- 1State Key Laboratory of Biocatalysis and Enzyme Engineering, Environmental Microbial Technology Center of Hubei Province, School of Life Sciences, Hubei University, Wuhan, China
- 2School of Environmental and Biological Engineering, Nanjing University of Science and Technology, Nanjing, China
- 3China Biotech Fermentation Industry Association, Beijing, China
- 4Zhejiang Huakang Pharmaceutical Co., Ltd., Quzhou, China
Zymomonas mobilis is a model ethanologenic bacterium for diverse biochemical production. Rich medium (RM) is a complex medium that is routinely used to cultivate Z. mobilis, which contains carbon sources such as glucose, nitrogen sources such as yeast extract (YE), and KH2PO4. Glucose consumption and cell growth of Z. mobilis is usually coupled during ethanol fermentation. However, sometimes glucose was not consumed during the exponential growth phase, and it took extended time for cells to consume glucose and produce ethanol, which eventually reduced the ethanol productivity. In this study, the effects of different nitrogen sources, as well as the supplementation of an additional nitrogen source into RM and minimal medium (MM), on cell growth and glucose consumption of Z. mobilis were investigated to understand the uncoupled cell growth and glucose consumption. Our results indicated that nitrogen sources such as YE from different companies affected cell growth, glucose utilization, and ethanol production. We also quantified the concentrations of major ion elements in different nitrogen sources using the quantitative analytic approach of Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES), and demonstrated that magnesium ion in the media affected cell growth, glucose consumption, and ethanol production. The effect of magnesium on gene expression was further investigated using RNA-Seq transcriptomics. Our results indicated that the lack of Mg2+ triggered stress responses, and the expression of genes involved in energy metabolism was reduced. Our work thus demonstrated that Mg2+concentration in nitrogen sources is essential for vigorous cell growth and ethanol fermentation, and the difference of Mg2+concentration in different YE is one of the major factors affecting the coupled cell growth, glucose consumption and ethanol fermentation in Z. mobilis. We also revealed that genes responsive for Mg2+ deficiency in the medium were majorly related to stress responses and energy conservation. The importance of magnesium on cell growth and ethanol fermentation suggests that metal ions should become one of the parameters for monitoring the quality of commercial nitrogen sources and optimizing microbial culture medium.
Introduction
The Gram-negative bacterium Zymomonas mobilis is a natural ethanologen with many desirable industrial biocatalyst characteristics, which include high glucose consumption rate, high specific ethanol productivity, high ethanol tolerance, a broad pH range for production (pH 3.5–7.5), and its status being generally regarded as safe (GRAS) (Rogers et al., 1984, 2007; Panesar et al., 2006; He et al., 2014; Yang et al., 2016a; Wang et al., 2018). Z. mobilis utilizes glucose for ethanol production faster than Saccharomyces cerevisiae because of its high cell surface area which leads to higher ethanol productivity (Conway, 1992; Lawford and Rousseau, 1997; Rogers et al., 2007; He et al., 2014; Yang et al., 2016a; Wang et al., 2018).
Additionally, Compared with S. cerevisiae, the preferred biocatalyst using the Embden-Meyerhof-Parnas (EMP) pathway for industrial ethanol production with mature infrastructures, Z. mobilis has improved ethanol yield by using the Entner-Doudoroff (ED) pathway with 50% less ATP produced relative to the EMP pathway (Conway, 1992; Kingston et al., 1996; Dien et al., 2003). Recently, Jacobson et al. (2019) established a network-level approach that integrates quantitative metabolomics with 2H and 13C metabolic flux analysis to investigate the in vivo thermodynamics of the ED pathway and central carbon metabolism in Z. mobilis. Their result is consistent with previous in silico prediction (Flamholz et al., 2013) and demonstrated that ED pathway is twice as thermodynamically favorable as the EMP pathway in E. coli or S. cerevisiae (Jacobson et al., 2019).
Nitrogen sources in the medium are reported to affect the growth of Z. mobilis. For example, the morphology of Z. mobilis CP3 changes when cultured in a medium with a low nitrogen source (Ju et al., 1983). A medium commonly used to culture Z. mobilis is rich medium (RM). RM contains carbon sources such as glucose, nitrogen sources such as yeast extract (YE), and KH2PO4 that is often used as the phosphorus and potassium source and a buffering agent. Although nitrogen sources like peptone, corn steep liquid, and even N2 can be used to sustain the cell growth of Z. mobilis (Ju et al., 1983; Lawford and Rousseau, 1997; Kremer et al., 2015), YE is the preferred nitrogen source because it can provide nitrogenous compounds, carbon, sulfur, trace nutrients, vitamin B complex and other important growth factors for various microorganisms (Zarei et al., 2016).
Despite the fact that manufacturers produce different YE to have the same composition of total nitrogen content and free amino acid nitrogen, YE produced by different companies has different trace components such as growth factors and metal ions due to the differences in their production processes. This then affects the microbial cell growth because vitamins and metal ions (e.g., Mg2+, Cu2+, Zn2+, and Fe2+) are cofactors of enzymes involved in various metabolic activities. For example, Kosaka et al. (2020) investigated the effect of different metal ions on the thermotolerance of Z. mobilis TISTR548 and related mutants, and demonstrated that the addition of Mg2+ and K+ reduced intracellular reactive oxygen species (ROS) accumulation at critical high temperature (CHT) with an increase of CHT by 1°C, which is probably due to the stabilization of both outer and inner membranes as well as the maintenance of homeostasis for cellular metabolism by the addition of Mg2+ and K+. In addition, the effect of supplementation of zinc on the ethanol fermentation performance of the self-flocculating yeast in the continuous ethanol fermentation was studied (Zhao et al., 2009; Xue et al., 2010; Ismail et al., 2014), and the roles of zinc and zinc containing proteins in yeast metabolism and cellular stress responses were summarized (Zhao and Bai, 2012).
With all nutrients needed for cell growth in the culture medium including a nitrogen source from YE, cell growth of Z. mobilis ZM4 is generally expected to be coupled with its glucose consumption and ethanol production. However, results and literature reports in a few studies from different research groups indicated that cell growth of Z. mobilis ZM4 and its glucose consumption were uncoupled, glucose was not completely consumed for ethanol production when cells reached stationary phase (Jones and Doelle, 1991; He et al., 2012; Yang et al., 2014a; Xia et al., 2018; Duan et al., 2019). This uncoupling phenomenon between growth and fermentation performance has also been reported in other microorganisms, including yeast, resulting in a prolonged fermentation time and decreased ethanol productivity (Cot et al., 2007; Pagliardini et al., 2013). For example, ethanol production of yeast cells was reported to be related to the length of the uncoupling phase during the batch fermentation process (Pagliardini et al., 2013).
A previous study in E. coli demonstrated that the inadequate amount of magnesium in the rich medium of buffered tryptone broth supplemented with glucose (TB7-glucose) is the major determinant leading to the uncoupled cell growth and glucose consumption (Christensen et al., 2017). They further demonstrated that the supplementation of sufficient magnesium can increase cell growth yield in other peptide-based media when carbon is in excess. In addition, their study also showed that magnesium can increase cell growth of multiple E. coli strains and other bacterial species such as Bacillus subtilis and Vibrio fischeri (Christensen et al., 2017). Recently, Lozano Terol et al. (2019) studied protein overexpression for E. coli BL21 and five derived mutants in M9 minimal medium and TB7 complex medium, and discovered a similar phenomenon that carbon source of glucose or glycerol was not consumed during the exponential growth phase in TB7 complex medium but not M9.
The uncoupling of growth and fermentation in the late growth phase could be beneficial for ethanol fermentation since carbon source will be channeled into ethanol production instead of biomass buildup especially for yeast ethanol production. However, the uncoupling of growth and glucose consumption at early growth phase is unfavorable since there will be no sufficient cell biomass for efficient sugar fermentation to ethanol, which is mostly due to stressful physical and chemical growth conditions, such as extreme temperature, extreme pH, and toxic compounds in the medium. Limited nutrient sources of carbon, nitrogen and metal ions used for fermentation were also reported to affect cell growth and fermentation performance (Jones and Doelle, 1991; Cot et al., 2007; Pagliardini et al., 2013; Yang Y. et al., 2018). Although the effect of diverse nitrogen sources on cell growth and fermentation performance has been reported in various microorganisms, such as the ethanologenic yeast S. cerevisiae and lactate-producer Sporolactobacillus inulinus (Martinez-Moreno et al., 2012; Kemsawasd et al., 2015; Klotz et al., 2017), metal ions within nitrogen sources and their impact on cell growth and fermentation have not been investigated extensively.
To understand the uncoupling phenomenon of cell growth and glucose consumption for both efficient carbon utilization and maximum ethanol productivity, we investigated the effects of different nitrogen sources on cell growth, glucose consumption and ethanol production of Z. mobilis in this study to help achieve the goal of optimal titer, rate, and yield for economic bioethanol production using Z. mobilis. Our results demonstrated that glucose consumption and ethanol production can be coupled with cell growth by changing the nitrogen sources in the rich medium, and the concentration of metal ions such as Mg2+ in the nitrogen source is a major factor affecting cell growth and ethanol production.
Results and Discussion
Effect of Supplementation of Nitrogen Sources Into RM
To understand the uncoupling of cell growth, glucose consumption and ethanol production in Z. mobilis, we evaluated factors in the medium affecting cell growth. The recipe of RM for Z. mobilis is relatively simple with carbon sources such as glucose, phosphate sources of KH2PO4, and YE as the source of nitrogen, minerals, and vitamins. Thus, 10 mM NH4Cl was added into RM using YE from the company OXOID (RMOXOID) to increase inorganic nitrogen content. However, extra NH4Cl supplemented into RMOXOID did not reduce the time for glucose utilization and ethanol production of ZM4 (data not shown).
The effect of supplementing different organic nitrogen sources such as peptone and tryptone were then tested. Our results showed that the supplementation of peptone did not reduce the time of glucose utilization. It still took more than 24 h for cells to consume all glucose in the media with 20 g/L peptone supplemented into RMOXOID. The final biomass in terms of OD600 value did increase slightly in correspondence to the increase of peptone added (Figure 1).
