolated from a culture broth of Penicillium citrinum. Cyclopiamines A and B are extracts from a toxinogenic strain of Penicillium cyclopium. The Notoamides A and B are key members of paraherquamide family which belongs to prenylated indole alkaloids that exhibit various bioactivities including antitumor, antibacterial, and insecticidal properties. Therefore, the development of efficient methods for the preparation of spiro(cyclopentane-1,3'-indoline) derivatives has attracted much interest. Success within this field has been achieved through both organocatalysis (Chen et al., 2009; Antonchick et al., 2010; Tan et al., 2011; Tian and Melchiorre, 2013; Zhang et al., 2016; Chaudhari et al., 2017) and transition metal catalysis (Trost et al., 2007; Brazeau et al., 2012; Ball-Jones et al., 2014; Deiana et al., 2014; Afewerki et al., 2015; Frost et al., 2015; Qiu et al., 2019). Despite of the great achievement obtained in the synthesis of spiro(cyclopentane-1,3'-indoline) molecules, the development of green and economical methods for efficient and stereoselective synthesis of them is still of great interest.
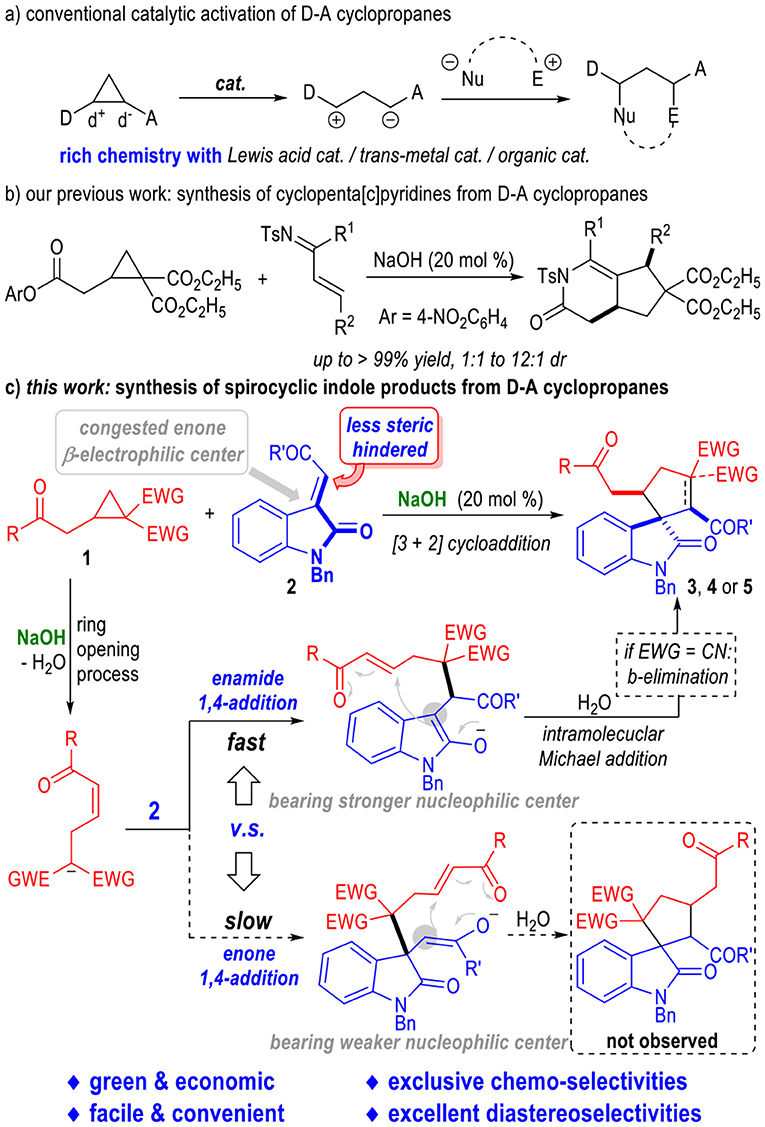
Cyclopropanes are important building blocks in organic synthesis (Sohn and Bode, 2006; Bode and Sohn, 2007; Li et al., 2009; Lv et al., 2011; Sparr and Gilmour, 2011; Halskov et al., 2015; Sanchez-Diez et al., 2016; Blom et al., 2017; Apel et al., 2019). Especially, the cyclopropanes bearing both an electron-donating and an electron-withdrawing group on their cyclic structures, which are commonly named as Donor-Acceptor (D-A) cyclopropanes (Danishefsky, 1979; Wenkert, 1980; Reissig and Zimmer, 2003; Carson and Kerr, 2009; Cavitt et al., 2014; Nanteuil et al., 2014; Schneider et al., 2014; Grover et al., 2015; Talukdar et al., 2016; Wang and Tang, 2016; Werz and Biju, 2019), have been extensively studied in the construction of various functional molecules. D-A Cyclopropanes are conventionally activated by transition metal catalysts (Nanteuil et al., 2014), Lewis acids (Reissig and Zimmer, 2003; Carson and Kerr, 2009; Cavitt et al., 2014; Schneider et al., 2014; Grover et al., 2015; Talukdar et al., 2016; Wang and Tang, 2016; Werz and Biju, 2019) or amine-based organic catalysts (Halskov et al., 2015; Sanchez-Diez et al., 2016; Blom et al., 2017) (Figure 2a). Efficient activation of D-A cyclopropanes by simple, inexpensive and readily available bases has been much less developed.
