- 1South China Institute of Environmental Sciences, The Ministry of Environment Protection of PRC, Guangzhou, China
- 2Graduate School at Shenzhen, Research Institute of Environmental Engineering and Nano-Technology, Tsinghua University, Shenzhen, China
- 3Shenzhen Environmental Science and New Energy Technology Engineering Laboratory, Tsinghua-Berkeley Shenzhen Institute, Shenzhen, China
Hypophosphite wastewater treatment is still a critical issue in metallurgical processes and the oxidation of hypophosphite to phosphate followed by the precipitation of phosphate is an important strategy for hypophosphite wastewater treatment. Herein, Ti4O7/g-C3N4 photocatalysts with various mass ratios (Ti4O7 (m): g-C3N4 (m) = 0.5, 0.2, 0.1, and 0.05) were synthesized by a hydrolysis method and the effect of the mass ratio of Ti4O7 (m): g-C3N4 (m) on Ti4O7/g-C3N4 visible light photocatalytic oxidation of hypophosphite was evaluated. The as-prepared Ti4O7/g-C3N4 were characterized and confirmed by SEM, XPS, XRD and FTIR. Moreover, the specific surface area and the distribution of pore size of Ti4O7/g-C3N4 was also analyzed. Our results showed that Ti4O7/g-C3N4 exhibited remarkably improved photocatalytic performance on hypophosphite oxidation compared with g-C3N4 and meanwhile 1:2-Ti4O7/g-C3N4 with a mass ratio of 0.5 showed the best photocatalytic performance with the highest oxidation rate constant (17.7-fold and 91.0-fold higher than that of pure g-C3N4 and Ti4O7, respectively). The enhanced performance of photocatalytic oxidation of hypophosphite was ascribed to the heterojunction structure of Ti4O7/g-C3N4 with broader light absorption and significantly enhanced efficiency of the charge carrier (e−-h+) generation and separation. Additionally, the generated ·OH and · radicals contributed to the hypophosphite oxidation during the photocatalytic system.
Introduction
Hypophosphite wastewater is produced in metallurgical processes where hypophosphite is a widely used reducing reagent for chemical nickel deposition (Gan et al., 2007; Huang et al., 2009). The discharge of hypophosphite wastewater may result in eutrophication and therefore the further treatment is required (Piveteau et al., 2017; Tian et al., 2017). Coagulants such as Fe have been widely used for phosphorus removal (Shih et al., 2013), however, the hypophosphite precipitants are not stable due to the high solubility constant (Zhao et al., 2017). As such, the pre-oxidation of hypophosphite to phosphate is very important for hypophosphite wastewater treatment so as to facilitate the following precipitation of phosphate in the form of insoluble salts precipitates.
Photocatalysis is considered to be a useful technology for water treatment with advantages of energy-free by using solar energy and high oxidation efficiency of pollutants by hydroxyl radicals (·OH) and superoxide radicals (·) generated during the photocatalytic process (Hao et al., 2017). The most commonly used TiO2 photocatalyst, however, is greatly limited in wide applications especially under visible light or sunlight due to its main drawback of wide band gap (3.2 eV) (Hao et al., 2016; Ma et al., 2018). Therefore, photocatalysts with wide range of response wavelength as well as good photogenerated charge separation properties are urgently required for photocatalytic applications.
Recently, graphite-like carbon nitride (g-C3N4) has attracted lots of attentions due to its advantages including small band gap of 2.73 eV, robust chemical stability over a wide pH range of 0–14 (Zhang et al., 2014; Li et al., 2016a,b). Nevertheless, limitations including low surface area and poor photogenerated charge separation properties still hinder the photocatalytic applications of g-C3N4 (Shao et al., 2017). Preparation of g-C3N4-based heterojunction is an alternative pathway to facilitate the enhancement of charge separation and photocatalytic performance (Masih et al., 2017). For instance, photocatalysts with p–n junction exhibited excellent photocatalytic performance in environmental and energy applications (Huang et al., 2015a,b, 2016; Zhang et al., 2018).
Magnéli phase titanium suboxides (TinO2n−1) are substoichiometric titanium oxides, where n is an integer between 4 and 10 (i.e., 4, 5, 6, and 8) (Zaky and Chaplin, 2014). Among various compositions of TinO2n−1, Ti4O7 has the properties of best conductivity (1,500 S cm−1) and the robust resistance to aggressive chemical conditions (Ganiyu et al., 2016). Construction of g-C3N4/Ti4O7 heterojunction significantly improved the photocatalytic performance as a result of the effective enhancement of charge separation was reported in our previous work (Guan et al., 2018). However, the effect of the mass ratio of Ti4O7 (m): g-C3N4 (m) of the g-C3N4/Ti4O7 heterojunction on photocatalytic performance is still unknown. In this study, the effect of the mass ratio of Ti4O7/g-C3N4 (Ti4O7 (m): g-C3N4 (m) = 0.5, 0.2, 0.1 and 0.05) on photocatalytic oxidation of hypophosphite was evaluated in view of the photocatalytic performance, optical and electrochemical properties as well as the contributions of ·OH and · radicals generated during the photocatalytic process. Our results indicated that Ti4O7/g-C3N4 with a mass ratio of 0.5 showed the highest rate constant of photocatalytic oxidation of hypophosphite (17.7-fold and 91.0-fold higher than that of pure g-C3N4 and Ti4O7, respectively).
Materials and Methods
Chemicals
The reagents used for the preparation and performance characterization of g-C3N4/Ti4O7 were analytical grade and included sub-stoichiometric titanium oxide, melamine, urea, sodium hypophosphite, sodium sulfate, isopropanol, sodium hydroxide, sulfuric acid. All chemicals were bought from Sinopharm Chemical Reagent Co., Ltd. (Beijing, China). In addition, all solutions were prepared using freshly prepared Milli-Q water (Millipore, 18.2 MΩ cm).
Preparation of Ti4O7/g-C3N4 Photocatalysts
Graphite-like carbon nitride was prepared first using a liquid-based growth method (Sun et al., 2018). Then, g-C3N4 (2 g) and Ti4O7 (1, 0.4, 0.2, 0.1 g) were well mixed in the 0.1 mol/L NaOH solution (100 mL) using ultrasonication following by the annealing procedure at 160°C for 20 h. Subsequently, the as-prepared g-C3N4/Ti4O7 with various mass ratios (Ti4O7 (m): g-C3N4 (m) = 0.5, 0.2, 0.1, and 0.05) were dried at 60°C for 12 h before usage and noted as 1:2-Ti4O7/g-C3N4, 1:5-Ti4O7/g-C3N4, 1:10-Ti4O7/g-C3N4 and 1:20-Ti4O7/g-C3N4, respectively.
