- 1Faculty of Dentistry, McGill University, Montréal, QC, Canada
- 2Shriners Hospitals for Children – Canada, Montréal, QC, Canada
- 3Department of Surgery, Mansoura University Cancer Center, Mansoura, Egypt
Metastatic bone disease is generally incurable and leads to pathological fractures, pain, hypercalcemia, spinal cord compression and decreased mobility. The skeleton is the major site of bone metastases from solid cancers, including breast and prostate carcinoma. Bone metastasis is facilitated by activation of bone-resorbing osteoclasts, terminally differentiated multinucleated cells formed by fusion from monocytic precursors. Cancer cells are known to produce specific factors that stimulate osteoclast differentiation and function. Of interest, cancer cells are also known to alter their own bioenergetics increasing the use of glycolysis for their survival and function. Such change in energy utilization by cancer cells would result in altered levels of cell-permeable metabolites, including glucose, lactate, and pyruvate. Osteoclast resorption is energy-expensive, and we have previously demonstrated that during differentiation osteoclasts actively adapt to their bioenergetics microenvironment. We hypothesize that altered bioenergetics state of cancer cells will also modify the bioenergetics substrate availability for the tissue-resident bone cells, potentially creating a favorable milieu for pathological osteolysis. The goals of this review are to analyze how metastasizing cancer cells change the availability of energy substrates in bone microenvironment; and to assess how the altered bioenergetics may affect osteoclast differentiation and activity.
Introduction
Bone is a preferred organ for metastasis from many tumors, including breast, prostate, and lung carcinomas (Hernandez et al., 2018). Establishment of metastatic bone lesions is facilitated by resident osteoclasts, cells that specialize in bone destruction. Molecular signatures that allow successful integration of cancer cells in the bone microenvironment have been extensively investigated (Olechnowicz and Edwards, 2014; Hiraga, 2019), however, none of the identified factors fully explains the success of tumors in thriving in the bone. In this mini-review, we will explore if tumor-mediated changes in bioenergetic environment may contribute to supporting osteoclast formation and function.
Cancer cells are different from their somatic counterparts in many factors, including their bioenergetics. Warburg effect, an increased use of anaerobic glycolysis by cancer cells, has re-gained much attention in the recent years (Lunt and Vander Heiden, 2011; Liberti and Locasale, 2016). The benefits of upregulating glycolysis for cancer cells are not fully understood, since oxidation of one molecule of glucose into pyruvate and 36 molecules of ATP per glucose are produced lactate during glycolysis generates 2 molecules of ATP, while 36 molecules of ATP per glucose are produced during oxidative phosphorylation. However, glycolysis is also important for biosynthesis of nucleotides, lipids and amino acids, all required for cellular proliferation (Lunt and Vander Heiden, 2011). Many metabolites involved in glycolysis and Krebs cycle are transported by the solute-carrier gene (SLC) family of membrane-bound transporters (Markovich and Murer, 2004). Glucose transporters that belong to 2A family of SLCs, represent a rate-limiting step in glycolysis and are known to be strongly dysregulated in cancer cells (Adekola et al., 2012). Lactate and pyruvate are transported by monocarboxylate transporters MCT1-4 that belong to the 16A family of SLCs, and MCT1 and MCT4 are upregulated in several cancers (Jones and Morris, 2016; Li et al., 2018). Importantly, intracellular and extracellular pools of lactate and pyruvate interchange relatively fast (Quek et al., 2016), therefore changes in intracellular metabolite levels lead to corresponding changes in the extracellular environment of cancer cells.
All cells adapt their energy metabolism to changing levels of energy demands, as well as availability of energy substrates. AMP-activated protein kinase (AMPK) is stimulated by an increase in AMP/ATP ratio due to cells inability to meet the current energy demand (Finley and Haigis, 2009). AMPK acts to decrease metabolic expenditure and increase energy production (Gwinn et al., 2008). Mammalian target of rapamycin (mTOR) generally acts downstream of AMPK. Two mTOR complexes, mTORC1 (with raptor and PRAS40) and mTORC2 (with rictor, mSIN1, and proctor) have distinct roles. While mTORC1 regulates protein synthesis (Foster and Toschi, 2009) and the SLC-mediated metabolite transport (Taylor, 2014), mTORC2 is linked to cytoskeletal dynamics and cell survival (Gaubitz et al., 2015). The metabolic sensors, AMPK and mTOR are critical players in cellular adaptation to a varying bioenergetics environment.
The goal of this review is to examine how changes in extracellular glycolytic metabolites due to the presence of actively proliferating cancer cells may alter osteoclast metabolic support, differentiation and function.
Bioenergetics Requirements of Osteoclasts
To understand how osteoclasts can be affected by the metabolic substrates, we need to consider the normal bioenergetic requirements of these cells at different stages of their differentiation and function. Osteoclasts are multinucleated cells formed by fusion of monocytes. Mature osteoclasts attach to bone matrix, forming a sealing zone, where proton pumps lower the extracellular pH to dissolve hydroxyapatite, and proteolytic enzymes are secreted to digest the organic matrix (Stenbeck, 2002). Osteoclasts survive for ∼7–10 days, after which they die primarily by apoptosis (Akchurin et al., 2008; Kopesky et al., 2014). Osteoclast differentiation and function place significant and varied demands for energy required for migration of monocytes for cell fusion, phospholipid synthesis for cell membrane growth, protein synthesis to gain resorptive capacity, action of ion pumps and secretion of proteolytic enzymes. To provide this energy, monocytes increase glucose and oxygen consumption within 24–48 h of exposure to RANKL (Kim et al., 2007), up-regulate metabolic enzymes involved in energy production (Czupalla et al., 2005), and generate abundant large mitochondria (Dudley and Spiro, 1961; Lemma et al., 2016; Figure 1). Mitochondrial biogenesis stimulated by peroxisome proliferator–activated receptor-c coactivator 1β (PGC-1β) is a pre-requisite of successful osteoclastogenesis (Ishii et al., 2009; Wei et al., 2010; Zeng et al., 2015; Zhang et al., 2018). During resorption, osteoclast glucose transport increases 2-fold (Williams et al., 1997) and mitochondria locate near resorption surface (Kawahara et al., 2009). ATP levels markedly increase during osteoclastogenesis (Le Nihouannen et al., 2010). AMPK and mTOR are important for osteoclast differentiation and function. Osteoclastogenesis is associated with changes in AMPK isoform composition (Fong et al., 2013) and AMPK negatively regulates early stages of osteoclast differentiation (Lee et al., 2010; Shah et al., 2010; Kang et al., 2013). Signaling through mTOR is critical for osteoclast formation and survival (Glantschnig et al., 2003; Sugatani and Hruska, 2005; Hu et al., 2016; Dai et al., 2017), while osteoclast fusion and cytoplasmic growth depend on mTOR-mediated Akt signaling (Tiedemann et al., 2017). Importantly, nutrient availability during osteoclast differentiation was shown to significantly affect AMPK, mTORC1 and mTORC2 complexes (Fong et al., 2013; Tiedemann et al., 2017). Thus, it is conceivable that changes in metabolic substrate accessibility due to the presence of proliferating cancer cells may directly affect osteoclast differentiation and function.