Very recently, we have disclosed that the D-A cyclopropanes could be activated by simple NaOH and reacted with α,β-unsaturated imines to give a variety of bioactive cyclopenta(c)pyridine derivatives in generally excellent yields and moderate diastereoselectivities (Pan et al., 2019) (Figure 2b). This approach has provided us with a green and facile method for the construction of structurally complex molecules from D-A cyclopropanes with simple and inexpensive reaction catalysts. Therefore, it is interesting and important to extend the border of this strategy to a wide range of substrates in order to get access to a broad scope of complex functional molecules in a green, facile, and economic fashion.
Herein, we disclose that the D-A cyclopropanes 1 can react with α,β-unsaturated enamide substrates 2 under basic conditions in chemoselective fashion (Figure 2c). Heavily substituted spiro(cyclopentane-1,3'-indoline) derivatives could be afforded in good to excellent yields. NaOH was used as the sole reaction catalyst. All the spirocyclic products bearing up to 3 adjacent chiral centers were afforded as single diastereomers. It is worth noting that both an enone and an enamide motif exist in the electrophilic substrate 2. After deprotonation of the D-A cyclopropane substrate 1, the afforded ring-opening intermediate I could selectively react with the electrophile 2 through an enamide 1,4-addition process and gave intermediate II bearing a highly reactive nucleophilic carbon center. The enamide 1,4-addition reaction was believed to go faster than the enone 1,4-addition reaction because that there were less steric hindrance around the enamide β-carbon. After an intramolecular Michael addition process, the spiral cyclopentane products 3 or 4 were afforded in excellent diastereoselectivities. Interestingly, an additional β-elemination could happen during this catalytic transformation when using the D-A cyclopropyl ketone bearing gem-dicyano groups as the reaction substrate. Spiral cyclopentenes 5 were afforded as the final products in this case. Product 6 that might be formed from the enone 1,4-addition intermediate III were not observed. The less nucleophilicity of the enol moiety of the intermediate III might be another reason for the difficult formation of the enone 1,4-addition products.
