- 1Materials Center for Energy and Photoelectrochemical Conversion, School of Material Science and Engineering, University of Jinan, Jinan, China
- 2Key Laboratory of Micro-Nano Powder and Advanced Energy Material of Anhui Higher Education Institutes, Chizhou University, Chizhou, China
- 3School of Physics and Physical Engineering, Qufu Normal University, Qufu, China
Supercapacitors (SCs) have attracted widespread attention due to their short charging/discharging time, long cycle life, and good temperature characteristics. Electrolytes have been considered as a key factor affecting the performance of SCs. They largely determine the energy density based on their decomposition voltage and the power density from their ionic conductivity. In recent years, redox electrolytes obtained a growing interest due to an additional redox activity from electrolytes, which offers an increased charge storage capacity in SCs. This article summarizes the latest progress in the research of redox electrolytes, and focuses on their properties, mechanisms, and applications based on different solvent types available. It also proposes potential solutions for how to effectively increase the energy density of the SCs while maintaining their high power and long life.
Introduction
Supercapacitors (SCs) are a new type of energy storage equipment filling the gap between secondary batteries and traditional capacitors (Hui et al., 2019; Poonam et al., 2019; Zhi-yu et al., 2019; Alipoori et al., 2020; Cheng et al., 2020; Iqbal et al., 2020; Li and Liang, 2020; Mohd Abdah et al., 2020; Panda et al., 2020; Yi et al., 2020). SCs are believed to be one of the most promising candidates due to their fast charging/discharging capability, long cycle life, high power density, and high safety (Zhang W. et al., 2017; Zhang et al., 2018; Afif et al., 2019). In the course of decades of their development, the research on SCs has mainly focused on the preparation and modification of electrode materials to improve capacity (Zhao and Zheng, 2015). As an important part of SCs, electrolytes provide ionic conductivity and promote the charge compensation of electrodes (Wang Y. et al., 2016), so the performance of the SCs is determined by the electrolyte together with the electrode material. The electrolyte has two key parameters: (1) Electrochemical stability window. If the electrode material doesn't undergo any decomposition reaction within the voltage range of the SCs, then the output voltage of the device largely depends on the decomposition voltage of the electrolyte (Schütter et al., 2016). (2) Ionic conductivity. It affects the dynamic process and determines the rate capability of the SCs. It is related to the number of carriers, the ionic charge, and carrier mobility. The SCs electrolytes mainly have the following types: aqueous electrolytes, organic electrolytes, ionic liquid electrolytes, all solid electrolytes, gel electrolytes, and redox electrolytes (Panda et al., 2020). Several reviews concerning electrolytes for SCs have been published previously (Zhao and Zheng, 2015; Zhong et al., 2015; Pal et al., 2019; Li et al., 2020). However, none of the previous reviews concentrated on the dependence of properties, mechanisms, and applications of redox electrolytes on the different solvent types available.
Redox electrolytes are a specific type of electrolyte, in which redox active species were added. They can greatly increase the electrochemical performance of SCs for two reasons: (1) The electrolyte additive is an active part of the SCs in redox reactions during charge and discharge processes (Sankar and Selvan, 2015; Sun et al., 2015b; Fan et al., 2016; Wang C. et al., 2016). (2) The redox reactions in the electrolyte are conducive to electron transfer between the electrode material and the redox species in the electrolyte (Dai et al., 2016; Vlad et al., 2016; Gao et al., 2017; Mourad et al., 2017; Xiong et al., 2017). This review mainly summarizes the latest research results of various redox electrolytes based on aqueous, organic, ionic, and gel solvents.
Redox Mediated Aqueous Electrolytes
Aqueous electrolytes can be divided into three types: acidic, alkaline, and neutral solutions. As a commonly used electrolyte, sulfuric acid aqueous solution not only has high ion conductivity/concentration but also low equivalent series resistance. Therefore, adding redox additives to sulfuric acid aqueous solution is a good way to optimize the electrolyte and improve the performance of SCs. Some typical redox additives contain KI (Zhang Y. et al., 2017; Gao et al., 2018), Na2MoO4 (Xu et al., 2017a), Ce2(SO4)3 (Díaz et al., 2015), Fe3+/Fe2+ (Ren et al., 2017), viologen substances (Sathyamoorthi et al., 2016), 1,4-dihydroxyanthraquinone (Xu et al., 2017b), hydroquinone (HQ) (Pham et al., 2015; Chen and Lin, 2019), and so on.
Generally, the energy density based on multiple redox additives is higher than that based on a single redox additive in aqueous electrolytes (Lee et al., 2016b; Teng et al., 2016). When using mixed electrolytes, their ratio is a key factor for the performance of SCs. Xu et al. (2017a) adjusted the overlapping redox voltage windows by the ratio of Na2MoO4 to KI. The optimal system (Na2MoO4: KI = 1:1) shows higher capacitance (the capacitance increased by 17.4 times) and better rate performance than other systems (Na2MoO4: KI ≠ 1:1) due to a synergistic effect between Na2MoO4 and KI. Sathyamoorthi reported on a viologen-based redox active electrolyte, in which the redox behavior of bromide and 1,1′-diethyl-4,4′-bipyridinium ions boosted both anode and cathode performance (Sathyamoorthi et al., 2016). Interestingly, the specific capacitance of the SCs increases continuously during the charge and discharge cycle, and a 30% increase is observed at the end of the 1,000 cycles. Hu et al. (2017) reported on redox additives 4-hydroxybenzoic acid (HBA), 3,4-dihydroxybenzoic acid (DHBA), and 3,4,5-trihydroxybenzoic acid (THBA) in H2SO4. SCs with HBA and DHBA exhibit higher capacitances because of their functional hydroxyl groups in the benzene ring.
