- Department of Applied Chemistry, Faculty of Engineering, Osaka University, Suita, Japan
Catalytic liquid-phase oxidation using a catalyst and oxygen gas (Catalytic wet air oxidation, CWAO) is one of the most promising technology to remove hazardous organic compounds in wastewater. Up to now, various heterogeneous catalysts have been reported for phenolic compounds decomposition. The CeO2-ZrO2 based catalysts have been recently studied, because CeO2-ZrO2 works as a promoter which supplies active oxygen species from inside the lattice to the active sites. Since it is difficult to dissolve oxygen gas into water, the use of the promoter is effective for realizing the high catalytic activity at moderate conditions. Also, CeO2-ZrO2 shows high resistance for the metal leaching during the catalytic reaction in the liquid-phase. This article reviews the studies of the catalytic liquid-phase oxidation of phenolic compounds using CeO2-ZrO2 based catalysts.
Introduction
To date, industrial sector has been rapidly growing and consequently contributing to an increase in hazardous waste. Phenolic compounds, e.g., phenol, chlorophenol, and bisphenol-A, are well-known organic pollutants encountered in the wastewater. They have been used as raw material in many kinds of industries for the production, such as phenol resins, polycarbonate plastics, and acetylsalicylic acid. However, phenolic compounds are toxic substances for human health and lethal to aquatic life in water. Especially, bisphenol-A has identified as an endocrine-disrupting chemical (EDC) and functions as an estrogenic substance in living organisms even at very low concentration level (Geens et al., 2012). To protect the environment and our health, it is necessary to remove these pollutants from wastewater.
The conventional treatments are activated carbon treatment, coagulating sedimentation process, and biodegradation process (Chung et al., 2003; Villegas et al., 2016; Karri et al., 2017); however, these processes have problems, such as replacement of activated carbon, post-treatment of sediments, and control of temperature and pH for microorganisms. Although advanced treatment technologies have been studied using UV irradiation, strong oxidizing agents (H2O2 or O3), Fenton reagent (H2O2 + Fe3+), and ultrasound (Rosenfeldt and Linden, 2004; Torres et al., 2008; Nidheesh, 2015; Zhang et al., 2018), they require constant supply of hazardous oxidizing additives, UV irradiation, or ultrasonic irradiation.
A liquid-phase oxidation with a gaseous oxygen, wet air oxidation (WAO), is the simple process for wastewater treatment without photoirradiation, sonication, nor addition of oxidizing agent. However, the effective removal requires elevated temperatures (125–320°C) and high pressures (0.5–20 MPa) in order to increase the solubility of oxygen molecule in the solution (Zimmermann, 1958; Li et al., 1991; Mishra et al., 1995). One of the most promising processes to realize the high efficiency under the mild conditions is a catalytic liquid-phase oxidation, generally denoted as catalytic wet air oxidation (CWAO). Homogeneous catalysts based on copper salts are reported to exhibit effective catalytic oxidation for treating wastewater (Imamura et al., 1982; Kulkarni and Dixit, 1991; Lin et al., 1996); however, the dissolved catalysts must be separated due to the toxicity of copper ions. Therefore, heterogeneous catalysts have received attention, because the additional separation process is not necessary. For the heterogeneous catalysts, metal oxides and supported noble metals have been extensively studied. Among the metal oxide catalysts, such as CuO, ZnO, and MnO2 (Katzer et al., 1976; Pintar and Levec, 1994; Chen et al., 2001; Santos et al., 2005), CeO2-based catalysts are effective for the oxidation of organic pollutants. In particular, CeO2-MnO2 solid solutions exhibited the high catalytic activity for decomposing organic compounds (Imamura et al., 1985, 1987; Ma et al., 2017), while these catalysts were deactivated because of the leaching of the active phase and the formation of carbonaceous deposits, likely due to the presence of manganese (Delgado et al., 2006). Noble metals (Pt, Ru, and Pd) have generally high catalytic activity and high resistance for metal leaching compared to metal oxides (Imamura et al., 1988; Masende et al., 2005). However, they still require severe reaction conditions compared to ambient temperature and pressure. Since it is difficult to dissolve oxygen gas into water at moderate conditions, a promoter which supplies active oxygen species from its lattice toward an activator is an important component. Recently, ceria-zirconia (CeO2-ZrO2) based catalysts have been paid attention for the wastewater treatment due to their high oxygen release and storage abilities.
In this mini-review, we provided the studies on the heterogeneous catalysts based on CeO2-ZrO2 solid solutions for catalytic liquid-phase oxidation of phenolic compounds.
