- 1Department of Oral Implantology, School and Hospital of Stomatology, Jilin University, Changchun, China
- 2Jilin Provincial Key Laboratory of Sciences and Technology for Stomatology Nanoengineering, Changchun, China
- 3Department of Periodontology, School and Hospital of Stomatology, Jilin University, Changchun, China
- 4Department of Prosthodontics, School and Hospital of Stomatology, Jilin University, Changchun, China
- 5State Key Laboratory on Integrated Optoelectronics, College of Electronic Science and Engineering, Jilin University, Changchun, China
Nowadays, the heavy burden of oral diseases such as dental caries, periodontitis, endodontic infections, etc., and their consequences on the patients’ quality of life indicate a strong need for developing effective therapies. Bacterial infections played an important role in the field of oral diseases, in-depth insight of such oral diseases have given rise to the demand for antibacterial therapeutic strategies. Recently, microporous frameworks have attracted tremendous interest in antibacterial application due to their well-defined porous structures for drug delivery. In addition, intensive efforts have been made to enhance the antibacterial performance of microporous frameworks, such as ion doping, photosensitizer incorporation as building blocks, and surface modifications. This review article aims on the major recent developments of microporous frameworks for antibacterial applications against oral diseases. The first part of this paper puts concentration on the cutting-edge researches on the versatile antibacterial strategies of microporous materials via drug delivery, inherent activity, and structural modification. The second part discusses the antibacterial applications of microporous frameworks against oral diseases. The applications of microporous frameworks not only have promising therapeutic potential to inhibit bacterial plaque-initiated oral infectious diseases, but also have a wide applicability to other biomedical applications.
Introduction
Oral diseases such as caries, periodontitis, and endodontic infections, attracted tremendous attentions all over the world since such diseases account for a vast burden of morbidity and healthcare spending. For example, an authoritative report claimed that dental caries and periodontitis were the first and 11th most prevalent causes of disease worldwide in 2016 (Vos et al., 2017), which was 5.4 million as a result of severe periodontitis, 4.6 million as a result of untreated caries in permanent teeth (Papapanou and Susin, 2017). The estimated costs of totaling worldwide in dental infectious diseases amounted to more than $540 billion dollars in 2015 (Righolt et al., 2018). Besides local destruction of teeth and supporting tissues, these bacterial-related oral diseases were closely related with pulmonary disease (Manger et al., 2017), cardiovascular disease (Sanz et al., 2020), and gastrointestinal cancer (Chen et al., 2019). Antibiotics are usually applied as major or auxiliary approach in treatment of such oral infectious diseases. Currently, the growing resistance of microorganisms to conventional antibiotics has become a public health problem and raises highly demand to look for more effective solutions (Wyszogrodzka et al., 2016). Amounts of antibacterial agents such as metallic ions and natural extracts were alternatively applied in the antibacterial field (Li et al., 2018). However, the inferior biocompatibility, short half-time period, and unexpected cytotoxicity limit the potential applications for these materials.
Nowadays, there has been a continuous and fast-paced emergence of new synthetic porous nanomaterials developed to meet pharmacological and biological requirements for antibacterial application over the past several decades. Thereinto, microporous solid is a category of nanomaterial with pore < 2 nm in diameter. On the basis of framework composition, there are three types of crystalline porous solids: hybrid inorganic–organic hybrids [for example, metal-organic frameworks (MOFs)], inorganic (for example, zeolites), and organic frameworks [for example, covalent organic frameworks (COFs)] (Xue et al., 2016). The well-ordered microporous structure of these frameworks was taken advantage in many applications in different fields, such as catalysts, sensors, purification, etc. (Wang et al., 2011; Beale et al., 2015; Zhang et al., 2018; Cui et al., 2019). With respect to antibacterial application, the porous feature also gives rise to the ability to serve as a carrier for drug and biological molecule delivery, since well-defined pores can provide the opportunity to store active antibacterial agents and then release them at the appropriate time and at the correct rate (Rimoli et al., 2008). Besides drug delivery, these microporous frameworks could also exert antibacterial effects via their own degradation along with the release of metal ions, ligands into saliva, gingival crevicular fluid, and other body liquid (Li X. et al., 2019). Furthermore, several promising molecules have potential as functional building blocks in COFs due to their planar geometry and rigidity coupled with inherent functionalities, such as photosensitizing and redox-active properties (Liu et al., 2017; Hynek et al., 2018). Upon external light radiation, the COFs with these active organic building blocks would be activated to generate reactive oxygen species (ROS) to exert photodynamic inactivation against microorganisms. The microporous structure could carry more oxygen to amplify the photodynamic inactivation efficacy (Restrepo et al., 2017).
Currently, several valuable reviews on microporous frameworks have described on their materials design (Mori et al., 2004; Zhao et al., 2018), processing approaches (Valtchev et al., 2013; Xue et al., 2016), catalysis (Beale et al., 2015), and purification (Krishna, 2018; Prasetya et al., 2019), which will not be repeated here. As shown in Figure 1, this article reviews the major new developments on microporous frameworks as promising platforms for antibacterial strategies including inherent property, drug delivery, and different modifications, particularly focusing on potential application against oral diseases.
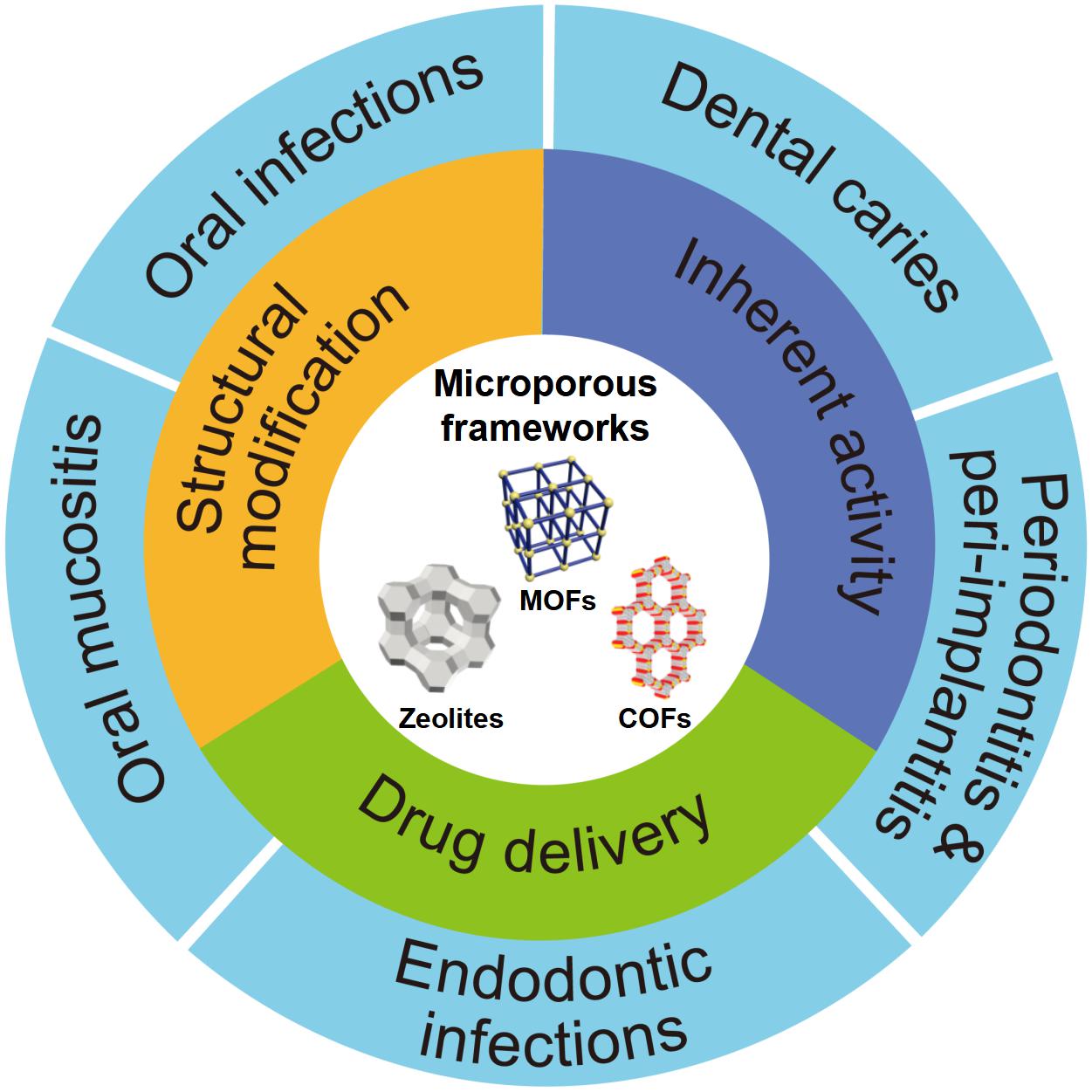
Figure 1. Schematic diagram of the application of microporous frameworks in dentistry. Notes: The microporous frameworks had potential to treat many oral diseases including dental caries, periodontitis, peri-implantitis, oral mucositis, and other oral infections. Abbreviations: COFs, covalent organic frameworks; MOFs, metal organic frameworks.
Microporous Materials With Different Antibacterial Strategies
The growing resistance of pathogens to antibiotic therapy has become a matter of concern. Because of the widespread use of antibiotics, major pathogens of infections such as Staphylococcus aureus are gradually developing drug resistance, leading to “super” infections. Hence, it is an urgent need to develop new and effective bactericidal agents to combat these drug-resistant microbes (Hughes, 2003). Therefore, developing antimicrobial nanomaterials is one of the most attractive approaches for eliminating the major perniciousness of pathogenic bacteria (Zhang Y. et al., 2019). Recently, the microporous materials have shown a promising perspective as an alternative method to combat the drug-resistant microbes by the following methods: (1) serving as drug reservoirs (Horcajada et al., 2010; Amorim et al., 2012); (2) acting as antimicrobial agents by degrading and releasing metal ions (Wyszogrodzka et al., 2016); and (3) turning into antibacterial drugs through different modifications (e.g., metal ions doping for bactericidal performance enhancement) (Li X. et al., 2019).