For alkaline electrolytes, K3Fe(CN)6 (Veerasubramani et al., 2016; Lamiel et al., 2017) and p-phenylenediamine (PPD) (Zhang et al., 2015) can improve the capacitance and stability of the SCs. Zhang et al. (2015) introduced PPD into KOH electrolytes to form a PPD-KOH electrolyte. As expected, the specific capacitance of the carbon sample in the PPD-KOH electrolyte was larger (501.4 F g−1 at 3 A g−1) than that of SCs using an electrolyte without PPD (119.2 F g−1 at 3 A g−1). Fic et al. (2015) demonstrated a new capacitor concept in which the positive electrode works in a KI solution and the negative electrode works in a KOH electrolyte. Because of the redox reactions of I−/I2, the capacitance and energy density of the SCs is improved.
For neutral electrolytes, K3Fe(CN)6 (Lee et al., 2016a), KI (Singh and Chandra, 2016) and other additives with redox properties (Chun et al., 2015) are usually added. Chun et al. (2015) found that a high energy density of about 14 Wh kg−1 was obtained under methyl viologen (MV)/bromide electrolytes due to the redox reactions of Br−/Br and MV2+/MV+. The stability was improved by substituting heptyl viologen (HV) for MV and did not degrade after 20,000 cycles. It is believed that this electrolyte system will gain a foothold in future advanced energy storage applications.
Despite making considerable progress, the low decomposition voltage of water (1.23 V) leads to a poor energy density of SCs (Yi et al., 2020). It is also reported that the cycle performance of SCs will deteriorate after adding redox additives to the aqueous electrolytes (Chodankar et al., 2016; Singh and Chandra, 2016). This is mainly because a strong redox reaction occurs at the electrode/electrolyte interfaces, which will affect the electroactive site to a certain extent (Chodankar et al., 2016).
Redox Mediated Organic Electrolytes
In order to increase the energy density, an organic electrolyte with a wide electrochemical stability window (around 3 V) is a good choice (Zhao and Zheng, 2015). The organic system consists of organic solvents and conductive salts. Propylene carbonate (PC) (Li et al., 2015; Salunkhe et al., 2016) and acetonitrile (AC) (Dall'Agnese et al., 2016; Jäckel et al., 2016; Singh and Chandra, 2016; Yang et al., 2017) are the most commonly used solvents in SCs. Tetraethyl ammonium tetrafluoroborate (TEABF4) (Li et al., 2015; Jäckel et al., 2016; Salunkhe et al., 2016; Singh and Chandra, 2016; Yang et al., 2017) and LiPF6 (Xie L. et al., 2016) are the most commonly used salts in SCs.
Kim et al. (2016) reported a high-performance flexible microcapacitor, which employed a poly(methyl methacrylate)-propylene carbonate-lithium perchlorate (PMMA-PC-LiClO4) electrolyte with hydroquinone (HQ) redox additive. The operating voltage of this system is up to 1.2 V, which is better than that of other flexible SCs under HQ-PVA-H2SO4 and PPD-PVA-KOH electrolytes (both below 1 V). The volumetric capacitance increased 35-fold due to the reversible redox reaction between hydroquinone (HQ) and benzoquinone (BQ). Also, a flexible SC with an extended operating voltage of 1.5 V, a specific capacitance of up to 363 F g−1, and an energy density of 27.4 Wh kg−1 was obtained under an organic electrolyte with ferrocene and 4-oxo-2, 2, 6, 6-tetramethylpiperidinooxy additive, due to the wide voltage of the organic electrolyte and the additional faraday capacitance from the redox mediator (Zhang et al., 2016). Dall'Agnese et al. (2016) studied the electrochemical behavior of two-dimensional titanium carbide (MXene) in acetonitrile solution with 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide (EMITFSI) additive. The capacitance of 85 F g−1 was obtained at 2 mV s−1, while a high rate capability and good cyclability appeared. Through in-situ X-ray diffraction studies, it was found that EMI+ cations are embedded in MXene, which results in increased capacitance.
Redox Mediated Ionic Liquid Electrolytes
Ionic liquids are generally composed of a bulky, asymmetric organic cation and a weakly coordinating inorganic/organic anion, which shows a wide electrochemical window (generally above 3.5 V), high electrochemical stability, good oxidation resistance, and so on (Brandt et al., 2013). In recent years, it was discovered that Quinones are excellent redox electrolyte additives in ionic liquid electrolytes. The introduction of hydroquinone (HQ) (Dubal et al., 2015; Sathyamoorthi et al., 2015; Xu et al., 2015) and benzoquinone (BQ) (Navalpotro et al., 2016) into the electrolyte as organic redox shuttles leads to low charge transfer resistance and contributes to the improvement of the specific capacitance and specific energy of SCs.
In addition, it is reported that the addition of tin sulfate (SnSO4) and vanadium sulfate (VOSO4) (Lee et al., 2016b) to the ionic liquid electrolyte can also significantly improve the overall performance of the SCs. Xie H. J. et al. (2016) reported two redox ionic liquids, [FcEIm][NTf2] and [EMIm][FcNTf], which were prepared by modifying either the [EMIm] cation or the [NTf2] anion with ferrocene. Based on [EMIm][FcNTf], the energy density is as high as 13.2 Wh kg−1, while the self-discharge at the positive electrode is fully suppressed due to the deposition of a film on the electrode. It can be seen that redox-mediated ionic liquid electrolytes are promising alternatives to conventional electrolytes. However, the problems of liquid electrolyte leakage and corrosion in liquid electrolytes have severely limited its application (Ma et al., 2015).
Redox Mediated Gel Electrolytes
GEL is a special material between liquid and solid, which exhibits the flexibility and stability of solid and the easy diffusion of liquid (Zhi-yu et al., 2019). It has a series of advantages such as a higher ionic conductivity than solid electrolytes and good mechanical and chemical stability, etc., which makes it a promising electrolyte (Batisse and Raymundo-Piñero, 2017; Qin and Panzer, 2017; Hui et al., 2019; Li et al., 2019). Recently, a novel redox-mediated strategy for SCs was reported, which can efficiently increase the ionic conductivity and produce additional capacitance by the quick reversible redox reaction introduced by the redox mediator (Alipoori et al., 2020). The redox additives in gel polymer electrolytes usually include indigo carmine (IC) (Ma et al., 2015), 2-mercaptopyridine (PySH) (Pan et al., 2015), 1-butyl-3-methylimidazolium iodide (BMIMI) (Tu et al., 2018), alizarin red S (ARS) (Sun et al., 2016), FeBr3 (Wang et al., 2019), 1,4 Naphthoquinone (Hashemi et al., 2018), 1-anthraquinone sulfonic acid sodium (AQQS) (Feng et al., 2016) and 1-ethyl-3-methylimidazolium tetrafluoroborate ([EMIM]BF4) (Seok Jang et al., 2016).