Results and Discussion
Reaction Condition Optimization and Large-Scale Reactions
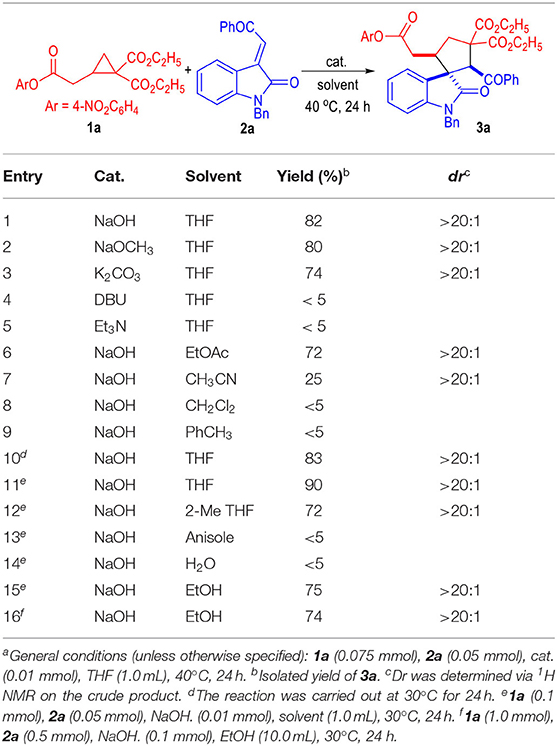
The 2-cyclopropyl acetate 1a and the oxindole-derived α,β-unsaturated enamide 2a were selected as the model substrates to test the catalytic conditions for this (3 + 2) cycloaddition reaction (Table 1). A variety of inorganic bases were found efficient for this 1,3-dipolar cycloaddition reaction between 2-cyclopropyl acetate 1a and the α,β-unsaturated enamide 2a (Table 1, entries 1 to 3). The organic bases we tested were not suitable for this reaction (entries 4 to 5). The catalytic cyclization process could also be carried out in several organic solvents with relatively high polarities, although the yields were generally decreased (entries 6 to 7). Solvents with low polarities could not be used for this transformation (entries 8 to 9). The reaction temperature could be slightly decreased to 30°C without erosion of the product yield (entry 10). Finally, the yield of the spiro(cyclopentane-1,3'-indoline) product 3a could be increased to 90% with a larger excess amount of 1a used under the catalysis of NaOH in THF at 30°C (entry 11). Note that, all the products afforded in these reactions were obtained as single diastereomers.
We were also very interested in developing a green and efficient method for the construction of the spiro(cyclopentane-1,3'-indoline) product 3a. Therefore, several green solvents were further examined after obtaining the optimized reaction condition (Table 1, entries 12 to 15). 2-Methyl-substituted THF could give the desired product in a good yield (entry 12). Anisole or water could not be used as the solvents for this transformation (entries 13 to 14). To our delight, the inexpensive and non-toxic ethanol could be used as a suitable medium for the construction of the spiro(cyclopentane-1,3'-indoline) products through this protocol (entry 15). Therefore, we carried out a large-scale reaction of the substrate 1a and 2a in ethanol, with the desired product 3a afforded in a 74% yield as a single diastereomer (entry 16).
Reaction Scope Investigation and Synthetic Application
With the optimized reaction conditions at hand (Table 1, entry 11), we then examined the substrate scope of this (3 + 2) cycloaddition reaction with respect to both D-A cyclopropyl acetates 1 and α,β-unsaturated enamides 2 (Tables 2–4).