Nanomaterials With Inherent Antibacterial Activity
Metal-organic frameworks, also known as porous coordination polymers, are a category of hybrid materials comprising metal ions/clusters linked by polydentate bridging ligands typically under mild conditions, bonding types of which include metal coordination, hydrogen bonding, electrostatic interactions, and p–p stacking (Carné et al., 2011; Della Rocca et al., 2011). Compared to conventional nanomedicines, nanoscale MOFs have greater advantages in the aspect of structural and chemical diversity, high loading capacity, and biodegradability (Wyszogrodzka et al., 2016). It was reported that the most probable mechanism of the inherent antibacterial effect for MOFs was the structural degradation, along with the release of metal ions and ligands (Berchel et al., 2011; Tamames-Tabar et al., 2015). A previous study demonstrated that Ag-based MOF, acting as a reservoir of bactericidal metal ions, was able to release Ag+ into solution steadily and subsequently and had the bactericidal effect against S. aureus, Escherichia coli, and Pseudomonas aeruginosa (Berchel et al., 2011). When contacting with Ag+, the bacterial envelopes were seriously destroyed, following by the loss of the cellular cohesion, ultimately leading to the bacterial cytoplasm drained and the bacteria dead (Lu et al., 2014). Besides Ag+-based MOFs, copper ion is also well−known as its antibacterial effect since it could induce damage to the outer membranes of bacteria (Santo et al., 2012). A copper-based MOF Cu3(BTC)2 (BTC = 1,3,5-benzenetricarboxylate), also known as CuBTC and HKUST-1 could be synthesized via an ultrasonic method at ambient temperature and atmospheric pressure (Li et al., 2009). The deposition of CuBTC on silk fibers by layer-by-layer technique exhibited a strong inhibitory activity against Gram-negative bacteria E. coli and Gram-positive bacteria S. aureus (Abbasi et al., 2012). It was also reported that CuBTC exerted a favorable antifungal capability against Saccharomyces cerevisiae (completely inhibition) and Geotrichum candidum (reduction from 6.16 to 1.29 CFU/mL) (Chiericatti et al., 2012). A recent study also indicated an acceptable antibacterial activity of manganese-based MOF (UoB-4) against both Gram-positive and Gram-negative bacteria (Aryanejad et al., 2020). Moreover, zeolitic imidazolate frameworks (ZIFs), a sub-family of MOFs, are constructed from transition metal ions (Zn2+, Co2+, etc.) and imidazolate linkers, the structures of which are similar to zeolite topologies (Aguado et al., 2014). ZIF-8 nanocomposite coatings showed excellent antibacterial activity against Gram−negative E. coli (Miao et al., 2018). Unlike silver-based and copper-based MOFs, the antibacterial mechanism of zinc-based ZIF-8 is the generation of ROS speeding up the inflammatory response (Li X. et al., 2019). In addition, MIL-100(Fe), MIL-88B, and MOF-53(Fe) are the representative iron-based MOFs nanoparticles. MOF-53(Fe) nanoparticles were composed with ferric ion clusters and ligands of the terephthalic acid. Lin S. et al. (2017) discovered the bactericidal viability of the group MOF-53(Fe) with concentrations in the range of 20–160 μg/mL was increasing against S. aureus comparing with that of the control group. Comparably, two cobalt-based MOF (ZIF-67 and Co-SIM-1) also exhibited bactericidal effects against Pseudomonas putida and E. coli with over 50% growth inhibition at the concentrations in the 5–10 mg/mL range (Aguado et al., 2014). Similarly, Zhuang et al. (2012) synthesized a novel cobalt-based MOF (Co-TDM) showing the strong killing effect on E. coli with minimal bactericidal concentration ranging from 10 to 15 mg/L. Although membrane damage was stated as the major reason, the following mechanism is comprehensively applicable for bacterial inactivation: (1) diffusion-directed lipid-oxidation, (2) cation transport interruption, (3) direct interaction, (4) ROS generation, (5) chelation effects, and (6) membrane depolarization (Zhuang et al., 2012). Several organic chemicals also possess antibacterial activity and could serve as ligands for MOF synthesis. Tamames-Tabar et al. (2015) formulated a bioactive BioMIL−5, synthesized from a Zn2+ salt and azelaic acid, both with interesting biocide properties. Afterward, Restrepo et al. (2017) also developed a zinc-based MOF with hydrazinebenzoate linkers. This novel MOF inhibited bacterial growth with a minimal bactericidal concentration of 20 μg/mL, which was mainly attributed to the release of 4-hydrazinebenzoate linker (Restrepo et al., 2017).
Zeolites, consisting of TO4 (T = Si and Al) tetrahedra linked to each other by oxygen atoms, are crystalline aluminosilicate materials with a three-dimensional microporous structure containing uniformly distributed channels (Fakin et al., 2015). According to the number of T-atoms in the ring, zeolites are conventionally classified into small pore opening (eight-membered ring), medium (10-membered ring), and large one (12-membered ring), the pore diameters of which range from 0.5 to 2.0 nm (McCusker and Baerlocher, 2005). In addition to natural sources, zeolites can be synthesized by sol–gel (Zhang et al., 2015), hydrothermal (Abdullahi et al., 2017), and microwave methods (Boosari et al., 2018). At present, zeolites are widely used in the field of biomedicine, which are able to act as antibacterial materials and drug carriers (Amorim et al., 2012; Ferreira et al., 2012). Zeolite exerts antibacterial effect mainly via an antibacterial ion-exchanging process, while zeolite per se does not possess any antibacterial activity. However, antibacterial and anti-adhesive zeolite coating was developed on titanium alloy surface by 2% Ag+ exchange. Interestingly, the bacteria adhered on zeolite-Ti was also remarkably reduced compared with the Ti surface without zeolites, indicating that non-silver containing zeolite coating possessed high hydrophilicity to donate Ti surface with certain antibacterial and antifouling properties (Wang et al., 2011).
Covalent organic frameworks, as novel crystalline porous organic compounds, consist of light atoms, like H, B, C, N, and O, through dynamic covalent bonds with periodic skeletons and ordered nanopores (Xue et al., 2016; Zhao et al., 2018). Furthermore, inherent properties, such as large accessible pore size, specific surface area, channel type-ordered structure, low density, crystallinity, and high thermal stability, can provide a unique advantage over MOFs (Bhanja et al., 2017). The feature of structural variability is beneficial to wide application in different fields through the design of holes and skeletons, such as semiconduction, photoconductor, gas adsorption and storage, diagnoses, and treatment (Wan et al., 2008, 2009; Fang et al., 2015; Huang et al., 2016; Mitra et al., 2017; Zhu and Zhang, 2017). Recently, nanomaterial-based antibacterial photodynamic therapy gradually gained increasing attention (Qi et al., 2019). The antibacterial photodynamic therapy bases on the interaction of harmless nanosized-photosensitizers, tissue oxygen, and visible light to yield high level of ROS, which has a strong oxidation and high reactivity, thus causing rapid lipid oxidation of the bacteria. COFs are promising for serving asnanosized-photosensitizers for antibacterial photodynamic therapy since traditional photosensitizers such as porphyrins are potential functional building blocks in COF structures. Before the year of 2017, the COF-based antibacterial photodynamic therapy function was well-reviewed in a previous paper by Li and Yang (2017), which were not repeated in the present article. In later 2017, Liu et al. (2017) fabricated two COFs (COF-SDU1 and COFs-Trif-Benz) by covalent linking benzidine or p-phenylenediamine in the structure. Both COF frameworks showed excellent photocatalytic antibacterial activity against S. aureus and E. coli via singlet oxygen (1O2) generation by visible light irradiation. A pioneering work by Hynek et al. (2018) designed and synthesized porphyrin-based COFs by Schiff-base chemistry. These porphyrinic COFs with high photostability and broad spectral efficiency exerted strong antibacterial effects toward P. aeruginosa and Enterococcus faecalis biofilms upon visible light radiation (460 or 525 nm).
Microporous Frameworks for Antimicrobial Drug Delivery
Besides inherent antimicrobial functions, the porous feature of microporous frameworks also gives rise to the ability to serve as carriers for drug and biological molecule delivery. To meet pharmacological and biological requirements, microporous materials serving as nanocarriers for antibacterial application need to possess following important properties: (1) well-control and release behavior without avoid initial burst, (2) high drug loading capability, (3) modifiable surface for targeted therapy, and (5) no cytotoxicity.
Metal-Organic Frameworks
In addition to their inherent antibacterial effects, MOFs have been broadly applied in the field of drug delivery due to their adjustable aperture, large surface area, large pore capacity, and easy modification (Horcajada et al., 2010; Simon-Yarza et al., 2018). MOFs were able to encapsulate antibacterial substances (such as antibiotics, metals and metal oxides, plant natural products, and nitric oxide) in their constructions, via the non-covalent connection between metal open sites in their structures and antibacterial agents (Guerra et al., 2016). Subsequently, the release of drug can be effectively controlled through fine-tuning of MOFs porosity, biodegradability, and external stimulus conditions, such as light, pH, etc. (Li et al., 1999; Cai et al., 2019; Liu Y. et al., 2019). In addition, the degradation of MOFs also leads to the release of metal ions, which exerts a synergistic antibacterial effect.
Since pure antibiotics are difficult to cross cell membranes and maintain effective antibacterial concentrations for long periods of time (Brown and Wright, 2016), MOFs have attracted much more attentions as antibiotic carriers. In a previous study, the antibiotics such as tetracycline hydrochloride and doxycycline monohydrate were encapsulated in iron-based MOF (nano-MIL-100), showing excellent controlled release behavior (Taherzade et al., 2017). Furthermore, a new method of using photo-responsive ZIF-8 as drug carriers for rifampicin has been reported (Song et al., 2018). A pH-jump reagent (2−nitrobenzaldehyde) was modified into the porous structure of ZIF-8 as a gatekeeper, allowing the UV-light (365 nm) responsive in situ production of acid, which subsequently induced pH-dependent degradation of ZIF-8 and promoted the release of the antibiotic loaded in the pores in a controlled manner (Song et al., 2018). The combination of the UV-light, the pH-triggered precise antibiotic release, and the zinc ions enabled the light-activated nanocomposite to significantly inhibit bacteria-induced wound infection and accelerate wound healing (Song et al., 2018). Based on the above researches, Zhang Y. et al. (2019) synthesized a novel tetracycline@ZIF-8@hyaluronic acid nanocomposite by wrapping tetracycline in ZIF-8 and hyaluronic acid and proved that the tetracycline@ZIF-8@hyaluronic acid nanocomposite was promising for eradication of pathogenic bacteria in vitro and in vivo experiments (Figure 2). The antibacterial activity in vitro of the tetracycline@ZIF-8@hyaluronic acid nanocomposite against S. aureus and Salmonella was evaluated by minimum inhibitory concentration. The result showed that fractional inhibitory concentration index calculated to be less than 0.5 verified the synergistic effect of the ZIF-8 and tetracycline components in the tetracycline@ZIF-8@hyaluronic acid nanocomposite (Zhang X. et al., 2019). In another research, incorporation of ciprofloxacin into zirconium-based MOF (UiO-66) had larger inhibitory ring range against S. aureus and E. coli in contrast to ciprofloxacin alone (Nasrabadi et al., 2019). Currently, different kinds of MOFs encapsulated varieties of antibacterial agents with dissimilar antibacterial efficient, which detailedly displayed in Table 1.
