
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Chem. , 10 January 2020
Sec. Medicinal and Pharmaceutical Chemistry
Volume 7 - 2019 | https://doi.org/10.3389/fchem.2019.00872
This article is part of the Research Topic The Chemistry of Biofilms and Their Inhibitors View all 10 articles
Many nanotechnology-based antimicrobials and antimicrobial-delivery-systems have been developed over the past decades with the aim to provide alternatives to antibiotic treatment of infectious-biofilms across the human body. Antimicrobials can be loaded into nanocarriers to protect them against de-activation, and to reduce their toxicity and potential, harmful side-effects. Moreover, antimicrobial nanocarriers such as micelles, can be equipped with stealth and pH-responsive features that allow self-targeting and accumulation in infectious-biofilms at high concentrations. Micellar and liposomal nanocarriers differ in hydrophilicity of their outer-surface and inner-core. Micelles are self-assembled, spherical core-shell structures composed of single layers of surfactants, with hydrophilic head-groups and hydrophobic tail-groups pointing to the micellar core. Liposomes are composed of lipids, self-assembled into bilayers. The hydrophilic head of the lipids determines the surface properties of liposomes, while the hydrophobic tail, internal to the bilayer, determines the fluidity of liposomal-membranes. Therefore, whereas micelles can only be loaded with hydrophobic antimicrobials, hydrophilic antimicrobials can be encapsulated in the hydrophilic, aqueous core of liposomes and hydrophobic or amphiphilic antimicrobials can be inserted in the phospholipid bilayer. Nanotechnology-derived liposomes can be prepared with diameters <100–200 nm, required to prevent reticulo-endothelial rejection and allow penetration into infectious-biofilms. However, surface-functionalization of liposomes is considerably more difficult than of micelles, which explains while self-targeting, pH-responsive liposomes that find their way through the blood circulation toward infectious-biofilms are still challenging to prepare. Equally, development of liposomes that penetrate over the entire thickness of biofilms to provide deep killing of biofilm inhabitants still provides a challenge. The liposomal phospholipid bilayer easily fuses with bacterial cell membranes to release high antimicrobial-doses directly inside bacteria. Arguably, protection against de-activation of antibiotics in liposomal nanocarriers and their fusogenicity constitute the biggest advantage of liposomal antimicrobial carriers over antimicrobials free in solution. Many Gram-negative and Gram-positive bacterial strains, resistant to specific antibiotics, have been demonstrated to be susceptible to these antibiotics when encapsulated in liposomal nanocarriers. Recently, also progress has been made concerning large-scale production and long-term storage of liposomes. Therewith, the remaining challenges to develop self-targeting liposomes that penetrate, accumulate and kill deeply in infectious-biofilms remain worthwhile to pursue.
The threat posed to mankind of hard to treat, antibiotic-resistant infectious biofilms is better realized world-wide than ever. With cancer being considered more and more as a chronic disease, infection by antibiotic-resistant bacteria is expected to become the number one cause of death by the year 2050 (Humphreys and Fleck, 2016). This frightening scenario has many reasons. First of all, infectious biofilms are tenacious by nature and antimicrobials have difficulty penetrating the biofilm matrix embedding its bacterial inhabitants (Gupta et al., 2018). The biofilm matrix is composed of Extracellular Polymeric Substances (EPS) (Bjarnsholt et al., 2013) containing proteins, polysaccharides, humic acids, and eDNA (Flemming et al., 2016). The EPS-matrix acts as a glue holding biofilm-bacteria together and protecting them against the host immune system and environmental challenges, amongst which antimicrobials (Liu et al., 2019a). Secondly, rampant overuse of antibiotics has yielded, and still is yielding new antibiotic-resistant strains that cannot be killed by known antibiotics (Neville and Jia, 2019). Thirdly, development of new antibiotics is stalling (N'Guessan et al., 2018; Jangra et al., 2019), because their effective life-time before the first resistant strains arise, is becoming shorter and shorter, decreasing the incentive for commercialization and therewith clinical use of new antibiotics (Liu et al., 2019b).
A first challenge in the development of new infection-control strategies, is to develop an antimicrobial or antimicrobial delivery-system that allows the antimicrobial to penetrate deeply into a biofilm and kill biofilm-bacteria across the entire thickness of the biofilm (Drbohlavova et al., 2013; Liu et al., 2019a). Many nanotechnology-based drugs and drug-delivery-systems have been developed over the past decades with the aim of self-targeting, penetrating and eradicating tumors (Kong et al., 2019; Majumder et al., 2019; Paunovska et al., 2019). Biofilms and tumors are on the one hand very different, yet are both characterized by a low pH environment, allowing self-targeting of pH adaptive, smart carriers (Liu et al., 2016). Also, their clinical treatment poses the same challenges, including prevention of resistance and recurrence. Not surprisingly, new strategies for infection-control are arising nowadays, that are derived from technologies initially designed for tumor treatment. Figure 1 gives an overview of nanotechnology-derived antimicrobial delivery-systems currently considered for infection-control, many of which are derived from new tumor treatment strategies.
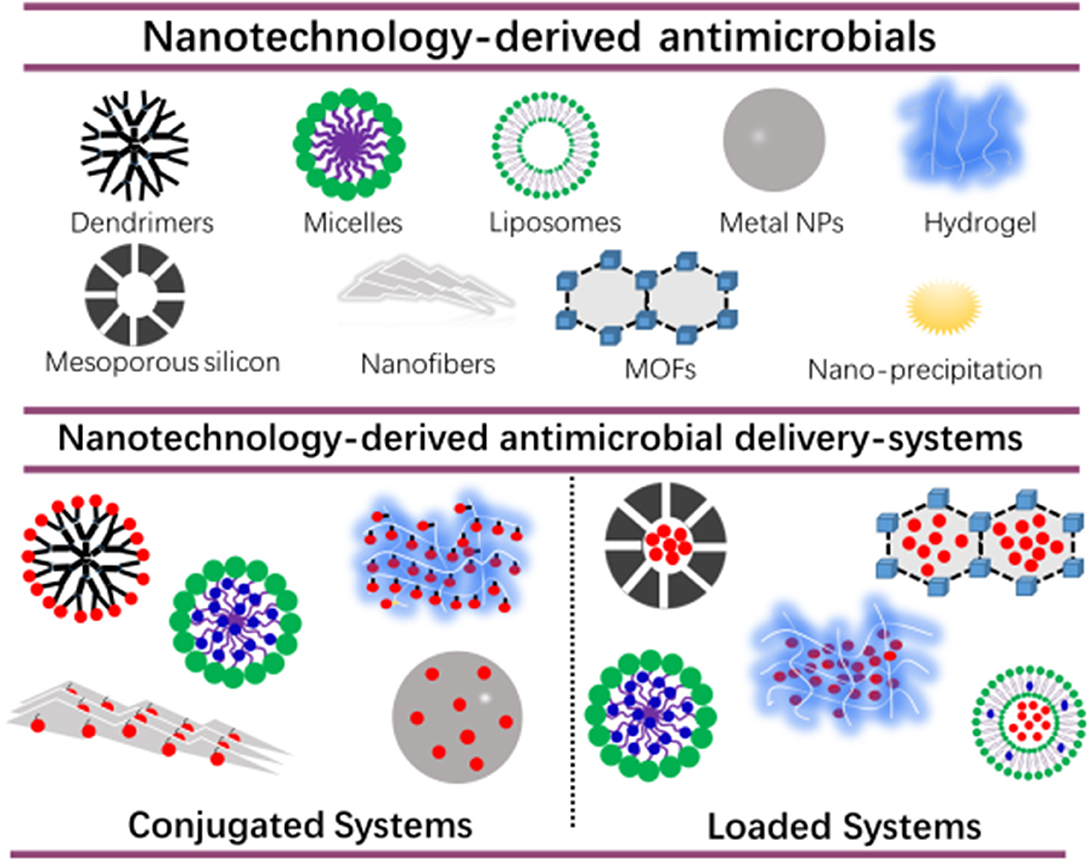
Figure 1. Nanotechnology-derived antimicrobial delivery-systems, including nanofiber-composed hydrogels. Delivery-systems are divided into systems in which antimicrobials are conjugated to a carrier or loaded into a carrier. Hydrophobic and hydrophilic antimicrobials are indicated in blue and red, respectively.
Nanotechnology-derived antimicrobial delivery systems have excellent biocompatibility, and can be designed to be environmentally-responsive and self-targeting (Lopes and Brandelli, 2018; Wolfmeier et al., 2018; Zhao et al., 2018), provided their diameter is below the limit for reticulo-endothelial rejection of around 100–200 nm (Wang et al., 2019). However, without suitable functionalization of their outermost surface or drug-loading (Figure 1), their antimicrobial efficacy is usually low. In conjugated systems, antibiotics, peptides or other antimicrobials are bound to dendrimers (Kumar et al., 2015; Xue et al., 2015), and hydrogels (Zendehdel et al., 2015) which should be done carefully in order not to sacrifice bio-active groups. To a certain extent, this restricts the application of antimicrobial-conjugated systems. Alternatively, antimicrobials can be loaded into nanotechnology-derived antimicrobial delivery-systems, to protect antimicrobials underway through the blood circulation from de-activation, reduce their toxicity and prevent potential, harmful side-effects of the antimicrobials. Moreover, antimicrobial nanocarriers can be equipped with stealth and pH-responsive features that allow self-targeting and accumulation in infectious biofilms at high concentrations. Micelles can be made for instance, consisting of a hydrophilic poly(ethylene glycol) (PEG)-shell and pH-responsive poly(β-amino ester) (PAE). This renders stealth properties to the micelles at physiological pH due to the exposure of the PEG-shell allowing their presence in the blood circulation without negative side-effects and penetration in a tumor or infectious biofilm. However, once in a more acidic, pathological site, such as in a tumor (Ray et al., 2019) or biofilm (Liu et al., 2016; Wu et al., 2019) (becoming even more acidic toward its bottom; Peeridogaheh et al., 2019), pH-responsive PAE groups become positively-charged causing self-targeting and accumulation (Liu et al., 2012, 2016). Micelles are more suitable for functionalizing of their surface without affecting their hydrophilicity ratio than liposomes, because of the relatively low molecular weight of the lipids involved in liposomes (1,200–1,800 g/mol) compared with the surfactants used in micelles (>8,000 g/mol). Inadvertent leakage remains a concern in antimicrobial-loaded systems (Kim et al., 2019).
