- 1Markey Cancer Center, University of Kentucky, Lexington, KY, United States
- 2Department of Pharmacology and Nutritional Sciences, University of Kentucky, Lexington, KY, United States
Collagen is the major component of extracellular matrix. Collagen cross-link and deposition depend on lysyl hydroxylation, which is catalyzed by procollagen-lysine, 2-oxoglutarate 5-dioxygenase (PLOD). Aberrant lysyl hydroxylation and collagen cross-link contributes to the progression of many collagen-related diseases, such as fibrosis and cancer. Three lysyl hydroxylases (LH1, LH2, and LH3) are identified, encoded by PLOD1, PLOD2, and PLOD3 genes. Expression of PLODs is regulated by multiple cytokines, transcription factors and microRNAs. Dysregulation of PLODs promotes cancer progression and metastasis, suggesting that targeting PLODs is potential strategy for cancer treatment. Here, we summarize the recent progress in the investigation of function and regulation of PLODs in normal tissue development and disease progression, especially in cancer.
Introduction
Collagen is one of the major components of extracellular matrix. The collagen-cell interaction induces biochemical and biophysical signals, which is essentially for normal tissue function and cancer progression (Egeblad et al., 2010; Xiong and Xu, 2016). The collagen family contains 28 members (Heino, 2007) and can be divided into two groups: fibrillar collagen (type I, II, III, V, XI) and non-fibrillar collagen (type IV, VIII, X, IX, XII, XIV, XV, XVIII, XIX, XXI). Collagen is the most abundant protein in our body, and presents in both normal tissues and cancer. Type I collagen, the most common type fibrillar, has been identified in many tissues, including skin, tendon, vascular ligature and bone; while type II collagen is the main collagenous component of cartilage. Non-fibrillar type IV collagen is required for basement membrane formation (Paulsson, 1992). Cell-collagen interaction induces cellular signaling via integrin [included α1β1 (Tulla et al., 2001; Hamaia et al., 2012), α2β1 (Tulla et al., 2001; Carafoli et al., 2013), α10β1 (Camper et al., 1998) and α11β1 (Tiger et al., 2001; Hamaia et al., 2012)], discoidin domain receptors (Leitinger, 2003, 2011) and Leukocyte-Associated Immunoglobulin-Like Receptor-1 (Rygiel et al., 2011; Kim et al., 2017). Collagen regulates tumor progression by modulating cancer cell migration, invasion (Xiong et al., 2014), proliferation (Pollard, 2004), survival (Cheon et al., 2014) and metastasis (Oudin et al., 2016; Sun et al., 2016).
All collagen is composed of a triple helix, and the most common motif of the triple helix sequence is Gly-X-Y (X and Y represent proline or hydroxyproline) (Albaugh et al., 2017). Collagen is synthesized in the rough endoplasmic reticulum (ER) as a precursor (Nimni, 1983). After peptide bond formation, proline and lysyl hydroxylation is catalyzed by prolyl 4-hydroxylase (P4H) and procollagen-lysine,2-oxoglutarate 5-dioxygenase (PLOD). The hydroxylation of lysyl residues is one of the critical steps of collagens biosynthesis. It usually occurs in the Y position of the repeating Gly-X-Y motif (Barnes et al., 1974; Valtavaara et al., 1998). Three PLODs (PLOD1, PLOD2 and PLOD3) has been identified, catalyzing the lysyl hydroxylation to hydroxylysine (Hausmann, 1967; Rhoads and Udenfriend, 1968; Kivirikko Ki, 1998; Rautavuoma et al., 2004).
Hydroxylysine residue is critical for the formation of covalent cross-links and collagen glycosylation (Valtavaara et al., 1998). PLODs catalyze hydroxylation of lysine (Lys) intracellularly before collagen is secreted, and then lysyl oxidase (LOX) binds to hydroxylysine (Hyl) residues in the extracellular collagen fibers and induces the cross-link formation (Saito and Marumo, 2010). This enzyme dependent collagen crosslinking stabilizes newly formed collagen fibers and enhances the stiffness of the matrix. During collagen maturation, the hydroxylysine residues in the helix region are often modified by the O-linked glycosylation. These reactions are catalyzed by hydroxylysine galactosyltransferase (GT) and galactosylhydroxylysine -glucosyltransferase (GGT) (Shinkai and Yonemasu, 1979; Yamauchi and Sricholpech, 2012). The enzymatic activities of GT and GGT are found in multifunctional PLOD3, but not in PLOD1 and PLOD2 (Heikkinen et al., 2000). Mutations of the human PLOD3 gene lead to congenital disorders affecting connective tissues of various organs (Salo et al., 2008), suggesting that GGT activity of PLOD3 is crucial for the normal function of collagen.
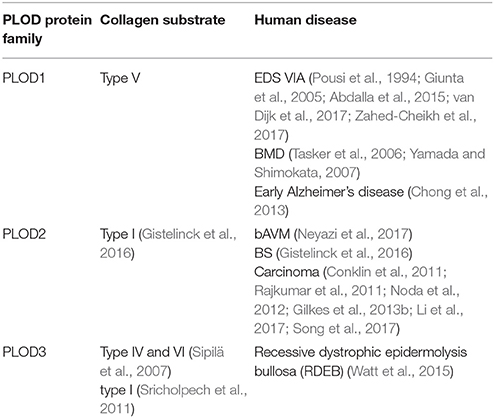
The mutation or overexpression of PLODs has been detected in many human diseases. The kyphoscoliotic type of Ehlers-Danlos syndrome (EDS type VIA) is due to a mutation in the PLOD1 gene (Rohrbach et al., 2011; Zahed-Cheikh et al., 2017). The reduction of PLOD3 protein at the basement membrane is associated with recessive dystrophic epidermolysis bullosa (RDEB) progression (Watt et al., 2015). The overexpression of PLOD2 is detected in many types of cancer. Therefore, investigating the function and the regulation of PLODs in normal organ development and disease progression may identify potential targets for the treatment of collagen-related diseases.