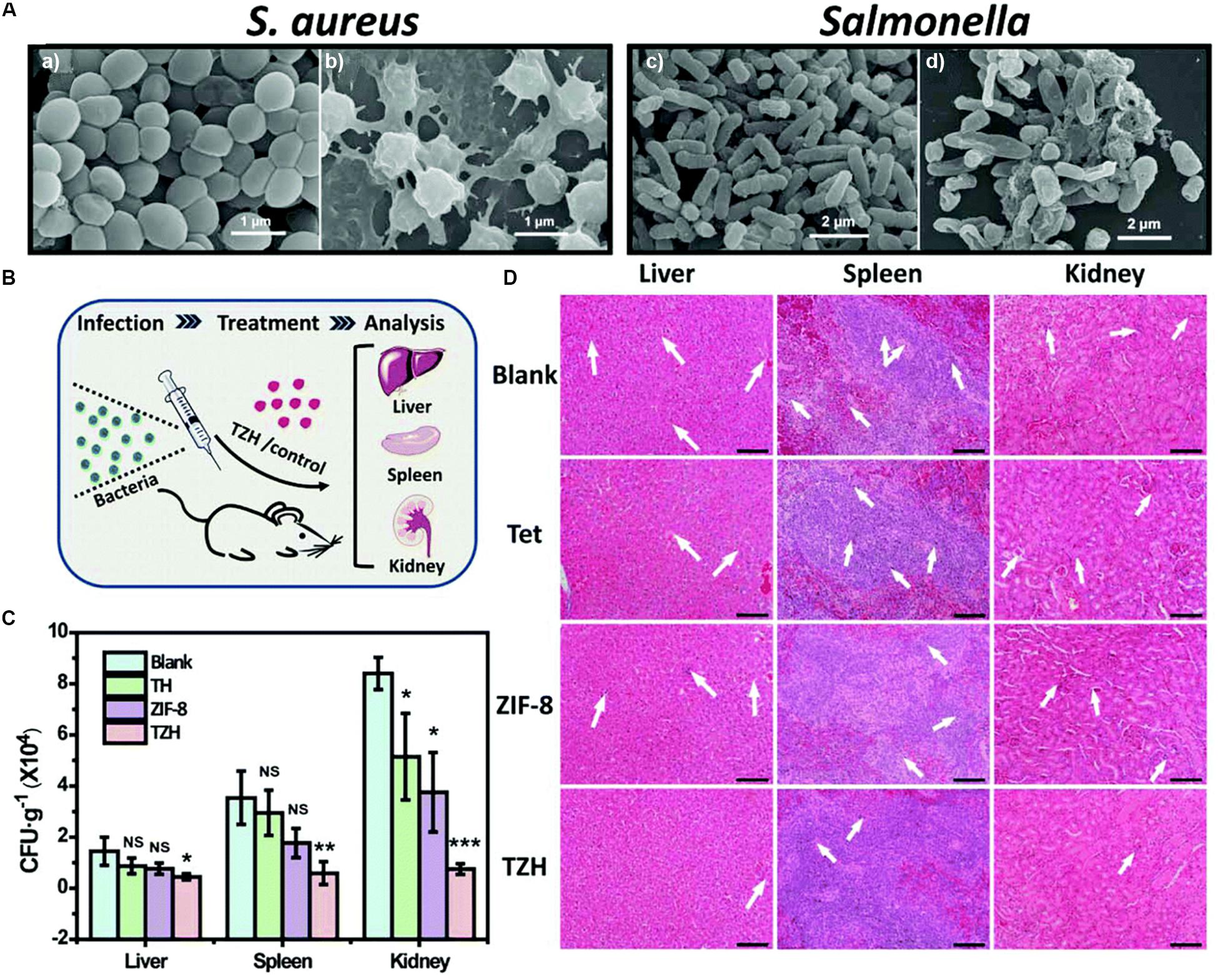
Figure 2. The sterilization situation of TZH in vitro and in vivo. Notes: (A) SEM images of untreated (a,c) and TZH-treated (50 μg/mL) (b,d) S. aureus and Salmonella. (B) The scheme for constructing in vivo animal experimental model. (C) CFU of bacteria in liver, spleen, and kidney after the treatment by TZH or other control groups (blank, Tet, ZIF-8). (*P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant.) (D) Pictures of histological sections with H&E stain in TZH and other control groups. The abnormal areas were shown by the white arrows. Scale bar = 50 μm. [Reprinted with permission from Zhang X. et al., 2019; Copyright (2019) Royal Society of Chemistry]. Abbreviations: CFU, colony forming unit; SEM, scanning electron microscopy; S. aureus, Staphylococcus aureus; Tet, tetracycline; TZH, tetracycline@ZIF-8@hyaluronic acid.
Moreover, the multifunctional combination of MOFs with metal/metal oxides nanoparticles further improves their bactericidal capacities. Ximing et al. (2017) successfully synthesized copper-based MOF (CuTCPP MOF) to encapsulate silver nanoparticles and prepared a new composite material Ag-CuTCPP MOF. The inhibition rates on E. coli, Bacillus subtilis, S. aureus, and their mixed strains were 82.18, 72.8, 89.1, and 80.4%, respectively, which were significantly higher than positive control penicillin (Ximing et al., 2017). The bactericidal principle was that the silver nanoparticles encapsulated in MOFs contacted with oxygen to form Ag+, which destroyed cell membrane permeability and caused bacterial death (Ximing et al., 2017). Similar results about Ag nanoparticles-MOF as antibacterial hybrid could also be found in recent studies (Zhu et al., 2015; Thakare and Ramteke, 2017; Abd El Salam et al., 2018). Beyond that, zinc oxide (ZnO) showed excellent antibacterial properties, and could be combined with other materials (such as gelatin, hydrogels) to further develop its antibacterial ability (Lin J. et al., 2017; Li et al., 2018). Recently, Redfern et al. successfully synthesized ZnO@ZIF-8 composite by spontaneously forming ZnO nanorods on the surface of ZIF-8 nanocrystals exposed to an aqueous solution of silver nitrate at room temperature. The authors drew a conclusion that catheter-associated urinary tract infection pathogens could be eliminated by ZnO@ZIF-8 composite (Redfern et al., 2018). The strong bactericidal effect was not only attributed to the action of ZnO, but also related to the direct attack and killing of pathogenic microorganisms by zinc ions and imidazole ligands of ZIF-8 (Redfern et al., 2018).
Furthermore, some plant natural products showed satisfactory antibacterial properties. Liu X. et al. (2019) developed the ultrathin two-dimensional MOF nanosheet [two-dimensional Cu-TCPP(Fe)] as a physical adsorption model of glucose oxidase for peroxidase simulators (enzyme catalyst). The designed system could self-activate the peroxidase-like activity of two-dimensional MOF nanosheets owing to the formation of gluconic acid, which significantly improved the generation rate of toxic ∙OH and enhanced the antibacterial effects. Besides, Naseri et al. (2018) have described the antibacterial effect of Cu-H2bpdc-cy MOF by mixing cytosine with copper-based MOF (Cu-H2bpdc) on Proteus strangis. When Cu-H2bpdc-cy MOF contacted with bacteria, the released copper ions were combined with negatively charged lipoproteins in bacterial cell wall, then entered the cell, and finally damaged the cell wall.
In addition, the antibacterial properties of nitric oxide were widely reported since it played a pivotal role in the body’s immune response to pathogens. The antibacterial mechanism of nitric oxide was due to the nitrosative and oxidative stress imposed by its reactive byproducts (e.g., nitrous oxide and pernitrite), which eventually led to the rupture of the bacterial membrane (Fang, 1997). Actually, nitrogen atoms are able to interact with the exoskeleton cations in MOFs to realize nitric oxide loading. The small molecule of nitric oxide would be released by exchanging with cations in the interstitial fluid. Consequently, the high concentrations of nitric oxide could promote the bactericidal effect of macrophages toward the pathogens (Horcajada et al., 2012). Therefore, nano-MOF materials make use of their gas storage capacity to carry nitric oxide, which can play an antibacterial role (Zhu et al., 2015). Another study reported that Staphylococcus epidermidis, S. aureus, and E. coli of 1 × 1010 CFU/mL in PBS solution (pH = 6) were totally killed at ZIF-8@I (iodine loaded ZIF-8) dosage of 0.2 g/L within 3 min (Au-Duong and Lee, 2017). Its bactericidal activity probably depended on the irreversible damage to bacterial cells caused by the release of iodine and zinc in the framework.
Yet some MOF nanomaterials are able to biodegrade and release metal ions making it possible for biological toxicity to occur. In addition, recent toxicological studies have shown that their toxicity is closely related to organic ligands in their structures (Tamames-Tabar et al., 2014; Shearier et al., 2016). The hydrophobic–hydrophilic balance of the constituent organic ligands may be responsible for cytotoxicity. To solve these problems, the cytotoxicity of MOF nanoparticles can be minimized by using biocompatible cations to construct MOFs and improving the hydrophilicity of organic ligands.
Zeolites
Zeolites have also appeared in drug controlled release systems in recent years due to their orderly and uniform pore shape and highly ion-exchanging capability (Rimoli et al., 2008). The basic chemical composition of zeolites is based on a silica framework (SiO2) where a proportion of the silicon atoms are be substituted by aluminum. Then replacing Si4+ with Al3+ produces a negatively charged aluminosilicate skeleton, requiring the exchange of additional skeleton cations (such as Na+) to keep the overall skeleton neutral (Torres-Giner et al., 2017). Those cations are not part of the frame but are located in the pores of the structure and they are exchanged out of the material since replaced by other cations (such as Ag+) (Hedström, 2001). A large number of studies have reported on the antibacterial properties of metal ions-loaded zeolites. Ag+ is one of the most antimicrobial metal ions exchanging in zeolites, because of its good stability and broad-spectrum antibacterial properties (Abe et al., 2004). Fortunately, zeolites have strong affinity for Ag+, which can be electrostatically combined to make the ratio of Ag+ to the weight of the frame up to 40% (Uchida, 1995). Afterward, Ag+ could be released from the zeolites by exchanging with the cations in external environment. Thereafter, it enters into the bacterial cell by penetrating through the cell wall and consequently changes the DNA into condensed form, finally causing the cell death (Rai et al., 2009). A previous study recently developed a new method for the green synthesis of highly stable zeolites such as green β-zeolite based on the seed−assisted synthesis, without the use of any organic structure−directing agent. After exchanging with Ag+, the green β-zeolite had the ability to inhibit the activity of E. coli (Saint-Cricq et al., 2012). After adding 20 mg of silver-loaded green β-zeolite into culture medium (10 mL, 108CFU/mL) for 1 h, no colonies of E. coli were found by agar plate counting. Besides, other types of zeolites, such as zeolite X and EMT zeolite, were also reported to exchange with Ag+ for antibacterial application (Dong et al., 2014; Chen S. et al., 2017; Tosheva et al., 2017). In addition to Ag+, other metal ions including zinc ions, copper ions, etc., were also exchanged into different types of zeolites to exert antibacterial properties. All exchanging zeolites exhibited favorable bacterial killing efficacy against different pathogens. The details could be found in Table 2.