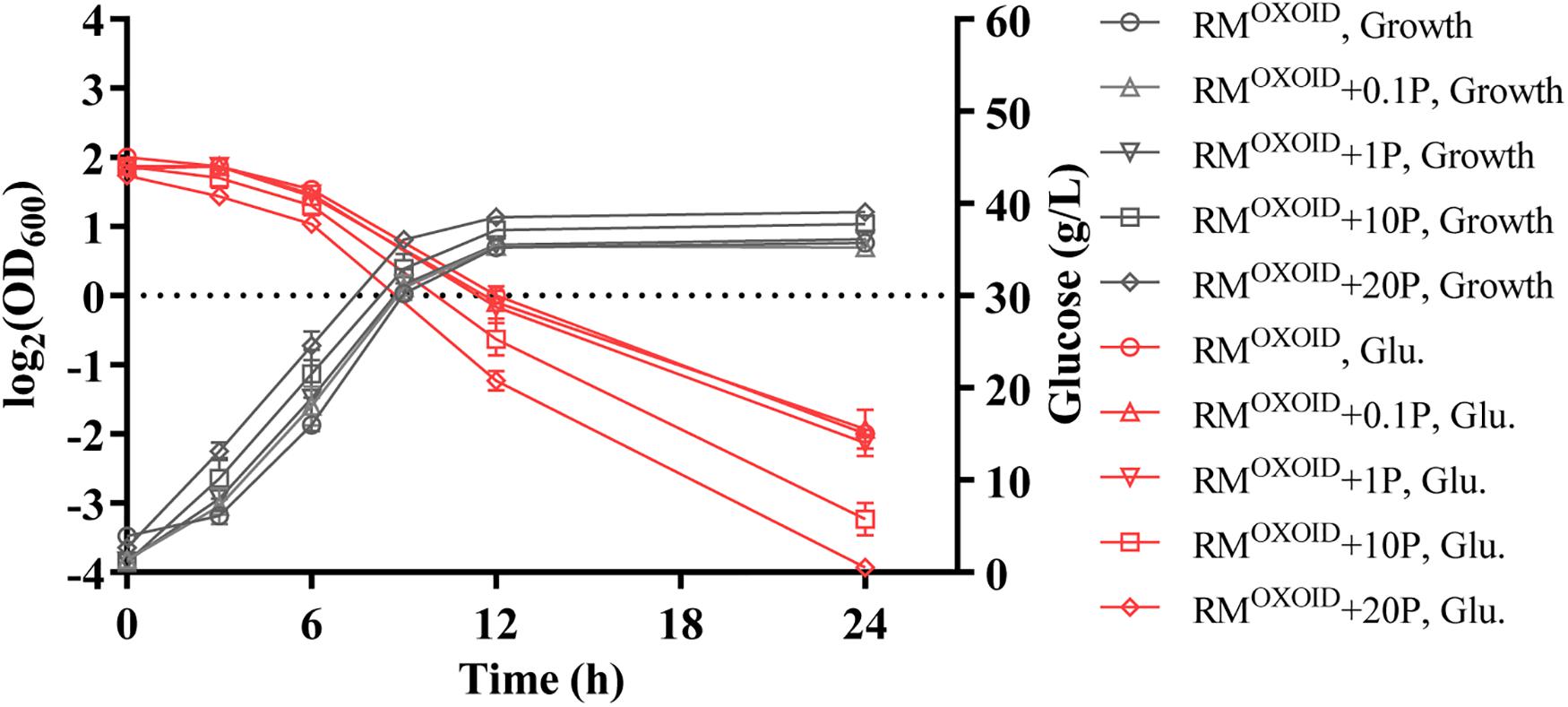
Figure 1. Cell growth and glucose (Glu) consumption of ZM4 in rich medium (RM) using yeast extract from OXOID company (RMOXOID) with extra peptone (P) supplemented at concentrations of 0.1, 1, 10, and 20 g/L.
Subsequently, different concentrations of tryptone were added in RMOXOID in a separate experiment. The results showed that the addition of extra tryptone could significantly increase both cell biomass and glucose consumption of ZM4 (Figure 2). The addition of more than 5 g/L tryptone in RMOXOID not only enhanced both cell growth and glucose utilization of ZM4, but also coupled the growth and ethanol fermentation of ZM4. The final highest OD600 value of ZM4 in RMOXOID with 5 g/L tryptone increased from 1.8 to 4.7, and all glucose was completely consumed within 12 h. Z. mobilis in medium without tryptone only utilized half the glucose at the same time point of 12-h post-inoculation (Figure 2).
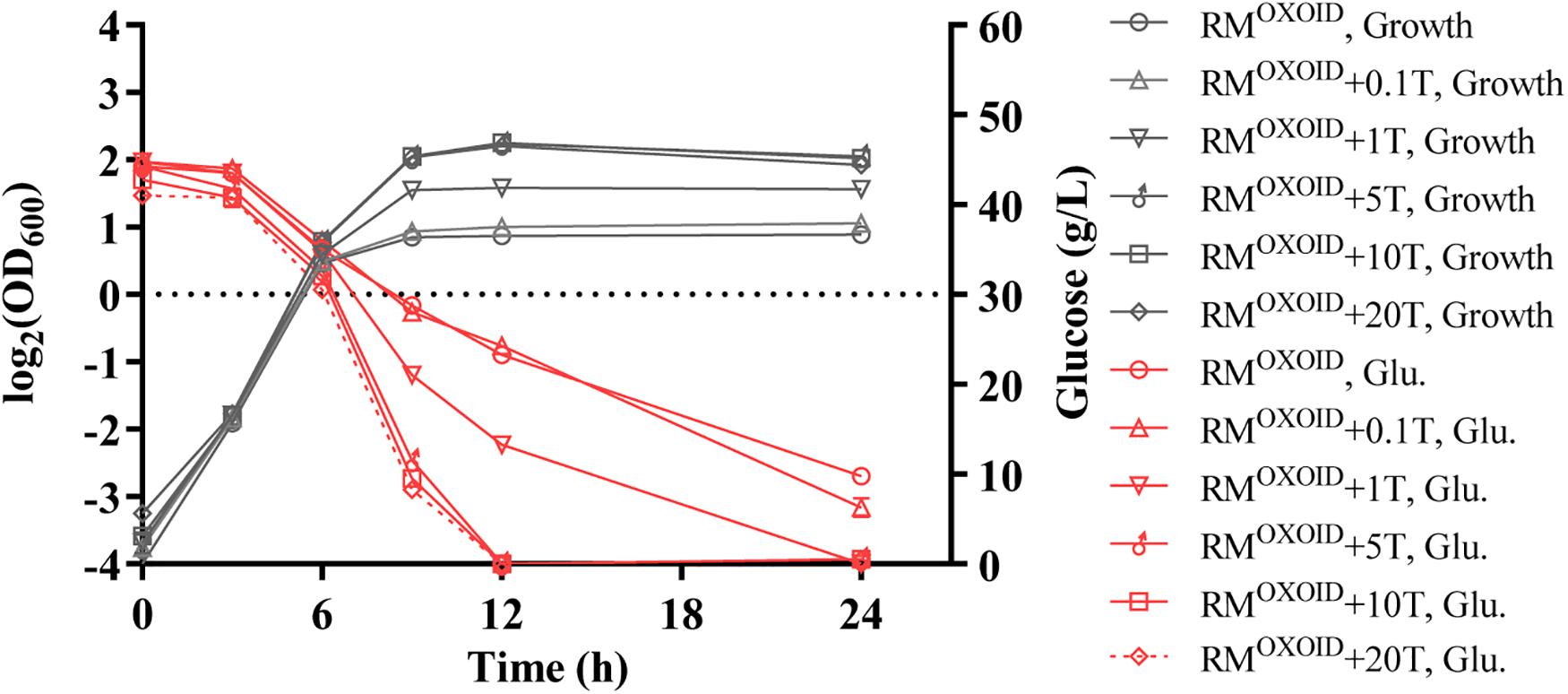
Figure 2. Cell growth and glucose (Glu) consumption of ZM4 in rich medium (RM) using yeast extract from OXOID company (RMOXOID) with tryptone (T) supplemented at concentrations of 0.1, 1, 5, 10, and 20 g/L.
The positive effect of adding tryptone into RMOXOID was further evaluated by comparing cell growth along with glucose utilization and ethanol production of ZM4 in both RMOXOID and RMOXOID + 5T at different temperatures of 30, 36, and 40°C. The results demonstrated that the supplementation of tryptone increased the growth and fermentation performance of ZM4 at each of these different temperatures, including the specific growth rate (μ), ethanol yield (Yp/s), and productivity (QP) (Table 1).
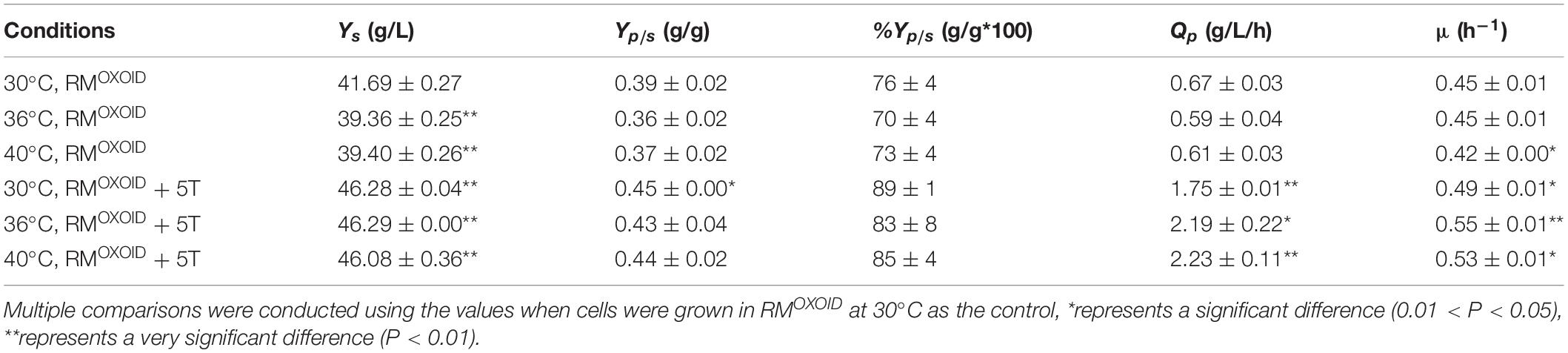
Table 1. Glucose consumption (Ys), ethanol yield (Yp/s), theoretical ethanol yield (%Yp/s), ethanol productivity (QP), and specific growth rate (μμ) of ZM4 in RMOXOID and RMOXOID supplemented with 5 g/L tryptone at different temperatures within 24 h.
It also increased cell growth, glucose consumption rate, and ethanol productivity of ZM4 at higher temperatures of 36 and 40°C than those at the normal temperature of 30°C in RMOXOID supplemented with 5 g/L tryptone, although ethanol yields were similar among these conditions (Table 1 and Figure 3). Compared with a normal temperature of 30°C, the time that ZM4 utilized all glucose at 36 and 40°C in RMOXOID with extra tryptone was reduced by about 3 h, but 1 day was not sufficient for ZM4 to completely consume glucose at any of the aforementioned temperatures in RMOXOID (Figure 3). ZM4 took less time to completely utilize glucose with maximum ethanol production achieved in RMOXOID + 5T at 36 and 40°C (9 h) than at 30°C (12 h) with a corresponding higher growth rate and ethanol productivity, which was increased from 1.75 ± 0.01 at 30°C to 2.19 ± 0.22 and 2.23 ± 0.11 at 36 and 40°C, respectively (Table 1 and Figure 3).