The two most common nanocarriers considered for drug loading are micelles and lipid-based liposomes. The structure and composition of liposomes, also known as vesicles, bear similarity to the one of cell membranes. The main difference between micelles and liposomes is the hydrophilicity of their outer surface and inner core (Table 1). Micelles are self-assembled, spherical core-shell structures composed of a single layer of surfactants, with a hydrophilic head-group and a hydrophobic tail-group pointing to the micellar core. Liposomes are composed of lipids and due to their amphiphilic nature can assemble into bilayers, similar to the structure and composition of cell membranes. The hydrophilic head of the lipids determines the surface properties of liposomes, while the hydrophobic tail, internal to the bilayer, determines the fluidity of liposomal membranes. Therefore, whereas micelles can only be loaded with hydrophobic antimicrobials of which there are few candidates, hydrophilic antimicrobials can be encapsulated in the hydrophilic, aqueous core of liposomes and hydrophobic or amphiphilic antimicrobials can be inserted in the phospholipid bilayer. As a consequence, the number of candidate antimicrobials for liposome-loading, is relatively large, while the loading capacity of liposomes is relatively high (Ehsan and Clancy, 2015; Liu et al., 2019a; see also Table 1).
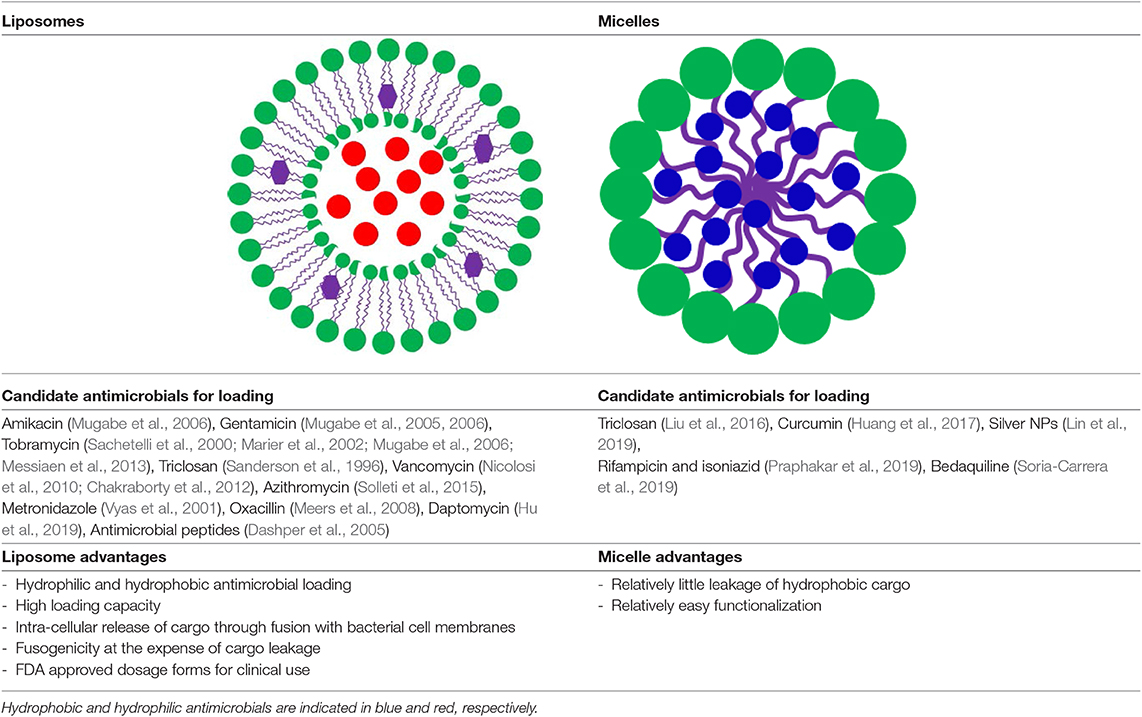
Table 1. Main differences between liposomal and micellar drug carriers, candidate antimicrobials for loading into liposomes or micelles and the relative advantages of both types of nanocarriers.
Apart from offering a wider choice of candidate antimicrobials for loading and higher loading, another advantage of lipid-based antimicrobial delivery-system is their fusogenicity, i.e., the ability of liposomes to fuse with the outer membrane of bacteria (see also Table 1), due to the fluidity of their phospholipid bilayer structure. The liposomal phospholipid bilayer resembles the structure of bacterial cell membranes, which facilitates fusion based on similarity (Figure 2). Upon fusion, high antimicrobial-doses are directly available inside a bacterium (Akbarzadeh et al., 2013).
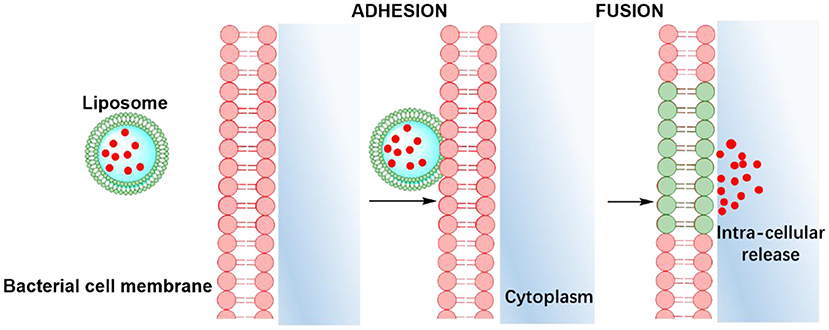
Figure 2. Similarity-mediated fusion of liposomes into bacterial cell membranes and release of antimicrobial cargo into a bacterium.
In this review, we summarize the different types of lipid-based antimicrobial delivery-systems according to their lipid bilayer composition, membrane fluidity, outer surface properties and ability to trigger the release of the encapsulated antimicrobials upon fusion. Applications and perspectives of liposomal, antimicrobial delivery-systems for the treatment of bacterial infections will be discussed.
Liposome preparation method is an important factor affecting the structure and size of liposomes. Although liposome preparation methods have been well-established, a short but comprehensive summary of the most used methods will be given to allow better understanding by a multi-disciplinary readership (Figure 3; Pick et al., 2018). In situ lipid synthesis and formation of liposomes by self-assembly into bilayered lipid structures yields liposomes of widely varying size. Liposomes can also be prepared by rehydration of dried lipid films, which spontaneously yields liposomes, with an enhanced yield when performed on conducting electrodes in the presence of an applied electric field. Liposomes size can be well-controlled by filtering, while sonication can be applied to decrease liposome size. Proteolipids can be applied in identical ways to create liposomes. Finally, large liposomes can be used to contain lipids and proteins to form proteoliposomes in situ, i.e., inside the larger liposomes.
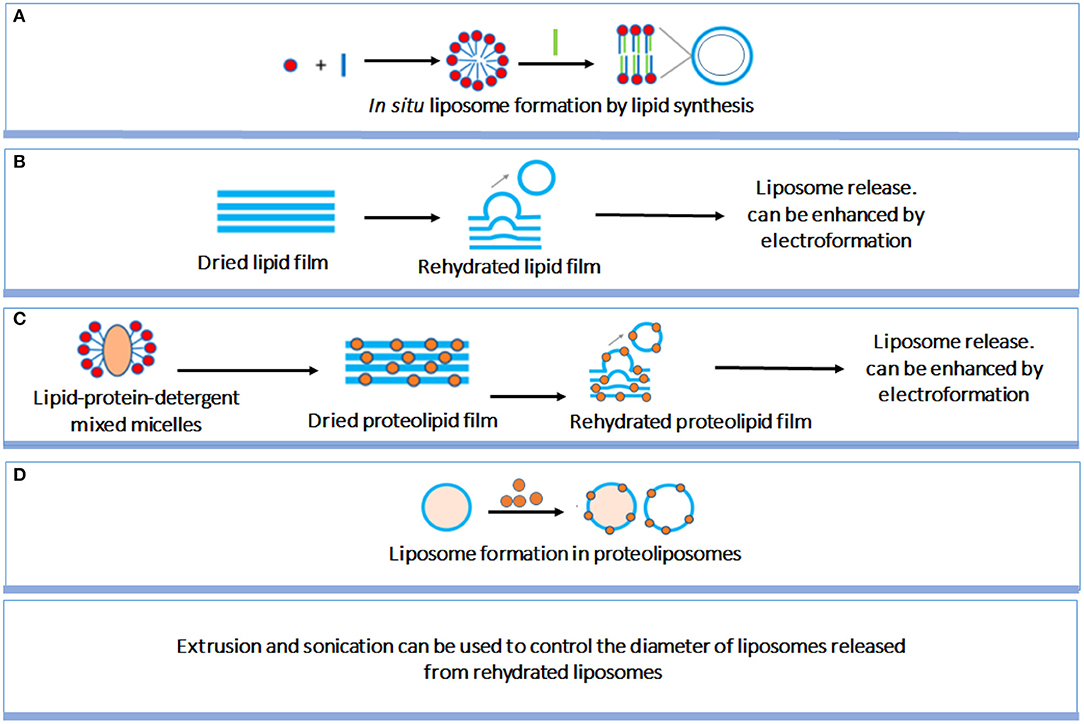
Figure 3. Summary of different liposome preparation methods. (A) in situ liposome formation by lipid synthesis; (B) Rehydration of dried lipid films yielding release of liposomes; (C) Similar as (B), now for dried proteolipid films; (D) Liposome formation in proteoliposomes (Pick et al., 2018) (with permission of American Chemical Society).
Liposomes can be classified according to different criteria. Based on diameter, small (<50 nm), large (50–500 nm) and giant (>500 nm) liposomes can be distinguished (Banerjee, 2001; Morton et al., 2012). Alternatively, a classification can be made on the basis of whether a liposome possesses uni-, oligo-, or multi-lamellar bilayers (Morton et al., 2012; Manaia et al., 2017). Liposomes can consist of naturally-occurring lipids or synthetically-made lipids (sometimes called “artificial” liposomes). Accordingly, liposomes can have widely different properties and for the purpose of infection-control (i.e., interaction with negatively-charged bacterial cell surfaces; Nederberg et al., 2011; Ng et al., 2013), it is relevant to classify them into natural lipid-based, cationic, anionic, zwitterionic liposomes, and fusogenic liposomes. Diameter and diameter distribution are the most important factors for in vivo use of liposomes (Malekar et al., 2015) and in order to prevent rejection by the reticulo-endothelial system (Wang et al., 2019) and allow penetration through water channels (Greiner et al., 2005) in infectious biofilms, liposomes for infection-control should preferentially have diameters that maximally range up to 100–200 nm (Liu et al., 2019a). Therefore, we will now confine this review to smaller liposomes with diameters of maximally 200 nm and briefly summarize the physico-chemistry underlying these liposomes.