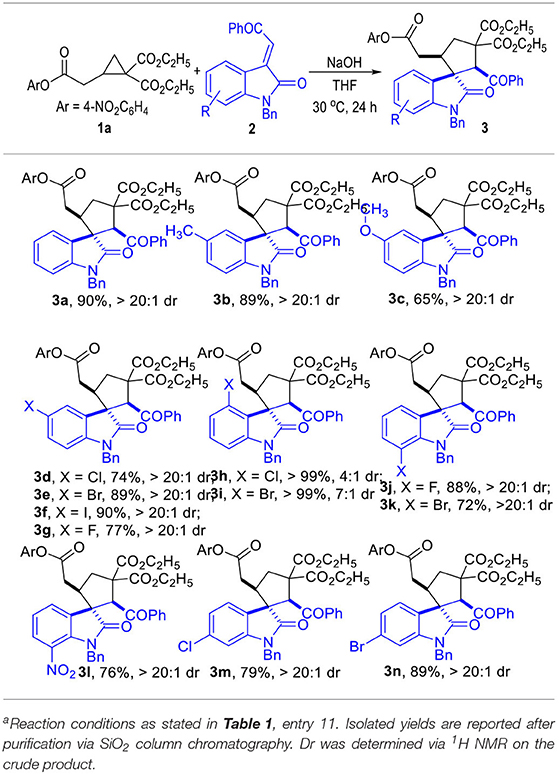
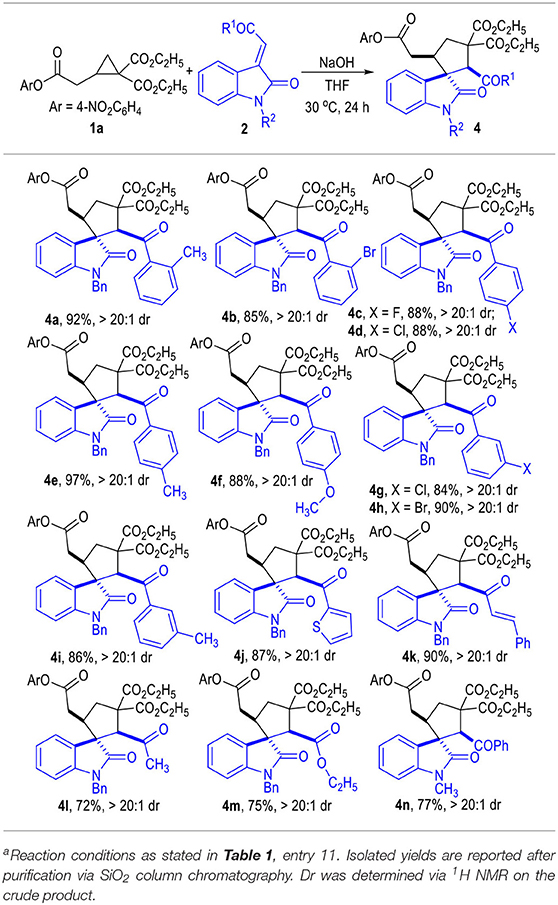
The R substituent on the phenyl group of the indoline motif of the α,β-unsaturated enamides 2 could be either electron-donating groups (Table 2, 3b and 3c) or electron-withdrawing groups (3d to 3n), with most of the spirocyclic products being afforded in good to excellent yields and diastereo-selectivities.
The R1 group of ketone moieties could be phenyl rings of different substitution patterns, with the corresponding products being afforded in excellent yields as single diastereomers (Table 3, 4a to 4i). Moreover, the R1 group could also be switched to a heteroaromatic group or a vinylogous phenyl group without erosion on the product yields or diastereoselectivities (4j to 4k). Interestingly, the R1 group of the ketones 2 could even be replaced with a simple methyl or ethyoxyl group, and the corresponding products 4l and 4m could be afforded in good yields as single diastereomers. The N-protecting benzyl group of indoline motif could be replaced with an N-methyl group, and the desired product 4n could also be afforded in a good yield as a single diastereomer. Unprotected isatin-derived enamide substrates were not effective in this transformation.
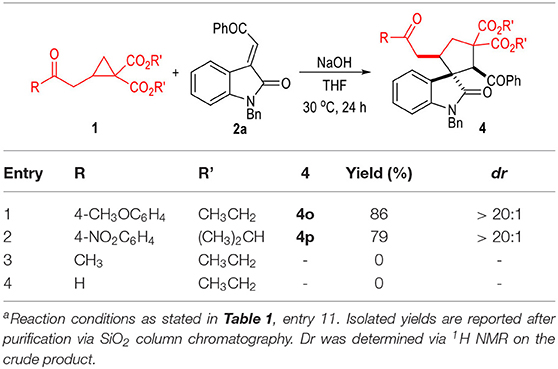
The scope of the D-A cyclopropyl acetates 1 was also examined (Table 4). The electron deficient 4-nitrophenol group on 1a could be switched to an electron rich aromatic group (1b) without erosion on the reaction diastereoselectivity, although the yield of the product was slightly decreased to 86%. Replacing the R group on ester substrate 1 with a simple methyl group (1c) led to only trace formation of the target product. The sterically bulkier isopropyl ester (1d) also worked well in this transformation and afforded the desired product in a good yield as a single diastereomer. It is worth noting that the cyclopropyl aldehyde 1e was not a suitable substrate for this (3 + 2) cycloaddition reaction.