The redox-mediated gel polymer electrolyte (PVA-H2SO4-IC) was prepared by adding indigo carmine (IC) to a mixture of polyvinyl alcohol (PVA) and sulfuric acid (H2SO4). Its ionic conductivity is increased by 188%, reaching 20.27 mS cm−1. Due to the reversible redox reaction of the IC, the specific capacitance of the device was increased by 112.2% (382 F g−1), and the energy density also increased to 13.26 Wh kg−1. It also shows excellent cycling stability (80.3% capacitance retention after 3,000 cycles) (Ma et al., 2015). When alizarin red S (ARS) was added into polyvinyl alcohol-sulfuric acid (PVA-H2SO4), a new type of electrolyte (PVA-H2SO4-ARS) was obtained (Figure 1). Its conductivity reached 33.3 mS cm−1, due to ARS acting as a redox shuttle in the electrolyte. Compared with ARS-free SCs (160 F g−1 at 0.5 A g−1), the specific capacitance of SCs using a PVA-H2SO4-ARS gel polymer electrolyte is larger (441 F g−1 at 0.5 A g−1). At the same time, its energy density is as high as 39.4 Wh kg−1 and it has a good cycling stability. Therefore, the redox-mediated electrolyte has a good application prospect in improving the electrochemical performance of SCs (Sun et al., 2016).
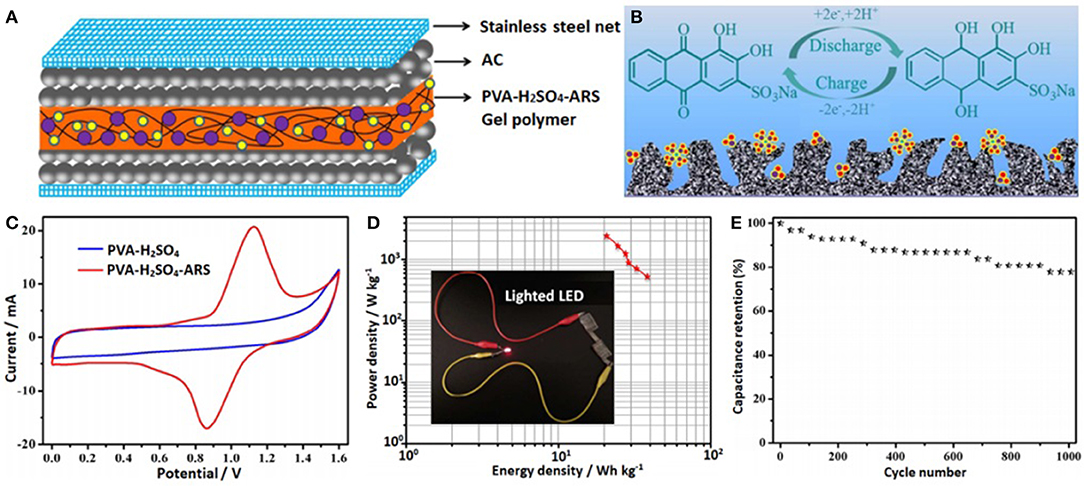
Figure 1. (A) The fabrication model of the SCs with PVA-H2SO4-ARS electrolyte, (B) double-layer formation and redox reaction on the carbon surface, (C) CV curves for the SCs at 10 mV s−1, (D) Ragone plots of the SCs with PVA-H2SO4-ARS electrolyte, (E) cyclic performances of the SCs with PVA-H2SO4-ARS electrolyte at 1 A g−1. Reproduced by permission of The Royal Society of Chemistry from Sun et al. (2016).
Sun et al. (2015a) prepared a redox-mediated gel polymer-polyvinyl alcohol-orthophosphate 2-mercaptopyridine (PVA-H3PO4-PySH) by introducing PySH into PVA-H3PO4. The ionic conductivity of the PVA-H3PO4-PySH system was increased by 92% to 22.57 mS cm−1. As a result, a high specific capacitance (1,128 F g−1) and energy density (39.17 Wh kg−1) were obtained. These improved properties are attributed to the redox reaction between PySH and 2,2′-bipyridine redox couple in PVA-H3PO4-PySH (Ye et al., 2018). These results undoubtedly indicate that redox-mediated gel polymers are promising electrolyte candidates for advanced flexible SCs (Aljafari et al., 2019).
Although redox electrolytes have greatly contributed to the improvement of the performance of SCs, it's worth noting that self-discharge (SD) is a fatal weakness for most redox electrolytes. So, many pieces of research have focused on this problem recently. Fan et al. (2020) lowered self-discharge and improved energy density and cycling stability (capacitance retention 87.9% after 10,000 cycles) by the addition of Li2SO4-BMIMBr-carbon nanotubes in the PVA solution, in which the 3D carbon nanotubes networks provide fast ion transmission channels. Chen et al. (2014) blocked the migration of the active electrolyte between two electrodes and suppressed the self-discharge though inhibiting BQ shuttle with Nafion®177 membrane or suppressing shuffle effect with a CuSO4 active electrolyte. It is believed that these results will guide the further design of SCs with both a high energy density and good energy retention.
In the end, a table (Table 1) was given, in which several typical redox electrolyte-based SCs are summarized and compared for clarity.