Natural liposomes are composed of naturally-occurring phospholipids, such as phosphatidylcholine, phosphatidylserine, soybean lecithin, or egg yolk lecithin, sometimes complemented with other lipids. Natural lipids contain a polar, hydrophilic head, and several hydrophobic lipid chains. Since the hydrophilic head of natural phospholipids is electrically neutral (Smith et al., 2017), the surface potential of lipids is electrically neutral, corresponding in general with zeta potentials between −10 and +10 mV (Smith et al., 2017; Figure 4). Liposomes in suspension require zeta potentials more negative than −30 mV or more positive than +30 mV in order to experience sufficient electrostatic double-layer repulsion to create stable suspensions. Given the importance of zeta potentials for the stability of liposome suspensions and interaction with their environment, including proteins or bacteria, liposomes have been equipped with several cationic and anionic functionalities to adjust their surface charge (see also Figure 5; Kamaly et al., 2012). In addition to their stability in suspension, also the stability of the lipid bilayer in a liposome sometimes needs enforcement, such as when highly charged lipids are used (Kaszuba et al., 2010) or due to oxidation of the membrane lipids. Oxidation induced instability of liposomes can be prevented by adding reductants to the membrane lipids (Khan et al., 1990).
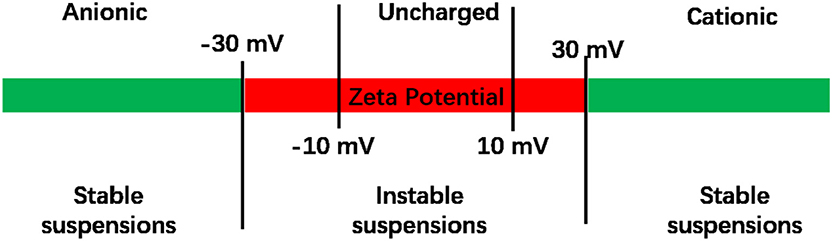
Figure 4. Zeta potentials of liposomes. Liposome suspensions are considered to be unstable when their zeta potential is between −30 and +30 mV (Manaia et al., 2017). Zeta potentials between −10 and +10 mV are considered to represent uncharged liposomes.
Cationic liposomes can be made using natural or synthetic lipids with cationic functionalities, such as ammonium (Jacobs et al., 1916; Gottenbos et al., 2001; Lu et al., 2007), sulfonium (Ghattas and Leroux, 2009), or phosphonium ions (Popa et al., 2003; Chang et al., 2010; Figure 5). As an example, Figure 6 presents the zeta potentials of cholesterol DSPC liposomes made positively-charged through DOPA, containing positively-charged ammonium groups. Within the range of DOPA concentrations applied, zeta potentials remained below the critical limit of +30 mV required for stable suspensions and accordingly these liposome suspensions were mentioned to aggregate within 24 h of processing. Interestingly, addition of 1.6 mol% lipid-PEG yielded a zeta potential of nearly zero. Yet, lipid-PEG containing liposome suspensions were described to be stable and stealth (Kataria et al., 2011), presumably due to steric stabilization and repulsion. Cationic liposomes have been suggested as a drug-releasing coating of natural surfaces, such as skin-associated bacteria (Sanderson and Jones, 1996) or teeth (Nguyen et al., 2013), both bearing a negative charge.
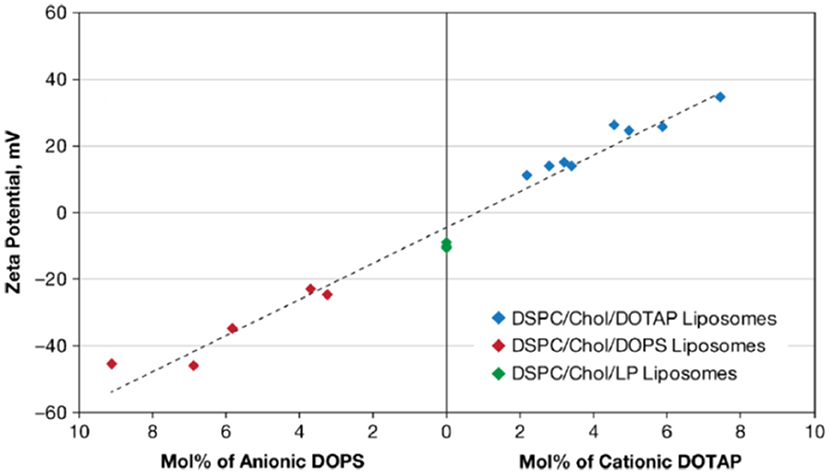
Figure 6. Zeta potentials in 0.01 mol/L NaCl (pH 7.4–7.7) of cholesterol (Chol), 1,2-distearoylsn-glycero-3-phosphocholine (DSPC) liposomes. Liposomes were made positively-charged with varying mol% of DOTAP (1,2-dioleoyl-3-trimethylammonium-propane) or negatively-charged with DOPS (1,2-dioleoyl-sn-glycero-3-phospho-L-serine). Liposomes indicated as DSPC/Chol/LP liposomes were prepared with lipid-PEG (poly-ethylene glycol) added (Smith et al., 2017) (with permission of Springer).
Instability of the liposomal bilayer structure in drug-loaded liposomes can result in inadvertent drug leakage (Drulis-Kawa and Dorotkiewicz-Jach, 2010). The stability of the lipid bilayer of cationic liposomes can be increased by coating with bacterial S-layer proteins. Zeta potentials of cationic liposomes composed of dipalmitoylphosphatidylcholine (DPPC), cholesterol and hexadecylamine [HDA: (+29.1 mV)] became negatively-charged (−27.1 mV) upon coating with S-layer proteins, which increased their stability against mechanical challenges (Figure 7; Mader et al., 1999).
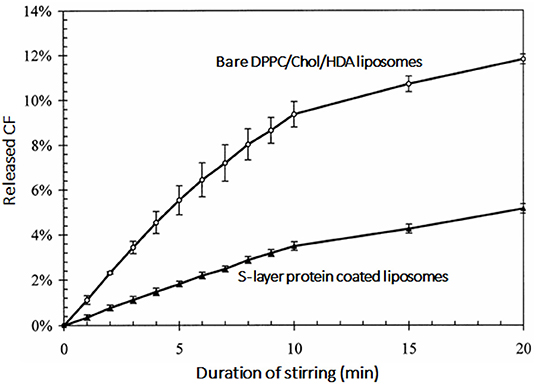
Figure 7. Release of fluorescent carboxyfluoresceine (CF) as an indication of the lipid bilayer stability of dipalmitoylphosphatidylcholine (DPPC), cholesterol and hexadecylamine (HDA) liposomes as a function of stirring time in the absence and presence of a bacterial S-layer coating on the liposomes (Mader et al., 1999) (with permission of Elsevier).
Anionic liposomes bear negatively-charged functional groups (Figure 5), such as carboxylic (Cheow et al., 2011), phosphoric or sulfonic acid (Derbali et al., 2019; Zhang and Lemay, 2019). Cholesterol-DSPC liposomes could be made positively-charged using DOTAP, but using DOPS, negative charge could be conveyed to these liposomes in a concentration dependent fashion (Figure 6; Smith et al., 2017). As a main advantage of anionic liposomes, opposite to cationic liposomes, anionic liposomes can more effectively encapsulate positively charged antimicrobials (Messiaen et al., 2013) and prolong their release time (Kaszuba et al., 1995; Robinson et al., 1998, 2000; Tang et al., 2009). Anionic liposomes composed of DPPG and DOPC could be loaded with eight-fold higher amounts of antibiotic than uncharged, natural-lipid based liposomes (Table 2; Messiaen et al., 2013).
Whereas, cationic and anionic liposomes usually demonstrate pH-dependent zeta potentials, they do not show complete charge reversal from being positively to negatively charged. Zwitterionic lipids have both acidic and alkaline functional groups (Figure 5; Hu et al., 2019; Makhathini et al., 2019) that allow full charge reversal below and above their iso-electric point (Figure 8A; Vila-Caballer et al., 2016; Liu et al., 2018). This feature allows the fabrication of liposomes that are negatively-charged under physiological pH conditions and become positively-charged under more acidic conditions, such as poly(methacryloyl sulfadimethoxine) (PSD) liposomes (Figure 8B; Couffin-Hoarau and Leroux, 2004; Ghattas and Leroux, 2009; Lu et al., 2018). Negative charge at physiological pH values aids transport of liposomes through the blood circulation without major interaction with other negatively-charged blood components (Hamal et al., 2019), while adaptation of a positive charge inside the acidic environment of a biofilm facilitates better interaction with negatively-charged bacteria (Robinson et al., 2001; Nederberg et al., 2011; Ng et al., 2013) in the biofilm.
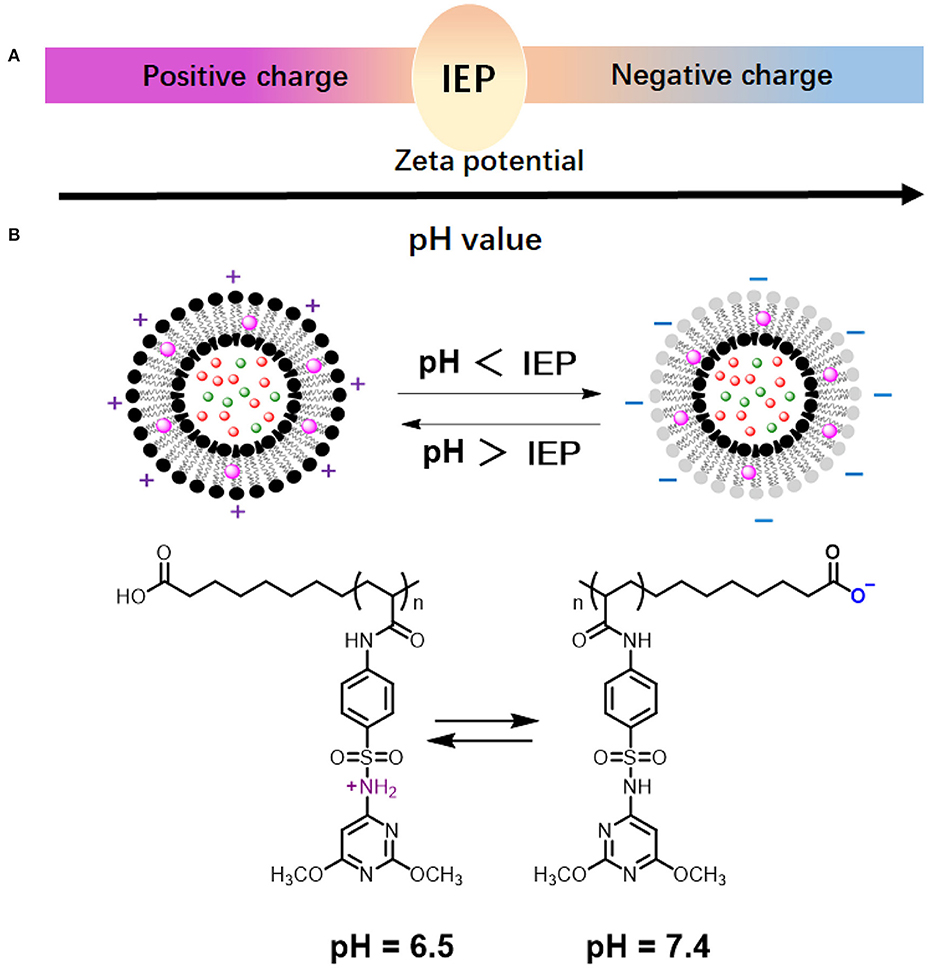
Figure 8. pH-dependent behavior of zwitterionic lipids and liposomes. (A) Zwitterionic liposomes reverse their charge from cationic to anionic when suspension pH increases from below to above the Iso-Electric Point (IEP) of the constituting lipids or vice versa. (B) Charge reversal of poly(methacryloyl sulfadimethoxine) (PSD) liposomes (Chen et al., 2018) (with permission of Elsevier).