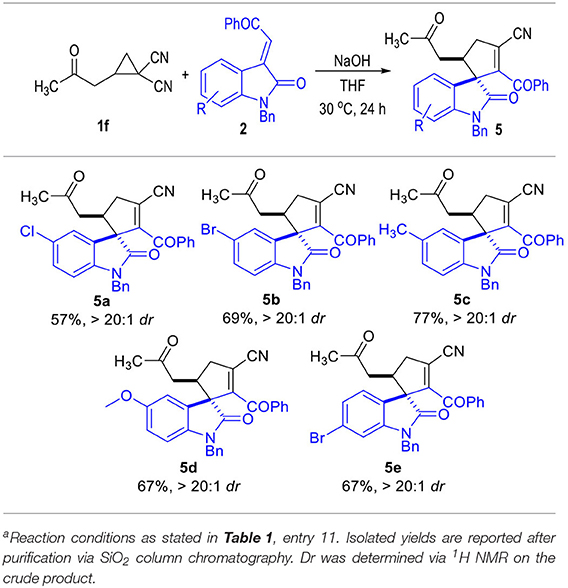
To our great delight, the D-A cyclopropyl acetone 1f bearing two cyano groups also worked well in the (3 + 2) cylcoaddition reaction with the oxindole-derived α,β-unsaturated enamide 2 under the current catalytic conditions (Table 5). The spirocyclopentenes 5 were afforded as the final products with the elimination of one equiv. of HCN. Both electron-donating and electron-withdrawing groups could be installed on the indoline moieties of the α,β-unsaturated enamides 2, with the corresponding products being afforded in moderate yields as single diastereomers.
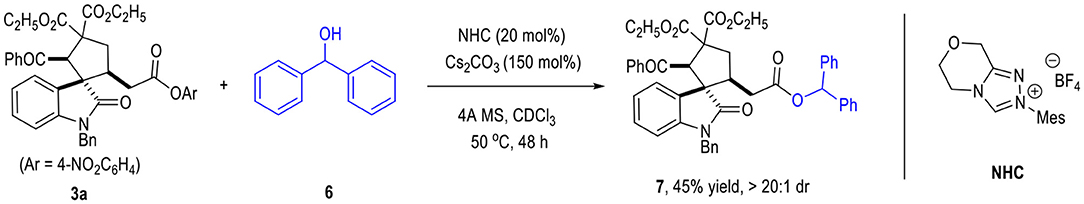
The afforded spiro(cyclopentane-1,3'-indoline) product 3a could be used as the reaction material for further transformations (Figure 3). For example, a trans-esterification reaction of 3a could give other ester products (e.g., 7) in moderate yields without erosion of the diastereomeric ratio.
Conclusion
In conclusion, we have developed a chemo- and diastereo-selective (3 + 2) cycloaddition between D-A cyclopropanes and α,β-unsaturated enones. Green, inexpensive, and readily available NaOH was used as the sole catalyst to promote this transformation. Structurally sophisticated spiro(cyclopentane-1,3'-indoline) derivatives bearing up to 3 adjacent chiral centers were afforded as the final products in generally good to excellent yields as single diastereomers. This study could provide us with a green, facile and economic approach in preparing complex functional molecules through simple operations. Further investigations on the development of efficient methods for the construction of complex molecules are in progress in our laboratory.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
XZ and DP conducted most of the experiments. CM, LP, and ZJ conceptualized and directed the whole project. ZJ drafted the manuscript. BZ participated in some experimental work and manuscript writing. All of the authors contributed in scientific discussions. All authors contributed to the article and approved the submitted version.
Funding
We acknowledge financial support from the National Natural Science Foundation of China (Nos. 21801051 and 21961006), Department of Science and Technology of Guizhou Province (Nos. (2019)1020 and (2009)700122), Undergraduate Innovation and Pioneer Training Program, Guizhou University of Traditional Chinese Medicine (Nos. (2018)31 and (2017)37) and Guizhou University (Nos. GZU(2017)34 and KY(2017)376).
