- Centre for Craniofacial and Regenerative Biology, King’s College London, London, United Kingdom
The novel mammalian jaw joint, known in humans as the temporomandibular joint or TMJ, is cushioned by a fibrocartilage disc. This disc is secondarily absent in therian mammals that have lost their dentition, such as giant anteaters and some baleen whales. The disc is also absent in all monotremes. However, it is not known if the absence in monotremes is secondary to the loss of dentition, or if it is an ancestral absence. We use museum held platypus and echidna histological sections to demonstrate that the developing monotreme jaw joint forms a disc primordium that fails to mature and become separated from the mandibular condyle. We then show that monotreme developmental anatomy is similar to that observed in transgenic mouse mutants with reduced cranial musculature. We therefore suggest that the absence of the disc on monotremes is a consequence of the changes in jaw musculature associated with the loss of adult teeth. Taken together, these data indicate that the ancestors of extant monotremes likely had a jaw joint disc, and that the disc evolved in the last common ancestor of all mammals.
Introduction
The temporomandibular joint (TMJ) is the one of the most used joints in the body, articulating the upper and lower jaw in mammals. A fibrous articular disc sits between the skeletal elements of the joint and acts as a cushion.
TMJ development occurs by the coming together of two membranous bones: the condylar process of the dentary bone in the mandible and the squamosal bone in the skull. The interaction of the condylar with the squamosal induces the formation of a glenoid (or mandibular) fossa on the latter (Wang et al., 2011). The articular disc sits between the two within a synovial capsule. The TMJ disc attaches to the superior head of the lateral pterygoid muscle anteriorly, and to ligaments posteriorly including the disco-mallear ligament that runs thought the capsule of the middle ear, joining the malleus to the TMJ disc. The TMJ articulates the jaw in all mammals and is referred to as the squamosal dentary joint (SDJ) in those mammals without a fused temporal bone. In non-mammals the upper and lower jaw articulate via the endochondral quadrate and articular, known as the primary jaw joint (Wilson and Tucker, 2004). TMJ developmental anatomy reflects its evolutionary history as this novel jaw joint forms after the development of the primary joint, which, in mammals, is integrated into the middle ear (Takechi and Kuratani, 2010; Anthwal et al., 2013; Maier and Ruf, 2016; Tucker, 2017). In recent years, a number of studies have advanced the understanding of middle ear evolution in the context of anatomical development (Luo, 2011; Anthwal et al., 2013, 2017; Urban et al., 2017; Wang et al., 2019), but little work has sought to understand the TMJ in an evolutionary and comparative developmental biology context. This is despite the crucial role that the formation of the TMJ has in mammalian evolution.
An important part of the TMJ is the disc that cushions its action. The origin of the disc is uncertain. The insertion of the lateral pterygoid muscle into the disc on the medial aspect, and the presence of the disco-malleolar ligament, has led to speculation that the disc represents a fibrocartilage sesamoid within a tendon (Herring, 2003). According to this hypothesis, this tendon, originally associated with the musculature of the articular (homologous to the malleus) of the primary jaw joint, would have become trapped as the dentary and squamosal moved together to create the mammalian jaw joint. However, studies in mice indicate that the disc develops from a region of flattered mesenchyme cells adjacent to, or possibly part of, the perichondrium of the developing condylar cartilage (Purcell et al., 2009, 2012; Hinton et al., 2015). Formation of the disc condensation is dependent on Ihh signaling from the cartilage (Shibukawa et al., 2007; Purcell et al., 2009; Yang et al., 2016), and Fgf signaling via Spry 1 and 2 genes from the adjacent muscles (Purcell et al., 2012). Therefore, the disc may have its origins in either a tendon, the novel secondary cartilage of the condylar process, or a combination of the two.
Interestingly the disc is absent in extant monotremes (Sprinz, 1964). Monotremes and therian mammals (marsupials and eutherians) are evolutionary distant, with the common ancestor of the two subclasses being a mammal-like reptile from around 160 million years ago (Kemp, 2005; Luo et al., 2015). Monotremes have a number of “reptile” like anatomical features such as a cloaca, external embryonic development in an egg, a straight cochlea in the inner ear and a sprawling posture (Griffiths, 1978). The absence of a disc in both echidna and platypus suggests that the disc evolved after the split between monotremes and therian mammals, and is therefore a therian novelty. Alternatively, absence of the TMJ disc in extant monotremes might be due to a secondary loss linked to the loss of teeth, and associated changes in the muscles of mastication. Extant adult monotremes are edentulous, possibly due to the expansion of the trigeminal during the evolution of electroreceptivity limiting the available space for tooth roots within the maxilla (Asahara et al., 2016). The juvenile platypus has rudimentary teeth that regress (Green, 1937), while the echidna shows only thickening of the dental epithelium during development. In contrast, fossil monotremes have a mammalian tribosphenic dentition, a structure unique to the mammal lineage that allows occlusion of upper and lower molar teeth for grinding of food in addition to crushing and shearing during mastication (Kemp, 2005). This indicates that extant monotremes evolved from animals with the ability to chew in the mammalian manner, involving lateral and rotational movements. The presence or absence of a disc in such fossils is difficult to ascertain due to lack of preservation of soft tissue. In support of mastication playing a role in disc formation, edentulous therian mammals, or those lacking enamel, often lack a disc. These species include some (but not all) baleen whales (El Adli and Deméré, 2015), giant ant eaters and sloths (Naples, 1999).
In order to discriminate between these two scenarios, we have examined the development of the TMJ in monotremes and made comparison with mouse developmental models where muscle development is perturbed.
Materials and Methods
Platypus (Ornithorhynchus anatinus) and short-beaked echidna (Tachyglossus aculeatus) slides were imaged from the collections at the Cambridge University Museum of Zoology. Details of samples imaged are in Table 1. All museum samples have been studied in previously published works (Watson, 1916; Green, 1937; Presley and Steel, 1978). Stages for platypus are after Ashwell (2012). Staging of echidna H.SP EC5 is estimated by cross-referencing previous studies (Griffiths, 1978; Rismiller and McKelvey, 2003). CT scans of adult platypus were a gift of Anjali Goswami, the Natural History Museum, London.
Mesp1Cre;Tbx1flox (Tbx1CKO) mice were derived as previously described (Anthwal et al., 2015).
Tissue processing and histological staining: embryonic samples for histological sectioning were fixed overnight at 4°C in 4% paraformaldehyde (PFA), before being dehydrated through a graded series of ethanol and stored at −20°C. For tissue processing, samples were cleared with Histoclear II, before wax infiltration with paraffin wax at 60°C. Wax embedded samples were microtome sectioned at 8 μm thickness, then mounted in parallel series on charged slides.
