- 1State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, China
- 2Collaborative Innovation Center of Chemical Science and Engineering, Nankai University, Tianjin, China
Carbon dioxide, as a promising C1 synthon, has attracted great interest in organic synthesis. Due to the thermodynamic stability and kinetic inertness of CO2, developing efficient strategies for CO2 activation and subsequent conversion is very crucial. In this context, Ionic liquids (ILs) show great potential for capturing and activating CO2 owing to their unique structures and properties, making them become ideal alternatives to volatile organic solvents and/or catalysts for CO2 transformation. This minireview aims at summarizing ILs-promoted reactions of CO2 with N-nucleophiles (primary amines)/O-nucleophiles (primary alcohols, water). Two catalytic systems i.e., metal/ILs binary systems such as Cu/ILs systems and Ag/ILs systems as well as single ILs systems including anion-functionalized ILs and bifunctionalized ILs have been developed for CO2 catalytic conversion, for instance, carboxylative cyclization of nucleophiles e.g., propargylic alcohols, amines, 2-aminobenzonitriles and o-aminobenzenethiol, and formylation of amines or 2-aminothiophenols with hydrosilanes to afford various value-added chemicals e.g., cyclic carbamates, unsymmetrical organic carbonates, α-hydroxyl ketones, and benzimidazolones. In a word, IL could provide a powerful tool for efficient CO2 utilization.
Introduction
CCS strategy, carbon capture and storage/sequestration, has been proposed as a most potential invention to reduce or mitigate CO2 emissions, including the capture of waste CO2, the transportation and deposition of CO2 in a safe place. Nevertheless, high cost and energy consumption of CCS process are the main obstacles. Carbon dioxide, as an abundant and non-poisonous C1 resource, has shown significant potential for constructing new C–C, C–O, and C–N bond in chemical synthesis (Shi et al., 2003; Zhang et al., 2008; He et al., 2009, 2010; Aresta et al., 2014; Liu et al., 2015, 2016a, 2017a,b; Song et al., 2017). However, the inherent thermodynamic stability and kinetic limitation of CO2 become the main barriers in transforming CO2 into high value-added chemicals, fuels, and materials. Therefore, developing efficient strategies for CO2 activation and conversion from environmental protection and economic perspectives is crucial. Carbon capture and utilization (CCU) strategy have been proposed by He group, which could be an ideal alternative to address the energy consumption problem in CCS (Yang et al., 2011a,b, 2012).
Ionic liquids (ILs) have attracted widespread attention as promising alternatives to solvents and catalysts on account of their unique properties such as the low melting point, unlimited tunability, negligible vapor pressure, and high stability (Zhang et al., 2006). As a novel green medium, ILs have been identified the outstanding performance in the absorption and conversion of CO2 under mild conditions through tunning the structures of cations and anions (Jutz et al., 2011; Yang et al., 2011b; Liu et al., 2016b). ILs-promoted CCU processes have attracted numerous attentions owning to ILs' unique properties. In most cases, ILs can be used as solvent, dehydrate, or catalyst, and these roles are similar in CCU processes. Thus, we hope to be able to shed light on all ILs-promoted CCU processes by using these limited but systematic examples.
In this minireview, we aim at summarizing ILs-promoted reactions of CO2 with some nucleophiles. We would like to divide this review into two parts that are metal/ILs binary systems such as Cu/ILs systems and Ag/ILs systems as well as single ILs systems including conventional ILs, anion-functionalized ILs, and bifunctionalized ILs. Selected carboxylative cyclization of nucleophiles, e.g., propargylic alcohols, amines, 2-aminobenzonitriles and o-aminobenzenethiol, and formylation of amines or 2-aminothiophenols with hydrosilanes, to afford various value-added chemicals are taken into consideration.
Metal/Ionic Liquid Binary Catalytic System
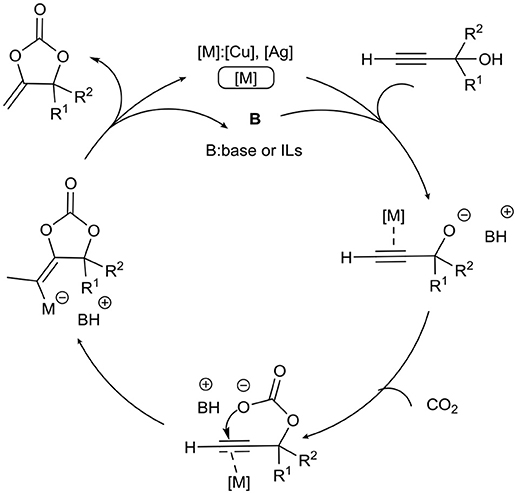
Metal (Cu, Ag)-ILs system is an important part of the CO2 capture and utilization. Since its ability to activate carbon-carbon triple bond, Cu/Ag has been used in various cyclization reactions of CO2 as shown in Scheme 1.
Cu/Ionic Liquid Catalysis
The first example of the synthesis of α-methylene cyclic carbonates from CO2 and propargylic alcohols in ILs is reported by Deng and co-workers (Gu et al., 2004; Figure S1). By screening of commercially available transition metal salts and ILs, [BMIm][PhSO3]/CuCl system exhibits the best performance in this reaction with a yield of 97%. Thanks to the reusability of ILs, CuCl can be reused 3 times without losing activity after immobilized in [BMIm][PhSO3].
A similar system, [BMIm]BF4/CuCl, is then used by the same group in a similar reaction (Figure S1; Gu et al., 2005). IL is used as the “green” reaction media and believed as the promoter for this three-component reaction of propargylic alcohols, primary amines and CO2.