In addition, metal and metal oxides nanoparticles supported into zeolites are widely used in antibacterial field. Recently, ZnO and titanium dioxide (TiO2) nanoparticles have attracted considerable attentions due to the characteristic that they can produce ROS to exert their bactericidal capacity (Dizaj et al., 2014). Azizi-Lalabadi et al. (2019) assessed the antimicrobial activity of 4A zeolite loading with TiO2, ZnO, and TiO2/ZnO nanoparticles and concluded that TiO2/ZnO nanoparticles loaded with 4A zeolite had the superior antibacterial effects on S. aureus, Pseudomonas fluorescens, Listeria monocytogenes, and E. coli. Although 4A zeolite per se has no antibacterial ability, it can be used as a carrier for ZnO and TiO2 nanoparticles and control the release of them to improve the bactericidal ability. After ZnO and TiO2 nanoparticles were released, they could produce metal ions and ROS together, and then destroyed the bacterial cells structure and inhibited their growth, thus playing a synergistic bactericidal effect (Azizi-Lalabadi et al., 2019). Moreover, another study reported that zeolite A loading with cuprous oxide possessed the antibacterial efficiency of more than 96% against E. coli (Du et al., 2017). The antibacterial mechanisms of zeolites with metal/metal oxides nanoparticles were attributed to the connection with microbial DNA and proteins, following by prevention of bacterial replication and inactivation of the bacterial electron transport chain, finally leading to the death of bacteria (Dizaj et al., 2014).
Besides, zeolites can also act as vehicles for other antimicrobial agents, such as gentamicin (GM). GM has broad spectrum antibacterial function, but high dose of which might lead to serious side-effects such as nephrotoxicity. Therefore, a study synthesized ZSM-5 zeolites loaded with GM with the hydrogen bond interaction between the functional group of GM and ZSM-5 zeolites, the purpose of which was to avoid the side effects of high doses of GM (Guo et al., 2014). For ZSM-5 zeolites containing GM, continuous release of drug minimized bacterial adhesion and prevented biofilm formation of S. epidermidis.
Despite of the promising perspective of zeolites in antibacterial application, some of the zeolites have certain biotoxicity to limit their adhibition in biomedical field. One study showed that the non-functionalized nanoscale zeolite L possessed higher cytotoxic with increasing concentrations, which might be due to the presence of a large number of surface acid sites on pure zeolites that catalyzed certain chemical reactions on cells (Li et al., 2013). Moreover, Kihara et al. (2011) also proved that the cytotoxicity of zeolites might be related to its surface morphology. Therefore, more studies are urgently needed on how to reduce the toxicity of zeolites so as to make safe utilization of zeolites in the field of drug delivery.
Covalent Organic Frameworks
Recently, COFs have become a hot topic because of their high load capacity and biocompatibility as the drug delivery vehicles, which can be connected to guest molecules by non-covalent action (Vyas et al., 2016). However, they are not yet widely used for antimicrobial delivery. Hence, COFs as antibacterial agent delivery carriers are a very promising research direction.
The Modified Nanomaterials Possessing Excellent Antibacterial Effects
Modifiability is one of the most remarkable properties of microporous nanomaterials, which can improve their stability, adjust their structural characters, and feature them with antimicrobial functions. Hence, modification is an effective way to broaden the application range of microporous nanomaterials and adapts them to biomedical applications, which can be achieved by doping metal ions, surface functionalization and incorporating with other organic or inorganic substances by functionalized skeleton or added substituents to form new antibacterial nanocomposites.
On the basis of a large number of reports, researchers attempted to understand the role of metal-doped microporous nanomaterials in antimicrobial applications. Studies have shown that when MOF materials were doped with a certain proportion of metal ions that were close to the metal ion radius in the structure of MOFs, the structure of could be kept stable. At the same time, MOF materials doped with other metal ions could be endowed with new functions (e.g., anti-inflammatory effect, osteogenesis) or enhanced certain abilities (e.g., antibacterial ability) to some extent (Horike et al., 2015; Schejn et al., 2015; Shen et al., 2019). Recently, zinc-based MOFs have received more attention due to the favorable bactericidal performance. Li P. et al. (2019) doped cerium ions into antibacterial ZIF-8 to endow with new anti-inflammatory properties. For the past few years, intensive studies tried to incorporate metal ions such as Co, Ag, Mn, into the vacant T-atom sites (T = Al) of the BEA zeolite (zeolite-β) (Dzwigaj and Che, 2006; Baran et al., 2016; Popovych et al., 2016). However, the corresponding antibacterial evaluations were not carried out.
As the therapeutic agents for infectious diseases, microporous nanomaterials could play their roles relying on surface modification with biocompatible parts. It has been reported that amino modified zeolite L can increase its ability of targeted binding to the surface of non-pathogenic E. coli (Popović et al., 2007). Butthis property had not been applied for therapeutic purposes. Whereafter, Strassert et al. (2009) synthesized a novel multifunctional nano-hybrid for targeting, labeling, and inactivating the antibiotic resistance bacteria (including E. coli and Neisseria gonorrhoeae). DXP [N,N’-bis(2,6-dimethylphenyl)perylene-3,4,9,10-tetracarbodiimide] was encapsulated in zeolite L, which was functionalized with photosensitizer phthalocyanine dihydroxide. Finally, multifunctional zeolites were coated with amino groups on their surface to promote adhesion to bacteria. The sterilized function mainly was attributed to the production of singlet oxygen on the surface of the photosensitizer under light. The result showed that the inactivation efficiency of E. coli and N. gonorrhoeae reached to 95% after 2 h of exposure to light radiation.
The latest study found a surprising improvement in the bactericidal activity of ZIF-8 functionalized with graphene oxide (GO) (Ahmad et al., 2020). When the ratio of GO to ZIF-8 was 1–100, ZIF-8/GO composites exhibited fivefolds of the antibacterial properties of the original ZIF-8 at the same concentration against S. aureus and E. coli. Moreover, a recent pioneer study attempted to introduce the GO to the silver-based MOF to form a new nanocomposite (GO-Ag-MOF) (Firouzjaei et al., 2018). The result showed that antibacterial effect of GO-Ag-MOF was more prominent than that of silver-based MOF on B. subtilis and E. coli (Figure 3). Furthermore, Hatamie et al. (2019) prepared a novel microporous hybrid material (GO/cobalt-based MOF) by modifying cobalt-based MOF with GO, and demonstrated that the antimicrobial activity of cobalt-based MOF was significantly enhanced by modified with GO. The result showed that the growth inhibition of the GO/cobalt-based MOF against E. coli and S. aureus could exceed 99% at the concentration of 100 μg/mL. The major antibacterial mechanisms of the nanocomposites synthesized from MOFs and GO are summarized as follows: (1) interactions between metal ions released by MOFs and bacterial cells are described in Section “Nanomaterials With Inherent Antibacterial Activity,” (2) the sharp edges of GO nanosheets scratch the cell walls of bacteria and then initiate to destroy their membranes (Akhavan and Ghaderi, 2010), and (3) GO produces superoxide anions that damage bacterial cell membranes (Kotchey et al., 2011). Moreover, using photocatalyst to synergistically fight bacteria to antibacterial application is a novel and important approach. Li J. et al. (2020) prepared a novel bactericidal nanocomposite by using (3-aminopropyl) triethoxysilane mounted on the surface of ZIF-8 as an intermediate junction to realize the connection between ZIF-8 and the photosensitizer chloroethane. It has been proved that ZIF-8 nanomaterial conjugated with chloroethane had synergistically antibacterial effect on S. aureus and methicillin-resistant S. aureus upon light triggering. The sterilization mechanisms include (1) the production of ROS stimulated by light, (2) zinc ions released out of ZIF-8 framework as a toxin to inhibit bacterial growth, and (3) the fact that nanocomposites with a rough surface could greatly influence the interactions between bacterial cells and thus play a bactericidal role. Besides, MOFs also had the ability to combine with other materials, such as activated carbon (Azad et al., 2016), polyvinylidene fluoride/perfluorooctyltriethoxysilane (Miao et al., 2018), sodium alginate/niflumic acid (Luo et al., 2020), and humic acid (Liu et al., 2020), to form hybrids to improve their antibacterial performance. All of them have greatly expanded the application potential and efficiency of MOF materials as antimicrobial agents. These hybrids have showed excellent bactericidal effect on common wound infection pathogenic bacteria S. aureus or E. coli, which can be used for the treatment of wound infection. Especially in the study of the Miao et al. (2018) the compound of polyvinylidene fluoride/perfluorooctyltriethoxysilane and ZIF-8 also possessed a good self-cleaning function, to some extent inhibiting bacterial adhesion.