For histological examination of bone and cartilage, the slides were then stained with picrosirius red and alcian blue trichrome stain using standard techniques.
This work was carried out under UK Home Office license and regulations in line with the regulations set out under the United Kingdom Animals (Scientific Procedures) Act 1986 and the European Union Directive 2010/63/EU.
Results
If the TMJ disc is a therian novelty, then no evidence of a disc would be expected in extant monotremes during development of the TMJ. The development of the jaw joint was therefore examined in museum held histological sections of developing post-hatching platypus and compared with the mouse.
As other authors have previously described (Purcell et al., 2009; Hinton, 2014), in embryonic day (E) 16.5 mice the disc anlage is observed as thickened layer of mesenchyme connected to the superior aspect of the condylar cartilage (Figure 1A). At postnatal day (P) 0, the disc has separated from the condylar process and sits within the synovial cavity of the jaw joint (Figure 1B). In a platypus sample estimated to be 6.5 days post-hatching, the TMJ had been initiated, but the joint cavity had not yet formed (Figures 1C,D). Close examination of the superior surface of the condylar cartilage revealed a double layer of thickened mesenchyme in the future fibrocartilage layer of the condylar (Figures 1C’,D’). The outer layer is similar to that known to develop into the articular disc in therian mammals (Purcell et al., 2009). This thickened mesenchyme persisted in older samples, estimated to be 10 days post-hatching, where the synovial cavity of the TMJ was beginning to form above (Figures 1E,E’). In the most mature platypus sample examined (around P80) the fibrocartilage layer of the condylar process was thick and had a double-layered structure (Figure 1F). The outer layer was connected via a tendon to the lateral pterygoid muscle. At this late stage of postnatal development, the platypus puggle would have been expected to start leaving the burrow and to be eating a mixed diet, although full weaning does not occur until around 205 days post-hatching (Rismiller and McKelvey, 2003). In the mature platypus, the condylar process sits within a glenoid fossa (Figure 1G), which was not fully formed at earlier stages. A disc-like structure lying over the condylar and connected to the adjacent muscles was therefore evident in the platypus postnatally but did not lift off the condylar at any stage.
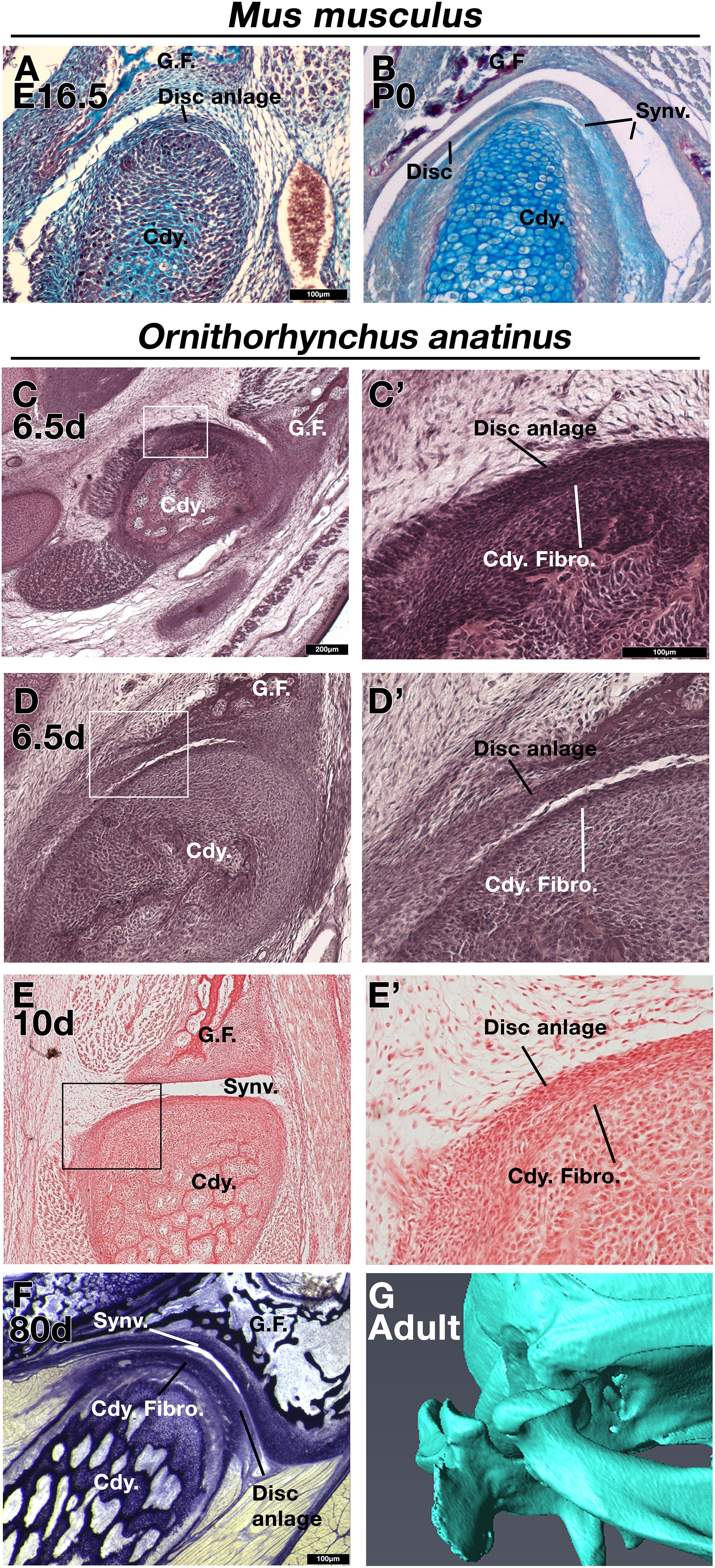
Figure 1. Comparison of mouse (Mus musculus) and platypus (Ornithorhynchus anatinus) developing jaw joint reveals the presence of a jaw joint disc anlage in early post-hatching platypus despite absence of the disc in adults. (A,B) Histological sections of mouse jaw joint disc development at embryonic day 16.5 (A) and postnatal day 0 (B). (C–D’) Histological sections of estimated post-hatching day 6.5 jaw joint at two different levels (C,D) Note that the separation between the disc anlage and condylar in D is probably a processing artifact. (E,E’) Histological sections of estimated post-hatching day 10 jaw joint. (F) Histological section of mature jaw joint in a juvenile platypus estimated post-hatching day 80. (G) μCT scan of jaw joint region of adult platypus. G.F., glenoid fossa; Cdy., condylar process; Cdy. Fibro., condylar fibrocartilage; Synv., synovial cavity of the jaw joint.