The synthesis of α-methylene oxazolidinones only from propargylic amines and CO2 is reported by our group, in which bifunctional Cu(II)-polyoxometalate-based ILs is used as catalyst (Figure S2; Wang et al., 2016a). According to the experimental results and NMR studies, this IL is found to be able to activate propargylic amine and CO2 at the same time (Figure S3). And both terminal and internal propargylic amines successfully deliver the corresponding 2-oxazolidinones in excellent yields.
Ag/Ionic Liquids Catalysis
Compared with copper, silver displays better reactivity in activation of carbon-carbon triple bond of propargylic amine/ alcohol. Therefore, Ag-IL catalyst systems have also been widely applied for catalyzing cyclization propargylic amine/alcohols with CO2.
In 2015, He et al. successfully developed a dual-component catalytic system comprising AgOAc and [(n-C7H15)4N][Br], which could effectively catalyze CO2 fixation with propargylic alcohols/amines to produce various cyclic carbonates/oxazolidinones in the absence of solvent, ligand or organic base (Figure S4; Song and He, 2016). This elegant system can achieve the TON of 6024. Through experimental results and DFT calculations, the cation with a longer alkyl chain can enhance the nucleophilicity of anion resulting in improving its activity.
Besides of cations, tuning structures of anions in ILs is another way that can control the activity of ILs. Recently, Wang group has developed an efficient AgOAc/ [P66614][DEIm] (trihexyltetradecylphosphonium dimethyl 4,5-imidazoledicarboxylate) system for the synthesis of cyclic carbonates from propargylic alcohols with CO2 under mild conditions (Figure S5; Chen et al., 2016a). The basicity of the IL is found to play a dramatic role: only when IL with moderate basicity, can it have excellent activity. According to DFT calculation and NMR spectroscopic analyzing, weaker basicity shows poor activity but stronger basicity will reduce the yield because of the polymerization of propargylic alcohols.
Similarly, AgI/[OAc] is disclosed as a robust catalyst system in the same reaction (Figure S5; Yuan et al., 2017). Only 1% of the catalyst is needed to obtain excellent yield of cyclic carbonates. The high concentration of [OAc] in this system is favorable for the activation of hydroxyl in the substrate and CO2, which is also identified by Steckel (Steckel, 2012). Another Ag binary catalyst system, AgCl/[OAc], is reported by Han group in the reaction of CO2, propargylic alcohols, and primary alcohols (Figure S6; Hu et al., 2017). AgCl/[BMIm][OAc] serves as both catalyst and solvent, and can be easily reused at least five times without reducing notably catalytic activity. The activation of alcoholic hydroxyl by [OAc] and the activation of triple bond by Ag salt are supposed as important steps in this reaction.
Ionic Liquid Catalysis for CO2 Conversion
Traditional Ionic Liquids
It has been a long time since ILs have been discovered the good solubility for CO2 (Blanchard et al., 1999; Bates et al., 2002; Jessop et al., 2005). And this property can also make IL a nice catalyst for CO2 conversion (Ma et al., 2017; Wang et al., 2018). Recently, two similar nonfunctional IL dual systems (CsOH or Co(acac)3/[BMMIm][Cl]) have been reported by Deng et al. in the synthesis of symmetric urea derivatives from CO2 and amines (Figure S7; Shi et al., 2003; Li et al., 2010). In the CsOH/[BMMIm][Cl] system, symmetric urea derivatives can be obtained in high yields, but no product is produced when without IL, indicating that IL is indispensable for this reaction. However, as a strong base, CsOH suffers from many weakness, such as corrosion, deactivation, and even destructive action to [BMMIm][Cl] under high temperature. In order to overcome these disadvantages, Co(acac)3/[BMIm][Cl] system is developed for this reaction. In this catalyst system, [BMIm][Cl] is believed as a physical dehydrant, importantly, it can be reused while keeping high activity.
IL, such as [BMIm][Br], is also believed as an efficient dehydrant in the synthesis of cyclic urethanes from 2-aminoethanol and CO2 (Figure S8; Fujita et al., 2006). K2CO3 is the catalyst in this reaction, while [BMIm][Br] acts as a recyclable dehydrant and can activate the carbonyl group. In another work to obtain oxazolidinone, Deng et al. developes an ILs-catalyzed efficient three-component reaction of propargylic alcohols, amines, and CO2 (Zhang et al., 2005). Among all of the solvents they use, [DMIm][BF4] exhibits the best performance, while traditional solvents like DMSO and toluene have no activity (Figure S9). Therefore, it is indicated that IL has a strong impact on this reaction. After recycling three times, high catalytic activity can be maintained for the [DMIm][BF4].
Imidazole IL ([BMIm][Cl]) is also applied by Liu group in the reductive functionalization of CO2 with amines to afford formamide (Hao et al., 2015). The experimental results imply that both anions and cations of ILs play important roles in their activity. [BMIm][Cl] can activate not only the Si–H bond of phenylsilane to react with CO2, but the amine through the hydrogen bond. In addition, [BMIm][Cl] can be reused for five times with high activity (Figure S10).
Anion-Functionalized Ionic Liquids
Although many CCU processes can be promoted by traditional ILs, most of them suffer from low ability for CO2 activation, in which high pressure (>1 MPa) or additional metal catalysts will be needed. Recently, anion-functioanlized ILs are designed for CO2 absorption and some of their CO2 absorption capacities are even 100 times higher than traditional organic solvent (Gurkan et al., 2010; Wang et al., 2011b; Yang et al., 2011a,b; Lei et al., 2014; Cui et al., 2016; Song et al., 2017). In addition, compared with traditional ILs, anion-functioanlized ILs also exhibit higher catalytic activities even without metal catalyst (Scheme 2).