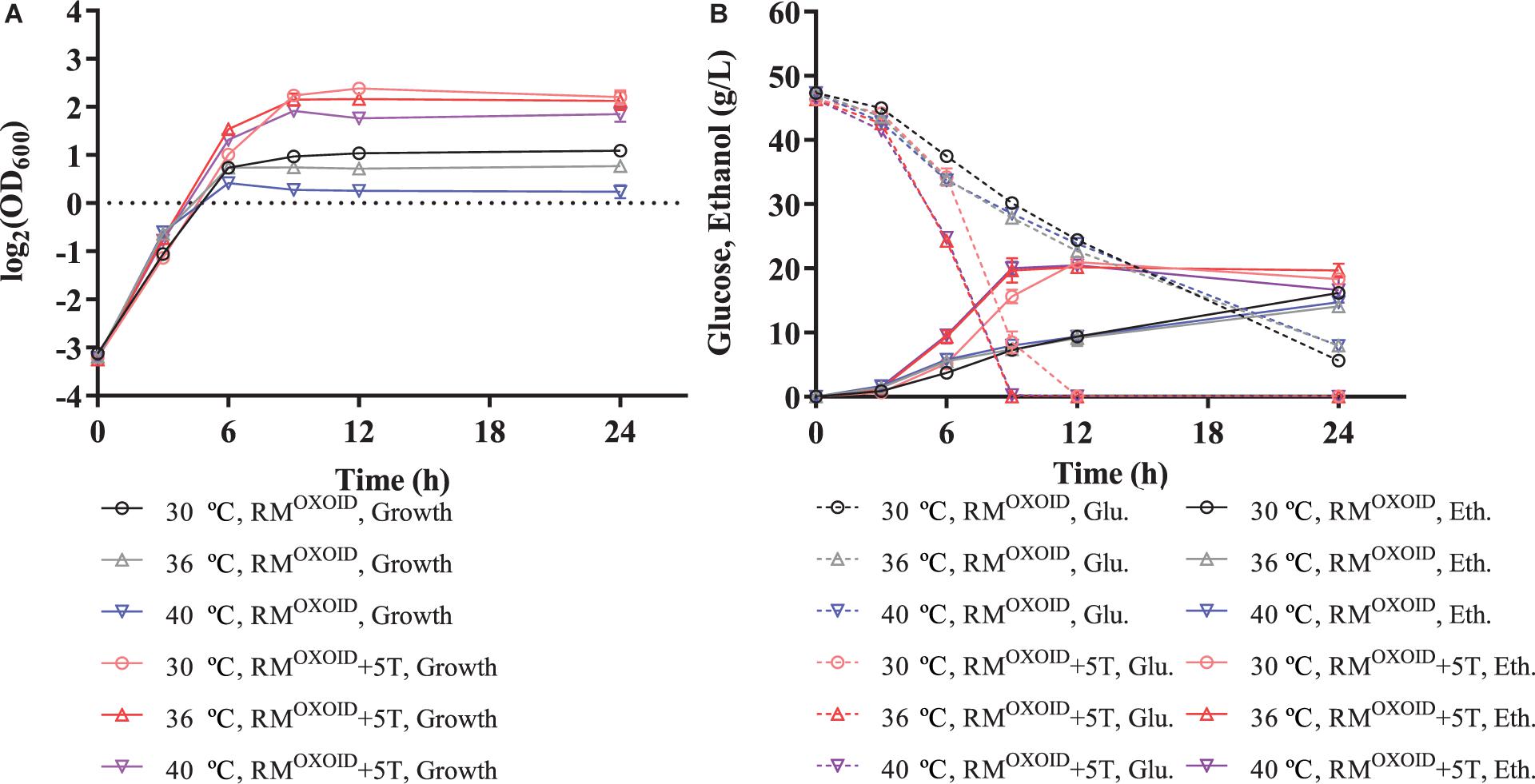
Figure 3. Cell growth (A), glucose (Glu) consumption and ethanol (Eth) production (B) of ZM4 in rich medium (RM) using yeast extract from OXOID company (RMOXOID) at different temperatures of 30, 36, and 40°C with extra 5 g/L tryptone added.
Effect of Exchanging YE From OXOID With Different Brand Ones in RM
As briefly mentioned above, YE distributed by different companies are produced with different processes which may lead to different amounts of total nitrogen and trace elements such as growth factors and metal ions. Besides adding extra nitrogen sources into the RMOXOID as discussed above (Figures 1–3), we also tested the effect of YE from different companies, including those from Becton Dickinson (YEBD) and Sangon Biotech Co., Ltd. (YESG, Shanghai, China). In addition, another industrial nitrogen source of corn steep liquid (CSL) from Macklin was evaluated at the same time as the two yeast-based nitrogen sources discussed above. All media used in this research are listed in Tables 5, 6.
YEOXOID in RMOXOID was replaced with different nitrogen sources including peptone (P), tryptone (T), CSL, YEBD, and YESG. Cell growth, glucose utilization, and ethanol production of ZM4 cultured in these media were then examined. Our results showed ZM4 performed differently in these media (Figure 4). The biomass of ZM4 in RM(T) was about two times higher than that in RMOXOID, while the biomass of ZM4 in RM(P) was the lowest (Figure 4B). Tryptone appeared to be better than YEOXOID and peptone for cell growth, which was consistent with the aforementioned experiments of supplementation of nitrogen source into RMOXOID (Figures 1, 2).
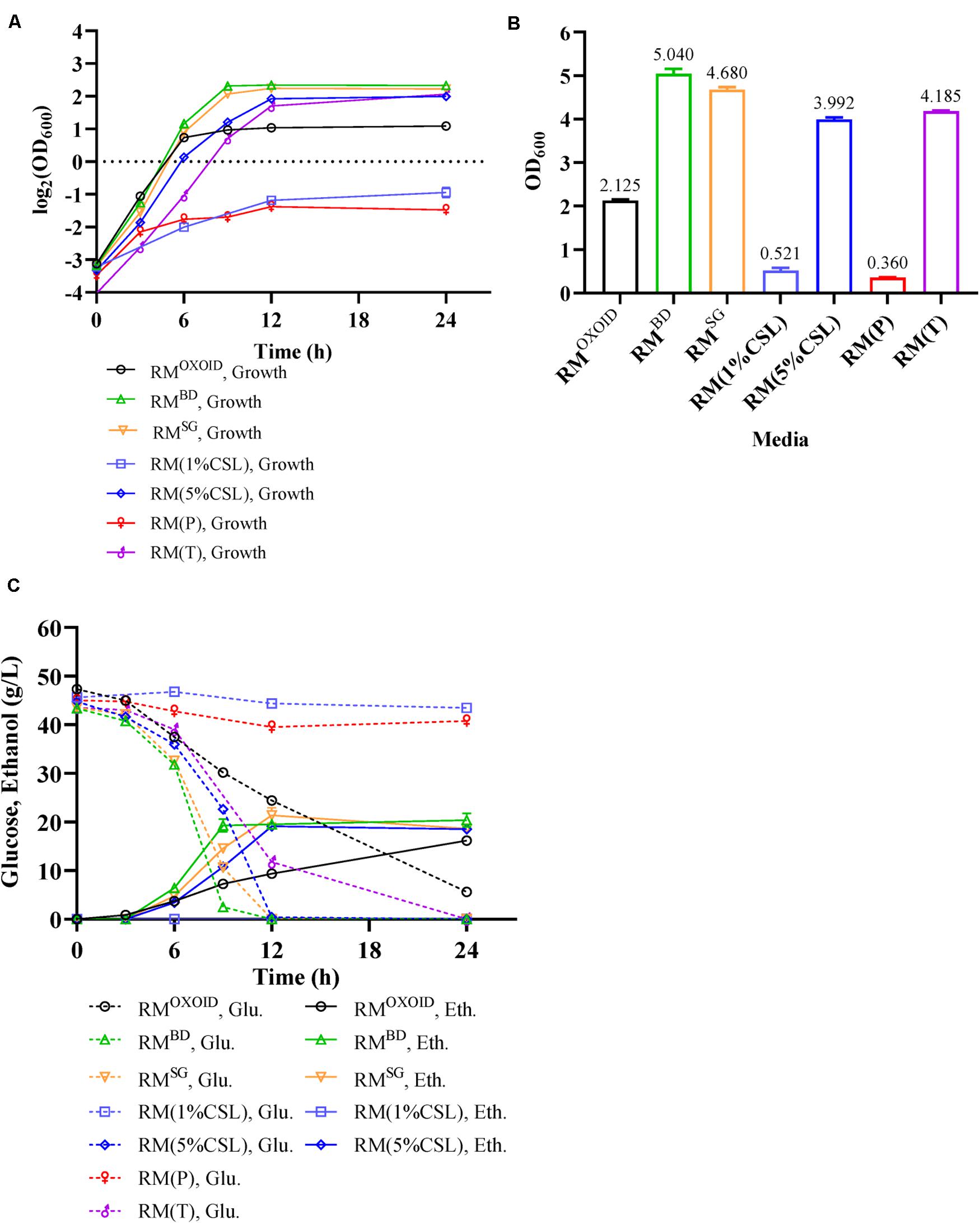
Figure 4. Cell growth (A), final OD600 value (B), as well as glucose (Glu) consumption and ethanol (Eth) production (C) of ZM4 in RM with different nitrogen sources.
Except for the fact that the specific growth rates of ZM4 in RM with 1% of peptone or CSL as the sole nitrogen source were lower, which can be seen from the slopes of the lines in the exponential phase, the specific growth rates of ZM4 in other RM were almost the same (Figure 4A). Compared with YEOXOID as the sole nitrogen source, YEBD, YESG, and 5% CSL all increased the final biomass of ZM4 (Figure 4B), reduced the time of glucose consumption (Figure 4C), and coupled cell growth and ethanol production. The biomass of ZM4 in RMBD was more than two times of that in RMOXOID and the time for glucose consumption in RMBD was about two-thirds shorter than that in RMOXOID (Figures 4B,C). YESG, though cheaper than YEBD, was almost just as good. In the future, YESG could be used in large-scale fermentation for ethanol production, and the appropriate nitrogen source can be chosen as needed based on the results above (Figures 1–4).
Effect of Exchanging (NH4)2SO4 in MM With Different Organic Nitrogen Sources
Although the RM recipe is relatively simple containing only three ingredients as discussed above, the composition of organic nitrogen sources such as YE is still complicated containing nitrogen, vitamins, metal ions, etc. Because the only nitrogen source in minimal medium (MM) is the inorganic nitrogen source (NH4)2SO4, it was selected to further examine the effect of different organic nitrogen sources on ZM4. The representative organic nitrogen sources used to replace (NH4)2SO4 in MM included YEBD, YEOXOID, and 5%CSL.
Our results demonstrated that the replacement of inorganic nitrogen source (NH4)2SO4 with organic nitrogen source enhanced cell growth, glucose utilization, and ethanol productivity (Figure 5). Interestingly, ZM4 had similar growth and glucose consumption rates in two MM media with either YEBD or YEOXOID as the sole nitrogen source (Figure 5). These results differed from those when YEBD or YEOXOID was used as the sole nitrogen source in RM where ZM4 grew better in RM with YEBD than in RM with YEOXOID as the nitrogen source (Figure 4). Therefore, some ingredients must exist in MM which RM lacks when YEOXOID is used as the nitrogen source. This difference is contingent on compositions of NaCl, Na2MoO4, and MgSO4 which differ in the ingredients of the two media (Tables 5, 6).
