- 1Key Laboratory of Jiangxi Province for Persistent Pollutants Control and Resources Recycle, Nanchang, China
- 2College of Environmental and Chemical Engineering, Nanchang Hangkong University, Nanchang, China
No visible light activity is the bottle neck for wide application of TiO2, and Boron doping is one of the effective way to broaden the adsorption edge of TiO2. In this study, several Boron doped TiO2 materials were prepared via a facile co-precipitation and calcination process. The B doping amounts were optimized by the degradation of rhodamine B (Rh B) under visible light irradiation, which indicated that when the mass fraction of boron is 6% (denoted as 6B-TiO2), the boron doped TiO2 materials exhibited the highest activity. In order to investigate the enhanced mechanism, the difference between B-doped TiO2 and bare TiO2 including visible light harvesting abilities, separation efficiencies of photo-generated electron-hole pairs, photo-induced electrons generation abilities, photo-induced charges transferring speed were studied and compared in details. h+ and · were determined to be the two main responsible active species in the photocatalytic oxidation process. Besides the high degradation efficiency, 6B-TiO2 also exhibited high reusability in the photocatalysis, which could be reused at least 5 cycles with almost no active reduction. The results indicate that 6B-TiO2 has high photocatalytic degradation ability toward organic dye of rhodamine B under visible light irradiation, which is a highly potential photocatalyst to cope with organic pollution.
Introduction
Environmental problems are global issues and effect all of human kind. These issues and pressures increase in severity as society continues to develop at a very fast pace (Samanta et al., 2002; Shao et al., 2017; Chen et al., 2018; Chowdhary et al., 2018; Tian et al., 2018; Hong et al., 2020). Water pollution is one of the most serious environmental problems and attracts much attention (Wang and Yang, 2016; Jiang et al., 2019b; Kapelewska et al., 2019; Quesad et al., 2019; Wu et al., 2019; Zhao et al., 2019b). Organic dyes have been synthesized on a large scale and are widely applied in our daily lives, resulting in tons of dyes being discharged into the aqueous environment every year, causing many serious environmental problems (Sohni et al., 2019; Tu et al., 2019; Zhan et al., 2019; Zhou X. et al., 2019). Rhodamine B is a toxic alkaline cationic dye, which was used as a food additive, but has been forbidden due to its high carcinogenic potential (Wu et al., 2018; Lops et al., 2019; Tian et al., 2019; Guo et al., 2020). Furthermore, it can also cause other serious diseases such as visceral disease and red skin staining (Alcocer et al., 2018; Liu et al., 2019; Maria Magdalane et al., 2019). It is very difficult to degrade rhodamine B under natural conditions. Methods for the effective removal of rhodamine B are therefore of great importance.
In recent decades, photocatalysis has exhibited its high potential in waste water treatment, due to its inherent merits including low costs, renewability, being environment-friendly, and its high efficiency. TiO2 is a widely used photocatalyst because of its chemical stability, high redox reactivity, easy preparation, and low cost. However, it can not adsorb and use visible light because of its wide energy band gap. So only ~4% solar energy of ultraviolet light can be used by TiO2, and 45% solar energy of visible light can not be used (Jiang et al., 2018a, 2019a; Yin et al., 2018; Ahadi et al., 2019; Zhou F. et al., 2019; Komtchoua et al., 2020). In order to broaden the light adsorption of TiO2 to visible light, many efforts have been conducted (Zhao et al., 2019a; Zhang et al., 2020). Doing has attracted increasing interest in recent years (Jiang et al., 2018a; Kamaludin et al., 2019; Lu et al., 2019; Xiu et al., 2019; Yan et al., 2019).
In this study, boron was used to dope into TiO2 to prepare the photocatalyst of B-TiO2. B-TiO2 shows high photocatalytic degradation ability toward rhodamine B under visible light. The preparation conditions were optimized, and the structure and photocatalytic performance of B-TiO2 were carefully investigated. Based on the experimental results, the photocatalytic degradation mechanism was discussed. This study indicates that B-TiO2 has the potential to treat dye pollution through visible light irradiation.
Experiment
Materials
All the chemicals used in this study were of analytical pure grade. Boric acid and aqueous ammonia were purchased from Xilong Science Co., Ltd, China. Rhodamine B and tetrabutyl titanate were bought from the Aladdin reagent company, China. Other chemicals are all commercial. Deionized water (DI water) was used throughout the study. The materials were directly used without any treatment.
