- 1Key Laboratory for Large-Format Battery Materials and System, Ministry of Education, School of Chemistry & Chemical Engineering, Huazhong University of Science and Technology, Wuhan, China
- 2Analysis Center of College of Science & Technology, Hebei Agricultural University, Huanghua, China
Complex mixtures were often observed when we attempted to synthesize 4-thio- and 2,4-dithio-glycoside derivatives by double parallel and double serial inversion, thus leading to no or low yields of target products. The reason was later found to be that many unexpected side products were produced when a nucleophile substituted the leaving group on the substrate containing the thioacetate group. We hypothesized that thioacetyl migration is prone to occur due to the labile thioacetate group even under weak basic conditions caused by the nucleophile, leading to this result. Therefore, we managed to inhibit the generation of thiol groups from thioacetate groups by the addition of an appropriate amount of conjugate acid/anhydride, successfully improving the synthesis of 4-thio- and 2,4-dithio-glycoside derivatives. The target products which were previously difficult to synthesize, were herein obtained in relatively high yields. Finally, 4-deoxy- and 2,4-dideoxy-glycoside derivatives were efficiently synthesized through the removal of thioacetate groups under UV light, starting from 4-thio- and 2,4-dithio-glycoside derivatives.
Graphical Abstract. Improved synthesis of sulfur-containing glycosides and the further synthesis of deoxyglycosides using them.
Introduction
The synthesis of deoxysugars has drawn increasing attention due to their biological importance (Weymouth-Wilson, 1997; Langenhan et al., 2005; Li et al., 2010; Zou et al., 2012; Balmond et al., 2014; Issa and Bennett, 2014; Thoden and Holden, 2014; Zhu et al., 2014; Elshahawi et al., 2015; Sau et al., 2017; Zhang et al., 2019). The synthesis of 4-deoxysugars drew attention because they are expected to express a variety of biological activities including angiogenesis inhibitory activities (Furuta et al., 1979; van Wijk et al., 2010, 2013; Valueva et al., 2011). 4-Deoxysugars are capable of acting as chain terminators for oligosaccharide biosynthesis with 1–4 glycosidic linkages. In order to achieve a siteselective deoxy product starting from a naturally abundant sugar, multistep protection/deprotection sequences and harsh reduction conditions are usually required (Arita et al., 1972; Rasmussen, 1980; Haque et al., 1986; Lin et al., 1989; Raju et al., 2009; Zou et al., 2012). A method has been developed toward direct synthesis of 4-deoxy pyranosides by two steps, site-selective toluoylation of 4-OH of free pyranosides and subsequent reductive deacyloxylation (Yanagi et al., 2019). However, the catalyst for the toluoylation is not readily available, and the yield for deacyloxylation is low (38–61%). Recently, we have developed an efficient method for the synthesis of deoxy glycosides through UV light promoted desulfurization of sulfur-containing glycosides (Ge et al., 2017a, 2019). The efficiency of obtaining siteselective sulfur-containing glycosides is the key to this approach (Ge et al., 2017a,b). We have been developing methods for the synthesis of sulfur-containing carbohydrate (Ren et al., 2015; Wu et al., 2015; Ge et al., 2017a,b; Norberg et al., 2017), since they can be used as tools for model studies or even therapeutic intervention (Crich and Li, 2007; Sakamoto et al., 2009; Caraballo et al., 2010; Baryal et al., 2013; Daly et al., 2013; Jana and Misra, 2013; Zeng et al., 2013). The introduction of sulfur into a carbohydrate molecule usually proceed through substitution of the leaving group with a thioacetate nucleophile. However, unexpected side reactions were often observed during the substitution process due to the existence of thioacetyl group (Knapp et al., 1992; Pei et al., 2006, 2007; Chen and Withers, 2010), which puzzled us until we thought that thioacetyl group migration cause these side reactions (Zhou et al., 2014). In this study, we initially attempted to synthesize 4-thio- and 2,4-dithio-glycoside derivatives by double parallel and double serial inversion (Dong et al., 2007a). However, no or low yields of target products due to complex side reactions. With methyl 3,6-di-OAc-α-mannoside as a starting material, E2 elimination products were obtained due to the axial 2-OTf leaving group and the steric hindrance of 1-OMe groups. With methyl 3,6-di-OAc-β-mannoside as a starting material, the inversion reactivity at the 2-position showed slightly higher than that at the 4-position due to that the axial 2-OTf leaving group can be attacked directly, leading to the failure of the double serial inversion. With methyl 3,6-di-OAc glucosides and galactosides as starting meterials, it was found that many unexpected side products were produced when a nucleophile substituted the leaving group on the substrate containing an thioacetate group. We hypothesized that thioacetyl group migration causes these unexpected side products and are due to the labile thioacetate group even under weak basic conditions caused by the nucleophile. Therefore, we managed to inhibit the generation of thiol groups from thioacetate groups by the addition of an appropriate amount of conjugate acid/anhydride to the reaction system, successfully improving the synthesis of 4-thio- and 2,4-dithio-glycoside derivatives (Scheme 1). Finally, 4-deoxy- and 2,4-dideoxy-glycoside derivatives were efficiently synthesized through the removal of thioacetate groups under UV light, starting from 4-thio- and 2,4-dithio-glycoside derivatives.
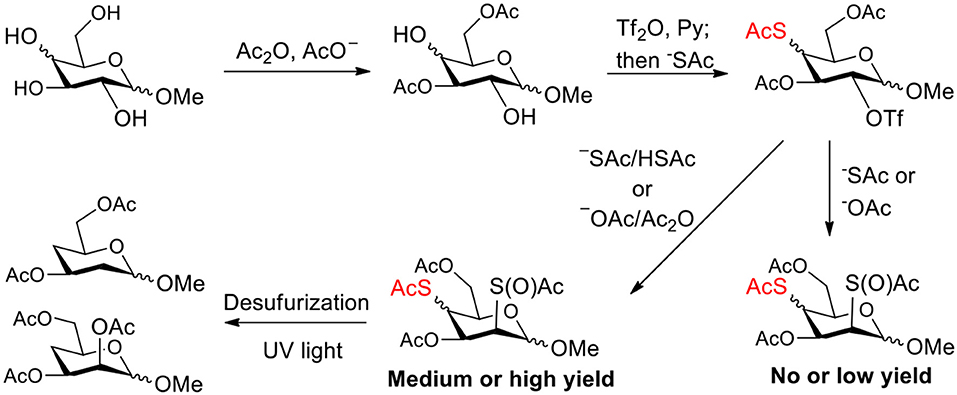
Scheme 1. Improving the synthesis of 4-thio- and 2,4-dithio-glycoside derivatives by suppressing thioacetyl migration.