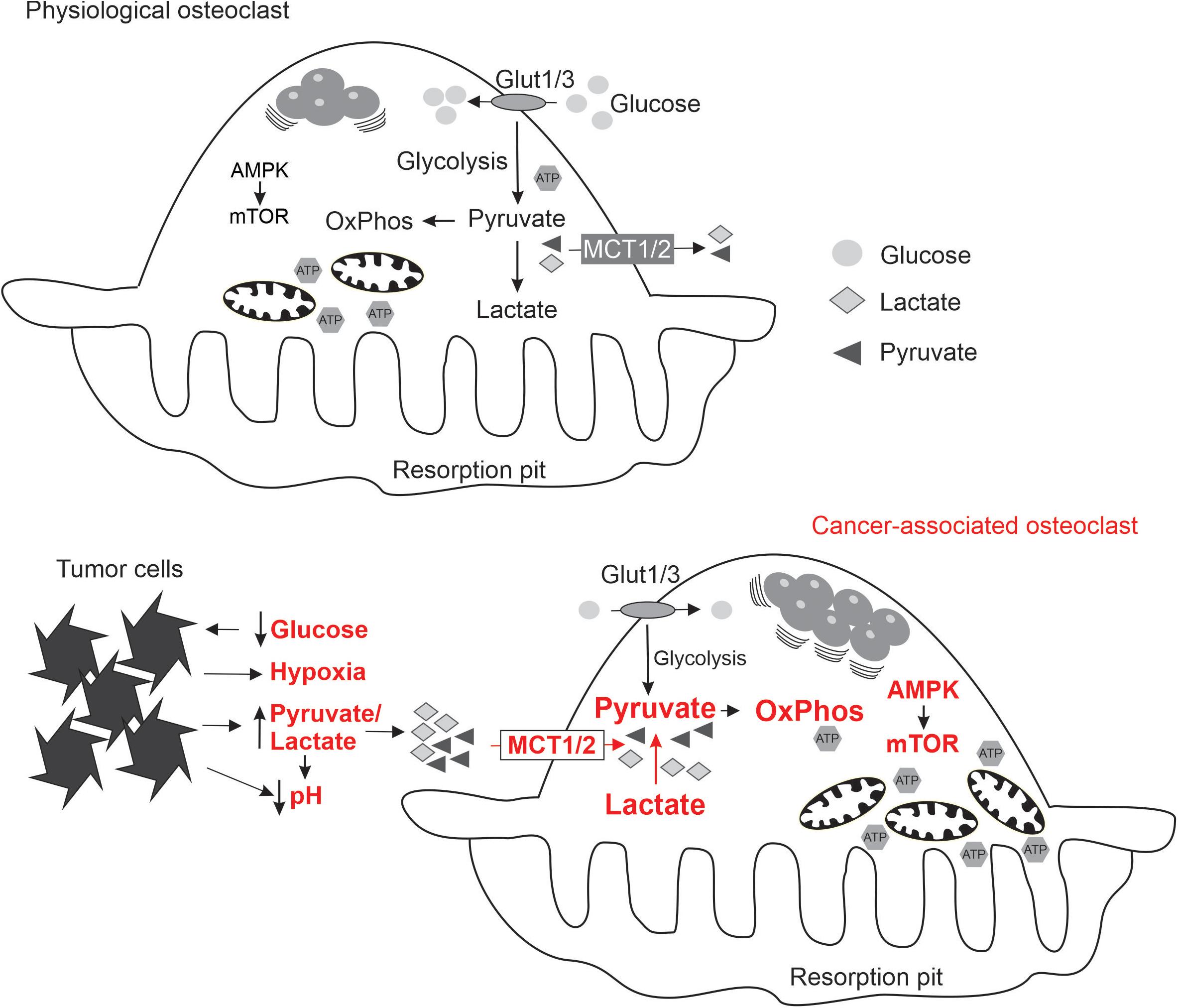
Figure 1. Schematics summarizing the state of energy metabolism in osteoclasts formed under physiological conditions (top), and the adaptive changes in osteoclasts (indicated in red) exposed to the local microenvironment modified by metastasizing cancer cells (bottom).
Potential Effects of Alterations in Metabolic Environment on Osteoclasts
Glucose
Glucose, transported by glucose transporters 1 and 3 (Kim et al., 2007), is the most effective bioenergetics substrate for supporting bone resorption (Williams et al., 1997). In the absence of glucose, fatty acids, ketone bodies, and lactate can support bone resorption at 20–30% of the levels achievable with glucose (Williams et al., 1997). Nevertheless, the dose-dependence of glucose effects is complex. An increase from less than 1 mM to 5–10 mM glucose was demonstrated to stimulate osteoclastogenesis (Kim et al., 2007), resorption (Williams et al., 1997), and osteoclastogenic signaling through p38 mitogen-activated protein kinase (Larsen et al., 2002) and calcium/calmodulin-dependent kinase II (CaMK II) (Larsen et al., 2005). In a mouse model of type 2 diabetes, moderate hyperglycemia [∼10 mM circulating glucose (Fernandez et al., 2001)] was associated with increased osteoclastogenesis (Kawashima et al., 2009). In contrast, high glucose concentrations inhibit osteoclastogenesis (Kim et al., 2007; Wittrant et al., 2008), which could be explained by metabolic effects, such as decreased oxygen consumption at higher glucose level [similar to the Crabtree effect observed in yeasts (Pfeiffer and Morley, 2014)], as well as osmotic effects (Botolin and McCabe, 2006). In the environment of highly glycolytic cancer cells, the ambient glucose levels would likely decrease, reducing its availability for osteoclastogenesis. Thus, decrease in glucose is unlikely to contribute to osteoclastogenic effects of cancer cells.
Pyruvate
Several studies have investigated how pyruvate affects osteoclast formation. Addition of small amounts of pyruvate to media containing normal levels of glucose significantly increased osteoclastogenesis (Kim et al., 2007; Fong et al., 2013), resulting in formation of large osteoclasts that contained more nuclei per cell (Fong et al., 2013; Tiedemann et al., 2017). Of interest, only when added in relatively small amounts, between 1 and 2 mM (Fong et al., 2013; Tiedemann et al., 2017) and 5 mM (Kim et al., 2007), pyruvate was effective in promoting osteoclast formation. Addition of low pyruvate concentrations stimulated osteoclast mitochondrial activity, leading to a metabolic shift toward oxidative phosphorylation, and an increase in cellular [ATP] (Kim et al., 2007; Fong et al., 2013). Pyruvate caused an inhibition of AMPK and an activation of mTOR/raptor complex leading to facilitated protein synthesis and cytoplasmic growth (Fong et al., 2013; Tiedemann et al., 2017). MCT1, 2, and 4 for lactate and pyruvate are expressed by osteoclasts (Imai et al., 2019). MCT2 has the highest affinity for both pyruvate (Km ∼0.1 mM) and lactate (Km ∼0.7 mM), compared to MCT1 that has a Km value in millimolar range, and MCT4, affinity of which is even lower (Halestrap, 2012). Low concentration of MCT inhibitor or deletion of MCT1 were shown to potentiate osteoclastogenesis, while high concentration of MCT inhibitor or deletion of MCT2 prevented osteoclast formation (Imai et al., 2019). Another important issue with the interpretation of pyruvate effects was highlighted by Long and Halliwell (2009), who demonstrated that addition of pyruvate dramatically affects the media levels of hydrogen peroxide, which in turn affects osteoclastogenesis (Le Nihouannen et al., 2010). Nevertheless, no anti-oxidative effects were observed after addition of small amounts of pyruvate (Fong et al., 2013). Increase in glycolysis due to Warburg effect in cancer cells can lead to increased production of pyruvate that can in turn be transported to the extracellular space (Doherty and Cleveland, 2013; Quek et al., 2016), and provide increased bioenergetic support for osteoclast formation.