The fusogenicity of liposomes with cellular membranes is a most distinguishing feature of liposomes and is related with the fluidity of the lipid bilayer. Generally, lower melting temperatures of the lipids imply higher fluidity of the liposome membrane and therewith a greater fusogenicity (Zora and Željka, 2016). Figure 9 summarizes the relation between melting temperatures and structure/composition of lipids. Both location of unsaturated bonds (Figure 9A; Nagahama et al., 2007) and alkyl chain length (Figure 9B) influence lipid melting temperatures (Feitosa et al., 2006) and therewith the fusogenicity of liposomes. Cholesterol hemisuccinate for instance, combined with dioleoylphosphatidylethanolamine (DOPE) and dipalmitoylphosphatidylcholine (DPPC) in a 4:2:4 molar ratio yielded highly fusogenic liposomes (Figure 9C). Increasing fusogenicity however, may go at the expense of the stability of the lipid bilayer constituting the membrane and liposomes with increased fusogenicity are more prone to bilayer membrane instability, rupture, and inadvertent cargo release (Marier et al., 2002; Li et al., 2013; Figure 9D).
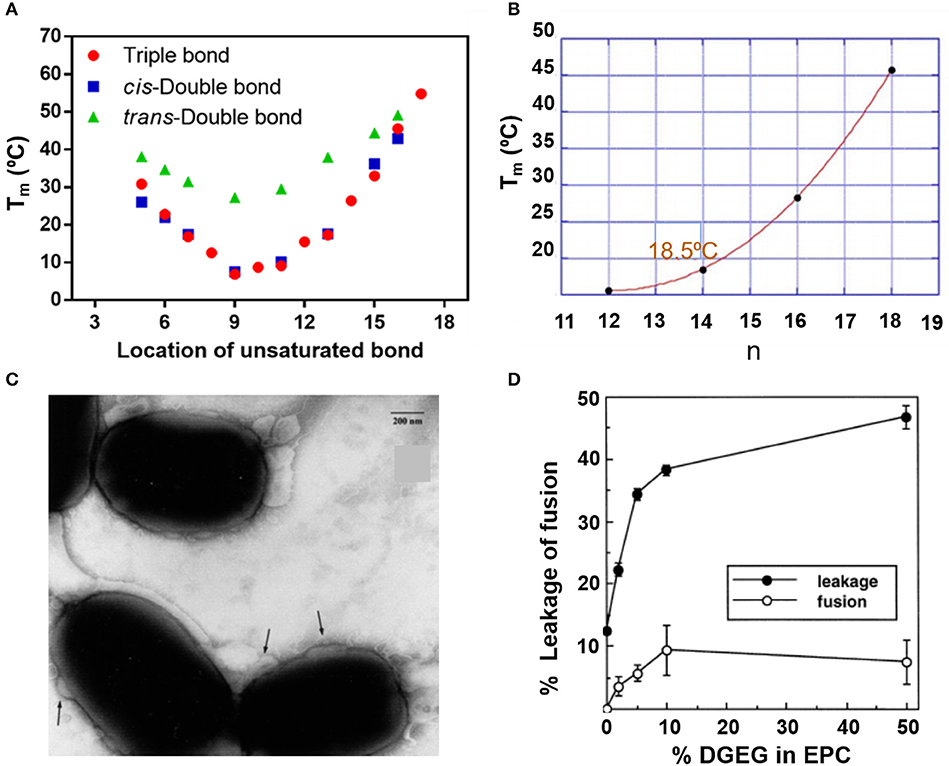
Figure 9. Fluidity of liposomes in relation with their lipid structure. Melting temperature Tm of lipids as an indication of fluidity. (A) Melting temperature as a function of unsaturated bond location in (f sn-1 saturated/sn-2 monosaturated phosphatidylcholine) (Nagahama et al., 2007). (B) Melting temperature of 5.0 mM dialkyldimethylammonium bromide in water as a function of the number (n) of carbon atoms in the alkyl chains (Feitosa et al., 2006) (with permission of Elsevier). (C) Transmission electron micrographs of the fusion (indicated by the arrows) of fusogenic, DOPE-DPPC-cholesterol hemisuccinate liposomes with E. coli. Bar marker equals 200 nm (Nicolosi et al., 2010) (with permission of Elsevier). (D) The % fused lipsosomes and % release of fluorescent carboxyfluorescein as a function of the % digalactosyldiacylglycerol (DGDG) in egg phosphatidylcholine (EPC) liposomes (Hincha et al., 1998) (with permission of Elsevier).
The problems to be overcome for the successful treatment of infectious biofilms in the human body are many-fold and some of them have persisted for centuries. Rather than aiming for a comprehensive overview of all studies attempting to apply liposomal antimicrobial-loaded nanocarriers for infection-control, we first present a brief overview of the problems encountered in the treatment of infectious biofilms using antimicrobials. Next, it will be addressed which problems can probably be successfully addressed using liposomal antimicrobial-loaded nanocarriers, and the steps that need to be taken for successful downward clinical translation.
Eradication of infectious biofilms is a highly complicated process for which there is no adequate treatment available ever since Van Leeuwenhoek noticed that the vinegar which he used to clean his teeth from oral biofilm killed only bacteria residing at the outside of the biofilm, but left the ones in the depth of a biofilm alive (Van Leewenhoek, 1684). One of the current struggles indeed, still is the penetration, accumulation and killing of antimicrobials over the entire thickness of an infectious biofilm, as noticed by Van Leeuwenhoek over three centuries ago (Figure 10). This includes prevention of wash-out of an antimicrobial in the dynamic environment of the human body. In addition, antimicrobials may be enzymatically de-activated underway to a biofilm in the blood circulation or once inside a biofilm (Albayaty et al., 2018). Taken together, these factors make bacterial killing into the depth of a biofilm impossible (Sutherland, 2001), contributing to recurrence of infection after treatment (Wolfmeier et al., 2018).
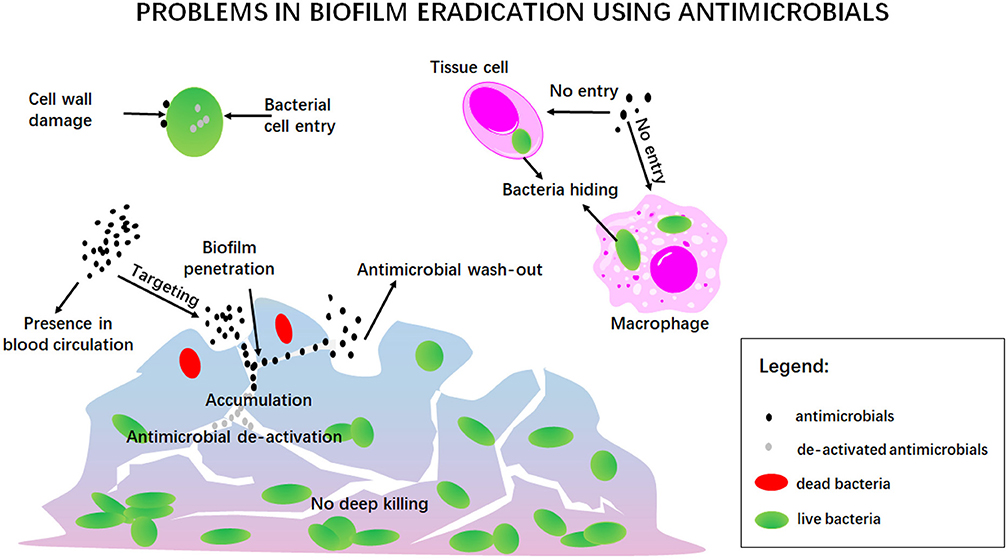
Figure 10. A summary of the traditional problems involved in antimicrobial treatment of infectious biofilms.
Penetration and accumulation can only occur once the antimicrobial has “found its way,” often from within the blood circulation, to the infectious biofilm. Since it may be undesirable to have high concentrations of an antimicrobial circulating through the body due to potential collateral tissue damage, self-targeting carriers are under design that can find their way at low blood concentrations to accumulate in sufficiently high amounts in an infectious biofilm (Forier et al., 2014). Once accumulated inside a biofilm, the antimicrobial should perform its antimicrobial action, which can either be based on generating cell wall damage, or entry into a bacterium to interfere with vital metabolic processes. Both can be difficult, especially since bacteria have developed a large array of protective mechanisms, that we summarize under the common denominator of antimicrobial resistance (Kumar et al., 2016). Adding to this, is the problem of bacteria seeking shelter against antimicrobials in mammalian cells (Mantovani et al., 2011), in which many antimicrobials cannot enter. Bacteria have even been found sheltering in macrophages intended by nature to kill them, de-activating macrophageal killing mechanisms (Knodler et al., 2001).
There are no antimicrobials or antimicrobial carriers that solve all the issues summarized above (see also Figure 10). Liposomal nanocarriers constitute no exception to this. Yet, liposomes possess a number of unique qualities, like stealth properties, protection of encapsulated antimicrobials against de-activation and entry in tissue cells and bacteria, as will be summarized below.