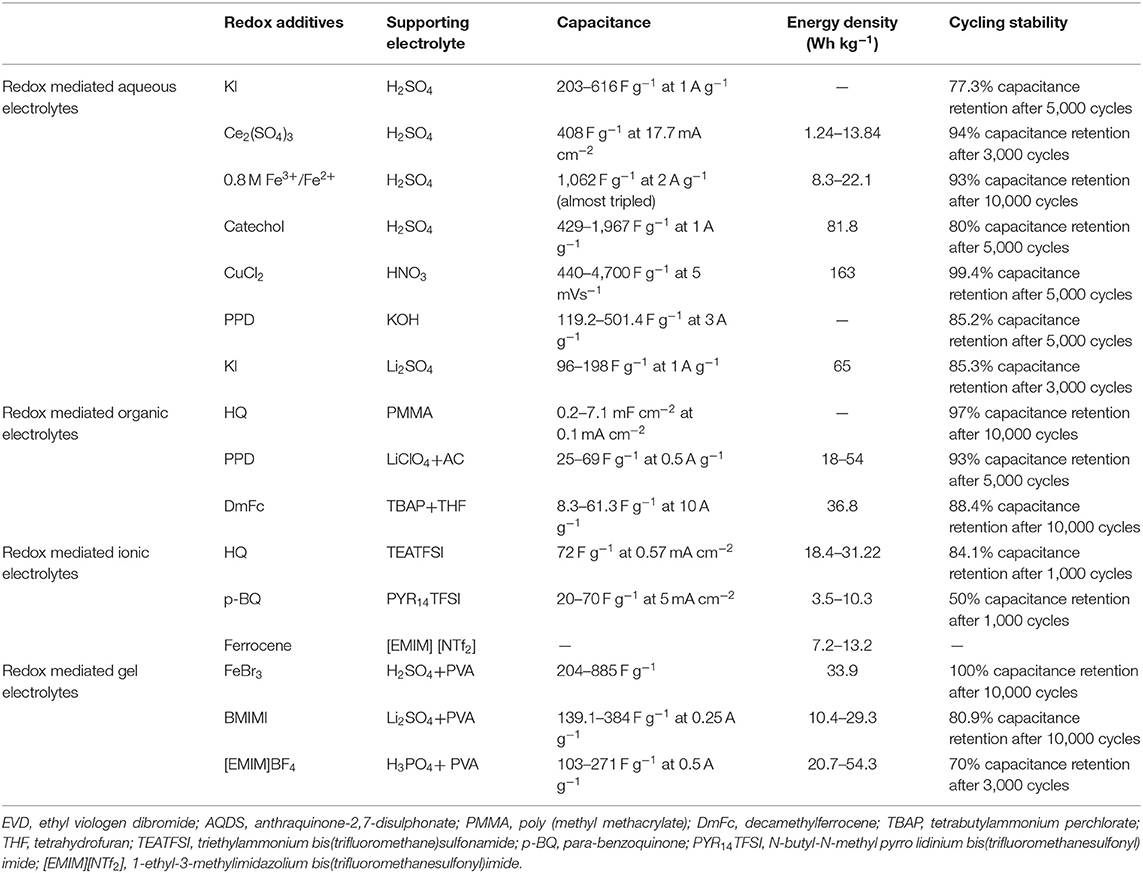
Table 1. Redox electrolyte-based SCs and their performance (Mai et al., 2013; Park et al., 2014; Yu et al., 2014; Díaz et al., 2015; Sathyamoorthi et al., 2015; Zhang et al., 2015; Kim et al., 2016; Navalpotro et al., 2016; Seok Jang et al., 2016; Singh and Chandra, 2016; Xie H. J. et al., 2016; Mousavi et al., 2017; Ren et al., 2017; Gao et al., 2018; Tu et al., 2018; Wang et al., 2019).
Conclusions and Perspectives
Each redox electrolyte has its own advantages and disadvantages. The two most important criteria for selecting an electrolyte are the operating voltage and the ionic conductivity. The higher the operating voltage and the ionic conductivity is, the greater the energy density and power density of the SCs. In order to further develop high-performance SCs electrolytes and improve the overall performance of SCs, we can start from the following aspects: (1) The introduction of redox active materials that can produce reversible redox reactions in the electrolyte is an effective way to increase the capacity and energy density of SCs; (2) Investigating the interaction mechanism between the electrode material and the electrolyte, and optimizing the matching relationship between them. Taken together, these redox electrolytes pave the way for high-performance SCs applications.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This work was supported by National Natural Science Foundation (51702123, 51872161), Shandong Province Higher Educational Youths Innovation Science and Technology Program (2019KJA018), and the Educational Commission of Anhui Province of China (KJ2019A0868). SY thanks University of Jinan Start-up Research Funding.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Afif, A., Rahman, S. M. H., Tasfiah Azad, A., Zaini, J., Islan, M. A., and Azad, A. K. (2019). Advanced materials and technologies for hybrid supercapacitors for energy storage - a review. J. Energy Storage 25:100852. doi: 10.1016/j.est.2019.100852
Alipoori, S., Mazinani, S., Aboutalebi, S. H., and Sharif, F. (2020). Review of PVA-based gel polymer electrolytes in flexible solid-state supercapacitors: Opportunities and challenges. J. Energy Storage 27:101072. doi: 10.1016/j.est.2019.101072
Aljafari, B., Alamro, T., Ram, M. K., and Takshi, A. (2019). Polyvinyl alcohol-acid redox active gel electrolytes for electrical double-layer capacitor devices. J. Solid State Electrochem. 23, 125–133. doi: 10.1007/s10008-018-4120-y
Batisse, N., and Raymundo-Piñero, E. (2017). A self-standing hydrogel neutral electrolyte for high voltage and safe flexible supercapacitors. J. Power Sources 348, 168–174. doi: 10.1016/j.jpowsour.2017.03.005
Brandt, A., Pohlmann, S., Varzi, A., Balducci, A., and Passerini, S. (2013). Ionic liquids in supercapacitors. MRS Bull. 38, 554–559. doi: 10.1557/mrs.2013.151
Chen, L., Bai, H., Huang, Z., and Li, L. (2014). Mechanism investigation and suppression of self-discharge in active electrolyte enhanced supercapacitors. Energy Environ. Sci. 7, 1750–1759. doi: 10.1039/C4EE00002A
Chen, Y.-C., and Lin, L.-Y. (2019). Investigating the redox behavior of activated carbon supercapacitors with hydroquinone and p-phenylenediamine dual redox additives in the electrolyte. J. Coll. Interface Sci. 537, 295–305. doi: 10.1016/j.jcis.2018.11.026
Cheng, Y., Xiao, X., Pan, K., and Pang, H. (2020). Development and application of self-healing materials in smart batteries and supercapacitors. Chem. Eng. J. 380:122565. doi: 10.1016/j.cej.2019.122565
Chodankar, N. R., Dubal, D. P., Lokhande, A. C., Patil, A. M., Kim, J. H., and Lokhande, C. D. (2016). An innovative concept of use of redox-active electrolyte in asymmetric capacitor based on MWCNTs/MnO2 and Fe2O3 thin films. Sci. Rep. 6, 1–14. doi: 10.1038/srep39205
Chun, S.-E., Evanko, B., Wang, X., Vonlanthen, D., Ji, X., Stucky, G. D., et al. (2015). Design of aqueous redox-enhanced electrochemical capacitors with high specific energies and slow self-discharge. Nat. Commun. 6:7818. doi: 10.1038/ncomms8818
Dai, S., Xu, W., Xi, Y., Wang, M., Gu, X., Guo, D., et al. (2016). Charge storage in KCu7S4 as redox active material for a flexible all-solid-state supercapacitor. Nano Energy 19, 363–372. doi: 10.1016/j.nanoen.2015.11.025