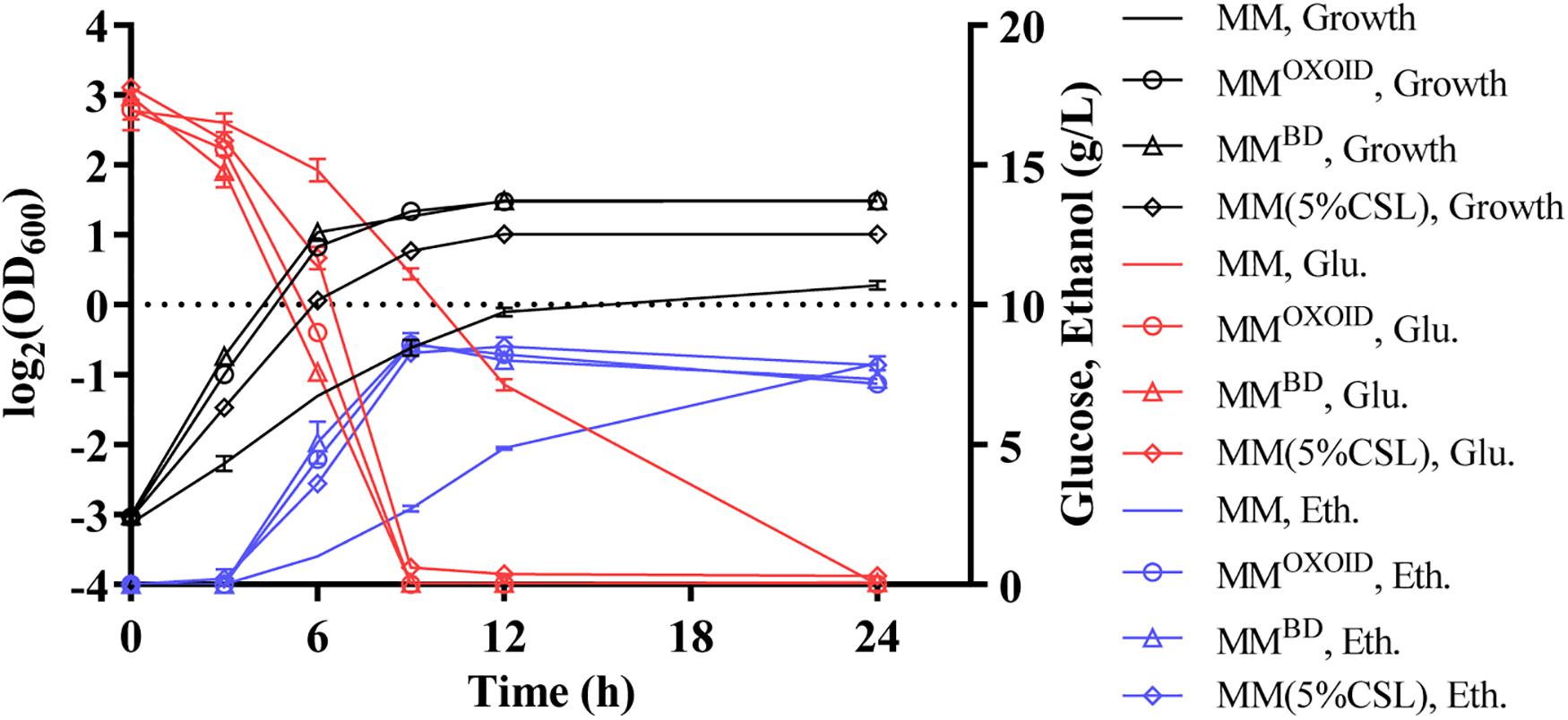
Figure 5. Cell growth, glucose (Glu) consumption and ethanol (Eth) production of ZM4 in MM containing 20 g/L glucose with different nitrogen sources.
Since magnesium ion is a cofactor of diverse enzymes involved in various metabolic activities, we investigated the effect of removing the magnesium ion from both MMBD and MMOXOID to test the growth and fermentation performance of ZM4. The lack of Mg2+ in MMBD had no effect on the growth of ZM4, but the biomass decreased in MMOXOID lacking Mg2+ (Figure 6). This result indicates that the concentration of Mg2+ in YEOXOID is different from that in YEBD, and Mg2+ plays important roles in cell growth of Z. mobilis, and Mg2+ was likely one of the factors affecting the coupling of cell growth, glucose consumption and ethanol production.
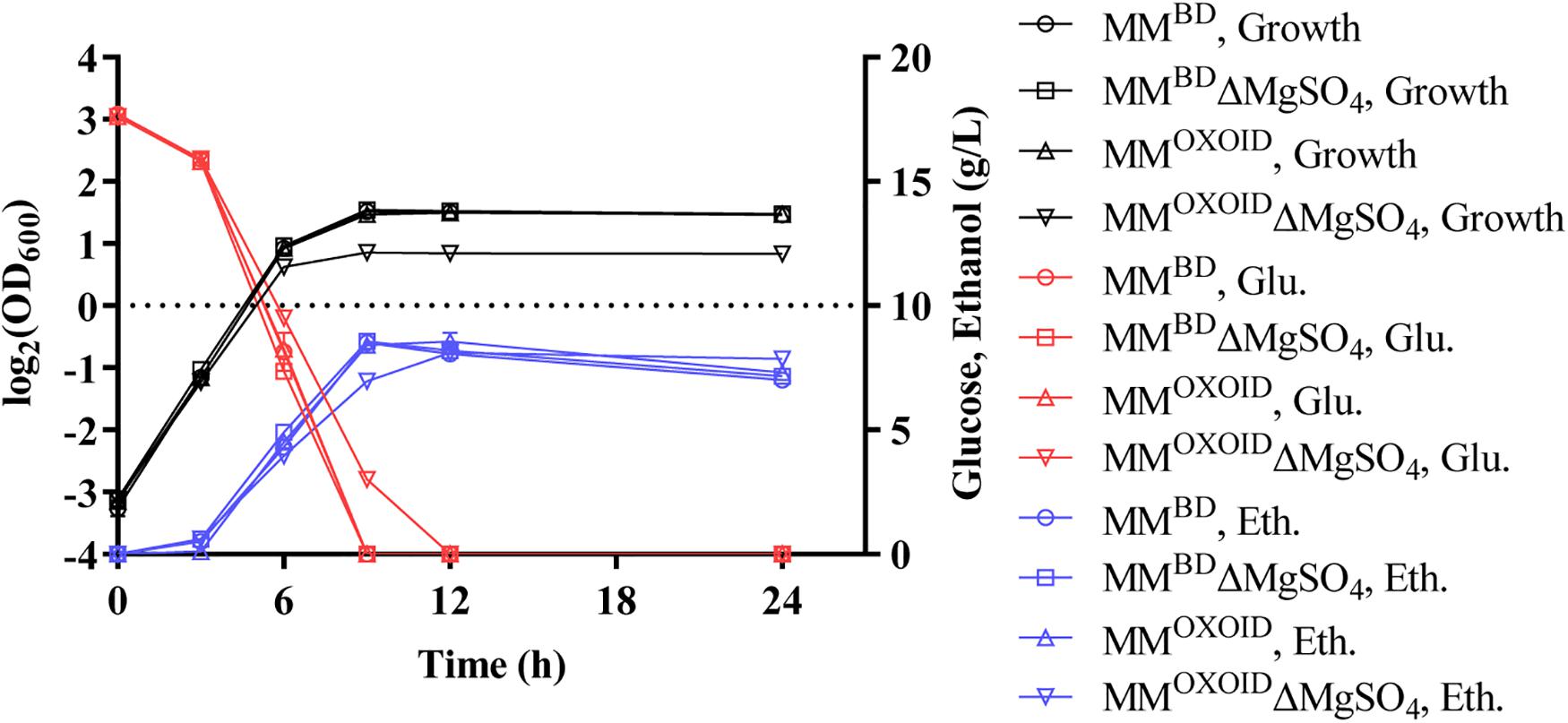
Figure 6. Cell growth, glucose (Glu) consumption and ethanol (Eth) production of ZM4 in MM containing 20 g/L glucose with original inorganic nitrogen sources replaced by YEBD and YEOXOID, respectively, as well as in above two media with Mg2+ removed.
Determination of Concentrations of Ions in Different Nitrogen Sources and the Impact of the Mg2+ on Fermentation Performance
In order to verify this hypothesis, the concentrations of several major ion elements in different organic nitrogen sources were then measured by ICP-OES. The result showed that the concentrations of these ion elements (Fe, K, Mg, P, S) were different among different nitrogen sources with peptone and tryptone containing fewer of these ions (Table 2). This may be one reason why cell growth and fermentation performance of Z. mobilis in media using tryptone and peptone as the sole nitrogen source was not as efficient as in other media using YE (Figure 4).
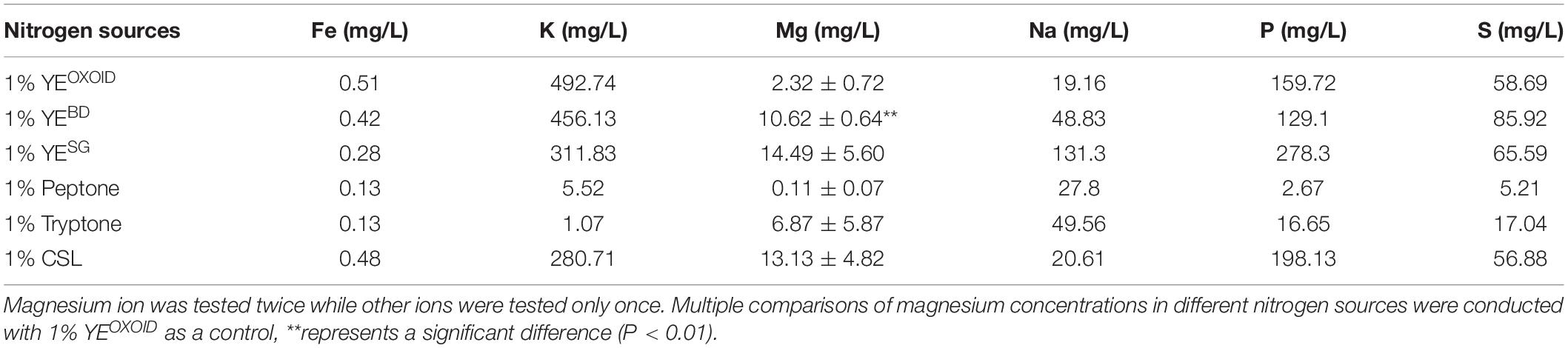
Table 2. Concentrations of different ion elements in different nitrogen sources measured by ICP-OES.
At the same concentration of nitrogen sources, the Mg2+ in the YE from OXOID was 8.3 mg/L less than that from Becton Dickinson. Mg2+ is the component with the major difference between these two nitrogen sources of YE from OXOID and BD companies, and therefore could be the reason that cell growth and ethanol fermentation of Z. mobilis in RMOXOID was not as efficient as in RMBD (Table 2). Since 0.5 g/L MgSO4⋅7H2O containing 49 mg/L Mg2+ was provided in MM which was sufficient for cell growth (Tables 2, 4), the growth difference of Z. mobilis in MMOXOID and MMBD was therefore not as obvious as that in RMOXOID and RMBD (Figures 4, 5). The concentration of Mg2+ in peptone was the lowest, which could also be one reason that ZM4 grew poorly in RM(P) compared to other nitrogen sources (Figure 4). These results further confirm the importance of sufficient Mg2+ concentration in media for optimal cell growth, glucose consumption and ethanol fermentation. The result that the concentration of Mg2+ in tryptone was higher than that in YEOXOID was consistent with the results of the nitrogen source supplement experiment (Kosaka et al., 2020), and provided a conjecture that perhaps the concentration of magnesium ion in nitrogen sources plays an important role in promoting temperature tolerance and cell growth (Figures 2, 3).
To verify the impact of Mg2+ on cell growth, different concentrations of Mg2+ were then added into the RMOXOID and MMOXOID without MgSO4 (MMOXOIDΔMgSO4), and the growth of ZM4 in these media was measured by the Bioscreen C (Figure 7). Our results indicated that in both RMOXOID and MMOXOIDΔMgSO4, even a small amount of Mg2+ at 4 mg/L could boost cell growth significantly.