Analysis and Test Methods
The as-prepared g-C3N4/Ti4O7 were characterized by scanning electron microscopy (SEM) (JEOL JSM-6701F), X-ray photoelectron spectroscopy (XPS) (Phi Quantern instrument with C 1s peak (284.8 eV) as the calibrated reference), X-ray diffraction (XRD) (model D/max RA, Rigaku Co., Japan), and fourier transforms infrared spectroscopy spectra (FTIR) (Bruker Tensor-27). The specific surface area and the pore size distribution of the as-prepared g-C3N4/Ti4O7 were calculated by Brunauer-Emmett-Teller (BET) equation and Barrett-Joyner-Halenda (BJH) method according to the N2 adsorption/desorption isotherms. The optical and electrochemical properties of the as-prepared g-C3N4/Ti4O7 were evaluated by Ultraviolet-visible diffraction spectra (UV-vis DRS) (UV-2450, Shimadzu, Japan) and CHI 660B electrochemical system in view of photocurrent (PC), cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). The contributions of ·OH and · radicals generated during the visible light photocatalytic process were identified by comparing the efficiencies of hypophosphite oxidation in the absence and presence of isopropanol (IPA) and N2 purging.
Analysis of Photocatalytic Performance
The photocatalytic performance of g-C3N4/Ti4O7 was characterized by photocatalytic oxidation of hypophosphite with the concentration of hypophosphite measured by ion chromatography using a 732 IC detector. A metal-halide lamp (35 W, Philips) with the light strength of ~5 mW cm−2 was used as the light source and a UV-cutoff filter of 420 nm was used to provide the visible light with the wavelength over 420 nm. Before the photocatalytic experiments, g-C3N4/Ti4O7 with various mass ratios (10 mg) dispersed in 100 mg L−1 hypophosphite aqueous solution (100 mL) were continuously stirred for 30 min in the dark to achieve the adsorption–desorption equilibrium. After that, the visible light photocatalytic oxidation of hypophosphite was conducted with the above solutions exposing to the visible light irradiation and sampling conducted at 1 h intervals over 6 h experimental period. More detailed information about the photocatalytic experiment can be found in our most recent paper (Guan et al., 2018). The oxidation efficiency of hypophosphite (η) was calculated using the following equation (Guan et al., 2017):
where C0 and Ct represent the concentrations of hypophosphite at initial and given time, respectively.
Results and Discussion
Materials Characterization
The SEM images of pure g-C3N4 and Ti4O7/g-C3N4 photocatalysts were shown in Figure 1 that g-C3N4 had a sheet-like structure (Figure 1A) and spheroidal Ti4O7 crystals were deposited on the surface of C3N4 (Figure 1B). The corresponding XPS high resolution spectra of Ti4O7/g-C3N4 were analyzed as shown in Figure 1. There were two components for the XPS spectra of C 1s core level (Figure 1C), the standard reference carbon (284.8 eV) and the sp2 bonded C in N=C(–N)2 (288.3 eV) (Jo and Natarajan, 2015). With regard to the N 1s spectra, there were three peaks (Figure 1D), the main peak at 398.8 eV assigned to sp2 nitrogen (C=N–C) involved in triazine rings, the peak at 400.0 eV originated from the tertiary nitrogen bonded to carbon atoms in the form of N–(C)3 (Wu et al., 2013) and the peak at 401.3 eV ascribed to amino functions (C–N–H) (Gao et al., 2014). These assignments of C 1s and N 1s were agreed well with the XPS results of g-C3N4 reported previously. Meanwhile, Ti4O7 is a mixed-valence compound with two evenly occupied Ti4+ (3d0) and Ti3+ (3d1) configurations. Four peaks were observed for Ti 2p spectra (Figure 1E) that two broad peaks at 458.6 eV and 464.7 eV were respectively assigned to Ti 2p1/2 and Ti 2p3/2 peaks of Ti4+, and another two peaks at 457.9 eV and 463.8 eV were assigned to Ti3+ (Zeng et al., 2017). In terms of the O 1s spectra, three peaks were observed (Figure 1F) that the peak at 533.5 eV was assigned to the C–O functional groups, and the peaks at 531.8 and 529.7 eV were ascribed to the OH–Ti and O–Ti bonds (Li et al., 2017). The XPS results confirmed the presence of Ti4O7 on the surface of g-C3N4 with covalent bonds.
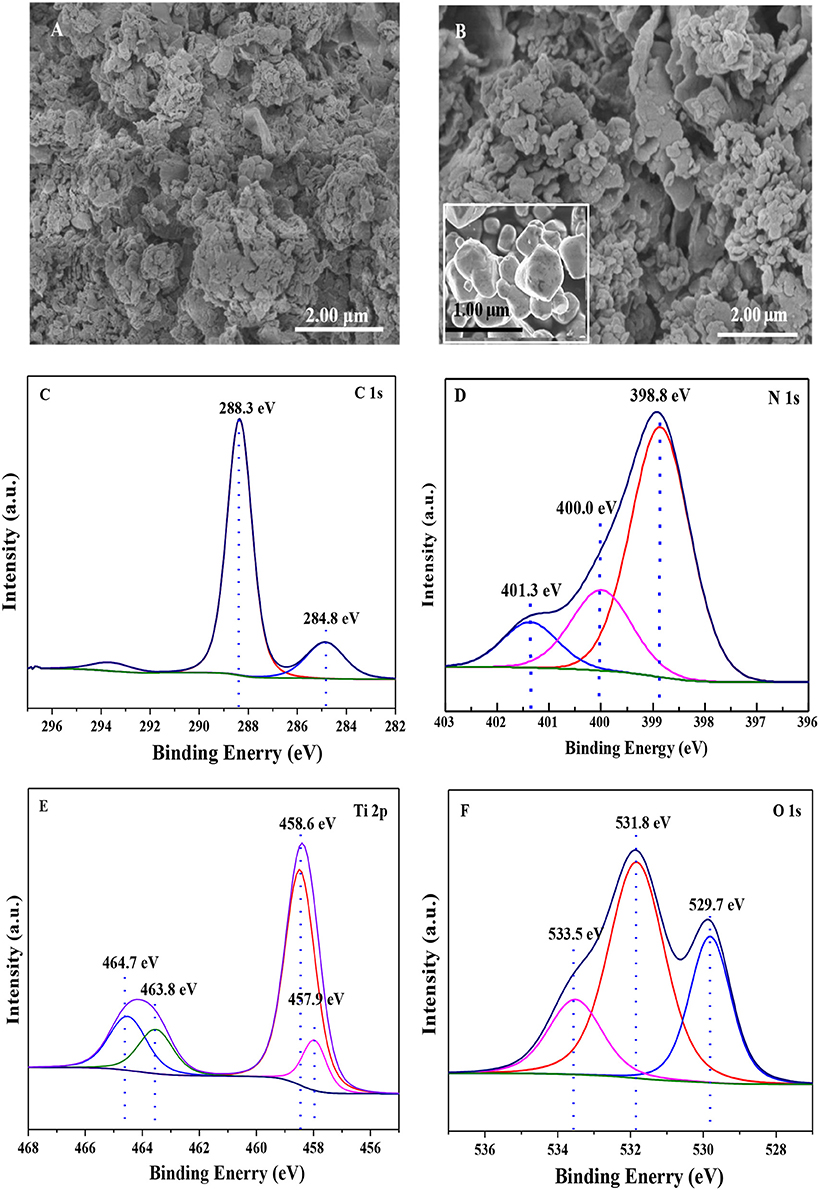
Figure 1. The SEM images of (A) g-C3N4, (B) Ti4O7/g-C3N4 photocatalysts; and XPS spectrum of (C) C 1s, (D) N 1s, (E) Ti 2p, (F) O 1s of Ti4O7/g-C3N4 photocatalyst.