Results and Discussion
The synthesis efficiencies of 2-thio-, 4-thio- and 2,4-dithio-glycosides are key to obtaining 2-deoxy-, 4-deoxy- and 2,4-dideoxy-glycosides by desulfurization (Ren et al., 2015; Wu et al., 2015; Ge et al., 2017a,b; Norberg et al., 2017). We have developed several efficient methods to synthesize glycosides in which both 3- and 6-positions were protected (Ren et al., 2014b; Xu et al., 2016; Zhang et al., 2016; Lv et al., 2018a,b, 2019). One of the methods using acetate (Ren et al., 2014b) or benzoate (Zhang et al., 2016) as a catalyst is particularly convenient and environmentally friendly. Based on this, we conceived to synthesize 4-thio- and 2,4-dithio-glycosides by double parallel or double serial inversion strategies (Dong et al., 2007a) starting from methyl 3,6-OAc glycosides. Methyl 3,6-OAc glycosides can be efficently obtained by selective acetylation of free methyl glycosides catalyzed by the acetate anion (Ren et al., 2014b), and be triflated to afford 2,4-OTf intermediates. Then the intermediates can be allowed to react with thioacetate anion in the double parallel inversion to give 2,4-dithio-glycoside derivatives, or sequentially react with thioacetate/acetate anion or acetate/thioacetate anion in the double serial inversion to give 2-thio- or 4-thio-glycoside derivatives. In our previous attempts to obtain 2-thio-, 4-thio- and 2,4-dithio-mannosides (Scheme 2) (Wu et al., 2015), the 2,4-dithio- and 2-thio-α/β-D-mannoside derivatives 9/10 and 11/12 were efficiently synthesized while the attempts to synthesize 4-thio-mannoside derivatives 5/6 failed. A complex mixture was obtained when the triflated intermediate 3/4 was treated with KSAc to substitute its 4-OTf, followed by the substitution of its 2-OTf with KOAc. In order to investigate the cause, we repeated this reaction. The investigation indicated that the substitution of the 4-OTf of 3/4 with KSAc in acetonitrile proceeded very well so as to afford the 4-thioacetate intermediate 7/8 in a high yield. However, A complex mixture was observed when the intermediate 7/8 was treated with KOAc whether in aceonitrile or in DMF. Withers encountered a similar dilemma when he attempted to synthesize p-nitrophenyl 4-thio-β-D-mannopyranoside by the double serial inversion (Chen and Withers, 2010). He proposed that the thioacetate group is usually labile even under weak basic conditions so as to cause a number of side reactions. Based on our previous studies on thioacetyl migration (Zhou et al., 2014), we guessed that thio group should be readily produced from the deacetylation of thioacetate under basic condition and further lead to acetyl migration, oxidation, and inversion products.
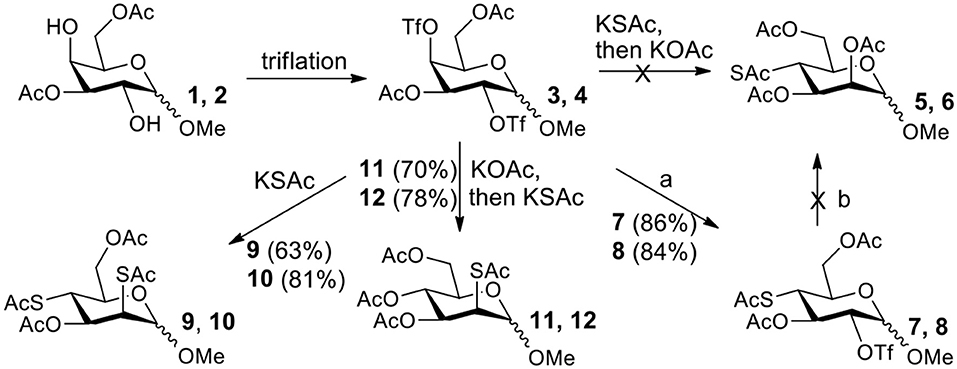
Scheme 2. Attempts to synthesize methyl 2-thio-, 4-thio- and 2, 4-dithio-a/β-D-mannopyranosides (Wu et al., 2015): (a) KSAc, MeCN, rt, 0.5 h; (b) KOAc, DMF or MeCN, rt, 48 h, a complex mixture.
It is more difficult to synthesize methyl 2-thio-, 4-thio- and 2, 4-thio-α/β-D-talosides through the double parallel and double serial inversion (Scheme 3). Methyl 3,6-di-OAc-α/β-D-glucoside 13/14 can be synthesized in a high yield by regioselective acetylation of free methyl α/β-D-glucoside (Ren et al., 2014b), followed by triflation to give triflated intermediate 15/16. The intermediate 15/16 was expected to be sequentially substituted with KOAc and KSAc to give 2-thio-α/β-D-taloside 17/18, to be substituted with an excess amount of KSAc to give 2,4-dithio-α/β-D-taloside 19/20, and to be sequentially substituted with KSAc and KOAc to give 4-thio-α/β-D-taloside 21/22. However, no or very low yields were obtained in all these reactions due to the formation of complex mixtures. The reason was supposed to be due to the neighboring group participation (3-OAc attacking 2 or 4-position) (Dong et al., 2007a, 2008b) and the instability of the thioacetate group under even weak basic conditions. Then the intermediate 15/16 was substituted with TBASAc in toluene to supress neighboring group participation, affording 4-SAc intermediate 23/24 in a high yield. The isolated 23/24 further reacted with KSAc in DMF to give the 2,4-di-SAc taloside derivative 19/20 in a yield of 33/36%. The attempts to obtain 21/22 by the inversion of 23/24 with KOAc in DMF still failed.