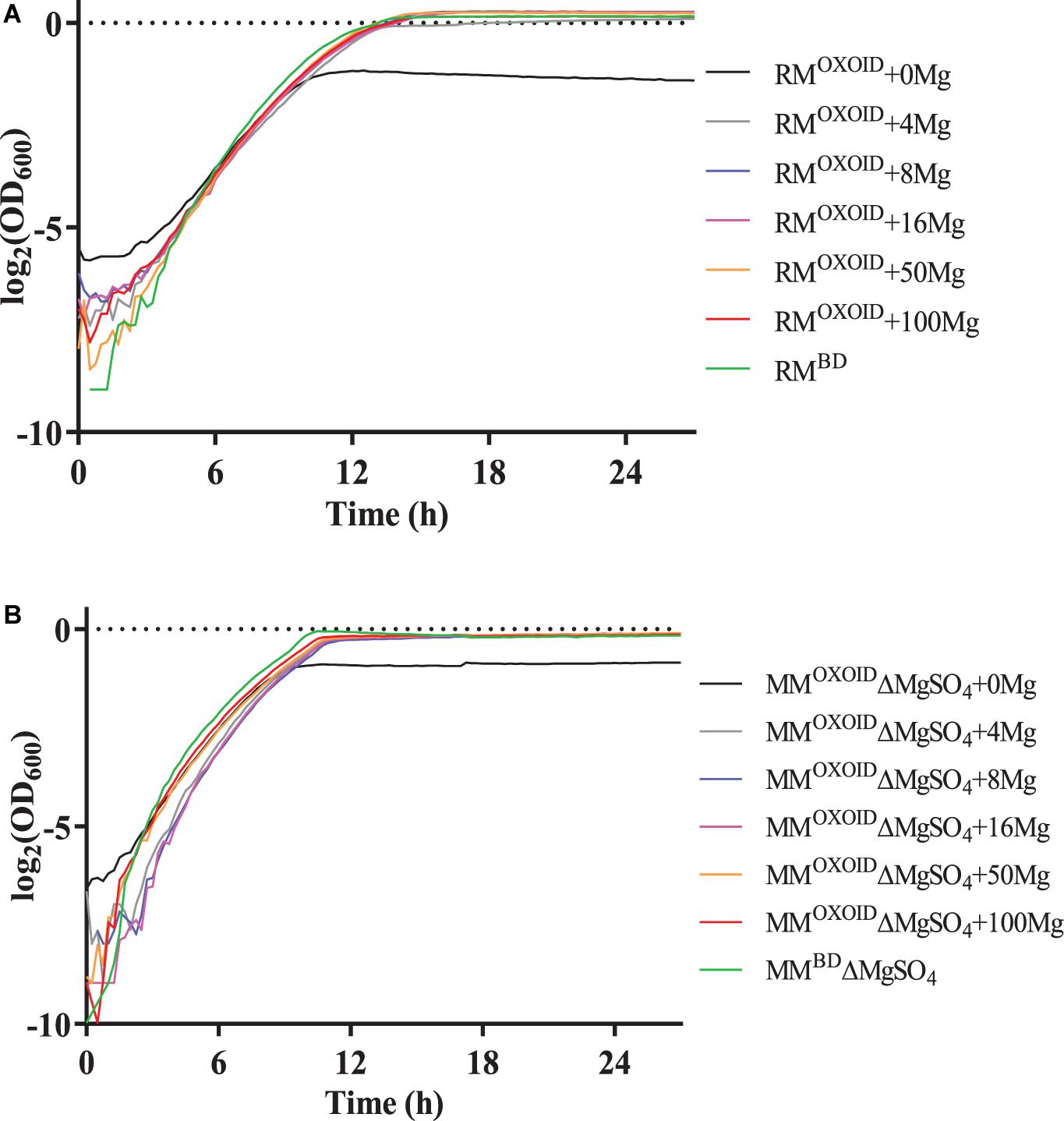
Figure 7. Cell growth of ZM4 in RMOXOID (A) or MMOXOID without MgSO4 (MMOXOIDΔMgSO4) (B) with Mg2+ supplemented at different concentrations of 0, 4, 8, 16, 50, and 100 mg/L. RMBD and MMBDΔMgSO4 were included as controls.
Cell growth, glucose utilization, and ethanol production were further investigated using the shake flask experiment with OD600 values, glucose and ethanol concentrations measured (Figure 8). Consistent with the result of Bioscreen C (Figure 7), the supplementation of at least 8 mg/L of Mg2+ in RMOXOID or MMOXOIDΔMgSO4 allowed ZM4 to grow as well as in RMBD or MMBDΔMgSO4. At the same time, cell growth, glucose consumption, and ethanol production of ZM4 in RMOXOID with 8 mg/L Mg2+ added (RMOXOID + 8Mg) were coupled as in RMBD (Figure 8). This suggests that Mg2+ is crucial for cell growth, glucose consumption and fermentation performance of Z. mobilis, and a minimum concentration of at least 8 mg/L is needed for optimal cell growth and ethanol fermentation.
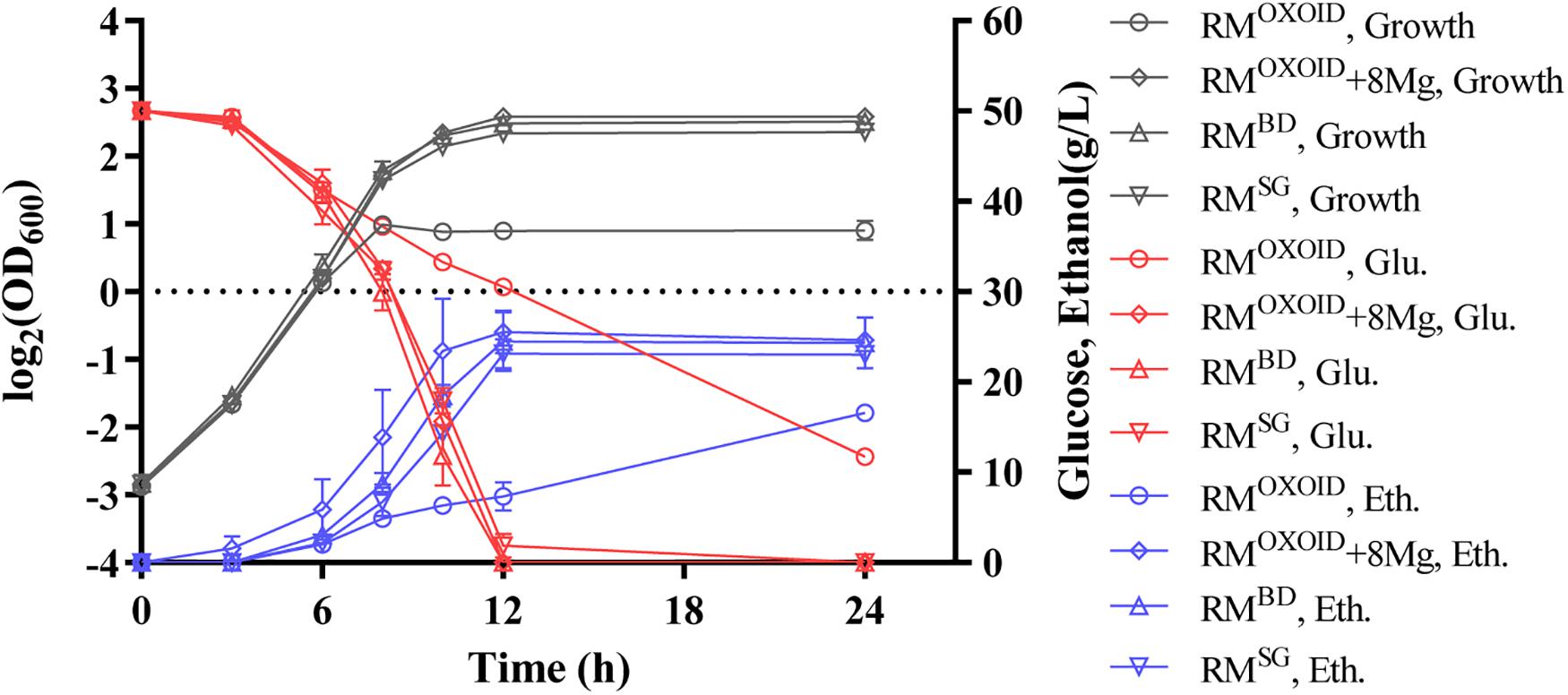
Figure 8. Cell growth, glucose (Glu) consumption, and ethanol (Eth) production of ZM4 in RMOXOID, RMOXOID + 8Mg, RMBD, and RMSG. At least two independent experiments were carried out with similar results. Values are the mean of one representative experiment with two or more technical replicates. Error bars represent standard deviations.
Effects of Mg2+ on Gene Expression of Z. mobilis
After confirmation of the impact of Mg2+ on cell growth and ethanol fermentation of Z. mobilis (Figure 8), next-generation sequencing (NGS)-based RNA-Seq transcriptomics was further applied to identify genes responsive for the uncoupling of cell growth, glucose consumption and ethanol fermentation due to the difference of Mg2+ concentration in the medium.
Despite that cell growth, glucose consumption and ethanol production exhibited apparent differences (Figure 8), RNA-Seq results showed that only a few genes were differentially expressed. There were seven and four genes down-regulated when Z. mobilis was cultured in RMOXOID and RMBD (RMOXOID/RMBD, Figure 9A and Table 3) as well as cultured in RMOXOID and RMOXOID + 8Mg (RMOXOID/RMOXOID + 8Mg, Figure 9B and Table 4), respectively. Although genes up-regulated in RMOXOID were more than those down-regulated (Figure 9 and Tables 3, 4), there were only 11 and 10 up-regulated genes identified when Z. mobilis was cultured in RMOXOID and RMBD (RMOXOID/RMBD, Figure 9A and Table 3) as well as cultured in RMOXOID and RMOXOID + 8Mg (RMOXOID/RMOXOID + 8Mg, Figure 9B and Table 4), respectively. Two and eight of these down-regulated and up-regulated ones are common between these two comparisons (Figure 9 and Tables 3, 4).