The XRD phase structures of Ti4O7/g-C3N4 photocatalysts with various mass ratios were shown in Figure 2A. Peaks at 13.10° and 27.40° were indexed as (1 0 0) plane of tri-s-triazine units and (0 0 2) plane of the conjugated aromatic system of g-C3N4, respectively (Liang and Zhu, 2016). Meanwhile, major peaks of Ti4O7 including 20.7, 26.3, 29.5, 31.7, 34.0, 36.3, 40.5, 53.1, 55.0, 63.8, and 66.4° were also found (Guo et al., 2016). With increase of the mass ratio of Ti4O7 (m): g-C3N4 (m), the intensity of the Ti4O7 peaks became stronger while that of the C3N4 peaks became weaker. Furthermore, Ti4O7/g-C3N4 especially 1:2-Ti4O7/g-C3N4 (mass ratio of Ti4O7/g-C3N4 of 0.5) matched well with the reference of pure Ti4O7 and g-C3N4, indicating that the main structures of Ti4O7 and g-C3N4 were not destroyed during the synthesis process of Ti4O7/g-C3N4.
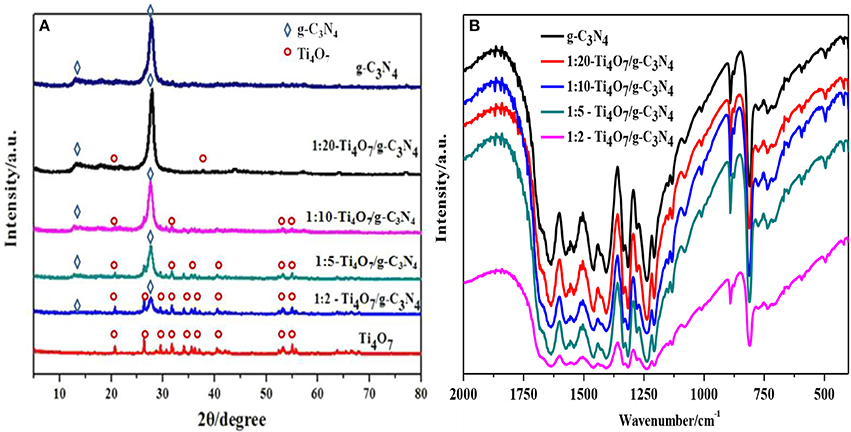
Figure 2. Structure and morphology analysis of g-C3N4 and Ti4O7/g-C3N4 photocatalysts: (A) XRD patterns analysis; (B) FTIR spectrometer analysis.
The as-prepared Ti4O7/g-C3N4 photocatalysts with various mass ratios were further characterized by FTIR as shown in Figure 2B. Typical absorption peaks of 1,230–1,630 cm−1 were attributed to tri-s-triazine ring moieties of g-C3N4. For example, the absorption peaks of 1,638 and 1,570 cm−1 were related to C = N stretching, and the peaks of 1,474, 1,410, 1,322, 1,241 cm−1 were attributed to C–N stretching. Meanwhile, the sharp peak of 810 cm−1 was due to the bending vibration of heptazine rings, indicating that the heptazine units might exist in C3N4 (Hatamie et al., 2018). With increase of the mass ratio of Ti4O7/g-C3N4, the absorption intensity related to the vibrational bands of g-C3N4 became weaker. The presence of Ti4O7 in Ti4O7/g-C3N4 restricted the evolution trend of g-C3N4 and thus alleviated the severe stacking of aromatic units of g-C3N4. Additionally, the delocalized π-π conjugated electronic system of g-C3N4 facilitated the transfer of photogenerated electron-hole pairs during the photocatalytic process (Jourshabani et al., 2018).
The nitrogen adsorption-desorption isotherms and pore size distribution of pure Ti4O7, g-C3N4 and 1:2-Ti4O7/g-C3N4 photocatalysts were shown in Figure 3. The specific surface area calculated according to Figure 3A was 116.0, 56.5, and 174.0 m2·g−1 for Ti4O7, g-C3N4 and 1:2-Ti4O7/g-C3N4, respectively. Generally, a catalyst with larger surface area could offer more active sites for adsorption and photodegradation toward organic pollutants, resulting in an enhanced photodecomposition activity (Dong et al., 2015). As such, compared with pure Ti4O7 and g-C3N4, 1:2-Ti4O7/g-C3N4 with larger specific surface area would facilitate photocatalytic oxidation of hypophosphite. Moreover, the pore size distribution of 1:2-Ti4O7/g-C3N4 was between 5 and 15 nm and that of pure Ti4O7 was mainly in the range of 10–25 nm, while no obvious mesopores were observed for g-C3N4 (Figure 3B).