CeO2-ZrO2 Solid Solutions
CeO2 is one of the most famous promoters, because of its unique non-stoichiometric characteristics resulting from the ability of easily transition between reduced and oxidized states (Ce3+⇄ Ce4+ + e−) (Trovarelli, 1996; Montini et al., 2016). This allows CeO2 to store gaseous oxygen into its crystal lattice and subsequently release active oxygen from the bulk material. Therefore, CeO2 can work as a promoter to facilitate the oxidation of organic compounds by supplying active oxygen to the activator. Zirconium ions (Zr4+) are generally introduced into the CeO2 lattice to increase the oxygen release and storage abilities (Fornasiero et al., 1995). In the CeO2-ZrO2 binary system, various crystal structure have been reported. Yashima et al. proposed the phase diagram of the CeO2-ZrO2 system by using X-ray powder diffraction measurement and Raman spectroscopy (Yashima et al., 1994; Varez et al., 2006; Montini et al., 2016). The phase diagram shows three different tetragonal phases (t, t′, and t′′), in addition to the monoclinic ZrO2 phase and the cubic CeO2 phase. The monoclinic and/or t phases were formed for CeO2 content less than ca. 20 mol%, where the monoclinic phase is thermodynamically stable for pure ZrO2 at room temperature. In the CeO2 rich area above 90 mol%, the cubic phase was detected. The t′ and t′′ phases were regarded as the metastable phase at intermediate compositions of 20–90 mol% CeO2. For the 50 mol% CeO2 content, other metastable structure has been identified to be pyrochlore, κ and t* phases, which are cation-ordered phases (Montini et al., 2016). According to Fornasiero et al. (1995), the highest oxygen storage value is found for the Rh supported on cubic Ce0.5Zr0.5O2 sample. By applying the oxygen release and storage abilities, the CeO2-ZrO2 solid solution has been commercialized as promoters in automotive exhaust catalysts.
Catalytic Liquid-Phase Oxidation of Phenol
As described in the Introduction section, phenol has received attention due to its toxicity and common pollutant in wastewater. In addition, since phenol is the simplest class among phenolic compounds, phenol is considered to be an intermediate in the catalytic oxidation of phenolic compounds. Therefore, phenol is generally selected as a model compound for the wastewater treatment using heterogeneous catalysts.
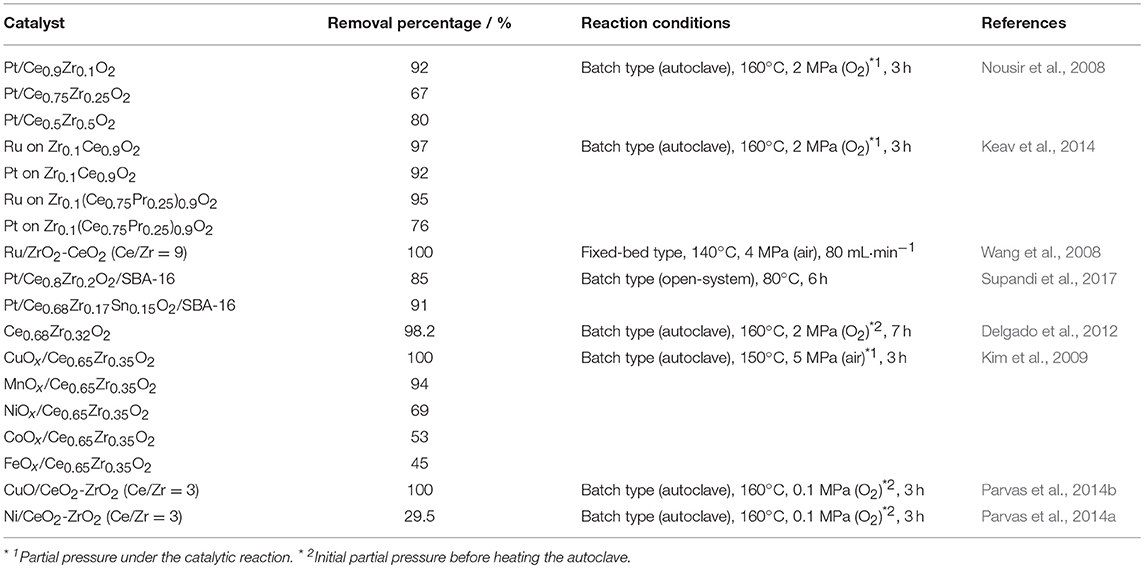
For catalytic phenol oxidation, noble metal loaded catalysts have been extensively studied due to their high catalytic activity and high resistance for the metal leaching. As for the noble metal activator, Barbier et al. (2005) investigated the Ru, Pd, or Pt supported on CeO2 catalysts, and confirmed that the order of phenol conversion was: Ru > Pd > Pt. For the Pd activator, the abrupt deactivation was also observed due to the deposition of carbonaceous species. Lee et al. (2010) studied the deactivation of catalyst during the phenol oxidation, and demonstrated that the deactivation was accelerated especially for Pt/Al2O3 compared to Pt/CeO2. They discussed that CeO2 might have promoted the oxidation of the carbonaceous deposits on the catalyst. According to Nousir et al. (2008), when introducing ZrO2 into the CeO2 lattice, oxygen storage capacity (OSC) of Pt/Ce0.9Zr0.1O2 was significantly enhanced compared to that of Pt/CeO2, indicating an increase in the oxygen species mobility. Although the phenol conversion of Pt/Ce0.9Zr0.1O2 (92%) was comparable with that of Pt/CeO2 (94%) after the reaction at 160°C and 2 MPa for 3 h, the selectivity in the complete oxidation product (CO2) for Pt/Ce0.9Zr0.1O2 (61%) was higher than that for Pt/CeO2 (50%); i.e., the formation of carbonaceous species on Pt/Ce0.9Zr0.1O2 was suppressed compared to the Pt/CeO2 case.
