
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Cell Dev. Biol. , 09 July 2020
Sec. Cell Adhesion and Migration
Volume 8 - 2020 | https://doi.org/10.3389/fcell.2020.00626
This article is part of the Research Topic The Cytoskeleton and Cellular Compartmentation: Cilia as Specialized Cellular Domains View all 18 articles
The vascular barrier between blood and tissues is a highly selective structure that is essential to maintain tissue homeostasis. Defects in the vascular barrier lead to a variety of cardiovascular diseases. The maintenance of vascular barriers is largely dependent on endothelial cells, but the precise mechanisms remain elusive. Recent studies reveal that primary cilia, microtubule-based structures that protrude from the surface of endothelial cells, play a critical role in the regulation of vascular barriers. Herein, we discuss recent advances on ciliary functions in the vascular barrier and suggest that ciliary signaling pathways might be targeted to modulate the vascular barrier.
Blood vessels are found throughout the body to deliver oxygen and nutrients to body tissues, and remove metabolic waste and carbon dioxide from tissues. In some organs in mammals, there are distinct boundaries between blood and tissues. These barriers separate blood from tissues and are composed of endothelial cells, pericytes, other vascular mural cells, and intercellular connections. In contrast to the common vascular barriers, there are extremely strict vascular barriers in some important organs, such as the blood-brain barrier (Chow and Gu, 2015; Yang Z. et al., 2019), the blood-retinal barrier (Diaz-Coranguez et al., 2017; Naylor et al., 2019), and the blood-testis barrier (Mruk and Cheng, 2015). Compared with the common vascular barriers, these barriers involve some special cells and intercellular connections. For example, 85% of the surface area of the brain capillary wall is surrounded by the astrocytic end-feet, and in the testicle, the supporting cells adjacent to the basement membrane of spermatogenic tubules are connected in a special structure (Gerber et al., 2016), which makes them less penetrating. The vascular barrier controls the exchange of molecules and ions between blood and tissues, prevents harmful substances from entering the tissue and causing damage, and maintains a stable internal tissue environment. In addition, in some tissues, the vascular barrier gives them immune privileges to avoid the occurrence of autoimmunity. However, once these vital organs have lesions, treatment is challenging as the vascular barrier prevents drugs from targeting the relevant tissues, making them ineffective (Pardridge, 2012).
The vascular barrier has been shown to be regulated by a number of signaling pathways, such as Mfsd2a, lipolysis stimulated lipoprotein receptor, angiopoietin-2, Wnt, and Norrin pathways (Liebner et al., 2008; Stenman et al., 2008; Yen et al., 2008; Daneman et al., 2009; Gao et al., 2010; Ye et al., 2010; Rangasamy et al., 2011; Ben-Zvi et al., 2014; Yu et al., 2017). In addition, the intestinal flora has been reported to affect the vascular barrier (Braniste et al., 2014; Al-Asmakh and Hedin, 2015). Some of the signaling mechanisms regulate initial angiogenesis, while others maintain the mature vascular barrier. Therefore, it is important to understand the molecular mechanisms that maintain vascular barriers and to develop new medicines to regulate the barriers. Recent evidence suggests that the primary cilia of endothelial cells play a critical role in regulating vascular barriers. In this review, we describe the functions of endothelial cilia in the development and maintenance of vascular barriers and discuss the potential of targeting cilium-related molecules to modulate the barriers.
Vascular endothelial cells play an important role in vascular barrier function. Recent studies demonstrate that primary cilia are present on vascular endothelial cells with typical “9 + 0” axonemes (Figure 1). Primary cilia are microtubule-based organelles, which are ubiquitous in mammals (Satir and Christensen, 2008; Yang et al., 2014; Yang Y. et al., 2019; Yu et al., 2016, 2019; Ran et al., 2020). Cilia are mainly composed of the basal body, the axoneme, the ciliary matrix, and the ciliary membrane. Transport proteins, ion channels, and receptors are embedded in the membrane (Satir et al., 2010). The basal body is localized at the base of the cilium and is derived from the mother centriole of the centrosome. In addition, the basal body consists of various appendages, as well as the pericentriolar material. The formation and maintenance of cilia depend on intraflagellar transport (IFT) proteins, motor proteins, and structural components.