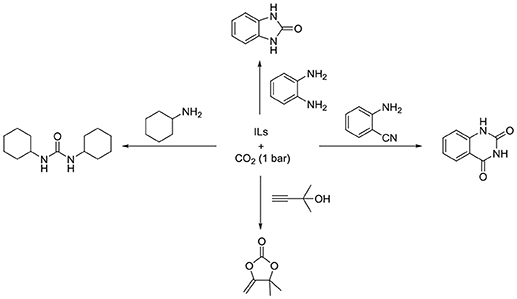
In 2009, Patil et al. developed an alternative method that can obtain quinazoline-2,4(1H,3H)-diones easily from CO2 and 2-aminobenzonitriles by using [BMIm][OH] as catalyst (Patil et al., 2009). Except for [BMIm][OH], many inorganic bases, Et3N or traditional ILs ([BMIm][BF4] and [BMIm][HSO4]) show weak or no activity (Figure S11). For the role of [BMIm][OH] in this process, they propose that [OH] will activate the 2-aminobenzonitrile to initiate the reaction, while [BMIm] can stabilize the intermediate.
To elucidate the mechanism of this reaction, Wu et al. conducts a systematic DFT calculation (Ren et al., 2011). In the beginning, the mechanism proposed by Patil et al. is calculated to be energy unfeasible. Inspired by NHCs–CO2 adducts, they think these adducts might be a potential catalyst. Although the overall barrier is lower than the mechanism suggested by Patil et al. it is also too high to be taken into consideration. Then, a new mechanism with a lower energy barrier (42.5 kcal/mol) is proposed that [OH]− initiated the reaction and the NHC, which is generated from [BMIm][OH], is the real catalyst for this process (Figure S12).
After that, in order to make catalyst's recycling easier, SiO2 supported [HMIm][OH] is used by Bhanage group in the reaction of 2-aminobenzonitriles and CO2 (Nale et al., 2014; Figure S11). This supported IL can be separated easily and only a minor change is found through FT-IR spectrum and surface areas analysis after using 3 times. Under optimized conditions, various electron-rich and electron-deficient groups on 2-aminobenzonitriles can react to give moderate to good yield.
Actually, imidazolium ILs are not stable under strong basicity conditions (Wang et al., 2017). Thus, many anions with weak basicity, such as acetate and azoles, are used instead of hydroxide. Han et al. discovers [Bmim][OAc] can catalyze the reaction of CO2 and 2-aminobenzonitriles even under atmospheric pressure of CO2 (Figure S13; Lu et al., 2014). Through screening different ILs, the anion in ILs is found to play a more significant role than the cation in this transformation. On the other hand, traditional organic/inorganic bases or non-functional ILs have no activity in this reaction.
Recently, Liu and coworkers utilize [BMIm][OAc] as catalyst in the cyclization of 2-aminothiophenols with CO2 and hydrosilane (Gao et al., 2015). A variety of benzothiazoles are obtained in moderate to excellent yield in the presence of [BMIm][OAc] and CO2 (Figure S13). Moreover, benzimidazoles can be also obtained in excellent yield by the same IL. Through 1H NMR analysis, [BMIm][OAc] is found to be capable of activating CO2, substrates and hydrosilane at the same time.
[BMIm][OAc] is found to promote the cross-link of chitosan by Guazzelli's group [Figure S13; (Mezzetta et al., 2017)]. Two amino groups in different chitosan chains can react with CO2 to form urea in [BMIm][OAc]. However, no cross-linked chitosan is found when using N-methyl-2-pyrrolidone as solvent, showing the importance of ILs. Moreover, the resulting products have good stability under different conditions that would have potential utility in drug delivery.
Azole-functionalized ILs are another kind of versatile ILs that can be utilized in the absorption of different gases (Wang et al., 2011a,b; Chen et al., 2015, 2016c; Cui et al., 2016). Also in the formation of urea, Deng and co-workers investigate the performance of various ILs in the reaction of CO2 with 1,6-hexamethylenediamine to form polyurea (Wang et al., 2016b). Since the importance of basicity of ILs is realized, [P4446][ATriz] is designed for this polymerization, and exhibits the best activity among the ILs used in this work (Figure S14).
Previous to this work, by using azole-functionalized ILs as catalysis, the group of Liu developes an efficient method to easily obtain α-ahydroxy ketones by the hydration of propargylic alcohol (Zhao et al., 2015; Figure S15). Azole-functionalized ILs are disclosed can promote this reaction even under atmospheric pressure of CO2. Based on the experimental and NMR investigations, the anion in IL, [Im]−, is proposed to capture CO2 at the first and then attack the triple bond of the propargylic alcohol to start the reaction.
In this mechanism, α-alkylidene cyclic carbonate is one of intermediates. Recently, the same group discovers that this reaction can stop at α-alkylidene cyclic carbonate when in an anhydrous conditions, while [P4444][2-MIm] showes the highest activity (Zhao et al., 2016; Figure S15). More importantly, the similar reaction condition can be also utilized in other reactions, such as CO2 with 2-aminobenzonitriles, o-phenylenediamines or 2-aminothiophenol. Since the ability to capture CO2 from atmosphere, [P4444][2-MIm] is believed to absorb CO2, then the formed carbamate intermediate would further react with substrates.
Although various kinds of substrates are suitable for this system, a high temperature (353 K) is needed to get α-alkylidene cyclic carbonate in that work. To lower down the energy demanding, they then use a new IL, [Bu4P]3[2,4-OPym-5-Ac], as catalyst in the cyclization reaction of propargylic alcohols with CO2 at ambient conditions (Wu et al., 2017; Figure S15). In addition, cations in ILs also exhibit a significant effect on this transformation, only the quaternary ammonium with C4 chain can it have good activity.