Blood circulation times of liposomes have become much longer since the inclusion of lipid-PEG in the bilayer membrane. Liposomes without lipid-PEG were rapidly removed from the circulation by macrophageal uptake (Hofmann et al., 2010) but stealth (Romberg et al., 2008) liposomes containing lipid-PEG demonstrated reduced reticulo-endothelial uptake.
Generally, cationic liposomes demonstrate better interaction with negatively charged bacterial cell surfaces (Robinson et al., 2001; Nederberg et al., 2011; Ng et al., 2013). However, pH-responsive liposomes that self-target from the blood circulation toward bacteria in an infectious biofilm have not been extensively explored. Zwitterionic liposomes prepared from pH-responsive quaternary ammonium chitosan with charge reversal from −9.08 mV at pH 7.4 to +8.92 mV at pH 4.5 have been described for the treatment of periodontal infection (Zhou et al., 2018; Hu et al., 2019). However, according to Figure 4 this change does not qualify as a charge reversal as these liposomes would have to be classified as uncharged at both pH values. Moreover, periodontal application does not imply self-targeting from the blood circulation, as required for the treatment of many other, internal infections. Interestingly, these zwitterionic liposomes were highly biocompatible and disruptive to periodontal biofilm.
Many Gram-negative and Gram-positive bacterial strains, resistant to a specific antibiotic free in solution, have been demonstrated to be susceptible to these antibiotics when encapsulated in a liposomal nanocarrier (Table 3). This may arguably be considered as the biggest advantage of liposomes over other nanocarriers. Although some have suggested that this must be attributed to the protection offered by liposomal encapsulation against enzymatic de-activation (Nacucchio et al., 1985), fusogenicity (Mugabe et al., 2006; Halwani et al., 2007) of liposomes can also significantly improve the antibacterial activity of antibiotics (Beaulac et al., 1996; Sachetelli et al., 1999; Li et al., 2013). Liposomes with enhanced fusogenicity possessing cholesterol hemisuccinate (Nicolosi et al., 2010) loaded with vancomycin for instance, had much lower minimal inhibitory concentrations (MIC) than vancomycin free in solution against a variety of Gram-negative bacterial strains, that would be considered vancomycin-resistant based on their MIC (see also Table 3). Also fusogenic liposomes composed of dipalmitoylphosphatidylcholine (DPPC) and dimiristoylphosphatidylglycerol (DMPG) in a ratio of 18:1 (w/w) loaded with tobramycin eradicated a mucoid chronic, pulmonary Pseudomonas aeruginosa infection, whereas tobramycin free in solution was not effective (Beaulac et al., 1996, 1998).
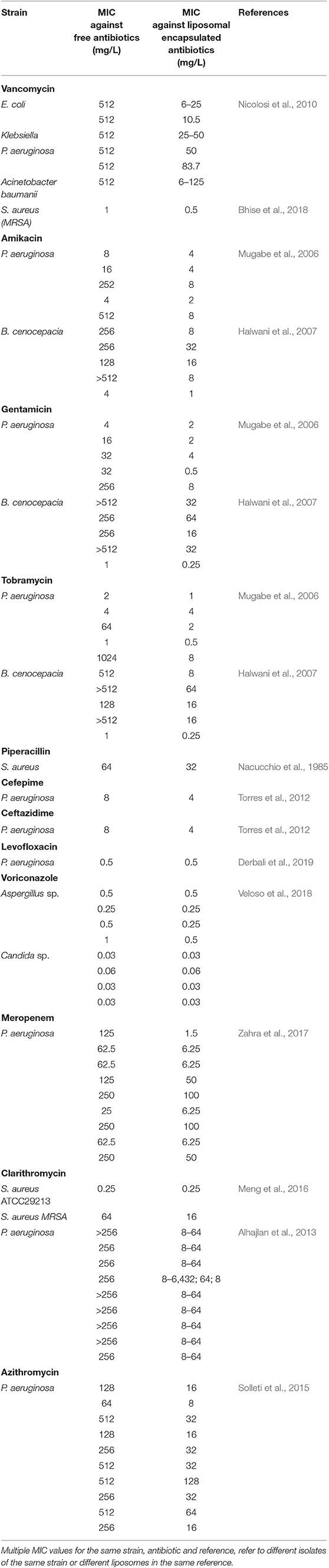
Table 3. Minimal inhibitory concentrations of different bacterial strains against antibiotic-loaded liposomes.
Protection of antibiotics against enzymatic de-activation and fusogenicity to enhance antibiotic efficacy, constitute unique advantages of liposomal antimicrobial nanocarriers that justify further research. Challenges in the ongoing development of liposomal antimicrobial nanocarriers include the realization of biofilm targeting from the blood circulation, penetration, and accumulation over the entire thickness of an infectious biofilm, associated with deep killing in the biofilm. Deep killing is necessary in order to prevent recurrence of infection, one of the troublesome features of clinical infection treatment. In this respect, it is also worthwhile to investigate whether liposomal antimicrobial nanocarriers can be designed that aid in the killing of bacteria seeking shelter in mammalian cells, impenetrable to many antimicrobials.
Downward clinical translation of liposomal drug nanocarriers has long been hampered for difficulties in large-scale production and storage. However, ethanol injection, membrane dispersion, and Shirasu porous glass membranes have enabled large-scale production of liposomes (Laouini et al., 2012). Equally, liposome storage problems are on their way to be solved. For commercial liposome products, storage in the fluid form is preferred since lyophilization and subsequent rehydration may lead to size changes and cargo leakage (Stark et al., 2010). Addition of stabilizers such as 2-morpholinoethansulfonic acid yielded low phospholipid degradation in liposomes after 12 months storage at 2–8°C (Doi et al., 2019).
Owing to these developments, liposomes are nowadays an FDA approved form of drug delivery and liposome encapsulated tobramycin, marketed under the name Fluidosomes™ is clinically applied for the treatment of chronic pulmonary infections in cystic fibrosis patients. A phase II clinical study is ongoing in Europe (Zora and Željka, 2016).
In conclusion, the challenges to further develop liposomes as a novel infection-control strategy supplementing antibiotic treatment are highly worthwhile to pursue.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
This work was financially supported by the National Natural Science Foundation of China (21620102005, 51933006, 51773099).
HB is also director of a consulting company, SASA BV.
The remaining authors declare no conflicts of interest with respect to authorship and/or publication of this article. Opinions and assertions contained herein are those of the authors and are not construed as necessarily representing views of their respective employers.
CF, carboxyfluoresceine; Chol, cholesterol; DGDG, digalactosyldiacylglycerol; DMPC, dimyristoyl phosphatidylcholine; DMPG, dimyristoyl phosphatidylglycerol; DOPA, 3,4-dihydroxyphenylalanine; DOPC, 1, 2-dioleoyl-sn-glycero-3-phosphocholine; DOPE, 1,2-dioleoyl-3-trimethylammonium-propane; DOPS, 1,2-dioleoyl-sn-glycero-3-phospho-L-serine; DOTAP, 1,2-dioleoyl-3-trimethylammonium-propane; DPPC, dipalmitoyl phosphatidylcholine; DPPG, dipalmitoylphosphatidylglycerol; DSPC, 1,2-distearoylsn-glycero-3-phosphocholine; DSPE, distearoyl phosphoethanolamine; EPC, egg phosphatidylcholine; EPS, extracellular polymeric substances; FDA, food and drug administration; HAD, hexadecylamine; IEP, iso-electric point; LP, lipid-PEG; MOFs, metal organic framework; MIC, minimal inhibitory concentration; MRSA, methicillin-resistant Staphylococcus aureus; NPs, nanoparticles; PAE, poly(β-amino ester); PEG, polyethylene glycol; PSD, poly(methacryloyl sulfadimethoxine).