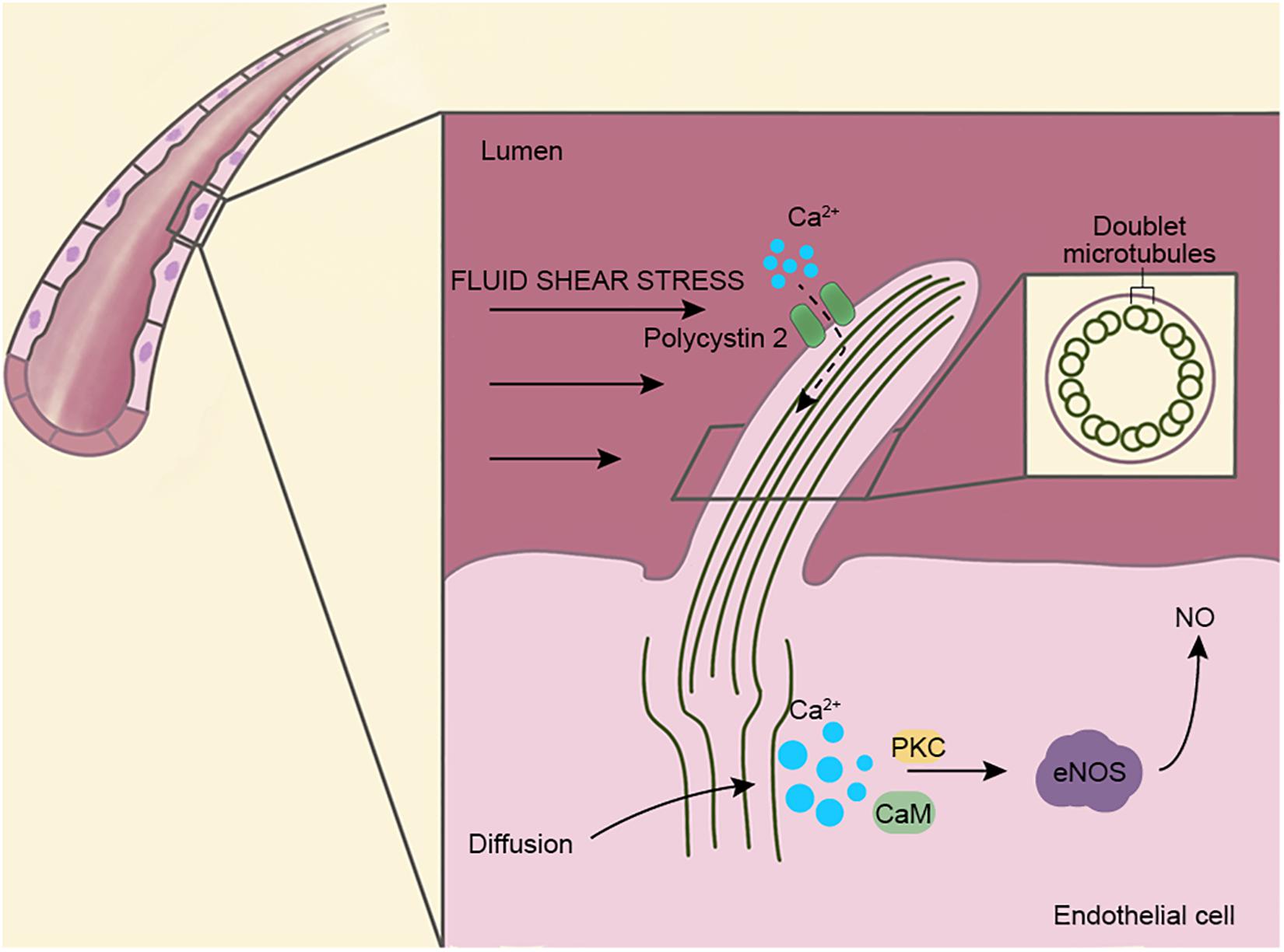
Figure 1. Structure and function of vascular endothelial cilia. Primary cilia are present on the surfaces of vascular endothelial cells and extend into the lumen. Cilia are used as mechanoreceptors and signaling centers to regulate vascular functions. The ciliary axenome is composed of nine pairs of doublet microtubules and surrounded by a ciliary membrane, where several transport proteins and ion channels are present. Fluid shear stress is known to open Ca2+ channels on cilia (dotted line), but recent studies show that Ca2+ originates in the cytoplasm instead of cilia. Via the actions of protein kinase C (PKC) and calmodulin (CaM), endothelial nitric oxide synthase (eNOS) is activated and leads to the upregulation of NO, which dilates blood vessels and prevents vascular rupture caused by excessive blood flow.
The primary cilia on the vascular endothelial cell extend into the lumen of the blood vessel and act as sensors and transmit extracellular signals into the cell (Pala et al., 2017). In addition, endothelial cilia regulate blood vessel function through blood flow sensing, cell migration, and calcium and nitric oxide (NO) signaling (Nauli et al., 2008; Jones et al., 2012). Ca2+ is an important second messenger that regulates many signaling pathways and regulates vasoconstriction and vasodilation through NO in vascular endothelial cells. A number of previous studies have shown that fluid shear stress triggers ciliary calcium signaling, and this process is mediated by polycystin 2 (PC2) (Jin et al., 2014). Recently, however, Delling et al. (2016) have proposed the opposite, suggesting that Ca2+ are produced in the cytoplasm and rapidly diffuse into the cilia and that the primary cilia may not be involved in the influx of Ca2+ (Figure 1). Thus, the source of Ca2+ in endothelial cells needs to be investigated further.
Different blood flow or velocity has different effects on endothelial cells, resulting in changes in blood vessel phenotypes and functions, a process called hemodynamic stimulation (Chistiakov et al., 2017). The primary cilia of endothelial cells appear in the early stages of angiogenesis, indicating that cilia are related to early angiogenesis. Interestingly, this functionality does not depend on hemodynamic stimulation (Eisa-Beygi et al., 2018). This is different from the role of cilia in regulating blood vessels by blood flow stimulation after angiogenesis. In addition, the cilia of vascular endothelial cells can detect the extracellular pH and regulate related pathways to maintain the acid-base homeostasis of endothelial cells (Atkinson et al., 2019). Damage to the primary cilia of endothelial cells causes several vascular diseases (Mohieldin et al., 2016; Luu et al., 2018). The recent evidence on the correlation between endothelial cilia and the vascular barrier suggests that endothelial cilia may be a potential therapeutic target for vascular barrier-related diseases.