Krebs Cycle Metabolites
Krebs cycle occurs in the mitochondria, however, several of its metabolites, including citrate, succinate, malate, oxaloacetate, fumarate, and α-ketoglutarate can be transported through the cell membrane by sodium-dependent SLC13 transporters (Markovich and Murer, 2004; Pajor, 2014). Citrate in particular gained a lot of interest, since its extracellular levels vary in diseases (Huang et al., 2020). Of particular interest is reported reduction in plasma citrate levels in prostate and lung cancers that readily metastasize to bone (Rocha et al., 2011; Dittrich et al., 2012), as well as in osteoporosis, in which citrate is also reduced in bone (major citrate reservoir) (Chen et al., 2018). Extracellular citrate affects osteoclastogenesis, however, contradictory outcomes were reported. Similar to pyruvate, 1–2 mM of sodium citrate was shown to enhance osteoclastogenesis (Fong et al., 2013). However, potassium citrate dose-dependently inhibited osteoclast formation at similar concentrations (Granchi et al., 2017). Importantly, osteoclast inhibition was also observed upon addition of potassium ion K+ (KCl) (Yeon et al., 2015), suggesting that the effect of citrate may depend on media composition. Another potentially important link to Krebs cycle metabolites was proposed through glutamate metabolism. The glutamine transporter from SLC family 1a5 and glutaminase-1 converting glutamine to glutamate were shown to increase during osteoclastogenesis, leading authors to speculate that glutamate can be converted to α-ketoglutarate, which fuels energy metabolism (Indo et al., 2013). However, actively secretion of glutamate by osteoclasts was also demonstrated (Morimoto et al., 2006; Seidlitz et al., 2010). Thus, while glutamate likely plays an important role during osteoclastogenesis, it is difficult to conclude if its main action is relevant to energy metabolism. No information about other Krebs cycle intermediary is currently available. Thus, while the decreased citrate levels associated with cancer may affect osteoclastogenesis, the outcome of these interactions is uncertain and likely influenced by the localized cell microenvironment.
Mitochondria
The presence of highly proliferative cancer cells results in hypoxic microenvironment (Al Tameemi et al., 2019), which stimulates osteoclast differentiation and supports resorption (Arnett, 2010; Knowles, 2015). Hypoxic environment leads to a surprising improvement of mitochondrial function and ATP production in osteoclasts (Knowles, 2015), which may be due to reduction in proton leak and uncoupled respiration noted in mitochondria exposed to low oxygen tension (Gnaiger et al., 2000). Mitochondria activity is also linked to the production of reactive oxygen species (ROS) such as peroxide and superoxide (Knowles, 2015). ROS generate oxidative stress, which is counteracted by cellular glutathione (GSH) producing its oxidized form, glutathione disulfide (GSSG). Oxidative stress has a bimodal effect on osteoclasts: while moderate stress resulting in GSH/GSSG decrease is stimulatory for osteoclastogenesis, severe stress leading to glutathione depletion inhibits resorption and limits osteoclast lifespan (Kim et al., 2006; Le Nihouannen et al., 2010; Domazetovic et al., 2017). Cancer cells also actively modulate their oxidative microenvironment by secreting antioxidants, such as peroxiredoxin 4 (Rafiei et al., 2015; Tiedemann et al., 2019), suggesting tumor-associated oxidative stress may differ for tumor types and stages of their growth. Additionally, oxidative stress is also induced by chemotherapy, such as doxorubicin (Rana et al., 2013). Thus, hypoxia and potentially oxidative stress generated by cancer cells may provide a microenvironment that supports osteoclastogenesis.
pH and Lactate
Changes in pH are integral to the metabolic glucose processing. Anaerobic glycolysis results in acidification due to production of two molecules of lactic acid per each glucose (lactic acidosis), while complete mitochondrial oxidation of glucose generates six protons per glucose. Active metabolism of proliferating cancer cells is well recognized to produce acidic extracellular environment (Corbet and Feron, 2017). Acidification is also known to be a prerequisite of successful osteoclastogenesis (Arnett, 2010; Yuan et al., 2016; Arnett and Orriss, 2018). Osteoclasts sense extracellular acidosis through the G-protein coupled receptors, including ovarian cancer G-protein-coupled receptor 1 (OGR1) (Yang et al., 2006; Pereverzev et al., 2008; Li et al., 2009; Yuan et al., 2014) and T cell death-associated gene 8 (TDAG8) (Hikiji et al., 2014). In addition, osteoclasts express acid-sensitive ion channels (ASIC) (Jahr et al., 2005; Li et al., 2013). Acidosis was demonstrated to induce nuclear translocation of key osteoclastogenic transcription factor, nuclear factor of activated T cells 1c (NFATc1) (Komarova et al., 2005; Li et al., 2013) resulting in improved osteoclast formation (Granchi et al., 2017), resorptive activity (Komarova et al., 2005; Ahn et al., 2016), and survival (Pereverzev et al., 2008). Lactate was shown to be taken up by osteoclast precursors via MCT1 and to drive oxidative phosphorylation thereby facilitating bone resorption (Lemma et al., 2017). Thus, tumor-associated tissue acidosis and increased extracellular lactate can be expected to promote osteoclast differentiation and activity.
Metabolic Adaptation of Osteoclasts to Cancer Microenvironment
Metastasizing cancer cells generate unique bioenergetics microenvironment: while normal substrates, glucose and oxygen, are consumed by cancer cells, and therefore not available for osteoclasts, cancer cells generate alternative substrates such as pyruvate and lactate. In addition, acidic, hypoxic and potentially oxidative environment is uniquely supportive for osteoclastogenesis. To successfully perform in this altered microenvironment, osteoclasts need metabolic sensors to adapt their energy metabolism (Figure 1). We have shown that soluble factors produced by breast cancer cells induce a change in osteoclast mTOR signaling (Hussein et al., 2012). Moreover, targeting mTOR with rapamycin in the mouse model of experimental bone metastases resulted in a significant attenuation of cancer-induced osteolysis (Hussein et al., 2012; Abdelaziz et al., 2014), but had minimal effect on osteoclasts in the cancer-free bones of the same animals (Abdelaziz et al., 2015). These findings suggest that metabolic sensors are central for osteoclast adaptation to the metastatic microenvironment, and may represent therapeutic targets reviewed in the following section.