Synthesis of B-TiO2
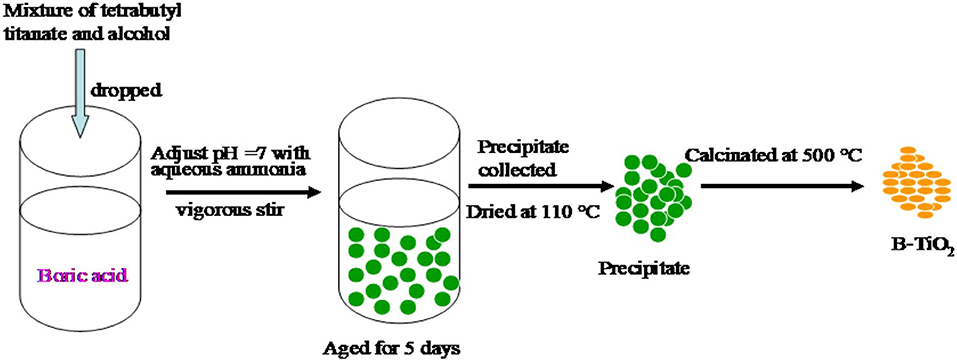
0.1 mol tetrabutyl titanate was dissolved into 100 ml absolute alcohol to form a clean solution. Boric acid was dissolved into a solution containing 2 ml nitric acid, 50 ml absolute alcohol and 50 ml DI water. After that, the tetrabutyl titanate solution described above was dripped into the boric acid solution and vigorously stirred. At the same time, aqueous ammonia was dripped into the mixture described above to adjust the pH value to 7. The formation of precipitation was found in the process. After being aged for 5 days, the precipitation was separated and dried at 110°C. Finally, it was calcinated at 500°C to obtain the B doped TiO2 denoted as B-TiO2. The feeding amounts of boric acid were changed to obtain B-TiO2 with a B mass ratio of 0, 3, 6, 9, 12, and 15%, and are denoted as TiO2, 3B-TiO2, 6B-TiO2, 9B-TiO2, 12B-TiO2, and 15 B-TiO2, respectively. The methodology is shown in Scheme 1.
Photocatalytic Degradation
Ten milligrams of photocatalyst was added into 25 ml rhodamine B solution at a concentration of 5 mg/L. The mixture was stirred in the dark for 30 min to obtain adsorption equilibrium. After that, the mixture was irradiated under visible light by a 500 W Xe lamp (Perfectlight, Beijing, China) with λ ≦ 400 nm cutoff, and sampled at determined intervals to examine the rhodamine B concentration in the suspension, which was determined by the adsorption at 552 nm.
Characterization
The morphology of the sample was investigated by scanning electron microscopy (SEM) (Hitachi-4800, Japan) and a transmission electron microscope (TEM) (JEM-2100, Japan). An X-ray powder diffractometer (XRD, Rigaku III/B max, Cu Ka) was used to analyze the samples. The pH values of solutions were determined by a JENCO 6175 pH meter (Renshi electronics Co. Ltd. USA). Electrochemical impedance spectroscopy (EIS) and photocurrent response analysis were performed by CHI660C electrochemical workstation (Shanghai Chenhua, China). Photoluminescence (PL) spectra were recorded on a F-7000 fluorescence spectrophotometer (Hitachi, Japan). UV-vis diffuse-reflectance spectra (DRS) of samples were obtained on a UV-vis-NIR spectrometer (Lambda 900).
Results and Discussion
Optimization of B Doping Amount
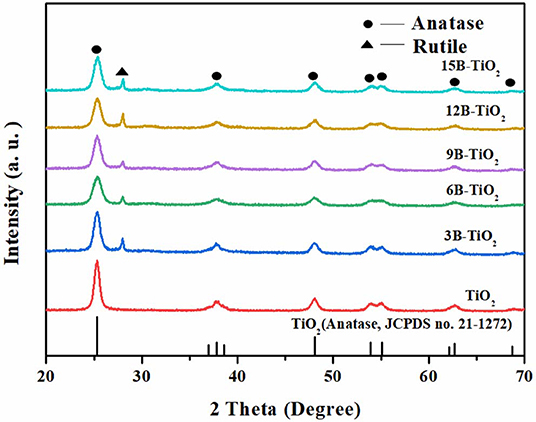
The bare TiO2 and B doping TiO2 were investigated by XRD analysis, and the results are shown in Figure 1. It can be seen that the samples show similar XRD characteristics. However, it is notable that there is a new peak at ~25.5°, which is attributable to rutile TiO2 (Wang et al., 2015; Warkhade et al., 2017), appears in the spectra of B doping TiO2, but is not the case in the spectrum of bare TiO2. This phenomenon indicates that under the experimental conditions of this study, the doping of B, no matter how much the doping amount is, can induce the pure anatase TiO2 to transform to mixed crystal phases of anatase and rutile (Cui et al., 2017). These results indicate that B has been successfully doped into the crystal lattice of TiO2.
All the samples, including the bare TiO2 and the B doping TiO2, were used to degrade rhodamine B under visible light irradiation. As shown in Figure 2, the degradation ability first increases with the B doping amount, and when the B doping amount reaches 6%, the degradation ability decreases with the rise of B doping amount. This phenomenon indicates that 6% of the B mass ratio is the optimal value. Thereafter, 6B-TiO2 was determined as the optimal sample, and 6% was determined as the optimal feeding amount of B in the preparation.
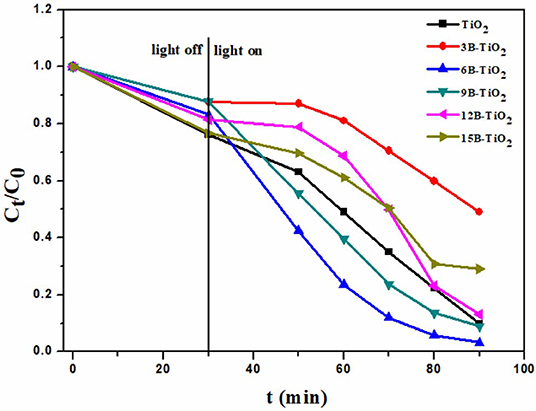
Figure 2. The photocatalytic degradation toward rhodamine B over B-TiO2 with different B doping amount.