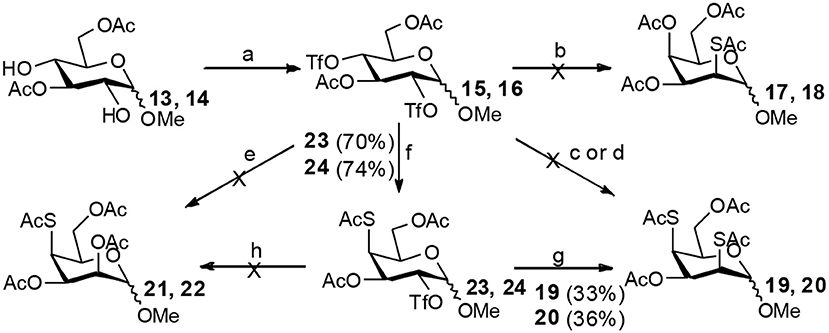
Scheme 3. Attempts to synthesize methyl 2-thio-, 4-thio- and 2, 4-thio-a/β-D-talosides: (a) i: TBAOAc, Ac2O, MeCN, rt, 24 h; ii: Tf2O, pyridine, DCM, −20–10°C, 3 h; (b) i: KOAc, DMF, rt, 1 h; ii: KSAc, DMF, 40°C, overnight (17, 11%, 18, <5%); (c) KSAc, DMF, rt, 48 h, complex mixture; (d) TBASAc, PhMe, rt, 7 d, <10% yield; (e) i: KSAc, DMF, rt, 1 h; ii: KOAc, DMF, 40°C, overnight, complex mixture; (f) TBASAc, PhMe, rt, 1 h, over 3 steps; (g) KSAc, DMF, 40°C, overnight; (h) KOAc, DMF, 40°C, overnight, complex mixture.
The attempt to synthesize methyl 2-thio-, 4-thio- and 2, 4-thio-α-D-galactosides through double parallel and double serial inversion failed (Scheme 4). The 3,6-di-OAc-α-D-mannoside 26 was obtained in 80% yield by organotin-mediated regioselective acetylation (Dong et al., 2007b) of free methyl α-D-mannoside 25. Triflation of 26 afforded triflated intermediate 27. Treatment of the intermediate 27 with 5.0 equiv of KSAc in DMF for 12 h provided a major product 28 and a minor product 29. However, it was observed (by TLC plate) that 29 was first formed and then slowly converted to 28 with time. Treatment of 27 with 2.0 equiv of TBASAc in toluene for 48 h gave a mixture of half and half of 28 and 29. Treatment of 27 with 1.2 equiv of KSAc in DMF for 48 h provided a major product 29. Similarly, when 27 was treated with 1.2 equiv of KOAc in DMF for 48 h, a major product 30 was isolated. The isolated other product proven to be a mixture containing 31 (Figure S1 in Supplementary Material). Obviously, the substitution of the 4-OTf of 27 by thioacetate could successfully produce the intermediate 32. However, the substitution of the axial 2-OTf of 32 by thioacetate or acetate was difficult due to the steric hindrance of 1-OMe in this case, thus leading to the E2 elimination and the elimination product 29 under basic conditions. Under this basic conditions, 29 slowly converted to 28 going through migration intermediates B and C with time.
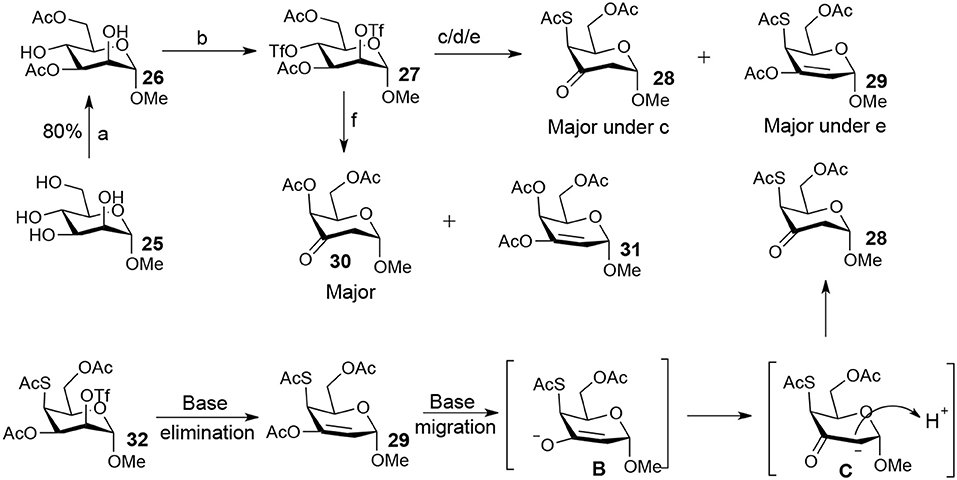
Scheme 4. Attempt to synthesize methyl 2-thio-, 4-thio- and 2, 4-dithio-α-D-galactosides starting from methyl α-D-mannoside: (a) i: Bu2SnO, MeOH, reflux 2 h; ii: Ac2O, MeCN, 0°C to rt, 12 h; (b) Tf2O, pyridine, DCM, −20–10°C, 3 h; (c) KSAc (5.0 eq), DMF, rt, 24 h; (d) TBASAc (2.0 eq), PhMe, rt, 48 h; (e) KSAc (1.2 eq), DMF, rt, 48 h; (f) KOAc (1.2 eq), DMF, rt, 48 h.