Effect of Bioenergetics Targeting Therapies on Bone Metastasis
Therapeutics targeting metabolic sensors, such as metformin for AMPK and rapamycin for mTOR, have been successfully used for many years in a number of conditions including diabetes (Kezic et al., 2018) and organ transplantation (Augustine et al., 2007; Nguyen et al., 2019). In this section we attempted to review available evidence for the effectiveness of metformin and rapamycin and their analogs in preventing and/or controlling bone metastases.
Metformin
Metformin is an anti-diabetic drug that activates AMPK (Faubert et al., 2015). In cancer cells, loss of AMPK induced a typical Warburg effect in transformed and non-transformed cells (Faubert et al., 2013), and promoted unchecked mTORC1 activity (Inoki et al., 2003). Activation of AMPK has multiple anti-tumor effects (Schulten, 2018), particularly in colorectal and prostate cancer patients (Coyle et al., 2016). In bone, in addition to its role in osteoclastogenesis, AMPK reduced the expression of osteoclastogenic cytokine RANKL (Lee et al., 2010; Wang et al., 2013; Cuyàs et al., 2017). While reports of treatment of bone metastases with metformin are sparse (Wang et al., 2013), a reduction in growth of primary tumor and metastases was demonstrated in a model of castration-resistant prostatic carcinoma upon treatment with metformin and simvastatin (Babcook et al., 2014). Limited number of reports regarding the effectiveness of metformin can be explained by the study that demonstrated that metformin looses its ability to activate AMPK in hypoxic conditions, which are commonly associated with growing tumor (Garofalo et al., 2013).
Rapamycin and Its Analogs
In preclinical models of breast cancer bone metastases, rapamycin reduced osteolysis and bone pain, and improved animal survival (Hussein et al., 2012; Abdelaziz et al., 2014). Everolimus, a rapamycin analog more selective toward mTORC1 pathway, was also effective in preventing or treating experimental bone metastases from breast (Simone et al., 2015; Browne et al., 2017), prostate (Morgan et al., 2008), and lung (Yu et al., 2014) cancers. Several clinical trials evaluated the effectiveness of everolimus therapy in the treatment of hormone-receptor positive, Her2/Neu negative advanced breast cancer patients. A phase III, double-blind, randomized international BOLERO-2 trial compared the combination of anti-estrogen aromatase inhibitor exemestane with everolimus or placebo in postmenopausal women with advanced breast cancer. In addition to increasing progression-free survival (Yardley et al., 2013), everolimus markedly decreased levels of bone resorption biomarkers in patients with or without bone metastases (Gnant et al., 2013). RADAR clinical trial reported the effectiveness of everolimus in increasing the time to progression in a phase II double-blind, placebo-controlled, randomized discontinuation study in advanced breast cancer patients with bone metastases only (Maass et al., 2013). Thus, targeting mTOR appears promising in preclinical and clinical studies.
Overall Conclusion
The presence of cancer cells in the bone microenvironment likely results in local hypoglycemia and hypoxia. However, an increased glycolysis due to the Warburg effect in cancer cells may provide alternative metabolic substrates such as superfluous pyruvate and lactate. Adaptation of osteoclasts to such environment likely require the activity of metabolic sensors AMPK and mTOR. Importantly, osteoclasts are known to successfully adapt their mitochondrial function to conditions of hypoxia, which in osteoclasts stimulates ATP production, differentiation and function (Knowles, 2015). Acidification is another cancer-driven change in the microenvironment that is known to be specifically stimulatory for osteoclast formation and function (Arnett and Orriss, 2018). Thus, osteoclasts formed in the osteolytic tumor lesions are likely different from physiologically formed in their reliance on alternative metabolic substrates, adjusted activity of metabolic sensors, and unusual mitochondria function. Of interest, the combination of syrosingopine-mediated inhibition of MCT1 and 2 with metformin was recently demonstrated to result in synthetic lethality for cancer cells (Benjamin et al., 2018). We suggest that such drug combinations may target both cancer cells and cancer-supportive osteoclasts alleviating destructive and painful bone metastasis.
Author Contributions
KT and SK conceived the study, researched, and summarized the preclinical studies. OH researched and summarized the clinical studies. All authors contributed to manuscript writing and approved the final version of the manuscript.
Funding
This work was supported by the Canadian Institutes of Health Research (MOP-77643 and PJT-152926 to SK).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
AMPK, AMP-activated protein kinase; mTOR, Mammalian target of rapamycin; MCT, Monocarboxylate transporters; PGC-1 β, Peroxisome proliferator–activated receptor-c coactivator 1 β; RANKL, Receptor activator of nuclear factor kappa B-ligand; SLC, Solute carrier transporters.