Dall'Agnese, Y., Rozier, P., Taberna, P.-L., Gogotsi, Y., and Simon, P. (2016). Capacitance of two-dimensional titanium carbide (MXene) and MXene/carbon nanotube composites in organic electrolytes. J. Power Sources 306, 510–515. doi: 10.1016/j.jpowsour.2015.12.036
Díaz, P., González, Z., Santamaría, R., Granda, M., Menéndez, R., and Blanco, C. (2015). Enhanced energy density of carbon-based supercapacitors using Cerium (III) sulphate as inorganic redox electrolyte. Electrochim. Acta 168, 277–284. doi: 10.1016/j.electacta.2015.03.187
Dubal, D. P., Suarez-Guevara, J., Tonti, D., Enciso, E., and Gomez-Romero, P. (2015). A high voltage solid state symmetric supercapacitor based on graphene-polyoxometalate hybrid electrodes with a hydroquinone doped hybrid gel-electrolyte. J. Mater. Chem. A 3, 23483–23492. doi: 10.1039/C5TA05660H
Fan, L.-Q., Tu, Q.-M., Geng, C.-L., Huang, J.-L., Gu, Y., Lin, J.-M., et al. (2020). High energy density and low self-discharge of a quasi-solid-state supercapacitor with carbon nanotubes incorporated redox-active ionic liquid-based gel polymer electrolyte. Electrochim. Acta 331:135425. doi: 10.1016/j.electacta.2019.135425
Fan, L.-Q., Zhong, J., Zhang, C.-Y., Wu, J.-H., and Wei, Y-L. (2016). Improving the energy density of quasi-solid-state supercapacitors by assembling two redox-active gel electrolytes. Int. J. Hydrogen Energy 41, 5725–5732. doi: 10.1016/j.ijhydene.2016.02.052
Feng, E., Ma, G., Sun, K., Yang, Q., Peng, H., and Lei, Z. (2016). Toughened redox-active hydrogel as flexible electrolyte and separator applying supercapacitors with superior performance. RSC Adv. 6, 75896–75904. doi: 10.1039/C6RA14149H
Fic, K., Meller, M., and Frackowiak, E. (2015). Interfacial redox phenomena for enhanced aqueous supercapacitors. J. Electrochem. Soc. 162, A5140–A5147. doi: 10.1149/2.0251505jes
Gao, Z., Liu, X., Chang, J., Wu, D., Xu, F., Zhang, L., et al. (2017). Graphene incorporated, N doped activated carbon as catalytic electrode in redox active electrolyte mediated supercapacitor. J. Power Sources 337, 25–35. doi: 10.1016/j.jpowsour.2016.10.114
Gao, Z., Zhang, L., Chang, J., Wang, Z., Wu, D., Xu, F., et al. (2018). Catalytic electrode-redox electrolyte supercapacitor system with enhanced capacitive performance. Chem. Eng. J. 335, 590–599. doi: 10.1016/j.cej.2017.11.037
Hashemi, M., Rahmanifar, M. S., El-Kady, M. F., Noori, A., Mousavi, M. F., and Kaner, R. B. (2018). The use of an electrocatalytic redox electrolyte for pushing the energy density boundary of a flexible polyaniline electrode to a new limit. Nano Energy 44, 489–498. doi: 10.1016/j.nanoen.2017.11.058
Hu, W., Xu, D., Sun, X. N., Xiao, Z. H., Chen, X. Y., and Zhang, Z. J. (2017). Template synthesis of nitrogen-doped carbon nanosheets for high-performance supercapacitors improved by redox additives. ACS Sustain. Chem. Eng. 5, 8630–8640. doi: 10.1021/acssuschemeng.7b01189
Hui, C.-y., Kan, C.-w., Mak, C.-I., and Chau, K.-h. (2019). Flexible energy storage system-an introductory review of textile-based flexible supercapacitors. Processes 7:922. doi: 10.3390/pr7120922
Iqbal, M. Z., Zakar, S., and Haider, S. S. (2020). Role of aqueous electrolytes on the performance of electrochemical energy storage device. J. Electroanal. Chem. 858:113793. doi: 10.1016/j.jelechem.2019.113793
Jäckel, N., Weingarth, D., Schreiber, A., Krüner, B., Zeiger, M., Tolosa, A., et al. (2016). Performance evaluation of conductive additives for activated carbon supercapacitors in organic electrolyte. Electrochim. Acta 191, 284–298. doi: 10.1016/j.electacta.2016.01.065
Kim, D., Lee, G., Kim, D., Yun, J., Lee, S.-S., and Ha, J. S. (2016). High performance flexible double-sided micro-supercapacitors with an organic gel electrolyte containing a redox-active additive. Nanoscale 8, 15611–15620. doi: 10.1039/C6NR04352F
Lamiel, C., Lee, Y. R., Cho, M. H., Tuma, D., and Shim, J-J. (2017). Enhanced electrochemical performance of nickel-cobalt-oxide@reduced graphene oxide//activated carbon asymmetric supercapacitors by the addition of a redox-active electrolyte. J. Colloid Interface Sci. 507, 300–309. doi: 10.1016/j.jcis.2017.08.003
Lee, J., Choudhury, S., Weingarth, D., Kim, D., and Presser, V. (2016a). High performance hybrid energy storage with potassium ferricyanide redox electrolyte. ACS Appl. Mater. Interfaces 8, 23676–23687. doi: 10.1021/acsami.6b06264