Structure of PLODs
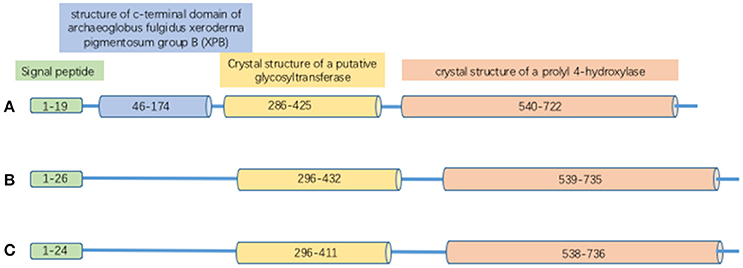
Proteins in the PLOD family are highly homologous; the overall identity in protein sequences among PLOD1, 2 and 3 is 47% (Valtavaara et al., 1998). PLOD protein has binding sites for cofactor Fe2+ and L-ascorbate. It also contains 26 amino acid signal peptide and a Prolyl 4-hydroxylase alpha subunit homologs domain (Figure 1). PLOD1 gene locates on chromosome 1p36 (Tasker et al., 2006) and is composed by 19 exons (Giunta et al., 2005). Collagen hydroxylation catalyzed by PLOD1 is crucial for bone mineral density (BMD) and bone quality (Tasker et al., 2006). PLOD2 gene is at chromosome 3q23-q24 (Szpirer et al., 1997) and also contains 19 exons. Two splice variants (LH2a and LH2b) have been identified in the PLOD2 gene; LH2b differs from LH2a by incorporating the small exon 13A (Valtavaara, 1999). PLOD2 plays a key role in formation of stabilized collagen cross-links (Gilkes et al., 2013a). PLOD3 gene is localized to chromosome 7q36 (Hautala et al., 1992; Szpirer et al., 1997; Valtavaara et al., 1998), and PLOD3 activity is important for the biosynthesis of type IV and VI collagen (Rautavuoma et al., 2004; Sipilä et al., 2007). PLOD1 and PLOD3 hydroxylate lysyl residues in the collagen triple helix, whereas PLOD2 (LH2b) hydroxylate lysyl residues in the telopeptides of collagen (Valtavaara, 1999). PLOD3 has glycosylation activity that induces either monosaccharide or disaccharide attaching to collagen hydroxylysines (Valtavaara, 1999).
Regulation of PLOD Expression
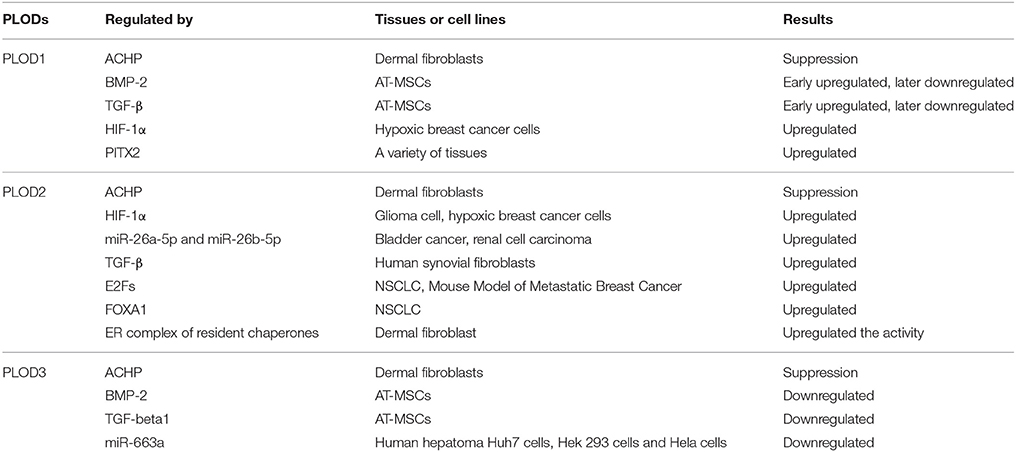
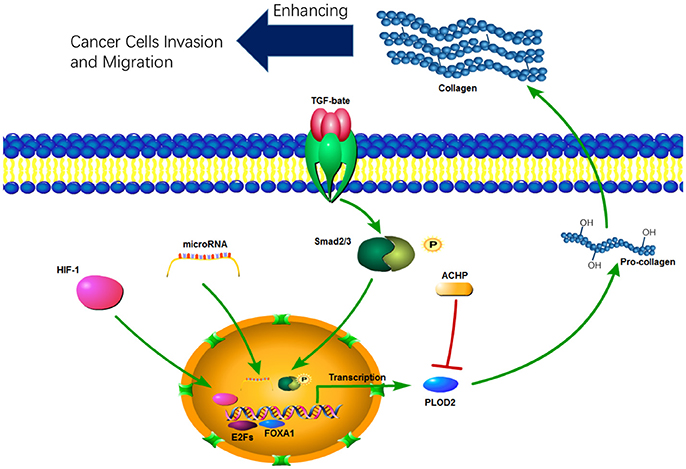
PLOD expression is mainly regulated at the transcription level. A number of cytokines, signaling pathways, and microRNAs have been identified to be involved in transcriptional regulation of PLODs (Table 1). PLOD2 is induced by hypoxia-inducible factor-1α (HIF-1α) under hypoxia condition, which in turn enhance hypoxia-induced Epithelial-Mesenchymal Transition (EMT) phenotypes in glioma cells (Song et al., 2017) and breast cancer cells (Gilkes et al., 2013b). In addition, hypoxia-inducible factor 1 (HIF-1) also activates transcription of PLOD1 in breast cancer cells; however, function PLOD2 is more important for HIF-1-induced cancer progression (Gilkes et al., 2013b). PLOD2 is also directly regulated by miR-26a-5p and miR-26b-5p, and PLOD2 expression is a potential prognostic marker for patients with bladder cancer (Miyamoto et al., 2016) and renal cell carcinoma (Kurozumi et al., 2016). TGF-β signaling is another important regulator of PLOD2 expression (Remst et al., 2014). SP1 and SMAD3, as downstream targets of TGF-β signaling, recruit histone modifying enzymes to the PLOD2 promoter region and induced PLOD2 transcription (Gjaltema et al., 2015). In addition, transcription factor E2Fs (Hollern et al., 2014) and FOXA1 (Du et al., 2017) have been identified as regulators of PLOD2 during cancer progression (Figure 2).