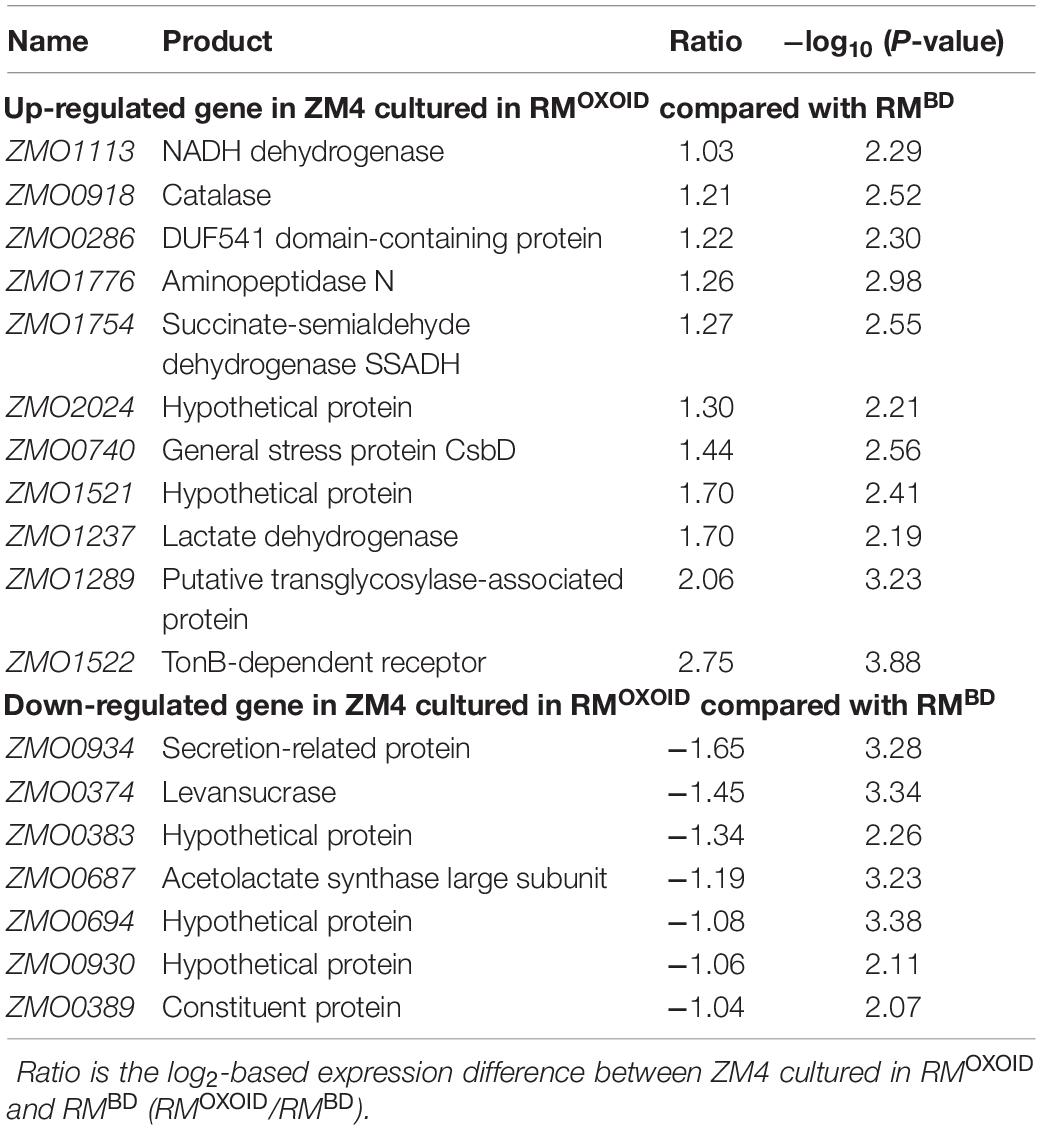
Table 3. List of significantly differentially expressed genes between ZM4 cultured in RMBD and RMOXOID.
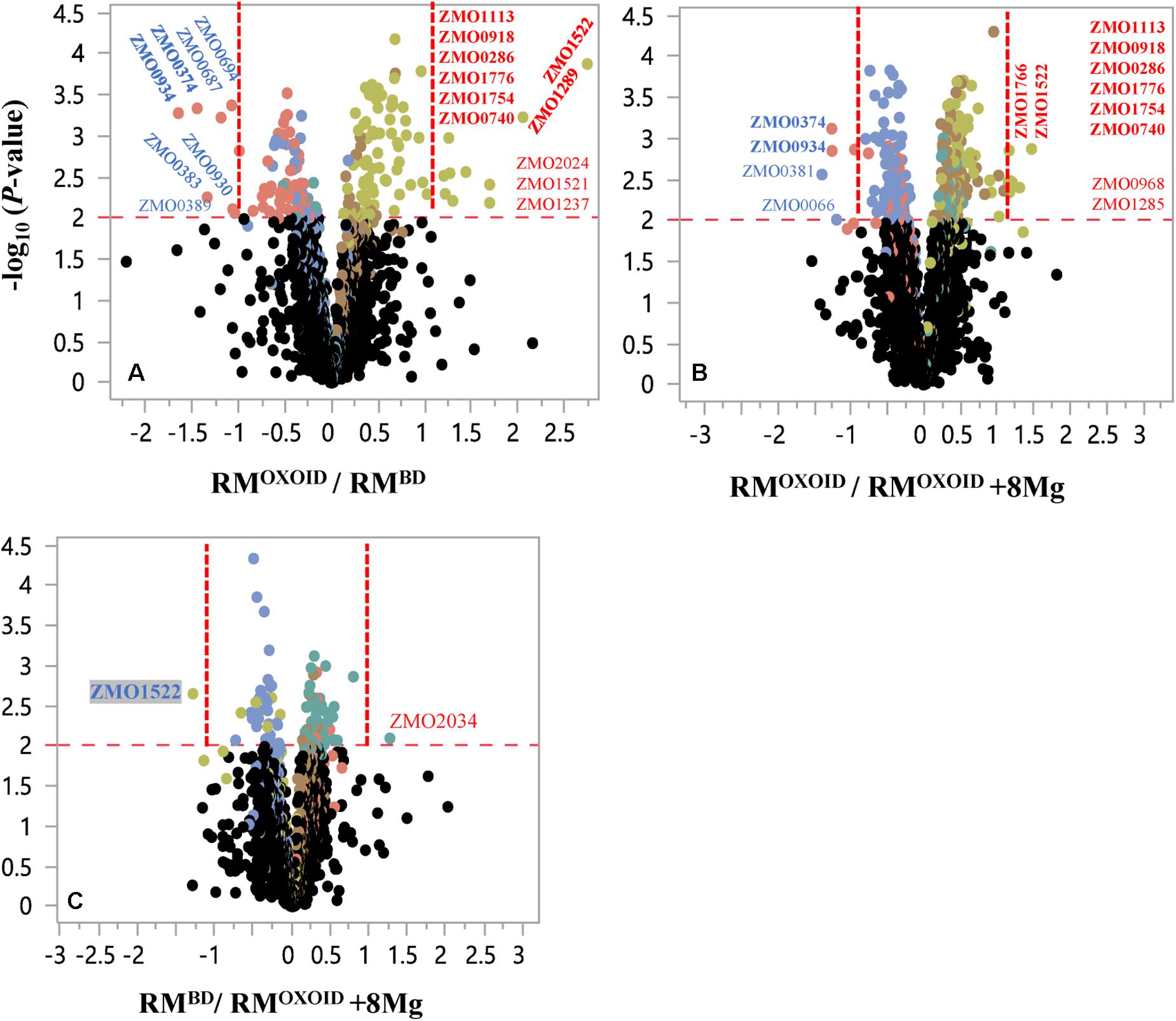
Figure 9. Volcano plots of significantly differentially expressed genes of Z. mobilis cultured in RMOXOID and RMBD (RMOXOID/RMBD, (A), RMOXOID and RMOXOID + 8Mg (RMOXOID/RMOXOID + 8Mg, (B), as well as RMBD and RMOXOID + 8Mg (RMBD/RMOXOID + 8Mg, (C). X-axis is the log2-based ratios between two conditions examined, and Y-axis is the -log10(P-value) of the difference. The dots above the horizontal red dash line indicate genes significantly differentially expressed, and the vertical red dash line indicate genes significantly differentially expressed with ratio greater than 2 (log2-based ratio greater than 1). Gene name with red and blue color font indicates up-regulated and down-regulated ones, respectively. Gene names with bold font indicate common ones between different comparisons of RMOXOID/RMBD, RMOXOID/RMOXOID + 8Mg, and RMBD/RMOXOID + 8Mg.
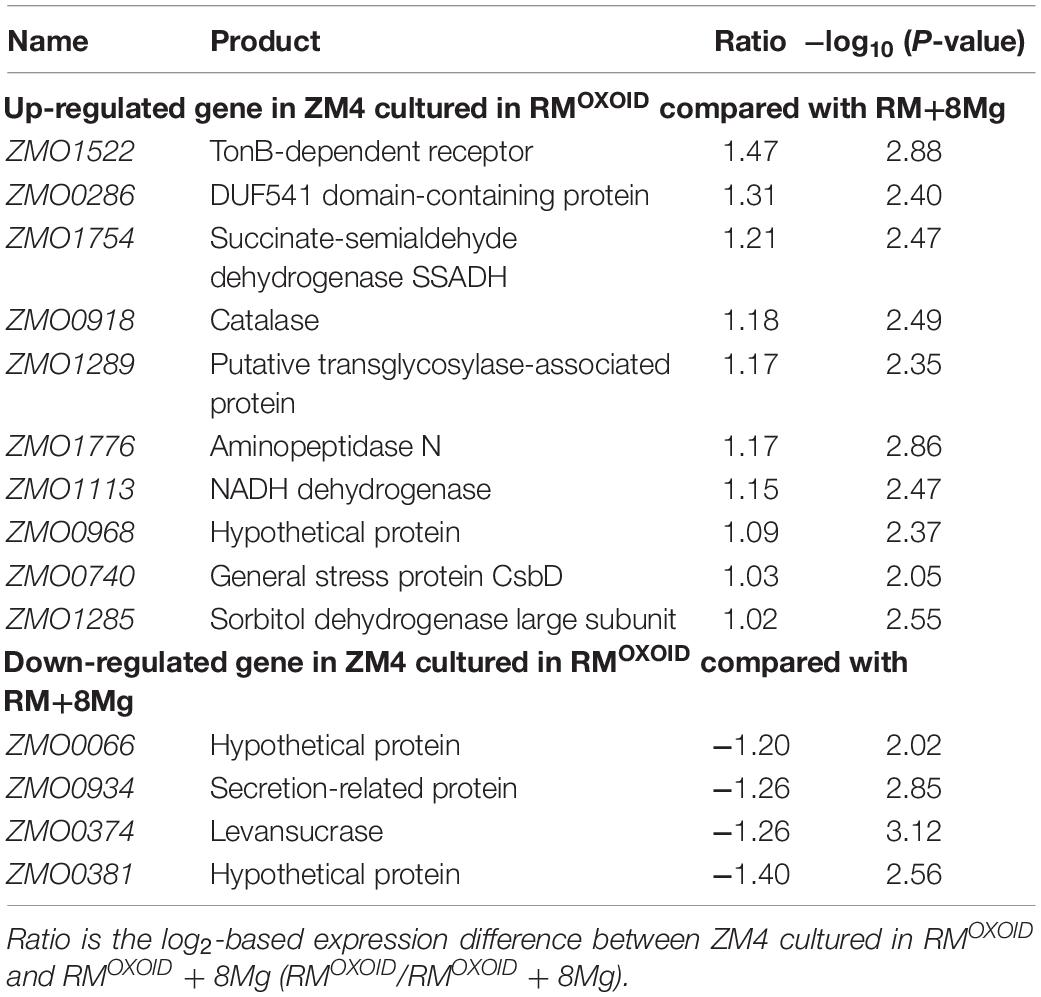
Table 4. List of significantly differentially expressed genes between ZM4 cultured in RMOXOID and RMOXOID + 8Mg.
Genes related to stress responses were up-regulated while genes related to protein secretion were down-regulated in RMOXOID medium compared with in RMOXOID + 8Mg or RMBD medium (Figure 9 and Tables 3, 4). For example, genes encoding catalase (ZMO0918), general stress protein CsbD (ZMO0740), NADH dehydrogenase (ZMO1113), and succinate-semialdehyde dehydrogenase SSADH (ZMO1754) were up-regulated when RMOXOID was used compared with those in RMOXOID + 8Mg or RMBD (Figure 9 and Tables 3, 4). These results indicate that Mg2+, a key element of cofactor, is essential for vigorous cell growth, and the lack of Mg2+ will trigger energy-consuming stress responses while slowing down energy-consuming metabolism with genes encoding levansucrase (ZMO0374) and secretion-related protein (ZMO0934) being down-regulated (Figure 9 and Tables 3, 4).
Considering that the supplementation of 8 mg/L Mg2+ into RMOXOID (RMOXOID + 8Mg) could restore the coupling of cell growth, glucose consumption and ethanol fermentation of Z. mobilis (Figure 8) and that there was only one gene up-regulated (ZMO2034) and one down-regulated (ZMO1522) when Z. mobilis grew in RMBD compared with RMOXOID + 8Mg (Figure 9C), the transcriptomics study further confirmed our hypothesis that the difference of Mg2+ concentrations in different nitrogen sources is one of the determinants affecting the coupling of cell growth, glucose consumption and ethanol fermentation in Z. mobilis.