Lee, J., Krüner, B., Tolosa, A., Sathyamoorthi, S., Kim, D., Choudhury, S., et al. (2016b). Tin/vanadium redox electrolyte for battery-like energy storage capacity combined with supercapacitor-like power handling. Energy Environ. Sci. 9, 3392–3398. doi: 10.1039/C6EE00712K
Li, H., and Liang, J. (2020). Recent development of printed micro-supercapacitors: printable materials, printing technologies, and perspectives. Adv. Mater. 32:1805864. doi: 10.1002/adma.201805864
Li, H., Lv, T., Sun, H., Qian, G., Li, N., Yao, Y., et al. (2019). Ultrastretchable and superior healable supercapacitors based on a double cross-linked hydrogel electrolyte. Nat. Commun. 10, 1–8. doi: 10.1038/s41467-019-08320-z
Li, J., Qiao, J., and Lian, K. (2020). Hydroxide ion conducting polymer electrolytes and their applications in solid supercapacitors: a review. Energy Storage Mater. 24, 6–21. doi: 10.1016/j.ensm.2019.08.012
Li, S.-M., Yang, S.-Y., Wang, Y.-S., Tsai, H.-P., Tien, H.-W., Hsiao, S.-T., et al. (2015). N-doped structures and surface functional groups of reduced graphene oxide and their effect on the electrochemical performance of supercapacitor with organic electrolyte. J. Power Sources 278, 218–229. doi: 10.1016/j.jpowsour.2014.12.025
Ma, G., Dong, M., Sun, K., Feng, E., Peng, H., and Lei, Z. (2015). A redox mediator doped gel polymer as an electrolyte and separator for a high performance solid state supercapacitor. J. Mater. Chem. A 3, 4035–4041. doi: 10.1039/C4TA06322H
Mai, L.-Q., Minhas-Khan, A., Tian, X., Hercule, K. M., Zhao, Y.-L., Lin, X., et al. (2013). Synergistic interaction between redox-active electrolyte and binder-free functionalized carbon for ultrahigh supercapacitor performance. Nat. Commun. 4, 1–7. doi: 10.1038/ncomms3923
Mohd Abdah, M. A. A., Azman, N. H. N., Kulandaivalu, S., and Sulaiman, Y. (2020). Review of the use of transition-metal-oxide and conducting polymer-based fibres for high-performance supercapacitors. Materials & Design 186:108199. doi: 10.1016/j.matdes.2019.108199
Mourad, E., Coustan, L., Lannelongue, P., Zigah, D., Mehdi, A., Vioux, A., et al. (2017). Biredox ionic liquids with solid-like redox density in the liquid state for high-energy supercapacitors. Nat. Mater. 16, 446–453. doi: 10.1038/nmat4808
Mousavi, M. F., Hashemi, M., Rahmanifar, M. S., and Noori, A. (2017). Synergistic effect between redox additive electrolyte and PANI-rGO nanocomposite electrode for high energy and high power supercapacitor. Electrochim. Acta 228, 290–298. doi: 10.1016/j.electacta.2017.01.027
Navalpotro, P., Palma, J., Anderson, M., and Marcilla, R. (2016). High performance hybrid supercapacitors by using para-Benzoquinone ionic liquid redox electrolyte. J. Power Sources 306, 711–717. doi: 10.1016/j.jpowsour.2015.12.103
Pal, B., Yang, S., Ramesh, S., Thangadurai, V., and Jose, R. (2019). Electrolyte selection for supercapacitive devices: a critical review. Nanoscale Adv. 1, 3807–3835. doi: 10.1039/C9NA00374F
Pan, S., Deng, J., Guan, G., Zhang, Y., Chen, P., Ren, J., et al. (2015). A redox-active gel electrolyte for fiber-shaped supercapacitor with high area specific capacitance. J. Mater. Chem. A 3, 6286–6290. doi: 10.1039/C5TA00007F
Panda, P. K., Grigoriev, A., Mishra, Y. K., and Ahuja, R. (2020). Progress in supercapacitors: roles of two dimensional nanotubular materials. Nanoscale Adv. 2, 70–108. doi: 10.1039/C9NA00307J
Park, J., Kim, B., Yoo, Y.-E., Chung, H., and Kim, W. (2014). Energy-density enhancement of carbon-nanotube-based supercapacitors with redox couple in organic electrolyte. ACS Appl. Mater. Interfaces 6, 19499–19503. doi: 10.1021/am506258s
Pham, V. H., Gebre, T., and Dickerson, J. H. (2015). Facile electrodeposition of reduced graphene oxide hydrogels for high-performance supercapacitors. Nanoscale 7, 5947–5950. doi: 10.1039/C4NR07508K
Poonam Sharma, K., Arora, A., and Tripathi, S. K. (2019). Review of supercapacitors: materials and devices. J. Energy Storage 21, 801–825. doi: 10.1016/j.est.2019.01.010
Qin, H., and Panzer, M. J. (2017). Chemically cross-linked poly (2-hydroxyethyl methacrylate)-supported deep eutectic solvent gel electrolytes for eco-friendly supercapacitors. ChemElectroChem 4, 2556–2562. doi: 10.1002/celc.201700586
Ren, L., Zhang, G., Yan, Z., Kang, L., Xu, H., Shi, F., et al. (2017). High capacitive property for supercapacitor using Fe3+/Fe2+ redox couple additive electrolyte. Electrochim. Acta 231:705–712. doi: 10.1016/j.electacta.2017.02.056
