- 1Department of Experimental Medicine, Sapienza University of Rome, Rome, Italy
- 2Clinical Department of Internal Medicine and Specialistics, Department of Advanced Clinical and Surgical Sciences, Università della Campania “Luigi Vanvitelli”, Naples, Italy
- 3IRCCS SDN, Naples, Italy
Adipose-derived stem cells (ASCs) represent a promising tool for soft tissue engineering as well as for clinical treatment of inflammatory and autoimmune pathologies. The well-characterized multi-differentiation potential and self-renewal properties of ASCs are coupled with their immunomodulatory ability in providing therapeutic efficacy. Yet, their impact in immune or inflammatory disorders might rely both on cell contact-dependent mechanisms and paracrine effects, resulting in the release of various soluble factors that regulate immune cells functions. Despite the widespread use of ASCs in clinical trials addressing several pathologies, the pathophysiological mechanisms at the basis of their clinical use have been not yet fully investigated. In particular, a thorough analysis of ASC immunomodulatory potential is mandatory. Here we explore such molecular mechanisms involved in ASC immunomodulatory properties, emphasizing the relevance of the milieu composition. We review the potential clinical use of ASC secretome as a mediator for immunomodulation, with a focus on in vitro and in vivo environmental conditions affecting clinical outcome. We describe some potential strategies for optimization of ASCs immunomodulatory capacity in clinical settings, which act either on adult stem cells gene expression and local microenvironment. Finally, we discuss the limitations of both allogeneic and autologous ASC use, highlighting the issues to be fixed in order to significantly improve the efficacy of ASC-based cell therapy.
Introduction
Adipose-derived mesenchymal stem cells (ASCs) represent a population of self-renewing multipotent adult cells in the vascular stroma of adipose tissues, playing important roles in development, post-natal growth, maintenance of tissue homeostasis and tissue repair and regeneration (Shingyochi et al., 2015; Dai et al., 2016). Significant amount of ASCs can be readily accessible from subcutaneous liposuction and, when appropriately stimulated, they can further differentiate into several cell-like types, including adipocytes, osteocytes, neural cells, vascular endothelial cells, cardiomyocytes, pancreatic cells, and hepatocytes (Zuk et al., 2001, 2002; Planat-Bénard et al., 2004; Aurich et al., 2009; Dave et al., 2014; Sommese et al., 2017). To date, mechanisms responsible for ASC therapeutic efficacy have been only partially investigated. The common belief is that their action relies on three major events: (1) multi-differentiation potential, (2) self-renewal properties and (3) immunomodulatory capacity. In particular, the first could account for their impact on tissue engineering applications, due to the ability to differentiate into tissue-specific cells and into endothelial cells, thus stimulating both tissue regeneration and arteriogenesis. Indeed, several researchers have analyzed the efficacy of ASC-based therapy in restoring cardiac function in ischemic heart diseases (IHD) (Valina et al., 2007; Naaijkens et al., 2014), providing evidence of an extremely low rate of survival and cardiac retention of ASCs after transplantation. Such indications suggest that ASC therapeutic efficacy in IHD should be ascribed mainly to their paracrine effects rather than the direct differentiation into cardiovascular lineage cells (Yang et al., 2013). So, evaluation of ASC therapeutic potential should take into account both cell retention and survival after transplantation and production of active paracrine factors (Li et al., 2019).
The proliferative potential could represent a key element to consolidate the results obtained by ASC injection, leading to the generation of a relatively stable clone of self-renewing cells within the target tissue. Regarding the immunomodulatory capacity, it has been demonstrated that ASCs, as well as BMSCs, have a hypoimmunogenic phenotype, since they lack the major MHC class II molecules and express only low levels of MHC class I, this allowing them to evade immune recognition (Puissant et al., 2005; McIntosh et al., 2006). Furthermore, ASCs can act as modulators of the host response, showing a greater in vitro immunomodulatory ability than BMSCs derived from age-matched donors (Melief et al., 2013), since they are able to partially suppress lymphocytes proliferation, as well as to inhibit differentiation of monocyte-derived immature dendritic cells and NK cell cytotoxic activity (Russell et al., 2016; Valencia et al., 2016). Such effects are likely to depend on both cell contact-dependent mechanisms and paracrine effects through the production of cytokines and various soluble factors that regulate immune cells functions (Sotiropoulou et al., 2006), improve the microenvironment for tissue healing (Burlacu et al., 2013) and exert strong immunosuppressive effects by decreasing inflammatory cytokine production (Zhao et al., 2010). Indeed, higher immunomodulatory potential of ASCs is also related to higher levels of cytokine production (Melief et al., 2013).
These findings contributed to make ASCs a viable option in regenerative medicine and a powerful tool in cell-based therapy for restoring damaged tissues and decreasing inflammatory/immune response, opening the way to their application in the treatment of a wide panel of pathologies, including inflammatory and autoimmune diseases (De Miguel et al., 2012; Scuderi et al., 2013; Onesti et al., 2016). In preclinical studies, ASCs have been successfully used to reduce chronic disability in ischemic stroke in rats (Gutiérrez-Fernández et al., 2013; Oh et al., 2015; Chen et al., 2016), to delay onset and slow disease progression in murine and rat models of multiple sclerosis (Yousefi et al., 2013; Semon et al., 2014; Bowles et al., 2017) and to limit structural changes in the lung parenchyma by reducing inflammation and neutrophils number in the airways in chronic obstructive pulmonary disease in mice and guinea pig models (Ghorbani et al., 2014; Hong et al., 2016). Preclinical studies on ASCs, performed in swine and rodent models, also showed promising results across a wide range of cardiovascular therapeutic applications (Hashemi et al., 2008; Cai et al., 2009; Madonna et al., 2009; Bai et al., 2010; Grimaldi et al., 2013; Sommese et al., 2017), due to both stimulation of angiogenesis and potent anti-inflammatory paracrine effect eventually favoring the cardiac healing process (Gnecchi et al., 2005). ASC-based cellular therapy has been further considered for the treatment of neurodegenerative diseases, including mouse models of Alzheimer’s disease or Parkinson’s disease and amyotrophic lateral sclerosis (ALS) patients (McCoy et al., 2008; Yan et al., 2014; Fontanilla et al., 2015; Staff et al., 2016), as well as, in humans, for immunological disorders, such as graft versus host disease (GvHD) (Yañez et al., 2006; Fang et al., 2007; Tholpady et al., 2009) and autoimmune pathologies, such as type I diabetes mellitus (Vanikar et al., 2010; Lin et al., 2015), systemic sclerosis (Scuderi et al., 2013), rheumatoid arthritis (El-Jawhari et al., 2014; Ueyama et al., 2020) and systemic lupus erythematosus (SLE) (Liang et al., 2010; Park et al., 2015). A consistent number of clinical trials using ASCs are ongoing for the treatment of some of these disorders1, even though only some complete clinical results are now available, but the greatest number of human studies are in patients with osteoarthritis and inflammatory bowel disease (IBD) (González et al., 2009a; Sovrea et al., 2019). In particular, multiple Phase I clinical trials assessed the efficacy of intra articular injection of ASCs in improving pain, function and mobility of affected joints, with no major adverse effects (Jo et al., 2014; Pers et al., 2016; Yokota et al., 2017). As for IBD, in a phase III study, ASCs have been shown to be effective and safe for the treatment of complex perianal fistulas in Crohn’s disease patients who did not respond to conventional and/or biological treatments (Panés et al., 2016). The most relevant studies on ASCs-based treatment in different pathologies are summarized in Table 1.
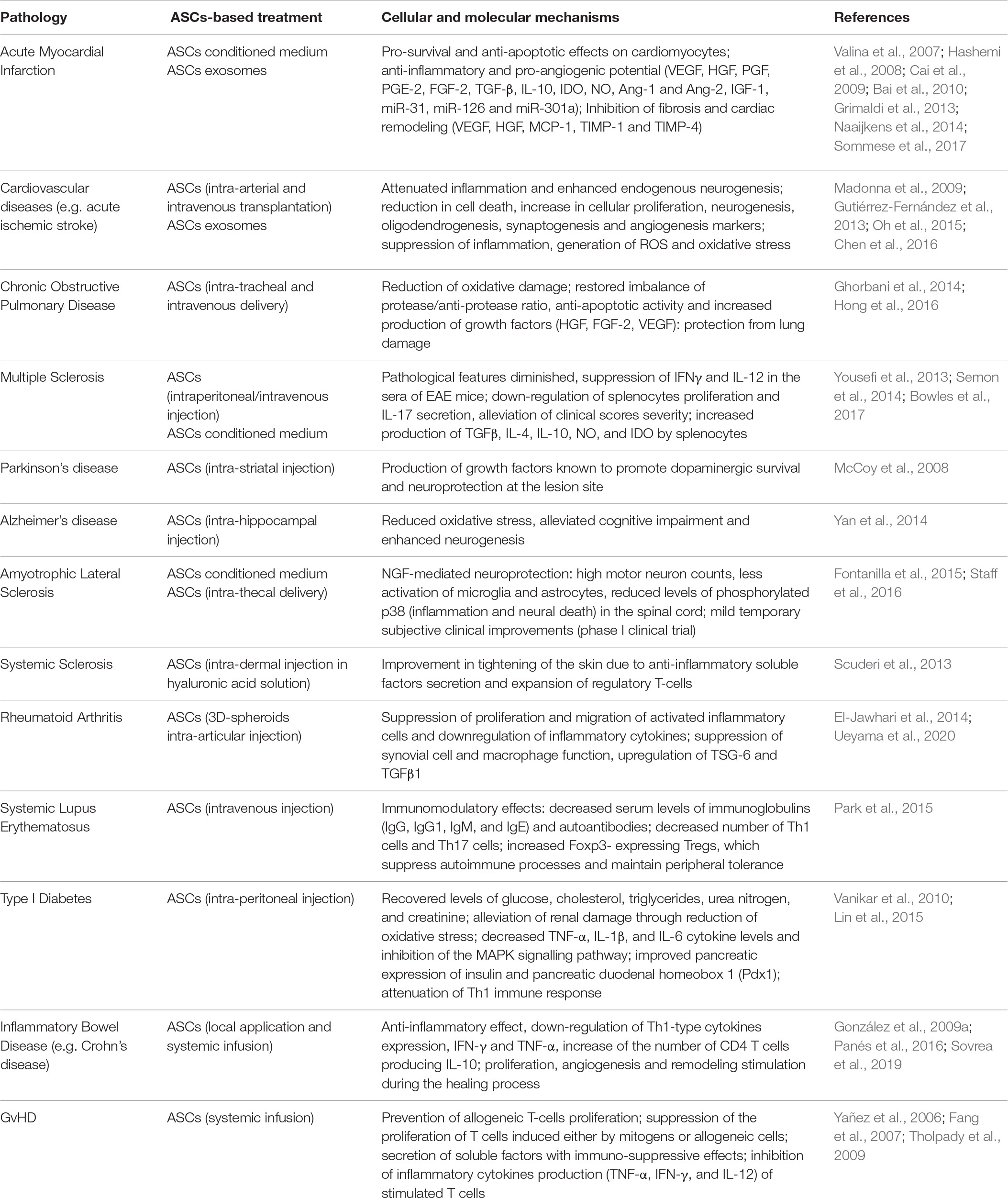
Table 1. The most relevant studies on ASCs-based treatment in different pathologies are highlighted, indicating the type of treatment and the cellular and molecular mechanisms involved in ASCs or ASC secretome effect in vivo.