Akbarzadeh, A., Rezaei-Sadabady, R., Davaran, S., Joo, S. W., Zarghami, N., Hanifehpour, Y., et al. (2013). Liposome: classification, preparation, and applications. Nanoscale Res. Lett. 8:102. doi: 10.1186/1556-276X-8-102
Albayaty, Y. N., Thomas, N., Hasan, S., and Prestidge, C. A. (2018). Penetration of topically used antimicrobials through Staphylococcus aureus biofilms: a comparative study using different models. J. Drug Del. Sci. Technol. 48, 429–436. doi: 10.1016/j.jddst.2018.10.015
Alhajlan, M., Alhariri, M., and Omri, A. (2013). Efficacy and safety of liposomal clarithromycin and its effect on Pseudomonas aeruginosa virulence factors. Antimicrob. Agents Chemother. 57, 2694–2704. doi: 10.1128/AAC.00235-13
Banerjee, R. (2001). Liposomes: applications in medicine. J. Biomater. Appl. 16, 3–21. doi: 10.1106/RA7U-1V9C-RV7C-8QXL
Beaulac, C., Clément-Major, S., Hawari, J., and Lagacé, J. (1996). Eradication of mucoid Pseudomonas aeruginosa with fluid liposome-encapsulated tobramycin in an animal model of chronic pulmonary infection. Antimicrob. Agents Chemother. 40, 665–669. doi: 10.1128/AAC.40.3.665
Beaulac, C., Sachetelli, S., and Lagace, J. (1998). In-vitro bactericidal efficacy of sub-MIC concentrations of liposome-encapsulated antibiotic against gram-negative and gram-positive bacteria. J. Antimicrob. Chemother. 41:35. doi: 10.1093/jac/41.1.35
Bhise, K., Sau, S., Kebriaei, R., Rice, S. A., Stamper, K. C., Alsaab, H. O., et al. (2018). Combination of vancomycin and cefazolin lipid nanoparticles for overcoming antibiotic resistance of MRSA. Materials 11:1245. doi: 10.3390/ma11071245
Bjarnsholt, T., Alhede, M., Alhede, M., Eickhardt-Soerensen, S. R., Moser, C., Kuhl, M., et al. (2013). The in vivo biofilm. Trends Microbiol. 21, 466–474. doi: 10.1016/j.tim.2013.06.002
Chakraborty, S. P., Sahu, S. K., Pramanik, P., and Roy, S. (2012). In vitro antimicrobial activity of nanoconjugated vancomycin against drug resistant Staphylococcus aureus. Int. J. Pharmaceut. 436, 659–676. doi: 10.1016/j.ijpharm.2012.07.033
Chang, H.-I., Yang, M.-S., and Liang, M. (2010). The synthesis, characterization and antibacterial activity of quaternized poly(2,6-dimethyl-1,4-phenylene oxide)s modified with ammonium and phosphonium salts. React. Funct. Polym. 70, 944–950. doi: 10.1016/j.reactfunctpolym.2010.09.005
Chen, M.-M., Song, F.-F., Feng, M., Liu, Y., Liu, Y.-Y., Tian, J., et al. (2018). pH-sensitive charge-conversional and NIR responsive bubble-generating liposomal system for synergetic thermo-chemotherapy. Coll. Surf. B Biointer. 167, 104–114. doi: 10.1016/j.colsurfb.2018.04.001
Cheow, W. S., Chang, M. W., and Hadinoto, K. (2011). The roles of lipid in anti-biofilm efficacy of lipid–polymer hybrid nanoparticles encapsulating antibiotics. Coll. Surf. A Physicochem. Eng. Aspects 389, 158–165. doi: 10.1016/j.colsurfa.2011.08.035
Couffin-Hoarau, A.-C., and Leroux, J.-C. (2004). Report on the use of poly(organophosphazenes) for the design of stimuli-responsive vesicles. Biomacromolecules 5, 2082–2087. doi: 10.1021/bm0400527
Dashper, S. G., O'Brien-Simpson, N. M., Cross, K. J., Paolini, R. A., Hoffmann, B., Catmull, D. V., et al. (2005). Divalent metal cations increase the activity of the antimicrobial peptide kappacin. Antimicrob. Agents Chemother. 49, 2322–2328. doi: 10.1128/AAC.49.6.2322-2328.2005
Derbali, R. M., Aoun, V., Moussa, G., Frei, G., Tehrani, S. F., Del'Orto, J. C., et al. (2019). Tailored nanocarriers for the pulmonary delivery of levofloxacin against Pseudomonas aeruginosa: a comparative study. Mol. Pharmaceut. 16, 1906–1916. doi: 10.1021/acs.molpharmaceut.8b01256
Doi, Y., Shimizu, T., Ishima, Y., and Ishida, T. (2019). Long-term storage of PEGylated liposomal oxaliplatin with improved stability and long circulation times in vivo. Int. J. Pharm. 564, 237–243. doi: 10.1016/j.ijpharm.2019.04.042
Drbohlavova, J., Chomoucka, J., Adam, V., Ryvolova, M., Eckschlager, T., Hubalek, J., et al. (2013). Nanocarriers for anticancer drugs–new trends in nanomedicine. Curr. Drug Metab. 14, 547–564. doi: 10.2174/1389200211314050005
Drulis-Kawa, A., and Dorotkiewicz-Jach, A. (2010). Liposomes as delivery systems for antibiotics. Int. J. Pharm. 387, 187–198. doi: 10.1016/j.ijpharm.2009.11.033
Ehsan, Z. E., and Clancy, J. P. (2015). Management of Pseudomonas aeruginosa infection in cystic fibrosis patients using inhaled antibiotics with a focus on nebulized liposomal amikacin. Fut. Microbiol. 10, 1901–1912. doi: 10.2217/fmb.15.117
Feitosa, E., Jansson, J., and Lindman, B. (2006). The effect of chain length on the melting temperature and size of dialkyldimethylammonium bromide vesicles. Chem. Phys. Lipids 142, 128–132. doi: 10.1016/j.chemphyslip.2006.02.001
Flemming, H. C., Wingender, J., Szewzyk, U., Steinberg, P., Rice, S. A., and Kjelleberg, S. (2016). Biofilms: an emergent form of bacterial life. Nat. Rev. Microbiol. 14, 563–575. doi: 10.1038/nrmicro.2016.94
Forier, K., Raemdonck, K., De Smedt, S. C., Demeester, J., Coenye, T., and Braeckmans, K. (2014). Lipid and polymer nanoparticles for drug delivery to bacterial biofilms. J. Control. Rel. 190, 607–623. doi: 10.1016/j.jconrel.2014.03.055
Ghattas, D., and Leroux, J.-C. (2009). Amphiphilic Ionizable Polyphosphazenes for The Preparation of pH-Responsive Liposomes. Hoboken, NJ: John Wiley & Sons, Inc. 227–247.
Gottenbos, B., Grijpma, D. W., Van der Mei, H. C., Feijen, J., and Busscher, H. J. (2001). Antimicrobial effects of positively charged surfaces on adhering gram-positive and gram-negative bacteria. J. Antimicrob. Chemother. 48, 7–13. doi: 10.1093/jac/48.1.7
Greiner, L. L., Edwards, J. L., Shao, J., Rabinak, C., Entz, D., and Apicella, M. A. (2005). Biofilm formation by Neisseria gonorrhoeae. Infect. Immun. 73, 1964–1970. doi: 10.1128/IAI.73.4.1964-1970.2005
Gupta, T. T., Karki, S. B., Fournier, R., and Ayan, H. (2018). Mathematical modelling of the effects of plasma treatment on the diffusivity of biofilm. Appl. Sci. 8:1729. doi: 10.3390/app8101729
Halwani, M., Mugabe, C., Azghani, A. O., Lafrenie, R. M., Kumar, A., and Omri, A. (2007). Bactericidal efficacy of liposomal aminoglycosides against Burkholderia cenocepacia. J. Antimicrob. Chemother. 60, 760–769. doi: 10.1093/jac/dkm289
Hamal, P., Nguyenhuu, H., Subasinghege Don, V., Kumal, R. R., Kumar, R., McCarley, R. L., et al. (2019). Molecular adsorption and transport at liposome surfaces studied by molecular dynamics simulations and second harmonic generation spectroscopy. J. Phys. Chem. B 123, 7722–7730. doi: 10.1021/acs.jpcb.9b05954
Hincha, D. K., Oliver, A. E., and Crowe, J. H. (1998). The effects of chloroplast lipids on the stability of liposomes during freezing and dryin. Biochim. Biophys. Acta Biomembr. 1368, 150–156. doi: 10.1016/S0005-2736(97)00204-6
Hofmann, A. M., Wurm, F., Hühn, E., Nawroth, T., Langguth, P., and Frey, H. (2010). Hyperbranched polyglycerol-based lipids via oxyanionic polymerization: toward multifunctional stealth liposomes. Biomacromolecules 11, 568–574. doi: 10.1021/bm901123j
Hu, F., Zhou, Z., Xu, Q., Fan, C., Wang, L., Ren, H., et al. (2019). A novel pH-responsive quaternary ammonium chitosan-liposome nanoparticles for periodontal treatment. Int. J. Biol. Macromol. 129, 1113–1119. doi: 10.1016/j.ijbiomac.2018.09.057
Huang, F., Gao, Y., Zhang, Y., Cheng, T., Ou, H., Yang, L., et al. (2017). Silver-decorated polymeric micelles combined with curcumin for enhanced antibacterial activity. ACS Appl. Mater. Interfaces 9, 16880–16889. doi: 10.1021/acsami.7b03347
Humphreys, G., and Fleck, F. (2016). United nations meeting on antimicrobial resistance. Bull. World Health Organ. 94, 638–639. doi: 10.2471/BLT.16.020916
Jacobs, W. A., Heidelberger, M., and Bull, C. G. (1916). Bactericidal properties of the quaternary salts of hexamethylenetetramine. III. Relation between constitution and bactericidal action in the quaternary salts obtained from haloacetyl compounds. J. Exp. Med. 23, 577–601. doi: 10.1084/jem.23.5.577
Jangra, M., Kaur, M., Tambat, R., Rana, R., Maurya, S. K., Khatri, N., et al. (2019). Tridecaptin M, a new variant discovered in mud bacterium, shows activity against colistin- and extremely drug-resistant enterobacteriaceae. Antimicrob. Agents Chemother. 63, e00338–e00319. doi: 10.1128/AAC.00338-19
Kamaly, N., Xiao, Z., Valencia, P. M., Radovic-Moreno, A. F., and Farokhzad, O. C. (2012). Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem. Soc. Rev. 41, 2971–3010. doi: 10.1039/c2cs15344k
Kaszuba, M., Corbett, J., Watson, F. M., and Jones, A. (2010). High-concentration zeta potential measurements using light-scattering techniques. Philos. Trans. R. Soc. A 368, 4439–4451. doi: 10.1098/rsta.2010.0175
Kaszuba, M., Lyle, I. G., and Jones, M. N. (1995). The targeting of lectin-bearing liposomes to skin-associated bacteria. Colloids Surf. B 4, 151–158. doi: 10.1016/0927-7765(95)01168-I
Kataria, S., Sandhu, P., Bilandi, A., Middha, A., and Kapoor, B. (2011). Stealth liposomes: a review. Int. J. Res. Ayurveda Pharm. 2, 1534–1538.