The attempt to abtain methyl 2-thio-, 4-thio- and 2, 4-thio-β-D-galactosides starting from methyl 3,6-di-OAc-β-manopyranoside 33 showed better results (Scheme 5). Substitution of 34 (the triflated product of 33) with 5.0 equiv of TBASAc in MeCN at room temperature led to a 84% yield of methyl 2,4-di-thioacetate-galactoside 35. Unexpectedly, neither intermediaite 36a (the 4-OTf of 34 substituted by thioacetate) nor intermediate 36b (the 2-OTf of 34 substituted by thioacetate) could be observed under the conditions that 1.0 equiv of TBASAc was used instead, and 35 was still obtained. Usually, 4-OTf showed higher reactivity than 2-OTf when substituted on a glycoside ring. However, the substitution of 4-OTf of 34 is disfavored due to steric hindrance of 2-OTf in this case. Once 2-OTf of 34 had been substituted by thioacetate, the substitution of 4-OTf would occur immediately due to the disappearance of the steric hindrance from 2-OTf, leading to the formation of 35. Similarly, the treatment of 34 with 1.0 equiv of TBAOAc in MeCN at room temperature mainly gave product 37. However, when this reaction was performed at 0°C, intermediate 38 was formed. Consequently, the following addition of 3 equiv of TBASAc led to 4-thioacetate galactoside 39 in 48% yield. However, the treatment of 34 with 1.0 equiv of TBASAc in MeCN at 0°C did not give intermediate 36b, but gave 35 in 38% yield. While axial triflates can be attacked directly (the antibonding orbital can be approached), the sugar ring has to adopt a different conformation to allow attack on the equatorial triflate (since the antibonding orbital is shielded by the axial substituents on the ring). Lowering the temperature thus may slow down the interconversion of the ring. Thus, the 2-OTf of 34 showed high reactivity on substitution by acetate due to the axial ttiflate leaving group. When the reaction proceeded at room temperature, the product 37 was formed immediately from the intermediate 38. However, when the reaction proceeded at 0°C, the interconversion of the sugar ring turned very slow, which thus restrained the further substitution of 4-OTf by the acetate. However, thioacetate showed much higher nucleophilicity than acetate since sulfur is a big atom and it will therefore readily have a productive overlap with the antiboding orbital. This high nucleophilicity of thioacetate flattened the difference in reactivity between 2-OTf and 4-OTf. We also proposed that a supramolecular control effect perhaps plays a key role in this process, in which an acetate ion can be accommodated at the center of the β-pyranoside face to produce an anion-carbohydrate complex (Dong et al., 2008a; Ren et al., 2014a), resulting in a higher reactivity of 2-OTf than that of 4-OTf. However, the poor or no supramolecular effect in polar solvent acetonitrile can not fully support this result.
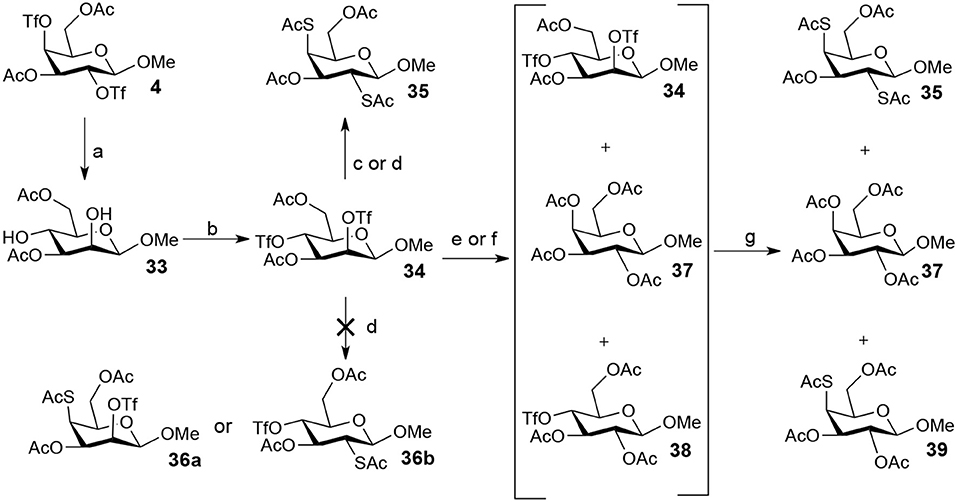
Scheme 5. Attempt to synthesize methyl 2-thio-, 4-thio- and 2, 4-thio-β-D-galactoside: (a) TBANO2, PhMe, rt, 6 h, 70%; (b) Tf2O, pyridine, DCM, −20–10°C, 3 h; (c) TBASAc (5.0 eq), MeCN, rt, 2 h, 35 (84%); (d) TBASAc (1.0 eq), MeCN, 0°C, 4 h, 35 (38%); (e) TBAOAc (2.0 eq), MeCN, rt, 2 h; (f) TBAOAc (2.0 eq), MeCN, 0°C, 4 h; (g) TBASAc (3.0 eq), MeCN, 0°C-rt, 3 h, 39 (8% from b-e-g, 48% from b-f-g).
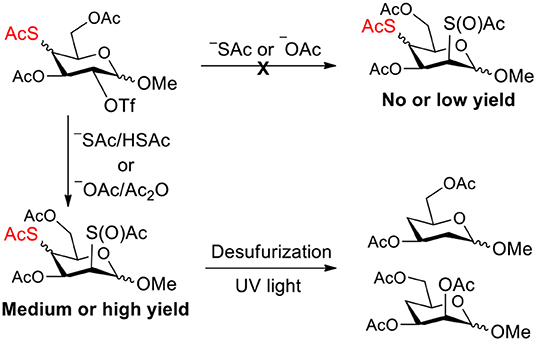
From these experiments, we noticed that the substitution on a substrate which have already contained a thioacetate group usually led to unexpected side-products. The previous studies (Chen and Withers, 2010; Zhou et al., 2014) have suggested that thioacetate groups are usually labile even under weak basic conditions, and then produce thiol groups, leading to acetyl migration, oxidation, and inversion products (Figure 1). The initial thiol might be generated by intermolecular acetyl migration to a nucleophile (trace of water, dimethylamine in DMF, acetate, or thioacetate) in the reaction mixture. Once a thiol group was formed, the intramolecular acetyl migration from an adjacent acetyl group to the thiol group would occur under even weak basic conditions. If the complex mixtures were indeed caused by thiol group and acetyl migration in these reactions, suppressing such formation of thiol and such migration by adjusting acidic/basic condition may improve these reactions.