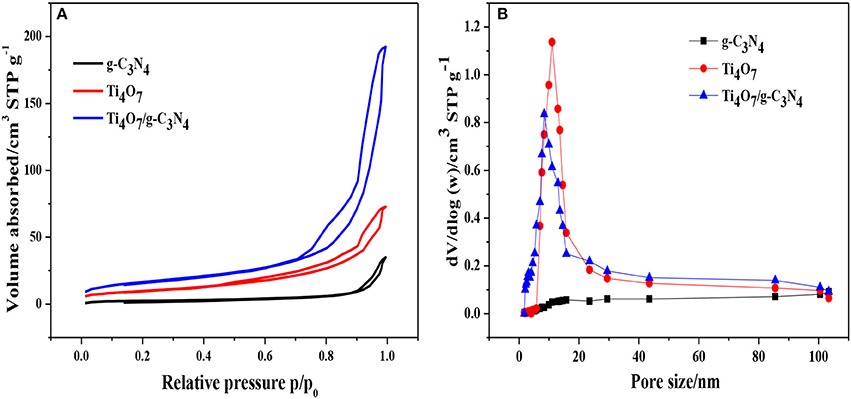
Figure 3. (A) N2 adsorption-desorption isotherms and (B) pore size distribution of g-C3N4, pure Ti4O7 and 1:2-Ti4O7/g-C3N4 photocatalysts.
Photocatalytic Performance Analysis
The photocatalytic performance of different photocatalysts was analyzed and compared as shown in Figure 4A. Ti4O7/g-C3N4 exhibited the significantly improved efficiency in hypophosphite oxidation under visible light irradiation (λ > 420 nm) in comparison to the pure g-C3N4 and Ti4O7. Additionally, the oxidation efficiency of hypophosphite was increased with increase of the mass ratio of Ti4O7/g-C3N4 and 1:2-Ti4O7/g-C3N4 illustrated the best photocatalytic performance with the highest oxidation efficiency of 83.6%. Furthermore, the photocatalytic oxidation reaction of hypophosphite was well fitted by pseudo first-order kinetic (R2 > 0.95) as shown in Figure 4B and the rate constant of 1:2-Ti4O7/g-C3N4 was 17.7-fold and 91.0-fold higher than that of pure g-C3N4 and Ti4O7, respectively. The low efficiency of photocatalytic oxidation of hypophosphite for pure g-C3N4 was possibly because of the rapid combination of electron-hole pairs (Yan et al., 2018). Similarly, the photocatalytic performance of pure Ti4O7 was extremely limited herein, which was mainly due to the wide band gap of 2.9 eV of pure Ti4O7 (Maragatha et al., 2017). Nevertheless, the efficiency of photocatalytic oxidation of hypophosphite was remarkably improved especially for 1:2-Ti4O7/g-C3N4 possibly as a result of the Ti4O7/g-C3N4 heterojunction with effectively increased recombination lifetime of electron-hole pairs and better charge transfer during the photocatalytic process, which was discussed in the following parts.
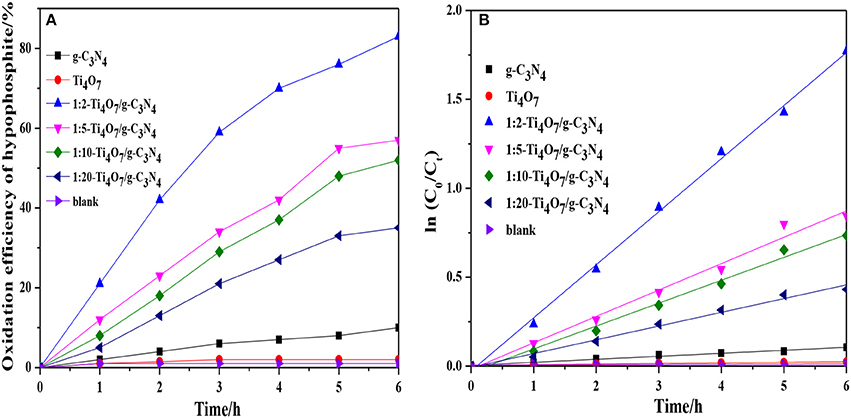
Figure 4. (A) The oxidation efficiency of hypophosphite for different photocatalysts; (B) Plot of ln(C0/Ct) against reaction time for the catalytic oxidation of hypophosphite using different photocatalysts.
Optical Properties Analysis
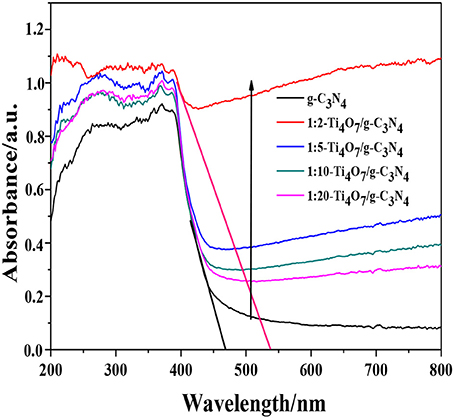
Generally, the band gap of a semiconductor is related to its photocatalytic performance, because it determines the absorption properties of the incident photon, the recombination lifetime of the electron-hole pairs and transfer of charge carriers. As shown in Figure 5, Ti4O7/g-C3N4 showed a distinct red-shift in comparison to pure g-C3N4 in the UV–vis diffuse reflectance spectra. Moreover, the absorption intensities ranging from 450 to 800 nm gradually strengthened with increase of the mass ratio of Ti4O7 (m): g-C3N4 (m), indicating that the Ti4O7/g-C3N4 heterojunction with good interaction between Ti4O7 and g-C3N4 facilitated the enhancement of visible-light harvesting (Chang et al., 2018). The band gap of Ti4O7/g-C3N4 was determined such that (Ai et al., 2018):
where α is the optical absorption coefficient; h is the Plank's constant; ν is the photonic frequency; A is the proportionality constant; Eg is the band gap.
The band gap followed the order: 1:2-Ti4O7/g-C3N4 (2.07 eV) < 1:5-Ti4O7/g-C3N4 (2.25 eV) < 1:10-Ti4O7/g-C3N4 (2.33 eV) < 1:20-Ti4O7/g-C3N4 (2.43 eV) < pure g-C3N4 (2.70 eV). As such, the narrowed band gap of Ti4O7/g-C3N4 would effectively enhance the photoabsorption efficiency, which thus contributed to the improved efficiency of photocatalytic oxidation of hypophosphite as shown in Figure 4.