Salunkhe, R. R., Young, C., Tang, J., Takei, T., Ide, Y., Kobayashi, N., et al. (2016). A high-performance supercapacitor cell based on ZIF-8-derived nanoporous carbon using an organic electrolyte. Chem. Commun. 52, 4764–4767. doi: 10.1039/C6CC00413J
Sankar, K. V., and Selvan, R. K. (2015). Improved electrochemical performances of reduced graphene oxide based supercapacitor using redox additive electrolyte. Carbon 90, 260–273. doi: 10.1016/j.carbon.2015.04.023
Sathyamoorthi, S., Kanagaraj, M., Kathiresan, M., Suryanarayanan, V., and Velayutham, D. (2016). Ethyl viologen dibromide as a novel dual redox shuttle for supercapacitors. J. Mater. Chem. A 4, 4562–4569. doi: 10.1039/C6TA00858E
Sathyamoorthi, S., Suryanarayanan, V., and Velayutham, D. (2015). Organo-redox shuttle promoted protic ionic liquid electrolyte for supercapacitor. J. Power Sources 274, 1135–1139. doi: 10.1016/j.jpowsour.2014.10.166
Schütter, C., Neale, A. R., Wilde, P., Goodrich, P., Hardacre, C., Passerini, S., et al. (2016). The use of binary mixtures of 1-butyl-1-methylpyrrolidinium bis {(trifluoromethyl) sulfonyl} imide and aliphatic nitrile solvents as electrolyte for supercapacitors. Electrochim. Acta 220, 146–155. doi: 10.1016/j.electacta.2016.10.088
Seok Jang, H., Justin Raj, C., Lee, W.-G., Chul Kim, B., and Hyun Yu, K. (2016). Enhanced supercapacitive performances of functionalized activated carbon in novel gel polymer electrolytes with ionic liquid redox-mediated poly(vinyl alcohol)/phosphoric acid. RSC Adv. 6, 75376–75383. doi: 10.1039/C6RA15070E
Singh, A., and Chandra, A. (2016). Enhancing specific energy and power in asymmetric supercapacitors-a synergetic strategy based on the use of redox additive electrolytes. Sci. Rep. 6:25793. doi: 10.1038/srep25793
Sun, K., Dong, M., Feng, E., Peng, H., Ma, G., Zhao, G., et al. (2015a). High performance solid state supercapacitor based on a 2-mercaptopyridine redox-mediated gel polymer. RSC Adv. 5, 22419–22425. doi: 10.1039/C4RA15484C
Sun, K., Feng, E., Peng, H., Ma, G., Wu, Y., Wang, H., et al. (2015b). A simple and high-performance supercapacitor based on nitrogen-doped porous carbon in redox-mediated sodium molybdate electrolyte. Electrochim. Acta 158, 361–367. doi: 10.1016/j.electacta.2015.01.185
Sun, K., Ran, F., Zhao, G., Zhu, Y., Zheng, Y., Ma, M., et al. (2016). High energy density of quasi-solid-state supercapacitor based on redox-mediated gel polymer electrolyte. RSC Adv. 6, 55225–55232. doi: 10.1039/C6RA06797B
Teng, Y., Liu, E., Ding, R., Liu, K., Liu, R., Wang, L., et al. (2016). Bean dregs-based activated carbon/copper ion supercapacitors. Electrochim. Acta 194, 394–404. doi: 10.1016/j.electacta.2016.01.227
Tu, Q.-M., Fan, L.-Q., Pan, F., Huang, J.-L., Gu, Y., Lin, J.-M., et al. (2018). Design of a novel redox-active gel polymer electrolyte with a dual-role ionic liquid for flexible supercapacitors. Electrochim. Acta 268, 562–568. doi: 10.1016/j.electacta.2018.02.008
Veerasubramani, G. K., Krishnamoorthy, K., and Kim, S. J. (2016). Improved electrochemical performances of binder-free CoMoO4 nanoplate arrays@Ni foam electrode using redox additive electrolyte. J. Power Sources 306, 378–386. doi: 10.1016/j.jpowsour.2015.12.034
Vlad, A., Singh, N., Melinte, S., Gohy, J.-F., and Ajayan, P. (2016). Carbon redox-polymer-gel hybrid supercapacitors. Sci. Rep. 6, 1–6. doi: 10.1038/srep22194
Wang, C., Xi, Y., Wang, M., Zhang, C., Wang, X., Yang, Q., et al. (2016). Carbon-modified Na2Ti3O7∙2H2O nanobelts as redox active materials for high-performance supercapacitor. Nano Energy 28, 115–123. doi: 10.1016/j.nanoen.2016.08.021
Wang, Y., Chang, Z., Qian, M., Zhang, Z., Lin, J., and Huang, F. (2019). Enhanced specific capacitance by a new dual redox-active electrolyte in activated carbon-based supercapacitors. Carbon 143, 300–308. doi: 10.1016/j.carbon.2018.11.033
Wang, Y., Song, Y., and Xia, Y. (2016). Electrochemical capacitors: mechanism, materials, systems, characterization and applications. Chem. Soc. Rev. 45, 5925–5950. doi: 10.1039/C5CS00580A
Xie, H. J., Gélinas, B., and Rochefort, D. (2016). Redox-active electrolyte supercapacitors using electroactive ionic liquids. Electrochem. Commun. 66, 42–45. doi: 10.1016/j.elecom.2016.02.019
Xie, L., Sun, G., Su, F., Guo, X., Kong, Q., Li, X., et al. (2016). Hierarchical porous carbon microtubes derived from willow catkins for supercapacitor applications. J. Mater. Chem. A 4, 1637–1646. doi: 10.1039/C5TA09043A