Characterization of B Doping TiO2 Samples
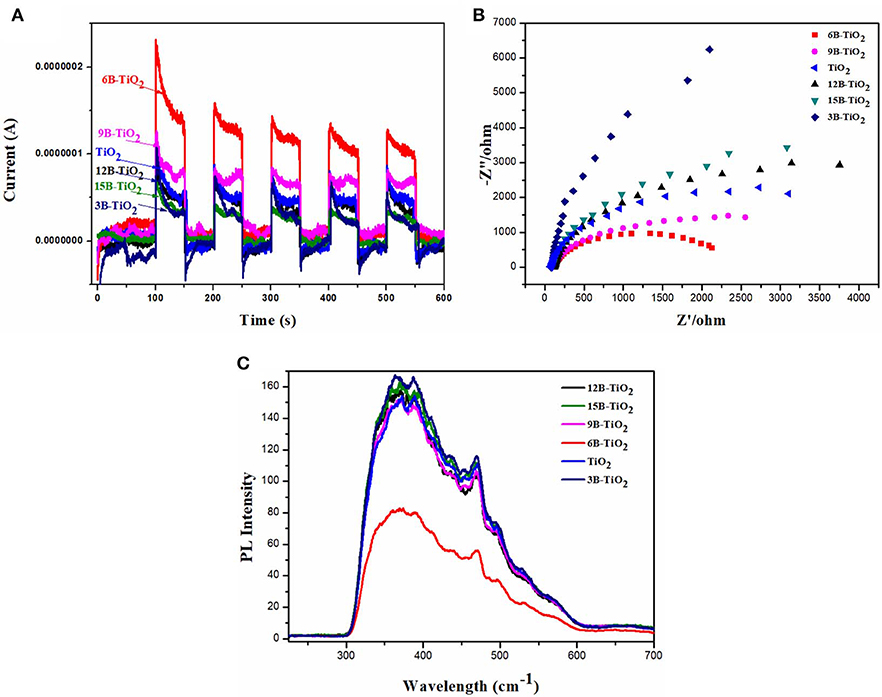
In order to investigate the mechanism of the enhanced photocatalytic performance of 6B-TiO2, the samples of bare TiO2 and B doping TiO2 were investigated by photocurrent response, EIS and PL spectrum, and the results are shown in Figure 3. It can be seen in Figure 3A that 6B-TiO2 shows highest photocurrent response, indicating that more electrons can be generated in 6B-TiO2 by visible light irradiation (Hu et al., 2019; Murali et al., 2019; Wang et al., 2019). In the EIS analysis (Figure 3B), 6B-TiO2 exhibits a semicircle of the EIS Nyquist plot with the smallest radius, indicating the smallest interfacial charge transference impedance as compared to those of bare TiO2 and other B doping TiO2 (Dai et al., 2015; Zou et al., 2016; Manwar et al., 2019). As shown in Figure 3C, 6B-TiO2 indicates the lowest photoluminescence intensity, which suggests that 6B-TiO2 has the lowest recombination rate of electron-hole pairs (Cai et al., 2019; Huang et al., 2019; Yuan et al., 2019). It can be found from the above analysis, that the most electrons can be generated by visible light in 6B-TiO2, and the charge carriers can transfer in 6B-TiO2 with the lowest impedance and recombination rate. All of these characteristic can favor the subsequent photocatalytic reaction, so there is no doubt that 6B-TiO2 exhibits the best photocatalytic performance as compared to bare TiO2 and other B doping TiO2.
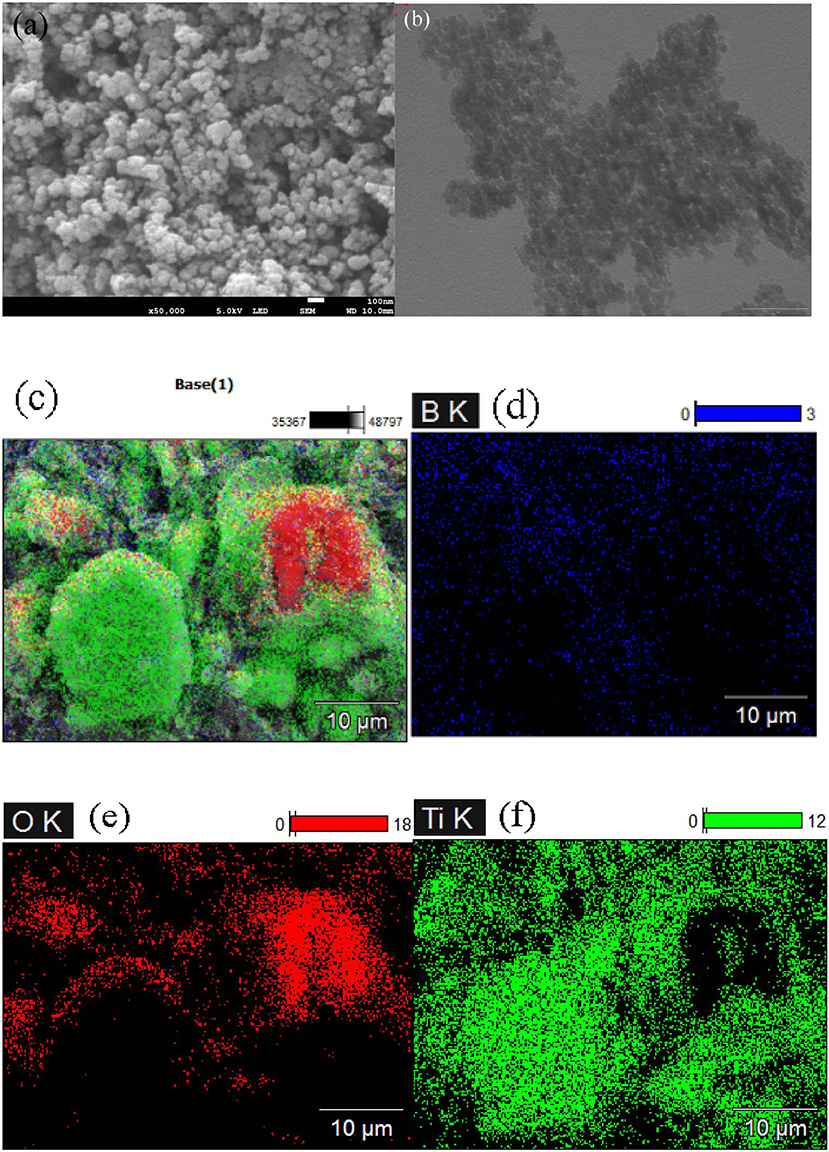
The morphology of 6B-TiO2 was investigated by SEM and TEM. As shown in Figures 4a,b, 6B-TiO2 exhibits nano spheral morphology with a litter aggregation. Elemental mapping indicates that elements of B, O, and Ti homogeneously distribute on the surface of 6B-TiO2, confirming the successful doping of B (Figures 4c–f).
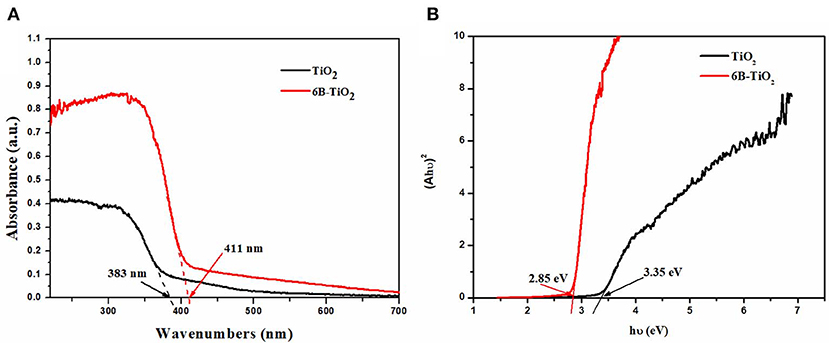