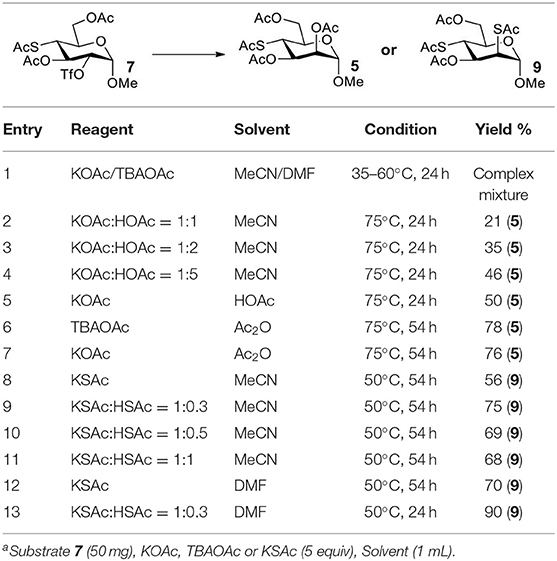
Therefore, the substitution on 2-triflated intermediate 7 by acetate/thioacetate was used as a model reaction to test under various acidic/basic conditions (Table 1). Substitution of 7 with acetate in acetonitrile or DMF led to a complex mixture (entry 1). To our delight, with the addition of more and more acetic acid to this reaction system, the target 5 was isolated for better and better yield (entries 2–5). Especially, the yield of 5 when using Ac2O as the solvent (entries 6 and 7) was 76–78% as compared with the 50% yield when using acetic acid as the solvent (entry 5). This must be because Ac2O greatly inhibited the generation of thiol groups by reacting with thiol groups to form thioacetates. Substitution of 7 with 5 equiv of thioacetate in acetonitrile yielded the target 9 in 56% yield (entry 8). The yield of 9 was increased to 75% when 1.5 equiv of thioacetic acid was added to this reaction system (entry 9). However, the addition of more thioacetic acid decreased the yield of 9 (entries 10 and 11). Substitution of 7 with 5 equiv of thioacetate in DMF in the absence/presence of 1.5 equiv of thioacetic acid yielded 9 in 70/90% yield, respectively (entries 12 and 13). These results indicated that our hypothesis was reasonable. The substitution on a substrate containing a thioacetate group by thioacetate/acetate could be improved by suppressing the formation of thiol and by adjusting the acidity/basicity of the reaction system.
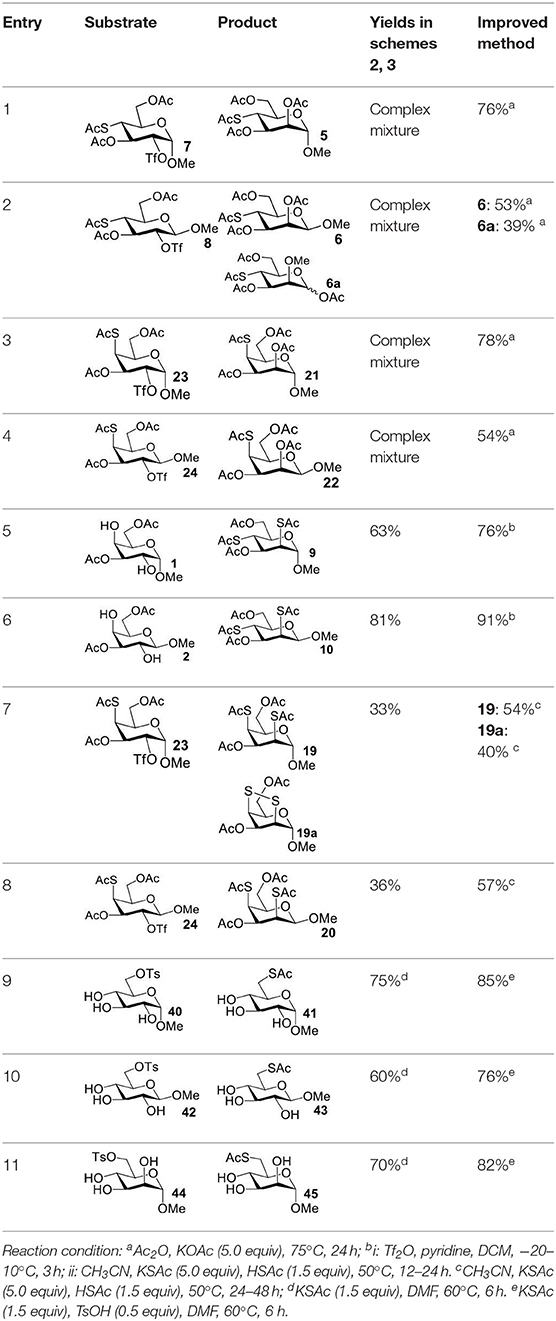
The synthesis of 2-OAc-4-SAc methyl glycosides 5, 6, 21, and 22 failed in Schemes 2, 3. However, with Ac2O used as the reaction solvent instead, 5, 6, 21, and 22 were successfully synthesized in medium to high yields (47–78%) starting from their 2-OTf intermediates 7, 8, 23, and 24 (entries 1–4 in Table 2). The main side-products seemed to be caused by 1-OMe group participation, such as 6a (Figure S2 in Supplementary Material). The yields of 2,4-di-SAc glycosides 9, 10, 19, and 20 were increased by 9–13% compared to the reaction without the addition of 1.5 equiv of thioacetic acid (entries 5–8). The main side-products seemed to be caused by the oxidation of intra-molecular thiol groups, such as 19a. Substitution of 6-OTs glycosides 40, 42, and 44 with potassium thioacetate in DMF, the yields of 6-SAc glycosides 41, 43, and 45 were increased by 10–16% compared to the reaction without the addition of 0.5 equiv of toluene sulfonic acid (entries 9–11).