Conflict of Interest
BZ was employed by the company Shenzhen AmTech Bioengineering Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2020.00542/full#supplementary-material
References
Afewerki, S., Ma, G., Ibrahem, I., Liu, L., Sun, J., and Córdova, A. (2015). Highly enantioselective control of dynamic cascade transformations by dual catalysis: asymmetric synthesis of polysubstituted spirocyclic oxindoles. ACS Catal. 5, 1266–1272. doi: 10.1021/cs501975u
Antonchick, A. P., Gerding-Reimers, C., Catarinella, M., Schürmann, M., Preut, H., Ziegler, S., et al. (2010). Highly enantioselective synthesis and cellular evaluation of spirooxindoles inspired by natural products. Nat. Chem. 2, 735–740. doi: 10.1038/nchem.730
Apel, C., Hartmann, S. S., Lentz, D., and Christmann, M. (2019). Dienamine-induced divinylcyclopropane-cycloheptadienerearrangements. Angew. Chem. Int. Ed. 58, 5075–5133. doi: 10.1002/anie.201813880
Ball-Jones, N. R., Badillo, J. J., Tran, N. T., and Franz, A. K. (2014). Catalytic enantioselective carboannulation with allylsilanes. Angew. Chem. Int. Ed. 53, 9462–9465. doi: 10.1002/anie.201403607
Blom, J., Vidal-Albalat, A., Jørgensen, J., Barløse, C. L., Jessen, K. S., Iversen, M. V., et al. (2017). Directing the activation of donor-acceptor cyclopropanes towards stereoselective 1,3-dipolar cycloaddition reactions by brønsted base catalysis. Angew. Chem. Int. Ed. 56, 11831–11835. doi: 10.1002/anie.201706150
Bode, J. W., and Sohn, S. S. (2007). N-Heterocyclic carbene-catalyzed redox amidations of α-functionalized aldehydes with amines. J. Am. Chem. Soc. 129, 13798–13799. doi: 10.1021/ja0768136
Boeyens, J. C. A., Holzapfel, C. W., and Steyn, P. S. (1979). Cyclopiamines A and B, novel oxindole metabolites of penicillium cyclopium westling. J. Chem. Soc. Perkin 1, 1751–1761. doi: 10.1039/p19790001751
Brazeau, J.-F., Zhang, S., Colomer, I., Corkey, B. K., and Toste, F. D. (2012). Enantioselective cyclizations of silyloxyenynes catalyzed by cationic metal phosphine complexes. J. Am. Chem. Soc. 134, 2742–2749. doi: 10.1021/ja210388g
Carson, C. A., and Kerr, M. A. (2009). Heterocycles from cyclopropanes: applications in natural product synthesis. Chem. Soc. Rev. 38, 3051–3060. doi: 10.1039/b901245c
Cavitt, M. A., Phun, L. H., and France, S. (2014). Intramolecular donor–acceptor cyclopropane ring-opening cyclizations. Chem. Soc. Rev. 43, 804–818. doi: 10.1039/C3CS60238A
Chaudhari, P. D., Hong, B.-C., and Lee, G.-H. (2017). Organocatalytic enantioselective michael–michael–michael–aldol condensation reactions: control of six stereocenters in a quadruple-cascade asymmetric synthesis of polysubstituted spirocyclic oxindoles. Org. Lett. 19, 6112–6115. doi: 10.1021/acs.orglett.7b02962
Chen, X.-H., Wei, Q., Luo, S.-W., Xiao, H., and Gong, L.-Z. (2009). Organocatalytic synthesis of spiro (pyrrolidin-3,3′-oxindoles) with high enantiopurity and structural diversity. J. Am. Chem. Soc. 131, 13819–13825. doi: 10.1021/ja905302f
Danishefsky, S. (1979). Electrophilic cyclopropanes in organic synthesis. Acc. Chem. Res. 12, 66–72. doi: 10.1021/ar50134a004