Based on the results mentioned above, the catalytic liquid-phase oxidation activities of phenol over the CeO2-ZrO2 based catalysts are tabulated in Table 1. Nousir et al. (2008) also compared the Pt/Ce1−xZrxO2 (x = 0.9, 0.75, 0.5) catalysts, and demonstrated that the phenol conversion was: Pt/Ce0.9Zr0.1O2 (92%) > Pt/Ce0.5Zr0.5O2 (80%) > Pt/Ce0.75Zr0.25O2 (67%), and the CO2 selectivity was: Pt/Ce0.5Zr0.5O2 (71%) > Pt/Ce0.9Zr0.1O2 (61%) > Pt/Ce0.75Zr0.25O2 (53%). Among them, the Pt/Ce0.5Zr0.5O2 catalyst exhibited the highest OSC value, which caused the high CO2 selectivity due to an easier elimination of the surface carbonaceous deposits. In addition, they assumed that the selectivity was also affected by the large interface between Pt and the support for Pt/Ce0.5Zr0.5O2. Keav et al. (2014) reported that Ru supported on Zr0.1Ce0.9O2 showed the higher phenol conversion (ca. 97%) than the Pt supported case (ca. 92%) after the reaction at 160°C and 2 MPa for 3 h, similar to the pure ceria supported catalysts (Barbier et al., 2005). They also demonstrated that Zr0.1(Ce0.75Pr0.25)0.9O2 exhibited the high OSC value compared to Zr0.1Ce0.9O2, because two kinds of valence states (Pr3+ and Pr4+) modified the kinetics of oxygen transfer, leading to promoting the redox process. However, the phenol conversions using Pt or Ru supported on Zr0.1(Ce0.75Pr0.25)0.9O2 was lower than each catalyst without Pr ion (Table 1), due to the formation of carbonaceous deposits. One possible reason of the low activity was the reduction treatment using hydrogen gas (30 mL·min−1) at 350–800°C for 3 h before the catalytic phenol oxidation. According to Wang et al. (2008), a complete removal of phenol was realized for a Ru/ZrO2-CeO2 (Ce/Zr = 9) catalyst in the fixed-bed flow reactor by applying conditions of 4 MPa and 140°C. In a recent study, the effective phenol removal was demonstrated even at the moderate conditions by using the catalyst, which consists of Pt and Ce0.68Zr0.17Sn0.15O2 dispersed on mesoporous silica SBA-16 (Santa Barbara Amorphous No. 16) with large surface area (Supandi et al., 2017). They reported that the phenol conversion of Pt/Ce0.68Zr0.17Sn0.15O2/SBA-16 reached up to 91% after the reaction at moderate temperature of 80°C and the atmospheric pressure for 6 h. Here, only ca. 4% of phenol was adsorbed into the SBA-16 support, indicating that the catalytic reaction was proceeded. They also revealed that the conversion of Pt/Ce0.68Zr0.17Sn0.15O2/SBA-16 was also higher than that of Pt/Ce0.8Zr0.2O2/SBA-16 (85%). Based on the literature (Yasuda et al., 2012), the introduction of SnO2 into the CeO2-ZrO2 lattice enhanced the oxygen release and storage abilities, because of the synergistically redox reaction between Ce4+/3+ and Sn4+/2+.