Intraflagellar transport is a two-way transport system located between axonemal microtubules and ciliary membrane. It is the main transport system of cilia, and also transports proteins during cilium-related signaling, such as Hedgehog (HH) and Wnt signaling (Goetz and Anderson, 2010). The model proposed by Haycraft et al. (2005) suggests that IFT is indirectly involved in HH signal transduction, and that the absence of IFT leads to the failure of GLI protein processing, thus affecting the downstream signal transduction of HH. However, the function of cilia in Wnt signaling is controversial. Other studies show normal Wnt signaling in mouse mutants that lack cilia due to Ift172 mutation (Eggenschwiler and Anderson, 2007). Normal Wnt signaling has also been shown in zebrafish mutants that lack all cilia, suggesting that cilia are not necessary for Wnt signaling (Huang and Schier, 2009). Therefore, whether cilia play a role in Wnt signaling requires further study. In addition, proteins in the cilia can be transported back to the cytoplasm through IFT to ensure the protein balance in the cilia (Lechtreck et al., 2013). IFT is essential for cilium assembly, maintenance, and perception. The loss of IFT leads to abnormal cilia. A zebrafish embryo model study demonstrates that cilia are present in the vascular endothelial cells of the zebrafish brain, and after IFT protein mutations, the probability of intracranial hemorrhage increases compared with the control group. However, no changes in blood vessel morphology and cell connections are observed. After re-expressing the relevant IFT protein, the bleeding phenotype improves significantly (Kallakuri et al., 2015).
In the mouse embryo model, Ift172 and Ift122 mutants show cranial neural tube defects and bleeding (Cortellino et al., 2009; Gorivodsky et al., 2009), but the molecular mechanism of the bleeding phenotype is still unclear. Another study has evaluated the permeability of endothelial cells from Ift88 mutant mice with polycystic kidney disease (PKD). Compared with wild-type cells, Ift88 mutant endothelial cells have reduced stress fibers and focal adhesion, and higher permeability to dextran, indicating that cilium defects cause damage to the vascular barrier. The expression of heat shock protein 27 in Ift88 mutant endothelial cells is inhibited by approximately 90%, and the phosphorylation level of its downstream target, focal adhesion kinase, is significantly reduced (Jones et al., 2012). These studies indicate that IFT proteins play a potential role in the vascular barrier.
In addition to IFT proteins, polycystin 1 (PC1), is another protein related to cilia that show a correlation with the vascular barrier. PC1 usually forms a channel-receptor complex with PC2. These transmembrane proteins regulate cell activity by detecting external stimuli and transmitting calcium-mediated signals (Qian et al., 1997). PC1 is expressed in endothelial cells and smooth muscle cells and is involved in cell-matrix connectivity. PKD1 (the gene encoding PC1) mutations cause autosomal dominant PKD, and vascular abnormalities are usually observed in these patients. Due to vascular leakage and hemorrhage, PKD1 knockout mice are embryonic lethal at embryonic day 15.5 (Kim et al., 2000). However, the mechanism is not clear and further studies are required. In zebrafish embryos, PKD2 (the gene encoding PC2) mutations demonstrate no bleeding phenotype (Kallakuri et al., 2015), suggesting that PC1, but not PC2, plays a major role in the maintenance of the vascular barrier.
Hedgehog signaling is critical for vascular development, maturity and integrity (Chapouly et al., 2019), and is one of the signaling pathways closely related to the function of the primary cilia (Chow and Gu, 2015). HH signaling elements, including patched 1 (PTCH1), G protein-coupled receptor 161 (GPR161), smoothened (SMO), suppressor of fused protein (SUFU), GLI family zinc finger 2 (GLI2), and GLI3, are located on primary cilia (Figure 2) (Bangs and Anderson, 2017). HH is a lipoprotein morphogenetic factor that participates in the development of vertebrate tissues and the maintenance of stem cell homeostasis, both essential for central nervous system development (Briscoe and Therond, 2013). Sonic HH (SHH) signaling, one of the HH signaling pathways, plays an important role to maintain the blood-brain barrier in embryos and adults. Specifically, astrocytes that comprise the blood-brain barrier structure, secrete SHH, and promote the expression of GLI1 and Sox18 in endothelial cells to upregulate the expression of occludin and claudin-5 and reduce endothelial cell permeability (Alvarez et al., 2011). Using zinc-finger nuclease-mediated or morpholino oligo-mediated mutagenesis to destroy genes encoding ciliary proteins, such as IFT proteins, Talpid3, and Dzip1/Iguana, Bangs and Anderson show characteristics of SHH deficiency (Bangs and Anderson, 2017). Together, these results indicate that cilia play a critical role in HH signaling.