Despite the widespread use of ASCs in cell therapy trials, the pathophysiological mechanisms at the basis of their clinical use have been not yet fully investigated. Here, we focused on the immunomodulatory properties of ASCs, through a comprehensive description of the molecular mechanisms and factors involved and the importance of milieu chemical-physical characteristics. Indeed, a plethora of paracrine signals are involved in ASC-mediated immunomodulation, thus suggesting the potential clinical use of ASC secretome, and the importance of environmental stimuli (culture conditions as well as local in vivo microenvironment) in determining clinical outcome. We also shed light on the potential strategies for optimization of ASCs efficacy in clinical settings, especially those related to boosting immunomodulatory capacity, acting both on local microenvironment and directly on ASCs through genetic or epigenetic regulation of gene expression, and to improving ASC characterization in order to standardize clinical outcome.
Immunomodulatory Properties of ASCs
ASCs are considered more powerful suppressors of immune response than mesenchymal stem cells (MSCs) derived from different tissue sources, including trabecular bone, bone marrow, dental pulp, and umbilical cord (Ribeiro et al., 2013; Nancarrow-Lei et al., 2017). In particular, ASCs immunomodulatory effects exceeds that of bone marrow MSCs, which are currently considered the gold standard, due to a higher level of cytokines secretion (Melief et al., 2013). Indeed, a small number of highly metabolically active ASCs secrete higher amount of immune suppressive cytokines, such as IL-6 and transforming growth factor-β1 (TGF-β1) (Soleymaninejadian et al., 2012; Melief et al., 2013; Montespan et al., 2014). In the allogeneic setting, where HLA mismatch occurs, such as in mixed lymphocyte reactions (MLR), ASCs suppress T cell allo-proliferation, because they express low levels of MHC-I, are deficient in major MHC-II and in costimulatory molecules, like CD80, CD86, CD40 and its ligand, CD40L (Strioga et al., 2012; Machado et al., 2013). ASCs also inhibit B lymphocyte proliferation and differentiation into plasmocytic cells, unable to produce antibodies (Franquesa et al., 2015). This characteristic could be important for their use in the treatment of B-cell mediated disorders and GvHD during organ transplantation (Franquesa et al., 2012, 2015). B-cells are antigen-presenting cells (APCs) that produce pro- and anti-inflammatory cytokines. When both ASCs and B-cells are in a co-culture setting, the former cells have an inhibitory effect on the chemotactic properties of the latter, by downregulating chemokine receptors on the B cells, such as CXCR4, CXCR5 (Corcione et al., 2006). In contrast, ASCs may induce proliferation of a subset of CD5 + regulatory B cells that secrete immunosuppressive IL-10. This cytokine inhibits the production of other inflammatory cytokines by activated T cells and could be relevant in the therapeutic treatment of autoimmune diseases (Kalampokis et al., 2013; Peng et al., 2015). In addition, a negative feedback loop between activated T cells-producing interferon γ (IFNγ) and ASCs exists (Machado et al., 2013). The secretion of IFNγ primes ASCs against T cells proliferation (Machado et al., 2013). Consequently, ASCs are able to escape immune surveillance and simultaneously, they are endowed with ability to self-renew and differentiate into other cell-like types, hence facilitating allogeneic tissue regeneration (Machado et al., 2013; Bateman et al., 2018). A relevant example of the immunomodulatory potential of allogeneic ASCs is the injection of human ASCs (hASCs) in a DBA/1 mouse model with collagen-induced arthritis (González et al., 2009b). Administration of hASCs provoked a decrease of several inflammatory cytokines and chemokines, resulting in reduction of antigen-specific Th1/Th17 cell proliferation. In contrast, IL-10 production was induced in lymph nodes and joints. Yet, antigen-specific Tregs were also produced and as a consequence, self-reactive T effector responses were suppressed (González et al., 2009b).
The stromal vascular fraction (SVF) of an adipose tissue contains not only ASCs but also different cell types, such as endothelial cells, pericytes, lymphocytes, monocytes macrophages, fibroblasts, and smooth muscle cells (Dominici et al., 2006; Bourin et al., 2013; Nürnberger et al., 2019). In vitro, freshly isolated SVF containing ASCs secrete trophic and pro-regenerative factors, such as cytokines, growth factors, anti-inflammatory factors and extracellular vesicles harboring proteins or even microRNAs, collectively known as secretome (Fu et al., 2017). In fact, several studies have shown that the paracrine effects of ASCs and not the cells themselves, are pivotal players for tissue repair along with angiogenic and immunomodulatory properties occurring at the site of the damaged tissue (Tögel et al., 2005; Krampera, 2011; Eleuteri and Fierabracci, 2019). For example, high levels of two immunomodulatory mediators, indoleamine-pyrrole 2,3-dioxygenase (IDO) and Prostaglandin E2 (PGE2), were detected at 24 h in the supernatants from freshly isolated SVF (Nürnberger et al., 2019). It is known that IDO is an immunomodulatory enzyme produced by macrophages with immunosuppressive functions for T-cells and natural killers (NK). Moreover, ASCs inhibit maturation of dendritic cells and induce macrophage to differentiate into anti-inflammatory regulatory cells (Kim and Hematti, 2009; Sun et al., 2019). PGE2 is a major mediator of ASCs immunomodulation having multiple functions (Gao et al., 2016; Najar et al., 2016; Kota et al., 2017). One of these is the induction of IL-10 by macrophages, which in turn inhibits NK cells and T helper cells (Eleuteri and Fierabracci, 2019). Human leukocyte antigen-G5 (HLA-G5), galectins and programmed cell death ligand (PD-L1) are other factors involved in immunomodulation by ASCs (Gieseke et al., 2010; Yang et al., 2012; Zhou et al., 2018). HLA-G5 is a non-classical MHC- class I molecule, expressed on the surface of ASCs, which exert immunogenic tolerance through inhibition of NK, allogeneic T-cell responses and dendritic cells (DC) (Nasef et al., 2007). Galectin-1 and PD-L1 are negative regulators of immune responses, known as immune checkpoints (ICs) and are expressed on the surface of ASCs (Najar et al., 2010; Zhou et al., 2018). A milestone study conducted by Sheng et al. (2008) showed that PD-L1 expression is induced on the surface of MSCs isolated from mouse bone marrow by T cell secreted IFNγ. This study provided further insights as to the role of PD-L1 and other ICs in the immunosuppressive potential of ASCs. Zhou et al. (2018) co-cultured human ASCs that express PD-L1 and galectin 9 (Gal-9), with allogeneic peripheral blood mononuclear cells (PBMCs). The two ICs bound the corresponding ligands on the T cell, PD-L1 to PD-1 and Gal-9 to TIMP-3. In these experimental conditions, ASCs evoked T cell suppression through inhibition of the transcription factor NF-κB activation in TCR-stimulated T cells via the PD-L1/PD-1 and Gal-9/TIMP-3 pathways (Zhou et al., 2018). However, the interaction between ICs and ASCs has not been explored fully and this could be an exciting area of further investigation.
The immunosuppressive ability of ASCs is both dose and cell passage dependent (Waldner et al., 2018). After the isolation of ASCs from adipose tissues, these cells undergo multiple culture passages in vitro, since significant numbers are needed for autologous clinical applications. Critically, during serial passaging, ASCs become more differentiated and simultaneously they start losing their immunosuppressive properties. For instance, HLA-G expression dramatically decreases in adult ASCs (Teklemariam et al., 2014). Furthermore, the expression of HLA class-II and the other co-stimulatory molecules, CD80 and CD86, increases in very late passaged ASCs differentiating in mature adipocytes then causing T-cell proliferation in a direct contact-dependent manner (Poloni et al., 2015). Changes in cytokine milieu chemical-physical characteristics with subsequent passages of ASCs, also need to be taken into consideration (Leto Barone et al., 2013). Therapeutic applications of ASCs are influenced by hypoxic conditions, 3D cell culture scaffolds and growth factors added in culture, that could modify cell proliferation and ultimately their secretome (Madrigal et al., 2014). Once the ASCs or their secretome are injected into an injured tissue, they might interact with an unfavorable microenvironment, provoking different therapeutic responses than expected. For this reason, it has been suggested to evaluate the inflammatory status and the disease stage of a patient prior to the recruitment and treatment for clinical trials (Eleuteri and Fierabracci, 2019). The safety and timing of ASCs administration are also important requirements for the favorable outcome of cell therapy (Leto Barone et al., 2013).
Approaches for Optimization of ASCs Immunomodulatory Efficacy
Although ASCs demonstrated higher immunomodulatory potential with respect to other kind of MSCs (Ivanova-Todorova et al., 2009; Najar et al., 2010, 2013; Ribeiro et al., 2013), their use as a gold standard for cell therapy of immune-related disorders needs further elucidations. Once ASCs are isolated and expanded in 2D cultures in vitro, they lose the stem cell niche environmental protection and sustainment, which is important to maintain ASC pluripotency, but also their immunomodulatory properties (Jones and Wagers, 2008; Napoli et al., 2008; Voog and Jones, 2010; Kaewsuwan et al., 2012). To date, different ASC enhancement strategies have been proposed, based on external stimuli changes and interventions focused on cells themselves.