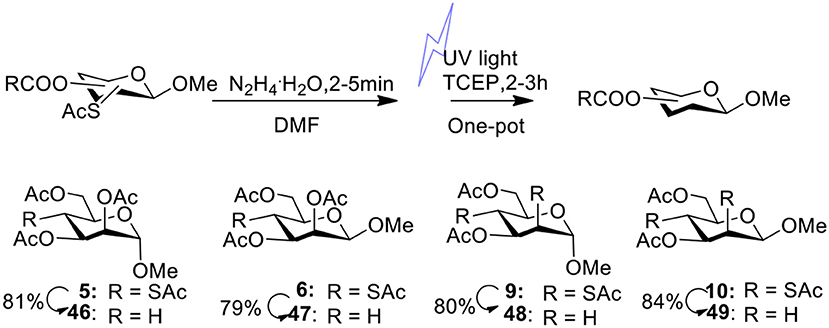
In our previous studies on the synthesis of deoxyglycosides by desulfurization under UV light (Ge et al., 2017b, 2019), we didn't obtain 4-deoxymannosidic derivatives because we were unable to obtain 4-SAc mannosides efficiently. With 4-SAc mannosides 5 and 6 in the hands, 4-deoxymannosidic derivatives 46 and 47 were obtained in 81 and 79% yields by our one-pot method removing thioacetate group (Figure 2), respectively. With 2,4-di-SAc mannosides 9 and 10 in the hands, we started to test if 2,4-di-deoxy glycosides could be obtained by simultaneously removing two thioacetate groups in a one-pot method. After optimizing the reaction conditions, substrates 9 and 10 were treated with 2.5 equiv of N2H4·H2O in DMF at room temperature for 4 min, followed by the addition of 3.0 equiv of TCEP.HCl, and desulfurization under UV light led to 2,4-di-deoxy glycosides 48 and 49 in 80 and 84% yields, respectively.
Conclusion
It was attempted to synthesize methyl 2-thio-, 4-thio- and 2,4-dithio-glycosides starting from methyl 3,6-di-OAc glycosides by a double parallel and double serial inversion strategy in this study. Complex mixtures were often observed, thus leading to no or low yields of target products. With methyl 3,6-di-OAc-α-mannoside as a starting material in this strategy, elimination products were obtained due to the steric hindrance of 1-OMe group. With methyl 3,6-di-OAc-β-mannoside as a starting material in this strategy, the slightly higher reactivity of 2-OTf than that of 4-OTf due to the axial 2-OTf leaving group, leads to the failure of the double serial inversion. With methyl 3,6-di-OAc glucosides and galactosides as starting materials, it was found that many unexpected side products were produced when a nucleophile substituted the leaving group on the substrate containing an thioacetate group. The reason is hypothesized that thioacetyl migration is prone to occur due to the labile thioacetate group even under weak basic conditions caused by the nucleophile. Therefore, when substitution of the substrate with an acetate anion, Ac2O was used as a solvent to inhibited the generation of thiol groups by reacting with thiol groups to form thioacetates; when substitution of the substrate with a thioacetate anion, an appropriate amount of thioacetic acid was added to the reaction system to adjust the basicity. Consequently, the synthesis of target 4-thio- and 2,4-dithio-glycoside products was successfully improved due to suppressing thioacetyl migration. Finally, 4-deoxy- and 2,4-dideoxy-glycoside derivatives were efficiently synthesized through the removal of thioacetate groups under UV light, starting from 4-thio- and 2,4-dithio-glycoside derivatives.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Author Contributions
TL performed the experiments and analyzed the data. YZ and JX prepared some related substrates and analyzed the data. YL and HD came up with the original idea, conceptualized and directed the project, and drafted the paper with the assistance from all co-authors.
Funding
This study was supported by the National Nature Science Foundation of China (Nos. 21772049, 21708010), Specialized Research Fund for the Doctoral Program of Hebei Agricultural University (No. 2D201712), and Natural Science Foundation of Hebei Province (No. B2019204243).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are also grateful to the staff in the Analytical and Test Center of HUST for support with the NMR and HRMS instruments.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2020.00319/full#supplementary-material
References
Arita, H., Fukukawa, K., and Matsushima, Y. (1972). Studies on amino-hexoses. XVI. synthesis of deoxy-analogues of N-acetyl-D-glucosamine. Bull. Chem. Soc. Jpn. 45, 3614–3619. doi: 10.1246/bcsj.45.3614
Balmond, E. I., Benito-Alifonso, D., Coe, D. M., Alder, R. W., McGarrigle, E. M., and Galan, M. C. (2014). A 3,4-trans-fused cyclic protecting group facilitates α-selective catalytic synthesis of 2-deoxyglycosides. Angew. Chem., Int. Ed. 53, 8190–8194. doi: 10.1002/anie.201403543
Baryal, K. N., Zhu, D., Li, X., and Zhu, J. (2013). Umpolung reactivity in the stereoselective synthesis of S-linked 2-deoxyglycosides. Angew. Chem. Int. Ed. 52:8012. doi: 10.1002/anie.201301682
Caraballo, R., Dong, H., Ribeiro, J. P., Jimenez-Barbero, J., and Ramström, O. (2010). Direct STD NMR identification of β-galactosidase inhibitors from a virtual dynamic hemithioacetal system. Angew. Chem. Int. Ed. 49, 589–593. doi: 10.1002/anie.200903920
Chen, H-M., and Withers, S. G. (2010). Syntheses of p-nitrophenyl 3- and 4-thio-β-d-glycopyranosides Carbohydr. Res. 345, 2596–2604. doi: 10.1016/j.carres.2010.10.001
Crich, D., and Li, M. (2007). Revisiting the armed–disarmed concept: the importance of anomeric configuration in the activation of S-benzoxazolyl glycosides. Org. Lett. 9, 4115–4118. doi: 10.1021/ol701466u
Daly, R., McCabe, T., and Scanlan, E. M. (2013). Development of fully and partially protected fucosyl donors for oligosaccharide synthesis. J. Org. Chem. 78, 1080–1090. doi: 10.1021/jo302487c