Regulation of PLOD1 and PLOD3 expression is not well-investigated compared to PLOD2. Differential display analysis identified PLOD1 as a potential target gene of TNFα in human chondrocyte-like cells (Ah-Kim et al., 2000). Using chromatin immunoprecipitation and luciferase reporter assay, Hjalt showed that PITX2 directly regulates PLOD1 expression by binding to the promoter region. Inactivation of this pathway may cause the Rieger syndrome (Hjalt et al., 2001). One report show that miR-663a reduces PLOD3 expression by targeting to 3'-UTR of PLOD3 mRNA, subsequently reducing extracellular accumulation of type IV collagen (Amodio et al., 2016).
ACHP (2-Amino-6-[2-(cyclopropylmethoxy)-6-hydroxyphenyl]-4-(4-piperidinyl)−3 pyridinecarbonitrile), a selective inhibitor of IκB kinase, suppresses expression of all three PLOD genes in dermal fibroblasts, but not in lung fibroblasts (Mia and Bank, 2015). Therefore, activation of NF-kB pathway may induce PLOD expression in certain types of cells. Treatment with bone morphogenetic protein-2 (BMP-2) and TGF-β1 induces PLOD1 expression in adipose tissue-derived mesenchymal stem cells (AT-MSCs). Interestingly, neither BMP-2 nor TGF-β1 can induce PLOD2 expression (Knippenberg et al., 2009). Given the crucial function of PLODs in collagen synthesis, further defining the molecular mechanisms by which PLOD expression is regulated may significantly expand our understanding of collagen-related disease progression.
Physiological Functions of PLODs
Collagen is the major component of connective tissues and maintains the structural integrity and the stability of tissues and organs (Patino et al., 2002). The hydroxylysine residues provide attachment sites for the carbohydrates and tensile strength and mechanical stability for the collagen fibrils (Rautavuoma et al., 2004). The abnormal expression or mutation of PLODs is associated with collagen-related diseases, such as Kyphoscoliotic type of EDS VIA (Pousi et al., 1994; Giunta et al., 2005; Abdalla et al., 2015; van Dijk et al., 2017; Zahed-Cheikh et al., 2017), Bruck Syndrome (BS) (Gistelinck et al., 2016) and RDEB (Watt et al., 2015) (Table 2). PLOD1 regulates the hydroxylation of lysyl residues on collagen type V. The duplication of the exon 10 to exon 16 region of PLOD1 (p.Glu326_Lys585dup) gene (Pousi et al., 1994; Giunta et al., 2005) and two mutations on Gln208 and Tyr675 cause the loss function of PLOD1, which may lead to EDS VIA (Abdalla et al., 2015; van Dijk et al., 2017). In addition, PLOD1 has been identified as a susceptibility gene for reduced BMD (Tasker et al., 2006; Yamada and Shimokata, 2007).
Dysregulation of PLOD2 is associated with brain arteriovenous malformations and cancer progression. PLOD2 is overexpressed in brain arteriovenous malformations (bAVM), and the levels of PLOD2 expression correlated with bAVM size (Neyazi et al., 2017). PLOD2 mutant zebrafish display molecular and tissue abnormalities in the musculoskeletal system that are concordant with clinical findings in BS patients (Gistelinck et al., 2016). There is evidence that the levels of mature hydroxylysine aldehyde-derived cross-links typical for skeletal tissues is increased in vein graft disease, this is accompanied by upregulation of PLOD2 (Kahle et al., 2016). Furthermore, increased PLOD2 expression has been detected in the macroscopically injured region of the capsule, and upregulation of TGF-β1, TGFβR1, and PLOD2 is likely related to the disease progression (Belangero et al., 2016).
It has been shown that PLOD3 mutations are associated with the connective tissue disorder (Salo et al., 2008). Analysis of PLOD3 knock-out embryos and cells indicate that loss of PLOD2 reduces glycosylated hydroxylysines on type IV and VI collagen with abnormal distribution (Sipilä et al., 2007). Reduced glycosylation may inhibit the tetramerization and secretion of type VI collagen. Another function of PLOD3 is to glucosylate galactosylhydroxylysine residues in type I collagen in osteoblasts. The G-Hyl glucosylation induced by PLOD3 is crucial for collagen fibrillogenesis in vitro (Sricholpech et al., 2011).
PLODs in Cancer Progression and Metastasis
Increased collagen deposition and cross-linking promote cancer development and progression by enhancing cancer cell migration, invasion and proliferation (Provenzano et al., 2006, 2008; Levental et al., 2009; Zhu et al., 2015). Therefore, PLODs may contribute to cancer progression by modulating collagen cross-link and maturation.
Increased PLOD expression has been detected in many types of cancer. The PLOD2 expression level is significantly upregulated in breast cancer compared to normal mammary tissue, and the upregulation correlates with short disease-related survival (Gjaltema et al., 2015). In esophageal squamous-cell carcinoma (ESCC), expression of the tumor suppressor gene esophageal cancer-related gene 4 has a negative association with PLOD1 and PLOD2 (Li et al., 2017). The PLOD2 expression is significantly correlated disease-free survival and tumor size in hepatocellular carcinoma (HCC) (Noda et al., 2012). PLOD3 is overexpressed in HCC (Elsemman et al., 2016; Shen et al., 2018) and is a potential diagnosis marker for early-stage HCC (Shen et al., 2018). Knockdown of PLOD3 suppresses liver tumor incidence as well as tumor growth rates in spontaneous mouse HCC model (Shen et al., 2018). Nicastri used a quantitative proteomic technique and identified 54 up-regulated glycoproteins in colorectal cancer samples, including PLOD2 and PLOD3 (Nicastri et al., 2014).