Noble-metal free catalysts for phenol removal have been also studied, as listed in Table 1. Delgado et al. (2012) investigated the catalytic activity of CeO2-ZrO2 without activators. Compared to pure CeO2, Ce0.68Zr0.32O2 showed the high performance, where the phenol conversions of CeO2 and Ce0.68Zr0.32O2 were 88.4 and 98.2%, respectively, after the reaction at 160°C and 2 MPa for 7 h. The enhancement in the activity was explained by the increase in oxide ion mobility; i.e., the vacancies or structural defects might form by the incorporation of Zr4+ into the fluorite CeO2 structure. In the case of the Zr-rich sample (Ce0.15Zr0.85O2), the activity decreased to 89.6% due to the segregation of ZrO2. They also reported that a carbonaceous deposit was not observed after the reaction at 160°C, while the deposit covered the catalyst surface in the case of the reaction at 120°C, resulting the deactivation (phenol conversion of Ce0.68Zr0.32O2: 60.9%). In addition, they revealed the high resistance for metal leaching of CeO2-ZrO2 after the reaction. Kim et al. (2009) developed various CeO2-ZrO2 supported metal oxide activators, and demonstrated that CuOx/Ce0.65Zr0.35O2 showed the high catalytic activity for phenol oxidation compared to MnOx/Ce0.65Zr0.35O2, FeOx/Ce0.65Zr0.35O2, CoOx/Ce0.65Zr0.35O2, and NiOx/Ce0.65Zr0.35O2. The complete phenol conversion was confirmed for CuOx/Ce0.65Zr0.35O2 after the reaction at 150°C under 5 MPa for 3 h. The high activity of CuOx/Ce0.65Zr0.35O2 was attributed to the leached copper ions, which caused a homogeneous oxidation reaction. In the case of MnOx/Ce0.65Zr0.35O2, while the high conversion of phenol (94%) was obtained, phenol was mainly converted to carbonaceous deposits on the surface of the catalyst, which caused the deactivation. The high activity for the CuO supported on CeO2-ZrO2 was also reported by Parvas et al. (2014b), while Ni/CeO2-ZrO2 showed low activity (Parvas et al., 2014a).
Catalytic Liquid-Phase Oxidation of Phenol Derivatives
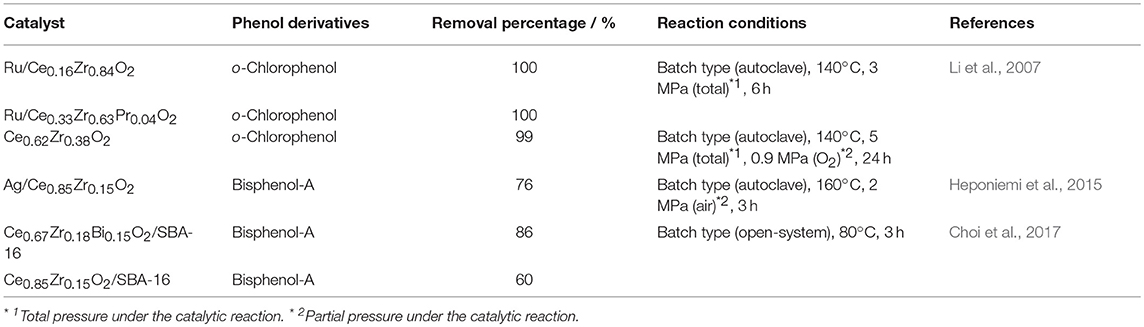
Up to date, effective oxidation of phenol derivatives has also been demonstrated by using CeO2-ZrO2 based catalysts, and their activities are summarized in Table 2. Li et al. (2007) studied the oxidation of o-chlorophenol over Ru/CeO2-ZrO2 solid solutions. The Ru/CeO2-ZrO2 catalysts have higher catalytic activities than Ru/CeO2 and Ru/ZrO2, because the oxygen supply to the active site from liquid phase was expected to be accelerated, where CeO2 supported catalyst might also be affected by the formation of carbonates at the catalyst surface. Among the Ru/CeO2-ZrO2 systems, Ru/Ce0.16Zr0.84O2 showed the highest activity, and the complete o-chlorophenol conversion was realized (140°C, total pressure 3 MPa, 6 h). They also reported that the introduction of a small amount of Pr or Nd into the CeO2-ZrO2 lattice enhanced the catalytic activity, because of the formation of oxygen vacancies, which increased the reducibility and facilitated the oxygen mobility. In particular, Ru/Ce0.33Zr0.63Pr0.04O2 showed the high total organic carbon removal of 95% compared to the Ru/Ce0.16Zr0.84O2 case (91%) (140°C, total pressure 3 MPa, 6 h), while both catalysts could completely convert o-chlorophenol. In the absence of Ru, Ce0.62Zr0.38O2 exhibited the high o-chlorophenol conversion of 99% under the severe conditions (140°C, total pressure 5 MPa, 24 h).