The bare TiO2 and 6B-TiO2 were investigated by DRS, and the results are shown in Figure 5A. It can be seen that the light adsorption edge of bare TiO2 is about 383 nm, indicating no visible light adsorption activity. As B was doped to form 6B-TiO2, the light adsorption edge significantly red shift to about 411 nm, which indicates that 6B-TiO2 can adsorb visible light. The band gap energies of the two samples were calculated by Tauc plot according to the DRS results by Jiang et al. (2018b); Kato et al. (2019); Khan et al. (2019), and are shown in Figure 5B. As one can see, bare TiO2 has a wide band gap of 3.35 eV, which is too high to be excited by visible light. However, after B doping, the band gap was narrowed to 2.85 eV, and can be excited to produce electrons by visible light. The results clearly show that B doping significantly narrows the band gap of TiO2, and broadens the light adsorption edge to a visible light range.
Investigation of the Active Species in the Photocatalytic Degradation
In order to study the photocatalytic mechanism, the potential active species in the photocatalytic degradation course were investigated. There are usually four active species involved in the photocatalytic degradation course. They are ·, ·OH, e−, and h+. 0.05 mmol different scavengers were added, respectively, in the photocatalytic degradation system, and other conditions were the same as described in “2.3 Photocatalytic degradation.” The scavengers are i-propanol (·OH scavenger), triethanolamine (h+ scavenger), 1, 4-Benzoquinone (·), and AgNO3 (e− scavenger) (Asmus et al., 1967; Zhang et al., 2013; Huyen et al., 2018). It can be seen in Figure 6 that h+ and · play the most important roles in the degradation of rhodamine B, because the addition of the scavengers for these two active species can dramatically decrease the degradation capacity. One can also find that ·OH takes part in the degradation, because the addition of i-propanol can impress the degradation too. h+, · and ·OH are three highly oxidative species. The results indicate that their high oxidative activities may be used to degrade rhodamine B in this photocatalysis course. It is notable that the addition of AgNO3 can promote the degradation. The reason is that AgNO3 can consume e− as an e− scavenger, which depress the recombination of e−-h+ pairs, and enhance the photocatalytic ability.