Increased PLOD2 expression is crucial for tumor invasion and metastasis (Figure 2). For instance, silencing PLOD2 expression in the breast cancer cell line MDA-MB 231 reduces cancer metastasis and collagen deposition in the primary tumor tissue; interestingly, PLOD2 expression has little effect on the primary tumor growth (Gilkes et al., 2013b). Hypoxia- and TGF-β1-induced PLOD2 expression promotes the migratory, invasive and adhesive capacities of cervical cancer cells by promoting EMT and the formation of focal adhesion (Remst et al., 2014; Xu et al., 2017). In HIF-1α-deficient tumors, ectopic PLOD2 expression restores the migration and metastatic potential, and inhibition of PLOD2 activity suppresses the tumor metastases (Eisinger-Mathason et al., 2013). Although HIF-1 induces expression of PLOD1 and PLOD2, PLOD2 expression in breast cancer cells is more important for fibrillary collagen formation, tumor stiffness and cancer metastasis to lymph nodes and lungs (Gilkes et al., 2013b).
Function of PLOD2 in lung cancer progression differs slightly from breast cancer; ectopic expression of PLOD2 enhances both primary cancer growth and metastasis (Chen et al., 2015). PLOD2 hydroxylates telopeptidyl lysine residues on collagen, subsequently increasing the level of hydroxylysine aldehyde–derived collagen cross-links (HLCCs) and lowering levels of lysine aldehyde–derived cross-links in lung cancer tissue (Chen et al., 2015). Recent study also reveal that PLOD2 expression induces PI3K/AKT signaling in glioma (Song et al., 2017) and non-small-cell lung cancer (NSCLC) (Du et al., 2017); activation of the PI3K pathway may contribute to increased cell proliferation, migration and invasion.
It is well established that PLOD2 protein locates in ER (Liefhebber et al., 2010). However, a recent study shows that PLOD2 protein can be secreted by lung cancer cells and induce collagen remodeling (Chen et al., 2016). Addition of recombinant PLOD2 to the extracellular space promotes HLCC formation in the extracellular matrix, suggesting that secreted PLOD2 is functional (Chen et al., 2016). However, function of secreted PLOD2 in cancer development and progression remains to be determined.
Cancer associated fibroblasts (CAFs) and stellate cells, as the major source of ECM production in the tumor microenvironment, promote tumor cell invasion and migration through the PLOD2-induced collagen cross-link (Bozóky et al., 2013; Pankova et al., 2016). PLOD2 is highly expressed in CAFs; silencing PLOD2 expression in CAFs significantly reduced the tumor invasion and metastasis (Pankova et al., 2016). Knockdown of PLOD2 in pancreatic stellate cells inhibits directional migration of cancer cells within the matrices by constructing an insensitive microenvironment of three-dimensional (3D) matrices to tumor migration (Sada et al., 2016). These results indicate that PLOD2 expressed in stromal cells is crucial for cancer progression.
Future Direction
Loss of function mutations and abnormal PLOD expression are involved in many collagen-related diseases. Impairment of PLOD1 function contributes the development of Kyphoscoliotic type of EDS. Mutations of PLOD3 cause the connective tissue disorder (Salo et al., 2008). Many studies demonstrate that increased PLOD2 and PLOD3 expression is required for cancer progression and metastasis. Therefore, targeting PLOD is a potential therapeutic strategy for cancer and other collagen-related diseases. Unfortunately, there is no potent PLOD inhibitor available. Since the client protein and function of PLOD1, PLOD2, and PLOD3 in collagen synthesis are different, it is important to develop specific inhibitors for PLOD to halt cancer progression. Another strategy to inhibit PLOD activity in cancer tissue is to reduce PLOD expression; therefore, further understanding how PLOD is regulated during cancer development may identify signaling pathways to target PLOD.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors acknowledge the cancer center's Research Communications Office for assistance with manuscript preparation. This study was supported by funding support from NCI (1R01CA207772, 1R01CA215095, and 1R21CA209045 to RX) and United States Department of Defense (W81XWH-15-1-0052 to RX).
Abbreviations
PLOD, procollagen-lysine, 2-oxoglutarate 5-dioxygenase; LH, lysyl hydroxylases; EDS, Ehlers-Danlos syndrome; RDEB, recessive dystrophic epidermolysis bullosa; BMD, bone mineral density; GT, galactosyltransferase; GGT, galactosylhydroxylysine -glucosyltransferase; BS, Bruck Syndrome; bAVM, brain arteriovenous malformations; HIF-1α, hypoxia-inducible factor-1α; EMT, Epithelial-Mesenchymal Transition; HIF-1, hypoxia-inducible factor 1; ACHP, 2-Amino-6-[2-(cyclopropylmethoxy)-6-hydroxyphenyl]-4-(4-piperidinyl)−3 pyridinecarbonitrile; BMP-2, bone morphogenetic protein-2; AT-MSCs, adipose tissue-derived mesenchymal stem cells; ESCC, esophageal squamous-cell carcinoma; HCC, hepatocellular carcinoma; HLCCs, hydroxylysine aldehyde–derived collagen cross-links; NSCLC, non-small-cell lung cancer; ER, endoplasmic reticulum; CAFs, Cancer associated fibroblasts; ECM, extracellular matrix; 3D, three-dimensional.