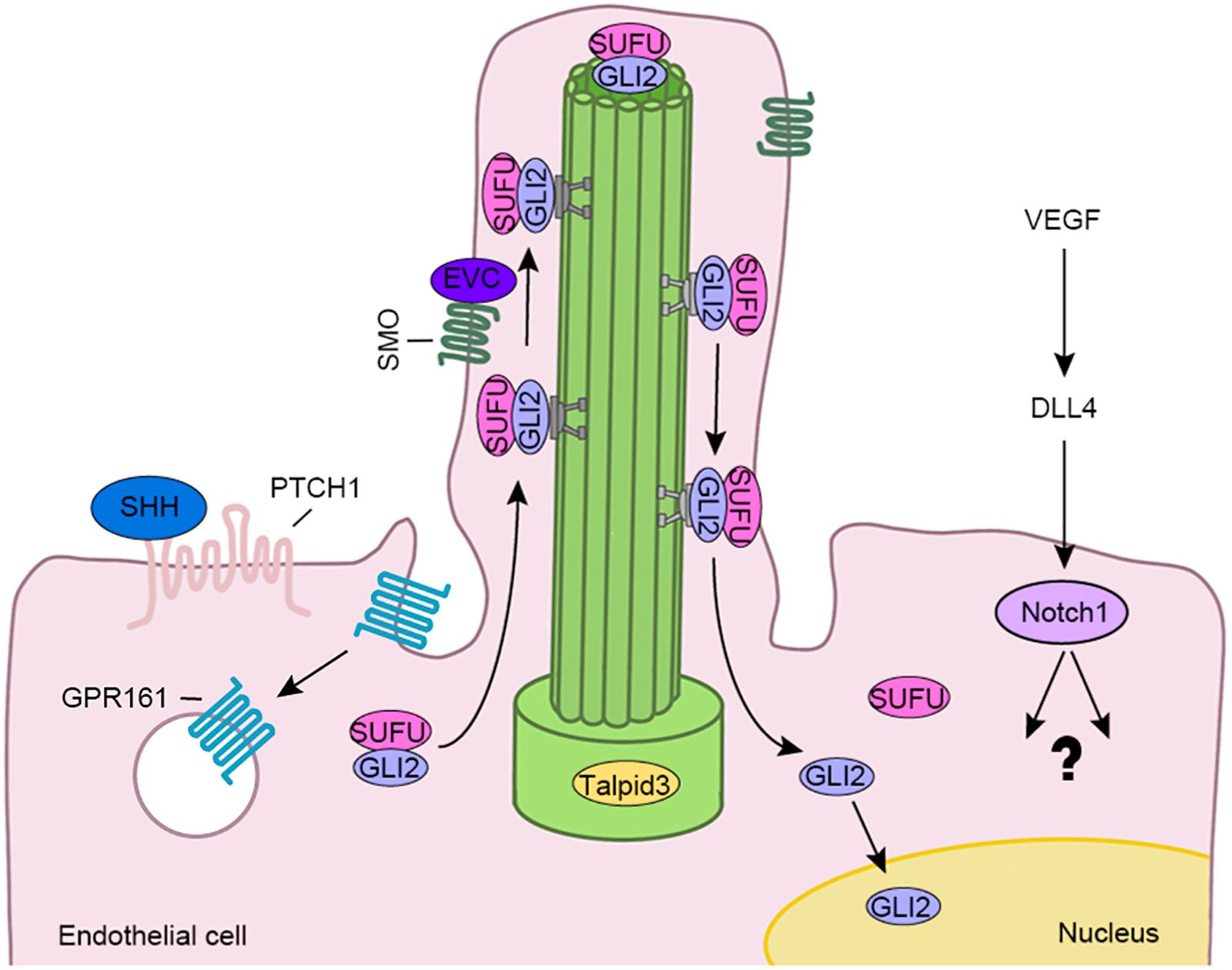
Figure 2. The endothelial cilium regulates various signaling pathways involved in the vascular barrier, such as HH and Notch pathways. The binding of SHH to PTCH1 leads to SMO activation and enrichment at the cilium. GPR161, the negative regulator of the HH pathway, then is internalized from the cilium. Subsequently, the GLI2/SUFU complex is transported to the ciliary tip by the IFT complex. After the complex is dissociated, GLI2 is activated and translocated to the nucleus. In addition, vascular endothelial growth factor (VEGF) induces the expression of delta like canonical Notch ligand 4 (DLL4), which then binds to Notch1 to activate downstream events.
The zebrafish model study shows that when the HH signaling is inhibited due to ciliary defects, the possibility of intracranial hemorrhage increases. Conversely, activation of this pathway reduces the risk of cerebral hemorrhage (Kallakuri et al., 2015), indicating that endothelial cilia may regulate vascular integrity through the HH signaling pathway. Furthermore, Talpid3 is an essential protein in ciliogenesis, and its mutation in zebrafish causes abnormal HH signaling. Embryos exhibit cerebral hemorrhage symptoms (Ben et al., 2011), which confirms the connection between cilia and vascular integrity. HH signaling is also related to retinal angiogenesis, because treatment with cyclopamine, an HH signal inhibitor, inhibits retinal angiogenesis. HH signaling is required for survival of retinal endothelial cells and pericytes (Surace et al., 2006).