An intuitive solution could be to directly recapitulate stem cell niche setting through 3D cultures. In these multicellular structures, adherent cells aggregate with each other through suspension culture system, generating spheroids (Kapur et al., 2012; Daquinag et al., 2013; Duval et al., 2017). As such, paracrine signaling is promoted by the proximity of the cells and the interaction of ASCs with extra cellular matrix (ECM)-like structures or other cell types (co-cultures) increases the secretion level of anti-inflammatory and pro-angiogenic molecules (Amos et al., 2010; Bartosh et al., 2010; Mineda et al., 2015). The employment of ASCs spheroids in the treatment of experimental in vivo disease models such as elastase-induced emphysema, hindlimb ischemia, acute kidney ischemia and diabetic skin wound healing demonstrated encouraging results regarding differentiation, pro-angiogenic and regenerative capacity of ASCs (Amos et al., 2010; Xu et al., 2016; Cho et al., 2017; Park et al., 2017). Currently, chitosan-coated culture plates, concaved bottom wells, ultra-low attachment plates, the hanging drop technique and spinner flask are the most widely used methods for spheroid generation (Duval et al., 2017). In particular, Regmi et al. (2019) showed that 3D-ASCs obtained through hanging drop method have enhanced immunomodulatory effects in systemic inflammatory response syndrome frequently observed in severe fulminant hepatic failure. Limitations of 3D spheroids may be related to the fact that in vitro nutrients and oxygen may be less available at the core of the 3D spheres, inducing necrosis and altering ASCs functions (Cheng et al., 2013; Edmondson et al., 2014). An alternative strategy to obtain 3D ASCs cell cultures is simulate microgravity in a Rotary Cell Culture System (RCCS) bioreactor (Yu et al., 2011; Kang et al., 2015). The RCCS system can be useful to overcome the oxygen and nutrient gradient problems (Costantini et al., 2019). Anyway, in these experimental conditions, some other factors involved in immunomodulation could be harder to control. An easier strategy may be to dissect these mechanisms and apply single level interventions.
As for the external stimuli, it has been demonstrated that the presence of growth factors in the culture medium could enhance ASCs differentiation (Ceccarelli et al., 2018). Likewise, pre-conditioning of ASCs with cytokines or other bioactive molecules represents one of the main approaches to boost ASCs immune regulatory functions before their therapeutic administration. IFN-γ and tumor necrosis factor α (TNF-α) are the major inflammatory cytokines used in MSC functional enhancement. After IFN-γ priming, ASCs showed overexpression of IDO, together with Cyclooxygenase 2 (COX2), TGF-β and Hepatocyte Growth Factor (HGF) (Ryan et al., 2007; Delarosa et al., 2009; Kronsteiner et al., 2011), central factors in immunological tolerance and anti-inflammatory mechanisms. TNF-α treatment, not only increased the production of IDO, PGE2, and HGF, but also of the pro-inflammatory cytokines IL-6 and IL-8, promoting endothelial progenitor cell migration and angiogenesis (Crop et al., 2010; Kwon et al., 2013), a primary goal of regenerative medicine. Furthermore, ASCs primed with IL-17, TNF-α, and IFN-γ have increased T cell immunosuppressive capacity mediated by inducible nitric oxide synthase (iNOS) production and have been shown to reduce inflammation and tissue injury in murine model of hepatitis (Han et al., 2014). An additional strategy is the pre-conditioning of ASCs with toll-like receptors (TLRs) agonists. ASCs express TLRs, cell membrane sensors that play a pivotal role in innate immune system mechanisms (Hwa Cho et al., 2006; Lombardo et al., 2009). Although TLRs agonists seem to be mainly implicated in ASCs multi-lineage differentiation capacity (Seo et al., 2019), studies demonstrated that TLR3 activation might induce suppressive phenotype (Hwa Cho et al., 2006; Bunnell et al., 2010; Waterman et al., 2010). By mimicking an in vivo inflammatory milieu, the combination of cytokines and TLRs ligands seemed to generate addictive effects on MSCs immunomodulatory properties, enhancing their therapeutic efficacy more than the use of a single molecular category (Gu et al., 2015). The downside of this approach may be that cytokine priming can confer immunogenicity to the ASCs, exposing them to host immune responses (Galipeau, 2017).
To date, only few studies have been performed on the induction of epigenetic modification to enhance immunomodulatory capacity of ASCs. Recent findings have provided insight into the exposure to 5-Aza-2′-deoxycytidine (5-AZA-dC) during in vitro expansion of ASCs that resulted in the upregulation of HLA-G gene, whose sustained expression is crucial to maintain immunomodulatory capabilities in adult stem cells (Teklemariam et al., 2014). MicroRNAs are also implicated in epigenetic regulation of ASCs immunomodulatory properties, as proven by Wang et al. (2019) in a mouse model of colitis. Mysm1, a histone deubiquitinase, is induced by TNF-α and IFNγ in ASCs and promotes miR-150 transcription, which enhances iNOS production. Nitric oxide is catalyzed by iNOS that is essential for the immunosuppressive capacity of ASCs. Therefore, miRNA-based strategies could be promising in enhancing therapeutic ASCs efficacy. Because both epigenetic modulators and ASCs have common aspects in immune modulation (Lee et al., 2015; Sabia et al., 2017), their respective contributions should be established and it is important to pay attention to possible cross-interactions.
A straightforward approach is to modify MSCs in order to increase the expression of genes such as IL-10 (Min et al., 2007), HGF (Bian et al., 2009), IDO (Kim et al., 2018), and FOXP3 (Qi et al., 2015) to obtain therapeutic anti-inflammatory effects. Genetically engineered ASCs have demonstrated efficacy in the treatment of inflammatory disease in vivo. Several studies reported the clinical success of different approaches to ASC genetic modification. The administration of human ASCs transduced with a bicistronic lentiviral vector encoding mouse IL-4 in C57Bl/6 mice with experimental autoimmune encephalomyelitis (EAE), a model of multiple sclerosis, results in a reduction of antigen-specific T-cell responses, thus attenuating clinical disease (Payne et al., 2012). Human ASCs transduced with a lentiviral vector for CTLA-4 Ig overexpression, when transplanted into a mouse model of sustained severe collagen induced arthritis, are able to enable CTLA-4 binding to CD28, which results in the induction of T-cell clonal anergy and the amelioration of autoimmune disease (Choi et al., 2016). The introduction of IL-1 receptor-like-1 (sST2), a decoy receptor for IL-33, in ASCs using a bicistronic lentiviral vector encoding the sST2–C-terminal promotes inflammation suppression and alleviation of the pathological events in acute lung injury (ALI) mouse model (Martínez-González et al., 2013). Furthermore, overexpression of IL-35, a recently discovered anti-inflammatory cytokine, represent a powerful tool to potentiate ASC-based cell therapy approach for auto-immune diseases. Results obtained by Zhao et al. demonstrated that murine ASCs transduced with a recombinant lentiviral vector to overexpress IL-35 are able to decrease CD4 + T-cell proliferation and IL-17 secretion in an in vitro co-culture model (Zhao et al., 2017).
The Road Ahead in Immunomodulatory Potential of ASCs
Much effort has been done regarding the use of autologous versus allogeneic cells for in vivo applications (Feisst et al., 2015), with the first being preferred especially for chronic pathologies since the time required for the isolation and expansion of cells is not a limit given to the non-acute nature of the diseases. However, by using autologous ASCs there were consistent variations in clinical outcome, since cell characteristics may vary between patients, not only according to biological factors as age, sex, body mass index but also depending upon the disease (Varghese et al., 2017).
In this regard, contradictory results are available about the properties of ASCs isolated from patients affected by autoimmune and chronic inflammatory diseases. For example, some reports demonstrated that ASCs from patients affected by systemic sclerosis show the same phenotypical and functional characteristics of their healthy counterparts (Scuderi et al., 2013; Capelli et al., 2017; Velier et al., 2019), while Griffin et al. (2017) observed that ASCs from sclerodermic patients showed identical phenotype and differentiation capacity of those from healthy donors, but displayed reduced proliferation and migration capacity. Whilst, the presence of cardiovascular risk factors in cardiac patients seems to reduce ASC pluripotency and self-renewal, thus discouraging their autologous use in the clinical setting (Dimmeler and Leri, 2008; Krawiec et al., 2016). In particular, microenvironmental factors and metabolic disorders may impact the functionality of these cells. ASCs derived from obese subjects and from patients affected by type 2 diabetes showed increased expression of inflammatory markers with respect to those derived from lean donors, as well as a remarkable reduction in their immunosuppressive activities (Serena et al., 2016). Moreover, ASCs from obese patients also display an impaired angiogenic potential (Oñate et al., 2012, 2013). Other groups observed that diabetes did not alter ASC isolation efficiency, growth curves and angiogenic potential, but ASCs from diabetic patients showed a delay in the acquisition of endothelial cell markers, thus suggesting an impaired differentiation (Policha et al., 2014).
The use of allogeneic stem cells may overcome these limitations, but it has to be considered that adipose tissue availability is dependent on surgical procedures, thus limiting the occurrence of overall healthy donors. More attention should be also paid to the origin of allogeneic ASCs, since several studies underlined a cellular and molecular variability depending on the donor age, sex and tissue source even under similar genetic and environmental conditions (Shu et al., 2012; Bodle et al., 2014; Ock et al., 2016; Abbo et al., 2017). In particular, ASCs derived from different donors might have significant variations in the chemical-physical characteristics of their secretome (Alicka et al., 2019), thus affecting their immunomodulatory capacity (Bunnell et al., 2010). In order to avoid donor-to-donor heterogeneity, a strategy with pooled ASCs of different allogeneic donors could be proposed, as previously reported for BMSCs (Kuçi et al., 2016), but immunogenic stimuli could increase (Ankrum et al., 2014; Patrikoski et al., 2014). Indeed, although ASCs have been shown to possess a low immunogenic profile, the potential immunogenicity of allogeneic cells, which might determine their rejection after infusion, cannot be excluded. In fact, it has been observed that the immunogenicity of ASCs decreases with cell passaging, so that cells at low passages are more immunogenic than those at higher passages (McIntosh et al., 2006), and that ASCs are not fully immune privileged, since they elicit both humoral and cellular immune response in vivo, depending on the microenvironment (Ankrum et al., 2014). Also ASC differentiation may alter their immunogenic phenotype, increasing HLA class-I and HLA class-II expression (García-Sancho et al., 2017), as well as culturing condition (e.g., use of human serum or serum-free conditions) (Patrikoski et al., 2014).
Conclusion
ASCs represent a valuable treatment option for a wide range of inflammatory or autoimmune diseases, which therapeutic efficacy relies primarily on immunomodulatory activities mediate by paracrine effects. An increasing number of preclinical studies and clinical trials are being developed to assess ASCs safety and efficacy. However, although there are promising results and increasing knowledge in the in vivo applications of ASCs, unfortunately, several clinical trials have failed due to differences in experimental protocols, read-out, animal models and variability in ASCs characteristics. So, the clinical translation of ASCs still requires a proper validation in large controlled trials. For a more successful outcome of ASC based therapies, thorough investigations with more standardized protocols are urgently required, as well as a better understand of ASC immunomodulatory network and the identification of key molecules and/or regulatory mechanisms responsible for ASC effects in chronic inflammatory diseases.