References
Abdelaziz, D. M., Stone, L. S., and Komarova, S. V. (2014). Osteolysis and pain due to experimental bone metastases are improved by treatment with rapamycin. Breast Cancer Res. Treat. 143, 227–237. doi: 10.1007/s10549-013-2799-0
Abdelaziz, D. M., Stone, L. S., and Komarova, S. V. (2015). Localized experimental bone metastasis drives osteolysis and sensory hypersensitivity at distant non-tumor-bearing sites. Breast Cancer Res. Treat. 153, 9–20. doi: 10.1007/s10549-015-3517-x
Adekola, K., Rosen, S. T., and Shanmugam, M. (2012). Glucose transporters in cancer metabolism. Curr. Opin. Oncol 24, 650–654. doi: 10.1097/CCO.0b013e328356da72
Ahn, H., Lee, K., Kim, J. M., Kwon, S. H., Lee, S. H., Lee, S. Y., et al. (2016). Accelerated lactate dehydrogenase activity potentiates osteoclastogenesis via NFATc1 signaling. PLoS One 11:e0153886. doi: 10.1371/journal.pone.0153886
Akchurin, T., Aissiou, T., Kemeny, N., Prosk, E., Nigam, N., and Komarova, S. V. (2008). Complex dynamics of osteoclast formation and death in long-term cultures. PLoS One 3:e2104. doi: 10.1371/journal.pone.0002104
Al Tameemi, W., Dale, T. P., Al-Jumaily, R. M. K., and Forsyth, N. R. (2019). Hypoxia-modified cancer cell metabolism. Front. Cell Dev. Biol. 7:4. doi: 10.3389/fcell.2019.00004
Arnett, T. R. (2010). Acidosis, hypoxia and bone. Arch. Biochem. Biophys. 503, 103–109. doi: 10.1016/j.abb.2010.07.021
Arnett, T. R., and Orriss, I. R. (2018). Metabolic properties of the osteoclast. Bone 115, 25–30. doi: 10.1016/j.bone.2017.12.021
Augustine, J. J., Bodziak, K. A., and Hricik, D. E. (2007). Use of sirolimus in solid organ transplantation. Drugs 67, 369–391. doi: 10.2165/00003495-200767030-00004
Babcook, M. A., Shukla, S., Fu, P., Vazquez, E. J., Puchowicz, M. A., Molter, J. P., et al. (2014). Synergistic simvastatin and metformin combination chemotherapy for osseous metastatic castration-resistant prostate cancer. Mol. Cancer Ther. 13, 2288–2302. doi: 10.1158/1535-7163.mct-14-0451
Benjamin, D., Robay, D., Hindupur, S. K., Pohlmann, J., Colombi, M., El-Shemerly, M. Y., et al. (2018). Dual inhibition of the lactate transporters MCT1 and MCT4 is synthetic lethal with metformin due to NAD+ depletion in cancer cells. Cell Rep. 25, 3047.e4–3058.e4. doi: 10.1016/j.celrep.2018.11.043
Botolin, S., and McCabe, L. R. (2006). Chronic hyperglycemia modulates osteoblast gene expression through osmotic and non-osmotic pathways. J. Cell. Biochem. 99, 411–424. doi: 10.1002/jcb.20842
Browne, A. J., Kubasch, M. L., Gobel, A., Hadji, P., Chen, D., Rauner, M., et al. (2017). Concurrent antitumor and bone-protective effects of everolimus in osteotropic breast cancer. Breast Cancer Res. 19:92. doi: 10.1186/s13058-017-0885-7
Chen, H., Wang, Y., Dai, H., Tian, X., Cui, Z. K., Chen, Z., et al. (2018). Bone and plasma citrate is reduced in osteoporosis. Bone 114, 189–197. doi: 10.1016/j.bone.2018.06.014
Corbet, C., and Feron, O. (2017). Tumour acidosis: from the passenger to the driver’s seat. Nat. Rev. Cancer 17, 577–593. doi: 10.1038/nrc.2017.77
Coyle, C., Cafferty, F. H., Vale, C., and Langley, R. E. (2016). Metformin as an adjuvant treatment for cancer: a systematic review and meta-analysis. Ann. Oncol. 27, 2184–2195. doi: 10.1093/annonc/mdw410
Cuyàs, E., Martin-Castillo, B., Bosch-Barrera, J., and Menendez, J. A. (2017). Metformin inhibits RANKL and sensitizes cancer stem cells to denosumab. Cell Cycle 16, 1022–1028. doi: 10.1080/15384101.2017.1310353
Czupalla, C., Mansukoski, H., Pursche, T., Krause, E., and Hoflack, B. (2005). Comparative study of protein and mRNA expression during osteoclastogenesis. Proteomics 5, 3868–3875. doi: 10.1002/pmic.200402059
Dai, Q., Xie, F., Han, Y., Ma, X., Zhou, S., Jiang, L., et al. (2017). Inactivation of regulatory-associated Protein of mTOR (Raptor)/mammalian target of rapamycin complex 1 (mTORC1) signaling in osteoclasts increases bone mass by inhibiting osteoclast differentiation in mice. J. Biol. Chem. 292, 196–204. doi: 10.1074/jbc.M116.764761
Dittrich, R., Kurth, J., Decelle, E. A., DeFeo, E. M., Taupitz, M., Wu, S., et al. (2012). Assessing prostate cancer growth with citrate measured by intact tissue proton magnetic resonance spectroscopy. Prostate Cancer Prostatic. Dis. 15, 278–282. doi: 10.1038/pcan.2011.70
Doherty, J. R., and Cleveland, J. L. (2013). Targeting lactate metabolism for cancer therapeutics. J. Clin. Invest. 123, 3685–3692. doi: 10.1172/jci69741
Domazetovic, V., Marcucci, G., Iantomasi, T., Brandi, M. L., and Vincenzini, M. T. (2017). Oxidative stress in bone remodeling: role of antioxidants. Clin. Cases Miner. Bone Metab. 14, 209–216. doi: 10.11138/ccmbm/2017.14.1.209
Dudley, H. R., and Spiro, D. (1961). The fine structure of bone cells. J. Biophys. Biochem. Cytol 11, 627–649. doi: 10.1083/jcb.11.3.627
Faubert, B., Boily, G., Izreig, S., Griss, T., Samborska, B., Dong, Z., et al. (2013). AMPK is a negative regulator of the warburg effect and suppresses tumor growth in vivo. Cell Metab. 17, 113–124. doi: 10.1016/j.cmet.2012.12.001
Faubert, B., Vincent, E. E., Poffenberger, M. C., and Jones, R. G. (2015). The AMP-activated protein kinase (AMPK) and cancer: many faces of a metabolic regulator. Cancer Metab. 356(2, Pt A), 165–170. doi: 10.1016/j.canlet.2014.01.018
Fernandez, A. M., Kim, J. K., Yakar, S., Dupont, J., Hernandez-Sanchez, C., Castle, A. L., et al. (2001). Functional inactivation of the IGF-I and insulin receptors in skeletal muscle causes type 2 diabetes. Genes Dev. 15, 1926–1934. doi: 10.1101/gad.908001
Finley, L. W., and Haigis, M. C. (2009). The coordination of nuclear and mitochondrial communication during aging and calorie restriction. Ageing Res. Rev. 8, 173–188. doi: 10.1016/j.arr.2009.03.003
Fong, J. E., Le Nihouannen, D., Tiedemann, K., Sadvakassova, G., Barralet, J. E., and Komarova, S. V. (2013). Moderate excess of pyruvate augments osteoclastogenesis. Biol. Open 2, 387–395. doi: 10.1242/bio.20133269