For the catalytic oxidation of bisphenol-A, most of catalysts were used in combination with strong oxidizing reagent (H2O2 etc.) or UV irradiation (Ohko et al., 2001; Mayani et al., 2014; Liu et al., 2016; Zhou et al., 2016). Heponiemi et al. (2015) reported the catalytic decomposition of bisphenol-A using Ag supported on CeO2-based catalysts. The bisphenol-A abatement of Ag/CeO2 was 51% after the reaction at 160°C for 3 h under a pressure of 2 MPa, where ca. 12% of bisphenol-A was adsorbed on the catalyst. By adding a small amount of ZrO2 to CeO2, the bisphenol-A removal percentage was enhanced; i.e., the removal percentage of Ag/Ce0.85Zr0.15O2 was 76% with 1% adsorption of bisphenol-A at the same condition. Choi et al. (2017) demonstrated that the CeO2-ZrO2-Bi2O3 supported on SBA-16 catalyst exhibited the catalytic activity for bisphenol-A oxidation, and 86% of bisphenol-A conversion was achieved for Ce0.67Zr0.18Bi0.15O2/SBA-16 even with the moderate reaction conditions of 80°C for 3 h under atmospheric pressure. Here, this conversion of Ce0.67Zr0.18Bi0.15O2/SBA-16 was higher than that of Ce0.85Zr0.15O2/SBA-16 (60%). This enhancement was explained by the high oxygen release and storage abilities of Ce0.67Zr0.18Bi0.15O2, because the introduction of Bi2O3 into the CeO2-ZrO2 lattice facilitated the oxygen supply via oxygen vacancies, formed by the replacement of Ce4+ and Zr4+ sites by the lower-valent Bi3+ ion (Imanaka et al., 2007). Choi et al. (2017) also reported that the Ce0.67Zr0.18Bi0.15O2/SBA-16 catalyst maintained a high conversion after three consecutive reactions without the deactivation.
Conclusion and Perspectives
In this mini-review, recent studies on catalytic liquid-phase oxidation of phenolic compounds using CeO2-ZrO2 based catalysts are summarized. By using CeO2-ZrO2 as a promoter, the catalytic activity was facilitated compared to the bare oxides, such as Al2O3 and CeO2. Pt/CeO2-ZrO2 and Ru/CeO2-ZrO2 catalysts exhibited high activities for phenol conversion, and the complete phenol decomposition was realized for the Ru/ZrO2-CeO2 (Ce/Zr = 9) catalyst under conditions of 140°C and 4 MPa. Even at the moderate conditions of 80°C and atmospheric pressure, the high phenol conversion of 91% was demonstrated by dispersing Pt and CeO2-ZrO2-SnO2 into SBA-16. Noble metal free catalysts have been also developed, and phenol was completely removed using the CuOx/CeO2-ZrO2 catalyst, in which the leached Cu2+ ions promoted the phenol oxidation by work as a homogeneous catalyst.
CeO2-ZrO2 was also effective for the removal of phenol derivatives. Ru/Ce0.33Zr0.63Pr0.04O2 completely converted o-chlorophenol, and the total organic carbon removal of 95% was realized. For the bisphenol-A removal, the conversions of Ag/CeO2-ZrO2 and CeO2-ZrO2-Bi2O3/SBA-16 were 76% (reaction conditions: 160°C, 2 MPa, 3 h) and 86% (reaction conditions: 80°C, atmospheric pressure, 3 h), respectively.
For future perspective, it is necessary to realize complete oxidation of phenolic compounds in facile operating conditions at room temperature and under atmospheric pressure, from the viewpoint of economically, safety, and sustainably. In addition, the suppression of the deactivation, caused by the carbonaceous deposit and the metal leaching, should be considered. Since one effective method to remove the carbonaceous deposit is the complete oxidation of phenols to carbon dioxide and water, the improvement in the oxygen release ability of the promoter would be a key factor, which also leads to the high catalytic activity. For enhancing the ability of the promoter, the formation of the oxygen vacancies in the lattice might lead to the increase in reducibility and the oxygen mobility. Here, to avoid the metal leaching, the composition of the promoter should be carefully selected. Further investigations would lead to the development of novel catalysts with high efficiency for practical applications.