The Notch pathway is the main signaling pathway that coordinates angiogenesis with angiogenin signaling and cell metabolism (Tetzlaff and Fischer, 2018). Notch signaling promotes the formation of functional blood vessels by regulating the differentiation of endothelial cells. Recent studies show that cilium-specific genes are required for the development of hematopoietic stem cells and progenitor cells in endothelial cells during zebrafish embryogenesis, which is primarily mediated by Notch signaling (Liu et al., 2019). Disassembly of cilia in Ift88 knockout mice could reduce Notch activity, resulting in thickening of corneal epithelial cells (Grisanti et al., 2016). Limited data about the function of Notch signaling in the formation and maintenance of the vascular barrier is available. Cilium-related proteins may regulate the vascular barrier function through various signaling pathways. A better understanding of the upstream effector molecules and downstream targets of these signaling pathways will help elucidate the molecular mechanisms regulating vascular barriers (Figure 2).
Due to the high selectivity of the vascular barrier, it is challenging to deliver drugs to certain tissues. It is therefore important to modulate the vascular barrier. Some solutions have been documented. For example, in the blood-brain barrier, the use of mannitol or focused ultrasound can temporarily open the junction between endothelial cells, so that molecules unable to pass through the barrier can temporarily enter the brain tissue. However, simultaneously, substances such as plasma proteins in blood vessels that may cause harm to brain tissue, are able to enter (Rodriguez et al., 2015). In vitro studies show that glucocorticoids improve the integrity of the blood-brain barrier in patients with multiple sclerosis, but this method alone can cause serious adverse reactions (Blecharz et al., 2010). Stimulants such as histamine, atrial natriuretic peptide, or thrombin increase the permeability of blood vessels (Konstantoulaki et al., 2003; Ashina et al., 2015).
Maintenance of the vascular barrier depends on the regulation of various signaling pathways. Cilia play an important role in some of these signaling pathways, especially in HH signaling, and indirectly regulates the formation and maintenance of the vascular barrier. A number of studies have shown that there is a certain relationship between cilia and the integrity of the vascular barrier. In addition, the target of certain drugs, such as cytochalasin D, is part of the signaling pathway regulated by cilia (Kim et al., 2010). Thus, targeting cilia (e.g., delivery of cilium-related genes with adenovirus vectors) is a potential strategy for treating diseases related to the vascular barrier (Kotterman and Schaffer, 2014), which requires a joint effort among clinicians and scientists.
The vascular barrier plays a key role to maintain tissue homeostasis, while cilia are involved in the generation and maintenance of the vascular barrier. Although more evidence is available, several aspects have not been resolved. For example, it is not clear whether cilia play different roles in different tissues and how cilia regulate various parts of the vascular barrier through different signaling pathways. The molecules responsible for regulating the formation and maturation of the vascular barrier, the method used to assess the vascular barrier and how to target cilia to regulate the vascular barrier with treatments still need to be investigated. Further research will provide new strategies for the treatment of vascular diseases caused by ciliary defects.
Destruction of cilium-related proteins can damage the vascular barrier, which may be mediated by HH, Notch, Wnt, and other signaling pathways. It is possible that cilium defects cause damage to intercellular junctions and paracellular transport, such as tight junctions and adherens junctions, which are important in vascular barriers (Xie et al., 2017; Dong et al., 2020). Alternatively, cilium defects may cause blood vessels to inhibit the recruitment of vascular mural cells, such as pericytes and smooth muscle cells, leading to damage to the vascular barrier (Chen et al., 2017). Knowledge of the link between cilium defects and human diseases continues to expand, and vascular dysfunction is present in some ciliary diseases. Maintenance of the vascular barrier involves several connexins, transmembrane proteins, and related signaling pathways. Defects in one of these proteins may lead to the destruction of the vascular barrier, and the same protein may play a role in ciliary signaling. In addition, compensation mechanisms in some signaling pathways may be present, which makes it difficult to study the specific role of cilia in the regulation of vascular barriers. At present, the mechanisms of how cilia are involved in maintaining vascular barriers remain elusive. A better understanding of this question will be undoubtedly helpful to the treatment of vascular barrier-related diseases.
NM wrote the manuscript and drew the figures. JZ conceived the study and revised the manuscript. Both authors read and approved the final version of the manuscript.