The in-depth analysis of the potential strategies aimed to boost ASC-mediated immunomodulation (summarized in Figure 1) will foster new targeted approaches for cell therapy applications in the field of immune diseases. Yet, such interventions need to be accurately considered, since any alteration of immune system sensitivity can be dangerous: weakening the capacity of recognize transformed cells or pathogens, or vice versa exacerbating immune response, could lead to adverse events. As for genetic modification, the engineered cells persisting in the host could generate undesirable effects, such as potential tumorigenicity related to genetic instability (Heslop et al., 2015; Neri, 2019).
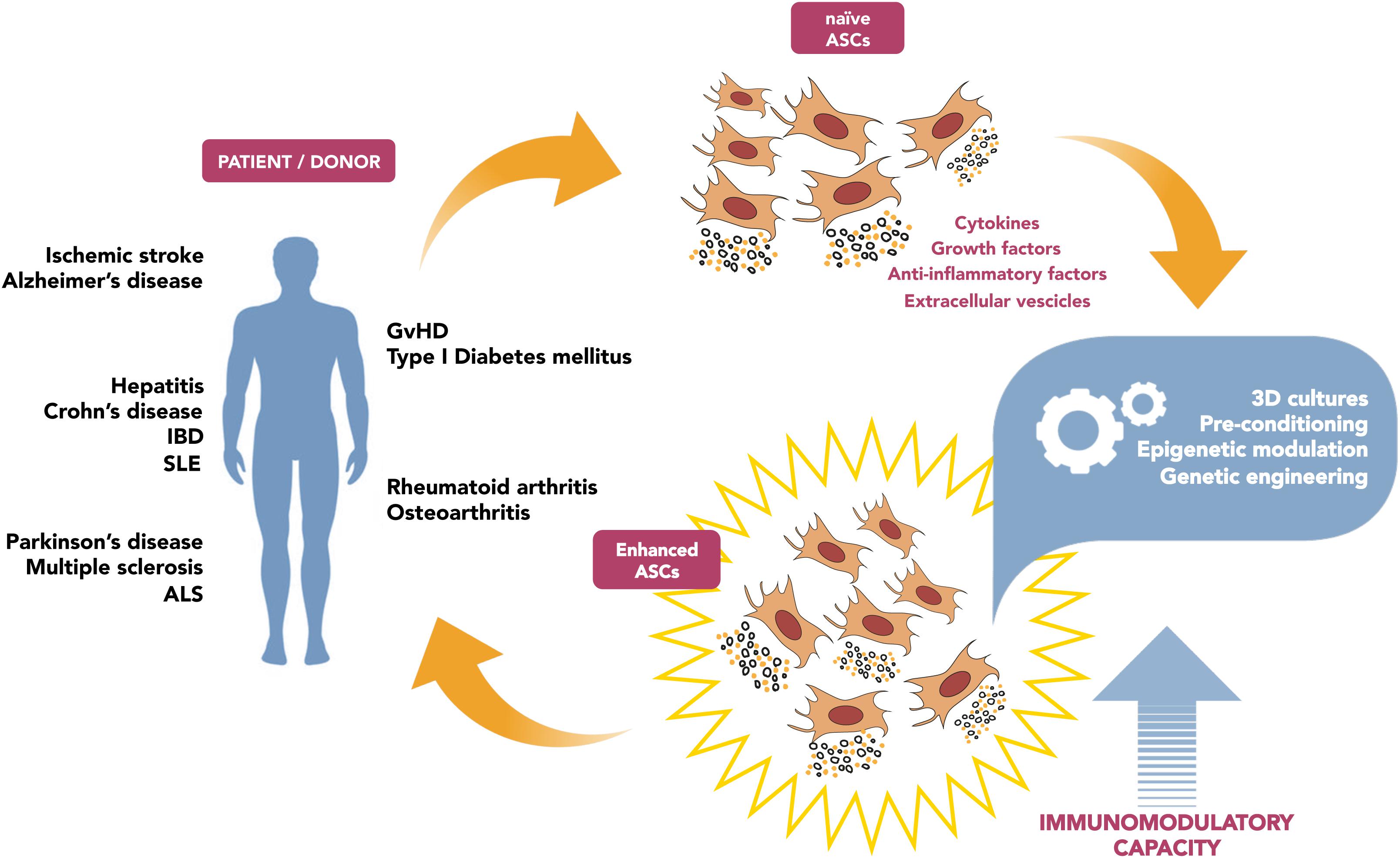
Figure 1. Schematic diagram illustrating the potential strategies aimed to boost ASC-mediated immunomodulation, in order to improve clinical outcome.
Finally, standardization of both ASC and patient’s characteristics prior to clinical use are necessary to avoid that the donor phenotype might compromise their immunomodulatory properties, thus impairing their therapeutic efficacy.
Author Contributions
SC conceived the review and wrote the manuscript. PP and EA revised the literature and helped to writing the manuscript. CN edited the manuscript. CM supervised the overall project and edited the manuscript. All authors had the opportunity to discuss and comment on the manuscript.
Funding
This research was conducted with the help of the PRIN 2017 supporting grant no. F8ZB89.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
References
Abbo, O., Taurand, M., Monsarrat, P., Raymond, I., Arnaud, E., De Barros, S., et al. (2017). Comparison between pediatric and adult adipose mesenchymal stromal cells. Cytotherapy 19, 395–407. doi: 10.1016/j.jcyt.2016.11.012
Alicka, M., Major, P., Wysocki, M., and Marycz, K. (2019). Adipose-derived mesenchymal stem cells isolated from patients with type 2 diabetes show reduced “Stemness” through an altered secretome profile. impaired anti-oxidative protection, and mitochondrial dynamics deterioration. J. Clin. Med. 8:E765. doi: 10.3390/jcm8060765
Amos, P. J., Kapur, S. K., Stapor, P. C., Shang, H., Bekiranov, S., Khurgel, M., et al. (2010). Human adipose-derived stromal cells accelerate diabetic wound healing: impact of cell formulation and delivery. Tissue Eng. Part A 16, 1595–1606. doi: 10.1089/ten.TEA.2009.0616
Ankrum, J. A., Ong, J. F., and Karp, J. M. (2014). Mesenchymal stem cells: immune evasive, not immune privileged. Nat. Biotechnol. 32, 252–260. doi: 10.1038/nbt.2816
Aurich, H., Sgodda, M., Kaltwaßer, P., Vetter, M., Weise, A., Liehr, T., et al. (2009). Hepatocyte differentiation of mesenchymal stem cells from human adipose tissue in vitro promotes hepatic integration in vivo. Gut 58, 570–581. doi: 10.1136/gut.2008.154880
Bai, X., Yan, Y., Song, Y. H., Seidensticker, M., Rabinovich, B., Metzele, R., et al. (2010). Both cultured and freshly isolated adipose tissue-derived stem cells enhance cardiac function after acute myocardial infarction. Eur. Heart J. 31, 489–501. doi: 10.1093/eurheartj/ehp568
Bartosh, T. J., Ylöstalo, J. H., Mohammadipoor, A., Bazhanov, N., Coble, K., Claypool, K., et al. (2010). Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc. Natl. Acad. Sci. U.S.A. 107, 13724–13729. doi: 10.1073/pnas.1008117107
Bateman, M. E., Strong, A. L., Gimble, J. M., and Bunnell, B. A. (2018). Concise review: using fat to fight disease: a systematic review of nonhomologous adipose-derived stromal/stem cell therapies. Stem Cells 36, 1311–1328. doi: 10.1002/stem.2847
Bian, L., Guo, Z. K., Wang, H. X., Wang, J. S., Wang, H., Li, Q. F., et al. (2009). In vitro and in vivo immunosuppresive characteristics of hepatocyte growth factor-modified murine mesenchymal stem cells. In Vivo 23, 21–27.
Bodle, J. C., Teeter, S. D., Hluck, B. H., Hardin, J. W., Bernacki, S. H., and Loboa, E. G. (2014). Age-related effects on the potency of human adipose-derived stem cells: creation and evaluation of superlots and implications for musculoskeletal tissue engineering applications. Tissue Eng. Part C Methods 20, 972–983. doi: 10.1089/ten.TEC.2013.0683
Bourin, P., Bunnell, B. A., Casteilla, L., Dominici, M., Katz, A. J., March, K. L., et al. (2013). Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International So. Cytotherapy 15, 641–648. doi: 10.1016/j.jcyt.2013.02.006
Bowles, A. C., Strong, A. L., Wise, R. M., Thomas, R. C., Gerstein, B. Y., Dutreil, M. F., et al. (2017). Adipose stromal vascular fraction-mediated improvements at late-stage disease in a murine model of multiple sclerosis. Stem Cells 35, 532–544. doi: 10.1002/stem.2516
Bunnell, B. A., Betancourt, A. M., and Sullivan, D. E. (2010). New concepts on the immune modulation mediated by mesenchymal stem cells. Stem Cell Res. Ther. 1:34. doi: 10.1186/scrt34
Burlacu, A., Grigorescu, G., Rosca, A. M., Preda, M. B., and Simionescu, M. (2013). Factors secreted by mesenchymal stem cells and endothelial progenitor cells have complementary effects on angiogenesis in vitro. Stem Cells Dev. 22, 643–653. doi: 10.1089/scd.2012.0273
Cai, L., Johnstone, B. H., Cook, T. G., Tan, J., Fishbein, M. C., Chen, P. -S., et al. (2009). IFATS collection: human adipose tissue-derived stem cells induce angiogenesis and nerve sprouting following myocardial infarction, in conjunction with potent preservation of cardiac function. Stem Cells 27, 230–237. doi: 10.1634/stemcells.2008-0273
Capelli, C., Zaccara, E., Cipriani, P., Di Benedetto, P., Maglione, W., Andracco, R., et al. (2017). Phenotypical and functional characteristics of in vitro-expanded adipose-derived mesenchymal stromal cells from patients with systematic sclerosis. Cell Transplant. 26, 841–854. doi: 10.3727/096368917X694822
Ceccarelli, S., Nodale, C., Vescarelli, E., Pontecorvi, P., Manganelli, V., Casella, G., et al. (2018). Neuropilin 1 mediates keratinocyte growth factor signaling in adipose-derived stem cells: potential involvement in adipogenesis. Stem Cells Int. 2018:1075156. doi: 10.1155/2018/1075156
Chen, K. H., Chen, C. H., Wallace, C. G., Yuen, C. M., Kao, G. S., Chen, Y. L., et al. (2016). Intravenous administration of xenogenic adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes markedly reduced brain infarct volume and preserved neurological function in rat after acute ischemic stroke. Oncotarget 7, 74537–74556. doi: 10.18632/oncotarget.12902
Cheng, N.-C., Chen, S.-Y., Li, J.-R., and Young, T.-H. (2013). Short-Term spheroid formation enhances the regenerative capacity of adipose-derived stem cells by promoting stemness, angiogenesis, and chemotaxis. Stem Cells Transl. Med. 2, 584–594. doi: 10.5966/sctm.2013-0007
Cho, R. J., Kim, Y. S., Kim, J. Y., and Oh, Y. M. (2017). Human adipose-derived mesenchymal stem cell spheroids improve recovery in a mouse model of elastase-induced emphysema. BMB Rep. 50, 79–84. doi: 10.5483/bmbrep.2017.50.2.101
Choi, E. W., Shin, I. S., Song, J. W., Lee, M. J., Yun, T. W., Yang, J., et al. (2016). Effects of transplantation of CTLA4Ig-overexpressing adipose tissue-derived mesenchymal stem cells in mice with sustained severe rheumatoid arthritis. Cell Transplant. 25, 243–259. doi: 10.3727/096368915X688470
Corcione, A., Benvenuto, F., Ferretti, E., Giunti, D., Cappiello, V., Cazzanti, F., et al. (2006). Human mesenchymal stem cells modulate B-cell functions. Blood 107, 367–372.
Costantini, D., Overi, D., Casadei, L., Cardinale, V., Nevi, L., Carpino, G., et al. (2019). Simulated microgravity promotes the formation of tridimensional cultures and stimulates pluripotency and a glycolytic metabolism in human hepatic and biliary tree stem/progenitor cells. Sci. Rep. 9:5559. doi: 10.1038/s41598-019-41908-5
Crop, M. J., Baan, C. C., Korevaar, S. S., IJzermans, J. N. M., Pescatori, M., Stubbs, A. P., et al. (2010). Inflammatory conditions affect gene expression and function of human adipose tissue-derived mesenchymal stem cells. Clin. Exp. Immunol. 162, 474–486. doi: 10.1111/j.1365-2249.2010.04256.x
Dai, R., Wang, Z., Samanipour, R., Koo, K. I., and Kim, K. (2016). Adipose-derived stem cells for tissue engineering and regenerative medicine applications. Stem Cells Int. 2016:6737345. doi: 10.1155/2016/6737345
Daquinag, A. C., Souza, G. R., and Kolonin, M. G. (2013). Adipose tissue engineering in three-dimensional levitation tissue culture system based on magnetic nanoparticles. Tissue Eng. Part C Methods 19, 336–344. doi: 10.1089/ten.TEC.2012.0198
Dave, S. D., Vanikar, A. V., and Trivedi, H. L. (2014). In-vitro generation of human adipose tissue derived insulin secreting cells: up-regulation of Pax-6. Ipf-1 and Isl-1. Cytotechnology 66, 299–307. doi: 10.1007/s10616-013-9573-3
De Miguel, M. P., Fuentes-Julian, S., Blazquez-Martinez, A., Pascual, C. Y., Aller, M. A., Arias, J., et al. (2012). Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr. Mol. Med. 12, 574–591.
Delarosa, O., Lombardo, E., Beraza, A., Mancheño-Corvo, P., Ramirez, C., Menta, R., et al. (2009). Requirement of IFN-γ-mediated indoleamine 2,3-dioxygenase expression in the modulation of lymphocyte proliferation by human adipose-derived stem cells. Tissue Eng. Part A 15, 2795–2806. doi: 10.1089/ten.tea.2008.0630
Dimmeler, S., and Leri, A. (2008). Aging and disease as modifiers of efficacy of cell therapy. Circ. Res. 102, 1319–1330. doi: 10.1161/CIRCRESAHA.108.175943
Dominici, M., Le Blanc, K., Mueller, I., Slaper-Cortenbach, I., Marini, F. C., Krause, D. S., et al. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8, 315–317. doi: 10.1080/14653240600855905
Duval, K., Grover, H., Han, L. H., Mou, Y., Pegoraro, A. F., Fredberg, J., et al. (2017). Modeling physiological events in 2D vs. 3D cell culture. Physiology 32, 266–277. doi: 10.1152/physiol.00036.2016
Edmondson, R., Broglie, J. J., Adcock, A. F., and Yang, L. (2014). Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol. 12, 207–218. doi: 10.1089/adt.2014.573
Eleuteri, S., and Fierabracci, A. (2019). Insights into the secretome of mesenchymal stem cells and its potential applications. Int. J. Mol. Sci. 20:E4597. doi: 10.3390/ijms20184597
El-Jawhari, J. J., El-Sherbiny, Y. M., Jones, E. A., and McGonagle, D. (2014). Mesenchymal stem cells, autoimmunity and rheumatoid arthritis. QJM 107, 505–514. doi: 10.1093/qjmed/hcu033
Fang, B., Song, Y., Lin, Q., Zhang, Y., Cao, Y., Zhao, R. C., et al. (2007). Human adipose tissue-derived mesenchymal stromal cells as salvage therapy for treatment of severe refractory acute graft-vs.-host disease in two children. Pediatr. Transplant. 11, 814–817. doi: 10.1111/j.1399-3046.2007.00780.x
Feisst, V., Meidinger, S., and Locke, M. B. (2015). From bench to bedside: use of human adipose-derived stem cells. Stem Cells Cloning 8, 149–162. doi: 10.2147/SCCAA.S64373
Fontanilla, C. V., Gu, H., Liu, Q., Zhu, T. Z., Zhou, C., Johnstone, B. H., et al. (2015). Adipose-derived stem cell conditioned media extends survival time of a mouse model of amyotrophic lateral sclerosis. Sci. Rep. 5:16953. doi: 10.1038/srep16953
Franquesa, M., Hoogduijn, M. J., Bestard, O., and Grinyó, J. M. (2012). Immunomodulatory effect of mesenchymal stem cells on B cells. Front. Immunol. 3:212. doi: 10.3389/fimmu.2012.00212
Franquesa, M., Mensah, F. K., Huizinga, R., Strini, T., Boon, L., Lombardo, E., et al. (2015). Human adipose tissue-derived mesenchymal stem cells abrogate plasmablast formation and induce regulatory B cells independently of T helper cells. Stem Cells 33, 880–891. doi: 10.1002/stem.1881
Fu, Y., Karbaat, L., Wu, L., Leijten, J., Both, S. K., and Karperien, M. (2017). Trophic effects of mesenchymal stem cells in tissue regeneration. Tissue Eng. Part B Rev. 23, 515–528. doi: 10.1089/ten.TEB.2016.0365
Galipeau, J. (2017). Reply: “function of cryopreserved mesenchymal stromal cells with and without interferon-γ prelicensing is context dependent.”. Stem Cells 35, 1440–1441. doi: 10.1002/stem.2526
Gao, F., Chiu, S. M., Motan, D. A. L., Zhang, Z., Chen, L., Ji, H. L., et al. (2016). Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis. 7:e2062. doi: 10.1038/cddis.2015.327
García-Sancho, J., Sánchez, A., Vega, A., Noriega, D. C., and Nocito, M. (2017). Influence of HLA matching on the efficacy of allogeneic mesenchymal stromal cell therapies for osteoarthritis and degenerative disc disease. Transplant. Direct 3:e205. doi: 10.1097/TXD.0000000000000724
Ghorbani, A., Feizpour, A., Hashemzahi, M., Gholami, L., Hosseini, M., Soukhtanloo, M., et al. (2014). The effect of adipose derived stromal cells on oxidative stress level, lung emphysema and white blood cells of guinea pigs model of chronic obstructive pulmonary disease. DARU J. Pharm. Sci. 22:26. doi: 10.1186/2008-2231-22-26
Gieseke, F., Böhringer, J., Bussolari, R., Dominici, M., Handgretinger, R., and Müller, I. (2010). Human multipotent mesenchymal stromal cells use galectin-1 to inhibit immune effector cells. Blood 116, 3770–3779. doi: 10.1182/blood-2010-02-270777
Gnecchi, M., He, H., Liang, O. D., Melo, L. G., Morello, F., Mu, H., et al. (2005). Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells [2]. Nat. Med. 11, 367–368. doi: 10.1038/nm0405-367
González, M. A., Gonzalez-Rey, E., Rico, L., Büscher, D., and Delgado, M. (2009a). Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology 136, 978–989. doi: 10.1053/j.gastro.2008.11.041
González, M. A., González-Rey, E., Rico, L., Büscher, D., and Delgado, M. (2009b). Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum. 60, 1006–1019. doi: 10.1002/art.24405
Griffin, M., Ryan, C. M., Pathan, O., Abraham, D., Denton, C. P., and Butler, P. E. M. (2017). Characteristics of human adipose derived stem cells in scleroderma in comparison to sex and age matched normal controls: implications for regenerative medicine. Stem Cell Res. Ther. 8:23. doi: 10.1186/s13287-016-0444-7
Grimaldi, V., Mancini, F. P., Casamassimi, A., Al-Omran, M., Zullo, A., Infante, T., et al. (2013). Potential benefits of cell therapy in coronary heart disease. J. Cardiol. 10(Suppl. 20), S2412–S2422.
Gu, W., Song, L., Li, X. M., Wang, D., Guo, X. J., and Xu, W. G. (2015). Mesenchymal stem cells alleviate airway inflammation and emphysema in COPD through down-regulation of cyclooxygenase-2 via p38 and ERK MAPK pathways. Sci. Rep. 5:8733. doi: 10.1038/srep08733
Gutiérrez-Fernández, M., Rodríguez-Frutos, B., Ramos-Cejudo, J., Teresa Vallejo-Cremades, M., Fuentes, B., Cerdán, S., et al. (2013). Effects of intravenous administration of allogenic bone marrow- and adipose tissue-derived mesenchymal stem cells on functional recovery and brain repair markers in experimental ischemic stroke. Stem Cell Res. Ther. 4:11. doi: 10.1186/scrt159
Han, X., Yang, Q., Lin, L., Xu, C., Zheng, C., Chen, X., et al. (2014). Interleukin-17 enhances immunosuppression by mesenchymal stem cells. Cell Death Differ. 21, 1758–1768. doi: 10.1038/cdd.2014.85
Hashemi, S. M., Ghods, S., Kolodgie, F. D., Parcham-Azad, K., Keane, M., Hamamdzic, D., et al. (2008). A placebo controlled, dose-ranging, safety study of allogenic mesenchymal stem cells injected by endomyocardial delivery after an acute myocardial infarction. Eur. Heart J. 29, 251–259. doi: 10.1093/eurheartj/ehm559
Heslop, J. A., Hammond, T. G., Santeramo, I., Tort Piella, A., Hopp, I., Zhou, J., et al. (2015). Concise review: workshop review: understanding and assessing the risks of stem cell-based therapies. Stem Cells Transl. Med. 4, 389–400. doi: 10.5966/sctm.2014-0110
Hong, Y., Kim, Y. S., Hong, S. H., and Oh, Y. M. (2016). Therapeutic effects of adipose-derived stem cells pretreated with pioglitazone in an emphysema mouse model. Exp. Mol. Med. 48, e266. doi: 10.1038/emm.2016.93
Hwa Cho, H., Bae, Y. C., and Jung, J. S. (2006). Role of toll-like receptors on human adipose-derived stromal cells. Stem Cells 24, 2744–2752. doi: 10.1634/stemcells.2006-0189
Ivanova-Todorova, E., Bochev, I., Mourdjeva, M., Dimitrov, R., Bukarev, D., Kyurkchiev, S., et al. (2009). Adipose tissue-derived mesenchymal stem cells are more potent suppressors of dendritic cells differentiation compared to bone marrow-derived mesenchymal stem cells. Immunol. Lett. 126, 37–42. doi: 10.1016/j.imlet.2009.07.010
Jo, C. H., Lee, Y. G., Shin, W. H., Kim, H., Chai, J. W., Jeong, E. C., et al. (2014). Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells 32, 1254–1266. doi: 10.1002/stem.1634
Jones, D. L., and Wagers, A. J. (2008). No place like home: anatomy and function of the stem cell niche. Nat. Rev. Mol. Cell Biol. 9, 11–21. doi: 10.1038/nrm2319
Kaewsuwan, S., Song, S. Y., Kim, J. H., and Sung, J. H. (2012). Mimicking the functional niche of adipose-derived stem cells for regenerative medicine. Expert Opin. Biol. Ther. 12, 1575–1588. doi: 10.1517/14712598.2012.721763
Kalampokis, I., Yoshizaki, A., and Tedder, T. F. (2013). IL-10-producing regulatory B cells (B10 cells) in autoimmune disease. Arthritis Res. Ther. 15(Suppl. 1):S1. doi: 10.1186/ar3907
Kang, H., Lu, S., Peng, J., Yang, Q., Liu, S., Zhang, L., et al. (2015). Chondrogenic differentiation of human adiposederived stem cells using microcarrier and bioreactor combination technique. Mol. Med. Rep. 11, 1195–1199. doi: 10.3892/mmr.2014.2820
Kapur, S. K., Wang, X., Shang, H., Yun, S., Li, X., Feng, G., et al. (2012). Human adipose stem cells maintain proliferative, synthetic and multipotential properties when suspension cultured as self-assembling spheroids. Biofabrication 4:025004. doi: 10.1088/1758-5082/4/2/025004
Kim, D. S., Jang, I. K., Lee, M. W., Ko, Y. J., Lee, D. H., Lee, J. W., et al. (2018). Enhanced immunosuppressive properties of human mesenchymal stem cells primed by interferon-γ. EBioMedicine 28, 261–273. doi: 10.1016/j.ebiom.2018.01.002
Kim, J., and Hematti, P. (2009). Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp. Hematol. 37, 1445–1453. doi: 10.1016/j.exphem.2009.09.004
Kota, D. J., Prabhakara, K. S., Toledano-Furman, N., Bhattarai, D., Chen, Q., DiCarlo, B., et al. (2017). Prostaglandin E2 indicates therapeutic efficacy of mesenchymal stem cells in experimental traumatic brain injury. Stem Cells 35, 1416–1430. doi: 10.1002/stem.2603
Krampera, M. (2011). Mesenchymal stromal cell licensing: a multistep process. Leukemia 25, 1408–1414. doi: 10.1038/leu.2011.108
Krawiec, J. T., Weinbaum, J. S., Liao, H. T., Ramaswamy, A. K., Pezzone, D. J., Josowitz, A. D., et al. (2016). In vivo functional evaluation of tissue-engineered vascular grafts fabricated using human adipose-derived stem cells from high cardiovascular risk populations. Tissue Eng. Part A 22, 765–775. doi: 10.1089/ten.TEA.2015.0379
Kronsteiner, B., Wolbank, S., Peterbauer, A., Hackl, C., Redl, H., Van Griensven, M., et al. (2011). Human mesenchymal stem cells from adipose tissue and amnion influence T-cells depending on stimulation method and presence of other immune cells. Stem Cells Dev. 20, 2115–2126. doi: 10.1089/scd.2011.0031
Kuçi, Z., Bönig, H., Kreyenberg, H., Bunos, M., Jauch, A., Janssen, J. W. G., et al. (2016). Mesenchymal stromal cells from pooled mononuclear cells of multiple bone marrow donors as rescue therapy in pediatric severe steroid-refractory graft-versus-host disease: a multicenter survey. Haematologica 101, 985–994. doi: 10.3324/haematol.2015.140368
Kwon, Y. W., Heo, S. C., Jeong, G. O., Yoon, J. W., Mo, W. M., Lee, M. J., et al. (2013). Tumor necrosis factor-α-activated mesenchymal stem cells promote endothelial progenitor cell homing and angiogenesis. Biochim. Biophys. Acta 1832, 2136–2144. doi: 10.1016/j.bbadis.2013.08.002
Lee, S., Persson, P., and Mathews, R. D. (2015). an us cr ip t Ac ce pt us cr ip t Ac ce pt ed. Rev. Financ. Stud. 29, 2341–2386.
Leto Barone, A. A., Khalifian, S., Lee, W. P. A., and Brandacher, G. (2013). Immunomodulatory effects of adipose-derived stem cells: Fact or fiction? Biomed Res. Int. 2103:383685. doi: 10.1155/2013/383685
Li, X., Ma, T., Sun, J., Shen, M., Xue, X., Chen, Y., et al. (2019). Harnessing the secretome of adipose-derived stem cells in the treatment of ischemic heart diseases. Stem Cell Res. Ther. 10:196. doi: 10.1186/s13287-019-1289-7
Liang, J., Zhang, H., Hua, B., Wang, H., Lu, L., Shi, S., et al. (2010). Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: a pilot clinical study. Ann. Rheum. Dis. 69, 1423–1429. doi: 10.1136/ard.2009.123463
Lin, H. P., Chan, T. M., Fu, R. H., Chuu, C. P., Chiu, S. C., Tseng, Y. H., et al. (2015). Applicability of adipose-derived stem cells in type 1 diabetes mellitus. Cell Transplant. 24, 521–532. doi: 10.3727/096368915X686977
Lombardo, E., Delarosa, O., Mancheño-Corvo, P., Menta, R., Ramírez, C., and Büscher, D. (2009). Toll-like receptor-mediated signaling in human adipose-derived stem cells: implications for immunogenicity and immunosuppressive potential. Tissue Eng. Part A 15, 1579–1589. doi: 10.1089/ten.tea.2008.0340
Machado, C. D., da Silva Telles, P. D., and Nascimento, I. L. O. (2013). Immunological characteristics of mesenchymal stem cells. Rev. Bras. Hematol. Hemoter. 35, 62–67. doi: 10.5581/1516-8484.20130017
Madonna, R., Geng, Y. J., and De Caterina, R. (2009). Adipose tissue-derived stem cells: characterization and potential for cardiovascular repair. Arterioscler. Thromb. Vasc. Biol. 29, 1723–1729. doi: 10.1161/ATVBAHA.109.187179
Madrigal, M., Rao, K. S., and Riordan, N. H. (2014). A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J. Transl. Med. 12:260. doi: 10.1186/s12967-014-0260-8
Martínez-González, I., Roca, O., Masclans, J. R., Moreno, R., Salcedo, M. T., Baekelandt, V., et al. (2013). Human mesenchymal stem cells overexpressing the IL-33 antagonist soluble IL-1 receptor-like-1 attenuate endotoxin-induced acute lung injury. Am. J. Respir. Cell Mol. Biol. 49, 552–562. doi: 10.1165/rcmb.2012-0406OC
McCoy, M. K., Martinez, T. N., Ruhn, K. A., Wrage, P. C., Keefer, E. W., Botterman, B. R., et al. (2008). Autologous transplants of Adipose-Derived Adult Stromal (ADAS) cells afford dopaminergic neuroprotection in a model of Parkinson’s disease. Exp. Neurol. 210, 14–29. doi: 10.1016/j.expneurol.2007.10.011
McIntosh, K., Zvonic, S., Garrett, S., Mitchell, J. B., Floyd, Z. E., Hammill, L., et al. (2006). The immunogenicity of human adipose-derived cells: temporal changes in vitro. Stem Cells 24, 1246–1253. doi: 10.1634/stemcells.2005-0235
Melief, S. M., Zwaginga, J. J., Fibbe, W. E., and Roelofs, H. (2013). Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Transl. Med. 2, 455–463. doi: 10.5966/sctm.2012-0184
Min, C. K., Kim, B. G., Park, G., Cho, B., and Oh, I. H. (2007). IL-10-transduced bone marrow mesenchymal stem cells can attenuate the severity of acute graft-versus-host disease after experimental allogeneic stem cell transplantation. Bone Marrow Transplant. 39, 637–645. doi: 10.1038/sj.bmt.1705644
Mineda, K., Feng, J., Ishimine, H., Takada, H., Doi, K., Kuno, S., et al. (2015). therapeutic potential of human adipose-derived stem/stromal cell microspheroids prepared by three-dimensional culture in non-cross-linked hyaluronic acid gel. Stem Cells Transl. Med. 4, 1511–1522. doi: 10.5966/sctm.2015-0037
Montespan, F., Deschaseaux, F., Sensébé, L., Carosella, E. D., and Rouas-Freiss, N. (2014). Osteodifferentiated mesenchymal stem cells from bone marrow and adipose tissue express HLA-G and display immunomodulatory properties in Hla-mismatched settings: implications in bone repair therapy. J. Immunol. Res. 2014:230346. doi: 10.1155/2014/230346
Naaijkens, B. A., van Dijk, A., Kamp, O., Krijnen, P. A. J., Niessen, H. W. M., and Juffermans, L. (2014). Therapeutic application of adipose derived stem cells in acute myocardial infarction: lessons from animal models. Stem Cell Rev. Rep. 10, 389–398. doi: 10.1007/s12015-014-9502-7
Najar, M., Raicevic, G., Boufker, H. I., Kazan, H. F., De Bruyn, C., Meuleman, N., et al. (2010). Mesenchymal stromal cells use PGE2 to modulate activation and proliferation of lymphocyte subsets: combined comparison of adipose tissue. Wharton’s Jelly and bone marrow sources. Cell. Immunol. 264, 171–179. doi: 10.1016/j.cellimm.2010.06.006
Najar, M., Raicevic, G., Fayyad-Kazan, H., Bron, D., Toungouz, M., and Lagneaux, L. (2016). Mesenchymal stromal cells and immunomodulation: a gathering of regulatory immune cells. Cytotherapy 18, 160–171. doi: 10.1016/j.jcyt.2015.10.011
Najar, M., Raicevic, G., Fayyad-Kazan, H., De Bruyn, C., Bron, D., Toungouz, M., et al. (2013). Impact of different mesenchymal stromal cell types on T-cell activation, proliferation and migration. Int. Immunopharmacol. 15, 693–702. doi: 10.1016/j.intimp.2013.02.020
Nancarrow-Lei, R., Mafi, P., Mafi, R., and Khan, W. (2017). A systemic review of adult mesenchymal stem cell sources and their multilineage differentiation potential relevant to musculoskeletal tissue repair and regeneration. Curr. Stem Cell Res. Ther. 12, 601–610. doi: 10.2174/1574888X12666170608124303
Napoli, C., William-Ignarro, S., Byrns, R., Balestrieri, M. L., Crimi, E., Farzati, B., et al. (2008). Therapeutic targeting of the stem cell niche in experimental hindlimb ischemia. Nat. Clin. Pract. Cardiovasc. Med. 5, 571–579. doi: 10.1038/ncpcardio1214
Nasef, A., Mathieu, N., Chapel, A., Frick, J., François, S., Mazurier, C., et al. (2007). Immunosuppressive effects of mesenchymal stem cells: involvement of HLA-G. Transplantation 84, 231–237. doi: 10.1097/01.tp.0000267918.07906.08
Neri, S. (2019). Genetic stability of mesenchymal stromal cells for regenerative medicine applications: a fundamental biosafety aspect. Int. J. Mol. Sci. 20:E2406. doi: 10.3390/ijms20102406
Nürnberger, S., Lindner, C., Maier, J., Strohmeier, K., Wurzer, C., Slezak, P., et al. (2019). Adipose-tissue-derived therapeutic cells in their natural environment as an autologous cell therapy strategy: the microtissue-stromal vascular fraction. Eur. Cell Mater. 37, 113–133. doi: 10.22203/eCM.v037a08
Ock, S. A., Lee, Y. M., Park, J. S., Shivakumar, S. B., Moon, S. W., Sung, N. J., et al. (2016). Evaluation of phenotypic, functional and molecular characteristics of porcine mesenchymal stromal/stem cells depending on donor age, gender and tissue source. J. Vet. Med. Sci. 78, 987–995. doi: 10.1292/jvms.15-0596
Oh, S. H., Choi, C., Chang, D. J., Shin, D. A., Lee, N., Jeon, I., et al. (2015). Early neuroprotective effect with lack of long-term cell replacement effect on experimental stroke after intra-arterial transplantation of adipose-derived mesenchymal stromal cells. Cytotherapy 17, 1090–1103. doi: 10.1016/j.jcyt.2015.04.007
Oñate, B., Vilahur, G., Camino-López, S., Díez-Caballero, A., Ballesta-López, C., Ybarra, J., et al. (2013). Stem cells isolated from adipose tissue of obese patients show changes in their transcriptomic profile that indicate loss in stemcellness and increased commitment to an adipocyte-like phenotype. BMC Genomics 14:625. doi: 10.1186/1471-2164-14-625
Oñate, B., Vilahur, G., Ferrer-Lorente, R., Ybarra, J., Díez-Caballero, A., Ballesta-López, C., et al. (2012). The subcutaneous adipose tissue reservoir of functionally active stem cells is reduced in obese patients. FASEB J. 26, 4327–4336. doi: 10.1096/fj.12-207217
Onesti, M. G., Carella, S., Ceccarelli, S., Marchese, C., and Scuderi, N. (2016). The use of human adipose-derived stem cells in the treatment of physiological and pathological vulvar dystrophies. Stem Cells Int. 2016:2561461. doi: 10.1155/2016/2561461
Panés, J., García-Olmo, D., Van Assche, G., Colombel, J. F., Reinisch, W., Baumgart, D. C., et al. (2016). Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial. Lancet 388, 1281–1290. doi: 10.1016/s0140-6736(16)31203-x
Park, I. S., Chung, P. S., and Ahn, J. C. (2017). Adipose-derived stem cell spheroid treated with low-level light irradiation accelerates spontaneous angiogenesis in mouse model of hindlimb ischemia. Cytotherapy 19, 1070–1078. doi: 10.1016/j.jcyt.2017.06.005
Park, M. J., Kwok, S. K., Lee, S. H., Kim, E. K., Park, S. H., and Cho, M. L. (2015). Adipose tissue-derived mesenchymal stem cells induce expansion of interleukin-10-producing regulatory b cells and ameliorate autoimmunity in a murine model of systemic lupus erythematosus. Cell Transplant. 24, 2367–2377. doi: 10.3727/096368914X685645
Patrikoski, M., Sivula, J., Huhtala, H., Helminen, M., Salo, F., Mannerström, B., et al. (2014). Different culture conditions modulate the immunological properties of adipose stem cells. Stem Cells Transl. Med. 3, 1220–1230. doi: 10.5966/sctm.2013-0201
Payne, N. L., Dantanarayana, A., Sun, G., Moussa, L., Caine, S., McDonald, C., et al. (2012). Early intervention with gene-modified mesenchymal stem cells overexpressing interleukin-4 enhances anti-inflammatory responses and functional recovery in experimental autoimmune demyelination. Cell Adhes. Migr. 6, 179–189. doi: 10.4161/cam.20341
Peng, Y., Chen, X., Liu, Q., Zhang, X., Huang, K., Liu, L., et al. (2015). Mesenchymal stromal cells infusions improve refractory chronic graft versus host disease through an increase of CD5+ regulatory B cells producing interleukin 10. Leukemia 29, 636–646. doi: 10.1038/leu.2014.225
Pers, Y.-M., Rackwitz, L., Ferreira, R., Pullig, O., Delfour, C., Barry, F., et al. (2016). Adipose mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: a phase i dose-escalation trial. Stem Cells Transl. Med. 5, 847–856. doi: 10.5966/sctm.2015-0245
Planat-Bénard, V., Menard, C., André, M., Puceat, M., Perez, A., Garcia-Verdugo, J. M., et al. (2004). Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circ. Res. 94, 223–229. doi: 10.1161/01.res.0000109792.43271.47
Policha, A., Zhang, P., Chang, L., Lamb, K., Tulenko, T., and DiMuzio, P. (2014). Endothelial differentiation of diabetic adipose-derived stem cells. J. Surg. Res. 192, 656–663. doi: 10.1016/j.jss.2014.06.041
Poloni, A., Maurizi, G., Ciarlantini, M., Medici, M., Mattiucci, D., Mancini, S., et al. (2015). Interaction between human mature adipocytes and lymphocytes induces T-cell proliferation. Cytotherapy 17, 1292–1301. doi: 10.1016/j.jcyt.2015.06.007
Puissant, B., Barreau, C., Bourin, P., Clavel, C., Corre, J., Bousquet, C., et al. (2005). Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br. J. Haematol. 129, 118–129. doi: 10.1111/j.1365-2141.2005.05409.x
Qi, H., Chen, G., Huang, Y., Si, Z., and Li, J. (2015). Foxp3-modified bone marrow mesenchymal stem cells promotes liver allograft tolerance through the generation of regulatory T cells in rats. J. Transl. Med. 13:274. doi: 10.1186/s12967-015-0638-2
Regmi, S., Pathak, S., Thanh, T. P., Nguyen, T. T., Sung, J. H., Yook, S., et al. (2019). Intraportally delivered stem cell spheroids localize in the liver and protect hepatocytes against GalN/LPS-induced fulminant hepatic toxicity. Stem Cell Res. Ther. 10:230. doi: 10.1186/s13287-019-1337-3
Ribeiro, A., Laranjeira, P., Mendes, S., Velada, I., Leite, C., Andrade, P., et al. (2013). Mesenchymal stem cells from umbilical cord matrix, adipose tissue and bone marrow exhibit different capability to suppress peripheral blood B, natural killer and T cells. Stem Cell Res. Ther. 4:125. doi: 10.1186/scrt336
Russell, K. A., Chow, N. H. C., Dukoff, D., Gibson, T. W. G., La Marre, J., Betts, D. H., et al. (2016). Characterization and immunomodulatory effects of canine adipose tissue- and bone marrow-derived mesenchymal stromal cells. PLoS One 11:e0167442. doi: 10.1371/journal.pone.0167442
Ryan, J. M., Barry, F., Murphy, J. M., and Mahon, B. P. (2007). Interferon-γ does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin. Exp. Immunol. 149, 353–363. doi: 10.1111/j.1365-2249.2007.03422.x
Sabia, C., Picascia, A., Grimaldi, V., Amarelli, C., Maiello, C., and Napoli, C. (2017). The epigenetic promise to improve prognosis of heart failure and heart transplantation. Transplant. Rev. 31, 249–256. doi: 10.1016/j.trre.2017.08.004
Scuderi, N., Ceccarelli, S., Onesti, M. G., Fioramonti, P., Guidi, C., Romano, F., et al. (2013). Human adipose-derived stromal cells for cell-based therapies in the treatment of systemic sclerosis. Cell Transplant. 22, 779–795. doi: 10.3727/096368912x639017
Semon, J. A., Maness, C., Zhang, X., Sharkey, S. A., Beuttler, M. M., Shah, F. S., et al. (2014). Comparison of human adult stem cells from adipose tissue and bone marrow in the treatment of experimental autoimmune encephalomyelitis. Stem Cell Res. Ther. 5:2. doi: 10.1186/scrt391
Seo, Y., Shin, T. H., and Kim, H. S. (2019). Current strategies to enhance adipose stem cell function: an update. Int. J. Mol. Sci. 20:E3827. doi: 10.3390/ijms20153827
Serena, C., Keiran, N., Ceperuelo-Mallafre, V., Ejarque, M., Fradera, R., Roche, K., et al. (2016). Obesity and type 2 diabetes alters the immune properties of human adipose derived stem cells. Stem Cells 34, 2559–2573. doi: 10.1002/stem.2429
Sheng, H., Wang, Y., Jin, Y., Zhang, Q., Zhang, Y., Wang, L., et al. (2008). A critical role of IFNγ in priming MSC-mediated suppression of T cell proliferation through up-regulation of B7-H1. Cell Res. 18, 846–857. doi: 10.1038/cr.2008.80
Shingyochi, Y., Orbay, H., and Mizuno, H. (2015). Adipose-derived stem cells for wound repair and regeneration. Expert Opin. Biol. Ther. 15, 1285–1292. doi: 10.1517/14712598.2015.1053867
Shu, W., Shu, Y. T., Dai, C. Y., and Zhen, Q. Z. (2012). Comparing the biological characteristics of adipose tissue-derived stem cells of different persons. J. Cell. Biochem. 113, 2020–2026. doi: 10.1002/jcb.24070
Soleymaninejadian, E., Pramanik, K., and Samadian, E. (2012). Immunomodulatory properties of mesenchymal stem cells: cytokines and factors. Am. J. Reprod. Immunol. 67, 1–8. doi: 10.1111/j.1600-0897.2011.01069.x
Sommese, L., Zullo, A., Schiano, C., Mancini, F. P., and Napoli, C. (2017). Possible muscle repair in the human cardiovascular system. Stem Cell Rev. Rep. 13, 170–191. doi: 10.1007/s12015-016-9711-3
Sotiropoulou, P. A., Perez, S. A., Salagianni, M., Baxevanis, C. N., and Papamichail, M. (2006). Cell culture medium composition and translational adult bone marrow-derived stem cell research. Stem Cells 24, 1409–1410. doi: 10.1634/stemcells.2005-0654
Sovrea, A. S., Bosca, A. B., Constantin, A. M., Dronca, E., and Ilea, A. (2019). State of the art in human adipose stem cells and their role in therapy. Rom. J. Morphol. Embryol. 60, 7–31.