Author Contributions
NN collected and read papers and wrote the manuscript. AS and P-GC collected papers and contributed to the manuscript writing. NI contributed to the paper design and also wrote the manuscript. All authors read and approved the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Barbier, J. Jr., Oliviero, L., Renard, B., and Duprez, D. (2005). Role of ceria-supported noble metal catalysts (Ru, Pd, Pt) in wet air oxidation of nitrogen and oxygen containing compounds. Top. Catal. 33, 77–86. doi: 10.1007/s11244-005-2509-1
Chen, H., Sayari, A., Adnot, A., and Larachi, F. (2001). Composition-activity effects of Mn-Ce-O composites on phenol catalytic wet oxidation. Appl. Catal. B Environ. 32, 195–204. doi: 10.1016/S0926-3373(01)00136-9
Choi, P. G., Kamijo, A., Nunotani, N., Nakano, T., and Imanaka, N. (2017). Catalytic liquid-phase oxidation of bisphenol-A under moderate condition using CeO2-ZrO2-Bi2O3 supported on SBA-16. Chem. Lett. 46, 257–259. doi: 10.1246/cl.161011
Chung, T. P., Tseng, H. Y., and Juang, R. S. (2003). Mass transfer effect and intermediate detection for phenol degradation in immobilized Pseudomonas putida systems. Process Biochem. 38, 1497–1507. doi: 10.1016/S0032-9592(03)00038-4
Delgado, J. J., Chen, X., Pérez-Omil, J. A., Rodríguez-Izquierdo, J. M., and Cauqui, M. A. (2012). The effect of reaction conditions on the apparent deactivation of Ce-Zr mixed oxides for the catalytic wet oxidation of phenol. Catal. Today 180, 25–33. doi: 10.1016/j.cattod.2011.03.069
Delgado, J. J., Pérez-Omil, J. A., Rodríguez-Izquierdo, J. M., and Cauqui, M. A. (2006). The role of the carbonaceous deposits in the catalytic wet oxidation (CWO) of phenol. Catal. Commun. 7, 639–643. doi: 10.1016/j.catcom.2006.02.003
Fornasiero, P., Monte, R. D., Rao, G. R., Kašpar, J., Meriani, S., Trovarelli, A., et al. (1995). Rh-loaded CeO2-ZrO2 solid solutions as highly efficient oxygen exchangers: dependence of the reduction behavior and the oxygen strorage capacity on the structural properties. J. Catal. 151, 168–177. doi: 10.1006/jcat.1995.1019
Geens, T., Aerts, D., Berthot, C., Bourguignon, J. P., Goeyens, L., Lecomte, P., et al. (2012). A review of dietary and non-dietary exposure to bisphenol-A. Food Chem. Toxicol. 50, 3725–3740. doi: 10.1016/j.fct.2012.07.059
Heponiemi, A., Azalim, S., Hu, T., and Lassi, U. (2015). Cerium oxide based catalysts for wet air oxidation of bisphenol A. Top. Catal. 58, 1043–1052. doi: 10.1007/s11244-015-0457-y
Imamura, S., Doi, A., and Ishida, S. (1985). Wet oxidation of ammonia catalyzed by cerium-based composite oxides. Ind. Eng. Chem. Prod. Res. Dev. 24, 75–80. doi: 10.1021/i300017a014
Imamura, S., Fukuda, I., and Ishida, S. (1988). Wet oxidation catalyzed by ruthenium supported on cerium(IV) oxides. Ind. Eng. Chem. Res. 27, 718–721. doi: 10.1021/ie00076a033
Imamura, S., Nishimura, H., and Ishida, S. (1987). Preparation of Mn/Ce composite oxide catalysts for the wet oxidation of acetic acid and their catalytic activities. Sekiyu Gakkaishi 30, 199–202. doi: 10.1627/jpi1958.30.199
Imamura, S., Sakai, T., and Ikuyama, T. (1982). Wet-oxidation of acetic acid catalyzed by copper salts. J. Jpn. Petrol. Inst. 25, 74–80. doi: 10.1627/jpi1958.25.74
Imanaka, N., Masui, T., Koyabu, K., Minami, K., and Egawa, T. (2007). Significant low-temperature redox activity of Ce0.64Zr0.16Bi0.20O1.90 supported on γ-Al2O3. Adv. Mater. 19, 1608–1611. doi: 10.1002/adma.200502741
Karri, R. R., Jayakumar, N. S., and Sahu, J. N. (2017). Modelling of fluidised-bed reactor by differential evolution optimization for phenol removal using coconut shells based activated carbon. J. Mol. Liq. 231, 249–262. doi: 10.1016/j.molliq.2017.02.003
Katzer, J. R., Ficke, H. H., and Sadana, A. (1976). An evaluation of aqueous phase catalytic oxidation. J. Water Pollut. Control Fed. 48, 920–933