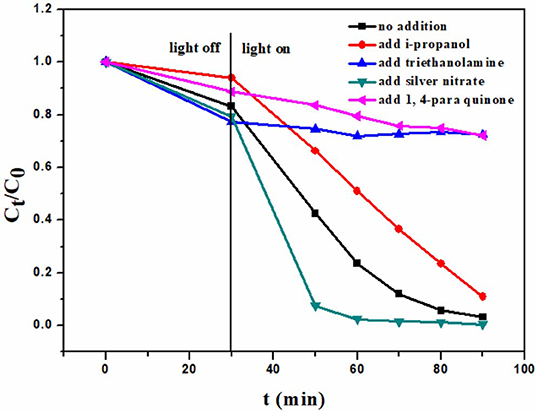
Figure 6. Photocatalytic degradation of rhodamine B over 6B-TiO2 in the presence of different scavengers.
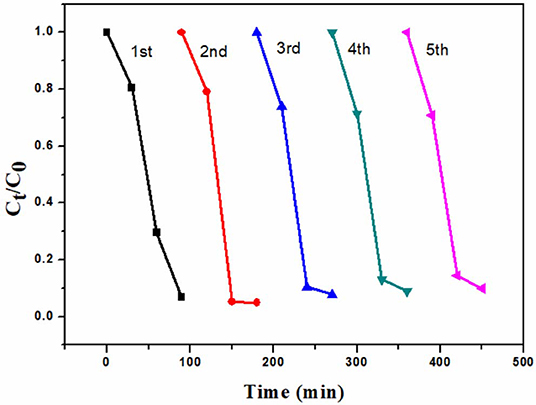
Investigation of Reusability
In order to evaluate of the reusability of 6B-TiO2, the used 6B-TiO2 was collected and washed wth DI water. After being dried, it was used in the photocatalytic degradation of rhodamine B again, and the experimental conditions are the same as descried in “2.3 Photocatalytic degradation” with little modification of sampling time. As one can see in Figure 7, 6B-TiO2 can be continuously used to effectively degrade rhodamine B at least in five cycles.
Conclusion
B doping TiO2 were successfully prepared in this study, and 6B-TiO2 was determined as the optimal B doping amount. 6B-TiO2 shows the best photocurrent response ability, fastest charge transference speed and lowest recombination rate of e−-h+ pairs, which significantly enhances its photocatalytic performance. B doping significantly narrows the band gap of TiO2, and therefore broaden the light adsorption edge to visible light range. h+ and · are the most important active species in the photocatalytic degradation, and ·OH is also involved in the degradation. 6B-TiO2 shows high reusability, which can be effectively used in at least five degradation cycles. 6B-TiO2 is a potential photocatalyst with visible light responsible ability, which can be used to effectively treat dye pollution.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Author Contributions
PN did the experiments. PC and HJ designed the experiments. GW, HZ, and QC were devoted to the discussion and analysis.
Funding
The work was supported by the National Natural Science Foundation of China (51568051, 51668046, and 51978323), the International Cooperation and Exchange NSFC (51720105001), and the Science Foundation for Young Scientists for Jiangxi Province-Key Project (2017ACB21034). Nanchang Hangkong University Innovation and Entrepreneurship Course Cultivation Project (KCPY1806).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Ahadi, S., Moalej, N. S., and Sheibani, S. (2019). Characteristics and photocatalytic behavior of Fe and Cu doped TiO2 prepared by combined sol-gel and mechanical alloying. Solid State Sci. 96:105975. doi: 10.1016/j.solidstatesciences.2019.105975
Alcocer, S., Picos, A., Uribe, A. R., Pérez, T., and Peraltahernández, J. M. (2018). Comparative study for degradation of industrial dyes by electrochemical advanced oxidation processes with BDD anode in a laboratory stirred tank reactor. Chemosphere 205, 682–689. doi: 10.1016/j.chemosphere.2018.04.155
Asmus, K. D., Cercek, B., Ebert, M., Henglein, A., and Wigger, A. (1967). Pulse radiolysis of nitrobenzene solutions. Trans. Faraday Soc. 63, 2435–2441. doi: 10.1039/tf9676302435
Cai, S. L., Lu, L., Wu, W. P., Wang, J., Sun, Y. C., Ma, A. Q., et al. (2019). A new mixed ligand based Cd(II) 2D coordination polymer with functional sites: photoluminescence and photocatalytic properties. Inorg. Chim. Acta. 484, 291–296. doi: 10.1016/j.ica.2018.09.066
Chen, P., Wang, T., Xiao, Y., Tian, E., Wang, W., Yule, Z., et al. (2018). Efficient fluoride removal from aqueous solution by synthetic Fe-Mg-La tri-metal nanocomposite and the analysis of its adsorption mechanism. J. Alloy. Compd. 738, 118–129. doi: 10.1016/j.jallcom.2017.12.142
Chowdhary, P., Raj, A., and Bharagava, R. N. (2018). Environmental pollution and health hazards from distillery wastewater and treatment approaches to combat the environmental threats: a review. Chemosphere 194, 229–246. doi: 10.1016/j.chemosphere.2017.11.163
Cui, Y., Ma, Q., Deng, X., Meng, Q., Cheng, X., Xie, M., et al. (2017). Fabrication of Ag-Ag2O/reduced TiO2 nanophotocatalyst and its enhanced visible light driven photocatalytic performance for degradation of diclofenac solution. Appl. Catal. B Environ. 206, 136–145. doi: 10.1016/j.apcatb.2017.01.014
Dai, Z., Qin, F., Zhao, H., Tian, F., Liu, Y., and Chen, R. (2015). Time-dependent evolution of the Bi3.64Mo0.36O6.55/Bi2MoO6 heterostructure for enhanced photocatalytic activity via the interfacial hole migration. Nanoscale 7, 11991–11999. doi: 10.1039/C5NR02745D