Foster, D. A., and Toschi, A. (2009). Targeting mTOR with rapamycin: one dose does not fit all. Cell Cycle 8, 1026–1029. doi: 10.4161/cc.8.7.8044
Garofalo, C., Capristo, M., Manara, M. C., Mancarella, C., Landuzzi, L., Belfiore, A., et al. (2013). Metformin as an adjuvant drug against pediatric sarcomas: hypoxia limits therapeutic effects of the drug. PLoS One 8:e83832. doi: 10.1371/journal.pone.0083832
Gaubitz, C., Oliveira, T. M., Prouteau, M., Leitner, A., Karuppasamy, M., Konstantinidou, G., et al. (2015). Molecular basis of the rapamycin insensitivity of target of rapamycin complex 2. Mol. Cell. 58, 977–988. doi: 10.1016/j.molcel.2015.04.031
Glantschnig, H., Fisher, J. E., Wesolowski, G., Rodan, G. A., and Reszka, A. A. (2003). M-CSF, TNFalpha and RANK ligand promote osteoclast survival by signaling through mTOR/S6 kinase. Cell Death. Differ. 10, 1165–1177. doi: 10.1038/sj.cdd.4401285
Gnaiger, E., Mendez, G., and Hand, S. C. (2000). High phosphorylation efficiency and depression of uncoupled respiration in mitochondria under hypoxia. Proc. Natl. Acad. Sci. U.S.A. 97, 11080–11085. doi: 10.1073/pnas.97.20.11080
Gnant, M., Baselga, J., Rugo, H. S., Noguchi, S., Burris, H. A., Piccart, M., et al. (2013). Effect of everolimus on bone marker levels and progressive disease in bone in BOLERO-2. J. Natl. Cancer Inst. 105, 654–663. doi: 10.1093/jnci/djt026
Granchi, D., Torreggiani, E., Massa, A., Caudarella, R., Di Pompo, G., and Baldini, N. (2017). Potassium citrate prevents increased osteoclastogenesis resulting from acidic conditions: implication for the treatment of postmenopausal bone loss. PLoS One 12:e0181230. doi: 10.1371/journal.pone.0181230
Gwinn, D. M., Shackelford, D. B., Egan, D. F., Mihaylova, M. M., Mery, A., Vasquez, D. S., et al. (2008). AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell. 30, 214–226. doi: 10.1016/j.molcel.2008.03.003
Halestrap, A. P. (2012). The monocarboxylate transporter family–Structure and functional characterization. IUBMB Life 64, 1–9. doi: 10.1002/iub.573
Hernandez, R. K., Wade, S. W., Reich, A., Pirolli, M., Liede, A., and Lyman, G. H. (2018). Incidence of bone metastases in patients with solid tumors: analysis of oncology electronic medical records in the United States. BMC Cancer 18:44. doi: 10.1186/s12885-017-3922-0
Hikiji, H., Endo, D., Horie, K., Harayama, T., Akahoshi, N., Igarashi, H., et al. (2014). TDAG8 activation inhibits osteoclastic bone resorption. FASEB J. 28, 871–879. doi: 10.1096/fj.13-233106
Hiraga, T. (2019). Bone metastasis: interaction between cancer cells and bone microenvironment. J. Oral. Biosci. 61, 95–98. doi: 10.1016/j.job.2019.02.002
Hu, Y., Carraro-Lacroix, L. R., Wang, A., Owen, C., Bajenova, E., Corey, P. N., et al. (2016). Lysosomal pH plays a key role in regulation of mtor activity in osteoclasts. J. Cell. Biochem. 117, 413–425. doi: 10.1002/jcb.25287
Huang, L., Wang, C., Xu, H., and Peng, G. (2020). Targeting citrate as a novel therapeutic strategy in cancer treatment. Biochim. Biophys. Acta Rev. Cancer 1873:188332. doi: 10.1016/j.bbcan.2019.188332
Hussein, O., Tiedemann, K., Murshed, M., and Komarova, S. V. (2012). Rapamycin inhibits osteolysis and improves survival in a model of experimental bone metastases. Cancer Lett. 314, 176–184. doi: 10.1016/j.canlet.2011.09.026
Imai, H., Yoshimura, K., Miyamoto, Y., Sasa, K., Sugano, M., Chatani, M., et al. (2019). Roles of monocarboxylate transporter subtypes in promotion and suppression of osteoclast differentiation and survival on bone. Sci. Rep. 9:15608. doi: 10.1038/s41598-019-52128-2
Indo, Y., Takeshita, S., Ishii, K. A., Hoshii, T., Aburatani, H., Hirao, A., et al. (2013). Metabolic regulation of osteoclast differentiation and function. J. Bone. Miner. Res. 28, 2392–2399. doi: 10.1002/jbmr.1976
Inoki, K., Zhu, T., and Guan, K.-L. (2003). TSC2 mediates cellular energy response to control cell growth and survival. Cell 115, 577–590.
Ishii, K. A., Fumoto, T., Iwai, K., Takeshita, S., Ito, M., Shimohata, N., et al. (2009). Coordination of PGC-1beta and iron uptake in mitochondrial biogenesis and osteoclast activation. Nat. Med. 15, 259–266. doi: 10.1038/nm.1910
Jahr, H., van Driel, M., van Osch, G. J., Weinans, H., and van Leeuwen, J. P. (2005). Identification of acid-sensing ion channels in bone. Biochem. Biophys. Res. Commun. 337, 349–354. doi: 10.1016/j.bbrc.2005.09.054
Jones, R. S., and Morris, M. E. (2016). Monocarboxylate transporters: therapeutic targets and prognostic factors in disease. Clin. Pharmacol. Ther 100, 454–463. doi: 10.1002/cpt.418
Kang, H., Viollet, B., and Wu, D. (2013). Genetic deletion of catalytic subunits of AMP-activated protein kinase increases osteoclasts and reduces bone mass in young adult mice. J. Biol. Chem. 288, 12187–12196. doi: 10.1074/jbc.M112.430389
Kawahara, I., Koide, M., Tadokoro, O., Udagawa, N., Nakamura, H., Takahashi, N., et al. (2009). The relationship between calcium accumulation in osteoclast mitochondrial granules and bone resorption. Bone 45, 980–986. doi: 10.1016/j.bone.2009.07.010
Kawashima, Y., Fritton, J. C., Yakar, S., Epstein, S., Schaffler, M. B., Jepsen, K. J., et al. (2009). Type 2 diabetic mice demonstrate slender long bones with increased fragility secondary to increased osteoclastogenesis. Bone 44, 648–655. doi: 10.1016/j.bone.2008.12.012
Kezic, A., Popovic, L., and Lalic, K. (2018). mTOR inhibitor therapy and metabolic consequences: where do we stand? Oxid Med. Cell Longev 2018:2640342. doi: 10.1155/2018/2640342
Kim, H., Kim, I. Y., Lee, S. Y., and Jeong, D. (2006). Bimodal actions of reactive oxygen species in the differentiation and bone-resorbing functions of osteoclasts. FEBS Lett. 580, 5661–5665. doi: 10.1016/j.febslet.2006.09.015
Kim, J. M., Jeong, D., Kang, H. K., Jung, S. Y., Kang, S. S., and Min, B. M. (2007). Osteoclast precursors display dynamic metabolic shifts toward accelerated glucose metabolism at an early stage of RANKL-stimulated osteoclast differentiation. Cell. Physiol. Biochem. 20, 935–946.