Keav, S., Monteros, A. E., Barbier, J. Jr., and Duprez, D. (2014). Wet air oxidation of phenol over Pt and Ru catalylsts supported on cerium-based oxides: resistance to fouling and kinetic modelling. Appl. Catal. B Environ. 150–151, 402–410. doi: 10.1016/j.apcatb.2013.12.028
Kim, K. H., Kim, J. R., and Ihm, S. K. (2009). Wet oxidation of phenol over transition metal oxide catalysts supported on Ce0.65Zr0.35O2 prepared by continuous hydrothermal synthesis in supercritical water. J. Hazard. Mater. 167, 1158–1162. doi: 10.1016/j.jhazmat.2009.01.110
Kulkarni, U. S., and Dixit, S. G. (1991). Destruction of phenol from wastewater by oxidation with sulfite-oxygen. Ind. Eng. Chem. Res. 30, 1916–1920. doi: 10.1021/ie00056a037
Lee, D. K., Kim, D. S., Kim, T. H., Lee, Y. K., Jeong, S. E., Le, N. T., et al. (2010). Deactivation of Pt catalysts during wet oxidation of phenol. Catal. Today 154, 244–249. doi: 10.1016/j.cattod.2010.03.052
Li, L., Chen, P., and Gloyna, E. F. (1991). Generalized kinetic model for wet oxidation of organic compounds. AIChE J. 37, 1687–1697. doi: 10.1002/aic.690371112
Li, N., Descorme, C., and Besson, M. (2007). Catalytic wet air oxidation of 2-chlorophenol over Ru loaded CexZr1−xO2 solid solutions. Appl. Catal. B Environ. 76, 92–100. doi: 10.1016/j.apcatb.2007.05.013
Lin, S. H., Ho, S. J., and Wu, C. L. (1996). Kinetic and performance characteristics of wet air oxidation of high-concentration wastewater. Ind. Eng. Chem. Res. 35, 307–314. doi: 10.1021/ie950251u
Liu, Y., Zhu, G., Gao, J., Hojamberdiev, M., Lu, H., Zhu, R., et al. (2016). A novel CeO2/Bi4Ti3O12 composite heterojunction structure with an enhanced photocatalytic activity for bisphenol A. J. Alloy. Compd. 688, 487–496. doi: 10.1016/j.jallcom.2016.07.054
Ma, C., Wen, Y., Yue, Q., Li, A., Fu, J., Zhang, et al. (2017). Oxygen-vacancy-promoted catalytic wet air oxidation of phenol from MnOx-CeO2. RSC Adv. 7, 27079–27088. doi: 10.1039/C7RA04037G
Masende, Z. P. G., Kuster, B. F. M., Ptasinski, K. J., Janssen, F. J. J. G., Katima, J. H. Y., and Schouten, J. C. (2005). Support and dispersion effects on activity of platinum catalysts during wet oxidation of organic wastes. Top. Catal. 33, 87–99. doi: 10.1007/s11244-005-2514-4
Mayani, S. V., Mayani, V. J., and Kim, S. W. (2014). SBA-15 supported Fe, Ni, Fe-Ni bimetallic catalysts for wet oxidation of bisphenol-A. Bull. Korean Chem. Soc. 35, 3535–3541. doi: 10.5012/bkcs.2014.35.12.3535
Mishra, V. S., Mahajani, V. V., and Joshi, J. B. (1995). Wet air oxidation. Ind. Eng. Chem. Res. 34, 2–48. doi: 10.1021/ie00040a001
Montini, T., Melchionna, M., Monai, M., and Fransiero, P. F. (2016). Fundamentals and catalytic applications of CeO2-based materials. Chem. Rev. 116, 5987–6041. doi: 10.1021/acs.chemrev.5b00603
Nidheesh, P. V. (2015). Heterogeneous Fenton catalysts for the abatement of organic pollutants from aqueous solution: a review. RSC Adv. 5, 40552–40577. doi: 10.1039/C5RA02023A
Nousir, S., Keav, S., Barbier, J. Jr., Bensitel, M., Brahmi, R., et al. (2008). Deactivation phenomena during catalytic wet air oxidation (CWAO) of phenol over platinum catalysts supported on ceria and ceria-zirconia mixed oxides. Appl. Catal. B Environ. 84, 723–731. doi: 10.1016/j.apcatb.2008.06.010
Ohko, Y., Ando, I., Niwa, C., Tatsuma, T., Yamamura, T., Nakashima, T., et al. (2001). Degradation of bisphenol A in water by TiO2 photocatalyst. Environ. Sci. Technol. 35, 2365–2368. doi: 10.1021/es001757t
Parvas, M., Haghighi, M., and Allahyari, S. (2014a). Catalytic wet air oxidation of phenol over ultrasound-assisted synthesized Ni/CeO2-ZrO2 nanocatalyst used in wastewater treatment. Arab. J. Chem. doi: 10.1016/j.arabjc.2014.10.043. [Epub ahead of print].