References
Abdalla, E. M., Rohrbach, M., Bürer, C., Kraenzlin, M., El-Tayeby, H., Elbelbesy, M. F., et al. (2015). Kyphoscoliotic type of Ehlers-Danlos Syndrome (EDS VIA) in six Egyptian patients presenting with a homogeneous clinical phenotype. Eur. J. Pediatr. 174, 105–112. doi: 10.1007/s00431-014-2429-9
Ah-Kim, H., Zhang, X., Islam, S., Sofi, J. I., Glickberg, Y., Malemud, C. J., et al. (2000). Tumour necrosis factor α enhances the expression of hydroxyl lyase, cytoplasmic antiproteinase-s and a dual specificity kinase TTK in human chondrocyte-like cells. Cytokine 12, 142–150. doi: 10.1006/cyto.1999.0539
Albaugh, V. L., Mukherjee, K., and Barbul, A. (2017). Proline precursors and collagen synthesis: biochemical challenges of nutrient supplementation and wound healing. J. Nutr. 147, 2011–2017. doi: 10.3945/jn.117.256404
Amodio, G., Sasso, E., D'ambrosio, C., Scaloni, A., Moltedo, O., Franceschelli, S., et al. (2016). Identification of a microRNA (miR-663a) induced by ER stress and its target gene PLOD3 by a combined microRNome and proteome approach. Cell Biol. Toxicol. 32, 285–303. doi: 10.1007/s10565-016-9335-z
Barnes, M. J., Constable, B. J., Morton, L. F., and Royce, P. M. (1974). Age-related variations in hydroxylation of lysine and proline in collagen. Biochem. J. 139, 461–468. doi: 10.1042/bj1390461
Belangero, P. S., Leal, M. F., Cohen, C., Figueiredo, E. A., Smith, M. C., Andreoli, C. V., et al. (2016). Expression analysis of genes involved in collagen cross-linking and its regulation in traumatic anterior shoulder instability. J. Orthop. Res. 34, 510–517. doi: 10.1002/jor.22984
Bozóky, B., Savchenko, A., Csermely, P., Korcsmáros, T., Dúl, Z., Pontén, F., et al. (2013). Novel signatures of cancer-associated fibroblasts. Int. J. Cancer 133, 286–293. doi: 10.1002/ijc.28035
Camper, L., Hellman, U., and Lundgren-Åkerlund, E. (1998). Isolation, cloning, and sequence analysis of the Integrin Subunit α10, a β1-associated collagen binding integrin expressed on chondrocytes. J. Biol. Chem. 273, 20383–20389. doi: 10.1074/jbc.273.32.20383
Carafoli, F., Hamaia, S. W., Bihan, D., Hohenester, E., and Farndale, R. W. (2013). An activating mutation reveals a second binding mode of the integrin α2 i domain to the GFOGER motif in collagens. PLoS ONE 8:e69833. doi: 10.1371/journal.pone.0069833
Chen, Y., Guo, H., Terajima, M., Banerjee, P., Liu, X., Yu, J., et al. (2016). Lysyl hydroxylase 2 is secreted by tumor cells and can modify collagen in the extracellular space. J. Biol. Chem. 291, 25799–25808. doi: 10.1074/jbc.M116.759803
Chen, Y., Terajima, M., Yang, Y., Sun, L., Ahn, Y.-H., Pankova, D., et al. (2015). Lysyl hydroxylase 2 induces a collagen cross-link switch in tumor stroma. J. Clin. Invest. 125, 1147–1162. doi: 10.1172/JCI74725
Cheon, D.-J., Tong, Y., Sim, M.-S., Dering, J., Berel, D., Cui, X., et al. (2014). A collagen-remodeling gene signature regulated by tgf-β signaling is associated with metastasis and poor survival in serous ovarian cancer. Clin. Cancer Res. 20, 711–723. doi: 10.1158/1078-0432.CCR-13-1256
Chong, M. S., Goh, L. K., Lim, W. S., Chan, M., Tay, L., Chen, G., et al. (2013). Gene expression profiling of peripheral blood leukocytes shows consistent longitudinal downregulation of TOMM40 and upregulation of KIR2DL5A, PLOD1, and SLC2A8 among fast progressors in early Alzheimer's disease. J. Alzheimers Dis. 34, 399–405. doi: 10.3233/JAD-121621
Conklin, M. W., Eickhoff, J. C., Riching, K. M., Pehlke, C. A., Eliceiri, K. W., Provenzano, P. P., et al. (2011). Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am. J. Pathol. 178, 1221–1232. doi: 10.1016/j.ajpath.2010.11.076
Du, H., Chen, Y., Hou, X., Huang, Y., Wei, X., Yu, X., et al. (2017). PLOD2 regulated by transcription factor FOXA1 promotes metastasis in NSCLC. Cell Death Dis. 8:e3143. doi: 10.1038/cddis.2017.553
Egeblad, M., Rasch, M. G., and Weaver, V. M. (2010). Dynamic interplay between the collagen scaffold and tumor evolution. Curr. Opin. Cell Biol. 22, 697–706. doi: 10.1016/j.ceb.2010.08.015
Eisinger-Mathason, T. S. K., Zhang, M., Qiu, Q., Skuli, N., Nakazawa, M. S., Karakasheva, T., et al. (2013). Hypoxia-dependent modification of collagen networks promotes sarcoma metastasis. Cancer Discov. 3, 1190–1205. doi: 10.1158/2159-8290.CD-13-0118
Elsemman, I. E., Mardinoglu, A., Shoaie, S., Soliman, T. H., and Nielsen, J. (2016). Systems biology analysis of hepatitis C virus infection reveals the role of copy number increases in regions of chromosome 1q in hepatocellular carcinoma metabolism. Mol. Biosyst. 12, 1496–1506. doi: 10.1039/C5MB00827A
Gilkes, D. M., Bajpai, S., Chaturvedi, P., Wirtz, D., and Semenza, G. L. (2013a). Hypoxia-inducible factor 1 (HIF-1) promotes extracellular matrix remodeling under hypoxic Conditions by Inducing P4HA1, P4HA2, and PLOD2 expression in fibroblasts. J. Biol. Chem. 288, 10819–10829. doi: 10.1074/jbc.M112.442939