Knowles, H. J. (2015). Hypoxic regulation of osteoclast differentiation and bone resorption activity. Hypoxia 3, 73–82. doi: 10.2147/hp.s95960
Komarova, S. V., Pereverzev, A., Shum, J. W., Sims, S. M., and Dixon, S. J. (2005). Convergent signaling by acidosis and receptor activator of NF-kappaB ligand (RANKL) on the calcium/calcineurin/NFAT pathway in osteoclasts. Proc. Natl. Acad. Sci. U.S.A. 102, 2643–2648. doi: 10.1073/pnas.0406874102
Kopesky, P., Tiedemann, K., Alkekhia, D., Zechner, C., Millard, B., Schoeberl, B., et al. (2014). Autocrine signaling is a key regulatory element during osteoclastogenesis. Biol. Open 3, 767–776. doi: 10.1242/bio.20148128
Larsen, K. I., Falany, M. L., Ponomareva, L. V., Wang, W., and Williams, J. P. (2002). Glucose-dependent regulation of osteoclast H(+)-ATPase expression: potential role of p38 MAP-kinase. J. Cell. Biochem. 87, 75–84. doi: 10.1002/jcb.10252
Larsen, K. I., Falany, M., Wang, W., and Williams, J. P. (2005). Glucose is a key metabolic regulator of osteoclasts; glucose stimulated increases in ATP/ADP ratio and calmodulin kinase II activity. Biochem. Cell Biol. 83, 667–673. doi: 10.1139/o05-136
Le Nihouannen, D., Barralet, J. E., Fong, J. E., and Komarova, S. V. (2010). Ascorbic acid accelerates osteoclast formation and death. Bone 46, 1336–1343. doi: 10.1016/j.bone.2009.11.021
Lee, Y. S., Kim, Y. S., Lee, S. Y., Kim, G. H., Kim, B. J., Lee, S. H., et al. (2010). AMP kinase acts as a negative regulator of RANKL in the differentiation of osteoclasts. Bone 47, 926–937. doi: 10.1016/j.bone.2010.08.001
Lemma, S., Di Pompo, G., Porporato, P. E., Sboarina, M., Russell, S., Gillies, R. J., et al. (2017). MDA-MB-231 breast cancer cells fuel osteoclast metabolism and activity: a new rationale for the pathogenesis of osteolytic bone metastases. Biochim. Biophys. Acta Mol. Basis Dis. 1863, 3254–3264. doi: 10.1016/j.bbadis.2017.08.030
Lemma, S., Sboarina, M., Porporato, P. E., Zini, N., Sonveaux, P., Di Pompo, G., et al. (2016). Energy metabolism in osteoclast formation and activity. Int. J. Biochem. Cell Biol. 79, 168–180. doi: 10.1016/j.biocel.2016.08.034
Li, H., Wang, D., Singh, L. S., Berk, M., Tan, H., Zhao, Z., et al. (2009). Abnormalities in osteoclastogenesis and decreased tumorigenesis in mice deficient for ovarian cancer G protein-coupled receptor 1. PLoS One 4:e5705. doi: 10.1371/journal.pone.0005705
Li, X., Xu, R. S., Jiang, D. L., He, X. L., Jin, C., Lu, W. G., et al. (2013). Acid-sensing ion channel 1a is involved in acid-induced osteoclastogenesis by regulating activation of the transcription factor NFATc1. FEBS Lett. 587, 3236–3242. doi: 10.1016/j.febslet.2013.08.017
Li, Z., Wu, Q., Sun, S., Wu, J., Li, J., Zhang, Y., et al. (2018). Monocarboxylate transporters in breast cancer and adipose tissue are novel biomarkers and potential therapeutic targets. Biochem. Biophys. Res. Commun. 501, 962–967. doi: 10.1016/j.bbrc.2018.05.091
Liberti, M. V., and Locasale, J. W. (2016). The warburg effect: how does it benefit cancer cells? Trends Biochem. Sci. 41, 211–218. doi: 10.1016/j.tibs.2015.12.001
Long, L. H., and Halliwell, B. (2009). Artefacts in cell culture: pyruvate as a scavenger of hydrogen peroxide generated by ascorbate or epigallocatechin gallate in cell culture media. Biochem. Biophys. Res. Commun. 388, 700–704. doi: 10.1016/j.bbrc.2009.08.069
Lunt, S. Y., and Vander Heiden, M. G. (2011). Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 27, 441–464. doi: 10.1146/annurev-cellbio-092910-154237
Maass, N., Harbeck, N., Mundhenke, C., Lerchenmuller, C., Barinoff, J., Luck, H. J., et al. (2013). Everolimus as treatment for breast cancer patients with bone metastases only: results of the phase II RADAR study. J. Cancer Res. Clin. Oncol. 139, 2047–2056. doi: 10.1007/s00432-013-1518-x
Markovich, D., and Murer, H. (2004). The SLC13 gene family of sodium sulphate/carboxylate cotransporters. Pflugers. Arch. 447, 594–602. doi: 10.1007/s00424-003-1128-6
Morgan, T. M., Pitts, T. E., Gross, T. S., Poliachik, S. L., Vessella, R. L., and Corey, E. (2008). RAD001 (Everolimus) inhibits growth of prostate cancer in the bone and the inhibitory effects are increased by combination with docetaxel and zoledronic acid. Prostate 68, 861–871. doi: 10.1002/pros.20752
Morimoto, R., Uehara, S., Yatsushiro, S., Juge, N., Hua, Z., Senoh, S., et al. (2006). Secretion of L-glutamate from osteoclasts through transcytosis. EMBO J. 25, 4175–4186. doi: 10.1038/sj.emboj.7601317
Nguyen, L. S., Vautier, M., Allenbach, Y., Zahr, N., Benveniste, O., Funck-Brentano, C., et al. (2019). Sirolimus and mTOR inhibitors: a review of side effects and specific management in solid organ transplantation. Drug Saf. 42, 813–825. doi: 10.1007/s40264-019-00810-9
Olechnowicz, S. W., and Edwards, C. M. (2014). Contributions of the host microenvironment to cancer-induced bone disease. Cancer Res. 74, 1625–1631. doi: 10.1158/0008-5472.Can-13-2645
Pajor, A. M. (2014). Sodium-coupled dicarboxylate and citrate transporters from the SLC13 family. Pflugers. Arch. 466, 119–130. doi: 10.1007/s00424-013-1369-y
Pereverzev, A., Komarova, S. V., Korcok, J., Armstrong, S., Tremblay, G. B., Dixon, S. J., et al. (2008). Extracellular acidification enhances osteoclast survival through an NFAT-independent, protein kinase C-dependent pathway. Bone 42, 150–161. doi: 10.1016/j.bone.2007.08.044
Pfeiffer, T., and Morley, A. (2014). An evolutionary perspective on the crabtree effect. Front. Mol. Biosci. 1:17. doi: 10.3389/fmolb.2014.00017
Quek, L. E., Liu, M., Joshi, S., and Turner, N. (2016). Fast exchange fluxes around the pyruvate node: a leaky cell model to explain the gain and loss of unlabelled and labelled metabolites in a tracer experiment. Cancer Metab. 4:3. doi: 10.1186/s40170-016-0153-9
Rafiei, S., Tiedemann, K., Tabaries, S., Siegel, P. M., and Komarova, S. V. (2015). Peroxiredoxin 4: a novel secreted mediator of cancer induced osteoclastogenesis. Cancer Lett. 361, 262–270. doi: 10.1016/j.canlet.2015.03.012