Apart from these ILs, tungstate ILs are found to be also able to catalyze this reaction under mild conditions (Kimura et al., 2012b). Mizuno group calculates the natural bond orbital (NBO) charges of O on different tungstates at the first, and [WO4]− is found to have more negative charges than others, suggesting that it should be the most basic among these tungstates. Then, in the reaction of 1,2-phenylenediamine with CO2 to give 2-benzimidazolone, [N4444]2[WO4] exhibits the best performance. Importantly, quinazoline-2,4(1H,3H)-diones, cyclic carbonates, and urea derivatives can be also obtained in good to excellent yield by using [N4444]2[WO4] as catalyst. Through 1H, 13C and 183W NMR spectra analysis, [WO4]− is identified can activate not only substrates but CO2. After that, this dual-functional role of [WO4] is also verified by DFT calculation in the reaction of CO2 and 2-aminobenzonitriles (Kimura et al., 2012a).
Bifunctionalized Ionic Liquids
As noted above, anion-functionalized ILs can promote the CO2 utilization because of their basicity. In many cases, however, cations in ILs are also helpful to lower down the energy barriers through forming hydrogen bond, and we call this kind of ILs as bifunctional ILs. Liu et al. have reported protic IL (PIL), [HDBU+][TFE], as catalyst that can simultaneously activate CO2 and aminobenzonitriles to synthesize of quinazoline-2,4(1H,3H)-diones (Zhao et al., 2014). In the mechanism analysis, the hydrogen bond between [HDBU] and substrates is found to facilitate the nucleophilic attack of substrates to CO2, which is activated by [TFE].
Using a similar IL, [DBUH][OAc], they disclose that o-phenylenediamines can react with CO2 to obtain benzimidazolones in mild condition (Yu et al., 2013). In this reaction, three DBU-ILs with different anion and [n-Bu-DBUH][OAc] are synthesized, then the activity is showed in the order: [DBUH][Cl] < [n-BuDBU][OAc] < [DBUH][Lac] < [DBUH][OAc]. NMR spectra demonstrate that [DBUH][OAc] acts as a bifunctional catalyst: the cation [HDBU] activated effectively CO2, while the nucleophilicity attack of o-phenylenediamine to CO2 is enhanced by hydrogen bond with [OAc] (Figure S16).
In 2014, Zheng et al. reported an IL-catalyzed route to fix CX2 (O, S) with 2-aminobenzonitriles for the synthesis of quinazoline-2,4(1H,3H)-diones and quinazoline-2,4(1H,3H)-dithiones (Zheng et al., 2014). The ILs can be formed via the mixture of DBU and ethanol under bubbling atmospheric pressure of CO2 or CS2 (Figure S17), which act as both catalyst and solvent in CX2 (O, S) conversion. As shown in previous, both of the cation and anion in this IL are significant in this reaction (Figure S18).
In 2015, Han and coworkers successfully developed a new method of synthesizing a series of 2-oxazolidinones from atmosphere CO2 and propargylic amines by utilizing [DBUH][MIm] as both catalyst and solvent under mild conditions [Figure S19; (Hu et al., 2015)]. Based on the DFT investigation, [MIm]− can capture and activate CO2, at the same time, the H atom on [DBUH]+ can attack the triple bond, which promotes effectively the intramolecular cyclization step (Figure S20).
Subsequently, Wang and co-workers firstly realize ILs-catalyzed one-pot domino hydration of diyne alcohols to synthesize 3-(2H)-furanones (Figure S21), where H2O acts as both a substrate and solvent under atmospheric pressure CO2 (Chen et al., 2016b). This is the first time to predict the catalytic activity of ILs through quantum calculation, and [HDBU][BenIm] shows more effective on catalytic activity than other ILs. Based on NMR spectroscopic investigation and DFT calculation, both cations and anions in ILs are important to keep a moderate basicity so as to reach an excellent reactivity.
Apart from DBU-based ILs, He et al. report carboxylative cyclization of 2-aminobenzonitriles with ambient CO2 by employing [HTMG][Im], as highly efficient and recyclable catalyst (Lang et al., 2016). This system has a broad substrate tolerance, and [HTMG][Im] exhibits excellent reusability.
Recently, Wang et al. developed an efficient strategy for generation of quinazoline-2,4(1H,3H)-diones from CO2 through varying the cations to design hydroxyl functionalized ILs, in which the catalytic activity is affected by the basicity of cation and the hydrogen bond from cation shows great promotion for this reaction (Shi et al., 2018). The aprotic IL (2-hydroxyethyl)-trimethyl-ammonium imidazole, [Ch][Im], gives the best catalytic activity. In addition, the high yield of quinazoline 2,4-(1H,3H)-dione can be obtained under one-gram scale and flue gas simulation system using [Ch][Im] as catalyst.
Conclusion and Outlook
As a sustainable C1 source, CO2 capture and utilization is an attractive field in view of environmental protection. Lots of strategies have been developed for the utilization of CO2, and the products have potential utility in biology and pharmacy. However, there is still a long way for most of these reactions to meet the requirement of industry, because of high cost and low efficiency. ILs-promoted CCU processes have attracted numerous attentions owning to ILs' unique properties. In most cases, ILs can be used as solvent, dehydrate, or catalyst, and these roles are similar in CCU processes. Thus, we hope to be able to shed light on all ILs-promoted CCU processes by using these limited but systematic examples. Owing to the nature of ILs, CO2-philic functional groups, such as oxygen atoms, and amine groups, could be incorporated into the anion and/or cation in ILs. Functional ILs show great potential for the absorption and conversion of CO2, and have been used as solvent, dehydrate, or catalyst in CCU processes. The anion-functionalized ILs exhibit better catalytic effect than the functionalization of cation part. We believe that ILs-promoted CCU protocol provides high efficient conversion of CO2 to synthesize a series of oxazolidinones, ureas, etc. We believe this ILs-promoted CCU protocol will be widely applied in CO2 chemistry, especially chemical utilization of CO2 to produce added-value commodity chemicals in industry.