Deiana, L., Jiang, Y., Palo-Nieto, C., Afewerki, S., Incerti-Pradillos, C. A., Verho, O., et al. (2014). Combined heterogeneous metal/chiral amine: multiple relay catalysis for versatile eco-friendly synthesis. Angew. Chem. Int. Ed. 53, 3447–3451. doi: 10.1002/anie.201310216
Frost, J. R., Huber, S. M., Breitenlechner, S., Bannwarth, C., and Bach, T. (2015). Enantiotopos-selective C-H oxygenation catalyzed by a supramolecular ruthenium complex angew. Chem. Int. Ed. 54, 691–695. doi: 10.1002/anie.201409224
Grover, H. K., Emmett, M. R., and Kerr, M. A. (2015). Carbocycles from donor–acceptor cyclopropanes. Org. Biomol. Chem. 13, 655–671. doi: 10.1039/C4OB02117G
Halskov, K. S., Kniep, F., Lauridsen, V. H., Iversen, E. H., Donslund, B. S., and Jørgensen, K. A. (2015). Organocatalytic enamine-activation of cyclopropanes for highly stereoselective formation of cyclobutanes. J. Am. Chem. Soc. 137, 1685–1691. doi: 10.1021/ja512573q
Li, G.-Q., Dai, L.-X., and You, S.-L. (2009). N-Heterocyclic carbene catalyzed ring expansion of formylcyclopropanes: synthesis of 3,4-dihydro-α-pyrone derivatives. Org. Lett. 11, 1623–1625. doi: 10.1021/ol9002898
Lv, H., Mo, J., Fang, X., and Chi, Y. R. (2011). Formal diels–alder reactions of chalcones and formylcyclopropanes catalyzed by chiral N-heterocyclic carbenes. Org. Lett. 13, 5366–5369. doi: 10.1021/ol202250s
Mugishima, T., Tsuda, M., Kasai, Y., Ishiyama, H., Fukushi, E., Kawabata, J., et al. (2005). Absolute stereochemistry of citrinadins A and B from marine-derived fungus. J. Org. Chem. 70, 9430–9435. doi: 10.1021/jo051499o
Nanteuil, F., De Simone, F., Frei, R., Benfatti, F., Serrano, E., and Waser, J. (2014). Cyclization and annulation reactions of nitrogen-substituted cyclopropanes and cyclobutanes. Chem. Commun. 50, 10912–10928. doi: 10.1039/C4CC03194F
Pan, D., Mou, C., Zan, N., Lv, Y., Song, B.-A., Chi, R. C., et al. (2019). NaOH-promoted chemoselective cascade cyclization of cyclopropyl esters with unsaturated imines: access to bioactive cyclopenta(c)pyridine derivatives. Org. Lett. 21, 6624–6627. doi: 10.1021/acs.orglett.9b02088
Qiu, B., Xu, D., Sun, Q., Lin, J., and Sun, W. (2019). Manganese-catalyzed asymmetric oxidation of methylene C–H of spirocyclic oxindoles and dihydroquinolinones with hydrogen peroxide. Org. Lett. 21, 618–622. doi: 10.1021/acs.orglett.8b03652
Reissig, H.-U., and Zimmer, R. (2003). Donor-acceptor-substituted cyclopropane derivatives and their application in organic synthesis. Chem. Rev. 103, 1151–1196. doi: 10.1021/cr010016n
Sanchez-Diez, E., Vesga, D. L., Reyes, E., Uria, U., Carrillo, L., and Vicario, J. L. (2016). Organocatalytically Generated Donor–Acceptor Cyclopropanes in Domino Reactions. One-Step Enantioselective synthesis of pyrrolo(1,2-a)quinolines. Org. Lett. 18, 1270–1273. doi: 10.1021/acs.orglett.6b00173
Schneider, T. F., Kaschel, J., and Werz, D. B. (2014). A new golden age for donor–acceptor cyclopropanes. Angew. Chem. Int. Ed. 53, 5504–5523. doi: 10.1002/anie.201309886
Sohn, S. S., and Bode, J. W. (2006). N-Heterocyclic carbene catalyzed C-C bond cleavage in redox esterifications of chiral formylcyclopropanes. Angew. Chem. Int. Ed. 45, 6021–6024. doi: 10.1002/anie.200601919