Parvas, M., Haghighi, M., and Allahyari, S. (2014b). Degradation of phenol via wet-air oxidation over CuO/CeO2-ZrO2 nanocatalyst synthesized employing ultrasoud energy: physicochemical characterizaton and catalytic performance. Environ. Technol. 35, 1140–1149. doi: 10.1080/09593330.2013.863952
Pintar, A., and Levec, J. (1994). Catalytic liquid-phase oxidation of phenol aqueous solutions. A kinetic investigation. Ind. Eng. Chem. Res. 33, 3070–3077. doi: 10.1021/ie00036a023
Rosenfeldt, E. J., and Linden, K. G. (2004). Degradation of endocrine disrupting chemicals bisphenol A, ethinyl estrodiol, and estradiol during UV photolysis and advanced oxidation processes. Environ. Sci. Technol. 38, 5476–5483. doi: 10.1021/es035413p
Santos, A., Yustos, P., Quintanilla, A., Ruiz, G., and Garcia-Ochoa, F. (2005). Study of the copper leaching in the wet oxidation of phenol with CuO-based catalysts: causes and effects. Appl. Catal. B Environ. 61, 323–333. doi: 10.1016/j.apcatb.2005.06.006
Supandi, A. R., Nunotani, N., and Imanaka, N. (2017). Liquid-phase oxidation of phenol in facile condition using Pt/CeO2-ZrO2-SnO2 catalyst supported on mesoporous silica SBA-16. J. Environ. Chem. Eng. 5, 3999–4003. doi: 10.1016/j.jece.2017.07.072
Torres, R. A., Sarantakos, G., Combet, E., Pétrier, C., and Pulgarin, C. (2008). Sequential helio-photo-Fenton and sonication processes for the treatment of bisphenol A. J. Photochem. Photobiol. A Chem. 199, 197–203. doi: 10.1016/j.jphotochem.2008.05.016
Trovarelli, A. (1996). Catalytic properties of ceria and CeO2-containing materials. Catal. Rev. Sci. Eng. 38, 439–520. doi: 10.1080/01614949608006464
Varez, A., Garcia-Gonzalez, E., and Sanz, J. (2006). Cation miscibility in CeO2-ZrO2 oxides with fluorite structure. A combined TEM, SAED and XRD Rietveld analysis. J. Mater. Chem. 16, 4249–4256. doi: 10.1039/B607778A
Villegas, L. G. C., Mashhadi, N., Chen, M., Mukherjee, D., Taylor, K. E., and Biswas, N. (2016). A short review of techniques for phenol removal from wastewater. Curr. Pollution Rep. 2, 157–167. doi: 10.1007/s40726-016-0035-3
Wang, J., Zhu, W., Yang, S., Wang, W., and Zhou, Y. (2008). Catalytic wet air oxidation of phenol with pelletized ruthenium catalysts. Appl. Catal. B Environ. 78, 30–37. doi: 10.1016/j.apcatb.2007.08.014
Yashima, M., Arashi, H., Kakihana, M., and Yoshimura, M. (1994). Raman scattering study of cubic-tetragonal phase transition in Zr1−xCexO2 solid solution. J. Am. Ceram. Soc. 77, 1067–1071. doi: 10.1111/j.1151-2916.1994.tb07270.x
Yasuda, K., Yoshimura, A., Katsuma, A., Masui, T., and Imanaka, N. (2012). Low-temperature complete combustion of volatile organic compounds over novel Pt/CeO2-ZrO2-SnO2/γ-Al2O3 catalysts. Bull. Chem. Soc. Jpn. 85, 522–526. doi: 10.1246/bcsj.20110382
Zhang, N., Xian, G., Li, X., Zhang, P., Zhang, G., and Zhu, J. (2018). Iron based catalysts used in water treatment assisted by ultrasoud: a mini review. Front. Chem. 6:12. doi: 10.3389/fchem.2018.00012
Zhou, Q., Xing, A., Li, J., Zhao, D., Zhao, K., and Lei, N. (2016). Synergistic enhancement in photoelectrocatalytic degradation of bisphenol A by CeO2 and reduces graphene oxide co-modified TiO2 nanotube arrays in combination with Fenton oxidation. Electrochim. Acta 209, 379–388. doi: 10.1016/j.electacta.2016.05.094
Keywords: catalyst, liquid-phase oxidation, phenolic compounds, ceria-zirconia, catalytic wet air oxidation
Citation: Nunotani N, Supandi AR, Choi P-G and Imanaka N (2018) Catalytic Liquid-Phase Oxidation of Phenolic Compounds Using Ceria-Zirconia Based Catalysts. Front. Chem. 6:553. doi: 10.3389/fchem.2018.00553
Received: 07 August 2018; Accepted: 29 October 2018;
Published: 15 November 2018.
Edited by:
Ramesh L. Gardas, Indian Institute of Technology Madras, IndiaReviewed by:
Guo-Hong Tao, Sichuan University, ChinaM. Hassan Beyzavi, University of Arkansas, United States
Trilochan Mishra, National Metallurgical Laboratory (CSIR), India
Copyright © 2018 Nunotani, Supandi, Choi and Imanaka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nobuhito Imanaka, aW1hbmFrYUBjaGVtLmVuZy5vc2FrYS11LmFjLmpw
†Present Address: Pil-Gyu Choi, National Institute of Advanced Industrial Science and Technology (AIST), Nagoya, Japan
 Naoyoshi Nunotani
Naoyoshi Nunotani Abdul Rohman Supandi
Abdul Rohman Supandi Pil-Gyu Choi
Pil-Gyu Choi Nobuhito Imanaka
Nobuhito Imanaka