Next we examined the development of the TMJ in a museum derived young short-beaked echidna puggle specimen with a crown-rump length of 83mm, which we estimate to be around 18 days post-hatching. The TMJ is not fully developed (Figure 2). The condylar process possessed a thick, doubled fibrocartilage outer layer (Figure 2), much as was observed in the platypus (Figure 1D). The outer fibrocartilage layer was connected by connective tissue to the lateral pterygoid muscle (Figure 2B’). Clear disc-like structures were therefore present during development in both extant monotremes.
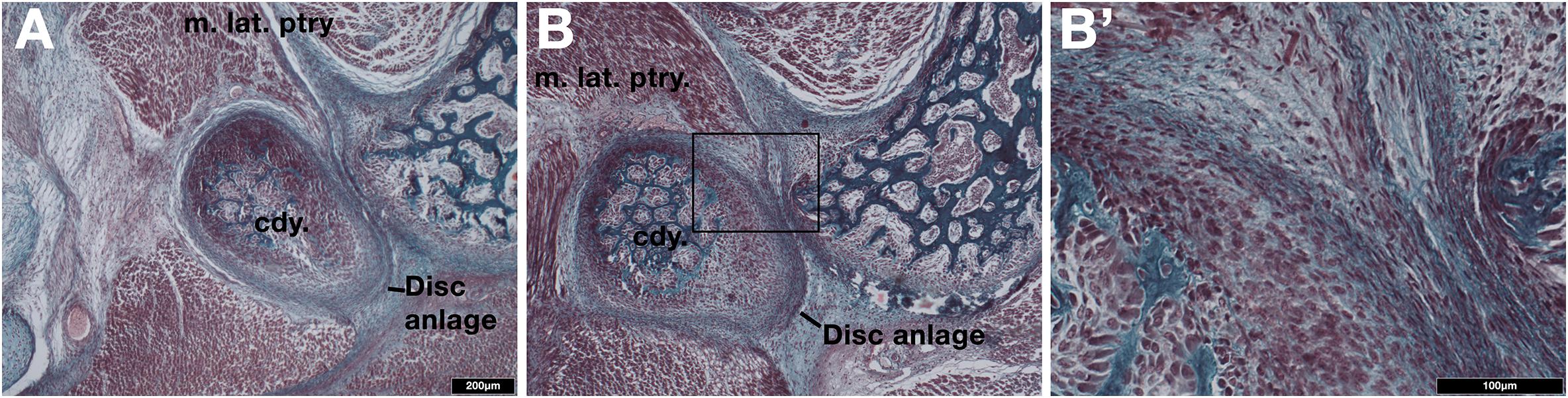
Figure 2. Examination of the developing jaw joint reveals the presence of a jaw joint disc anlage in post-hatching day 18 short-beaked echidna (Tachyglossus aculeatus). (A,B) Histological staining at the forming jaw articulation in echidna young estimated to be 18 days post-hatching at two different level. Fibrocartilage disc anlage superior to the condylar and connected by tendon to the lateral pterygoid muscle is observed. (B’) High-powered view of boxed region in B showing the connection between the muscle and the developing disc anlage. Cdy., condylar process; m. lat. ptry., lateral pterygoid muscle.
Taken together, the developmental evidence suggests that extant monotremes initiate a layer of fibrocartilage connected to the lateral pterygoid muscle, similar to the initiation of the TMJ disc in therian mammals. However, unlike in therian mammals, the monotreme fibrocartilage failed to separate from the condylar to form an articular disc in the TMJ. Interactions with musculature, both mechanical (Habib et al., 2007; Purcell et al., 2012; Jahan et al., 2014; Nickel et al., 2018) and molecular (Shibukawa et al., 2007; Gu et al., 2008; Purcell et al., 2009, 2012; Kinumatsu et al., 2011; Michikami et al., 2012; Yasuda et al., 2012; Kubiak et al., 2016), have been suggested to be responsible for the proper formation of the TMJ disc. Lack of mechanical force or changes in signaling from the muscle in monotremes might therefore result in the disc remaining attached to the condylar. In order to examine how changes in muscle might influence disc development, we next examined disc development in the Mesp1Cre;Tbx1flox conditional mutant mouse (Tbx1CKO). This mouse has a mesoderm specific deletion of the T-box transcription factor Tbx1, resulting in severely perturbed development of the pharyngeal arch mesoderm-derived muscles of the head, resulting in their significant reduction or absence (Grifone et al., 2008; Aggarwal et al., 2010; Anthwal et al., 2015).
We used alcian blue/alizarin red stained histological sections to investigate the development of the TMJ disc in TbxCKO mice at embryonic day 15.5. This is the stage when future disc mesenchyme is first observed. In wildtype embryos, the future disc mesenchyme was observed as a condensation attached to the superior surface of the condylar fibrocartilage (Figure 3A). A distinct disc-like mesenchyme was also observed superior to the condylar of the Tbx1CKO (Figure 3B). This mesenchyme and the fibrocartilage layer of the condylar cartilage both appeared thicker in the Tbx1CKO compared to its wildtype littermate. At E18.5, the wildtype TMJ disc had separated from the condylar process and sat within a synovial joint cavity (Figure 3C). In the Tbx1CKO an upper synovial cavity had formed, similar to the WT, but there was little evidence of the earlier disc with no separation from the condylar (Figure 3D). Instead, a thickened band of fibrocartilage was observed on the superior surface of the condylar process. The lateral pterygoid muscle was either massively reduced or absent in the Tbx1CKO, while other muscles, such as the temporalis, were present but much reduced in volume (see Anthwal et al., 2015).
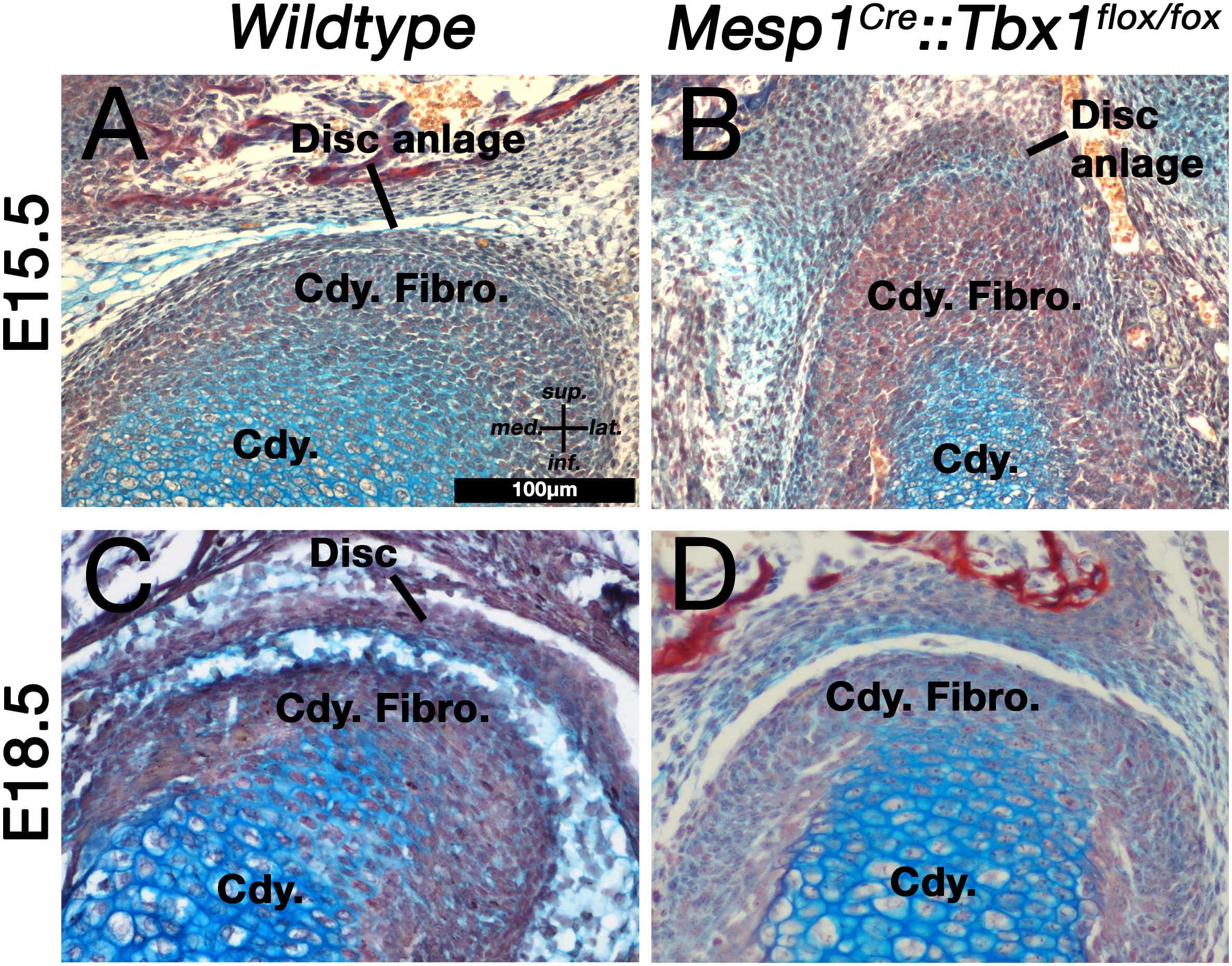
Figure 3. Muscle-disc interactions are required for the maturation and separation of the jaw joint articular disc. (A,B) The disc anlage is observed at E15.5 in both wildtype mice (A) and Mesp1Cre;Tbx1fl/fl mice with a hypomorphic muscle phenotype (B). (C,D) By E18.5 the disc has separated from the condylar process in wildtype mice (C), but not in Mesp1Cre;Tbx1fl/fl mice. Cdy., condylar process; Cdy. Fibro., condylar fibrocartilage.
Discussion
The absence of an articular disc in monotremes has been thought to be either a secondary loss related to the absence of a mature dentition, or the disc being a later acquisition in the therian clade. The data presented here show that a mesenchyme similar to the TMJ disc is initiated in both platypus and echidna jaws during post-hatching development, but fails to mature and separate from the dentary condyle. In the light of the failure of the disc to fully separate in transgenic mouse models with hypomorphic muscle development, it seems likely that the disc has been secondarily lost in edentulous mammals, including monotremes.
The earliest stem mammals, such as Morganuconodon, have a mandibular middle ear where the middle ear bones are fully attached to the mandible and have been proposed to act in both hearing and feeding. The secondary jaw joint of these animals were likely to be able to withstand the biomechanical stresses sufficient for feeding on the hard keratinized bodies of insects, while others such as Kuehneotherium could not (Gill et al., 2014). More crownward stem-mammals developed a range of mandibular movements during chewing, including rolling, yaw and front to back movements (Kemp, 2005; Luo et al., 2015; Grossnickle, 2017; Lautenschlager et al., 2017, 2018; Bhullar et al., 2019). It is not clear if these species had evolved an articular disc, since fibrocartilage is rarely fossilized. However, the synovial secondary jaw joint was likely present in stem mammals such as Morganuconodon (Allin and Hopson, 1992), and the lateral pterygoid has been proposed to have inserted into the condylar of the dentary forming the secondary articulation in basal mammaliforms (Lautenschlager et al., 2017). When this is considered alongside the presence of the first stages of disc formation during monotreme development, it is likely that the common stem Jurassic mammal-like reptilian ancestor of both monotremes and therian mammals had a disc. The data presented here confirms an essential biomechanical component in disc development. Therefore, we are able to consider when during mammalian evolution these forces were able to act to enable disc formation. For example, it is probable that many late Triassic and early Jurassic mammaliaforms such Hadrocodium (Luo, 2001) possessed an articular disc, since they possessed a well-formed squamosal dentary joint and occluding teeth capable of grinding food between the cusps of tribosphenic teeth during mastication.
One hypothesis for the origin of the articular disc is that it formed from the tendon of a muscle of jaw closure of the primary jaw joint interrupted by the formation of the novel mammalian jaw joint (Herring, 2003). The tendons and skeleton of the front of the head are derived from the cranial neural crest, whereas the musculature is mesoderm derived (Santagati and Rijli, 2003; Yoshida et al., 2008). Interactions between the mesoderm and neural crest co-ordinate the muscular skeletal development of the head (Grenier et al., 2009). A striking piece of evidence for the tendon origin of the disc is the expression in the developing articular disc of Scleraxis (Purcell et al., 2012; Roberts et al., 2019), a specific regulator of tendon and ligament development (Schweitzer et al., 2001; Sugimoto et al., 2013). If the disc is derived from a tendon, then it may be thought of as a fibrocartilage sesamoid. Such sesamoids are found in joints and in tendons that are subject to compression, like the tendons that pass around bony pulleys such as the flexor digitorum profundus tendon in quadrupeds, the patella tendon and ligament (Benjamin and Ralphs, 1998), and the cartilago transiliens in crocodilians (Tsai and Holliday, 2011). Fibrocartilages also form at the enthesis of long bones. Interestingly, it has been demonstrated that much like the TMJ disc, enthesis fibrocartilage cells are derived from Hh responsive cells and that these cells are responsive to mechanical loading (Schwartz et al., 2015). To support the tendon origin of the TMJ disc, our data show that the formation of the disc is dependent on interactions between the skeletal and muscle components of the TMJ. Such tissue interaction is also a key process in the formation of tendons and ligaments (Eloy-Trinquet et al., 2009; Huang, 2017).
The mechanism by which the disc fails to separate from the condylar in monotremes is not yet clear. Hh signaling is known to be involved in both the initiation of the disc, and the later separation from the condylar (Purcell et al., 2009). It is still possible that the role in Hh in separation of the disc is a therian innovation, and as such the reason that monotremes fail to do so is a lack of the later Hh dependent developmental program for disc separation. However, the absence of the disc in therian edentates, such as some whales and giant anteaters (Naples, 1999; El Adli and Deméré, 2015), strongly suggests that the loss is secondary. The absence of teeth and associated changes in jaw function in monotremes lends itself to the hypothesis that related changes in the lateral pterygoid muscle are responsible for the failure of disc maturation. Secondary loss, through changes in interactions between the developing disc and muscles, is supported by the failure of the disc to elevate off the condylar in Tbx1CKO mice that fail to form the lateral pterygoid muscle. These interactions may be either, or a combination of, biomechanical stimulation acting in addition to the compressive force of the TMJ joint, or molecular signaling from the muscles, such as Fgf and Tgf-beta signaling pathways that are known to act in the muscle-tendon-bone/cartilage axis (Purcell et al., 2012; Woronowicz et al., 2018; Roberts et al., 2019; Tan et al., 2020). The source of this signal is likely the lateral pterygoid muscle, which acts to abduct, protrude and laterally move the jaw. These movements are of decreased importance in extant tooth-less monotremes due to feeding modalities that do not rely on chewing with an occluded dentition. As such the formation and maturation of the disc is unlikely to be directly dependent on the presence of teeth, and its absence in edentates is instead a function of the associated changes in musculature. This is supported by the fact that the TMJ disc forms during embryonic development in mice, quite some time before the eruption of the teeth at the end of the second postnatal week. Baleen whales vary in the presence or absence of TMJ discs, and indeed TMJ synovial cavities (El Adli and Deméré, 2015). Significantly, the toothless gray whale has no disc and the lateral and medial pterygoid muscles are fused and function as the medial pterygoid (El Adli and Deméré, 2015), a situation also reported in the adult platypus (Edgeworth, 1931). In addition, although they have a full carnivore dentition, the marsupial Tasmanian Devil has a poorly developed lateral pterygoid muscle and completely lacks the TMJ disc (Hayashi et al., 2013). Evidence that disc maturation is, at least in part, dependent on biomechanical, rather than molecular signaling, cues is found in the disrupted development of the disc in mice after ex utero surgical manipulation, where the jaw is sutured closed at E15.5 but the muscle is unaffected (Habib et al., 2007).
Monotremes appear to have two distinct layers in the disc remnant attached to the upper surface of the condylar cartilage (Figure 1F), whereas the Tbx1CKO mouse has only one continuous fibrocartilage by E18.5 (Figure 3D). This may reflect the near total absence of the lateral pterygoid muscle in the mouse mutant, compared to its presence in a reduced form in monotremes. Unfortunately, due to the rarity of fresh material, it is not possible to further examine the mechanistic aspects of TMJ development in edentulous monotreme species at the present time.
Conclusion
In conclusion, we demonstrate that during development, monotremes show evidence of initiation of a fibrocartilage articular disc, despite all adult monotremes not having an articular TMJ disc. Maturation and separation of the disc is dependent on interaction with the developing musculature, either through biomechanical stimulation or molecular signals, as demonstrated by the failure of disc maturation and separation in mouse mutants with hypomorphic cranial muscles. Therefore, toothed ancestors of monotremes likely had a TMJ disc. Our research suggests that changes in the cranial musculature that occurred as a consequence of a move toward edentulous dietary niches resulted in absence of the TMJ disc in monotremes, a parallel loss occurring in edentulous therian mammals (Figure 4). Finally, the presence of the disc anlage in monotremes indicates that the mammal-like reptile ancestor of all modern mammals likely possessed a disc to cushion the novel jaw articulation.
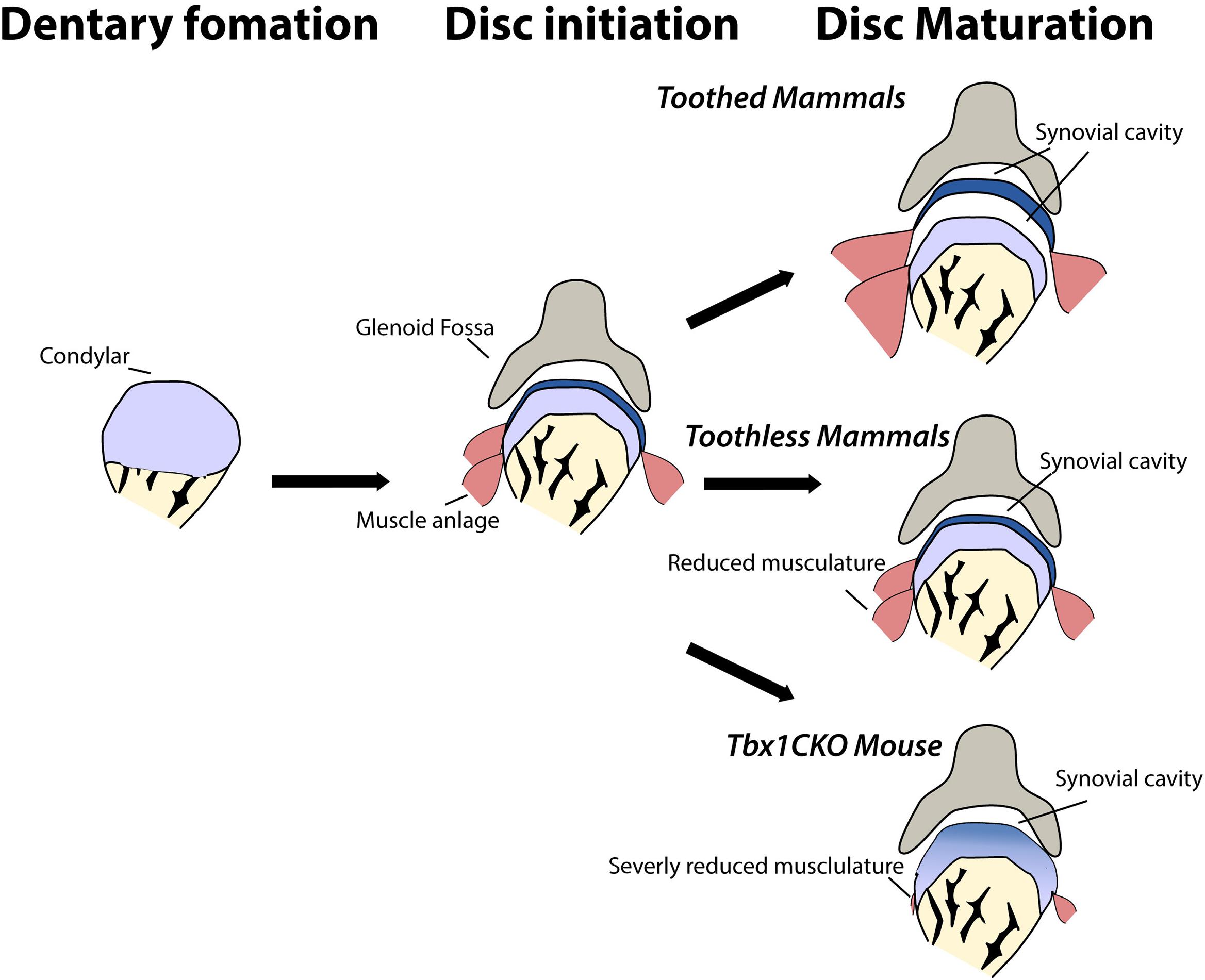
Figure 4. Maturation of the jaw joint articular disc in mammals is dependent on muscle interactions. In toothless mammals and in Tbx1CKO mice, reduction or loss of jaw musculature results in changes in muscle-disc interaction and so the disc does not separate from the mandibular condyle to sit within the synovial joint capsule.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
Ethical review and approval was not required for the animal study because all tissues used were archival, and in some cases museum specimens. No live studies were carried out.
Author Contributions
NA carried out all mouse work, imaged monotreme samples, and wrote the manuscript. AT imaged monotreme samples and critically appraised and edited the manuscript.
Funding
This work was supported by the Wellcome Trust (102889/Z/13/Z).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank and acknowledge the following people. Anjali Goswami provided μCT images of the adult platypus. Robert Asher provided access to samples held at the Zoological Museum in Cambridge University. Peter Giere provided access to the Hill Collection at the Berlin Museum für Naturkunde. Andrew Gillis provided assistance with imaging. This manuscript has been released as a pre-print at https://doi.org/10.1101/2020.01.17.910471 (Anthwal and Tucker, 2020).
References
Aggarwal, V. S., Carpenter, C., Freyer, L., Liao, J., Petti, M., and Morrow, B. E. (2010). Mesodermal Tbx1 is required for patterning the proximal mandible in mice. Dev. Biol. 344, 669–681. doi: 10.1016/j.ydbio.2010.05.496
Allin, E. F., and Hopson, J. A. (1992). “Evolution of the auditory system in Synapsida (“mammal-like reptiles” and primitive mammals) as seen in the fossil record,” in The Evolutionary Biology of Hearing, eds D. B. Webster, R. R. Fay, and A. N. Popper (Berlin: Springer-Verlag), 587–614.
Anthwal, N., Joshi, L., and Tucker, A. S. (2013). Evolution of the mammalian middle ear and jaw: adaptations and novel structures. J. Anat. 222, 147–160. doi: 10.1111/j.1469-7580.2012.01526.x
Anthwal, N., Peters, H., and Tucker, A. S. (2015). Species-specific modifications of mandible shape reveal independent mechanisms for growth and initiation of the coronoid. Evodevo 6:35. doi: 10.1186/s13227-015-0030-6
Anthwal, N., and Tucker, A. S. (2020). The TMJ disc is a common ancestral feature in all mammals, as evidenced by the presence of a rudimentary disc during monotreme development. bioRxiv. doi: 10.1101/2020.01.17.910471
Anthwal, N., Urban, D. J., Luo, Z.-X., Sears, K. E., and Tucker, A. S. (2017). Meckel’s cartilage breakdown offers clues to mammalian middle ear evolution. Nat. Ecol. Evol. 1:0093. doi: 10.1038/s41559-017-0093
Asahara, M., Koizumi, M., Macrini, T. E., Hand, S. J., and Archer, M. (2016). Comparative cranial morphology in living and extinct platypuses: feeding behavior, electroreception, and loss of teeth. Sci. Adv. 2:e1601329. doi: 10.1126/sciadv.1601329
Ashwell, K. W. S. (2012). Development of the hypothalamus and pituitary in platypus (Ornithorhynchus anatinus) and short-beaked echidna (Tachyglossus aculeatus). J. Anat. 221, 9–20. doi: 10.1111/j.1469-7580.2012.01508.x
Benjamin, M., and Ralphs, J. R. (1998). Fibrocartilage in tendons and ligaments–an adaptation to compressive load. J. Anat. 193(Pt 4), 481–494. doi: 10.1046/j.1469-7580.1998.19340481.x
Bhullar, B.-A. S., Manafzadeh, A. R., Miyamae, J. A., Hoffman, E. A., Brainerd, E. L., Musinsky, C., et al. (2019). Rolling of the jaw is essential for mammalian chewing and tribosphenic molar function. Nature 566, 528–532. doi: 10.1038/s41586-019-0940-x
Edgeworth, F. H. (1931). On the Development of the external ocular, masticatory, and hyoid muscles of monotremata. Proc. Zool. Soc. London 101, 809–815. doi: 10.1111/j.1096-3642.1931.tb01045.x
El Adli, J. J., and Deméré, T. A. (2015). On the Anatomy of the temporomandibular joint and the muscles that act upon it: observations on the gray whale. Eschrichtius robustus. Anat. Rec. 298, 680–690. doi: 10.1002/ar.23109
Eloy-Trinquet, S., Wang, H., Edom-Vovard, F., and Duprez, D. (2009). Fgf signaling components are associated with muscles and tendons during limb development. Dev. Dyn. 238, 1195–1206. doi: 10.1002/dvdy.21946
Gill, P. G., Purnell, M. A., Crumpton, N., Brown, K. R., Gostling, N. J., Stampanoni, M., et al. (2014). Dietary specializations and diversity in feeding ecology of the earliest stem mammals. Nature 512, 303–305. doi: 10.1038/nature13622
Green, H. L. H. H. (1937). VIII—The development and morphology of the teeth of ornithorhynchus ∗. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 228, 367–420. doi: 10.1098/rstb.1937.0015
Grenier, J., Teillet, M.-A., Grifone, R., Kelly, R. G., and Duprez, D. (2009). Relationship between neural crest cells and cranial mesoderm during head muscle development. PLoS One 4:e4381. doi: 10.1371/journal.pone.0004381
Grifone, R., Jarry, T., Dandonneau, M., Grenier, J., Duprez, D., and Kelly, R. G. (2008). Properties of branchiomeric and somite-derived muscle development in Tbx1 mutant embryos. Dev. Dyn. 237, 3071–3078. doi: 10.1002/dvdy.21718
Grossnickle, D. M. (2017). The evolutionary origin of jaw yaw in mammals. Sci. Rep. 7:45094. doi: 10.1038/srep45094
Gu, S., Wei, N., Yu, L., Fei, J., and Chen, Y. (2008). Shox2-deficiency leads to dysplasia and ankylosis of the temporomandibular joint in mice. Mech. Dev. 125, 729–742. doi: 10.1016/j.mod.2008.04.003
Habib, H., Hatta, T., Rahman, O. I. F., Yoshimura, Y., and Otani, H. (2007). Fetal jaw movement affects development of articular disk in the temporomandibular joint. Congenit. Anom. (Kyoto) 47, 53–57. doi: 10.1111/j.1741-4520.2007.00143.x
Hayashi, K., Sugisaki, M., Kino, K., Ishikawa, T., Sugisaki, M., and Abe, S. (2013). Absence of the articular disc in the Tasmanian devil temporomandibular joint. J. Vet. Med. Ser. C Anat. Histol. Embryol. 42, 415–419. doi: 10.1111/ahe.12031
Herring, S. W. (2003). TMJ anatomy and animals models. J. Musculos. Neur. Interact. 3, 997–1003. doi: 10.1016/j.biotechadv.2011.08.021.Secreted
Hinton, R. J. (2014). Genes that regulate morphogenesis and growth of the temporomandibular joint: a review. Dev. Dyn. 243, 864–874. doi: 10.1002/dvdy.24130
Hinton, R. J., Jing, J., and Feng, J. Q. (2015). Genetic influences on temporomandibular joint development and growth. Craniofacial Dev. 115, 85–109. doi: 10.1016/bs.ctdb.2015.07.008
Huang, A. H. (2017). Coordinated development of the limb musculoskeletal system: tendon and muscle patterning and integration with the skeleton. Dev. Biol. 429, 420–428. doi: 10.1016/j.ydbio.2017.03.028
Jahan, E., Matsumoto, A., Rafiq, A. M., Hashimoto, R., Inoue, T., Udagawa, J., et al. (2014). Fetal jaw movement affects Ihh signaling in mandibular condylar cartilage development: the possible role of Ihh as mechanotransduction mediator. Arch. Oral Biol. 59, 1108–1118. doi: 10.1016/j.archoralbio.2014.06.009
Kinumatsu, T., Shibukawa, Y., Yasuda, T., Nagayama, M., Yamada, S., Serra, R., et al. (2011). TMJ development and growth require primary cilia function. J. Dent. Res. 90, 988–994. doi: 10.1177/0022034511409407
Kubiak, M., Ditzel, M., Kubiak, M., and Ditzel, M. (2016). A joint less ordinary: intriguing roles for hedgehog signalling in the development of the temporomandibular synovial joint. J. Dev. Biol. 4:25. doi: 10.3390/jdb4030025
Lautenschlager, S., Gill, P., Luo, Z.-X., Fagan, M. J., and Rayfield, E. J. (2017). Morphological evolution of the mammalian jaw adductor complex. Biol. Rev. 92, 1910–1940. doi: 10.1111/brv.12314
Lautenschlager, S., Gill, P. G., Luo, Z.-X., Fagan, M. J., and Rayfield, E. J. (2018). The role of miniaturization in the evolution of the mammalian jaw and middle ear. Nature 561, 533–537. doi: 10.1038/s41586-018-0521-4
Luo, Z.-X. (2001). A new mammaliaform from the early jurassic and evolution of mammalian characteristics. Science 292, 1535–1540. doi: 10.1126/science.1058476
Luo, Z.-X. (2011). Developmental patterns in mesozoic evolution of mammal ears. Annu. Rev. Ecol. Evol. Syst. 42, 355–380. doi: 10.1146/annurev-ecolsys-032511-142302
Luo, Z.-X., Gatesy, S. M., Jenkins, F. A., Amaral, W. W., and Shubin, N. H. (2015). Mandibular and dental characteristics of Late Triassic mammaliaform haramiyavia and their ramifications for basal mammal evolution. Proc. Natl. Acad. Sci. U.S.A. 112, E7101–E7109. doi: 10.1073/pnas.1519387112
Maier, W., and Ruf, I. (2016). Evolution of the mammalian middle ear: a historical review. J. Anat. 228, 270–283. doi: 10.1111/joa.12379
Michikami, I., Fukushi, T., Honma, S., Yoshioka, S., Itoh, S., Muragaki, Y., et al. (2012). Trps1 is necessary for normal temporomandibular joint development. Cell Tissue Res. 348, 131–140. doi: 10.1007/s00441-012-1372-1
Naples, V. L. (1999). Morphology, evolution and function of feeding in the giant anteater (Myrmecophaga tridactyla). J. Zool. 249:S0952836999009036. doi: 10.1017/S0952836999009036
Nickel, J. C., Iwasaki, L. R., Gonzalez, Y. M., Gallo, L. M., and Yao, H. (2018). Mechanobehavior and ontogenesis of the temporomandibular joint. J. Dent. Res. 97, 1185–1192. doi: 10.1177/0022034518786469
Presley, R., and Steel, F. L. D. (1978). The pterygoid and ectopterygoid in mammals. Anat. Embryol. (Berl) 154, 95–110. doi: 10.1007/BF00317957
Purcell, P., Jheon, A., Vivero, M. P., Rahimi, H., Joo, A., Klein, O. D., et al. (2012). Spry1 and spry2 are essential for development of the temporomandibular joint. J. Dent. Res. 91, 387–393. doi: 10.1177/0022034512438401
Purcell, P., Joo, B. W., Hu, J. K., Tran, P. V., Calicchio, M. L., O’Connell, D. J., et al. (2009). Temporomandibular joint formation requires two distinct hedgehog-dependent steps. Proc. Natl. Acad. Sci. U.S.A. 106, 18297–18302. doi: 10.1073/pnas.0908836106
Rismiller, P. D., and McKelvey, M. W. (2003). Body mass, age and sexual maturity in short-beaked echidnas, Tachyglossus aculeatus. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 136, 851–865. doi: 10.1016/S1095-6433(03)00225-3
Roberts, R. R., Bobzin, L., Teng, C. S., Pal, D., Tuzon, C. T., Schweitzer, R., et al. (2019). FGF signaling patterns cell fate at the interface between tendon and bone. Development 146:dev170241. doi: 10.1242/dev.170241
Santagati, F., and Rijli, F. M. (2003). Cranial neural crest and the building of the vertebrate head. Nat. Rev. Neurosci. 4, 806–818. doi: 10.1038/nrn1221
Schwartz, A. G., Long, F., and Thomopoulos, S. (2015). Enthesis fibrocartilage cells originate from a population of Hedgehog-responsive cells modulated by the loading environment. Development 142, 196–206. doi: 10.1242/dev.112714
Schweitzer, R., Chyung, J. H., Murtaugh, L. C., Brent, A. E., Rosen, V., Olson, E. N., et al. (2001). Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development 128, 3855–3866.
Shibukawa, Y., Young, B., Wu, C., Yamada, S., Long, F., Pacifici, M., et al. (2007). Temporomandibular joint formation and condyle growth require Indian hedgehog signaling. Dev. Dyn. 236, 426–434. doi: 10.1002/dvdy.21036
Sprinz, R. (1964). A note on the mandibular intra-articular disc in the joints of marsupialia and monotremata. Proc. Zool. Soc. London 144, 327–337. doi: 10.1088/1751-8113/44/8/085201
Sugimoto, Y., Takimoto, A., Hiraki, Y., and Shukunami, C. (2013). Generation and characterization of ScxCre transgenic mice. Genesis 51, 275–283. doi: 10.1002/dvg.22372
Takechi, M., and Kuratani, S. (2010). History of studies on mammalian middle ear evolution: a comparative morphological and developmental biology perspective. J. Exp. Zool. B. Mol. Dev. Evol. 314, 417–433. doi: 10.1002/jez.b.21347
Tan, G.-K., Pryce, B. A., Stabio, A., Brigande, J. V., Wang, C., Xia, Z., et al. (2020). TGFβ signaling is critical for maintenance of the tendon cell fate. eLife 9:e52695. doi: 10.7554/eLife.52695
Tsai, H. P., and Holliday, C. M. (2011). Ontogeny of the alligator cartilago transiliens and its significance for sauropsid jaw muscle evolution. PLoS One 6:e24935. doi: 10.1371/journal.pone.0024935
Tucker, A. S. (2017). Major evolutionary transitions and innovations: the tympanic middle ear. Philos. Trans. R. Soc. B Biol. Sci. 372:20150483. doi: 10.1098/rstb.2015.0483
Urban, D. J., Anthwal, N., Luo, Z.-X., Maier, J. A., Sadier, A., Tucker, A. S., et al. (2017). A new developmental mechanism for the separation of the mammalian middle ear ossicles from the jaw. Proc. R. Soc. B Biol. Sci. 284:20162416. doi: 10.1098/rspb.2016.2416
Wang, H., Meng, J., and Wang, Y. (2019). Cretaceous fossil reveals a new pattern in mammalian middle ear evolution. Nature 576, 102–105. doi: 10.1038/s41586-019-1792-0
Wang, Y., Liu, C., Rohr, J., Liu, H., He, F., Yu, J., et al. (2011). Tissue interaction is required for glenoid fossa development during temporomandibular joint formation. Dev. Dyn. 240, 2466–2473. doi: 10.1002/dvdy.22748
Watson, D. M. S. (1916). The monotreme skull: a contribution to mammalian morphogenesis. Philos. Trans. R. Soc. London. Ser. B, Contain. Pap. Biol. Character 207, 311–374. doi: 10.2307/92025
Wilson, J., and Tucker, A. S. (2004). Fgf and Bmp signals repress the expression of Bapx1 in the mandibular mesenchyme and control the position of the developing jaw joint. Dev. Biol. 266, 138–150. doi: 10.1016/j.ydbio.2003.10.012
Woronowicz, K. C., Gline, S. E., Herfat, S. T., Fields, A. J., and Schneider, R. A. (2018). FGF and TGFβ Signaling Link Form and Function During Jaw Development and Evolution. Dev. Biol. 1(Suppl. 1), S219–S236. doi: 10.1016/J.YDBIO.2018.05.002
Yang, L., Gu, S., Ye, W., Song, Y., and Chen, Y. P. (2016). Augmented Indian hedgehog signaling in cranial neural crest cells leads to craniofacial abnormalities and dysplastic temporomandibular joint in mice. Cell Tissue Res. 364, 105–115. doi: 10.1007/s00441-015-2306-5
Yasuda, T., Nah, H. D., Laurita, J., Kinumatsu, T., Shibukawa, Y., Shibutani, T., et al. (2012). Muenke syndrome mutation, FgfR3 P244R, causes TMJ defects. J. Dent. Res. 91, 683–689. doi: 10.1177/0022034512449170
Keywords: TMJ disc, monotreme, mammalian evolution, jaw joint, evo devo, muscle, tendon
Citation: Anthwal N and Tucker AS (2020) The TMJ Disc Is a Common Ancestral Feature in All Mammals, as Evidenced by the Presence of a Rudimentary Disc During Monotreme Development. Front. Cell Dev. Biol. 8:356. doi: 10.3389/fcell.2020.00356
Received: 31 January 2020; Accepted: 21 April 2020;
Published: 19 May 2020.
Edited by:
Neva P. Meyer, Clark University, United StatesReviewed by:
Zerina Johanson, Natural History Museum, United KingdomPatrick Tschopp, University of Basel, Switzerland
Copyright © 2020 Anthwal and Tucker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Neal Anthwal, bi5hbnRod2FsQGtjbC5hYy51aw==
 Neal Anthwal
Neal Anthwal Abigail S. Tucker
Abigail S. Tucker