Electrochemical Properties Analysis
CV and EIS results can indicate the combination efficiency of electron-hole pairs during the photocatalytic process. As shown in Figure 6A, the transient photoelectrochemical response current density of Ti4O7/g-C3N4 increased with the increase of the mass ratio of Ti4O7/g-C3N4. 1:2-Ti4O7/g-C3N4 had the highest photocurrent density of 0.30 μA cm−2, while the photocurrent density for pure g-C3N4 was only 0.10 μA cm−2. The enlarged photocurrent density of Ti4O7/g-C3N4 suggested the more efficient charge carrier (e−-h+) generation on the Ti4O7/g-C3N4 surface (Liu et al., 2017). Herein, the electrons in the valence band of g-C3N4 were excited upon visible light irradiation and then migrated to the conduction band of Ti4O7 with effectively enhanced efficiency of the charge carrier (e−-h+) generation and separation (Samanta and Srivastava, 2017), which improved the photocatalytic performance as indicated in Figure 4. In addition, the photoelectrochemical response current density of Ti4O7/g-C3N4 switched reversibly and was unchanged after repetitive ON/OFF illumination cycles, indicating the good photoelectrochemical stability of Ti4O7/g-C3N4.
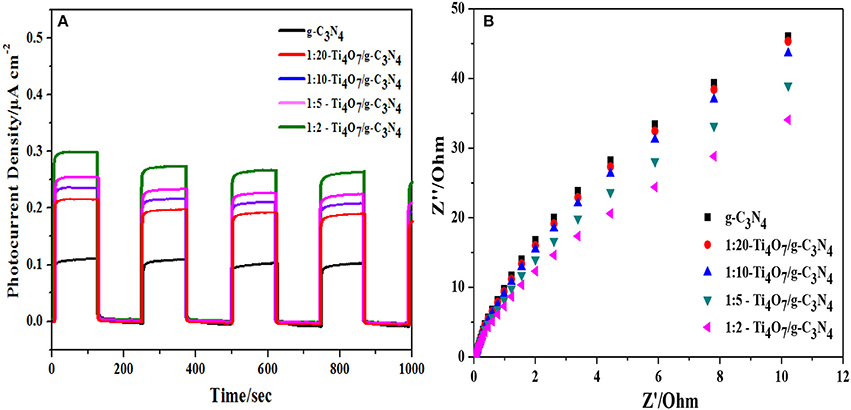
Figure 6. Electrochemical properties analysis: (A) photocurrent density and (B) EIS Nyquist plots of different photocatalysts.
Charge transfer of photocatalysts is another very important factor determining the photocatalytic performance (Kang et al., 2016). EIS was applied to analyze the photogenerated electron transfer process of Ti4O7/g-C3N4. As shown in Figure 6B, the arc radius decreased gradually with increase of the mass ratio of Ti4O7/g-C3N4 and the arc radius was lowest for 1:2-Ti4O7/g-C3N4. It is well known that the radius of Nyquist circle is related to the interfacial charge transfer that the smaller radius of Nyquist circle indicates a faster interfacial charge transfer and lower recombination rate of electron and hole (Guo et al., 2017). Herein, the decreased arc radius of Ti4O7/g-C3N4 indicated that 1:2-Ti4O7/g-C3N4 in comparison to pure g-C3N4 would also facilitate the photocatalytic oxidation of hypophosphite as evident in Figure 4.
Proposed Mechanism
Reactive oxygen species (ROS) including · and ·OH would be generated during the photocatalytic system (Huang et al., 2017a,b). Herein, the contributions of ·OH and · to the photocatalytic oxidation of hypophosphite were analyzed by evaluation of the photocatalytic oxidation efficiency of hypophosphite in the presence of isopropanol (IPA) acting as the scavenger of ·OH (Tian et al., 2015) and N2 purging applied to reduce the superoxide · radicals (Zhang et al., 2017). As shown in Figure 7, the oxidation efficiency of hypophosphite was decreased to 42 and 27% in the presence of IPA and N2, respectively. In contrast, that value was 83% without radical scavengers. These results indicated that ·OH and · radicals significantly contributed to the photocatalytic oxidation of hypophosphite and other radicals such as singlet oxygen (1O2) and peroxyl (RO2·) may also make a contribution as evident by the lowest efficiency of 27% rather than 0% (Huang et al., 2017a).
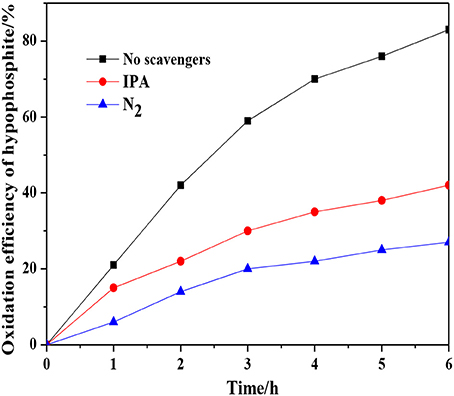
Figure 7. Effect of radical scavengers on the photocatalytic oxidation of hypophosphite by the 1:2-Ti4O7/g-C3N4 photocatalyst.
The photocatalytic mechanism of Ti4O7/g-C3N4 was proposed as shown in Figure 8. Under visible light irradiation, electrons in the valence band of g-C3N4 were excited and then the excited electrons migrated to the conduction band of Ti4O7 through the heterojunction surface of Ti4O7/g-C3N4 with the generation of holes in the valence band of g-C3N4 (Zhu et al., 2018). The electrons on the surface of Ti4O7 could easily react with O2 adsorbed on the Ti4O7/g-C3N4 surface to generate · radicals for hypophosphite oxidation. Meanwhile, the active ·OH radicals with high redox potential were generated through · radicals, which further facilitated the oxidation of hypophosphite (Nosaka and Nosaka, 2017; Guan et al., 2018). Therefore, it can be concluded that the oxidation of hypophosphite was mainly ascribed to ·OH and · radicals generated during the photocatalytic process as shown in Figure 8.
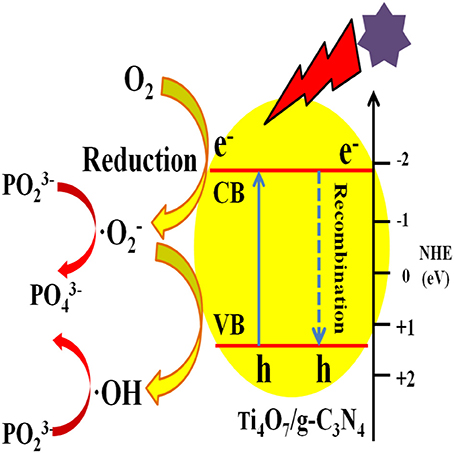
Figure 8. Schematic of the mechanism of photocatalytic oxidation of hypophosphite by Ti4O7/g-C3N4 photocatalysts.