Gilkes, D. M., Bajpai, S., Wong, C. C., Chaturvedi, P., Hubbi, M. E., Wirtz, D., et al. (2013b). Procollagen lysyl hydroxylase 2 is essential for hypoxia-induced breast cancer metastasis. Mol. Cancer Res. 11, 456–466. doi: 10.1158/1541-7786.MCR-12-0629
Gistelinck, C., Witten, P. E., Huysseune, A., Symoens, S., Malfait, F., Larionova, D., et al. (2016). Loss of type I collagen telopeptide lysyl hydroxylation causes musculoskeletal abnormalities in a zebrafish model of bruck syndrome. J. Bone Miner. Res. 31, 1930–1942. doi: 10.1002/jbmr.2977
Giunta, C., Randolph, A., and Steinmann, B. (2005). Mutation analysis of the PLOD1 gene: an efficient multistep approach to the molecular diagnosis of the kyphoscoliotic type of Ehlers-Danlos syndrome (EDS VIA). Mol. Genet. Metab. 86, 269–276. doi: 10.1016/j.ymgme.2005.04.014
Gjaltema, R., a, F., De Rond, S., Rots, M. G., and Bank, R.A. (2015). procollagen lysyl hydroxylase 2 expression is regulated by an alternative downstream transforming growth factor β-1 activation mechanism. J. Biol. Chem. 290, 28465–28476. doi: 10.1074/jbc.M114.634311
Hamaia, S. W., Pugh, N., Raynal, N., Némoz, B., Stone, R., Gullberg, D., et al. (2012). Mapping of potent and specific binding motifs, glogen and gvogea, for integrin α1β1 using collagen toolkits, I. I., and III. J. Biol. Chem. 287, 26019–26028. doi: 10.1074/jbc.M112.353144
Hausmann, E. (1967). Cofactor requirements for the enzymatic hydroxylation of lysine in a polypeptide precursor of collagen. Biochim. Biophys. Acta 133, 591–593. doi: 10.1016/0005-2795(67)90566-1
Hautala, T., Byers, M. G., Eddy, R. L., Shows, T. B., Kivirikko, K. I., and Myllyla, R. (1992). Cloning of human lysyl hydroxylase: complete cDNA-derived amino acid sequence and assignment of the gene (PLOD) to chromosome 1p36.3 → p36.2. Genomics 13, 62–69. doi: 10.1016/0888-7543(92)90202-4
Heikkinen, J., Risteli, M., Wang, C., Latvala, J., Rossi, M., Valtavaara, M., et al. (2000). Lysyl hydroxylase 3 is a multifunctional protein possessing collagen glucosyltransferase activity. J. Biol. Chem. 275, 36158–36163. doi: 10.1074/jbc.M006203200
Heino, J. (2007). The collagen family members as cell adhesion proteins. Bioessays 29, 1001–1010. doi: 10.1002/bies.20636
Hjalt, T. A., Amendt, B. A., and Murray, J. C. (2001). Pitx2 regulates procollagen lysyl hydroxylase (plod) gene expression: implications for the pathology of Rieger syndrome. J. Cell Biol. 152, 545–552. doi: 10.1083/jcb.152.3.545
Hollern, D. P., Honeysett, J., Cardiff, R. D., and Andrechek, E. R. (2014). The E2F transcription factors regulate tumor development and metastasis in a mouse model of metastatic breast cancer. Mol. Cell. Biol. 34, 3229–3243. doi: 10.1128/MCB.00737-14
Kahle, B., Schmidtke, C., Hunzelmann, N., Bartels, C., Sievers, H. H., Steenbock, H., et al. (2016). The extracellular matrix signature in vein graft disease. Can. J. Cardiol. 32, 1008e11–1008e17. doi: 10.1016/j.cjca.2015.11.014
Kim, S., Easterling, E. R., Price, L. C., Smith, S. L., Coligan, J. E., Park, J.-E., et al. (2017). The role of leukocyte-associated ig-like receptor-1 in suppressing collagen-induced arthritis. J. Immunol. 199, 2692–2700. doi: 10.4049/jimmunol.1700271
Kivirikko Ki, P. T. (1998). Collagen hydroxylases and the protein disulfide isomerase subunit of prolyl 4-hydroxylases. Adv. Enzymol. Relat. Areas Mol. Biol. 72, 325–398. doi: 10.1002/9780470123188.ch9
Knippenberg, M., Helder, M. N., Doulabi, B. Z., Bank, R. A., Wuisman, P. I. J. M., and Klein-Nulend, J. (2009). Differential effects of bone morphogenetic protein-2 and transforming growth factor-β1 on gene expression of collagen-modifying enzymes in human adipose tissue–derived mesenchymal stem cells. Tissue Eng. Part A 15, 2213–2225. doi: 10.1089/ten.tea.2007.0184
Kurozumi, A., Kato, M., Goto, Y., Matsushita, R., Nishikawa, R., Okato, A., et al. (2016). Regulation of the collagen cross-linking enzymes LOXL2 and PLOD2 by tumor-suppressive microRNA-26a/b in renal cell carcinoma. Int. J. Oncol. 48, 1837–1846. doi: 10.3892/ijo.2016.3440
Leitinger, B. (2003). Molecular analysis of collagen binding by the human discoidin domain receptors, DDR1 and DDR2: identification of collagen binding sites in DDR2. J. Biol. Chem. 278, 16761–16769. doi: 10.1074/jbc.M301370200
Leitinger, B. (2011). Transmembrane collagen receptors. Annu. Rev. Cell Dev. Biol. 27, 265–290. doi: 10.1146/annurev-cellbio-092910-154013
Levental, K. R., Yu, H., Kass, L., Lakins, J. N., Egeblad, M., Erler, J. T., et al. (2009). Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891–906. doi: 10.1016/j.cell.2009.10.027
Li, L., Wang, W., Li, X., and Gao, T. (2017). Association of ECRG4 with PLK1, CDK4, PLOD1 and PLOD2 in esophageal squamous cell carcinoma. Am. J. Transl. Res. 9, 3741–3748.
Liefhebber, J. M. P., Punt, S., Spaan, W. J. M., and Van Leeuwen, H. C. (2010). The human collagen beta(1-O)galactosyltransferase, GLT25D1, is a soluble endoplasmic reticulum localized protein. BMC Cell Biol. 11:33. doi: 10.1186/1471-2121-11-33
Paulsson, M. (1992). Basement membrane proteins: structure, assembly, and cellular interactions. Crit. Rev. Biochem. Mol. Biol. 27, 93–127.