However, a few of interesting or important fields are still needed to explore. At first, the efficiency of these processes needs to be improved, either new efficient CO2 utilized reaction or highly efficient and low-cost catalyst needs to be discovered. On the other hand, decreasing the cost of these reactions, such as lowering the pressure and temperature or using renewable energy (light or electricity), is a tendency for these researches. In addition, structures of ILs are related to their properties, which exhibit dramatically influence on their activity. Therefore, the investigation of the way structures affects their activity should have a profound significance in this field. Only when we obtain these answers, can we know how to design an efficient IL for specific reaction without basing on experience and experimental trial and error.
Author Contributions
All authors contributed for the writing of the manuscript. L-NH designed this proposal and determined the contents. S-MX wrote the Abstract, Introduction, Ag/Ionic Liquids Catalysis, and Bifunctionalized Ionic Liquids. H-CF wrote the Cu/Ionic Liquid Catalysis and Traditional Ionic Liquids Parts. K-HC wrote Anion-Functionalized Ionic Liquids, Conclusion and Outlook. K-HC, L-NH, and S-MX revised the manuscript.
Funding
This work was financially supported by National Key Research and Development Program (2016YFA0602900), the National Natural Science Foundation of China (21672119), the Natural Science Foundation of Tianjin Municipality (16JCZDJC39900).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2018.00462/full#supplementary-material
Abbreviations
[BMIm][PhSO3], 1-butyl-3-methylimidazolium benzenesulfonate; [BMIm][BF4], 1-n-butyl-3-methylimidazolium tetrafluoroborate; [(n-C7H15)4N][Br], tetraheptylammonium bromide; [P66614][DEIm], trihexyl(tetradecyl)phosphonium dimethyl 4,5-imidazoledicarboxylate; [Bmim][OAc], 1-butyl-3-methylimidazolium acetate; [Bmim][Cl], 1-n-butyl-3-methyl imidazolium chloride; [BMIm][Br], 1-n-butyl-3-methyl imidazolium bromide; [DMIm][BF4], 1-n-decyl-3-methylimidazolium tetrafluoroborate; [Bmim][OH], 1-n-butyl-3-methylimidazolium hydroxide; [BMIm][HSO4], 1-n-butyl-3-methylimidazolium hydrosulfate; [HMIm][OH], 1-hexyl-3-methylimidazolium hydroxide; [P4446][ATriz], hexyltributylphosphonium aminotriazole; [Bu4P]3[2,4-OPym-5-Ac], tritetrabutylphosphonium 2-oxidopyrimidine-5-carboxylate; [N4444]2[WO4], tetrabutylamine tungstate; DBU, 1,8-diazabicyclo[5.4.0]undec-7-ene; TFE, trifluoroethanol; [DBUH][TFE], 1,8-diazabicyclo[5.4.0]undec-7-ene trifluoroethanol; [DBUH][OAc], DBU acetate; [DBUH][Lac], DBU lactate; [DBUH][Cl], DBU chloride; [n-Bu-DBUH][OAc], n-butyl DBU acetate; [DBUH][MIm], DBU 2-methylimidazolide; [HDBU][BenIm], DBU Benzimidazole; [HTMG][Im], 1,1,3,3-tetramethylguanidinium imidazolide; [Ch][Im], (2-hydroxyethyl)-trimethyl-ammonium imidazole.
References
Aresta, M., Dibenedetto, A., and Angelini, A. (2014). Catalysis for the valorization of exhaust carbon: from CO2 to chemicals, materials, and fuels. Technological use of CO2. Chem. Rev. 114, 1709–1742. doi: 10.1021/cr4002758
Bates, E. D., Mayton, R. D., Ntai, I., and Davis, J. H. (2002). CO2 capture by a task-specific ionic liquid. J. Am. Chem. Soc. 124, 926–927. doi: 10.1021/ja017593d
Blanchard, L. A., Hancu, D., Beckman, E. J., and Brennecke, J. F. (1999). Green processing using ionic liquids and CO2. Nature 399, 28–29. doi: 10.1038/19887
Chen, K. H., Lin, W. J., Yu, X. N., Luo, X. Y., Ding, F., He, X., et al. (2015). Designing of anion-functionalized ionic liquids for efficient capture of SO2 from flue gas. AIChE J. 61, 2028–2034. doi: 10.1002/aic.14793
Chen, K. H., Shi, G. L., Dao, R. N., Mei, K., Zhou, X. Y., Li, H. R., et al. (2016a). Tuning the basicity of ionic liquids for efficient synthesis of alkylidene carbonates from CO2 at atmospheric pressure. Chem. Commun. 52, 7830–7833. doi: 10.1039/C6CC02853E
Chen, K. H., Shi, G. L., Zhang, W. D., Li, H. R., and Wang, C. M. (2016b). Computer-assisted design of ionic liquids for efficient synthesis of 3(2H)-furanones: a domino reaction triggered by CO2. J. Am. Chem. Soc. 138, 14198–14201. doi: 10.1021/jacs.6b08895
Chen, K. H., Shi, G. L., Zhou, X. Y., Li, H. R., and Wang, C. M. (2016c). Highly efficient nitric oxide capture by azole-based ionic liquids through multiple-site absorption. Angew. Chem. Int. Ed. 55, 14362–14366. doi: 10.1002/anie.201607528