Conclusion
The effects of nitrogen sources on cell growth, glucose consumption, and ethanol fermentation performance of Z. mobilis ZM4 were investigated to understand the uncoupling of cell growth, glucose consumption and ethanol fermentation of ZM4 in this study. Through the supplementation and replacement of inorganic or organic nitrogen sources in both RM and MM, we found that YEBD, YESG, or 5% CSL were better than YEOXOID. We also quantified the ion elements in different nitrogen sources using ICP-OES, and demonstrated that the difference of magnesium ion in YE is one of the major factors affecting cell growth and ethanol fermentation. We further verified the impact of Mg2+ on cell growth of ZM4 by supplementing various concentrations of Mg2+ into the medium, and used the RNA-Seq transcriptomics approach to identify genes responsive for the uncoupling of cell growth, glucose consumption and ethanol fermentation when the medium lacked Mg2+. These findings can be used as a reference for the selection and/or modification of nitrogen sources using Z. mobilis ZM4. The concentrations of ion elements in nitrogen sources affecting cell growth and fermentation performance can also be used as a parameter for optimizing and monitoring the components of nitrogen sources for efficient cell growth and fermentation using other microorganisms.
Materials and Methods
Bacterial Strain, Media, and Growth Conditions
Zymomonas mobilis ZM4 (ATCC 31821) was revived from frozen glycerol stocks in 10 mL RMOXOID (50 g/L glucose, 10 g/L YE, 2 g/L KH2PO4) at 30°C for about 6∼8 h prior to inoculating overnight seed cultures at 30°C at 100 rpm in RMOXOID using shake flasks with a sealing gas permeable membrane sealed filled to 80% capacity. Glucose, KH2PO4, K2HPO4, NaCl, MgSO4⋅7H2O, Na2MoO4⋅2H2O, and NH4Cl were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). YE was purchased from OXOID, Becton Dickinson, and Sangon Biotech (Shanghai) Co., Ltd. (Shanghai, China). Tryptone was purchased from OXOID. Peptone was purchased from Becton Dickinson. Corn steep liquid (CSL) was purchased from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China).
The recipes of different rich medium (RM) and minimal medium (MM) with different nitrogen sources used in this work were listed in Tables 5, 6, respectively.
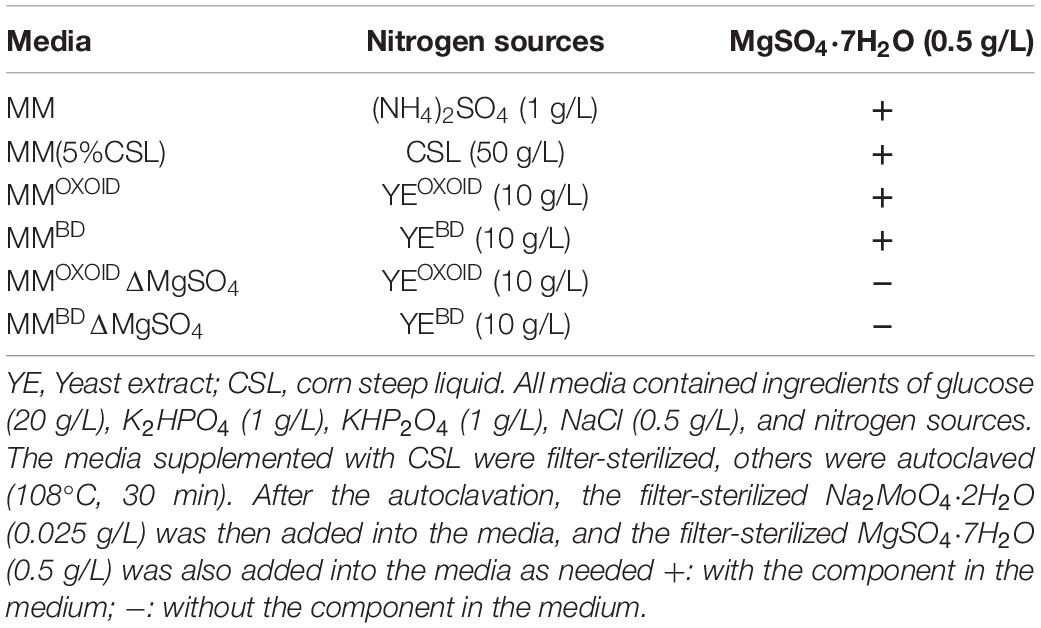
Table 6. Recipes of different minimal medium (MM) with different nitrogen sources and metal ions of Mg2+ and MoO42– used in this work.
Growth Test by Bioscreen C
The Bioscreen C automatic growth curve analyzer has the functions of culturing cells and measuring cell turbidity. It is similar to the plate reader, but can continuously measure the turbidity of cells, while maintaining a constant temperature and rotation speed to maintain normal cell growth.
The seed culture of Z. mobilis was centrifuged to remove RMOXOID. Cells were resuspended with test medium. Bioscreen C assays were carried out as described previously (Franden et al., 2009; Yang et al., 2010, 2014a,b, 2016b; Yang S. et al., 2018) except that cells were inoculated into Bioscreen C wells containing a total volume of 200 μL test medium at an initial OD600 value of 0.05 and incubated without shaking at 30°C. Triplicate were used for each condition, and turbidity measurements (OD600) were taken every 15 min till cells grew into stationary phase. At least two independent experiments were carried out with similar results. Values are the mean of one representative experiment with two or more technical replicates. Error bars represent standard deviations.
Flask Fermentation and Analytical Analysis
The seed culture of Z. mobilis was used to inoculate the shake flask containing 80% of test medium with a sealing gas permeable membrane sealed at an initial OD600 of 0.1, and cultured at 30°C, 100 rpm. At least two replicates were used for each condition.
The OD600 values of the bacterial culture was measured by UV-visible spectrophotometer UV-1800 (AoYi Instrument Co., Ltd., Shanghai, China) every 3 h. At the same time, 1-mL culture was centrifuged at 12,000 rpm for 1 min to obtain the supernatant for measuring the glucose and ethanol concentrations in the culture. Biosensor analyzer M-100 (Sieman Technology Co., Ltd., Shenzhen, China) was used for quick assessment of the concentrations of glucose and ethanol. The supernatant was also filtered through a 0.45 μm filter before applying on a Shimadzu LC-2030 high pressure liquid chromatography (HPLC) with refractive index detector (RID). Bio-Rad Aminex HPX-87H (300 × 7.8 mm) column was used to separate the fermentation products, and 0.005 M H2SO4 was used as the mobile phase at a flow rate of 0.5 mL/min. Temperatures of detector and column were 40 and 60°C, respectively. The measurement of glucose and ethanol by HPLC and Biosensor analyzer M-100 was compared, and the results showed that the measurement data of the two instruments on the same sample were close (data not shown). At least two independent experiments were carried out with similar results. Values are the mean of one representative experiment with two or more technical replicates. Error bars represent standard deviations.
One percent (w/v) of different organic nitrogen sources were prepared in ddH2O and then filter-sterilized. The concentrations of different ions in these samples were then measured by Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES, Wuhan SouSepad Testing Technology Co., Ltd., Wuhan, China).
Ethanol Production Calculations
Cell growth was monitored by optical density spectrophoto- metrically at 600 nm. The time points when cells reached stationary phase and when glucose was completely consumed with the maximum ethanol produced were recorded. The concentrations of glucose and ethanol determined by HPLC or Biosensor analyzer M-100 were then used for the calculation of the ethanol yield (Yp/s), theoretical ethanol yield (%Yp/s), ethanol productivity (QP), and specific growth rate (μ) using the formula below:
Yp/s = maximum ethanol (g)/consumed glucose (g);
%Yp/s = (ethanol(g)/(consumed sugar(g)*0.511))*100;
QP = maximum ethanol titer (g/L)/time to reach the maximum ethanol.
μ = ln((OD600 at t2)/(OD600 at t1))/(t2−t1), t1 and t2 are the time points in the log phase.
Transcriptomic Analysis
The transcriptomics study followed previous work (Franden et al., 2009; Zhao et al., 2009; Martinez-Moreno et al., 2012; Kemsawasd et al., 2015; Klotz et al., 2017; Yang S. et al., 2018; Lozano Terol et al., 2019). Briefly, cell culture samples were collected during the exponential phase with the OD600 values around 0.6–0.8. RNA-Seq was performed using paired-end sequencing technology according to standard Illumina protocols (Genewiz Inc., Suzhou, China). The quality of RNA-Seq fastq data was evaluated using FastQC software (Babraham Bioinformatics, United States). Data passing the quality control were imported into CLC Genomics Workbench (Ver. 11.0) for RNA-Seq analysis to get the RPKM value (reads mapping to the genome per kilobase of transcript per million reads sequenced) of each gene with the reference genome. Genome sequence of Z. mobilis was used as the reference for RPKM calculation (Yang et al., 2010; Martinez-Moreno et al., 2012). The RPKM value of each gene was then imported into JMP Genomics (Ver. 9.0); data normalization and statistical analysis were conducted to identify differentially expressed genes when three different media were used. Duplicate samples were used for each condition. RNA-Seq raw data were deposited at Sequence Read Archive (SRA) database with the Bioproject accession number of PRJNA601020.
Data Availability Statement
The RNA-Seq raw data was deposited at Sequence Read Archive (SRA) database with the BioProject accession number PRJNA601020.
Author Contributions
SY conceived and designed the experiments with inputs from RL, MJ, SC, ML, and JD. RL performed the experiments. RL and SY wrote the manuscript. MJ, ML, SC, and JD conducted the extensive manuscript review. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Key Research and Development Program of China (2018YFA0900300), the National Science Foundation of China (21978071 and U1932141), the Technical Innovation Special Fund of Hubei Province (2019AHB055 and 2018ACA149), and the Leading Innovative and Entrepreneur Team Introduction Program of Zhejiang Province (2018R01014). We also acknowledge the support from State Key Laboratory of Biocatalysis and Enzyme Engineering.
Conflict of Interest
JD was employed by China Biotech Fermentation Industry Association. ML was employed by Zhejiang Huakang Pharmaceutical Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer QG declared a past co-authorship with one of the authors SY to the handling editor.
Acknowledgments
This manuscript has been released as a pre-print at Research Square: RL, MJ, JD, SC, SY, Underlying Mechanism of Uncoupled Cell Growth and Ethanol Fermentation of Zymomonas mobilis using Different Nitrogen Sources, doi: 10.21203/rs.2.21812/v1. We appreciate the reviewers for their valuable advice.
Abbreviations
CSL, corn steep liquid; ED, Entner-Doudoroff; EMP, Embden-Meyerhof-Parnas; GRAS, generally regarded as safe status; MM, minimum medium; ICP-OES, Inductively Coupled Plasma Optical Emission Spectroscopy; P, peptone; RM, rich medium; RMBD, yeast extract in rich medium was from company Becton Dickinson (BD); RMOXOID, yeast extract in rich medium was from company OXOID; RPKM, reads mapping to the genome per kilobase of transcript per million reads sequenced; T, tryptone; TRY, titer, rate and yield; YE, yeast extract.