Sparr, C., and Gilmour, R. (2011). Cyclopropyl iminium activation: reactivity umpolung in enantioselective organocatalytic reaction design. Angew. Chem. Int. Ed. 50, 8391–8395. doi: 10.1002/anie.201103360
Talukdar, R., Saha, A., and Ghorai, M. K. (2016). Domino-ring opening-cyclization (DROC) of donor-acceptor (DA) cyclopropanes. Isr. J. Chem. 56, 445–453. doi: 10.1002/ijch.201500092
Tan, B., Candeias, N. R., and Barbas, C. F. III. (2011). Core-structure-motivated design of a phosphine-catalyzed (3+2) cycloaddition reaction: enantioselective syntheses of spirocyclopenteneoxindoles. J. Am. Chem. Soc. 133, 4672–4675. doi: 10.1021/ja110147w
Tian, X., and Melchiorre, P. (2013). Control of remote stereochemistry in the synthesis of spirocyclic oxindoles: vinylogous organocascade catalysis. Angew. Chem. Int. Ed. 52, 5360–5363. doi: 10.1002/anie.201301017
Trost, B. M., Cramer, N., and Silverman, S. M. (2007). Enantioselective construction of spirocyclic oxindolic cyclopentanes by palladium-catalyzed trimethylenemethane - (3+2) - cycloaddition. J. Am. Chem. Soc. 129, 12396–12397. doi: 10.1021/ja075335w
Tsuda, M., Kasai, Y., Komatsu, K., Sone, T., Tanaka, M., Mikami, Y., et al. (2004). A novel pentacyclic alkaloid from marine-derived fungus penicillium citrinum. Org. Lett. 6, 3087–3089. doi: 10.1002/chin.200452182
Wang, L., and Tang, Y. (2016). Asymmetric ring-opening reactions of donor-acceptor cyclopropanes and cyclobutanes. Isr. J. Chem. 56, 463–475. doi: 10.1002/ijch.201500094
Wenkert, E. (1980). Oxycyclopropanes in organochemical synthesis. Acc. Chem. Res. 13, 27–31. doi: 10.1021/ar50145a005
Werz, D. B., and Biju, A. T. (2019). Uncovering the neglected similarities of arynes and donor–acceptor cyclopropanes. Angew. Chem. Int. Ed. 59, 3385–3398. doi: 10.1002/anie.201909213
Zhang, J., Cao, D., Wang, H., Zheng, C., Zhao, G., and Shang, Y. (2016). Enantioselective construction of spirocyclic oxindoles via tandem michael/michael reactions catalyzed by multifunctional quaternary phosphonium salt. J. Org. Chem. 81, 10558–10568. doi: 10.1021/acs.joc.6b01553
Keywords: green, NaOH, donor-acceptor cyclopropane, (3 + 2) cycloaddition, spirocyclopentane, indole derivative
Citation: Zhu X, Pan D, Mou C, Zhou B, Pan L and Jin Z (2020) Green and Facile Synthesis of Spirocyclopentanes Through NaOH-Promoted Chemo- and Diastereo-Selective (3 + 2) Cycloaddition Reactions of Activated Cyclopropanes and Enamides. Front. Chem. 8:542. doi: 10.3389/fchem.2020.00542
Received: 07 April 2020; Accepted: 26 May 2020;
Published: 26 June 2020.
Edited by:
Yaqiong Su, Eindhoven University of Technology, NetherlandsReviewed by:
Huanzhen Ni, Georgia Institute of Technology, United StatesWai Lun Chan, Hong Kong Baptist University, Hong Kong
Copyright © 2020 Zhu, Pan, Mou, Zhou, Pan and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chengli Mou, bW91Y2hlbmdsaV9oJiN4MDAwNDA7MTYzLmNvbQ==; Zhichao Jin, emNqaW4mI3gwMDA0MDtnenUuZWR1LmNu
†These authors have contributed equally to this work
 Xun Zhu1†
Xun Zhu1† Zhichao Jin
Zhichao Jin