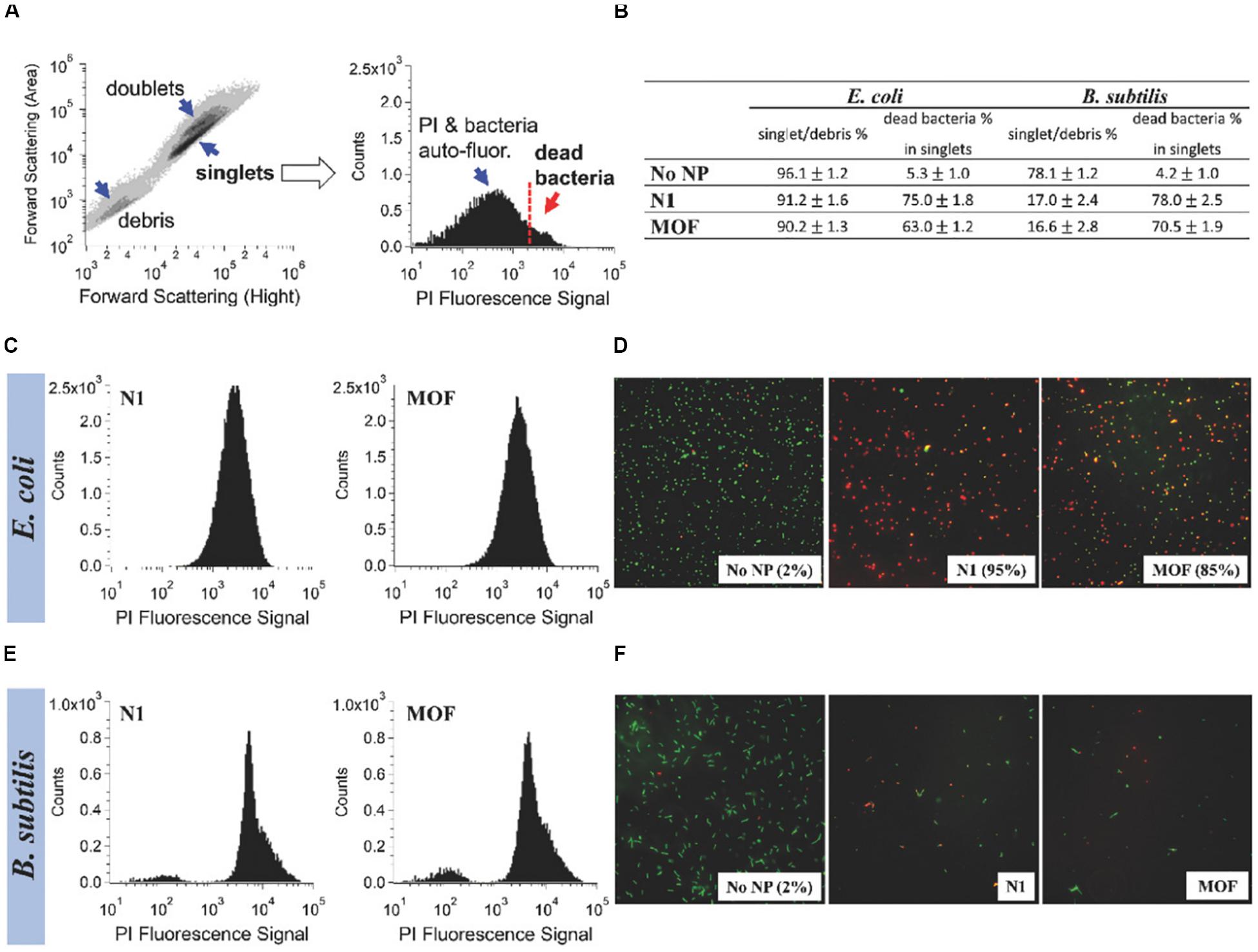
Figure 3. Comparison of the antibacterial activity of Ag-MOF and N1. Notes: The information shown in (B), (C), and (E) was obtained by taking the contour map and the PI fluorescence histogram of the untreated E. coli sample (A) as example. The flow cytometry of E. coli and B. subtilis after about 3 h of treatment with 100 μg/mL of complex and MOF were shown in (C) and (E). (D,F) The fluorescence images of the blank control group and the experimental groups. [Reprinted with permission from Firouzjaei et al., 2018; Copyright (2018) Wiley-Blackwell]. Abbreviations: Ag-MOF, silver-based metal organic framework; B. subtilis, Bacillus subtilis; E. coli, Escherichia coli; N1, the nanocomposite consisting of Ag-MOF and graphene oxide; PI, propidium iodide.
With respect to the modification of zeolites, Nosrati et al. (2015) immobilized TiO2 nanoparticles onto the surface of Ag-exchanged-zeolite-A to form a novel hybrid with enhanced antibacterial and photocatalytic properties. This nanoscale hybrid was used as additive in the matrix of polyacrylic latex to construct a promising nanocomposite coating, which exhibited outstanding advantage in the aspects of anti-microorganism, self-cleaning, and stability in water (Nosrati et al., 2015). The authors applied the novel coating to the sterile glass and placed it in a bacterial plate, and then found that the Gram-positive bacteria (S. aureus, L. monocytogenes, Salmonella thyphimurium) had no growth zone around it, while the Gram-negative bacteria (E. coli, Bacillus anthraci) had the opposite result. Recently, a new study hinted that NaA zeolites exchanged with silver and copper ions could combine with epoxy to form the high flux thin-film zeolite AgA/epoxy and zeolite CuA/epoxy nanocomposite membranes (Khademi et al., 2020). According to the inhibition experiment, zeolite AgA/epoxy nanocomposite membrane had better antibacterial capacity against E. coli and S. aureus compared with zeolite CuA/epoxy nanocomposite membrane. Similar results have been published by Ruparelia et al. (2008) where weaker biocidal effect of Cu nanoparticles in comparison with Ag nanoparticles was found. The antibacterial difference was due to the higher sensitivity of E. coli and S. aureus to Ag nanoparticles compared with the same concentration of Cu nanoparticles (Ruparelia et al., 2008).
In addition to aforementioned MOFs and zeolites, there are also increasing researches on antibacterial compounds based on COFs. Ionic covalent organic nanosheets (iCONs) are belonging to a kind of COFs with the morphology of nanofilm. By using three self-exfoliate guanidinium halide based porous iCONs (TpTGCl, TpTGBr, and TpTGI) and polysulfone, Mitra et al. (2016) produced iCONs@polysulfone mixed matrix membrane with excellent antimicrobial performance on porous non-woven support fabrics. It was reported that the interaction of positively charged guanidine units on the surface of the iCONs@polysulfone mixed matrix membrane with negatively charged phospholipid bilayer of Gram-positive (S. aureus) and Gram-negative (E. coli) bacteria was the main cause of sterilization. As shown in Figure 4, the iCONs@polysulfone mixed matrix membrane had destroyed the membranes of E. coli and S. aureus, resulting in bacterial cell death (Mitra et al., 2016).
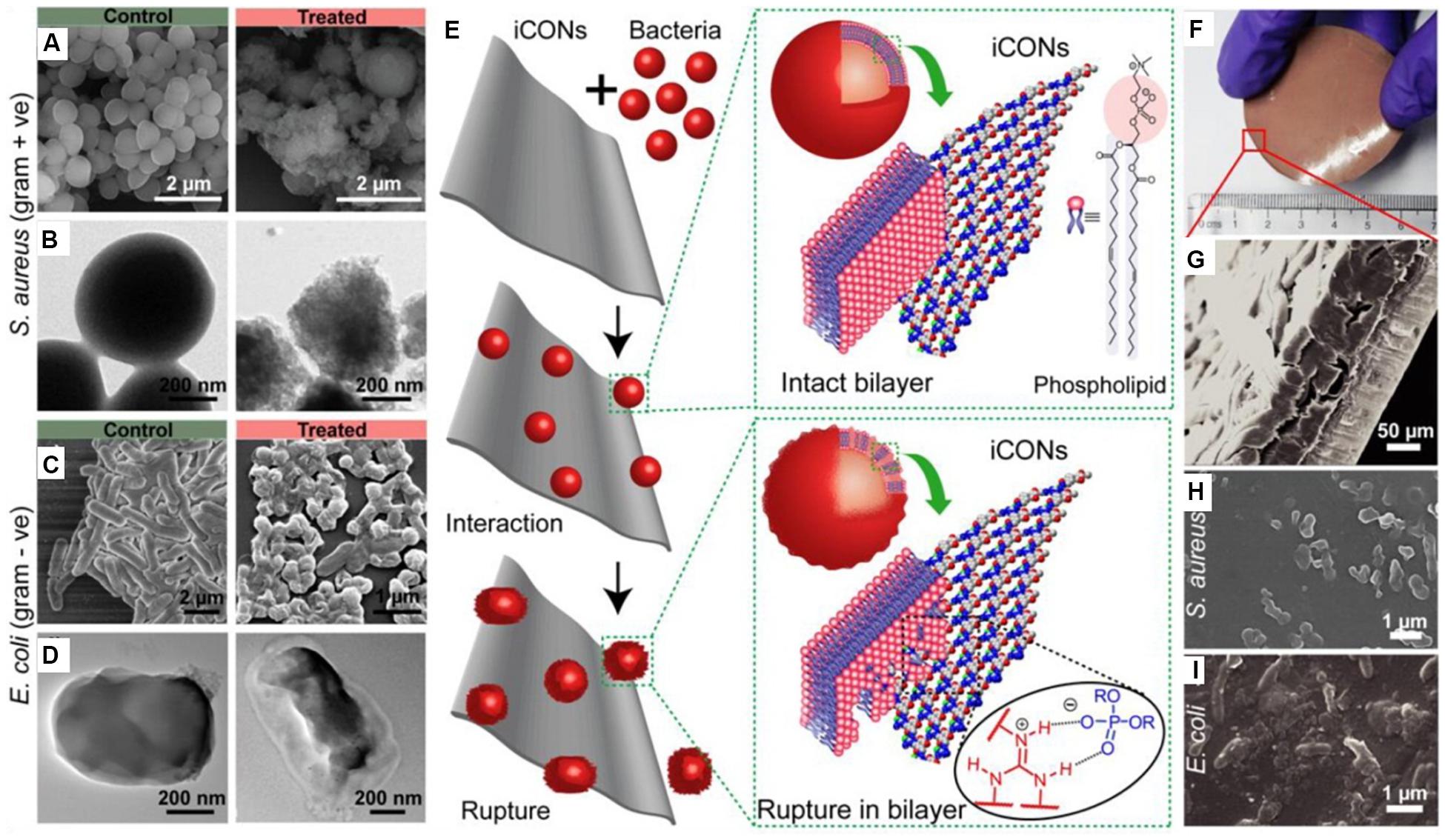
Figure 4. The antibacterial activity of iCONs@PSF mixed matrix membrane. Notes: (A,C) SEM images and (B,D) TEM images of control and TpTGCl treated S. aureus and E. coli. (E) The schematic illustration of the interaction between the bacteria and the iCONs. (F) Digital image and (G) SEM image of TpTGCl@PSF mixed matrix membrane. Growth inhibition of (H) S. aureus and (I) E. coli on the surface of TpTGCl@PSF mixed matrix membrane. [Reprinted with permission from Mitra et al., 2016; Copyright (2016) American Chemical Society]. Abbreviations: E. coli, Escherichia coli; iCONs, ionic covalent organic nanosheets; iCONs@PSF, the composite of ionic covalent organic nanosheets and polysulfone; SEM, scanning electron microscopy; S. aureus, Staphylococcus aureus; TEM, transmission electron microscopy; TpTGCl, self-exfoliate guanidinium halide-based ionic covalent organic nanosheets; TpTGCl@PSF, the composite of TpTGCl and polysulfone.
Antibacterial Microporous Nanomaterials Against Oral Diseases
This section describes a variety of oral infections and related pathogens. Moreover, it also reviews the frontier researches on the application of microporous nanomaterials in oral infectious diseases and the progress in the treatment of related pathogens.