Cui, G. K., Wang, J. J., and Zhang, S. J. (2016). Active chemisorption sites in functionalized ionic liquids for carbon capture. Chem. Soc. Rev. 45, 4307–4339. doi: 10.1039/C5CS00462D
Fujita, S., Kanamaru, H., Senboku, H., and Arai, M. (2006). Preparation of cyclic urethanes from amino alcohols and carbon dioxide using ionic liquid catalysts with alkali metal promoters. Int. J. Mol. Sci. 7, 438–450. doi: 10.3390/i7100438
Gao, X., Yu, B., Yang, Z. Z., Zhao, Y. F., Zhang, H. G., Hao, L. D., et al. (2015). Ionic liquid-catalyzed C-S bond construction using CO2 as a C1 building block under mild conditions: a metal-free route to synthesis of benzothiazoles. ACS Catal. 5, 6648–6652. doi: 10.1021/acscatal.5b01874
Gu, Y. L., Shi, F., and Deng, Y. Q. (2004). Ionic liquid as an efficient promoting medium for fixation of CO2: clean synthesis of alpha-methylene cyclic carbonates from CO2 and propargyl alcohols catalyzed by metal salts under mild conditions. J. Org. Chem. 69, 391–394. doi: 10.1021/jo0351365
Gu, Y. L., Zhang, Q. H., Duan, Z. Y., Zhang, J., Zhang, S. G., and Deng, Y. Q. (2005). Ionic liquid as an efficient promoting medium for fixation of carbon dioxide: a clean method for the synthesis of 5-methylene-1,3-oxazolidin-2-ones from propargylic alcohols, amines, and carbon dioxide catalyzed by CUM under mild conditions. J. Org. Chem. 70, 7376–7380. doi: 10.1021/jo050802i
Gurkan, B. E., de la Fuente, J. C., Mindrup, E. M., Ficke, L. E., Goodrich, B. F., Price, E. A., et al. (2010). Equimolar CO2 absorption by anion-functionalized ionic liquids. J. Am. Chem. Soc. 132, 2116–2117. doi: 10.1021/ja909305t
Hao, L. D., Zhao, Y. F., Yu, B., Yang, Z. Z., Zhang, H. Y., Han, B. X., et al. (2015). Imidazolium-based ionic liquids catalyzed formylation of amines using carbon dioxide and phenylsilane at room temperature. ACS Catal. 5, 4989–4993. doi: 10.1021/acscatal.5b01274
He, L. N., Wang, J. Q., and Wang, J. L. (2009). Carbon dioxide chemistry: examples and challenges in chemical utilization of carbon dioxide. Pure Appl. Chem. 81, 2069–2080. doi: 10.1351/PAC-CON-08-10-22
He, L. N., Yang, Z. Z., Liu, A. H., and Gao, J. (2010). “CO2 chemistry at nankai group: catalytic conversion of CO2 into value-added chemicals,” in Advances in CO2 Conversion and Utilization, ed Y. H. Hu (Washington, DC: American Chemical Society), 77–101.
Hu, J. Y., Ma, J., Lu, L. G., Qian, Q. L., Zhang, Z. F., Xie, C., et al. (2017). Synthesis of asymmetrical organic carbonates using CO2 as a feedstock in AgCl/ionic liquid system at ambient conditions. Chemsuschem 10, 1292–1297. doi: 10.1002/cssc.201601773
Hu, J. Y., Ma, J., Zhu, Q. G., Zhang, Z. F., Wu, C. Y., and Han, B. X. (2015). Transformation of atmospheric CO2 catalyzed by protic ionic liquids: efficient synthesis of 2-Oxazolidinones. Angew. Chem. Int. Ed. 54, 5399–5403. doi: 10.1002/anie.201411969
Jessop, P. G., Heldebrant, D. J., Li, X. W., Eckert, C. A., and Liotta, C. L. (2005). Green chemistry - reversible nonpolar-to-polar solvent. Nat. 436, 1102–1102. doi: 10.1038/4361102a
Jutz, F., Andanson, J. M., and Baiker, A. (2011). Ionic liquids and dense carbon dioxide: a beneficial biphasic system for catalysis. Chem. Rev. 111, 322–353. doi: 10.1021/cr100194q
Kimura, T., Kamata, K., and Mizuno, N. (2012a). A bifunctional tungstate catalyst for chemical fixation of CO2 at atmospheric pressure. Angew. Chem. Int. Ed. 51, 6700–6703. doi: 10.1002/anie.201203189
Kimura, T., Sunaba, H., Kamata, K., and Mizuno, N. (2012b). Efficient [WO4](2-)-catalyzed chemical fixation of carbon dioxide with 2-aminobenzonitriles to quinazoline-2,4(1H,3H)-diones. Inorg. Chem. 51, 13001–13008. doi: 10.1021/ic302110a
Lang, X. D., Yu, Y. C., Li, Z. M., and He, L. N. (2016). Protic ionic liquids-promoted efficient synthesis of quinazolines from 2-aminobenzonitriles and CO2 at ambient conditions. J. CO2 Util. 15, 115–122. doi: 10.1016/j.jcou.2016.03.002
Lei, Z. G., Dai, C. N., and Chen, B. H. (2014). Gas solubility in ionic liquids. Chem. Rev. 114, 1289–1326. doi: 10.1021/cr300497a
Li, J. A., Guo, X. G., Wang, L. G., Ma, X. Y., Zhang, Q. H., Shi, F., et al. (2010). Co(acac)3/BMMImCl as a base-free catalyst system for clean syntheses of N,N '-disubstituted ureas from amines and CO2. Sci. China Chem. 53, 1534–1540. doi: 10.1007/s11426-010-4026-8
Liu, M. S., Gao, K. Q., Liang, L., Sun, J. M., Sheng, L., and Arai, M. (2016a). Experimental and theoretical insights into binary Zn-SBA-15/KI catalysts for the selective coupling of CO2 and epoxides into cyclic carbonates under mild conditions. Catal. Sci. Technol. 6, 6406–6416. doi: 10.1039/C6CY00725B