Rana, T., Chakrabarti, A., Freeman, M., and Biswas, S. (2013). Doxorubicin-mediated bone loss in breast cancer bone metastases is driven by an interplay between oxidative stress and induction of TGFbeta. PLoS One 8:e78043. doi: 10.1371/journal.pone.0078043
Rocha, C. M., Carrola, J., Barros, A. S., Gil, A. M., Goodfellow, B. J., Carreira, I. M., et al. (2011). Metabolic signatures of lung cancer in biofluids: NMR-based metabonomics of blood plasma. J. Proteome Res. 10, 4314–4324. doi: 10.1021/pr200550p
Schulten, H. J. (2018). Pleiotropic effects of metformin on cancer. Int. J. Mol. Sci. 19:2850. doi: 10.3390/ijms19102850
Seidlitz, E. P., Sharma, M. K., and Singh, G. (2010). Extracellular glutamate alters mature osteoclast and osteoblast functions. Can. J. Physiol. Pharmacol. 88, 929–936. doi: 10.1139/y10-070
Shah, M., Kola, B., Bataveljic, A., Arnett, T. R., Viollet, B., Saxon, L., et al. (2010). AMP-activated protein kinase (AMPK) activation regulates in vitro bone formation and bone mass. Bone 47, 309–319. doi: 10.1016/j.bone.2010.04.596
Simone, V., Ciavarella, S., Brunetti, O., Savonarola, A., Cives, M., Tucci, M., et al. (2015). Everolimus restrains the paracrine pro-osteoclast activity of breast cancer cells. BMC Cancer 15:692. doi: 10.1186/s12885-015-1717-8
Stenbeck, G. (2002). Formation and function of the ruffled border in osteoclasts. Semin. Cell Dev. Biol. 13, 285–292. doi: 10.1016/s1084952102000587
Sugatani, T., and Hruska, K. A. (2005). Akt1/Akt2 and mammalian target of rapamycin/Bim play critical roles in osteoclast differentiation and survival, respectively, whereas Akt is dispensable for cell survival in isolated osteoclast precursors. J. Biol. Chem. 280, 3583–3589. doi: 10.1074/jbc.M410480200
Taylor, P. M. (2014). Role of amino acid transporters in amino acid sensing. Am. J. Clin. Nutr. 99, 223s–230s. doi: 10.3945/ajcn.113.070086
Tiedemann, K., Le Nihouannen, D., Fong, J. E., Hussein, O., Barralet, J. E., and Komarova, S. V. (2017). Regulation of osteoclast growth and fusion by mTOR/raptor and mTOR/rictor/Akt. Front. Cell Dev. Biol. 5:54. doi: 10.3389/fcell.2017.00054
Tiedemann, K., Sadvakassova, G., Mikolajewicz, N., Juhas, M., Sabirova, Z., Tabaries, S., et al. (2019). Exosomal release of L-plastin by breast cancer cells facilitates metastatic bone osteolysis. Transl. Oncol. 12, 462–474. doi: 10.1016/j.tranon.2018.11.014
Wang, J., Chen, T. Y., Qin, S., Duan, Y., and Wang, G. (2013). Inhibitory effect of metformin on bone metastasis of cancer via OPG/RANKL/RANK system. Med. Hypotheses 81, 805–806. doi: 10.1016/j.mehy.2013.08.032
Wei, W., Wang, X., Yang, M., Smith, L. C., Dechow, P. C., Sonoda, J., et al. (2010). PGC1beta mediates PPARgamma activation of osteoclastogenesis and rosiglitazone-induced bone loss. Cell Metab. 11, 503–516. doi: 10.1016/j.cmet.2010.04.015
Williams, J. P., Blair, H. C., McDonald, J. M., McKenna, M. A., Jordan, S. E., Williford, J., et al. (1997). Regulation of osteoclastic bone resorption by glucose. Biochem. Biophys. Res. Commun. 235, 646–651. doi: 10.1006/bbrc.1997.6795
Wittrant, Y., Gorin, Y., Woodruff, K., Horn, D., Abboud, H. E., Mohan, S., et al. (2008). High d(+)glucose concentration inhibits RANKL-induced osteoclastogenesis. Bone 42, 1122–1130. doi: 10.1016/j.bone.2008.02.006
Yang, M., Mailhot, G., Birnbaum, M. J., MacKay, C. A., Mason-Savas, A., and Odgren, P. R. (2006). Expression of and role for ovarian cancer G-protein-coupled receptor 1 (OGR1) during osteoclastogenesis. J. Biol. Chem. 281, 23598–23605. doi: 10.1074/jbc.M602191200
Yardley, D. A., Noguchi, S., Pritchard, K. I., Burris, H. A., Baselga, J., Gnant, M., et al. (2013). Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO-2 final progression-free survival analysis. Adv. Ther. 30, 870–884. doi: 10.1007/s12325-013-0060-1
Yeon, J. T., Kim, K. J., Chun, S. W., Lee, H. I., Lim, J. Y., Son, Y. J., et al. (2015). KCNK1 inhibits osteoclastogenesis by blocking the Ca2+ oscillation and JNK-NFATc1 signaling axis. J. Cell Sci. 128, 3411–3419. doi: 10.1242/jcs.170738
Yu, Y., Song, Z., Yang, S., Yang, X., Zhang, J., and Lu, S. (2014). Everolimus and zoledronic acid–a potential synergistic treatment for lung adenocarcinoma bone metastasis. Acta Biochim. Biophys. Sin. 46, 792–801. doi: 10.1093/abbs/gmu069
Yuan, F. L., Xu, M. H., Li, X., Xinlong, H., Fang, W., and Dong, J. (2016). The roles of acidosis in osteoclast biology. Front. Physiol. 7:222. doi: 10.3389/fphys.2016.00222
Yuan, F. L., Zhao, M. D., Jiang, L. B., Wang, H. R., Cao, L., Zhou, X. G., et al. (2014). Molecular actions of ovarian cancer G protein-coupled receptor 1 caused by extracellular acidification in bone. Int. J. Mol. Sci 15, 22365–22373. doi: 10.3390/ijms151222365
Zeng, R., Faccio, R., and Novack, D. V. (2015). Alternative NF-kappaB regulates RANKL-induced osteoclast differentiation and mitochondrial biogenesis via independent mechanisms. J. Bone Mineral Res. 30, 2287–2299. doi: 10.1002/jbmr.2584
Keywords: bioenergetics, metabolism, osteoclast, bone microenvironment, cancer, osteolysis, metabolic sensors
Citation: Tiedemann K, Hussein O and Komarova SV (2020) Role of Altered Metabolic Microenvironment in Osteolytic Metastasis. Front. Cell Dev. Biol. 8:435. doi: 10.3389/fcell.2020.00435
Received: 24 January 2020; Accepted: 08 May 2020;
Published: 05 June 2020.
Edited by:
Maria Teresa Valenti, University of Verona, ItalyReviewed by:
Sofia Avnet, Rizzoli Orthopedic Institute (IRCCS), ItalyHilary Ann Coller, University of California, Los Angeles, United States
Copyright © 2020 Tiedemann, Hussein and Komarova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Svetlana V. Komarova, c3ZldGxhbmEua29tYXJvdmFAbWNnaWxsLmNh
 Kerstin Tiedemann
Kerstin Tiedemann Osama Hussein
Osama Hussein Svetlana V. Komarova
Svetlana V. Komarova