References
Christensen, D. G., Orr, J. S., Rao, C. V., and Wolfe, A. J. (2017). Increasing growth yield and decreasing acetylation in Escherichia coli by optimizing the carbon-to-magnesium ratio in peptide-based media. Appl. Environ. Microbiol. 83:AEM.03034-16. doi: 10.1128/AEM.03034-16
Conway, T. (1992). The entner-doudoroff pathway. history, physiology and molecular biology. FEMS Microbiol. Rev. 9, 1–27.
Cot, M., Loret, M. O., Francois, J., and Benbadis, L. (2007). Physiological behaviour of Saccharomyces cerevisiae in aerated fed-batch fermentation for high level production of bioethanol. FEMS Yeast Res. 7, 22–32. doi: 10.1111/j.1567-1364.2006.00152.x
Dien, B. S., Cotta, M. A., and Jeffries, T. W. (2003). Bacteria engineered for fuel ethanol production. current status. Appl. Microbiol. Biotechnol. 63, 258–266. doi: 10.1007/s00253-003-1444-y
Duan, G., Wu, B., Qin, H., Wang, W., Tan, Q., Dai, Y., et al. (2019). Replacing water and nutrients for ethanol production by ARTP derived biogas slurry tolerant Zymomonas mobilis strain. Biotechnol. Biofuels 12:124.
Flamholz, A., Noor, E., Bar-Even, A., Liebermeister, W., and Milo, R. (2013). Glycolytic strategy as a tradeoff between energy yield and protein cost. Proc. Natl. Acad. Sci. U.S.A. 110, 10039–10044. doi: 10.1073/pnas.1215283110
Franden, M. A., Pienkos, P. T., and Zhang, M. (2009). Development of a high-throughput method to evaluate the impact of inhibitory compounds from lignocellulosic hydrolysates on the growth of Zymomonas mobilis. J. Biotechnol. 144, 259–267. doi: 10.1016/j.jbiotec.2009.08.006
He, M. X., Wu, B., Qin, H., Ruan, Z. Y., Tan, F. R., Wang, J. L., et al. (2014). Zymomonas mobilis. a novel platform for future biorefineries. Biotechnol. Biofuels 7:101. doi: 10.1186/1754-6834-7-101
He, M. X., Wu, B., Shui, Z. X., Hu, Q. C., Wang, W. G., Tan, F. R., et al. (2012). Transcriptome profiling of Zymomonas mobilis under ethanol stress. Biotechnol. Biofuels 5:75. doi: 10.1186/1754-6834-5-75
Ismail, K. S., Sakamoto, T., Hasunuma, T., Zhao, X. Q., and Kondo, A. (2014). Zinc, magnesium, and calcium ion supplementation confers tolerance to acetic acid stress in industrial Saccharomyces cerevisiae utilizing xylose. Biotechnol. J. 9, 1519–1525. doi: 10.1002/biot.201300553
Jacobson, T. B., Adamczyk, P. A., Stevenson, D. M., Regner, M., Ralph, J., Reed, J. L., et al. (2019). 2H and 13C metabolic flux analysis elucidates in vivo thermodynamics of the ED pathway in Zymomonas mobilis. Metab. Eng. 54, 301–316. doi: 10.1016/j.ymben.2019.05.006
Jones, C. W., and Doelle, H. W. (1991). Kinetic control of ethanol production by Zymomonas mobilis. Appl. Microbiol. Biotechnol. 35, 4–9.
Ju, N. H., Damiano, D., Shin, C. S., Kim, N. K., and Wang, S. S. (1983). Continuous ethanol fermentation of Zymomonas mobilis using soy flour as a protective agent. Biotechnol. Lett. 5, 837–842. doi: 10.1007/bf01386658
Kemsawasd, V., Viana, T., Ardo, Y., and Arneborg, N. (2015). Influence of nitrogen sources on growth and fermentation performance of different wine yeast species during alcoholic fermentation. Appl. Microbiol. Biotechnol. 99, 10191–10207. doi: 10.1007/s00253-015-6835-3
Kingston, R. L., Scopes, R. K., and Baker, E. N. (1996). The structure of glucose-fructose oxidoreductase from Zymomonas mobilis. an osmoprotective periplasmic enzyme containing non-dissociable NADP. Structure 4, 1413–1428. doi: 10.1016/s0969-2126(96)00149-9
Klotz, S., Kuenz, A., and Prüße, U. (2017). Nutritional requirements and the impact of yeast extract on the d-lactic acid production by Sporolactobacillus inulinus. Green Chem. 19, 4633–4641. doi: 10.1039/c7gc01796k
Kosaka, T., Nishioka, A., Sakurada, T., Miura, K., Anggarini, S., and Yamada, M. (2020). Enhancement of thermal resistance by metal ions in thermotolerant Zymomonas mobilis TISTR 548. Front. Microbiol. 11:502. doi: 10.3389/fmicb.2020.00502
Kremer, T. A., LaSarre, B., Posto, A. L., and McKinlay, J. B. (2015). N2 gas is an effective fertilizer for bioethanol production by Zymomonas mobilis. Proc. Natl. Acad. Sci. U.S.A. 112, 2222–2226. doi: 10.1073/pnas.1420663112
Lawford, H. G., and Rousseau, J. D. (1997). Corn steep liquor as a cost-effective nutrition adjunct in high-performance Zymomonas ethanol fermentations. Appl. Biochem. Biotechnol. 6, 287–304. doi: 10.1007/978-1-4612-2312-2_25
Lozano Terol, G., Gallego-Jara, J., Sola Martínez, R. A., Cánovas Díaz, M., and de Diego Puente, T. (2019). Engineering protein production by rationally choosing a carbon and nitrogen source using E. coli BL21 acetate metabolism knockout strains. Microbial. Cell Factories 18:151.
Martinez-Moreno, R., Morales, P., Gonzalez, R., Mas, A., and Beltran, G. (2012). Biomass production and alcoholic fermentation performance of Saccharomyces cerevisiae as a function of nitrogen source. FEMS Yeast Res. 12, 477–485. doi: 10.1111/j.1567-1364.2012.00802.x
Pagliardini, J., Hubmann, G., Alfenore, S., Nevoigt, E., Bideaux, C., and Guillouet, S. E. (2013). The metabolic costs of improving ethanol yield by reducing glycerol formation capacity under anaerobic conditions in Saccharomyces cerevisiae. Microb. Cell Fact. 12:29. doi: 10.1186/1475-2859-12-29
Panesar, P. S., Marwaha, S. S., and Kennedy, J. F. (2006). Zymomonas mobilis. an alternative ethanol producer. J. Chem. Technol. Biotechnol. 81, 623–635. doi: 10.1002/jctb.1448
Rogers, P. L., Goodman, A. E., and Heyes, R. H. (1984). Zymomonas ethanol fermentations. Microbiol. Sci. 1, 133–136.
Rogers, P. L., Jeon, Y. J., Lee, K. J., and Lawford, H. G. (2007). Zymomonas mobilis for fuel ethanol and higher value products. Adv. Biochem. Eng. Biotechnol. 108, 263–288. doi: 10.1007/10_2007_060
Wang, X., He, Q., Yang, Y., Wang, J., Haning, K., Hu, Y., et al. (2018). Advances and prospects in metabolic engineering of Zymomonas mobilis. Metab. Eng. 50, 57–73. doi: 10.1016/j.ymben.2018.04.001
Xia, J., Liu, C. G., Zhao, X. Q., Xiao, Y., Xia, X. X., and Bai, F. W. (2018). Contribution of cellulose synthesis, formation of fibrils and their entanglement to the self-flocculation of Zymomonas mobilis. Biotechnol. Bioeng. 115, 2714–2725. doi: 10.1002/bit.26806
Xue, C., Zhao, X. Q., and Bai, F. W. (2010). Effect of the size of yeast flocs and zinc supplementation on continuous ethanol fermentation performance and metabolic flux distribution under very high concentration conditions. Biotechnol. Bioeng. 105, 935–944.
Yang, S., Fei, Q., Zhang, Y., Contreras, L. M., Utturkar, S. M., Brown, S. D., et al. (2016a). Zymomonas mobilis as a model system for production of biofuels and biochemicals. Microb. Biotechnol. 9, 699–717. doi: 10.1111/1751-7915.12408
Yang, S., Mohagheghi, A., Franden, M. A., Chou, Y. C., Chen, X., Dowe, N., et al. (2016b). Metabolic engineering of Zymomonas mobilis for 2,3-butanediol production from lignocellulosic biomass sugars. Biotechnol. Biofuels 9:189.
Yang, S., Franden, M. A., Brown, S. D., Chou, Y. C., Pienkos, P. T., and Zhang, M. (2014a). Insights into acetate toxicity in Zymomonas mobilis 8b using different substrates. Biotechnol. Biofuels 7:140.
Yang, S., Pan, C., Hurst, G. B., Dice, L., Davison, B. H., and Brown, S. D. (2014b). Elucidation of Zymomonas mobilis physiology and stress responses by quantitative proteomics and transcriptomics. Front. Microbiol. 5:246. doi: 10.3389/fmicb.2014.00246
Yang, S., Franden, M. A., Yang, Q., Chou, Y. C., Zhang, M., and Pienkos, P. T. (2018). Identification of inhibitors in lignocellulosic slurries and determination of their effect on hydrocarbon-producing microorganisms. Front. Bioeng. Biotechnol. 6:23. doi: 10.3389/fbioe.2018.00023
Yang, S., Land, M. L., Klingeman, D. M., Pelletier, D. A., Lu, T. Y., Martin, S. L., et al. (2010). Paradigm for industrial strain improvement identifies sodium acetate tolerance loci in Zymomonas mobilis and Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U.S.A. 107, 10395–10400. doi: 10.1073/pnas.0914506107
Yang, Y., Hu, M., Tang, Y., Geng, B., Qiu, M., He, Q., et al. (2018). Progress and perspective on lignocellulosic hydrolysate inhibitor tolerance improvement in Zymomonas mobilis. Bioresour. Bioprocess. 5:6.
Zarei, O., Dastmalchi, S., and Hamzeh-Mivehroud, M. (2016). A simple and rapid protocol for producing yeast extract from Saccharomyces cerevisiae suitable for preparing bacterial culture media. Iran J. Pharm. Res. 15, 907–913.
Zhao, X. Q., and Bai, F. W. (2012). Zinc and yeast stress tolerance. micronutrient plays a big role. J. Biotechnol. 158, 176–183. doi: 10.1016/j.jbiotec.2011.06.038
Keywords: Zymomonas mobilis, ethanol fermentation, nitrogen sources, yeast extract, magnesium, RNA-Seq, stress responses
Citation: Li R, Jin M, Du J, Li M, Chen S and Yang S (2020) The Magnesium Concentration in Yeast Extracts Is a Major Determinant Affecting Ethanol Fermentation Performance of Zymomonas mobilis. Front. Bioeng. Biotechnol. 8:957. doi: 10.3389/fbioe.2020.00957
Received: 10 May 2020; Accepted: 23 July 2020;
Published: 31 August 2020.
Edited by:
Maizirwan Mel, International Islamic University Malaysia, MalaysiaReviewed by:
Pornthap Thanonkeo, Khon Kaen University, ThailandBing-Zhi Li, Tianjin University, China
Qiuqiang Gao, Columbia University, United States
Copyright © 2020 Li, Jin, Du, Li, Chen and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shihui Yang, U2hpaHVpLllhbmdAaHVidS5lZHUuY24=; c2hoeW91bmdAaG90bWFpbC5jb20=
 Runxia Li
Runxia Li Mingjie Jin
Mingjie Jin Jun Du3
Jun Du3 Mian Li
Mian Li Shouwen Chen
Shouwen Chen Shihui Yang
Shihui Yang