Staff, N. P., Madigan, N. N., Morris, J., Jentoft, M., Sorenson, E. J., Butler, G., et al. (2016). Safety of intrathecal autologous adipose-derived mesenchymal stromal cells in patients with ALS. Neurology 87, 2230–2234. doi: 10.1212/wnl.0000000000003359
Strioga, M., Viswanathan, S., Darinskas, A., Slaby, O., and Michalek, J. (2012). Same or not the same? comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 21, 2724–2752. doi: 10.1089/scd.2011.0722
Sun, M., Sun, L., Huang, C., Chen, B. C., and Zhou, Z. (2019). Induction of macrophage M2b/c polarization by adipose tissue-derived mesenchymal stem cells. J. Immunol. Res. 2019:7059680. doi: 10.1155/2019/7059680
Teklemariam, T., Purandare, B., Zhao, L., and Hantash, B. M. (2014). Inhibition of DNA methylation enhances HLA-G expression in human mesenchymal stem cells. Biochem. Biophys. Res. Commun. 452, 753–759. doi: 10.1016/j.bbrc.2014.08.152
Tholpady, S. S., Ogle, R. C., and Katz, A. J. (2009). Adipose stem cells and solid organ transplantation. Curr. Opin. Organ. Transplant. 14, 51–55. doi: 10.1097/MOT.0b013e328320d2cf
Tögel, F., Hu, Z., Weiss, K., Isaac, J., Lange, C., and Westenfelder, C. (2005). Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am. J. Physiol. Renal Physiol. 289, F31–F42.
Ueyama, H., Okano, T., Orita, K., Mamoto, K., Sobajima, S., Iwaguro, H., et al. (2020). Local transplantation of adipose-derived stem cells has a significant therapeutic effect in a mouse model of rheumatoid arthritis. Sci. Rep. 10:3076. doi: 10.1038/s41598-020-60041-2
Valencia, J., Blanco, B., Yáñez, R., Vázquez, M., Herrero Sánchez, C., Fernández-García, M., et al. (2016). Comparative analysis of the immunomodulatory capacities of human bone marrow– and adipose tissue–derived mesenchymal stromal cells from the same donor. Cytotherapy 18, 1297–1311. doi: 10.1016/j.jcyt.2016.07.006
Valina, C., Pinkernell, K., Song, Y. H., Bai, X., Sadat, S., Campeau, R. J., et al. (2007). Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur. Heart J. 28, 2667–2677. doi: 10.1093/eurheartj/ehm426
Vanikar, A. V., Dave, S. D., Thakkar, U. G., and Trivedi, H. L. (2010). Cotransplantation of adipose tissue-derived insulin-secreting mesenchymal stem cells and hematopoietic stem cells: a novel therapy for insulin-dependent diabetes mellitus. Stem Cells Int. 2010, 582382. doi: 10.4061/2010/582382
Varghese, J., Griffin, M., Mosahebi, A., and Butler, P. (2017). Systematic review of patient factors affecting adipose stem cell viability and function: implications for regenerative therapy. Stem Cell Res. Ther. 8:45. doi: 10.1186/s13287-017-0483-8
Velier, M., Simoncini, S., Abellan, M., Francois, P., Eap, S., Lagrange, A., et al. (2019). Adipose-derived stem cells from systemic sclerosis patients maintain pro-angiogenic and antifibrotic paracrine effects in vitro. J. Clin. Med. 8:E1979. doi: 10.3390/jcm8111979
Voog, J., and Jones, D. L. (2010). Stem cells and the niche: a dynamic duo. Cell Stem Cell 6, 103–115. doi: 10.1016/j.stem.2010.01.011
Waldner, M., Zhang, W., James, I. B., Allbright, K., Havis, E., Bliley, J. M., et al. (2018). Characteristics and immunomodulating functions of adipose-derived and bone marrow-derived mesenchymal stem cells across defined human leukocyte antigen barriers. Front. Immunol. 9:1642. doi: 10.3389/fimmu.2018.01642
Wang, Y. H., Huang, X. H., Yang, Y. M., He, Y., Dong, X. H., Yang, H. X., et al. (2019). Mysm1 epigenetically regulates the immunomodulatory function of adipose-derived stem cells in part by targeting miR-150. J. Cell. Mol. Med. 23, 3737–3746. doi: 10.1111/jcmm.14281
Waterman, R. S., Tomchuck, S. L., Henkle, S. L., and Betancourt, A. M. (2010). A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an immunosuppressive MSC2 phenotype. PLoS One 5:e10088. doi: 10.1371/journal.pone.0010088
Xu, Y., Shi, T., Xu, A., and Zhang, L. (2016). 3D spheroid culture enhances survival and therapeutic capacities of MSCs injected into ischemic kidney. J. Cell. Mol. Med. 20, 1203–1213. doi: 10.1111/jcmm.12651
Yan, Y., Ma, T., Gong, K., Ao, Q., Zhang, X., and Gong, Y. (2014). Adipose-derived mesenchymal stem cell transplantation promotes adult neurogenesis in the brains of Alzheimer’s disease mice. Neural Regen. Res. 9, 798–805.
Yañez, R., Lamana, M. L., García-Castro, J., Colmenero, I., Ramírez, M., and Bueren, J. A. (2006). Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells 24, 2582–2591. doi: 10.1634/stemcells.2006-0228
Yang, D., Wang, W., Li, L., Peng, Y., Chen, P., Huang, H., et al. (2013). The relative contribution of paracine effect versus direct differentiation on adipose-derived stem cell transplantation mediated cardiac repair. PLoS One 8:e59020. doi: 10.1371/journal.pone.0059020
Yang, H. M., Sung, J. H., Choi, Y. S., Lee, H. J., Roh, C. R., Kim, J., et al. (2012). Enhancement of the immunosuppressive effect of human adipose tissue-derived mesenchymal stromal cells through HLA-G1 expression. Cytotherapy 14, 70–79. doi: 10.3109/14653249.2011.613926
Yokota, N., Yamakawa, M., Shirata, T., Kimura, T., and Kaneshima, H. (2017). Clinical results following intra-articular injection of adipose-derived stromal vascular fraction cells in patients with osteoarthritis of the knee. Regen. Ther. 6, 108–112. doi: 10.1016/j.reth.2017.04.002
Yousefi, F., Ebtekar, M., Soleimani, M., Soudi, S., and Hashemi, S. M. (2013). Comparison of in vivo immunomodulatory effects of intravenous and intraperitoneal administration of adipose-tissue mesenchymal stem cells in experimental autoimmune encephalomyelitis (EAE). Int. Immunopharmacol. 17, 608–616. doi: 10.1016/j.intimp.2013.07.016
Yu, B., Yu, D., Cao, L., Zhao, X., Long, T., Liu, G., et al. (2011). Simulated microgravity using a rotary cell culture system promotes chondrogenesis of human adipose-derived mesenchymal stem cells via the p38 MAPK pathway. Biochem. Biophys. Res. Commun. 414, 412–418. doi: 10.1016/j.bbrc.2011.09.103
Zhao, N., Li, H., Yan, Y., Jiang, R., and He, X. (2017). Mesenchymal stem cells overexpressing IL-35 effectively inhibit CD4+ T cell function. Cell. Immunol. 312, 61–66. doi: 10.1016/j.cellimm.2016.12.001
Zhao, S., Wehner, R., Bornhäuser, M., Wassmuth, R., Bachmann, M., and Schmitz, M. (2010). Immunomodulatory properties of mesenchymal stromal cells and their therapeutic consequences for immune-mediated disorders. Stem Cells Dev. 19, 607–614. doi: 10.1089/scd.2009.0345
Zhou, K., Guo, S., Tong, S., Sun, Q., Li, F., Zhang, X., et al. (2018). Immunosuppression of human adipose-derived stem cells on T cell subsets via the reduction of NF-kappaB activation mediated by PD-L1/PD-1 and Gal-9/TIM-3 Pathways. Stem Cells Dev. 27, 1191–1202. doi: 10.1089/scd.2018.0033
Zuk, P. A., Zhu, M., Ashjian, P., De Ugarte, D. A., Huang, J. I., Mizuno, H., et al. (2002). Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 13, 4279–4295.
Keywords: adipose-derived stem cells, immunomodulation, microenvironment, cytokines, clinical application
Citation: Ceccarelli S, Pontecorvi P, Anastasiadou E, Napoli C and Marchese C (2020) Immunomodulatory Effect of Adipose-Derived Stem Cells: The Cutting Edge of Clinical Application. Front. Cell Dev. Biol. 8:236. doi: 10.3389/fcell.2020.00236
Received: 31 January 2020; Accepted: 20 March 2020;
Published: 17 April 2020.
Edited by:
Gianluca Carnevale, University of Modena and Reggio Emilia, ItalyReviewed by:
Paolo Di Nardo, University of Rome Tor Vergata, ItalyLorenzo Nevi, University of Milan, Italy
Copyright © 2020 Ceccarelli, Pontecorvi, Anastasiadou, Napoli and Marchese. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cinzia Marchese, Y2luemlhLm1hcmNoZXNlQHVuaXJvbWExLml0
 Simona Ceccarelli
Simona Ceccarelli Paola Pontecorvi
Paola Pontecorvi Eleni Anastasiadou
Eleni Anastasiadou Claudio Napoli2,3
Claudio Napoli2,3 Cinzia Marchese
Cinzia Marchese