Analysis of Stability
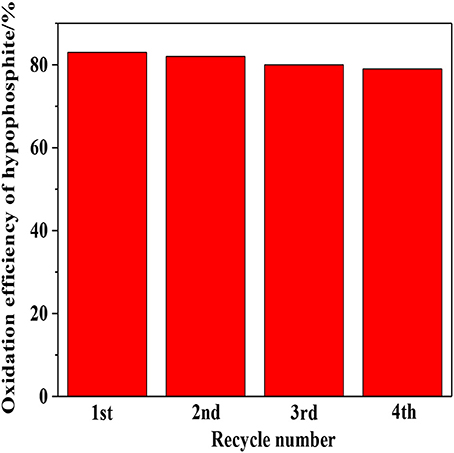
The stability of photocatalyst is another vital consideration to evaluate the photocatalytic performance. As shown in Figure 9, relatively robust reusability was exhibited by 1:2-Ti4O7/g-C3N4 photocatalyst that the efficiency of photocatalytic oxidation of hypophosphite was almost stable in the range of 79–84% after four repetitive experiments. The slight decrease of oxidation efficiency was possibly due to the inevitable mass loss of photocatalyst during the recycling process.
Conclusion
In this work, Ti4O7/g-C3N4 with various mass ratios (Ti4O7 (m): g-C3N4 (m) = 0.5, 0.2, 0.1, and 0.05) were synthesized by a hydrolysis method and the effect of the mass ratio of Ti4O7/g-C3N4 on Ti4O7/g-C3N4 visible light photocatalytic oxidation of hypophosphite was evaluated. Ti4O7/g-C3N4 exhibited remarkably improved photocatalytic performance on hypophosphite oxidation in comparison to pure g-C3N4 and 1:2-Ti4O7/g-C3N4 with a mass ratio of 0.5 showed the best photocatalytic performance with the highest oxidation rate constant of photocatalytic (17.7-fold and 91.0-fold higher than that of pure g-C3N4 and Ti4O7, respectively). The heterojunction structure of Ti4O7/g-C3N4 with broader light absorption significantly enhanced the efficiency of the charge carrier (e−-h+) generation and separation. ·OH and · radicals generated during the photocatalytic process were the main radicals contributing to the oxidation of hypophosphite.
Author Contributions
WG: experiment. ZZ: paper writing. ST: data analysis. JD: sample analysis.
Funding
This work was supported by National Natural Science Foundation of China (No. 51608086); Science and Technology Planning Project of Guangdong Province (2017A050506032); Science and Technology Project from Chongqing (CSTC2015JCYJA20027); Chongqing Key Laboratory of Environmental Materials and Remediation Technology, Chongqing University of Arts and Sciences (CKE1407); Scientific Research Foundation of Chongqing University of Arts and Sciences (R2014CH08).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Ai, Z. Z., Zhao, G., Zhong, Y. Y., Shao, Y. L., Huang, B. B., Wu, Y. Z., et al. (2018). Phase junction CdS: High efficient and stable photocatalyst for hydrogen generation. Appl. Catal. B: Environ. 221, 179–186. doi: 10.1016/j.apcatb.2017.09.002
Chang, F., Zheng, J. J., Wang, X. F., Xu, Q., Deng, B. Q., Hu, X. F., et al. (2018). Heterojuncted non-metal binary composites silicon carbide/g-C3N4 with enhanced photocatalytic performance. Mat. Sci. Semicon. Proc. 75, 183–192. doi: 10.1016/j.mssp.2017.11.043
Dong, G. H., Ho, W. K., Li, Y. H., and Zhang, L. Z. (2015). Facile synthesis of porous graphene-like carbon nitride (C6N9H3) with excellent photocatalytic activity for NO removal. Appl. Catal. B: Environ. 174–175, 477–485. doi: 10.1016/j.apcatb.2015.03.035
Gan, X. P., Wu, Y. T., Liu, L., Shen, B., and Hu, W. B. (2007). Electroless copper plating on PET fabrics using hypophosphite as reducing agent. Surf. Coat. Tech. 201, 7018–7023. doi: 10.1016/j.surfcoat.2007.01.006
Ganiyu, S. O., Oturan, N., Raffy, S., Cretin, M., Esmilaire, R., van Hullebusch, E. et al. (2016). Sub-stoichiometric titanium oxide (Ti4O7) as a suitable ceramic anode for electrooxidation of organic pollutants: a case study of kinetics, mineralization and toxicity assessment of amoxicillin. Water Res. 106, 171–182. doi: 10.1016/j.watres.2016.09.056
Gao, D. Q., Xu, Q., Zhang, J., Yang, Z. L., Si, M. S., Yan, Z. J., et al. (2014). Defect-related ferromagnetism in ultrathin metal-free g-C3N4 nanosheets. Nanoscale 6, 2577–2581. doi: 10.1039/c3nr04743a
Guan, W., Sun, G. G., Yin, L., Zhang, Z. H., and Tian, S. C. (2018). Ti4O7/g-C3N4 visible light photocatalytic performance on hypophosphite oxidation: effect of annealing temperature. Front. Chem. 6:37. doi: 10.3389/fchem.2018.00037
Guan, W., Tian, S. C., Cao, D., Chen, Y. T., and Zhao, X. (2017). Electrooxidation of nickel-ammonia complexes and simultaneous electrodeposition recovery of nickel from practical nickel-electroplating rinse wastewater. Electrochim. Acta 246, 1230–1236. doi: 10.1016/j.electacta.2017.06.121
Guo, L., Jing, Y., and Chaplin, B. P. (2016). Development and characterization of ultrafiltration TiO2 magnéli phase reactive electrochemical membranes. Environ. Sci. Technol. 50, 1428–1436. doi: 10.1021/acs.est.5b04366
Guo, W., Wang, J. Y., Fan, C., Chen, Z., Liu, P., Zhu, D. J., et al. (2017). Synthesis of carbon self-repairing porous g-C3N4 nanosheets/NiCo2S4 nanoparticles hybrid composite as high-performance electrode materials for supercapacitors. Electrochim. Acta 253, 68–77. doi: 10.1016/j.electacta.2017.09.025
Hao, R. R., Wang, G. H., Jiang, C. J., Tang, H., and Xu, Q. C. (2017). In situ hydrothermal synthesis of g-C3N4/TiO2 heterojunction photocatalysts with high specific surface area for rhodamine B degradation. Appl. Surf. Sci. 411, 400–410. doi: 10.1016/j.apsusc.2017.03.197
Hao, R. R., Wang, G. H., Tang, H., Sun, L. L., Xu, C., and Han, D. Y. (2016). Template-free preparation of macro/mesoporous g-C3N4/TiO2 heterojunction photocatalysts with enhanced visible light photocatalytic activity. Appl. Catal. B: Environ. 187, 47–58. doi: 10.1016/j.apcatb.2016.01.026
Hatamie, A., Marahel, F., and Sharifat, A. (2018). Green synthesis of graphitic carbon nitride nanosheet (g-C3N4) and using it as a label-free fluorosensor for detection of metronidazole via quenching of the fluorescence. Talanta 176, 518–525. doi: 10.1016/j.talanta.2017.08.059
Huang, H. W., Han, X., Li, X. W., Wang, S. C., Chu, P. K., and Zhang, Y. H. (2015a). Fabrication of multiple heterojunctions with tunable visible-light-active photocatalytic reactivity in BiOBr–BiOI full-range composites based on microstructure modulation and band structures. ACS Appl. Mater. Interfaces 7, 482–492. doi: 10.1021/am5065409
Huang, H. W., He, Y., Du, X., Chu, P. K., and Zhang, Y. H. (2015b). A general and facile approach to heterostructured core/shell BiVO4/BiOI p-n junction: room-temperature in situ assembly and highly boosted visible-light photocatalysis. ACS Sustainable Chem. Eng. 3, 3262–3273. doi: 10.1021/acssuschemeng.5b01038
Huang, H. W., Tu, S. C., Zeng, C., Zhang, T. R., Reshak, A. H., and Zhang, Y. H. (2017a). Macroscopic polarization enhancement promoting photo- and piezoelectric-induced charge separation and molecular oxygen activation. Angew. Chem. Int. Ed. 56, 11860–11864. doi: 10.1002/anie.201706549
Huang, H. W., Xiao, K., He, Y., Zhang, T. R., Dong, F., Du, X., et al. (2016). In situ assembly of BiOI@Bi12O17Cl2 p-n junction: charge induced unique front-lateral surfaces coupling heterostructure with high exposure of BiOI {001} active facets for robust and nonselective photocatalysis. Appl. Catal. B: Environ. 199, 75–86. doi: 10.1016/j.apcatb.2016.06.020
Huang, H. W., Xiao, K., Tian, N., Dong, F., Zhang, T. R., Du, X., et al. (2017b). Template-free precursor-surface-etching route to porous, thin g-C3N4 nanosheets for enhancing photocatalytic reduction and oxidation activity. J. Mater. Chem. A 5, 17452–17463. doi: 10.1039/c7ta04639a
Huang, Y. H., Su, H. T., and Lin, L. W. (2009). Removal of citrate and hypophosphite binary components using fenton, photo-fenton and electro-fenton processes. J. Environ. Sci. 21, 35–40. doi: 10.1016/S1001-0742(09)60008-5
Jo, W. K., and Natarajan, T. S. (2015). Influence of TiO2 morphology on the photocatalytic efficiency of direct Z-scheme g-C3N4/TiO2 photocatalysts for isoniazid degradation. Chem. Eng. J. 281, 549–565. doi: 10.1016/j.cej.2015.06.120
Jourshabani, M., Shariatinia, Z., and Badiei, A. (2018). Synthesis and characterization of novel Sm2O3/S-doped g-C3N4 nanocomposites with enhanced photocatalytic activities under visible light irradiation. Appl. Surf. Sci. 427, 375–387. doi: 10.1016/j.apsusc.2017.08.051
Kang, K., Watanabe, S., Broch, K., Sepe, A., Brown, A., Nasrallah, I., et al. (2016). 2D coherent charge transport in highly ordered conducting polymers doped by solid state diffusion. Nat. Mater. 15, 896–902. doi: 10.1038/NMAT4634
Li, Y. A., Wang, M. Q., Bao, S. J., Lu, S. Y., Xu, M. W., Long, D. B., et al. (2016b). Tuning and thermal exfoliation graphene-like carbon nitride nanosheets for superior photocatalytic activity. Ceram. Int. 42, 18521–18528. doi: 10.1016/j.ceramint.2016.08.190
Li, Y., Zhang, C., Shuai, D. M., Naraginti, S., Wang, D. W., and Zhang, W. L. (2016a). Visible-light-driven photocatalytic inactivation of MS2 by metal-free g-C3N4: virucidal performance and mechanism. Water Res. 106, 249–258. doi: 10.1016/j.watres.2016.10.009
Li, Z. Q., Qi, M. Y., Tu, C. Y., Wang, W. P., Chen, J. R., and Wang, A. J. (2017). Highly efficient removal of chlorotetracycline from aqueous solution using graphene oxide/TiO2 composite: properties and mechanism. Appl. Surf. Sci. 425, 765–775. doi: 10.1016/j.apsusc.2017.07.027
Liang, F. F., and Zhu, Y. F. (2016). Enhancement of mineralization ability for phenol via synergetic effect of photoelectrocatalysis of g-C3N4 film. Appl. Catal. B: Environ. 180, 324–329. doi: 10.1016/j.apcatb.2015.05.009
Liu, Q., Chen, T. X., Guo, Y. R., Zhang, Z. G., and Fang, X. M. (2017). Grafting Fe(III) species on carbon nanodots/Fe-doped g-C3N4 via interfacial charge transfer effect for highly improved photocatalytic performance. Appl. Catal. B: Environ. 205, 173–181. doi: 10.1016/j.apcatb.2016.12.028
Ma, L. N., Wang, G. H., Jiang, C. J., Bao, H. L., and Xu, Q. C. (2018). Synthesis of core-shell TiO2@g-C3N4 hollow microspheres for efficient photocatalytic degradation of rhodamine B under visible light. Appl. Surf. Sci. 430, 263–272. doi: 10.1016/j.apsusc.2017.07.282
Maragatha, J., Rani, C., Rajendran, S., and Karuppuchamy, S. (2017). Microwave synthesis of nitrogen doped Ti4O7 for photocatalytic applications. Physica. E 93, 78–82. doi: 10.1016/j.physe.2017.05.020
Masih, D., Ma, Y. Y., and Rohani, S. (2017). Graphitic C3N4 based noble-metal-free photocatalyst systems: a review. Appl. Catal. B: Environ. 206, 556–588. doi: 10.1016/j.apcatb.2017.01.061
Nosaka, Y., and Nosaka, A. Y. (2017). Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev. 117, 11302–11336. doi: 10.1021/acs.chemrev.7b00161
Piveteau, S., Picard, S., Dabert, P., and Daumer, M. L. (2017). Dissolution of particulate phosphorus in pig slurry through biological acidification: a critical step for maximum phosphorus recovery as struvite. Water Res. 124, 693–701. doi: 10.1016/j.watres.2017.08.017