This work was supported by the Taishan Scholars Program of Shandong Province (20161201).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Al-Asmakh, M., and Hedin, L. (2015). Microbiota and the control of blood-tissue barriers. Tissue Barriers 3:e1039691. doi: 10.1080/21688370.2015.1039691
Alvarez, J. I., Dodelet-Devillers, A., Kebir, H., Ifergan, I., Fabre, P. J., Terouz, S., et al. (2011). The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science 334, 1727–1731. doi: 10.1126/science.1206936
Ashina, K., Tsubosaka, Y., Nakamura, T., Omori, K., Kobayashi, K., Hori, M., et al. (2015). Histamine induces vascular hyperpermeability by increasing blood flow and endothelial barrier disruption in vivo. PLoS One 10:e0132367. doi: 10.1371/journal.pone.0132367
Atkinson, K. F., Sherpa, R. T., and Nauli, S. M. (2019). The role of the primary cilium in sensing extracellular pH. Cells 8:704. doi: 10.3390/cells8070704
Bangs, F., and Anderson, K. V. (2017). Primary cilia and mammalian hedgehog signaling. Cold Spring Harb. Perspect. Biol. 9:a028175. doi: 10.1101/cshperspect.a028175
Ben, J., Elworthy, S., Ng, A. S., van Eeden, F., and Ingham, P. W. (2011). Targeted mutation of the talpid3 gene in zebrafish reveals its conserved requirement for ciliogenesis and Hedgehog signalling across the vertebrates. Development 138, 4969–4978. doi: 10.1242/dev.070862
Ben-Zvi, A., Lacoste, B., Kur, E., Andreone, B. J., Mayshar, Y., Yan, H., et al. (2014). Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 509, 507–511. doi: 10.1038/nature13324
Blecharz, K. G., Haghikia, A., Stasiolek, M., Kruse, N., Drenckhahn, D., Gold, R., et al. (2010). Glucocorticoid effects on endothelial barrier function in the murine brain endothelial cell line cEND incubated with sera from patients with multiple sclerosis. Mult. Scler. 16, 293–302. doi: 10.1177/1352458509358189
Braniste, V., Al-Asmakh, M., Kowal, C., Anuar, F., Abbaspour, A., Toth, M., et al. (2014). The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 6:263ra158. doi: 10.1126/scitranslmed.3009759
Briscoe, J., and Therond, P. P. (2013). The mechanisms of Hedgehog signalling and its roles in development and disease. Nat. Rev. Mol. Cell Biol. 14, 416–429. doi: 10.1038/nrm3598
Chapouly, C., Guimbal, S., Hollier, P. L., and Renault, M. A. (2019). Role of hedgehog signaling in vasculature development, differentiation, and maintenance. Int. J. Mol. Sci. 20:3076. doi: 10.3390/ijms20123076
Chen, X., Gays, D., Milia, C., and Santoro, M. M. (2017). Cilia control vascular mural cell recruitment in vertebrates. Cell Rep. 18, 1033–1047. doi: 10.1016/j.celrep.2016.12.044
Chistiakov, D. A., Orekhov, A. N., and Bobryshev, Y. V. (2017). Effects of shear stress on endothelial cells: go with the flow. Acta Physiol. 219, 382–408. doi: 10.1111/apha.12725
Chow, B. W., and Gu, C. (2015). The molecular constituents of the blood-brain barrier. Trends Neurosci. 38, 598–608. doi: 10.1016/j.tins.2015.08.003
Cortellino, S., Wang, C., Wang, B., Bassi, M. R., Caretti, E., Champeval, D., et al. (2009). Defective ciliogenesis, embryonic lethality and severe impairment of the Sonic Hedgehog pathway caused by inactivation of the mouse complex A intraflagellar transport gene Ift122/Wdr10, partially overlapping with the DNA repair gene Med1/Mbd4. Dev. Biol. 325, 225–237. doi: 10.1016/j.ydbio.2008.10.020
Daneman, R., Agalliu, D., Zhou, L., Kuhnert, F., Kuo, C. J., and Barres, B. A. (2009). Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc. Natl. Acad. Sci. U.S.A. 106, 641–646. doi: 10.1073/pnas.0805165106
Delling, M., Indzhykulian, A. A., Liu, X., Li, Y., Xie, T., Corey, D. P., et al. (2016). Primary cilia are not calcium-responsive mechanosensors. Nature 531, 656–660. doi: 10.1038/nature17426
Diaz-Coranguez, M., Ramos, C., and Antonetti, D. A. (2017). The inner blood-retinal barrier: cellular basis and development. Vision Res. 139, 123–137. doi: 10.1016/j.visres.2017.05.009
Dong, D., Xie, W., and Liu, M. (2020). Alteration of cell junctions during viral infection. Thorac. Cancer 11, 519–525. doi: 10.1111/1759-7714.13344
Eggenschwiler, J. T., and Anderson, K. V. (2007). Cilia and developmental signaling. Annu. Rev. Cell Dev. Biol. 23, 345–373. doi: 10.1146/annurev.cellbio.23.090506.123249
Eisa-Beygi, S., Benslimane, F. M., El-Rass, S., Prabhudesai, S., Abdelrasoul, M. K. A., Simpson, P. M., et al. (2018). Characterization of endothelial cilia distribution during cerebral-vascular development in zebrafish (Danio rerio). Arterioscler. Thromb. Vasc. Biol. 38, 2806–2818. doi: 10.1161/ATVBAHA.118.311231
Gao, J., Sun, L., Huo, L., Liu, M., Li, D., and Zhou, J. (2010). CYLD regulates angiogenesis by mediating vascular endothelial cell migration. Blood 115, 4130–4137. doi: 10.1182/blood-2009-10-248526
Gerber, J., Heinrich, J., and Brehm, R. (2016). Blood-testis barrier and Sertoli cell function: lessons from SCCx43KO mice. Reproduction 151, R15–R27.