Xiong, T., Lee, W. S. V., Chen, L., Tan, T. L., Huang, X., and Xue, J. (2017). Indole-based conjugated macromolecules as a redox-mediated electrolyte for an ultrahigh power supercapacitor. Energy Environ. Sci. 10, 2441–2449. doi: 10.1039/C7EE02584J
Xu, D., Hu, W., Sun, X. N., Cui, P., and Chen, X. Y. (2017a). Redox additives of Na2MoO4 and KI: synergistic effect and the improved capacitive performances for carbon-based supercapacitors. J. Power Sources 341, 448–456. doi: 10.1016/j.jpowsour.2016.12.031
Xu, D., Sun, X. N., Hu, W., and Chen, X. Y. (2017b). Carbon nanosheets-based supercapacitors: design of dual redox additives of 1, 4-dihydroxyanthraquinone and hydroquinone for improved performance. J. Power Sources 357, 107–116. doi: 10.1016/j.jpowsour.2017.05.001
Xu, R., Guo, F., Cui, X., Zhang, L., Wang, K., and Wei, J. (2015). High performance carbon nanotube based fiber-shaped supercapacitors using redox additives of polypyrrole and hydroquinone. J. Mater. Chem. A 3, 22353–22360. doi: 10.1039/C5TA06165B
Yang, W., Yang, W., Song, A., Gao, L., Su, L., and Shao, G. (2017). Supercapacitance of nitrogen-sulfur-oxygen co-doped 3D hierarchical porous carbon in aqueous and organic electrolyte. J. Power Sources 359, 556–567. doi: 10.1016/j.jpowsour.2017.05.108
Ye, T., Li, D., Liu, H., She, X., Xia, Y., Zhang, S., et al. (2018). Seaweed biomass-derived flame-retardant gel electrolyte membrane for safe solid-state supercapacitors. Macromolecules 51, 9360–9367. doi: 10.1021/acs.macromol.8b01955
Yi, T. F., Wei, T. T., Mei, J., Zhang, W., Zhu, Y., Liu, Y. G., et al. (2020). Approaching high-performance supercapacitors via enhancing pseudocapacitive nickel oxide-based materials. Adv. Sustain. Syst. 4:1900137. doi: 10.1002/adsu.201900137
Yu, H., Wu, J., Fan, L., Hao, S., Lin, J., and Huang, M. (2014). An efficient redox-mediated organic electrolyte for high-energy supercapacitor. J. Power Sources 248, 1123–1126. doi: 10.1016/j.jpowsour.2013.10.040
Zhang, H., Li, J., Gu, C., Yao, M., Yang, B., Lu, P., et al. (2016). High performance, flexible, poly(3,4-ethylenedioxythiophene) supercapacitors achieved by doping redox mediators in organogel electrolytes. J. Power Sources 332, 413–419. doi: 10.1016/j.jpowsour.2016.09.137
Zhang, L., Hu, X., Wang, Z., Sun, F., and Dorrell, D. G. (2018). A review of supercapacitor modeling, estimation, and applications: a control/management perspective. Renew. Sustain. Energy Rev. 81, 1868–1878. doi: 10.1016/j.rser.2017.05.283
Zhang, W., Xu, C., Ma, C., Li, G., Wang, Y., Zhang, K., et al. (2017). Nitrogen-superdoped 3D graphene networks for high-performance supercapacitors. Adv. Mater. 29:1701677. doi: 10.1002/adma.201701677
Zhang, Y., Zu, L., Lian, H., Hu, Z., Jiang, Y., Liu, Y., et al. (2017). An ultrahigh performance supercapacitors based on simultaneous redox in both electrode and electrolyte. J. Alloys and Compounds 694, 136–144. doi: 10.1016/j.jallcom.2016.09.302
Zhang, Z. J., Zhu, Y. Q., Chen, X. Y., and Cao, Y. (2015). Pronounced improvement of supercapacitor capacitance by using redox active electrolyte of p-phenylenediamine. Electrochim. Acta 176, 941–948. doi: 10.1016/j.electacta.2015.07.136
Zhao, C., and Zheng, W. (2015). A review for aqueous electrochemical supercapacitors. Front. Energy Res. 3:23. doi: 10.3389/fenrg.2015.00023
Zhi-yu, X., Pu, H., Yang, L., Shou-peng, N., and Peng-fei, H. (2019). Research progress of polymer electrolytes in supercapacitors. J. Mater. Eng. 47, 71–83. doi: 10.11868/j.issn.1001-4381.2019.000346
Keywords: ionic conductivity, energy density, power density, redox electrolyte, supercapacitor
Citation: Zhang L, Yang S, Chang J, Zhao D, Wang J, Yang C and Cao B (2020) A Review of Redox Electrolytes for Supercapacitors. Front. Chem. 8:413. doi: 10.3389/fchem.2020.00413
Received: 01 March 2020; Accepted: 20 April 2020;
Published: 03 June 2020.
Edited by:
Kwan San Hui, University of East Anglia, United KingdomReviewed by:
Dmitry Medvedev, Institute of High Temperature Electrochemistry (RAS), RussiaLe-Qing Fan, Huaqiao University, China
Copyright © 2020 Zhang, Yang, Chang, Zhao, Wang, Yang and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuhua Yang, eWFuZ3NodWh1YTc4QDE2My5jb20=; Jie Chang, Y2hhbmdqaWVjekBob3RtYWlsLmNvbQ==; Bingqiang Cao, bXNlX2Nhb2JxQHVqbi5lZHUuY24=
 Le Zhang1
Le Zhang1 Shuhua Yang
Shuhua Yang Bingqiang Cao
Bingqiang Cao