Dental Caries
Dental caries is one of the most common diseases in the world (Cagetti et al., 2013). In a study in 2015 about Global Burden of Disease, the age-standardized prevalence rates of untreated caries in deciduous teeth and permanent teeth were estimated up to 7.8 and 34.1%, respectively (Kassebaum et al., 2017). Untreated caries in permanent teeth affect 2.5 billion people worldwide (Kassebaum et al., 2017) and accounts for 12% of global productivity losses due to dental diseases (Righolt et al., 2018). Dental caries is regarded to be caused by bacterial biofilms on the surfaces of the teeth, the formation of which is regulated by a complex interaction between pathogenic bacteria and their hosts, including teeth and saliva (Selwitz et al., 2007). The main pathogenic bacteria of dental caries, represented by Streptococcus mutans, tend to adhere to the teeth surfaces and produce organic acids to dissolve the mineralized tissues of the teeth, thus leading to the development of dental caries from the teeth surfaces to the insides (Rainey et al., 2018). The conventional treatment for dental caries is to remove the infectious tooth tissue and to restore with filling material such as polymeric resin (BaniHani et al., 2017). However, the traditional approach is apt to lead to the microleakage of the interface of dental tissue and restorations, which was consequent in the occurrence of secondary caries (Jokstad, 2016).
Secondary caries was reported as one of the main causes of restoration failures (Nedeljkovic et al., 2015). The formation of the biofilm between restoration or backfill and dental tissue is considered as a risk factor for secondary caries (Jokstad, 2016). It is generally believed that the pathogenic biofilm of secondary caries is similar to that of primary caries, mainly composed of bacteria represented by S. mutans (González-Cabezas et al., 1999). It has been reported that silver-containing EMT zeolite can be used to prevent the occurrence of secondary caries (Li W. et al., 2020). EMT type zeolite crystal with ultra-small nanoscale size is an ideal carrier for efficient and high volume silver ion exchange. Previous study showed that EMT zeolite containing Ag had good antibacterial activity against E. coli (Dong et al., 2014). Recently, based on the previous study, Li W. et al. (2020) added large amounts of nanoscale Ag-EMT zeolites into dental adhesive to prevent the development of secondary dental caries, as well as to avoid the color change caused by direct addition of silver ions. It could be further concluded that the antibacterial efficient was enhanced with the increasing exchange time of Ag+ (Figure 5). The sample with the longest exchange time reduced CFU counts of the biofilms of S. mutans, Streptococcus gordonii, and Streptococcus sanguinis bacteria by nearly two orders of magnitude.
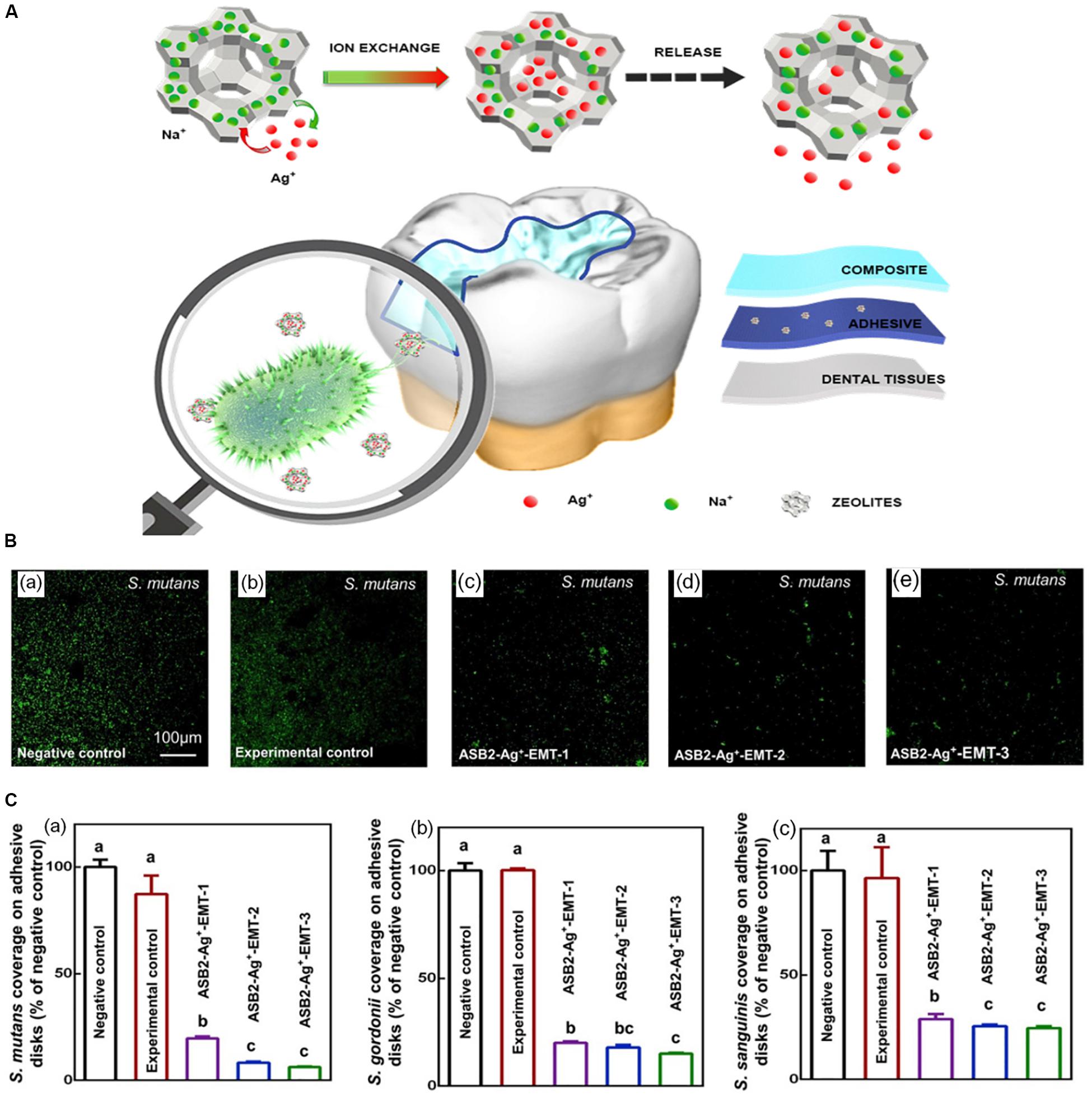
Figure 5. The schematic diagram of the synthesis and antimicrobial activity of ASB2 containing 5% Ag+-EMT. Notes: (A) The diagram of Ag+-EMT and their antibacteria performance in dental restoration. (B) Fluorescent images of S. mutans in the groups of (a) negative control (pure ASB2), (b) experimental control (ASB2 + 5% EMT zeolites), and (c–e) ASB2-Ag + -EMT (ASB2 + 5% EMT zeolites with silver ion exchange for 10, 20, and 40 min). (C) The histogram of early bacterial coverage for 4 h of (a) S. mutans, (b) S. gordonii, and (c) S. sanguinis. Different letters indicate that the difference between them is statistically significant. [Reprinted with permission from Li W. et al., 2020. Copyright (2020) Elsevier]. Abbreviations: Ag+-EMT, silver-exchanged EMT zeolites; ASB2, the dental adhesive; S. gordonii, Streptococcus gordonii; S. mutans, Streptococcus mutans; S. sanguinis, Streptococcus sanguinis.
In the research by Cao et al. (2020), three types of MOFs, [AgL]n⋅nH2O (p-MOF), MOF-5, and ZIF-8, were synthesized to fight S. mutans, Fusobacterium nucleatum, and Porphyromonas gingivalis bacteria, which possessed the slow-release sterilization abilities. The result indicated that MOFs were promising candidates in the field of the treatment of dental caries and periodontitis. Massoudinejad et al. (2018) also discovered that the UIO-66 could be applied to efficiently load fluoride. It is well known that fluoride ion can effectively prevent the occurrence of dental caries. Hence, it is a promising research direction to use MOFs to load fluoride to prevent dental caries.
Periodontitis and Peri-Implantitis
Periodontitis becomes a major global health care problem, with increasing costs to individuals and society (Tonetti et al., 2017), since it is regarded as one of the leading causes of tooth loss and ranks as the sixth most common disease in the world (Papapanou, 1996; Kassebaum et al., 2017). Periodontitis is caused by pathogenic microorganisms in biofilms or plaque that eventually destroy the periodontal tissue that supports the teeth (Pihlstrom et al., 2005). It is closely related to a defined microbial composition (the “red-complex” bacteria: P. gingivalis, Tannerella forsythia, and Treponema denticola) found on the surface and root of teeth (Darveau, 2010). They have significant virulence and can interfere with host’s defense ability through a variety of mechanisms, thus causing the destruction of periodontal tissues. Mechanical debridement is often used in conjunction with systemic or topical antibiotic therapy to treat periodontitis (Herrera et al., 2012). However, long-term use of antibiotics will lead to the rampant growth of drug-resistant strains, reducing the effectiveness of periodontitis treatment (Wyszogrodzka et al., 2016). Therefore, the development of microporous frame structure is particularly important in the treatment of periodontitis.
Kawahara et al. (2000) proposed that under the anaerobic conditions, silver-zeolite had prominent antibacterial effect on major periodontal pathogens such as P. gingivalis, Prevotella intermedia, and Actinobacillus actinomycetemcomitans. Therefore, silver zeolite could be applied to the anaerobic area of the mouth (periodontal pocket), and acts as an effective drug for periodontitis treatment. In addition, a recent study described ZIF-8 nanoparticles containing different proportions of cerium (Ce) for treating periodontitis (Li X. et al., 2019). The result showed that as the proportions of Ce increased, the antibacterial effect of nanoparticles slightly decreased against P. gingivalis and F. nucleatum. Even that, the antibacterial efficacy of 10% Ce doped ZIF-8 against these two pathogens was still beyond two orders of magnitude. Moreover, the addition of Ce could provide ZIF-8 nanoparticles with excellent anti-inflammatory properties. The anti-inflammatory effect is mainly achieved by the presence of a large number of oxygen vacancies in Ce-based nanomaterials and the reversible conversion between Ce(III) and Ce(IV) ions to eliminate excess ROS (Huang et al., 2018).
Peri-implantitis is an important cause of dental implant failure. The prevalence of peri−implantitis on implant level ranged from 1.1 to 85.0% and the incidence from 0.4% within 3 years, to 43.9% within 5 years, respectively (Dreyer et al., 2018). Similar to periodontitis, dental plaque was considered as the initiator of peri-implantitis. Treatment of mild and moderate forms of peri-implantitis is similar to that of periodontitis. The therapeutic methods included a variety of manual ablation, laser-supported systems, and photodynamic therapies, which can be extended with topical or systemic antibiotics (Smeets et al., 2014). However, severe peri−implantitis often requires surgical treatment (Smeets et al., 2014). As the treatment method for peri−implantitis, the microporous frameworks can not only reduce the use of antibiotics but also prevent the pain of patients caused by surgical treatment.