Mia, M. M., and Bank, R. A. (2015). The IκB kinase inhibitor ACHP strongly attenuates TGFβ1-induced myofibroblast formation and collagen synthesis. J. Cell. Mol. Med. 19, 2780–2792. doi: 10.1111/jcmm.12661
Miyamoto, K., Seki, N., Matsushita, R., Yonemori, M., Yoshino, H., Nakagawa, M., et al. (2016). Tumour-suppressive miRNA-26a-5p and miR-26b-5p inhibit cell aggressiveness by regulating PLOD2 in bladder cancer. Br. J. Cancer 115, 354–363. doi: 10.1038/bjc.2016.179
Neyazi, B., Tanrikulu, L., Wilkens, L., Hartmann, C., Stein, K.-P., Dumitru, C. A., et al. (2017). Procollagen-Lysine, 2-Oxoglutarate 5-Dioxygenase 2 expression in brain arteriovenous malformations and its association with brain arteriovenous malformation size. World Neurosurg. 102, 79–84. doi: 10.1016/j.wneu.2017.02.116
Nicastri, A., Gaspari, M., Sacco, R., Elia, L., Gabriele, C., Romano, R., et al. (2014). N-Glycoprotein analysis discovers new up-regulated glycoproteins in colorectal cancer tissue. J. Proteome Res. 13, 4932–4941. doi: 10.1021/pr500647y
Nimni, M. E. (1983). Collagen: structure, function, and metabolism in normal and fibrotic tissues. Semin. Arthritis Rheum. 13, 1–86. doi: 10.1016/0049-0172(83)90024-0
Noda, T., Yamamoto, H., Takemasa, I., Yamada, D., Uemura, M., Wada, H., et al. (2012). PLOD2 induced under hypoxia is a novel prognostic factor for hepatocellular carcinoma after curative resection. Liver Int. 32, 110–118. doi: 10.1111/j.1478-3231.2011.02619.x
Oudin, M. J., Jonas, O., Kosciuk, T., Broye, L. C., Guido, B. C., Wyckoff, J., et al. (2016). Tumor cell–driven extracellular matrix remodeling drives haptotaxis during metastatic progression. Cancer Discov. 6, 516–531. doi: 10.1158/2159-8290.CD-15-1183
Pankova, D., Chen, Y., Terajima, M., Schliekelman, M. J., Baird, B. N., Fahrenholtz, M., et al. (2016). Cancer-associated fibroblasts induce a collagen cross-link switch in tumor stroma. Mol. Cancer Res. 14, 287–295. doi: 10.1158/1541-7786.MCR-15-0307
Patino, M. G., Neiders, M. E., Andreana, S., Noble, B., and Cohen, R. E. (2002). Collagen: an overview. Implant Dent. 11, 280–285. doi: 10.1097/00008505-200207000-00014
Pollard, J. W. (2004). Tumour-educated macrophages promote tumour progression and metastasis. Nat. Rev. Cancer 4, 71–78. doi: 10.1038/nrc1256
Pousi, B., Hautala, T., Heikkinen, J., Pajunen, L., Kivirikko, K. I., and Myllyl,ä, R. (1994). Alu-Alu recombination results in a duplication of seven exons in the lysyl hydroxylase gene in a patient with the type VI variant of Ehlers-Danlos syndrome. Am. J. Hum. Genet. 55, 899–906. doi: 10.1016/0945-053X(94)90130-9
Provenzano, P. P., Eliceiri, K. W., Campbell, J. M., Inman, D. R., White, J. G., and Keely, P. J. (2006). Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 4:38. doi: 10.1186/1741-7015-4-38
Provenzano, P. P., Inman, D. R., Eliceiri, K. W., Knittel, J. G., Yan, L., Rueden, C. T., et al. (2008). Collagen density promotes mammary tumor initiation and progression. BMC Med. 6:11. doi: 10.1186/1741-7015-6-11
Rajkumar, T., Sabitha, K., Vijayalakshmi, N., Shirley, S., Bose, M. V., Gopal, G., et al. (2011). Identification and validation of genes involved in cervical tumourigenesis. BMC Cancer 11:80. doi: 10.1186/1471-2407-11-80
Rautavuoma, K., Takaluoma, K., Sormunen, R., Myllyharju, J., Kivirikko, K. I., and Soininen, R. (2004). Premature aggregation of type IV collagen and early lethality in lysyl hydroxylase 3 null mice. Proc. Natl. Acad. Sci. U.S.A. 101, 14120–14125. doi: 10.1073/pnas.0404966101
Remst, D. F. G., Blaney Davidson, E. N., Vitters, E. L., Bank, R. A., Van Den Berg, W. B., and Van Der Kraan, P. M. (2014). TGF-ß induces Lysyl hydroxylase 2b in human synovial osteoarthritic fibroblasts through ALK5 signaling. Cell Tissue Res. 355, 163–171. doi: 10.1007/s00441-013-1740-5
Rhoads, R. E., and Udenfriend, S. (1968). Decarboxylation of alpha-ketoglutarate coupled to collagen proline hydroxylase. Proc. Natl. Acad. Sci. U.S.A. 60, 1473–1478. doi: 10.1073/pnas.60.4.1473
Rohrbach, M., Vandersteen, A., Yi,ş, U., Serdaroglu, G., Ataman, E., Chopra, M., et al. (2011). Phenotypic variability of the kyphoscoliotic type of Ehlers-Danlos syndrome (EDS VIA): clinical, molecular and biochemical delineation. Orphanet J. Rare Dis. 6, 46–46. doi: 10.1186/1750-1172-6-46
Rygiel, T. P., Stolte, E. H., De Ruiter, T., Van De Weijer, M. L., and Meyaard, L. (2011). Tumor-expressed collagens can modulate immune cell function through the inhibitory collagen receptor LAIR-1. Mol. Immunol. 49, 402–406. doi: 10.1016/j.molimm.2011.09.006
Sada, M., Ohuchida, K., Horioka, K., Okumura, T., Moriyama, T., Miyasaka, Y., et al. (2016). Hypoxic stellate cells of pancreatic cancer stroma regulate extracellular matrix fiber organization and cancer cell motility. Cancer Lett. 372, 210–218. doi: 10.1016/j.canlet.2016.01.016
Saito, M., and Marumo, K. (2010). Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos. Int. 21, 195–214. doi: 10.1007/s00198-009-1066-z
Salo, A. M., Cox, H., Farndon, P., Moss, C., Grindulis, H., Risteli, M., et al. (2008). A connective tissue disorder caused by mutations of the Lysyl Hydroxylase 3 gene. Am. J. Hum. Genet. 83, 495–503. doi: 10.1016/j.ajhg.2008.09.004