Goetz, S. C., and Anderson, K. V. (2010). The primary cilium: a signalling centre during vertebrate development. Nat. Rev. Genet. 11, 331–344. doi: 10.1038/nrg2774
Gorivodsky, M., Mukhopadhyay, M., Wilsch-Braeuninger, M., Phillips, M., Teufel, A., Kim, C., et al. (2009). Intraflagellar transport protein 172 is essential for primary cilia formation and plays a vital role in patterning the mammalian brain. Dev. Biol. 325, 24–32. doi: 10.1016/j.ydbio.2008.09.019
Grisanti, L., Revenkova, E., Gordon, R. E., and Iomini, C. (2016). Primary cilia maintain corneal epithelial homeostasis by regulation of the Notch signaling pathway. Development 143, 2160–2171. doi: 10.1242/dev.132704
Haycraft, C. J., Banizs, B., Aydin-Son, Y., Zhang, Q., Michaud, E. J., and Yoder, B. K. (2005). Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 1:e53. doi: 10.1371/journal.pgen.0010053
Huang, P., and Schier, A. F. (2009). Dampened Hedgehog signaling but normal Wnt signaling in zebrafish without cilia. Development 136, 3089–3098. doi: 10.1242/dev.041343
Jin, X., Mohieldin, A. M., Muntean, B. S., Green, J. A., Shah, J. V., Mykytyn, K., et al. (2014). Cilioplasm is a cellular compartment for calcium signaling in response to mechanical and chemical stimuli. Cell. Mol. Life Sci. 71, 2165–2178. doi: 10.1007/s00018-013-1483-1
Jones, T. J., Adapala, R. K., Geldenhuys, W. J., Bursley, C., AbouAlaiwi, W. A., Nauli, S. M., et al. (2012). Primary cilia regulates the directional migration and barrier integrity of endothelial cells through the modulation of hsp27 dependent actin cytoskeletal organization. J. Cell. Physiol. 227, 70–76. doi: 10.1002/jcp.22704
Kallakuri, S., Yu, J. A., Li, J., Li, Y., Weinstein, B. M., Nicoli, S., et al. (2015). Endothelial cilia are essential for developmental vascular integrity in zebrafish. J. Am. Soc. Nephrol. 26, 864–875. doi: 10.1681/ASN.2013121314
Kim, J., Lee, J. E., Heynen-Genel, S., Suyama, E., Ono, K., Lee, K., et al. (2010). Functional genomic screen for modulators of ciliogenesis and cilium length. Nature 464, 1048–1051. doi: 10.1038/nature08895
Kim, K., Drummond, I., Ibraghimov-Beskrovnaya, O., Klinger, K., and Arnaout, M. A. (2000). Polycystin 1 is required for the structural integrity of blood vessels. Proc. Natl. Acad. Sci. U.S.A. 97, 1731–1736. doi: 10.1073/pnas.040550097
Konstantoulaki, M., Kouklis, P., and Malik, A. B. (2003). Protein kinase C modifications of VE-cadherin, p120, and beta-catenin contribute to endothelial barrier dysregulation induced by thrombin. Am. J. Physiol. Lung Cell. Mol. Physiol. 285, L434–L442. doi: 10.1152/ajplung.00075.2003
Kotterman, M. A., and Schaffer, D. V. (2014). Engineering adeno-associated viruses for clinical gene therapy. Nat. Rev. Genet. 15, 445–451. doi: 10.1038/nrg3742
Lechtreck, K. F., Brown, J. M., Sampaio, J. L., Craft, J. M., Shevchenko, A., Evans, J. E., et al. (2013). Cycling of the signaling protein phospholipase D through cilia requires the BBSome only for the export phase. J. Cell Biol. 201, 249–261. doi: 10.1083/jcb.201207139
Liebner, S., Corada, M., Bangsow, T., Babbage, J., Taddei, A., Czupalla, C. J., et al. (2008). Wnt/beta-catenin signaling controls development of the blood-brain barrier. J. Cell Biol. 183, 409–417. doi: 10.1083/jcb.200806024
Liu, Z., Tu, H., Kang, Y., Xue, Y., Ma, D., Zhao, C., et al. (2019). Primary cilia regulate hematopoietic stem and progenitor cell specification through Notch signaling in zebrafish. Nat. Commun. 10:1839.
Luu, V. Z., Chowdhury, B., Al-Omran, M., Hess, D. A., and Verma, S. (2018). Role of endothelial primary cilia as fluid mechanosensors on vascular health. Atherosclerosis 275, 196–204. doi: 10.1016/j.atherosclerosis.2018.06.818
Mohieldin, A. M., Zubayer, H. S., Al Omran, A. J., Saternos, H. C., Zarban, A. A., Nauli, S. M., et al. (2016). Vascular endothelial primary cilia: mechanosensation and hypertension. Curr. Hypertens. Rev. 12, 57–67. doi: 10.2174/1573402111666150630140615
Mruk, D. D., and Cheng, C. Y. (2015). The mammalian blood-testis barrier: its biology and regulation. Endocr. Rev. 36, 564–591. doi: 10.1210/er.2014-1101
Nauli, S. M., Kawanabe, Y., Kaminski, J. J., Pearce, W. J., Ingber, D. E., and Zhou, J. (2008). Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation 117, 1161–1171. doi: 10.1161/CIRCULATIONAHA.107.710111
Naylor, A., Hopkins, A., Hudson, N., and Campbell, M. (2019). Tight junctions of the outer blood retina barrier. Int. J. Mol. Sci. 21:211. doi: 10.3390/ijms21010211
Pala, R., Alomari, N., and Nauli, S. M. (2017). Primary cilium-dependent signaling mechanisms. Int. J. Mol. Sci. 18:2272. doi: 10.3390/ijms18112272
Pardridge, W. M. (2012). Drug transport across the blood-brain barrier. J. Cereb. Blood Flow Metab. 32, 1959–1972. doi: 10.1038/jcbfm.2012.126
Qian, F., Germino, F. J., Cai, Y., Zhang, X., Somlo, S., and Germino, G. G. (1997). PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat. Genet. 16, 179–183. doi: 10.1038/ng0697-179
Ran, J., Liu, M., Feng, J., Li, H., Ma, H., Song, T., et al. (2020). ASK1-mediated phosphorylation blocks HDAC6 ubiquitination and degradation to drive the disassembly of photoreceptor connecting cilia. Dev. Cell 53, 287–299. doi: 10.1016/j.devcel.2020.03.010
Rangasamy, S., Srinivasan, R., Maestas, J., McGuire, P. G., and Das, A. (2011). A potential role for angiopoietin 2 in the regulation of the blood-retinal barrier in diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 52, 3784–3791.