Dong, H., Pei, Z., Angelin, M., Bystrom, S., and Ramstrom, O. (2007a). Efficient synthesis of β-D-mannosides and β-D-talosides by double parallel or double serial inversion. J. Org. Chem. 72, 3694–3701. doi: 10.1021/jo062643o
Dong, H., Pei, Z., Bystrom, S., and Ramstrom, O. (2007b). Reagent-dependent regioselective control in multiple carbohydrate esterifications. J. Org. Chem. 72, 1499–1502. doi: 10.1021/jo0620821
Dong, H., Pei, Z.-C., and Ramström, O. (2008b). Supramolecular activation in triggered cascade inversion. Chem. Commun. 11, 1359–1361. doi: 10.1039/b717301f
Dong, H., Rahm, M., Brinck, T., and Ramstrom, O. (2008a). Supramolecular control in carbohydrate epimerization: discovery of a new anion host–guest system. J. Am. Chem. Soc. 130, 15270–15271. doi: 10.1021/ja807044p
Elshahawi, S. I., Shaaban, K. A., Kharel, M. K., and Thorson, J. S. (2015). A comprehensive review of glycosylated bacterial natural products. Chem. Soc. Rev. 44, 7591–7697. doi: 10.1039/C4CS00426D
Furuta, R., Naruto, S., Tamura, A., and Yokogawa, K. (1979). Neosidomycin, a new antibiotic of streptomyces. Tetrahedron Lett. 19, 1701–1704. doi: 10.1016/S0040-4039(01)93628-7
Ge, J-T., Li, Y-Y., Tian, J., Liao, R-Z., and Dong, H. (2017a). Synthesis of deoxyglycosides by desulfurization under UV light. J. Org. Chem. 82, 7008–7014. doi: 10.1021/acs.joc.7b00896
Ge, J-T., Zhou, L., Luo, T., Lv, J., and Dong, H. (2019). A one-pot method for removal of thioacetyl group via desulfurization under ultraviolet light to synthesize deoxyglycosides. Org. Lett. 21, 5903–5906. doi: 10.1021/acs.orglett.9b02033
Ge, J-T., Zhou, L., Zhao, F-L., and Dong, H. (2017b). Straightforward S-S bond formation via the oxidation of S-acetyl by iodine in the presence of N-iodosuccinimide. J. Org. Chem. 82, 12613–12623. doi: 10.1021/acs.joc.7b02367
Haque, M. E., Kikuchi, T., Kanemitsu, K., and Tsuda, Y. (1986). Utilization of sugars in organic syntheses. XVI. Synthesis of some deoxy, unsaturated, and dideoxy sugars via regioselective thioacylation of glycopyranosides by the dibutyltin oxide method. Chem. Pharm. Bull. 34, 430–433. doi: 10.1248/cpb.34.430
Issa, J. P., and Bennett, C. S. (2014). A reagent-controlled SN2-glycosylation for the direct synthesis of β-linked 2-deoxy-sugars. J. Am. Chem. Soc. 136, 5740–5744. doi: 10.1021/ja500410c
Jana, M., and Misra, A. K. (2013). Stereoselective synthesis of β-glycosyl thiols and their synthetic applications. J. Org. Chem. 78, 2680–2686. doi: 10.1021/jo302115k
Knapp, S., Naughton, A. B. J., Jaramillo, C., and Pipik, B. (1992). Reactions of some pyranoside diol monotriflates with nucleophiles and bases. J. Org. Chem. 57, 7328–7334. doi: 10.1021/jo00052a058
Langenhan, J. M., Griffith, B. R., and Thorson, J. S. (2005). Neo-glyco-randomization and chemoenzymic glyco-randomization: two complementary tools for natural product diversification. J. Nat. Prod. 68, 1696–1711. doi: 10.1021/np0502084
Li, Y., Yang, X., Liu, Y., Zhu, C., Yang, Y., and Yu, B. (2010). Gold(I)-catalyzed glycosylation with glycosyl ortho-alkynylbenzoates as donors: general scope and application in the synthesis of a cyclic triterpene saponin. Chem. Eur. J. 16, 1871–1882. doi: 10.1002/chem.200902548
Lin, T. H., Kovác, P., and Glaudemans, C. P. (1989). Improved synthesis of the 2-, 3-, and 4-deoxy derivatives from methyl β-D-galactopyranoside. Carbohydr. Res. 188, 228–238. doi: 10.1016/0008-6215(89)84076-5
Lv, J., Ge, J. T., Luo, T., and Dong, H. (2018a). An inexpensive catalyst, Fe(acac)3, for regio/site-selective acylation of diols and carbohydrates containing a 1,2-cis-diol. Green Chem. 20, 1987–1991. doi: 10.1039/C8GC00428E
Lv, J., Luo, T., Zhang, Y., Pei, Z., and Dong, H. (2018b). Regio/site-selective benzoylation of carbohydrates by catalytic amounts of FeCl3. ACS Omega 3, 17717–17723. doi: 10.1021/acsomega.8b02360
Lv, J., Luo, T., Zou, D., and Dong, H. (2019). Using DMF as both a catalyst and cosolvent for the regioselective silylation of polyols and diols. Eur J. Org. Chem. 37, 6383–6395. doi: 10.1002/ejoc.201901195
Norberg, O., Wu, B., Thota, N., Ge, J-T., Fauquet, G., Saur, A-K., et al. (2017). Synthesis and binding affinity analysis of α1-2- and α1-6-O/S-linked dimannosides for the elucidation of sulfur in glycosidic bonds using quartz crystal microbalance sensors. Carbohydr. Res. 452, 35–42. doi: 10.1016/j.carres.2017.09.015
Pei, Z., Dong, H., Caraballo, R., and Ramström, O. (2007). Synthesis of positional thiol analogs of β-D-galactopyranose. Eur. J. Org. Chem. 29, 4927–4934. doi: 10.1002/ejoc.200700364
Pei, Z., Larsson, R., Aastrup, T., Anderson, H., Lehn, J. M., and Ramström, O. (2006). Quartz crystal microbalance bioaffinity sensor for rapid identification of glycosyldisulfide lectin inhibitors from a dynamic combinatorial library. Biosens. Bioelectron. 22, 42–48. doi: 10.1016/j.bios.2005.11.024
Raju, R., Castillo, B. F., Richardson, S. K., Thakur, M., Severins, R., Kronenberg, M., et al. (2009). Synthesis and evaluation of 3”- and 4”-deoxy and -fluoro analogs of the immunostimulatory glycolipid, KRN7000. Bioorg. Med. Chem. Lett. 19, 4122–4125. doi: 10.1016/j.bmcl.2009.06.005