Liu, M. S., Lan, J. W., Liang, L., Sun, J. M., and Arai, M. (2017a). Heterogeneous catalytic conversion of CO2 and epoxides to cyclic carbonates over multifunctional tri-s-triazine terminal-linked ionic liquids. J.Catal. 347, 138–147. doi: 10.1016/j.jcat.2016.11.038
Liu, M. S., Liang, L., Li, X., Gao, X. X., and Sun, J. M. (2016b). Novel urea derivative-based ionic liquids with dual-functions: CO2 capture and conversion under metal- and solvent-free conditions. Green Chem. 18, 2851–2863. doi: 10.1039/C5GC02605A
Liu, M. S., Lu, X. Y., Shi, L., Wang, F. X., and Sun, J. (2017b). Periodic mesoporous organosilica with a basic urea-derived framework for enhanced carbon dioxide capture and conversion under mild conditions. ChemSusChem 10, 1110–1119. doi: 10.1002/cssc.201600973
Liu, Q., Wu, L. P., Jackstell, R., and Beller, M. (2015). Using carbon dioxide as a building block in organic synthesis. Nat. Commun. 6:5933. doi: 10.1038/ncomms6933
Lu, W. J., Ma, J., Hu, J. Y., Song, J. L., Zhang, Z. F., Yang, G. Y., et al. (2014). Efficient synthesis of quinazoline-2,4(1H,3H)-diones from CO2 using ionic liquids as a dual solvent-catalyst at atmospheric pressure. Green Chem. 16, 221–225. doi: 10.1039/C3GC41467A
Ma, Y., Chen, C., Wang, T. F., Zhang, J. S., Wu, J. J., Liu, X. D., et al. (2017). Dialkylpyrazolium ionic liquids as novel catalyst for efficient fixation of CO2 with metal- and solvent-free. Appl. Catal. A Gen. 547, 265–273. doi: 10.1016/j.apcata.2017.09.009
Mezzetta, A., Guazzelli, L., and Chiappe, C. (2017). Access to cross-linked chitosans by exploiting CO2 and the double solvent-catalytic effect of ionic liquids. Green Chem. 19, 1235–1239. doi: 10.1039/C6GC02935C
Nale, D. B., Saigaonkar, S. D., and Bhanage, B. M. (2014). An efficient synthesis of quinazoline-2,4(1H,3H)-dione from CO2 and 2-aminobenzonitrile using [Hmim]OH/SiO2 as a base functionalized supported ionic liquid phase catalyst. J. CO2 Util. 8, 67–73. doi: 10.1016/j.jcou.2014.08.001
Patil, Y. P., Tambade, P. J., Deshmukh, K. M., and Bhanage, B. M. (2009). Synthesis of quinazoline-2,4(1H,3H)-diones from carbon dioxide and 2-aminobenzonitriles using [Bmim]OH as a homogeneous recyclable catalyst. Catal. Today 148, 355–360. doi: 10.1016/j.cattod.2009.06.010
Ren, Y., Meng, T. T., Jia, J. F., and Wu, H. S. (2011). A computational study on the chemical fixation of carbon dioxide with 2-aminobenzonitrile catalyzed by 1-butyl-3-methyl imidazolium hydroxide ionic liquids. Comput. Theor. Chem. 978, 47–56. doi: 10.1016/j.comptc.2011.09.032
Shi, F., Deng, Y. Q., SiMa, T. L., Peng, J. J., Gu, Y. L., and Qiao, B. T. (2003). Alternatives to phosgene and carbon monoxide: synthesis of symmetric urea derivatives with carbon dioxide in ionic liquids. Angew. Chem. Int. Ed. 42, 3257–3260. doi: 10.1002/anie.200351098
Shi, G. L., Chen, K. H., Wang, Y. T., Li, H. R., and Wang, C. M. (2018). Highly efficient synthesis of quinazoline-2,4(1H,3H)-diones from CO2 by hydroxyl functionalized aprotic Ionic Liquids. ACS Sust. Chem. Eng. 6, 5760–5765. doi: 10.1021/acssuschemeng.8b01109
Song, Q. W., and He, L. N. (2016). Robust silver(I) catalyst for the carboxylative cyclization of propargylic alcohols with carbon dioxide under ambient conditions. Adv. Synth. Catal. 358, 1251–1258. doi: 10.1002/adsc.201500639
Song, Q. W., Zhou, Z. H., and He, L. N. (2017). Efficient, selective and sustainable catalysis of carbon dioxide. Green Chem. 19, 3707–3728. doi: 10.1039/C7GC00199A
Steckel, J. A. (2012). Ab initio calculations of the interaction between CO2 and the acetate ion. J. Phys. Chem. A 116, 11643–11650. doi: 10.1021/jp306446d
Wang, B. S., Qin, L., Mu, T. C., Xue, Z. M., and Gao, G. H. (2017). Are ionic liquids chemically stable? Chem. Rev. 117, 7113–7131. doi: 10.1021/acs.chemrev.6b00594
Wang, C. M., Cui, G. K., Luo, X. Y., Xu, Y. J., Li, H. R., and Dai, S. (2011a). Highly efficient and reversible SO2 capture by tunable azole-based ionic liquids through multiple-site chemical absorption. J. Am. Chem. Soc. 133, 11916–11919. doi: 10.1021/ja204808h
Wang, C. M., Luo, X. Y., Luo, H. M., Jiang, D. E., Li, H. R., and Dai, S. (2011b). Tuning the basicity of Ionic liquids for equimolar CO2 capture. Angew. Chem. Int. Ed. 50, 4918–4922. doi: 10.1002/anie.201008151