Rodriguez, A., Tatter, S. B., and Debinski, W. (2015). Neurosurgical techniques for disruption of the blood-brain barrier for glioblastoma treatment. Pharmaceutics 7, 175–187. doi: 10.3390/pharmaceutics7030175
Satir, P., and Christensen, S. T. (2008). Structure and function of mammalian cilia. Histochem. Cell Biol. 129, 687–693. doi: 10.1007/s00418-008-0416-9
Satir, P., Pedersen, L. B., and Christensen, S. T. (2010). The primary cilium at a glance. J. Cell Sci. 123(Pt 4), 499–503. doi: 10.1242/jcs.050377
Stenman, J. M., Rajagopal, J., Carroll, T. J., Ishibashi, M., McMahon, J., and McMahon, A. P. (2008). Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science 322, 1247–1250. doi: 10.1126/science.1164594
Surace, E. M., Balaggan, K. S., Tessitore, A., Mussolino, C., Cotugno, G., Bonetti, C., et al. (2006). Inhibition of ocular neovascularization by hedgehog blockade. Mol. Ther. 13, 573–579. doi: 10.1016/j.ymthe.2005.10.010
Tetzlaff, F., and Fischer, A. (2018). Control of blood vessel formation by Notch signaling. Adv. Exp. Med. Biol. 1066, 319–338. doi: 10.1007/978-3-319-89512-3_16
Xie, W., Yang, Y., Gao, S., Song, T., Wu, Y., Li, D., et al. (2017). The tumor suppressor CYLD controls epithelial morphogenesis and homeostasis by regulating mitotic spindle behavior and adherens junction assembly. J. Genet. Genomics 44, 343–353. doi: 10.1016/j.jgg.2017.06.002
Yang, Y., Hao, H., Wu, X., Guo, S., Liu, Y., Ran, J., et al. (2019). Mixed-lineage leukemia protein 2 suppresses ciliary assembly by the modulation of actin dynamics and vesicle transport. Cell Discov. 5:33.
Yang, Y., Ran, J., Liu, M., Li, D., Li, Y., Shi, X., et al. (2014). CYLD mediates ciliogenesis in multiple organs by deubiquitinating Cep70 and inactivating HDAC6. Cell Res. 24, 1342–1353. doi: 10.1038/cr.2014.136
Yang, Z., Huang, C., Wu, Y., Chen, B., Zhang, W., and Zhang, J. (2019). Autophagy protects the blood-brain barrier through regulating the dynamic of claudin-5 in short-term starvation. Front. Physiol. 10:2. doi: 10.3389/fphys.2019.00002
Ye, X., Wang, Y., and Nathans, J. (2010). The Norrin/Frizzled4 signaling pathway in retinal vascular development and disease. Trends Mol. Med. 16, 417–425. doi: 10.1016/j.molmed.2010.07.003
Yen, F. T., Roitel, O., Bonnard, L., Notet, V., Pratte, D., Stenger, C., et al. (2008). Lipolysis stimulated lipoprotein receptor: a novel molecular link between hyperlipidemia, weight gain, and atherosclerosis in mice. J. Biol. Chem. 283, 25650–25659. doi: 10.1074/jbc.M801027200
Yu, B., Liu, Z., Fu, Y., Wang, Y., Zhang, L., Cai, Z., et al. (2017). CYLD deubiquitinates nicotinamide adenine dinucleotide phosphate oxidase 4 contributing to adventitial remodeling. Arterioscler. Thromb. Vasc. Biol. 37, 1698–1709. doi: 10.1161/ATVBAHA.117.309859
Yu, F., Guo, S., Li, T., Ran, J., Zhao, W., Li, D., et al. (2019). Ciliary defects caused by dysregulation of O-GlcNAc modification are associated with diabetic complications. Cell Res. 29, 171–173. doi: 10.1038/s41422-018-0114-7
Keywords: vascular barrier, endothelial cell, primary cilium, signaling pathway, disease
Citation: Ma N and Zhou J (2020) Functions of Endothelial Cilia in the Regulation of Vascular Barriers. Front. Cell Dev. Biol. 8:626. doi: 10.3389/fcell.2020.00626
Received: 09 May 2020; Accepted: 23 June 2020;
Published: 09 July 2020.
Edited by:
Helena Soares, University of Lisbon, PortugalReviewed by:
Muqing Cao, Shanghai Jiao Tong University, ChinaCopyright © 2020 Ma and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Zhou, anVuemhvdUBuYW5rYWkuZWR1LmNu; anVuemhvdUBzZG51LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.