A paper published by Wuttke et al. (2017) assessed the safety of different MOFs [MIL-100(Fe), MIL-101(Cr), and Zr-fum MOF of different sizes] for dental applications, for instance, multifunctional surface coatings of implants (Wuttke et al., 2017). Notably, primary gingival fibroblasts had no significant toxic response to all MOF nanoparticles tested. In addition, there was no significant change in the morphology and metabolic activity of fibroblasts, suggesting that the MOF nanoparticles tested had good biocompatibility (Wuttke et al., 2017). The above experimental results demonstrated the feasibility of using MOF nanoparticles as dental implant coatings because of their excellent biosecurity and biocompatibility to human gingival fibroblasts. Recently, Chen J. et al. (2017) made the first attempt to prepare ZIF-8 nanofilm on the surface of titanium. It was found that the number of S. mutans was significantly reduced on the titanium surface with ZIF-8 nanofilm compared with the control group. Interestingly, in addition to the favorable antibacterial properties, ZIF-8 nanofilm could also play an osteogenic role by up-regulating the genes for alkaline phosphatase and the key osteogenic transcription factor Runx2 through the release of zinc ions after degradation (Yang et al., 2012; Yuan et al., 2016).
Endodontic Infections
In case of dental caries, when the enamel or cementum of the tooth is destroyed by bacteria, the bacteria can invade pulp through the dentin tubules and progress into infections of dental pulp, leading to endodontic infections. Endodontic infections are a common type of oral diseases. In the Brazilian adult population, the majority of endodontically treated teeth were found in 46–60 years of age (47.6%), and the prevalence increased with age (Hollanda et al., 2008). The most common biological cause of endodontic disease is the root canal biofilm, which is significantly formed by E. faecalis and other microorganisms (Mohammadi et al., 2014). Hence, the most important purpose for effective treatment of endodontic infections is to eliminate suspected pathogens from the root canal (Siqueira, 2002). Root canal therapy is the usual clinical treatment strategy for endodontic infections (Li and Wang, 2015). However, the failures of root canal therapy are often caused by the incomplete removal of infectious pathogens in the root canal (Siqueira and Rôças, 2004). In order to increase the cure rate of endodontic infections, the application of microporous frameworks was studied in root canal treatment including the endodontic cavity flushing and endodontic filling (Odabaş et al., 2011; Ghivari et al., 2017).
One study showed that the antibacterial ability of the root canal filling material glass ionomer cement was significantly enhanced due to the addition of silver-zeolite (Çınar et al., 2009). The experiment suggested that the inhibition diameters of Streptococcus milleri, S. aureus, and E. faecalis by glass ionomer cement with 2 and 0.2% mass fraction silver-zeolite, was nearly one to two times greater than that by glass ionomer cement without any additives. After that, Odabaş et al. (2011) attempted to incorporate silver-zeolite to the mineral trioxide aggregate as a root-end filling material with high antimicrobial activity in order to achieve a good effect of endodontic therapy. It was proved that compared with pure mineral trioxide aggregate, mineral trioxide aggregate with 0.2 and 2% silver-zeolite had increased inhibition of bacteria, especially S. aureus and E. faecalis, and also remarkably strengthened the sterilization effects of fungi (Odabaş et al., 2011). Moreover, Ghivari et al. (2017) first investigated the antibacterial effects of silver-zeolite as a root canal irrigant. The result showed that the silver-zeolite (2%) showed the lowest antibacterial activity against E. faecalis, S. aureus, and Candida albicans biofilm, compared with the commonly used root canal irrigants, sodium hypochlorite (5.25%), chlorhexidine (2%), and otinidine hydrochloride (0.10%). It was demonstrated that silver-zeolite needed to come into contact with bacterial cells to perform its bactericidal action (Matsumura et al., 2003). The reason for the decreased antibacterial activity of silver-zeolite may be due to the presence of biofilm reducing the interaction between silver-zeolite and bacteria.
In addition, the MIL family of MOF nanomaterials is crafted from trivalent metal centers and carboxylic acid bridging ligands, which has attracted much attention due to its considerable loading capacity (Huxford et al., 2010). On basis of this, Golmohamadpour et al. (2018) had synthesized three types of MOF nanomaterials including Al-MIL-101-NH2, Fe-MIL-88B-NH2, and Fe-MIL-101-NH2 loaded with indocyanine green, which showed obvious antibacterial activity on E. faecalis. Under the laser irradiation at wavelength of 810 nm, MOF nanomaterials loaded with indocyanine green significantly reduced the survival rate of E. faecalis compared with pure MOF nanomaterials. The enhancement of antibacterial and anti-biofilm capability of indocyanine green loaded MOF nanomaterials was due to the fact that indocyanine green could enhance the generation capacity of ROS after laser irradiation (Omar et al., 2008).
Oral Mucositis
Denture stomatitis is a common oral mucous membrane infection. The prevalence of denture stomatitis in the elderly population has been concluded to be between 15 and 71% (Gendreau and Loewy, 2011). It is generally believed to be caused by a number of factors (such as lack of clean dentures, dietary factors, continued use of dentures without removal, etc.) (Schneid, 1992). One of the causes of denture stomatitis is the presence of biofilm on the denture surface. This biofilm matrix created a physical barrier for oral microbes like C. albicans, which were thought to be the main pathogen in the pathogenesis of denture stomatitis, to protect them from the outside world (Ramage et al., 2004). Broad-spectrum antifungal agents are commonly used to treat denture stomatitis (Mima et al., 2012). Although the drugs can be used topically, however, they are difficult to be effective because of the large amount of saliva flowing and the protection of biofilm matrix. Therefore, Casemiro et al. (2008) added zeolites exchanged with silver and zinc ions at varying percentages into the denture base material acrylic resin to increase the antibacterial activity against C. albicans. As the percentage of silver-zinc zeolite increased from 2.5 to 10%, the diameters of the inhibitory zones for C. albicans and S. mutans increased linearly. Moreover, Nikawa et al. (1997) added silver zeolite to the tissue conditioner (soft lining material) for the first time, which could effectively control dental plaque and inhibit the growth of C. albicans. The inhibitory effect of C. albicans was studied by monitoring the change of pH in the growth medium. It was concluded that the soft lining materials had more obvious bacteriostatic efficacy after the addition of silver zeolites. In a previous study, Nikawa et al. (1993) also proposed that the presence of saliva promoted the colonization of multiple layers of C. albicans and the formation of biofilms, thereby reducing the antibacterial effect of soft lining materials on C. albicans. However, the effect of saliva on antibacterial efficacy of silver-zeolite doped soft lining materials had not been systematically addressed in this study (Nikawa et al., 1993). Subsequently, Abe et al. (2004) further studied the effect of saliva on the antibacterial efficiency of silver-zeolite doped tissue conditioner. It could be concluded that, after 28 days of treatment with water or saliva, there was no significant difference in the bactericidal efficacy of silver-zeolite doped tissue conditioner against C. albicans. Currently, intensive studies have been made on the resistance of MOFs to C. albicans (Wojciechowska et al., 2015; Emam et al., 2018). However, there have been no reports on the application of MOFs in the treatment of denture stomatitis.
In addition, oral ulcerative disease is one of the most common complaints of oral mucosa (Leão et al., 2007). The pathogenesis is complex, with local and systemic conditions as well as genetic, immune, and microbial factors that may play a role. The combination of Chinese and western medicine is often used to treat oral ulcerative disease. But it is difficult to achieve the desired results. It is still difficult to achieve a favorable and satisfied effect on oral mucosal ulcer by current available treatments. Salehi et al. (2017) prepared a starch-based nanocomposite hydrogel scaffold enhanced by zeolite nanoparticles. In addition, herbs (chamomile extract) were added into the matrix to promote wound healing (Salehi et al., 2017). Animal experiments and clinical trials both showed that the combination of starch/zeolite nanocomposites and extracts could preferably improve wound healing, which washighly expected to be applied to the treatment of intractable ulcers in the mouth.
Oral Infections
In addition to the treatment of the above diseases, microporous nanomaterials can also be used for the treatment of oral infections such as jaws osteomyelitis and oral multi-space infection. Conventional treatment for oral infections is the use of antibiotics (Levi and Eusterman, 2011). However, due to the emergence of drug-resistant strains, the satisfied therapeutic effect is difficult to achieve. Therefore, it is necessary to study novel antibacterial agents for oral infection treatments.
Jaws osteomyelitis is a devastating infection of bone and bone marrow, of which Gram-positive Staphylococcus is one of the most common pathogens (Kavanagh et al., 2018). In recent study, ZIF-8 nanocrystals loaded with vancomycin were embedded into chitosan scaffolds to obtain three-dimensional scaffolds with antibacterial properties and excellent osteogenesis, which could be used to treat severe bone tissue infections such as osteomyelitis (Karakeçili et al., 2019). The result showed that under the simulated inflammation acidic condition (pH = 5.4), the chitosan scaffold containing 5% vancomycin loaded ZIF-8 crystals had the superior antibacterial performance against S. aureus. The reason for its excellent antibacterial efficacy was to promote the degradation of ZIF-8 and the release of vancomycin under acidic condition. Furthermore, it could simultaneously promote the bone regeneration to restore the surrounding bone damage.
Similar to jaws osteomyelitis, S. aureus is also the prevalent pathogenic bacteria of oral multi-space infection. Methicillin-resistant S. aureus is an increasingly dangerous and antibiotic-resistant bacterium, which is able to lead to oral soft-tissue infections, such as cellulitis and multigap infections. Chen S. et al. (2017) quantified the inhibition and bactericidal activity of the silver-exchange nanostructured zeolite X on methicillin-resistant S. aureus, and compared it with the micro-sized zeolite. It was found that the antimicrobial diameter of the nanostructured zeolite was larger than that of the micro-sized zeolite, suggesting that nanostructured zeolite had superior antibacterial effect (Figure 6). The difference in the antibacterial effect between different sized zeolites was probably due to the fact that nanoscale silver-exchange zeolite X could release more silver ions. Due to the short diffusion lengths and higher external surface area of the nanostructured zeolites, compared with the traditional micro-scale zeolites, they have higher ion release efficiency (Izumi and Nakazawa, 1986).
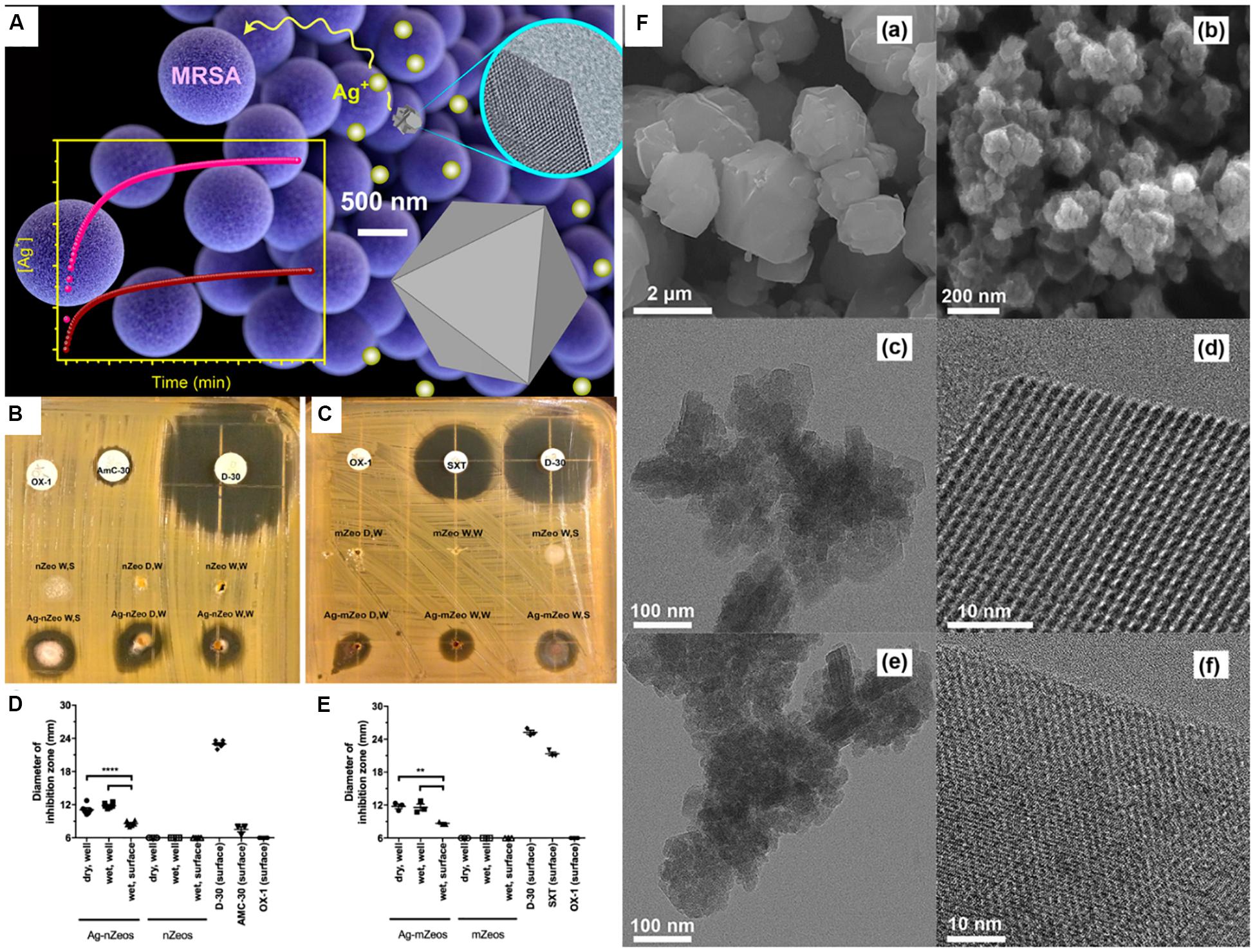
Figure 6. Comparison of Ag-nZeo and Ag-mZeo on growth inhibition of MRSA. Notes: (A) Schematic diagram of the bactericidal action of mZeo and nZeo on MRSA and their silver ion release rate. (B) Ag-nZeo and (C) Ag-mZeo ion diffusion and MRSA inhibition demonstrated by agar diffusion test. The bacteriostatic diameter of (D) Ag-nZeo and nZeo or (E) Ag-mZeo and mZeo compared with AmC-30, D-30, OX-1, and SXT (**P < 0.01; ****P < 0.0001). (F) SEM images of (a) mZeo and (b) nZeo; TEM and HRTEM images of (c,e) Ag–mZeo and (d,f) Ag–nZeo. [Reprinted with permission from Chen S. et al., 2017; Copyright (2017) American Chemical Society]. Abbreviations: Ag–mZeo, Ag-ion-exchanged microsized zeolites; Ag–nZeo, Ag-ion-exchanged nanostructured zeolites; AmC-30, amoxicillin with clavulanic acid; D-30, doxycycline; HRTEM, high resolution transmission electron microscopy; MRSA, methicillin-resistant Staphylococcus aureus; mZeo, microsized zeolites; nZeo, nanostructured zeolites; OX-1, oxacillin; SEM, scanning electron microscopy; SXT, trimethoprim/sulfamethoxazole; TEM, transmission electron microscopy.
Conclusion and Perspective
This article reviewed recent research progress of antibacterial microporous nanomaterials with respect to oral diseases. With the development of drug resistance, it is necessary to find a drug delivery system compatible with a variety of antimicrobial agents and new antimicrobial agents. Novel microporous nanomaterials and their composites offer a valuable opportunity and are compatible with a wide range of antibiotics for oral infectious diseases. In this paper, three types of microporous frameworks were summarized, including zeolites, metal organic frameworks, covalent organic frameworks, particularly on their potential of antibacterial effects via inherent properties, drug delivery, and modification. Importantly, their application in the treatment of oral infection diseases was detailedly reviewed. However, it is considered that most of these microporous frameworks and their nanocomposites are uncommercialized and their acquisition procedures are complex, which limits their wide range of clinical applications. Therefore, the faster and more efficient production of these nanomaterials remains to be further investigated. In addition, more and more studies should be focus on the biocompatibility improvement and evaluate the therapeutic efficiency against oral diseases in animal studies and clinical trials.
Author Contributions
YaW and WX wrote the manuscript. XR and YuW participated and helped with the final revision of the manuscript. LW and BD designed and critically revised the review manuscript. All the authors read and approved the final version of the manuscript prior to submission.
Funding
This study was supported by the Natural Science Foundation of China NSFC 81400487 (LW); China Postdoctoral Science Foundation 2015M581405 and 2017T100213 (LW); General program of Natural Science Foundation of Jilin Province 20200201356JC (LW) and 20200201317JC (CL); and seed grant from Jilin University School of Dentistry (LW).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abbasi, A. R., Akhbari, K., and Morsali, A. (2012). Dense coating of surface mounted CuBTC metal–organic framework nanostructures on silk fibers, prepared by layer-by-layer method under ultrasound irradiation with antibacterial activity. Ultrason. Sonochem. 19, 846–852. doi: 10.1016/j.ultsonch.2011.11.016
Abd El Salam, H. M., Nassar, H. N., Khidr, A. S. A., and Zaki, T. (2018). Antimicrobial activities of green synthesized Ag nanoparticles @ Ni-MOF nanosheets. J. Inorg. Organomet. Polym. 28, 2791–2798. doi: 10.1007/s10904-018-0950-4
Abdullahi, T., Harun, Z., and Othman, M. H. D. (2017). A review on sustainable synthesis of zeolite from kaolinite resources via hydrothermal process. Adv. Powder Technol. 28, 1827–1840. doi: 10.1016/j.apt.2017.04.028
Abe, Y., Ishii, M., Takeuchi, M., Ueshige, M., Tanaka, S., and Akagawa, Y. (2004). Effect of saliva on an antimicrobial tissue conditioner containing silver–zeolite. J. Oral Rehabil. 31, 568–573. doi: 10.1111/j.1365-2842.2004.01267.x
Aguado, S., Quirós, J., Canivet, J., Farrusseng, D., Boltes, K., and Rosal, R. (2014). Antimicrobial activity of cobalt imidazolate metal–organic frameworks. Chemosphere 113, 188–192. doi: 10.1016/j.chemosphere.2014.05.029
Ahmad, N., Md Nordin, N. A. H., Jaafar, J., Nik Malek, N. A. N., Ismail, A. F., Yahya, M. N. F., et al. (2020). Eco-friendly method for synthesis of zeolitic imidazolate framework 8 decorated graphene oxide for antibacterial activity enhancement. Particuology 49, 24–32. doi: 10.1016/j.partic.2019.04.007
Akhavan, O., and Ghaderi, E. (2010). Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano 4, 5731–5736. doi: 10.1021/nn101390x
Amorim, R., Vilaça, N. L., Martinho, O., Reis, R. M., Sardo, M., Rocha, J., et al. (2012). Zeolite structures loading with an anticancer compound as drug delivery systems. J. Phys. Chem. C 116, 25642–25650. doi: 10.1021/jp3093868
Aryanejad, S., Bagherzade, G., and Moudi, M. (2020). Green synthesis and characterization of novel Mn-MOFs with catalytic and antibacterial potentials. New J. Chem. 44, 1508–1516. doi: 10.1039/c9nj04977k
Au-Duong, A.-N., and Lee, C.-K. (2017). Iodine-loaded metal organic framework as growth-triggered antimicrobial agent. Mater. Sci. Eng. C Mater. Biol. Appl. 76, 477–482. doi: 10.1016/j.msec.2017.03.114
Azad, F. N., Ghaedi, M., Dashtian, K., Hajati, S., and Pezeshkpour, V. (2016). Ultrasonically assisted hydrothermal synthesis of activated carbon–HKUST-1-MOF hybrid for efficient simultaneous ultrasound-assisted removal of ternary organic dyes and antibacterial investigation: taguchi optimization. Ultrason. Sonochem. 31, 383–393. doi: 10.1016/j.ultsonch.2016.01.024
Azizi-Lalabadi, M., Ehsani, A., Divband, B., and Alizadeh-Sani, M. (2019). Antimicrobial activity of titanium dioxide and zinc oxide nanoparticles supported in 4A zeolite and evaluation the morphological characteristic. Sci. Rep. 9:17439. doi: 10.1038/s41598-019-54025-0
BaniHani, A., Deery, C., Toumba, J., Munyombwe, T., and Duggal, M. (2017). The impact of dental caries and its treatment by conventional or biological approaches on the oral health-related quality of life of children and carers. Int. J. Paediatr. Dent. 28, 266–276. doi: 10.1111/ipd.12350
Baran, R., Valentin, L., and Dzwigaj, S. (2016). Incorporation of Mn into the vacant T-atom sites of a BEA zeolite as isolated, mononuclear Mn: FTIR, XPS, EPR and DR UV-Vis studies. Phys. Chem. Chem. Phys. 18, 12050–12057. doi: 10.1039/c6cp01713d
Beale, A. M., Gao, F., Lezcano-Gonzalez, I., Peden, C. H., and Szanyi, J. (2015). Recent advances in automotive catalysis for NOx emission control by small-pore microporous materials. Chem. Soc. Rev. 44, 7371–7405. doi: 10.1039/c5cs00108k
Berchel, M., Le Gall, T., Denis, C., Le Hir, S., