
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell Dev. Biol. , 28 April 2020
Sec. Cellular Biochemistry
Volume 8 - 2020 | https://doi.org/10.3389/fcell.2020.00285
This article is part of the Research Topic Functional Heterogeneities in Biomembranes View all 26 articles
Leukocyte migration across vessels into and within peripheral and lymphoid tissues is essential for host defense against invading pathogens. Leukocytes are specialized in sensing a variety of guidance cues and to integrate environmental stimuli to navigate in a timely and spatially controlled manner. These extracellular signals must be transmitted across the leukocyte’s plasma membrane in a way that intracellular signaling cascades enable directional cell movement. Therefore, the composition of the membrane in concert with proteins that influence the compartmentalization of the plasma membrane or contribute to delineate intracellular signaling molecules are key in controlling leukocyte navigation. This becomes evident by the fact that mislocalization of membrane proteins is known to deleteriously affect cellular functions that may cause diseases. In this review we summarize recent advances made in the understanding of how membrane cholesterol levels modulate chemokine receptor signaling and hence leukocyte trafficking. Moreover, we provide an overview on the role of membrane scaffold proteins, particularly tetraspanins, flotillins/reggies, and caveolins in controlling leukocyte migration both in vitro and in vivo.
Cell migration is essential for a number of physiological and pathophysiological processes, such as embryogenesis, organogenesis, tissue homeostasis, but also cancer malignancy. In host defense, guided cell locomotion and positioning critically contributes to wound healing and cellular immune responses. Leukocytes are professional migratory cells that are able to sense various guidance cues and to integrate external signals to navigate through different types of tissue and to cross blood and lymph vessels (Nourshargh et al., 2010). Important guidance cues are provided by the chemokine network. Locally produced chemokines can form gradients in situ that migrating cells can sense through cognate chemokine receptors (Hughes and Nibbs, 2018). Chemokine receptors belong to the class A of G-protein coupled receptors (GPCRs) and possess seven α-helical domains that span the plasma membrane and are connected by extracellular and intracellular loops (Legler and Thelen, 2018; Lämmermann and Kastenmüller, 2019). Chemokine binding to the receptor induces conformational changes that markedly rearrange the positions of the transmembrane helices particularly at the cytoplasmic surface of the plasma membrane allowing G-protein coupling and signal transduction (Legler and Thelen, 2018; Weis and Kobilka, 2018). Chemokine receptors couple to heterotrimeric G-proteins of the Gi class and their activation promotes the exchange of GTP for GDP on the Gα-subunit resulting in its dissociation from the βγ-subunits (Figure 1). Notably, members of the small GTPase family transmit downstream signals and thereby link chemokine receptor activation to actin cytoskeleton rearrangements required for the induction of cell polarity and locomotion. Members of the Rho family GTPases, namely Rac1 (Benvenuti et al., 2004), RhoA (Pertz et al., 2006), and Cdc42 (Lämmermann et al., 2009), translocate to the plasma membrane upon activation (Collins, 2003). In general, Rac1 is known to control actin polymerization at the leading edge, while RhoA regulates myosin contraction at the rear of a migrating cell (Pertz et al., 2006; MacHacek et al., 2009).
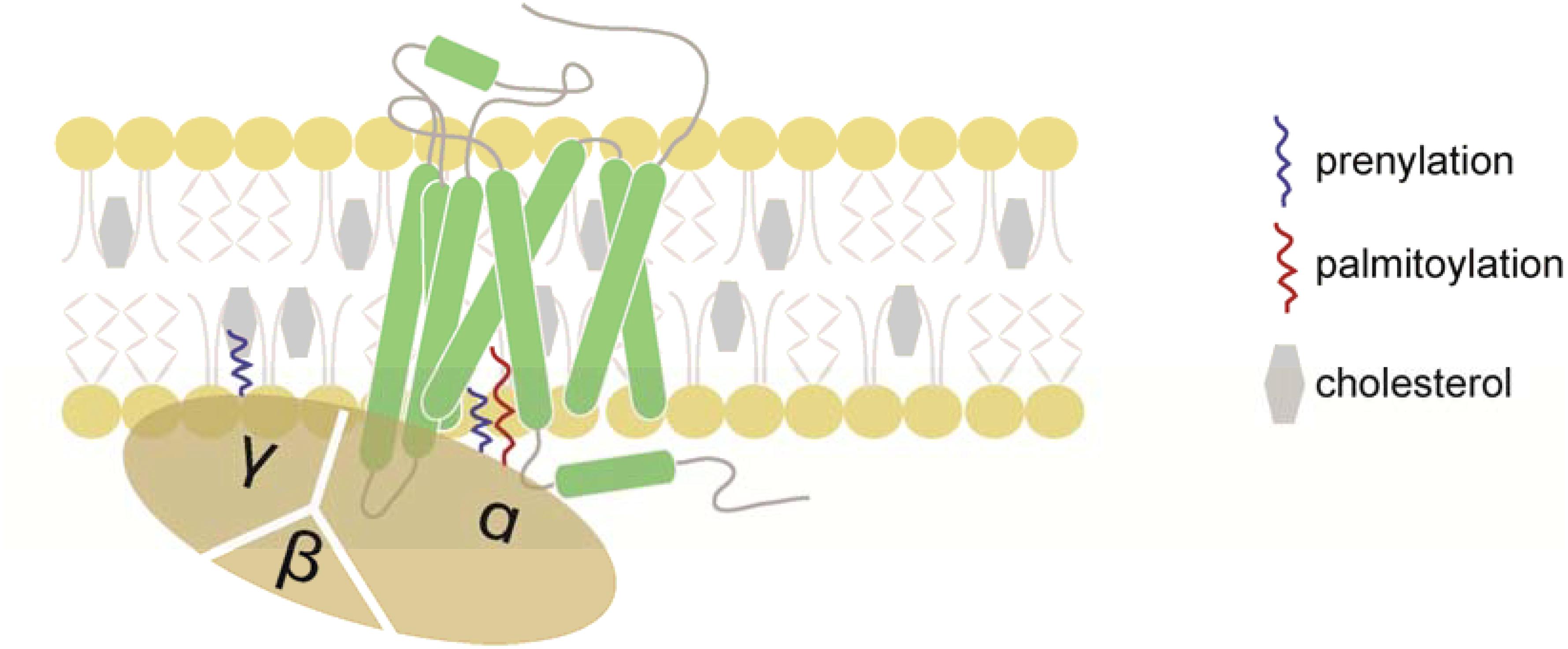
Figure 1. Schematic representation of a chemokine receptor and its associated heterotrimeric G-protein. Chemokine receptors belong to the GPCR family and possess seven-transmembrane domains. Chemokines initiate chemokine receptor activation by binding to the N-terminus and extracellular loops of the receptor. Once the chemokine is tethered to the receptor, the N-terminus enters the binding pocket where it interacts with the transmembrane domains of the chemokine receptor. The presence of cholesterol is critical for the stability of the chemokine receptor. Upon ligand binding, the receptor promotes the exchange of GDP for GTP on the Gα-subunit, resulting in the dissociation of the Gα- from the Gβγ-subunits and downstream signaling. The Gα- and Gγ-subunits are post-transcriptionally lipidated facilitating their association with the plasma membrane.
As guided cell migration depends on extracellular signals that must be transmitted across the plasma membrane, it became obvious that the organization of the plasma membrane and membrane compartmentalization influence the cell’s ability to sense extracellular cues and to migrate. One of the most prominent concept for membrane compartmentalization refers to as the “lipid raft” hypothesis first described in 1988 (Simons and Van Meers, 1988) proposing that specialized subcompartments or microdomains of the lipid bilayer of the membrane control different cellular functions such as receptor endocytosis and signaling (Simons and Ikonen, 1997). In the 1990s, different membrane residing scaffold protein families were discovered, that affect the composition of the membrane (Figure 2). Proteins of the tetraspanin family integrate into the membrane through four transmembrane domains, whereas the flotillin/reggie family represent small cytoplasmic proteins that are hooked to the membrane by means of fatty acid oxidation (Seigneuret et al., 2001; Ficht et al., 2019). Finally, proteins of the caveolin (cav) family penetrate from the cytoplasmic site into the membrane through a hairpin-like structure and are further anchored into the membrane through palmitoylation/myristoylation (Dietzen et al., 1995; Figure 2). Briefly, tetraspanins have the ability to interact with other members of their family or with partner proteins such as integrins, adhesion molecules or signaling receptors to form “tetraspanin enriched microdomains” or “TEMs” (Hemler, 2005). The flotillin/reggie family consists of two members, flotillin-1 (flot1), also known as reggie-2, and flotillin-2 (flot2)/reggie-1 (Bickel et al., 1997; Schulte et al., 1997). Flotillins are known to hetero-dimerize and to assemble into larger complexes to act as scaffold (Langhorst et al., 2007; Neumann-Giesen et al., 2007). For example, in T cells, flotillins were shown to pre-assembly in caps to stabilize the immunological synapse and to as scaffold for the T cell receptor (TCR) machinery (Slaughter et al., 2003; Langhorst et al., 2006; Compeer et al., 2018). Members of the cav family are best known for the formation of cave-like membrane structures termed caveolae, membrane invaginations involved in endocytosis and signaling (Lefkir et al., 2003; Collins et al., 2012; Sotgia et al., 2012; Wang et al., 2015). In this review, we summarize the current understanding how cholesterol modulates chemokine receptor signaling and how membrane scaffold proteins regulate leukocyte migration.
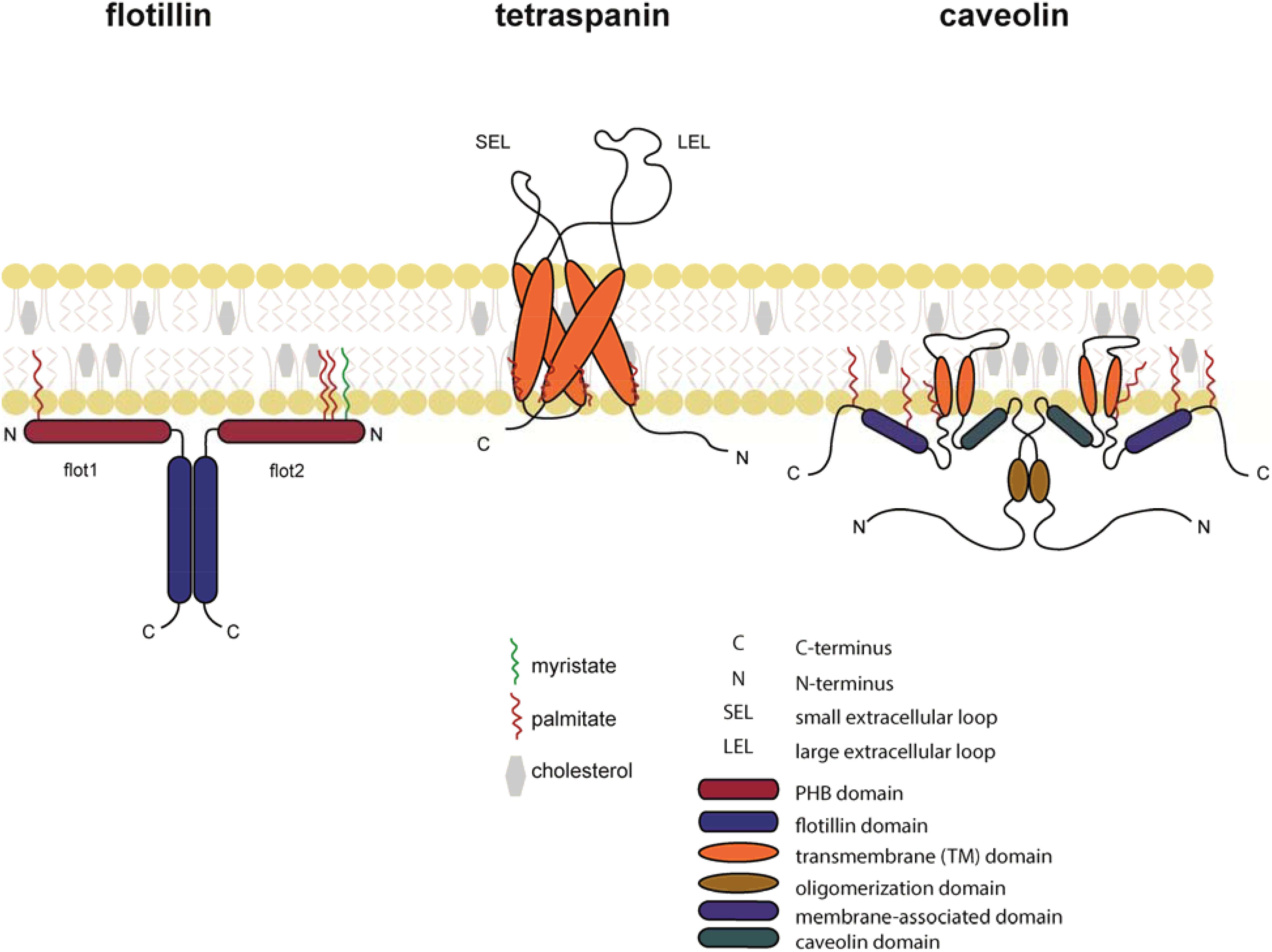
Figure 2. Schematic representation of flotillin, tetraspanin, and caveolin in the lipid bilayer. Flotillins are associated at the cytosolic leaflet of the plasma membrane through its N-terminal PHB domain. Membrane association is further assured through myristoylation (green) and palmitoylation (red). Flotillins form hetero-mers through specialized intracellular flotillin domains. Tetraspanins are composed of four transmembrane α-helices and two extracellular domains: the SEL (small extracellular loop) and the LEL (large extracellular loop). Tetraspanins are palmitoylated at a conserved CXXC motif in their transmembrane domains. Caveolins form hairpin loops that are inserted into the plasma membrane. Both N- and C-termini face the cytoplasmic side of the membrane.
Amphiphilic phospholipids represent the major building block of lipid bilayers of vertebrate membranes. Phospholipids are composed of a hydrophilic phosphate head and two hydrophobic fatty acid tails, which vary in length and saturation and thereby account for the broad range of phospholipid species (Simons and Toomre, 2000). The fatty acyl groups of the phospholipids influence the membrane fluidity and hence the lateral mobility of membrane associated proteins (Krapf, 2018). In addition, cell membranes also contain the sterol cholesterol. Cholesterol molecules preferentially interact with saturated fatty acyl groups of phospholipids and thereby shift the membrane structure from a heterogeneous fluid membrane with high mobility to a more rigid and stiff membrane with lipid and protein patches (Legler et al., 2017). The original concept of “lipid raft” or “membrane microdomains” (Simons and Ikonen, 1997) has been further developed and refined. Although direct microscopic visualization of lipids rafts at millisecond rates still remains challenging (Klymchenko and Kreder, 2014; Sezgin et al., 2017; Kinoshita et al., 2018), recent new biophysical techniques confirmed the presence of such domains in cells and provided new insights in to the cell membrane heterogeneity (Sezgin et al., 2017). In addition, studies on crystal structures of proteins clearly revealed that cholesterol molecules can directly interact with membrane associated scaffold proteins. Notably, solving the crystal structure of the tetraspanin CD81 revealed a cholesterol-binding pocket at the cavity between the four transmembrane helices situated at the inner leaflet of the membrane (Zimmerman et al., 2016). Importantly, the presence of cholesterol within the cavity keeps CD81 in a closed conformation. Molecular dynamics analysis revealed that cholesterol dissociation from the binding pocked results in an open conformation of CD81 that facilitates a tetraspanin-dependent transport of CD19 to the cell surface (Zimmerman et al., 2016). These findings are in line with an earlier study showing that membrane cholesterol contributes to the organization of tetraspanin microdomains (Charrin et al., 2003). More generally, this property of cholesterol to modulate the mode of action of tetraspanins not only affects protein transport [e.g., CD81:CD19 (Zimmerman et al., 2016); CD9:MHCII (Silvie et al., 2003; Rocha-Perugini et al., 2009; Banse et al., 2018)], but also malaria or cytomegalovirus infection [through CD81 (Silvie et al., 2003; Rocha-Perugini et al., 2009; Banse et al., 2018)], and cell migration as described later. Similarly, early electron microscopy studies identified an important role of cholesterol for the assembly of caveolae (Rothberg et al., 1992) whose major constituent, caveolin-1, contributes to dendritic cell migration as discussed in a subsequent paragraph.
Chemokine receptor activation is initiated by the binding of the chemokine to the extracellular N-terminus and extracellular loops of the receptors (Figure 1). Once the chemokine is tethered to its cognate receptor, its unstructured N-terminus is capable to enter the binding pocket where it interacts with the transmembrane bundles of the receptor. This leads to the rearrangement of the seven transmembrane helices of the receptor resulting in profound conformational changes across the plasma membrane (Kufareva et al., 2017; Legler and Thelen, 2018). Attempts to crystalize chemokine receptors, and GPCRs in general, revealed that addition of cholesterol is necessary to stabilize the receptor during the solubilization, purification, and crystallization processes (Wu et al., 2010; Qin et al., 2015). Evidence for a direct physical interaction between cholesterol and a GPCR has first been noted in the crystal structure of the β2-adrenergic receptor, where two cholesterol molecules were found to directly interact with a receptor monomer (Cherezov et al., 2007). Although chemokine receptors do not possess a consensus cholesterol binding motifs as many other GPCRs (Hanson et al., 2008; Thelen and Legler, 2018), chemokine receptors (i.e., CXCR4, CCR2, CCR5, and CCR7) had to be reconstituted into lipidic cubic phases containing at least 10% cholesterol prior to successful crystallization (Wu et al., 2010; Qin et al., 2015; Zheng et al., 2016, 2017; Jaeger et al., 2019). Importantly, cholesterol inclusion was shown to increase chemokine binding to solubilized CXCR4 (Babcock et al., 2003; Palmesino et al., 2016). By contrast, cholesterol depletion in cells reversibly inhibited ligand binding to the chemokine receptor CCR5 and resulted in attenuated signal transduction and cell migration (Mañes et al., 2000; Signoret et al., 2005). A regulatory role for cholesterol in chemokine receptor function derive from the discovery that CCR5 and CXCR4 serve as co-receptors for human immunodeficiency virus (HIV) infection and that cholesterol is essential for the budding and fusion of the virus envelope with the host plasma membrane (Hug et al., 2000; Simons and Ehehalt, 2002). In fact, the HIV glycoprotein gp120 binds to CXCR4 and CCR5 in cholesterol-enriched domains of the host cell (Mañes et al., 2000; Ono and Freed, 2001). The notion that changes in cholesterol levels in dendritic cells regulate their migratory capacity (Hauser et al., 2016) has gained significant attention. In fact, exposing dendritic cells to danger signals led to a marked downregulation of key enzymes involved in cholesterol biosynthesis, while proteins controlling cholesterol efflux were upregulated. Simultaneously, theses danger signals were shown to provoke oligomerization of the chemokine receptor CCR7 resulting in a pro-migratory dendritic cell phenotype (Hauser et al., 2016). Moderately modulating cholesterol levels using cholesterol lowering drugs not only affected CCR7 oligomerization, but also chemokine-driven migration (Hauser et al., 2016). By contrast, a complete depletion of cellular cholesterol interfered with the stability of the receptor manifested by impaired chemokine binding to the receptor and hampered chemotactic cell behavior (Nguyen and Taub, 2002). Molecularly, cholesterol-dependent CCR7 oligomerization enabled the activation of an additional oligomer-dependent Src kinase signal transduction pathway aside the classical G-protein-dependent signaling pathway. This conjoint signaling is possible as in a CCR7 dimer (or tetramer) scenario, one (or two) receptor-mer(s) are able to couple to the heterotrimeric G-protein, while the other (two) receptor-mer(s) interact with the Src kinase. Notably, Src is able to pre-associate with oligomeric CCR7, which upon chemokine activation, phosphorylates the receptor and creates a docking site for SH2-domain-bearing signaling molecules (Hauser et al., 2016). It is interesting to note that the α- and γ-subunits of heterotrimeric G-proteins, as well as Src, undergo lipid modification facilitating their association with cholesterol-rich membrane domains.
Interestingly, CXCR4 was found to form monomers, dimers and nanoclusters on T cells that own distinct lateral mobility characteristics (Martínez-Muñoz et al., 2018). Ligand binding was further shown to modulate CXCR4 dynamics leading to enhanced nanoclustering of the receptor that is controlled by the cortical actin, which in turn correlated with the strength of CXCR4 signaling. Consequently, cells expressing CXCR4 mutants with deficits in nanocluster formation showed impaired chemokine-driven signaling and leukocyte migration, both in vitro and in vivo (Martínez-Muñoz et al., 2018). Although not formally shown in this study, it is tempting to speculate that cholesterol molecules, by modulating the stiffness of membranes, are involved in controlling the lateral mobility of CXCR4 and the formation of nanoclusters.
Beside the above described role of plasma membrane cholesterol, altered extracellular cholesterol levels are observed under certain pathological conditions. High cholesterol levels in the blood (hypercholesterolemia) is a common risk factor for coronary heart diseases (Tall and Yvan-Charvet, 2015). Deposition of cholesterol in the subendothelial layer is effectively narrowing and hardening arteries leading to atherosclerosis. Importantly, cholesterol accumulation in atherosclerotic plaques gives rise to the formation of cholesterol crystals, which induce complement-dependent inflammasome activation (Samstad et al., 2014) and production of inflammatory chemokines (CCL2, CCL3, and CCL5), which results in leukocyte recruitment and a CCR2-driven chronic inflammatory disorder (Boring et al., 1998). Moreover, in a mouse model for atherosclerosis, namely in apolipoprotein E (ApoE)-deficient mice, cholesterol deposits and local dermal inflammation were observed to coincide with skin resident dendritic cells possessing a systemically reduced migratory behavior (Angeli et al., 2004). As dendritic cell emigration from the skin relies on CCR7-guided migration, it is tempting to speculate that extracellular cholesterol is taken up by dendritic cells and integrated into the plasma membrane where it interferes with CCR7 oligomerization and signaling. Pre-clinical studies using statins, which inhibit the HMG-CoA reductase to block cholesterol de novo synthesis, in ApoE-deficient mice revealed a marked regression of atherosclerosis through a CCR7-dependent emigration of foam cells from plaques (Feig et al., 2011) supporting this hypothesis.
The ubiquitously conserved membrane organizing proteins flotillin-1 (flot1), also known as reggie-2, and flotillin-2 (flot2)/reggie-1 have been reported as important regulators of leukocyte activities (Giri et al., 2007; Langhorst et al., 2007; Otto and Nichols, 2011; Guillaume et al., 2013; Bodin et al., 2014). The reggie proteins were originally described to be upregulated on goldfish retinal ganglion cells after nerve injury and subsequent axon regeneration (Schulte et al., 1997). Simultaneously, flotillins were identified as lipid raft proteins of detergent-resistant membrane fractions of marine lung tissue that float in sucrose density gradients (Bickel et al., 1997). Flotillins are also expressed at different levels in many leukocyte subsets, including neutrophils, monocytes, T cells and dendritic cells (Table 1). Both flot1 and flot2 possess N-terminal fatty acid modifications close to the prohibitin homology (PHB) domain (Figure 2) that allow their association with the plasma membrane and a direct interaction with F-actin (Langhorst et al., 2007; Guillaume et al., 2013; Bodin et al., 2014). Particularly, flotillins pile in actin-driven mobile membranes, such as lamellipodia and ruffles (Guillaume et al., 2013). The C-terminal part of flotillins contain an α-helical region required for their hetero-oligomerization, stabilization and lipid rafts association (Langhorst et al., 2007; Guillaume et al., 2013; Bodin et al., 2014). As flotillins are involved in cell-cell contacts (Guillaume et al., 2013; Bodin et al., 2014) and are able to interact with the actomyosin cytoskeleton of leukocytes (Ludwig et al., 2010), flotillins are predestinated to contribute to cell adhesion and migration processes. In neutrophils, both flotillins were found to interact with the adhesion molecule P-selectin glycoprotein ligand 1 (PSGL-1; Rossy et al., 2009). Upon chemokine stimulation, neutrophils polarized and flotillins together with other lipid raft associated signaling molecules (i.e., CD43 and ezrin/radixin/moesin proteins) accumulated at the cell’s uropod. Notably, the redistribution of flotillins preceded the one of CD43 and the ezrin/radixin/moesin proteins and required the integrity of the actin cytoskeleton, but not actin-myosin contraction (Rossy et al., 2009), suggesting that flotillins actively participate in neutrophil polarization. Spurred by these observations, Ludwig and colleagues found that flot1-deficient neutrophils and monocytes failed to efficiently migrate to inflammatory sites in vivo (Ludwig et al., 2010). Ex vivo analysis revealed that uropod formation and myosin IIa activity are compromised in flot1-deficient neutrophils (Ludwig et al., 2010).
In T cells, flotillins also accumulate at the uropod upon exposure to chemotactic signals. Moreover, flotillins in these cells were shown to bind to actin and to regulate the actin cytoskeleton (Langhorst et al., 2007; Neumann-Giesen et al., 2007), suggesting that flotillins are required for optimal T cell migration. Recently, Ficht and colleagues demonstrated that migrating CD8+ T cells retrieved from flot1-deficient mice indeed displayed significant altered shape changes and motility in vitro and in vivo (Ficht et al., 2019). Surprisingly, CD8+ T cell homing to lymphoid organs was comparable in wild-type and flot1-deficient mice (Ficht et al., 2019).
Box 1. | Role of tetraspanins in antigen presentation. Orchestrated leukocyte migration is essential to launch innate and adaptive immune responses. Homing of dendritic cells to draining lymph nodes and the presentation of peripherally acquired antigens derived from pathogens to T cells conjointly dictate the quality of an adaptive immune response. Importantly, T cells migrate within lymph nodes in search for cognate antigens presented by dendritic cells. Hence the role of tetraspanins in leukocyte migration must also be discussed in the light of antigen presentation by major histocompatibility complex (MHC) molecules. Notably, many tetraspanins expressed by dendritic cells not only influence their ability to migrate but also influence antigen presentation. In fact, CD9, CD53, CD81, CD151, and CD37 were shown to associate with MHCII molecules on the cell surface of dendritic cells to augment antigen presentation (Angelisová et al., 1994; Szöllósi et al., 1996; Engering and Pieters, 2001; Saiz et al., 2018). Other members of the tetraspanin family, namely, CD63 and CD82, regulate antigen processing, MHCII biosynthesis and/or transport to the cell surface (Hammond et al., 1998; Mantegazza et al., 2004; Untemaehrer et al., 2007; Saiz et al., 2018). Several members of the tetraspanin family (CD37, CD53, CD63, CD81, and CD82) are expressed by human antigen presenting cells (Escola et al., 1998; Hammond et al., 1998; Van Den Hoorn et al., 2012) and have therefore been proposed as potential target candidates for treating inflammation and immune-mediated chronic diseases (Jin et al., 2018). More information on the role of tetraspanins in antigen presentation can be found in a recent review by Saiz et al. (2018).
Box 2. | Role of scaffold proteins and cancer. Cancer progression and metastasis formation are clearly linked to migration. Although not discussed in this review, it is important to note that the expression of the three families of scaffold proteins discussed in this review are implicated in cancer. Enhanced expression of flot2 was detected in samples of breast cancer and mice lacking flot2 expression showed a significantly reduced tumorigenicity and metastatic capability (Berger et al., 2013). This finding is in line with other studies that proposed the presence of flotillins as a marker for poor prognosis in breast cancer (Banning et al., 2014; Ou et al., 2017), melanoma (Liu et al., 2015), and gastric cancer (Zhu et al., 2013). Similarly, high expression of the tetraspanin CD151 has been proposed as a maker for poor prognosis in a number of metastatic tumors (Franco et al., 2010; Voss et al., 2011; Deng et al., 2012; Kwon et al., 2012; Lee et al., 2013; Li et al., 2013; Sachs et al., 2014; Yu et al., 2018; Jiang et al., 2019). The role of CD9 in cancer remains controversial and seems to vary among different cancer types. Despite promising results obtained in pre-clinical mouse models (Beckwith et al., 2015), CD37 is the only targeted tetraspanin that has moved to clinical studies (de Winde et al., 2017). CD37 is highly expressed in malignant B cells, but not on solid tumors, which makes it suitable for immunotherapy (de Winde et al., 2017). Reduced or absent expression of cav-1 strongly correlated with a poor prognosis in cancer patients. This was attributed to altered signaling in tumor cells and changes in the metabolic tumor environment as reviewed elsewhere (Martinez-Outschoorn et al., 2015).
In conclusion, flot1 emerges to play a critical role in myeloid cell migration by facilitating cell polarization, whereas in CD8+ T cell migration flot1 plays an unexpectedly minor role. The contribution of flot2, i.e., using flot2-deficient mice, in leukocyte migration has not been studied yet. Further studies are hence mandatory to decipher the precise role of the two flotillin proteins in the migratory behavior of different leukocyte subsets.
The cav family constitutes of three isoforms, namely cav-1, cav-2, and cav-3, of which cav-1 is best characterized. The two splicing variants of cav-1, cav-1α, and cav-1β (Okamoto et al., 1998), not only localizes at the plasma membrane, but also at endomembranes, such as the ER, the Golgi, endosomes, and mitochondria, as well as at lipid droplets (Parton and Howes, 2010). Cav-1 is constituted of an N-terminal domain, followed by a scaffold domain, an integral membrane domain and a C-terminal domain (Root et al., 2015). The integral membrane domain includes two α-helices, which are connected by a linker region forming a U-shaped conformation that penetrates deep into the lipid bilayer of the membrane (Rui et al., 2014). Major post-translational modifications, including phosphorylations at the N-terminal domain (on tyrosine14 and serine80) and palmitoylations on three cysteine residues located at the C-terminal domain (Krishna and Sengupta, 2019), not only anchor cav-1 in the membrane but also facilitates cav oligomerization and cholesterol transport (Monier et al., 1996; Okamoto et al., 1998). Although cav proteins are predominately expressed in epithelial cells, endothelial cells, fibroblasts, and adipocytes, they are also present in leukocytes (Harris et al., 2002; Tomassian et al., 2011; Table 1). Importantly, cav-1 was reported to be upregulated in dendritic cells upon exposure to pathogen-derived danger signals (Oyarce et al., 2017). Pathogen encountering also provokes the induction of CCR7 and subsequent migration of dendritic cells to the draining lymph where the dendritic cells present pathogen-derived antigens to T cells to launch an adaptive immune response (Hauser and Legler, 2016). Notably, cav-1-deficient dendritic cells migrate significantly less towards the CCR7 chemokine ligand CCL21 compared to cav-1 proficient cells (Oyarce et al., 2017). Interestingly, the intrinsic random cell motility was not affected in dendritic cells lacking cav-1 (Oyarce et al., 2017), suggesting that cav-1 contributes to directional cell locomotion. Mechanistically, danger signal challenged dendritic cells retrieved from wild-type mice possessed significantly more actin-rich protrusions and filopodia than cav-1-deficient cells. In addition, CCR7-driven activation of the GTPases Rac1, known to promote actin protrusions, was impaired in dendritic cells lacking cav-1 (Oyarce et al., 2017). Collectively, this study suggests that cav-1 control chemokine-mediated Rac1 activation, cytoskeleton rearrangement and migration of dendritic cells in vitro and in vivo.
The family of tetraspanins, also known as the transmembrane 4 superfamily (TM4SF), comprises 34 members in mammals that are highly conserved among species (Adell et al., 2004; Huang et al., 2005). Tetraspanins are composed of four transmembrane domains, a small and large extracellular loop (termed SEL and LEL, respectively), and two intracellular tail domains (Seigneuret et al., 2001; Levy and Shoham, 2005; Figure 2). The LEL domain accounts for most interactions with environmental stimuli, while the cytoplasmic regions are linked to cytoskeletal and signaling molecules. The four transmembrane domains are quite flexible and facilitates the formation of the so-called tetraspanin webs or tetraspanin-enriched microdomains (TEMs) by neighboring tetraspanins (Kitadokoro et al., 2001; Hemler, 2005; Levy and Shoham, 2005; Seigneuret, 2006). Generally, tetraspanin webs act as important signaling platforms that control signaling, cell invasion, cell–cell fusion, cell adhesion, antigen presentation (Box 1), as well as cell migration.
Members of the tetraspanin family relevant for leukocyte migration, including information on the expression level, are listed in Table 1. In T cells, the tetraspanin CD151 was shown to form complexes with integrins (VLA-4 and LFA-1), and its activation was found to augment chemokine-mediated actin polymerization and migration in vitro (Zelman-Toister et al., 2016). Monocytes express the tetraspanins CD9 and CD81, and their cross-linking by specific antibodies was shown to significantly improve their ability to migrate across endothelial monolayers in vitro (Dijkstra et al., 2008; Schenk et al., 2013). Immature dendritic cells were shown to express the tetraspanins CD9, CD63, CD81, CD82, and CD151, of which CD9 and CD81 are mostly expressed at the cell surface, whereas CD63, CD82, and CD151 also localize in intracellular organelles (Mantegazza et al., 2004). Antibody-mediated cross-linking of CD9, CD63, CD81, and CD82 substantially enhanced immature dendritic cell migration in vitro towards the inflammatory chemokines CCL3 and CCL15, while cross-linking CD151 showed no effect (Mantegazza et al., 2004). Interestingly, cross-linking CD81 on mature, danger signal challenged dendritic cells inhibited their in vitro migration abilities towards the lymph-node homing chemokine CCL21 (Nattermann et al., 2006). Using gene-targeted mice revealed that CD9-deficient dendritic cells migrated readily towards CCL21 in vitro and migrated from the skin to inguinal lymph nodes in vivo (Rocha-Perugini et al., 2017). Collectively, these studies provide evidence that the tetraspanin CD9 contributes to, but is dispensable for dendritic cell migration. By contrast, migration of dendritic cells from the skin to draining lymph nodes in a contact sensitization model (FITC skin painting) was impaired in mice lacking CD37 (Gartlan et al., 2013), while mice lacking CD82 display the opposite phenotype (Jones et al., 2016). Both CD37 and CD82-deficient dendritic cells lack cellular projections. Nevertheless, CD37–/– dendritic cells poorly spread under low shear flow conditions on fibronectin, while CD82–/– dendritic cells showed increased cell spreading (Jones et al., 2016). Interestingly, immature dendritic cells, defined as CD37hiCD82lo (as found in the skin), are highly motile cells owing a limited ability to activate naive T cells, while matured dendritic cells, defined as CD37loCD82hi (which have been exposed to pathogens and homed to lymph nodes), are less motile but show a well-orchestrated antigen presentation machinery to efficiently activate naive T cells (Jones et al., 2016). These observations strengthen the notion that leukocyte migration, cell–cell interaction and antigen presentation are interconnected processes than conjointly regulate immunity (see Box 1).
Mechanistic insights into how tetraspanins regulate cell migration are sparse. However, dendritic cells and neutrophils lacking CD37 have deficits in actin polymerization, cell spreading and polarization, which can partially be attributed to deregulated Rac1 activation and accelerated β2-integrin internalization, which conjointly result in impaired cell adhesion (Jones et al., 2016). Generally speaking, the family of tetraspanins play versatile roles in modulating various leukocyte functions, including migration. More to that, a single member of the tetraspanin family fulfills distinct functions that depend on subcellular localization, the differentiation stage of the cell, as well as on the environmental context the cell is navigating through.
Accumulated evidence underpins the critical role of cellular cholesterol in regulating chemokine receptor signaling and functions. Particularly, the presence of cholesterol is essential for chemokine receptor stability, ligand binding, and hence receptor function. Moreover, recent advances indicate that pathogen-derived danger signals modulate cholesterol levels in dendritic cells, which in turn affects their migratory capacities. Similarly chemokine receptor nanocluster, which is presumably regulated by cholesterol, emerges to control the signaling strength and consequently lymphocyte migration. The three membrane scaffold protein families have in common that they contribute to the formation, organization and maintenance of specialized membrane compartments. Flotillins redistribute in migrating leukocytes. Notably, while chemokine-driven cell polarization and spatio-temporal redistribution of flotillins are observed in myeloid cells and lymphocytes, flotillins are fundamentally required for directional myeloid cell migration, but seem to be dispensable for T cell migration in vivo. This suggests that different leukocyte subsets possess alternative adaptation modes for efficient cell migration. Caveolin-1 controls chemokine-driven Rac1 activation to promote cytoskeleton rearrangements and migration of dendritic cells in vitro and in vivo. Several tetraspanins, CD37 and CD82 in particular, play a role in regulating leukocyte migration although the molecular mechanism(s) are far from being fully understood. Further studies are required for a more comprehensive understanding of how membrane compartmentalization and membrane scaffold proteins control cell migration in general. This becomes evident by the fact that these scaffold proteins also affect cancer cell migration and metastasis formation as briefly summarized in Box 2. In general, new knowledge will be key to understand how membrane compartments in concert with membrane-associated or – spanning proteins orchestrated cell migration in health and disease.
GS prepared the figures. Both authors wrote the manuscript.
This work is supported by the Swiss National Science Foundation (grant number 189144), the Thurgauische Stiftung für Wissenschaft und Forschung, and the State Secretariat for Education, Research and Innovation (all to DL).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Adell, T., Gamulin, V., Peroviæ-Ottstadt, S., Wiens, M., Korzhev, M., Müller, I. M., et al. (2004). Evolution of metazoan cell junction proteins: the scaffold protein MAGI and the transmembrane receptor tetraspanin in the demosponge Suberites domuncula. J. Mol. Evol. 59, 41–50. doi: 10.1007/s00239-004-2602-2
Angeli, V., Llodrá, J., Rong, J. X., Satoh, K., Ishii, S., Shimizu, T., et al. (2004). Dyslipidemia associated with atherosclerotic disease systemically alters dendritic cell mobilization. Immunity 21, 561–574. doi: 10.1016/j.immuni.2004.09.003
Angelisová, P., Hilgert, I., and Horejsí, V. (1994). Association of four antigens of the tetraspans family (CD37, CD53, TAPA-1, and R2/C33) with MHC class II glycoproteins. Immunogenetics 39, 249–256. doi: 10.1007/BF00188787
Babcock, G. J., Farzan, M., and Sodroski, J. (2003). Ligand-independent dimerization of CXCR4, a principal HIV-1 coreceptor. J. Biol. Chem. 278, 3378–3385. doi: 10.1074/jbc.M210140200
Banning, A., Kurrle, N., Meister, M., and Tikkanen, R. (2014). Flotillins in receptor tyrosine kinase signaling and cancer. Cells 3, 129–149. doi: 10.3390/cells3010129
Banse, P., Moeller, R., Bruening, J., Lasswitz, L., Kahl, S., Khan, A. G., et al. (2018). CD81 receptor regions outside the large extracellular loop determine hepatitis C virus entry into hepatoma cells. Viruses 10:E207. doi: 10.3390/v10040207
Beckwith, K. A., Byrd, J. C., and Muthusamy, N. (2015). Tetraspanins as therapeutic targets in hematological malignancy: a concise review. Front. Physiol. 6:91. doi: 10.3389/fphys.2015.00091
Benvenuti, F., Hugues, S., Walmsley, M., Ruf, S., Fetler, L., Popoff, M., et al. (2004). Requirement of Rac1 and Rac2 expression by mature dendritic cells for T cell priming. Science 305, 1150–1153. doi: 10.1126/science.1099159
Berger, T., Ueda, T., Arpaia, E., Chio, I. I. C., Shirdel, E. A., Jurisica, I., et al. (2013). Flotillin-2 deficiency leads to reduced lung metastases in a mouse breast cancer model. Oncogene 32, 4989–4994. doi: 10.1038/onc.2012.499
Bickel, P. E., Scherer, P. E., Schnitzer, J. E., Oh, P., Lisanti, M. P., and Lodish, H. F. (1997). Flotillin and epidermal surface antigen define a new family of caveolae-associated integral membrane proteins. J. Biol. Chem. 272, 13793–13802. doi: 10.1074/jbc.272.21.13793
Bodin, S., Planchon, D., Rios Morris, E., Comunale, F., and Gauthier-Rouviere, C. (2014). Flotillins in intercellular adhesion - from cellular physiology to human diseases. J. Cell Sci. 127, 5139–5147. doi: 10.1242/jcs.159764
Boring, L., Gosling, J., Cleary, M., and Charo, I. F. (1998). Decreased lesion formation in CCR2-/- mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 394, 894–897. doi: 10.1038/29788
Charrin, S., Manié, S., Thiele, C., Billard, M., Gerlier, D., Boucheix, C., et al. (2003). A physical and functional link between cholesterol and tetraspanins. Eur. J. Immunol. 33, 2479–2489. doi: 10.1002/eji.200323884
Cherezov, V., Rosenbaum, D. M., Hanson, M. A., Rasmussen, S. G. F., Thian, F. S., Kobilka, T. S., et al. (2007). High-resolution crystal structure of an engineered human β2-adrenergic G protein-coupled receptor. Science 318, 1258–1265. doi: 10.1126/science.1150577
Collins, B. M., Davis, M. J., Hancock, J. F., and Parton, R. G. (2012). Structure-based reassessment of the caveolin signaling model: do caveolae regulate signaling through caveolin-protein interactions? Dev. Cell 23, 11–20. doi: 10.1016/j.devcel.2012.06.012
Collins, R. N. (2003). ‘Getting it on’ - GDI displacement and small GTPase membrane recruitment. Mol. Cell 12, 1064–1066. doi: 10.1016/S1097-2765(03)00445-3
Compeer, E. B., Kraus, F., Ecker, M., Redpath, G., Amiezer, M., Rother, N., et al. (2018). A mobile endocytic network connects clathrin-independent receptor endocytosis to recycling and promotes T cell activation. Nat. Commun. 9:1597. doi: 10.1038/s41467-018-04088-w
de Winde, C. M., Elfrink, S., and van Spriel, A. B. (2017). Novel Insights into Membrane Targeting of B Cell Lymphoma. Trends Cancer 3, 442–453. doi: 10.1016/j.trecan.2017.04.006
Deng, X., Li, Q., Hoff, J., Novak, M., Yang, H., Jin, H., et al. (2012). Integrin-associated CD151 drives ErbB2-evoked mammary tumor onset and metastasis. Neoplasia 14, 678–689. doi: 10.1593/neo.12922
Dietzen, D. J., Hastings, W. R., and Lublin, D. M. (1995). Caveolin is palmitoylated on multiple cysteine residues. Palmitoylation is not necessary for localization of caveolin to caveolae. J. Biol. Chem. 270, 6838–6842. doi: 10.1074/jbc.270.12.6838
Dijkstra, S., Kooij, G., Verbeek, R., van der Pol, S. M. A., Amor, S., Geisert, E. E., et al. (2008). Targeting the tetraspanin CD81 blocks monocyte transmigration and ameliorates EAE. Neurobiol. Dis. 31, 413–421. doi: 10.1016/j.nbd.2008.05.018
Engering, A., and Pieters, J. (2001). Association of distinct tetraspanins with MHC class II molecules at different subcellular locations in human immature dendritic cells. Int. Immunol. 13, 127–134. doi: 10.1093/intimm/13.2.127
Escola, J. M., Kleijmeer, M. J., Stoorvogel, W., Griffith, J. M., Yoshie, O., and Geuze, H. J. (1998). Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J. Biol. Chem. 273, 20121–20127. doi: 10.1074/jbc.273.32.20121
Feig, J. E., Shang, Y., Rotllan, N., Vengrenyuk, Y., Wu, C., Shamir, R., et al. (2011). Statins promote the regression of atherosclerosis via activation of the CCR7-dependent emigration pathway in macrophages. PLoS One 6:e28534. doi: 10.1371/journal.pone.0028534
Ficht, X., Ruef, N., Stolp, B., Samson, G. P. B., Moalli, F., Page, N., et al. (2019). In vivo function of the lipid raft protein flotillin-1 during CD8+ T cell-mediated host surveillance. J. Immunol. 203, 2377–2387. doi: 10.4049/jimmunol.1900075
Franco, M., Muratori, C., Corso, S., Tenaglia, E., Bertotti, A., Capparuccia, L., et al. (2010). The tetraspanin CD151 is required for Met-dependent signaling and tumor cell growth. J. Biol. Chem. 285, 38756–38764. doi: 10.1074/jbc.M110.145417
Gartlan, K. H., Wee, J. L., Demaria, M. C., Nastovska, R., Chang, T. M., Jones, E. L., et al. (2013). Tetraspanin CD37 contributes to the initiation of cellular immunity by promoting dendritic cell migration. Eur. J. Immunol. 43, 1208–1219. doi: 10.1002/eji.201242730
Giri, B., Dixit, V. D. D., Ghosh, M. C. C., Collins, G. D. D., Khan, I. U. U., Madara, K., et al. (2007). CXCL12-induced partitioning of flotillin-1 with lipid rafts plays a role in CXCR4 function. Eur. J. Immunol. 37, 2104–2116. doi: 10.1002/eji.200636680
Guillaume, E., Comunale, F., Do Khoa, N., Planchon, D., Bodin, S., Gauthier-Rouviere, C., et al. (2013). Flotillin microdomains stabilize cadherins at cell–cell junctions. J. Cell Sci. 126, 5293–5304. doi: 10.1242/jcs.133975
Hammond, C., Denzin, L. K., Pan, M., Griffith, J. M., Geuze, H. J., and Cresswell, P. (1998). The tetraspan protein CD82 is a resident of MHC class II compartments where it associates with HLA-DR, -DM, and -DO molecules. J. Immunol. 161, 3282–3291.
Hanson, M. A., Cherezov, V., Griffith, M. T., Roth, C. B., Jaakola, V.-P., Chien, E. Y. T., et al. (2008). A specific cholesterol binding site is established by the 2.8 Å structure of the human β2-adrenergic receptor. Structure 16, 897–905. doi: 10.1016/j.str.2008.05.001
Harris, J., Werling, D., Hope, J. C., Taylor, G., and Howard, C. J. (2002). Caveolae and caveolin in immune cells: distribution and functions. Trends Immunol. 23, 158–164. doi: 10.1016/s1471-4906(01)02161-5
Hauser, M. A., and Legler, D. F. (2016). Common and biased signaling pathways of the chemokine receptor CCR7 elicited by its ligands CCL19 and CCL21 in leukocytes. 99, 1–14. doi: 10.1189/jlb.2MR0815-380R
Hauser, M. A., Schaeuble, K., Kindinger, I., Impellizzieri, D., Krueger, W. A., Hauck, C. R., et al. (2016). Inflammation-induced CCR7 oligomers form scaffolds to integrate distinct signaling pathways for efficient cell migration. Immunity 44, 59–72. doi: 10.1016/j.immuni.2015.12.010
Hemler, M. E. (2005). Tetraspanin functions and associated microdomains. Nat. Rev. Mol. Cell Biol. 6, 801–811. doi: 10.1038/nrm1736
Huang, S., Yuan, S., Dong, M., Su, J., Yu, C., Shen, Y., et al. (2005). The phylogenetic analysis of tetraspanins projects the evolution of cell-cell interactions from unicellular to multicellular organisms. Genomics 86, 674–684. doi: 10.1016/j.ygeno.2005.08.004
Hug, P., Lin, H.-M. J., Korte, T., Xiao, X., Dimitrov, D. S., Wang, J. M., et al. (2000). Glycosphingolipids promote entry of a broad range of human immunodeficiency virus type 1 isolates into cell lines expressing CD4, CXCR4, and/or CCR5. J. Virol. 74, 6377–6385. doi: 10.1128/jvi.74.14.6377-6385.2000
Hughes, C. E., and Nibbs, R. J. B. (2018). A guide to chemokines and their receptors. FEBS J. 285, 2944–2971. doi: 10.1111/febs.14466
Jaeger, K., Bruenle, S., Weinert, T., Guba, W., Muehle, J., Miyazaki, T., et al. (2019). Structural basis for allosteric ligand recognition in the human CC chemokine receptor 7. Cell 178:1222-1230.e10. doi: 10.1016/j.cell.2019.07.028
Jiang, L., Zhang, X., Geradts, J., Wei, Q., Hochwald, S., Xu, H., et al. (2019). Expression of tetraspanins NET-6 and CD151 in breast cancer as a potential tumor biomarker. Clin. Exp. Med. 19, 377–384. doi: 10.1007/s10238-019-00554-x
Jin, Y., Takeda, Y., Kondo, Y., Tripathi, L. P., Kang, S., Takeshita, H., et al. (2018). Double deletion of tetraspanins CD9 and CD81 in mice leads to a syndrome resembling accelerated aging. Sci. Rep. 8:5145. doi: 10.1038/s41598-018-23338-x
Jones, E. L., Wee, J. L., Demaria, M. C., Blakeley, J., Ho, P. K., Vega-Ramos, J., et al. (2016). Dendritic cell migration and antigen presentation are coordinated by the opposing functions of the tetraspanins CD82 and CD37. J. Immunol. 196, 978–987. doi: 10.4049/jimmunol.1500357
Kinoshita, M., Suzuki, K. G. N., Murata, M., and Matsumori, N. (2018). Evidence of lipid rafts based on the partition and dynamic behavior of sphingomyelins. Chem. Phys. Lipids 215, 84–95. doi: 10.1016/j.chemphyslip.2018.07.002
Kitadokoro, K., Bordo, D., Galli, G., Petracca, R., Falugi, F., Abrignani, S., et al. (2001). CD81 extracellular domain 3D structure: insight into the tetraspanin superfamily structural motifs. EMBO J. 20, 12–18. doi: 10.1093/emboj/20.1.12
Klymchenko, A. S., and Kreder, R. (2014). Fluorescent probes for lipid rafts: from model membranes to living cells. Chem. Biol. 21, 97–113. doi: 10.1016/j.chembiol.2013.11.009
Krapf, D. (2018). Compartmentalization of the plasma membrane. Curr. Opin. Cell Biol. 53, 15–21. doi: 10.1016/j.ceb.2018.04.002
Krishna, A., and Sengupta, D. (2019). Interplay between membrane curvature and cholesterol: role of Palmitoylated Caveolin-1. Biophys. J. 116, 69–78. doi: 10.1016/j.bpj.2018.11.3127
Kufareva, I., Gustavsson, M., Zheng, Y., Stephens, B. S., and Handel, T. M. (2017). What do structures tell us about chemokine receptor function and antagonism? Annu. Rev. Biophys. 46, 175–198. doi: 10.1146/annurev-biophys-051013-022942
Kwon, M. J., Park, S., Choi, J. Y., Oh, E., Kim, Y. J., Park, Y. H., et al. (2012). Clinical significance of CD151 overexpression in subtypes of invasive breast cancer. Br. J. Cancer 106, 923–930. doi: 10.1038/bjc.2012.11
Lämmermann, T., and Kastenmüller, W. (2019). Concepts of GPCR-controlled navigation in the immune system. Immunol. Rev. 289, 205–231. doi: 10.1111/imr.12752
Lämmermann, T., Renkawitz, J., Wu, X., Hirsch, K., Brakebusch, C., and Sixt, M. (2009). Cdc42-dependent leading edge coordination is essential for interstitial dendritic cell migration. Blood 113, 5703–5710. doi: 10.1182/blood-2008-11-191882
Langhorst, M. F., Reuter, A., Luxenhofer, G., Boneberg, E.-M., Legler, D. F., Plattner, H., et al. (2006). Preformed reggie/flotillin caps: stable priming platforms for macrodomain assembly in T cells. FASEB J. 20, 711–713. doi: 10.1096/fj.05-4760fje
Langhorst, M. F., Solis, G. P., Hannbeck, S., Plattner, H., and Stuermer, C. A. O. O. (2007). Linking membrane microdomains to the cytoskeleton: regulation of the lateral mobility of reggie-1/flotillin-2 by interaction with actin. FEBS Lett. 581, 4697–4703. doi: 10.1016/j.febslet.2007.08.074
Lee, D., Suh, Y. L., Park, T. I., Do, I. G., Seol, H. J., Nam, D. H., et al. (2013). Prognostic significance of tetraspanin CD151 in newly diagnosed glioblastomas. J. Surg. Oncol. 107, 646–652. doi: 10.1002/jso.23249
Lefkir, Y., Chassey, B., Dubois, A., Bogdanovic, A., Brady, R., Destaing, O., et al. (2003). The AP-1 Clathrin-adaptor is reguired for lysosomal enzymes sorting and biogenesis of the contractile vacuole complex in dictyostelium Cells. Mol. Biol. Cell 14, 1835–1851. doi: 10.1091/mbc.E02
Legler, D. F., Matti, C., Laufer, J. M., Jakobs, B. D., Purvanov, V., Allmen, E. U., et al. (2017). Modulation of chemokine receptor function by cholesterol: new prospects for pharmacological intervention. Mol. Pharmacol. 91, 331–338. doi: 10.1124/mol.116.107151
Legler, D. F., and Thelen, M. (2018). New insights in chemokine signaling. F1000Res. 7:95. doi: 10.12688/f1000research.13130.1
Levy, S., and Shoham, T. (2005). The tetraspanin web modulates immune-signalling complexes. Nat. Rev. Immunol. 5, 136–148. doi: 10.1038/nri1548
Li, Q., Yang, X., Xu, F., Sharma, C., Wang, H.-X., Knoblich, K., et al. (2013). Tetraspanin CD151 plays a key role in skin squamous cell carcinoma. Oncogene 32, 1772–1783. doi: 10.1038/onc.2012.205
Liu, R., Xie, H., Luo, C., Chen, Z., Zhou, X., Xia, K., et al. (2015). Identification of FLOT2 as a novel target for microRNA-34a in melanoma. J. Cancer Res. Clin. Oncol. 141, 993–1006. doi: 10.1007/s00432-014-1874-1
Ludwig, A., Otto, G. P., Riento, K., Hams, E., Fallon, P. G., and Nichols, B. J. (2010). Flotillin microdomains interact with the cortical cytoskeleton to control uropod formation and neutrophil recruitment. J. Cell Biol. 191, 771–781. doi: 10.1083/jcb.201005140
MacHacek, M., Hodgson, L., Welch, C., Elliott, H., Pertz, O., Nalbant, P., et al. (2009). Coordination of Rho GTPase activities during cell protrusion. Nature 461, 99–103. doi: 10.1038/nature08242
Mañes, S., del Real, G., Lacalle, R. A., Lucas, P., Gómez-Moutón, C., Sánchez-Palomino, S., et al. (2000). Membrane raft microdomains mediate lateral assemblies required for HIV-1 infection. EMBO Rep. 1, 190–196. doi: 10.1093/embo-reports/kvd025
Mantegazza, A. R., Barrio, M. M., Moutel, S., Bover, L., Weck, M., Brossart, P., et al. (2004). CD63 tetraspanin slows down cell migration and translocates to the endosomal-lysosomal-MIICs route after extracellular stimuli in human immature dendritic cells. Blood 104, 1183–1190. doi: 10.1182/blood-2004-01-0104
Martínez-Muñoz, L., Rodríguez-Frade, J. M., Barroso, R., Sorzano, C. ÓS., Torreño-Pina, J. A., Santiago, C. A., et al. (2018). Separating actin-dependent chemokine receptor nanoclustering from dimerization indicates a role for clustering in CXCR4 signaling and function. Mol. Cell 71:873. doi: 10.1016/j.molcel.2018.08.012
Martinez-Outschoorn, U. E., Sotgia, F., and Lisanti, M. P. (2015). Caveolae and signalling in cancer. Nat. Rev. Cancer 15, 225–237. doi: 10.1038/nrc3915
Monier, S., Dietzen, D. J., Hastings, W. R., Lublin, D. M., and Kurzchalia, T. V. (1996). Oligomerization of VIP21-caveolin in vitro is stabilized by long chain fatty acylation or cholesterol. FEBS Lett. 388, 143–149. doi: 10.1016/0014-5793(96)00519-4
Nattermann, J., Zimmermann, H., Iwan, A., Von Lilienfeld-Toal, M., Leifeld, L., Nischalke, H. D., et al. (2006). Hepatitis C virus E2 and CD81 interaction may be associated with altered trafficking of dendritic cells in chronic hepatitis C. Hepatology 44, 945–954. doi: 10.1002/hep.21350
Neumann-Giesen, C., Fernow, I., Amaddii, M., and Tikkanen, R. (2007). Role of EGF-induced tyrosine phosphorylation of reggie-1/flotillin-2 in cell spreading and signaling to the actin cytoskeleton. J. Cell Sci. 120, 395–406. doi: 10.1242/jcs.03336
Nguyen, D. H., and Taub, D. (2002). Cholesterol is essential for macrophage inflammatory protein 1β binding and conformational integrity of CC chemokine receptor 5. Blood 99, 4298–4306. doi: 10.1182/blood-2001-11-0087
Nourshargh, S., Hordijk, P. L., and Sixt, M. (2010). Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat. Rev. Mol. Cell Biol. 11, 366–378. doi: 10.1038/nrm2889
Okamoto, T., Schlegel, A., Scherer, P. E., and Lisanti, M. P. (1998). Caveolins, a family of scaffolding proteins for organizing ‘preassembled signaling complexes’ at the plasma membrane. J. Biol. Chem. 273, 5419–5422. doi: 10.1074/jbc.273.10.5419
Ono, A., and Freed, E. O. (2001). Plasma membrane rafts play a critical role in HIV-1 assembly and release. Proc. Natl. Acad. Sci. U.S.A. 98, 13925–13930. doi: 10.1073/pnas.241320298
Otto, G. P., and Nichols, B. J. (2011). The roles of flotillin microdomains - endocytosis and beyond. J. Cell Sci. 124, 3933–3940. doi: 10.1242/jcs.092015
Ou, Y.-X., Liu, F.-T., Chen, F.-Y., and Zhu, Z.-M. (2017). Prognostic value of Flotillin-1 expression in patients with solid tumors. Oncotarget 8, 52665–52677. doi: 10.18632/oncotarget.17075
Oyarce, C., Cruz-Gomez, S., Galvez-Cancino, F., Vargas, P., Moreau, H. D., Diaz-Valdivia, N., et al. (2017). Caveolin-1 expression increases upon maturation in dendritic cells and promotes their migration to lymph nodes thereby favoring the induction of CD8+ T cell responses. Front. Immunol. 8:1794. doi: 10.3389/fimmu.2017.01794
Palmesino, E., Apuzzo, T., Thelen, S., Mueller, B., Langen, H., and Thelen, M. (2016). Association of eukaryotic translation initiation factor eIF2B with fully solubilized CXCR4. J. Leukoc. Biol. 99, 971–978. doi: 10.1189/jlb.2MA0915-415R
Parton, R. G., and Howes, M. T. (2010). Revisiting caveolin trafficking: the end of the caveosome. J. Cell Biol. 191, 439–441. doi: 10.1083/jcb.201009093
Pertz, O., Hodgson, L., Klemke, R. L., and Hahn, K. M. (2006). Spatiotemporal dynamics of RhoA activity in migrating cells. Nature 440, 1069–1072. doi: 10.1038/nature04665
Qin, L., Kufareva, I., Holden, L. G., Wang, C., Zheng, Y., Zhao, C., et al. (2015). Crystal structure of the chemokine receptor CXCR4 in complex with a viral chemokine. Science 347, 1117–1122. doi: 10.1126/science.1261064
Rocha-Perugini, V., Lavie, M., Delgrange, D., Canton, J., Pillez, A., Potel, J., et al. (2009). The association of CD81 with tetraspanin-enriched microdomains is not essential for Hepatitis C virus entry. BMC Microbiol 9:111. doi: 10.1186/1471-2180-9-111
Rocha-Perugini, V., Martínez Del Hoyo, G., González-Granado, J. M., Ramírez-Huesca, M., Zorita, V., Rubinstein, E., et al. (2017). CD9 regulates major histocompatibility complex class II trafficking in monocyte-derived dendritic Cells. Mol. Cell. Biol. 37:e00202-17. doi: 10.1128/MCB.00202-17
Root, K. T., Plucinsky, S. M., and Glover, K. J. (2015). Recent progress in the topology, structure, and oligomerization of caveolin: a building block of caveolae. Curr. Top. Membr. 75, 305–336. doi: 10.1016/bs.ctm.2015.03.007
Rossy, J., Schlicht, D., Engelhardt, B., and Niggli, V. (2009). Flotillins interact with PSGL-1 in neutrophils and, upon stimulation, rapidly organize into membrane domains subsequently accumulating in the uropod. PLoS One 4:e5403. doi: 10.1371/journal.pone.0005403
Rothberg, K. G., Heuser, J. E., Donzell, W. C., Ying, Y. S., Glenney, J. R., and Anderson, R. G. W. (1992). Caveolin, a protein component of caveolae membrane coats. Cell 68, 673–682. doi: 10.1016/0092-8674(92)90143-Z
Rui, H., Root, K. T., Lee, J., Glover, K. J., and Im, W. (2014). Probing the U-shaped conformation of caveolin-1 in a bilayer. Biophys. J. 106, 1371–1380. doi: 10.1016/j.bpj.2014.02.005
Sachs, N., Secades, P., Van Hulst, L., Song, J. Y., and Sonnenberg, A. (2014). Reduced susceptibility to two-stage skin carcinogenesis in mice with epidermis-specific deletion of CD151. J. Invest. Dermatol. 134, 221–228. doi: 10.1038/jid.2013.280
Saiz, M. L., Rocha-Perugini, V., and Sánchez-Madrid, F. (2018). Tetraspanins as organizers of antigen-presenting cell function. Front. Immunol. 9:1074. doi: 10.3389/fimmu.2018.01074
Samstad, E. O., Niyonzima, N., Nymo, S., Aune, M. H., Ryan, L., Bakke, S. S., et al. (2014). Cholesterol crystals induce complement-dependent inflammasome activation and cytokine release. J. Immunol. 192, 2837–2845. doi: 10.4049/jimmunol.1302484
Schenk, G. J., Dijkstra, S., van het Hof, A. J., van der Pol, S. M. A., Drexhage, J. A. R., van der Valk, P., et al. (2013). Roles for HB-EGF and CD9 in multiple sclerosis. Glia 61, 1890–1905. doi: 10.1002/glia.22565
Schulte, T., Paschke, K. A., Laessing, U., Lottspeich, F., and Stuermer, C. A. (1997). Reggie-1 and reggie-2, two cell surface proteins expressed by retinal ganglion cells during axon regeneration. Development 124, 577–587.
Seigneuret, M. (2006). Complete predicted three-dimensional structure of the facilitator transmembrane protein and hepatitis C virus receptor CD81: conserved and variable structural domains in the tetraspanin superfamily. Biophys. J. 90, 212–227. doi: 10.1529/biophysj.105.069666
Seigneuret, M., Delaguillaumie, A., Lagaudrière-Gesbert, C., and Conjeaud, H. (2001). Structure of the tetraspanin main extracellular domain: a partially conserved fold with a structurally variable domain insertion. J. Biol. Chem. 276, 40055–40064. doi: 10.1074/jbc.M105557200
Sezgin, E., Levental, I., Mayor, S., and Eggeling, C. (2017). The mystery of membrane organization: composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 18, 361–374. doi: 10.1038/nrm.2017.16
Signoret, N., Hewlett, L., Wavre, S., Pelchen-Matthews, A., Oppermann, M., and Marsh, M. (2005). Agonist-induced endocytosis of CC chemokine receptor 5 is clathrin dependent. Mol. Biol. Cell 16, 902–917. doi: 10.1091/mbc.e04-08-0687
Silvie, O., Rubinstein, E., Franetich, J. F., Prenant, M., Belnoue, E., Rénia, L., et al. (2003). Hepatocyte CD81 is required for Plasmodium falciparum and Plasmodium yoelii sporozoite infectivity. Nat. Med. 9, 93–96. doi: 10.1038/nm808
Simons, K., and Ehehalt, R. (2002). Cholesterol, lipid rafts, and disease. J. Clin. Invest. 110, 597–603. doi: 10.1172/JCI16390
Simons, K., and Ikonen, E. (1997). Functional rafts in cell membranes. Nature 387, 569–572. doi: 10.1038/42408
Simons, K., and Toomre, D. (2000). Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1, 31–39. doi: 10.1038/35036052
Simons, K., and Van Meers, G. (1988). Lipid sorting in epithelial cells. Biochemistry 27, 6197–6202. doi: 10.1021/bi00417a001
Slaughter, N., Laux, I., Tu, X., Whitelegge, J., Zhu, X., Effros, R., et al. (2003). The flotillins are integral membrane proteins in lipid rafts that contain TCR-associated signaling components: implications for T-cell activation. Clin. Immunol. 108, 138–151. doi: 10.1016/S1521-6616(03)00097-4
Sotgia, F., Martinez-Outschoorn, U. E., Howell, A., Pestell, R. G., Pavlides, S., and Lisanti, M. P. (2012). Caveolin-1 and cancer metabolism in the tumor microenvironment: markers, models, and mechanisms. Annu. Rev. Pathol. 7, 423–467. doi: 10.1146/annurev-pathol-011811-120856
Szöllósi, J., Horejsí, V., Bene, L., Angelisová, P., and Damjanovich, S. (1996). Supramolecular complexes of MHC class I, MHC class II, CD20, and tetraspan molecules (CD53, CD81, and CD82) at the surface of a B cell line JY. J. Immunol. 157, 2939–2946.
Tall, A. R., and Yvan-Charvet, L. (2015). Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 15, 104–116. doi: 10.1038/nri3793
Thelen, M., and Legler, D. F. (2018). Membrane lipid environment: potential modulation of chemokine receptor function. Cytokine 109, 72–75. doi: 10.1016/j.cyto.2018.02.011
Tomassian, T., Humphries, L. A., Liu, S. D., Silva, O., Brooks, D. G., and Miceli, M. C. (2011). Caveolin-1 orchestrates TCR synaptic polarity, signal specificity, and function in CD8 T cells. J. Immunol. 187, 2993–3002. doi: 10.4049/jimmunol.1101447
Untemaehrer, J. J., Chow, A., Pypaert, M., Inaba, K., and Mellman, I. (2007). The tetraspanin CD9 mediates lateral association of MHC class II molecules on the dendritic cell surface. Proc. Natl. Acad. Sci. U.S.A. 104, 234–239. doi: 10.1073/pnas.0609665104
Van Den Hoorn, T., Paul, P., Janssen, L., Janssen, H., and Neefjes, J. (2012). Dynamics within tetraspanin pairs affect MHC class II expression. J. Cell Sci. 125, 328–339. doi: 10.1242/jcs.088047
Voss, M. A., Gordon, N., Maloney, S., Ganesan, R., Ludeman, L., McCarthy, K., et al. (2011). Tetraspanin CD151 is a novel prognostic marker in poor outcome endometrial cancer. Br. J. Cancer 104, 1611–1618. doi: 10.1038/bjc.2011.80
Wang, Z., Wang, N., Liu, P., Peng, F., Tang, H., Chen, Q., et al. (2015). Caveolin-1, a stress-related oncotarget, in drug resistance. Oncotarget 6, 37135–37150. doi: 10.18632/oncotarget.5789
Weis, W. I., and Kobilka, B. K. (2018). The molecular basis of G protein–coupled receptor activation. Annu. Rev. Biochem. 87, 897–919. doi: 10.1146/annurev-biochem-060614-033910
Wu, B., Chien, E. Y. T., Mol, C. D., Fenalti, G., Liu, W., Katritch, V., et al. (2010). Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science 330, 1066–1071. doi: 10.1126/science.1194396
Yu, Y., Liang, C., Wang, S., Zhu, J., Miao, C., Hua, Y., et al. (2018). CD151 promotes cell metastasis via activating TGF-β1/smad signaling in renal cell carcinoma. Oncotarget 9, 13313–13323. doi: 10.18632/oncotarget.24028
Zelman-Toister, E., Bakos, E., Cohen, S., Zigmond, E., Shezen, E., Grabovsky, V., et al. (2016). CD151 Regulates T-cell migration in health and inflammatory bowel disease. Inflamm. Bowel Dis. 22, 257–267. doi: 10.1097/MIB.0000000000000621
Zheng, Y., Han, G. W., Abagyan, R., Wu, B., Stevens, R. C., Cherezov, V., et al. (2017). Structure of CC chemokine receptor 5 with a potent chemokine antagonist reveals mechanisms of chemokine recognition and molecular mimicry by HIV. Immunity 46 1005–1017.e5. doi: 10.1016/j.immuni.2017.05.002
Zheng, Y., Qin, L., Zacarías, N. V. O., De Vries, H., Han, G. W., Gustavsson, M., et al. (2016). Structure of CC chemokine receptor 2 with orthosteric and allosteric antagonists. Nature 540, 458–461. doi: 10.1038/nature20605
Zhu, Z., Wang, J., Sun, Z., Sun, X., Wang, Z., and Xu, H. (2013). Flotillin2 expression correlates with HER2 levels and poor prognosis in gastric cancer. PLoS One 8:e62365. doi: 10.1371/journal.pone.0062365
Keywords: leukocyte migration, membrane compartmentalization, scaffold proteins, flotillin/reggie, tetraspanin, caveolin
Citation: Samson GPB and Legler DF (2020) Membrane Compartmentalization and Scaffold Proteins in Leukocyte Migration. Front. Cell Dev. Biol. 8:285. doi: 10.3389/fcell.2020.00285
Received: 23 January 2020; Accepted: 02 April 2020;
Published: 28 April 2020.
Edited by:
Falk Nimmerjahn, University of Erlangen–Nuremberg, GermanyReviewed by:
Ritva Tikkanen, University of Giessen, GermanyCopyright © 2020 Samson and Legler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel F. Legler, ZGFuaWVsLmxlZ2xlckBiaXRnLmNo
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.