Guo, N., Liu, H., Fu, Y., Hu, J. (2020). Preparation of Fe2O3 nanoparticles doped with In2O3 and photocatalytic degradation property for rhodamine B. Optik 201:163537. doi: 10.1016/j.ijleo.2019.163537
Hong, S. M., Yoon, H. J., Choi, Y., Cho, Y. Z., Mun, S., Pol, V. G., et al. (2020). Solving two environmental problems simultaneously: scalable production of carbon microsheets from structured packing peanuts with tailored microporosity for efficient CO2 capture. Chem. Eng. J. 379:122219. doi: 10.1016/j.cej.2019.122219
Hu, T., Wang, W., Han, D., and Dong, W. (2019). Enhanced photocurrent and photocatalytic degradation of methyl orange in cobalt hydroxide loaded titanium dioxide film. AIP Adv. 9:055122. doi: 10.1063/1.5098399
Huang, Y., Qin, J., Fan, Z., Wei, D., and Seo, H. J. (2019). Photoenergy conversion behaviors of photoluminescence and photocatalysis in silver-coated LiBaPO4:Eu2+. Inorg. Chem. 58, 13161–13169. doi: 10.1021/acs.inorgchem.9b02037
Huyen, T. T. T., Chi, T. T. K., Dung, N. D., Kosslick, H., and Liem, N. Q. (2018). Enhanced photocatalytic activity of {110}-faceted TiO2 rutile nanorods in the photodegradation of hazardous pharmaceuticals. Nanomaterials 8:276. doi: 10.3390/nano8050276
Jiang, H., Li, M., Liu, J., Li, X., Tian, L., and Chen, P. (2018a). Alkali-free synthesis of a novel heterostructured CeO2-TiO2 nanocomposite with high performance to reduce Cr(VI) under visible light. Ceram. Int. 44, 2709–2717. doi: 10.1016/j.ceramint.2017.10.225
Jiang, H., Li, X., Li, M., Niu, P., Wang, T., Chen, D., et al. (2019a). A new strategy for triggering photocatalytic activity of cytrochrome P450 by coupling of semiconductors. Chem. Eng. J. 358, 58–66. doi: 10.1016/j.cej.2018.09.199
Jiang, H., Li, X., Tian, L., Wang, T., Wang, Q., Pingping, N., et al. (2019b). Defluoridation investigation of yttrium by laminated Y-Zr-Al tri-metal nanocomposite and analysis of the fluoride sorption mechanism. Sci. Total Environ. 648, 1342–1353. doi: 10.1016/j.scitotenv.2018.08.258
Jiang, H., Liu, J., Li, M., Tian, L., Ding, G., Chen, P., et al. (2018b). Facile synthesis of C-decorated Fe, N co-doped TiO2 with enhanced visible-light photocatalytic activity by a novel co-precursor method. Chin. J. Catal. 39, 747–759. doi: 10.1016/S1872-2067(18)63038-4
Kamaludin, R., Puad, A. S. M., Othman, M. H. D., AbdulKadir, S. H. S., and Harun, Z. (2019). Incorporation of N-doped TiO2 into dual layer hollow fiber (DLHF) membrane for visible light-driven photocatalytic removal of reactive black 5. Polym. Test. 78:105939. doi: 10.1016/j.polymertesting.2019.105939
Kapelewska, J., Kotowska, U., Karpinska, J., Astel, A., Zielinski, P., Suchta, J., et al. (2019). Water pollution indicators and chemometric expertise for the assessment of the impact of municipal solid waste landfills on groundwater located in their area. Chem. Eng. J. 359, 790–800. doi: 10.1016/j.cej.2018.11.137
Kato, K., Vaucher, S., Hoffmann, P., Xin, Y., and Shirai, T. (2019). A novel single-mode microwave assisted synthesis of metal oxide as visible-light photocatalyst. Mater. Lett. 235, 125–128. doi: 10.1016/j.matlet.2018.09.132
Khan, M. S., Diao, Z., Osada, M., and Shen, S. (2019). Nitrogen doped ultrathin calcium/sodium niobate perovskite nanosheets for photocatalytic water oxidation. Sol. Energy Mater. Sol. Cells 205:110283. doi: 10.1016/j.solmat.2019.110283
Komtchoua, S., Delegan, N., Dirany, A., Drogui, P., Robert, D., and Khakani, M. A. E. (2020). Photo-electrocatalytic oxidation of atrazine using sputtured deposited TiO2: WN photoanodes under UV/visible light. Cataly. Today 340, 323–333. doi: 10.1016/j.cattod.2019.04.067
Liu, N., Jing, C., Li, Z., Huang, W., Gao, B., You, F., et al. (2019). Effect of synthesis conditions on the photocatalytic degradation of rhodamine B of MIL-53(Fe). Mater. Lett. 237, 92–95. doi: 10.1016/j.matlet.2018.11.079