Wang, M. Y., Song, Q. W., Ma, R., Xie, J. N., and He, L. N. (2016a). Efficient conversion of carbon dioxide at atmospheric pressure to 2-oxazolidinones promoted by bifunctional Cu(II)-substituted polyoxometalate-based ionic liquids. Green Chem. 18, 282–287. doi: 10.1039/C5GC02311D
Wang, P. X., Ma, X. Y., Li, Q. H., Yang, B. Q., Shang, J. P., and Deng, Y. Q. (2016b). Green synthesis of polyureas from CO2 and diamines with a functional ionic liquid as the catalyst. RSC Adv. 6, 54013–54019. doi: 10.1039/C6RA07452A
Wang, T. F., Zheng, D. N., Zhang, J. S., Fan, B. W., Ma, Y., Ren, T. G., et al. (2018). Protic pyrazolium ionic liquids: an efficient catalyst for conversion of CO2 in the absence of metal and solvent. ACS Sust. Chem. Eng. 6, 2574–2582. doi: 10.1021/acssuschemeng.7b04051
Wu, Y. Y., Zhao, Y. F., Li, R. P., Yu, B., Chen, Y., Liu, X. W., et al. (2017). Tetrabutylphosphonium-based ionic liquid catalyzed CO2 transformation at ambient conditions: a case of synthesis of alpha-alkylidene cyclic carbonates. ACS Catal. 7, 6251–6255. doi: 10.1021/acscatal.7b01422
Yang, Z. Z., He, L. N., Gao, J., Liu, A. H., and Yu, B. (2012). Carbon dioxide utilization with C–N bond formation: carbon dioxide capture and subsequent conversion. Energy Environ. Sci. 5, 6602. doi: 10.1039/c2ee02774g
Yang, Z. Z., He, L. N., Zhao, Y. N., Li, B., and Yu, B. (2011a). CO2 capture and activation by superbase/polyethylene glycol and its subsequent conversion. Energy Environ. Sci. 4, 3971–3975. doi: 10.1039/c1ee02156g
Yang, Z. Z., Zhao, Y. N., and He, L. N. (2011b). CO2 chemistry: task-specific ionic liquids for CO2 capture/activation and subsequent conversion. RSC Adv. 1, 545–567. doi: 10.1039/c1ra00307k
Yu, B., Zhang, H. Y., Zhao, Y. F., Chen, S., Xu, J. L., Hao, L. D., et al. (2013). DBU-based ionic-liquid-catalyzed carbonylation of o-phenylenediamines with CO2 to 2-benzimidazolones under solvent-free conditions. ACS Catal. 3, 2076–2082. doi: 10.1021/cs400256j
Yuan, Y., Xie, Y., Zeng, C., Song, D. D., Chaemchuen, S., Chen, C., et al. (2017). A recyclable AgI/OAc− catalytic system for the efficient synthesis of α-alkylidene cyclic carbonates: carbon dioxide conversion at atmospheric pressure. Green Chem. 19, 2936–2940. doi: 10.1039/C7GC00276A
Zhang, Q. H., Shi, F., Gu, Y. L., Yang, J., and Deng, Y. Q. (2005). Efficient and eco-friendly process for the synthesis of N-substituted 4-methylene-2-oxazolidinones in ionic liquids. Tetrahedron Lett. 46, 5907–5911. doi: 10.1016/j.tetlet.2005.06.116
Zhang, S. J., Sun, N., He, X. Z., Lu, X. M., and Zhang, X. P. (2006). Physical properties of ionic liquids: database and evaluation. J. Phys. Chem. Ref. Data 35, 1475–1517. doi: 10.1063/1.2204959
Zhang, Z. F., Xie, E., Li, W. J., Hu, S. Q., Song, J. L., Jiang, T., et al. (2008). Hydrogenation of carbon dioxide is promoted by a task-specific ionic liquid. Angew. Chem. Int. Ed. 47, 1127–1129. doi: 10.1002/anie.200704487
Zhao, Y. F., Wu, Y. Y., Yuan, G. F., Hao, L. D., Gao, X., Yang, Z. Z., et al. (2016). Azole-anion-based aprotic ionic liquids: functional solvents for atmospheric CO2 transformation into various heterocyclic compounds. Chem. Asian J. 11, 2735–2740. doi: 10.1002/asia.201600281
Zhao, Y. F., Yang, Z. Z., Yu, B., Zhang, H. Y., Xu, H. J., Hao, L. D., et al. (2015). Task-specific ionic liquid and CO2-cocatalysed efficient hydration of propargylic alcohols to alpha-hydroxy ketones. Chem. Sci. 6, 2297–2301. doi: 10.1039/C5SC00040H
Zhao, Y. F., Yu, B., Yang, Z. Z., Zhang, H. Y., Hao, L. D., Gao, X., et al. (2014). A protic ionic liquid catalyzes CO2 conversion at atmospheric pressure and room temperature: synthesis of quinazoline-2,4-(1H,3H)-diones. Angew. Chem. Int. Ed. 53, 5922–5925. doi: 10.1002/anie.201400521
Keywords: CO2 conversion, carboxylative cyclization, catalysis, ionic liquids, green chemistry
Citation: Xia S-M, Chen K-H, Fu H-C and He L-N (2018) Ionic Liquids Catalysis for Carbon Dioxide Conversion With Nucleophiles. Front. Chem. 6:462. doi: 10.3389/fchem.2018.00462
Received: 13 August 2018; Accepted: 14 September 2018;
Published: 08 October 2018.
Edited by:
Francesca D'Anna, Università degli Studi di Palermo, ItalyReviewed by:
Jianmin Sun, Harbin Institute of Technology, ChinaJinglai Zhang, Henan University, China
Copyright © 2018 Xia, Chen, Fu and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liang-Nian He, aGVsbkBuYW5rYWkuZWR1LmNu
 Shu-Mei Xia
Shu-Mei Xia Kai-Hong Chen
Kai-Hong Chen Hong-Chen Fu
Hong-Chen Fu Liang-Nian He
Liang-Nian He