Khan, M. R., Gray, A. I., Waterman, P. G., and Sadler, I. H. (1990). Clerodane diterpenes from Casearia corymbosa stem bark. Phytochemistry 29, 3591–3595. doi: 10.1016/0031-9422(90)85282-K
Kim, H. Y., Kang, M., Choo, Y. W., Go, S.-H., Kwon, S. P., Song, S. Y., et al. (2019). Immunomodulatory lipocomplex functionalized with photosensitizer-embedded cancer cell membrane inhibits tumor growth and metastasis. Nano Lett. 19, 5185–5193. doi: 10.1021/acs.nanolett.9b01571
Knodler, L. A., Celli, J., and Finlay, B. B. (2001). Pathogenic trickery: deception of host cell processes. Nat. Rev. Mol. Cell Biol. 2, 578–588. doi: 10.1038/35085062
Kong, X., Liu, Y., Huang, X., Huang, S., Gao, F., Rong, P., et al. (2019). Cancer therapy based on smart drug delivery with advanced nanoparticles. Anti-Cancer Agents Med. Chem. 19, 720–730. doi: 10.2174/1871520619666190212124944
Kumar, P., Shenoi, R. A., Lai, B. F. L., Nguyen, M., Kizhakkedathu, J. N., and Straus, S. K. (2015). Conjugation of aurein 2.2 to HPG yields an antimicrobial with better properties. Biomacromolecules 16, 913–923. doi: 10.1021/bm5018244
Kumar, S., He, G., Kakarla, P., Shrestha, U., Ranjana, K. C., Ranaweera, I., et al. (2016). Bacterial multidrug efflux pumps of the major facilitator superfamily as targets for modulation. Infect. Disord. Drug Targets 16, 28–43. doi: 10.2174/1871526516666160407113848
Laouini, A., Jaafar-Maalej, C., Gandoura-Sfar, S., Charcosset, C., and Fessi, H. (2012). Spironolactone-loaded liposomes produced using a membrane contactor method: an improvement of the ethanol injection technique. Prog. Colloid Polym. Sci. 139, 23–28. doi: 10.1007/978-3-642-28974-3_5
Li, C., Zhang, X., Huang, X., Wang, X., Liao, G., and Chen, Z. (2013). Preparation and characterization of flexible nanoliposomes loaded with daptomycin, a novel antibiotic, for topical skin therapy. Int. J. Nanomed. 8, 1285–1292. doi: 10.2147/IJN.S41695
Lin, W., Huang, K., Li, Y., Qin, Y., Xiong, D., Ling, J., et al. (2019). Facile in situ preparation and in vitro antibacterial activity of PDMAEMA-based silver-bearing copolymer micelles. Nanoscale Res. Lett. 14:256. doi: 10.1186/s11671-019-3074-z
Liu, K., Li, H., Williams, G. R., Wu, J., and Zhu, L.-M. (2018). pH-responsive liposomes self-assembled from electrosprayed microparticles, and their drug release properties. Colloids Surf. A 537, 20–27. doi: 10.1016/j.colsurfa.2017.09.046
Liu, X., Ma, R., Shen, J., Xu, Y., An, Y., and Shi, L. (2012). Controlled release of ionic drugs from complex micelles with charged channels. Biomacromolecules 13, 1307–1314. doi: 10.1021/bm2018382
Liu, Y., Busscher, H. J., Zhao, B., Li, Y., Zhang, Z., Van der Mei, H. C., et al. (2016). Surface-adaptive, antimicrobially loaded, micellar nanocarriers with enhanced penetration and killing efficiency in Staphylococcal biofilms. ACS Nano 10, 4779–4789. doi: 10.1021/acsnano.6b01370
Liu, Y., Shi, L., Su, L., Van der Mei, H. C., Jutte, P. C., Ren, Y., et al. (2019a). Nanotechnology-based antimicrobials and delivery systems for biofilm-infection control. Chem. Soc. Rev. 48, 428–446. doi: 10.1039/C7CS00807D
Liu, Y., Shi, L., Van der Mei, H. C., Ren, Y., and Busscher, H. J. (2019b). “Perspectives on and need to develop new infection control methods,” in Racing for the Surface: Advances in Antimicrobial and Osteoinductive Studies, eds B. Li, T. F. Moriarty, T. Webster, and M. Xing (Cham: Springer In press).
Lopes, N. A., and Brandelli, A. (2018). Nanostructures for delivery of natural antimicrobials in food. Crit. Rev. Food Sci. Nutr. 58, 2202–2212. doi: 10.1080/10408398.2017.1308915
Lu, G., Wu, D., and Fu, R. (2007). Studies on the synthesis and antibacterial activities of polymeric quaternary ammonium salts from dimethylaminoethyl methacrylate. React. Funct. Polym. 67, 355–366. doi: 10.1016/j.reactfunctpolym.2007.01.008
Lu, M.-M., Ge, Y., Qiu, J., Shao, D., Zhang, Y., Bai, J., et al. (2018). Redox/pH dual-controlled release of chlorhexidine and silver ions from biodegradable mesoporous silica nanoparticles against oral biofilms. Int. J. Nanomed. 13, 7697–7709. doi: 10.2147/IJN.S181168
Mader, C., Küpcü, S., Sára, M., and Sleytr, U. B. (1999). Stabilizing effect of an S-layer on liposomes towards thermal or mechanical stress. BBA-Biomembranes 1418, 106–116. doi: 10.1016/S0005-2736(99)00030-9
Majumder, J., Taratula, O., and Minko, T. (2019). Nanocarrier-based systems for targeted and site specific therapeutic delivery. Adv. Drug Delivery Rev. 144, 57–77. doi: 10.1016/j.addr.2019.07.010
Makhathini, S. S., Kalhapure, R. S., Jadhav, M., Waddad, A. Y., Gannimani, R., Omolo, C. A., et al. (2019). Novel two-chain fatty acid-based lipids for development of vancomycin pH-responsive liposomes against Staphylococcus aureus and methicillin-resistant Staphylococcus aureus (MRSA). J. Drug Target 27, 1094–1107. doi: 10.1080/1061186X.2019.1599380
Malekar, S. A., Sarode, A. L., Bach, A. C., Bose, A., Bothun, G., and Worthen, D. R. (2015). Radio frequency-activated nanoliposomes for controlled combination drug delivery. AAPS Pharmscitech. 16, 1335–1343. doi: 10.1208/s12249-015-0323-z
Manaia, E. B., Abuçafy, M. P., Chiari-Andréo, B. G., Silva, B. L., Oshiro-Júnior, J. A., and Chiavacci, L. (2017). Physicochemical characterization of drug nanocarriers. Int. J. Nanomed. 12, 4991–5011. doi: 10.2147/IJN.S133832
Mantovani, A., Cassatella, M. A., Costantini, C., and Jaillon, S. (2011). Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 11, 519–531. doi: 10.1038/nri3024
Marier, J. F., Lavigne, J., and Ducharme, M. P. (2002). Pharmacokinetics and efficacies of liposomal and conventional formulations of tobramycin after intratracheal administration in rats with pulmonary Burkholderia cepacia infection. Antimicrob. Agents Chemother. 46, 3776–3781. doi: 10.1128/AAC.46.12.3776-3781.2002
Meers, P., Neville, M., Malinin, V., Scotto, A. W., Sardaryan, G., Kurumunda, R., et al. (2008). Biofilm penetration, triggered release and in vivo activity of inhaled liposomal amikacin in chronic Pseudomonas aeruginosa lung infections. J. Antimicrob. Chemother. 61, 859–868. doi: 10.1093/jac/dkn059
Meng, Y., Hou, X., Lei, J., Chen, M., Cong, S., Zhang, Y., et al. (2016). Multi-functional liposomes enhancing target and antibacterial immunity for antimicrobial and anti-biofilm against methicillin-resistant Staphylococcus aureus. Pharm. Res. 33, 763–775. doi: 10.1007/s11095-015-1825-9
Messiaen, A. S., Forier, K., Nelis, H., Braeckmans, K., and Coenye, T. (2013). Transport of nanoparticles and tobramycin-loaded liposomes in Burkholderia cepacia complex biofilms. PLoS ONE 8:e79220. doi: 10.1371/journal.pone.0079220
Morton, L. A., Saludes, J. P., and Yin, H. (2012). Constant pressure-controlled extrusion method for the preparation of nano-sized lipid vesicles. J. Vis. Exp. 64:e4151. doi: 10.3791/4151
Mugabe, C., Azghani, A. O., and Omri, A. (2005). Liposome-mediated gentamicin delivery: development and activity against resistant strains of Pseudomonas aeruginosa isolated from cystic fibrosis patients. J. Antimicrob. Chemother. 55, 269–271. doi: 10.1093/jac/dkh518
Mugabe, C., Halwani, M., Azghani, A. O., Lafrenie, R. M., and Abdelwahab, O. (2006). Mechanism of enhanced activity of liposome-entrapped aminoglycosides against resistant strains of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 50, 2016–2022. doi: 10.1128/AAC.01547-05
Nacucchio, M. C., Gatto Bellora, M. J., Sordelli, D. O., and D'Aquino, M. (1985). Enhanced liposome-mediated activity of piperacillin against staphylococci. Antimicrob. Agents Chemother. 27, 137–139. doi: 10.1128/AAC.27.1.137
Nagahama, M., Otsuka, A., Oda, M., Singh, R. K., Ziora, Z. M., Imagawa, H., et al. (2007). Effect of unsaturated bonds in the sn-2 acyl chain of phosphatidylcholine on the membrane-damaging action of Clostridium perfringens alpha-toxin toward liposomes. Biochim. Biophys. Acta Biomembr. 1768, 2940–2945. doi: 10.1016/j.bbamem.2007.08.016
Nederberg, F., Zhang, Y., Tan, J. P., Xu, K., Wang, H., Yang, C., et al. (2011). Biodegradable nanostructures with selective lysis of microbial membranes. Nat. Chem. 3, 409–414. doi: 10.1038/nchem.1012
Neville, N., and Jia, Z. (2019). Approaches to the structure-based design of antivirulence drugs: therapeutics for the post-antibiotic era. Molecules 24:378. doi: 10.3390/molecules24030378
Ng, V. W. L., Ke, X., Lee, A. L. Z., Hedrick, J. L., and Yang, Y. Y. (2013). Synergistic co-delivery of membrane-disrupting polymers with commercial antibiotics against highly opportunistic bacteria. Adv. Mater. 25, 6730–6736. doi: 10.1002/adma.201302952
N'Guessan, D. U. J.-P., Ouattara, M., Coulibaly, S., Kone, M. W., and Sissouma, D. (2018). Antibacterial activity of imidazo [1,2-α] pyridinylchalcones derivatives against Enterococcus faecalis. World J. Pharm. Res. 7, 21–33. doi: 10.20959/wjpr201818-13501
Nguyen, S., Hiorth, M., Rykke, M., and Smistad, G. (2013). Polymer coated liposomes for dental drug delivery – Interactions with parotid saliva and dental enamel. Eur. J. Pharm. Sci. 50, 78–85. doi: 10.1016/j.ejps.2013.03.004
Nicolosi, D., Scalia, M., Nicolosi, V. M., and Pignatello, R. (2010). Encapsulation in fusogenic liposomes broadens the spectrum of action of vancomycin against gram-negative bacteria. Int. J. Antimicrob. Agents 35, 553–558. doi: 10.1016/j.ijantimicag.2010.01.015
Paunovska, K., Loughrey, D., Sago, C. D., Langer, R., and Dahlman, J. E. (2019). Using large datasets to understand nanotechnology. Adv. Mater. 31:1902798. doi: 10.1002/adma.201902798
Peeridogaheh, H., Pourhajibagher, M., Barikani, H. R., and Bahador, A. (2019). The impact of Aggregatibacter actinomycetemcomitans biofilm-derived effectors following antimicrobial photodynamic therapy on cytokine production in human gingival fibroblasts. Photodiagn. Photodyn. Ther. 27, 1–6. doi: 10.1016/j.pdpdt.2019.05.025
Pick, H., Alves, A. C., and Vogel, H. (2018). Single-vesicle assays using liposomes and cell-derived vesicles: from modeling complex membrane processes to synthetic biology and biomedical applications. Chem. Rev. 118, 8598–8654. doi: 10.1021/acs.chemrev.7b00777
Popa, A., Davidescu, C. M., Trif, R., Ilia, G., Iliescu, S., and Dehelean, G. (2003). Study of quaternary onium salts grafted on polymers: antibacterial activity of quaternary phosphonium salts grafted on ‘gel-type’ styrene-divinylbenzene copolymers. React. Funct. Polym. 55, 151–158. doi: 10.1016/S1381-5148(02)00224-9
Praphakar, R. A., Ebenezer, R. S., Vignesh, S., Shakila, H., and Rajan, M. (2019). Versatile pH-responsive chitosan-g-polycaprolactone/maleic anhydride-isoniazid polymeric micelle to improve the bioavailability of tuberculosis multidrugs. ACS Appl. Bio Mater. 2, 1931–1943. doi: 10.1021/acsabm.9b00003
Ray, K. J., Simard, M. A., Larkin, J. R., Coates, J., Kinchesh, P., Smart, S. C., et al. (2019). Tumor pH and protein concentration contribute to the signal of amide proton transfer magnetic resonance imaging. Cancer Res. 79, 1343–1352. doi: 10.1158/0008-5472.CAN-18-2168
Robinson, A. M., Bannister, M., Creeth, J. E., and Jones, M. N. (2001). The interaction of phospholipid liposomes with mixed bacterial biofilms and their use in the delivery of bactericide. Colloids Surf. A 186, 43–53. doi: 10.1016/S0927-7757(01)00481-2
Robinson, A. M., Creeth, J. E., and Jones, M. N. (1998). The specificity and affinity of immunoliposome targeting to oral bacteria. BBA-Biomembranes 1369, 278–286. doi: 10.1016/S0005-2736(97)00231-9
Robinson, A. M., Creeth, J. E., and Jones, M. N. (2000). The use of immunoliposomes for specific delivery of antimicrobial agents to oral bacteria immobilized on polystyrene. J. Biomater. Sci. Polym. Ed. 11, 1381–1393. doi: 10.1163/156856200744408
Romberg, B., Hennink, W. E., and Storm, G. (2008). Sheddable coatings for long-circulating nanoparticles. Pharmaceut. Res. 25, 55–71. doi: 10.1007/s11095-007-9348-7
Sachetelli, S., Beaulac, C., Riffon, R., and Lagacé, J. (1999). Evaluation of the pulmonary and systemic immunogenicity of fluidosomes, a fluid liposomal–tobramycin formulation for the treatment of chronic infections in lungs. Biochim. Biophys. Acta 1428, 334–340. doi: 10.1016/S0304-4165(99)00078-1
Sachetelli, S., Khalil, H., Chen, T., Beaulac, C., Senechal, S., and Lagace, J. (2000). Demonstration of a fusion mechanism between a fluid bactericidal liposomal formulation and bacterial cells. BBA-Biomembranes 1463, 254–266. doi: 10.1016/S0005-2736(99)00217-5
Sanderson, N. M., Guo, B., Jacob, A. E., Handley, P. S., Cunniffe, J. G., and Jones, M. N. (1996). The interaction of cationic liposomes with the skin-associated bacterium Staphylococcus epidermidis: effects of ionic strength and temperature. Biochim. Biophys. Acta 1283, 207–214. doi: 10.1016/0005-2736(96)00099-5
Sanderson, N. M., and Jones, M. N. (1996). Targeting of cationic liposomes to skin-associated bacteria. J. Pesticide Sci. 46, 255–261. doi: 10.1002/(SICI)1096-9063(199603)46:3<255::AID-PS345>3.0.CO;2-Y
Smith, M. C., Crist, R. M., Clogston, J. D., and McNeil, S. E. (2017). Zeta potential: a case study of cationic, anionic, and neutral liposomes. Anal. Bioanal. Chem. 409, 5779–5787. doi: 10.1007/s00216-017-0527-z
Solleti, V. S., Alhariri, M., Halwani, M., and Omri, A. (2015). Antimicrobial properties of liposomal azithromycin for Pseudomonas infections in cystic fibrosis patients. J. Antimicrob. Chemother. 70, 784–796. doi: 10.1093/jac/dku452
Soria-Carrera, H., Lucia, A., De Matteis, L., Ainsa, J. A., de la Fuente, J. M., and Martin-Rapun, R. (2019). Polypeptidic micelles stabilized with sodium alginate enhance the activity of encapsulated bedaquiline. Macromol. Biosci. 19:1800397. doi: 10.1002/mabi.201800397
Stark, B., Pabst, G., and Prassl, R. (2010). Long-term stability of sterically stabilized liposomes by freezing and freeze-drying: effects of cryoprotectants on structure. Eur. J. Pharm. Sci. 41, 546–555. doi: 10.1016/j.ejps.2010.08.010
Sutherland, I. W. (2001). The biofilm matrix. an immobilized but dynamic microbial environment. Trends Microbiol. 9, 222–227. doi: 10.1016/S0966-842X(01)02012-1
Tang, H., Xu, Y., Zheng, T., Li, G., You, Y., Jiang, M., et al. (2009). Treatment of osteomyelitis by liposomal gentamicin-impregnated calcium sulfate. Arch. Orthop. Trauma Surg. 129, 1301–1308. doi: 10.1007/s00402-008-0782-8
Torres, I. M. S., Bento, E. B., da Cunha Almeida, L., de Sá, L. Z., and Lima, E. M. (2012). Preparation, characterization and in vitro antimicrobial activity of liposomal ceftazidime and cefepime against Pseudomonas aeruginosa strains. Braz. J. Microbiol. 43, 984–992. doi: 10.1590/S1517-83822012000300020
Van Leewenhoek, A. (1684). An abstract of a letter from Mr. Anthony Leevvenhoeck at Delft, dated Sep. 17. 1683. containing some microscopical observations, about animals in the scurf of the teeth, the substance call'd worms in the nose, the cuticula consisting of scales. Philos. Trans. 14, 568–574. doi: 10.1098/rstl.1684.0030
Veloso, D. F. M. C., Benedetti, N. I. G. M., Avila, R. I., Bastos, T. S. A., Silva, T. C., Silva, M. R. R., et al. (2018). Intravenous delivery of a liposomal formulation of voriconazole improves drug pharmacokinetics, tissue distribution, and enhances antifungal activity. Drug Deliv. 25, 1585–1594. doi: 10.1080/10717544.2018.1492046
Vila-Caballer, M., Codolo, G., Munari, F., Malfanti, A., Fassan, M., Rugge, M., et al. (2016). A pH-sensitive stearoyl-PEG-poly(methacryloyl sulfadimethoxine)-decorated liposome system for protein delivery: an application for bladder cancer treatment. J. Control. Rel. 238, 31–42. doi: 10.1016/j.jconrel.2016.07.024
Vyas, S. P., Sihorkar, V., and Dubey, P. K. (2001). Preparation, characterization and in vitro antimicrobial activity of metronidazole bearing lectinized liposomes for intra-periodontal pocket delivery. Pharmazie 56, 554–560.
Wang, Y., Wang, Z., Xu, C., Tian, H., and Chen, X. (2019). A disassembling strategy overcomes the EPR effect and renal clearance dilemma of the multifunctional theranostic nanoparticles for cancer therapy. Biomaterials 197, 284–293. doi: 10.1016/j.biomaterials.2019.01.025
Wolfmeier, H., Pletzer, D., Mansour, S. C., and Hancock, R. E. W. (2018). New perspectives in biofilm eradication. ACS Infect. Dis. 4, 93–106. doi: 10.1021/acsinfecdis.7b00170
Wu, J., Li, F., Hu, X., Lu, J., Sun, X., Gao, J., et al. (2019). Responsive assembly of silver nanoclusters with a biofilm locally amplified bactericidal effect to enhance treatments against multi-drug-resistant bacterial infections. ACS Cent. Sci. 5, 1366–1376. doi: 10.1021/acscentsci.9b00359
Xue, X.-Y., Mao, X.-G., Li, Z., Chen, Z., Zhou, Y., Hou, Z., et al. (2015). A potent and selective antimicrobial poly(amidoamine) dendrimer conjugate with LED209 targeting QseC receptor to inhibit the virulence genes of gram negative bacteria. Nanomedicine 11, 329–339. doi: 10.1016/j.nano.2014.09.016
Zahra, M.-J., Hamed, H., Mohammad, R.-Y., Nosratollah, Z., Akbarzadeh, A., and Morteza, M. (2017). Evaluation and study of antimicrobial activity of nanoliposomal meropenem against Pseudomonas aeruginosa isolates. Artif. Cells Nanomed. Biotechnol. 45, 975–980. doi: 10.1080/21691401.2016.1198362
Zendehdel, M., Zendehnam, A., Hoseini, F., and Azarkish, M. (2015). Investigation of removal of chemical oxygen demand (COD) wastewater and antibacterial activity of nanosilver incorporated in poly (acrylamide-co-acrylic acid)/NaY zeolite nanocomposite. Polym. Bull. 72, 1281–1300. doi: 10.1007/s00289-015-1326-3
Zhang, M., and Lemay, S. G. (2019). Interaction of anionic bulk nanobubbles with cationic liposomes: evidence for reentrant condensation. Langmuir 35, 4146–4151. doi: 10.1021/acs.langmuir.8b03927
Zhao, D., Shuang, Y., Sun, B., Shuang, G., Guo, S., and Kai, Z. (2018). Biomedical applications of chitosan and its derivative nanoparticles. Polymers 10:462. doi: 10.3390/polym10040462
Zhou, Z., Fang, H., Hu, S., Ming, K., Chao, F., Liu, Y., et al. (2018). pH-activated nanoparticles with targeting for the treatment of oral plaque biofilm. J. Mater. Chem. B 6, 586–592. doi: 10.1039/C7TB02682J
Keywords: bacterial biofilm, micelles, zeta potentials, hydrophobicity, lipids, liposomes, infection, fusogenicity
Citation: Wang D-Y, van der Mei HC, Ren Y, Busscher HJ and Shi L (2020) Lipid-Based Antimicrobial Delivery-Systems for the Treatment of Bacterial Infections. Front. Chem. 7:872. doi: 10.3389/fchem.2019.00872
Received: 10 October 2019; Accepted: 03 December 2019;
Published: 10 January 2020.
Edited by:
Manuel Simões, University of Porto, PortugalReviewed by:
Yohann Corvis, Université de Paris, FranceCopyright © 2020 Wang, van der Mei, Ren, Busscher and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Henny C. van der Mei, aC5jLnZhbi5kZXIubWVpQHVtY2cubmw=; Henk J. Busscher, aC5qLmJ1c3NjaGVyQHVtY2cubmw=; Linqi Shi, c2hpbGlucWlAbmFua2FpLmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.