Lops, C., Ancona, A., Cesare, K. D., Dumontel, B., Garino, N., Canavese, G., et al. (2019). Sonophotocatalytic degradation mechanisms of rhodamine B dye via radicals generation by micro- and nano-particles of ZnO. Appl. Cata. B Environ. 243, 629–640. doi: 10.1016/j.apcatb.2018.10.078
Lu, X., Li, X., Chen, F., Chen, Z., Qian, J., and Zhang, Q. (2019). Biotemplating synthesis of N-doped two-dimensional CeO2-TiO2 nanosheets with enhanced visible light photocatalytic desulfurization performance. J. Alloy. Comp. 815:152326. doi: 10.1016/j.jallcom.2019.152326
Manwar, N. R., Jain, S. L., Rayalu, S., and Labhasetwar, N. K. (2019). Solar energy conversion: Pt-doped mesoporous ceria as an efficient electro-photocatalyst for hydrogen production from water splitting. Mater. Today Proc. 17, 277–287. doi: 10.1016/j.matpr.2019.06.431
Maria Magdalane, C., Kaviyarasu, K., Priyadharsini, G. M. A., Bashir, A. K. H., Mayedwa, N., Matinise, N., et al. (2019). Improved photocatalytic decomposition of aqueous rhodamine-B by solar light illuminated hierarchical yttria nanosphere decorated ceria nanorods. J. Mater. Res. Technol. 8, 2898–2909. doi: 10.1016/j.jmrt.2018.11.019
Murali, A., Sarswat, P. K., and Sohn, H. Y. (2019). Enhanced photocatalytic activity and photocurrent properties of plasma-synthesized indium-doped zinc oxide nanopowder. Mater. Today Chem. 11, 60–68. doi: 10.1016/j.mtchem.2018.10.007
Quesad, H. B., Baptista, A. T. A., Cusioli, L. F., Seibert, D., Bezerra, C. O., and Bergamasco, R. (2019). Surface water pollution by pharmaceuticals and an alternative of removal by low-cost adsorbents: a review. Chemosphere 222, 766–780. doi: 10.1016/j.chemosphere.2019.02.009
Samanta, S. K., Singh, O. V., and Jain, R. K. (2002). Polycyclic aromatic hydrocarbons: environmental pollution and bioremediation. Trends Biotechnol. 20, 243–248. doi: 10.1016/S0167-7799(02)01943-1
Shao, J., Sheng, W., Wang, M., Li, S., Chen, J., Zhang, Y., et al. (2017). In situ synthesis of carbon-doped TiO2 single-crystal nanorods with a remarkably photocatalytic efficiency. Appl. Catal. B Environ. 209, 311–319. doi: 10.1016/j.apcatb.2017.03.008
Sohni, S., Hashim, R., Nidaullah, H., Lamaming, J., and Sulaiman, O. (2019). Chitosan/nano-lignin based composite as a new sorbent for enhanced removal of dye pollution from aqueous solutions. Int. J. Biol. Macromol. 132, 1304–1317. doi: 10.1016/j.ijbiomac.2019.03.151
Tian, J., Shao, Q., Zhao, J., Pan, D., Dong, M., Jia, C., et al. (2019). Microwave solvothermal carboxymethyl chitosan templated synthesis of TiO2/ZrO2 composites toward enhanced photocatalytic degradation of rhodamine B. J. Colloid Interf. Sci. 541, 18–29. doi: 10.1016/j.jcis.2019.01.069
Tian, L., Jiang, H. L., Chen, P. H., Wang, Q., Niu, P. P., Shi, Y. M., et al. (2018). A novel GO/PNIPAm hybrid with two functional domains can simultaneously effectively adsorb and recover valuable organic and inorganic resources. Chem. Eng. J. 343, 607–618. doi: 10.1016/j.cej.2018.03.015
Tu, H., Li, D., Yi, Y., Liu, R., Wu, Y., Dong, X., et al. (2019). Incorporation of rectorite into porous polycaprolactone/TiO2 nanofibrous mats for enhancing photocatalysis properties towards organic dye pollution. Composite. Comm. 15, 58–63. doi: 10.1016/j.coco.2019.06.006
Wang, B., Zhang, G., Sun, Z., Zheng, S., and Frost, R. L. (2015). A comparative study about the influence of metal ions (Ce, La and V) doping on the solar-light-induced. J. Environ. Chem. Eng. 3, 1444–1451. doi: 10.1016/j.jece.2015.05.007
Wang, Q., and Yang, Z. (2016). Industrial water pollution, water environment treatment, and health risks in China. Environ. Epidemiol. 218, 358–365. doi: 10.1016/j.envpol.2016.07.011
Wang, Y., Shen, L., Wang, Y., Hou, B., Gibson, G. N., Poudel, N., et al. (2019). Hot electron-driven photocatalysis and transient absorption spectroscopy in plasmon resonant grating structures. Faraday Discuss. 214, 325–339. doi: 10.1039/C8FD00141C
Warkhade, S. K., Gaikwad, G. S., Zodape, S. P., Pratap, U., Maldhure, A. V., and Wankhade, A. V. (2017). Low temperature synthesis of pure anatase carbon doped titanium dioxide: an efficient visible light active photocatalyst. Mat. Sci. Semicon. Proc. 63, 18–24. doi: 10.1016/j.mssp.2017.01.011