Rasmussen, J. R. (1980). Synthesis of 4-deoxy-D-lyxo-hexose. J. Org. Chem. 45, 2725–2727. doi: 10.1021/jo01301a041
Ren, B., Dong, H., and Ramström, O. (2014a). A carbohydrate-anion recognition system in aprotic solvents. Chem. Asian J. 9, 1298–1304. doi: 10.1002/asia.201301617
Ren, B., Rahm, M., Zhang, X., Zhou, Y., and Dong, H. (2014b). Regioselective acetylation of diols and polyols by acetate catalysis: mechanism and application. J. Org. Chem. 79, 8134–8142. doi: 10.1021/jo501343x
Ren, B., Wang, M., Liu, J., Ge, J., Zhang, X., and Dong, H. (2015). Zempl'en transesterification: a name reaction that has misled us for 90 years. Green Chem. 17, 1390–1394. doi: 10.1039/C4GC02006E
Sakamoto, J., Koyama, T., Miyamoto, D., Yingsakmongkon, S., Hidari, K. I., Jampangern, W., et al. (2009). Systematic syntheses of influenza neuraminidase inhibitors: a series of carbosilane dendrimers uniformly functionalized with thioglycoside-type sialic acid moieties. Bioorg. Med. Chem. 17, 5451–5464. doi: 10.1016/j.bmc.2009.06.036
Sau, A., Williams, R., Palo-Nieto, C., Franconetti, A., Medina, S., and Galan, M. C. (2017). Palladium-catalyzed direct stereoselective synthesis of deoxyglycosides from glycals. Angew. Chem. Int. Ed. 56, 3640–3644. doi: 10.1002/anie.201612071
Thoden, J. B., and Holden, H. M. (2014). Production of a novel N-monomethylated dideoxysugar. Biochemistry 53, 1105–1107. doi: 10.1021/bi500098a
Valueva, O. A., Rakhuba, D., Shashkov, A. S., Zdorovenko, E. L., Kiseleva, E., Novik, G., et al. (2011). Structure of the major O-specific polysaccharide from the lipopolysaccharide of Pseudomonas fluorescens BIM B-582: identification of 4-deoxy-D-xylo-hexose as a component of bacterial polysaccharides. J. Nat. Prod. 74, 2161–2167. doi: 10.1021/np200472p
van Wijk, X. M. R., Oosterhof, A., van den Broek, S. A. M. W., Griffioen, A. W., ten Dam, G. B., Rutjes, F. P. J. T., et al. (2010). A 4-deoxy analogue of N-acetyl-D-glucosamine inhibits heparan sulphate expression and growth factor binding in vitro. Cell Res. 316, 2504–2512. doi: 10.1016/j.yexcr.2010.04.025
van Wijk, X. M. R., Thijssen, V. L., Lawrence, R., van den Broek, S. A., Dona, M., Naidu, N., et al. (2013). Interfering with UDP-GlcNAc metabolism and heparan sulfate expression using a sugar analogue reduces angiogenesis. ACS Chem. Biol. 8, 2331–2338. doi: 10.1021/cb4004332
Weymouth-Wilson, A. C. (1997). The role of carbohydrates in biologically active natural products. Nat. Prod. Rep. 14, 99–110. doi: 10.1039/np9971400099
Wu, B., Ge, J., Ren, B., Pei, Z., and Dong, H. (2015). Synthesis and binding affinity analysis of positional thiol analogs of mannopyranose for the elucidation of sulfur in different position. Tetrahedron 71, 4023–4030. doi: 10.1016/j.tet.2015.04.060
Xu, H., Ren, B., Zhao, W., Xin, X., Lu, Y., Pei, Y., et al. (2016). Regioselective mono and multiple alkylation of diols and polyols catalyzed by organotin and its applications on the synthesis of value-added carbohydrate intermediates. Tetrahedron 72, 3490–3499. doi: 10.1016/j.tet.2016.04.076
Yanagi, M., Ueda, Y., Ninomiya, R., Imayoshi, A., Furuta, T., Mishiro, K., et al. (2019). Synthesis of 4-deoxy pyranosides via catalyst-controlled site-selective toluoylation of abundant sugars. Org. Lett. 21, 5006–5009. doi: 10.1021/acs.orglett.9b01549
Zeng, X., Smith, R., and Zhu, X. (2013). Synthesis of thioglycoside analogs of maradolipid. J. Org. Chem. 78, 4165–4170. doi: 10.1021/jo400274s
Zhang, X., Ren, B., Ge, J., Pei, Z., and Dong, H. (2016). A green and convenient method for regioselective mono and multiple benzoylation of diols and polyols. Tetrahedron 72, 1005–1010. doi: 10.1016/j.tet.2015.12.074
Zhang, Y., Zhao, F-L., Luo, T., Pei, Z., and Dong, H. (2019). Regio/stereoselective glycosylation of diol and polyol acceptors in efficient synthesis of Neu5Ac-α-2, 3-LacNPhth trisaccharide. Chem. Asian J. 14, 223–234. doi: 10.1002/asia.201801486
Zhou, Y., Zhang, X., Ren, B., Wu, B., Pei, Z., and Dong, H. (2014). S-acetyl migration in synthesis of sulfur-containing glycosides. Tetrahedron 70, 5385–5390. doi: 10.1016/j.tet.2014.07.014
Zhu, D., Baryal, K. N., Adhikari, S., and Zhu, J. (2014). Direct synthesis of 2-deoxy-β-glycosides via anomeric O-alkylation with secondary electrophiles. J. Am. Chem. Soc. 136, 3172–3175. doi: 10.1021/ja4116956
Keywords: sulfur-containing glycoside, acetyl group migration, 4-deoxy glycoside, 2,4-dideoxy-glycoside, desulfurization
Citation: Luo T, Zhang Y, Xi J, Lu Y and Dong H (2020) Improved Synthesis of Sulfur-Containing Glycosides by Suppressing Thioacetyl Migration. Front. Chem. 8:319. doi: 10.3389/fchem.2020.00319
Received: 12 December 2019; Accepted: 30 March 2020;
Published: 23 April 2020.
Edited by:
Zhongping Tan, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaReviewed by:
George Kokotos, National and Kapodistrian University of Athens, GreeceMartin D. Witte, University of Groningen, Netherlands
Copyright © 2020 Luo, Zhang, Xi, Lu and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuchao Lu, THV5dWNoYW9AaGViYXUuZWR1LmNu; Hai Dong, aGRvbmdAbWFpbC5odXN0LmVkdS5jbg==
 Tao Luo1
Tao Luo1 Yuchao Lu
Yuchao Lu Hai Dong
Hai Dong