Shen, Q., Eun, J. W., Lee, K., Kim, H. S., Yang, H. D., Kim, S. Y., et al. (2018). BANF1, PLOD3, SF3B4 as early-stage cancer decision markers and drivers of hepatocellular carcinoma. Hepatology 67, 1360–1377. doi: 10.1002/hep.29606
Shinkai, H., and Yonemasu, K. (1979). Hydroxylysine-linked glycosides of human complement subcomponent C1q and various collagens. Biochem. J. 177, 847–852. doi: 10.1042/bj1770847
Sipilä, L., Ruotsalainen, H., Sormunen, R., Baker, N. L., Lamand,é, S. R., Vapola, M., et al. (2007). Secretion and assembly of type, i. v., and vi collagens depend on glycosylation of hydroxylysines. J. Biol. Chem. 282, 33381–33388. doi: 10.1074/jbc.M704198200
Song, Y., Zheng, S., Wang, J., Long, H., Fang, L., Wang, G., et al. (2017). Hypoxia-induced PLOD2 promotes proliferation, migration and invasion via PI3K/Akt signaling in glioma. Oncotarget 8, 41947–41962. doi: 10.18632/oncotarget.16710
Sricholpech, M., Perdivara, I., Nagaoka, H., Yokoyama, M., Tomer, K. B., and Yamauchi, M. (2011). Lysyl hydroxylase 3 glucosylates galactosylhydroxylysine residues in Type I collagen in osteoblast culture. J. Biol. Chem. 286, 8846–8856. doi: 10.1074/jbc.M110.178509
Sun, L., Wang, D., Li, X., Zhang, L., Zhang, H., and Zhang, Y. (2016). Extracellular matrix protein ITGBL1 promotes ovarian cancer cell migration and adhesion through Wnt/PCP signaling and FAK/SRC pathway. Biomed. Pharmacother. 81, 145–151. doi: 10.1016/j.biopha.2016.03.053
Szpirer, C., Szpirer, J., Rivière, M., Vanvooren, P., Valtavaara, M., and Myllyl,ä, R. (1997). Localization of the gene encoding a novel isoform of lysyl hydroxylase. Mamm. Genome 8, 707–708. doi: 10.1007/s003359900549
Tasker, P. N., Macdonald, H., Fraser, W. D., Reid, D. M., Ralston, S. H., and Albagha, O. M. E. (2006). Association of PLOD1 polymorphisms with bone mineral density in a population-based study of women from the UK. Osteoporos. Int. 17, 1078–1085. doi: 10.1007/s00198-006-0129-7
Tiger, C.-F., Fougerousse, F., Grundström, G., Velling, T., and Gullberg, D. (2001). α11β1 integrin is a receptor for interstitial collagens involved in cell migration and collagen reorganization on mesenchymal nonmuscle cells. Dev. Biol. 237, 116–129. doi: 10.1006/dbio.2001.0363
Tulla, M., Pentikäinen, O. T., Viitasalo, T., Käpylä, J., Impola, U., Nykvist, P., et al. (2001). Selective binding of collagen subtypes by integrin α1I, α2I, and α10I domains. J. Biol. Chem. 276, 48206–48212. doi: 10.1074/jbc.M104058200
Valtavaara, M., Szpirer, C., Szpirer, J., and Myllyl,ä, R. (1998). Primary structure, tissue distribution, and chromosomal localization of a novel isoform of Lysyl Hydroxylase (Lysyl Hydroxylase 3). J. Biol. Chem. 273, 12881–12886. doi: 10.1074/jbc.273.21.12881
van Dijk, F. S., Mancini, G. M. S., Maugeri, A., and Cobben, J. M. (2017). Ehlers Danlos syndrome, kyphoscoliotic type due to Lysyl Hydroxylase 1 deficiency in two children without congenital or early onset kyphoscoliosis. Eur. J. Med. Genet. 60, 536–540. doi: 10.1016/j.ejmg.2017.07.011
Watt, S. A., Dayal, J. H. S., Wright, S., Riddle, M., Pourreyron, C., Mcmillan, J. R., et al. (2015). Lysyl Hydroxylase 3 localizes to epidermal basement membrane and is reduced in patients with recessive dystrophic epidermolysis bullosa. PLoS ONE 10:e0137639. doi: 10.1371/journal.pone.0137639
Xiong, G., Deng, L., Zhu, J., Rychahou, P. G., and Xu, R. (2014). Prolyl-4-hydroxylase α subunit 2 promotes breast cancer progression and metastasis by regulating collagen deposition. BMC Cancer 14:1. doi: 10.1186/1471-2407-14-1
Xiong, G.-F., and Xu, R. (2016). Function of cancer cell-derived extracellular matrix in tumor progression. J. Cancer Metastas. Treat. 2, 357–364. doi: 10.20517/2394-4722.2016.08
Xu, F., Zhang, J., Hu, G., Liu, L., and Liang, W. (2017). Hypoxia and TGF-β1 induced PLOD2 expression improve the migration and invasion of cervical cancer cells by promoting epithelial-to-mesenchymal transition (EMT) and focal adhesion formation. Cancer Cell Int. 17:54. doi: 10.1186/s12935-017-0420-z
Yamada, Y. A. F, and Shimokata, H. (2007). Association of candidate gene polymorphisms with bone mineral density in community-dwelling Japanese women and men. Int. J. Mol. Med. 19, 791–801. doi: 10.3892/ijmm.19.5.791
Yamauchi, M., and Sricholpech, M. (2012). Lysine post-translational modifications of collagen. Essays Biochem. 52, 113–133. doi: 10.1042/bse0520113
Zahed-Cheikh, M. T. B, Coze, S., and Gire, C. (2017). Kyphoscolitic type of Ehlers-Danlos syndrome with prenatal stroke. Indian Pediatr. 54, 495–497. doi: 10.1007/s13312-017-1054-x
Keywords: collagen, extracellular matrix, lysyl hydroxylation, procollagen-lysine 2-oxoglutarate 5-dioxygenase, cancer progression
Citation: Qi Y and Xu R (2018) Roles of PLODs in Collagen Synthesis and Cancer Progression. Front. Cell Dev. Biol. 6:66. doi: 10.3389/fcell.2018.00066
Received: 13 December 2017; Accepted: 11 June 2018;
Published: 28 June 2018.
Edited by:
Catherine Park, University of California, San Francisco, United StatesReviewed by:
Ellis Fok, The Chinese University of Hong Kong, Hong KongKandice Tanner, National Institutes of Health (NIH), United States
Copyright © 2018 Qi and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ren Xu, cmVuLnh1MjAxMEB1a3kuZWR1
 Yifei Qi1
Yifei Qi1 Ren Xu
Ren Xu