Wu, X., Li, H., Su, J., Zhang, J., Feng, Y., Jia, Y., et al. (2019). Full spectrum responsive In2.77S4/WS2 p-n heterojunction as an efficient photocatalyst for Cr(VI) reduction and tetracycline oxidation. Appl. Surf. Sci. 473, 992–1001. doi: 10.1016/j.apsusc.2018.12.219
Wu, X., Sun, Y., Li, H., Wang, Y., Zhang, C., Zhang, J., et al. (2018). In-situ synthesis of novel p-n heterojunction of Ag2CrO4-Bi2Sn2O7 hybrids for visible-light-driven photocatalysis. J. Alloy Compd. 740, 1197–1203. doi: 10.1016/j.jallcom.2018.01.100
Xiu, Z., Guo, M., Zhao, T., Pan, K., Xing, Z., Zhenzi, L., et al. (2019). Recent advances in Ti3+ self-doped nanostructured TiO2 visible light photocatalysts for environmental and energy applications. Chem. Eng. J. 382:123011. doi: 10.1016/j.cej.2019.123011
Yan, J., Zha, J., Hao, L., Hu, Y., Liu, T., Guan, S., et al. (2019). Low-temperature S-doping on N-doped TiO2 films and remarkable enhancement on visible-light performance. Mater. Res. Bull. 120:110594. doi: 10.1016/j.materresbull.2019.110594
Yin, Z., Qiu, S., Chen, W., Li, H., Cheng, L., and Cao, S. (2018). Highly photocatalytic activity from tri-modified TiO2 hollow spheres. Mater. Lett. 214, 202–204. doi: 10.1016/j.matlet.2017.11.123
Yuan, H., Xu, M., Luo, K., and Hu, W. (2019). Relationships between defect-related photoluminescence and photocatalytic activity of (F, Na)-codoped ZnO nanocrystals. Ceram. Int. 45, 16694–16697. doi: 10.1016/j.ceramint.2019.05.136
Zhan, Y., Guan, X., Ren, E., Lin, S., and Lan, J. (2019). Fabrication of zeolitic imidazolate framework-8 functional polyacrylonitrile nanofibrous mats for dye removal. J. Polym. Res. 26:145. doi: 10.1007/s10965-019-1806-5
Zhang, S., Yi, J., Chen, J., Yin, Z., Tang, T., Wei, W., et al. (2020). Spatially confined Fe2O3 in hierarchical SiO2@TiO2 hollow sphere exhibiting superior photocatalytic efficiency for degrading antibiotics. Chem. Eng. J. 380:122583. doi: 10.1016/j.cej.2019.122583
Zhang, Y., Zhang, N., Tang, Z. R., and Xu, Y. J. (2013). Identification of Bi2WO6 as a highly selective visible-light photocatalyst toward oxidation of glycerol to dihydroxyacetone in water. Chem. Sci. 4, 1820–1824. doi: 10.1039/c3sc50285f
Zhao, S., Chen, J., Liu, Y., Jiang, Y., Jiang, C., Yin, Z., et al. (2019a). Silver nanoparticles confined in shell-in-shell hollow TiO2 manifesting efficiently photocatalytic activity and stability. Chem. Eng. J. 367, 249–259. doi: 10.1016/j.cej.2019.02.123
Zhao, S., Zhu, H., Wang, H., Rassu, P., Wang, Z., Song, P., et al. (2019b). Free-standing graphene oxide membrane with tunable channels for efficient water pollution control. J. Hazard. Mater. 366, 659–668. doi: 10.1016/j.jhazmat.2018.12.055
Zhou, F., Yan, C., Sun, Q., and Komarneni, S. (2019). TiO2/Sepiolite nanocomposites doped with rare earth ions: preparation, characterization and visible light photocatalytic activity. Micropor. Mesopor. Mater. 274, 25–32. doi: 10.1016/j.micromeso.2018.07.031
Zhou, X., Zhou, Y., Liu, J., Song, S., Sun, J., Zhu, G., et al. (2019). Study on the pollution characteristics and emission factors of PCDD/Fs from disperse dye production in China. Chemosphere 228, 328–334. doi: 10.1016/j.chemosphere.2019.04.136
Keywords: TiO2, doping, boron, dye pollution, photocatalytic degradation
Citation: Niu P, Wu G, Chen P, Zheng H, Cao Q and Jiang H (2020) Optimization of Boron Doped TiO2 as an Efficient Visible Light-Driven Photocatalyst for Organic Dye Degradation With High Reusability. Front. Chem. 8:172. doi: 10.3389/fchem.2020.00172
Received: 27 January 2020; Accepted: 26 February 2020;
Published: 13 March 2020.
Edited by:
Jinwen Shi, Xi'an Jiaotong University, ChinaReviewed by:
Xiang-Feng Wu, Shijiazhuang Tiedao University, ChinaShunsheng Cao, Jiangsu University, China
Huiquan Li, Fuyang Normal University, China
Copyright © 2020 Niu, Wu, Chen, Zheng, Cao and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pinghua Chen, cph1979@126.com; Hualin Jiang, hua20022000@126.com
 Pingping Niu1,2
Pingping Niu1,2 Hualin Jiang
Hualin Jiang