Samanta, S., and Srivastava, R. (2017). Thermal catalysis vs. photocatalysis: a case study with FeVO4/g-C3N4 nanocomposites for the efficient activation of aromatic and benzylic CH bonds to oxygenated products. Appl. Catal. B: Environ. 218, 621–636. doi: 10.1016/j.apcatb.2017.06.043
Shao, H. X., Zhao, X., Wang, Y. B., Mao, R., Wang, Y., Qiao, M., et al. (2017). Synergetic activation of peroxymonosulfate by Co3O4 modified g-C3N4 for enhanced degradation of diclofenac sodium under visible light irradiation. Appl. Catal. B: Environ. 218, 810–818. doi: 10.1016/j.apcatb.2017.07.016
Shih, Y. J., Lin, C. P., and Huang, Y. H. (2013). Application of fered-fenton and chemical precipitation process for the treatment of electroless nickel plating wastewater. Sep. Purif. Technol. 104, 100–105. doi: 10.1016/j.seppur.2012.11.025
Sun, X., Jiang, D., Zhang, L., and Wang, W. Z. (2018). Alkaline modified g-C3N4 photocatalyst for high selective oxide coupling of benzyl alcohol to benzoin. Appl. Catal. B: Environ. 220, 553–560. doi: 10.1016/j.apcatb.2017.08.057
Tian, Q., Zhuang, L. J., Ong, S. K., Wang, Q., Wang, K. W., Xie, X. H., et al. (2017). Phosphorus (P) recovery coupled with increasing influent ammonium facilitated intracellular carbon source storage and simultaneous aerobic phosphorus & nitrogen removal. Water Res. 119, 267–275. doi: 10.1016/j.watres.2017.02.050
Tian, S. C., Li, Y. B., and Zhao, X. (2015). Cyanide removal with a copper/active carbon fiber cathode via a combined oxidation of a fenton-like reaction and in situ generated copper oxides at anode. Electrochim. Acta 180, 746–755. doi: 10.1016/j.electacta.2015.09.006
Wu, G. S., Thind, S. S., Wen, J. L., Yan, K., and Chen, A. C. (2013). A novel nanoporous α-C3N4 photocatalyst with superior high visible light activity. Appl. Catal. B: Environ. 142–143, 590–597. doi: 10.1016/j.apcatb.2013.05.070
Yan, X. X., Jia, Z. Y., Che, H. B., Chen, S. Q., Hu, P., Wang, J. S., et al. (2018). A selective ion replacement strategy for the synthesis of copper doped carbon nitride nanotubes with improved photocatalytic hydrogen evolution. Appl. Catal. B: Environ. 234, 19–25. doi: 10.1016/j.apcatb.2018.04.020
Zaky, A. M., and Chaplin, B. P. (2014). Mechanism of p-substituted phenol oxidation at a Ti4O7 reactive electrochemical membrane. Environ. Sci. Technol. 48, 5857–5867. doi: 10.1021/es5010472
Zeng, X. K., Wang, Z. Y., Wang, G., Gengenbach, T. R., McCarthy, D. T., Deletic, A., et al. (2017). Highly dispersed TiO2 nanocrystals and WO3 nanorods on reduced graphene oxide: Z-scheme photocatalysis system for accelerated photocatalytic water disinfection. Appl. Catal. B: Environ. 218, 163–173. doi: 10.1016/j.apcatb.2017.06.055
Zhang, F. W., Wen, Q. J., Hong, M. Z., Zhuang, Z. Y., and Yu, Y. (2017). Efficient and sustainable metal-free GR/C3N4/CDots ternary heterostructrues for versatile visible-light-driven photoredox applications: toward synergistic interaction of carbon materials. Chem. Eng. J. 307, 593–603. doi: 10.1016/j.cej.2016.08.120
Zhang, L. P., Wang, G. H., Xiong, Z. Z., Tang, H., and Jiang, C. J. (2018). Fabrication of flower-like direct Z-scheme β-Bi2O3/g-C3N4 photocatalyst with enhanced visible light photoactivity for rhodamine B degradation. Appl. Surf. Sci. 436, 162–171. doi: 10.1016/j.apsusc.2017.11.280
Zhang, X. S., Hu, J. Y., and Jiang, H. (2014). Facile modification of a graphitic carbon nitride catalyst to improve its photoreactivity under visible light irradiation. Chem. Eng. J. 256, 230–237. doi: 10.1016/j.cej.2014.07.012
Zhao, Z. L., Dong, W. Y., Wang, H. J., Chen, G. H., Wang, W., Liu, Z. K., et al. (2017). Advanced oxidation removal of hypophosphite by O3/H2O2 combined with sequential Fe(II) catalytic process. Chemosphere 180, 48–56. doi: 10.1016/j.chemosphere.2017.04.003
Zhu, Z., Huo, P. W., Lu, Z. Y., Yan, Y. S., Liu, Z., Shi, W. D., et al. (2018). Fabrication of magnetically recoverable photocatalysts using g-C3N4 for effective separation of charge carriers through like-Z-scheme mechanism with Fe3O4 mediator. Chem. Eng. J. 331, 615–625. doi: 10.1016/j.cej.2017.08.131
Keywords: photocatalysts, hypophosphite oxidation, graphitic carbon nitride, sub-stoichiometric titanium oxides, visible light irradiation
Citation: Guan W, Zhang Z, Tian S and Du J (2018) Ti4O7/g-C3N4 for Visible Light Photocatalytic Oxidation of Hypophosphite: Effect of Mass Ratio of Ti4O7/g-C3N4 Front. Chem. 6:313. doi: 10.3389/fchem.2018.00313
Received: 07 May 2018; Accepted: 09 July 2018;
Published: 24 July 2018.
Edited by:
Fan Dong, Chongqing Technology and Business University, ChinaReviewed by:
Guohong Wang, Hubei Normal University, ChinaAihua Xu, Wuhan Textile University, China
Shaowen Cao, Wuhan University of Technology, China
Hongwei Huang, China University of Geosciences, China
Copyright © 2018 Guan, Zhang, Tian and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenghua Zhang, emhlbmdodWEuemhhbmdAc3oudHNpbmdodWEuZWR1LmNu
Shichao Tian, MTg4MTI2NzI2MTlAMTYzLmNvbQ==
†These authors have contributed equally to this work.
 Wei Guan1
Wei Guan1 Zhenghua Zhang
Zhenghua Zhang Shichao Tian
Shichao Tian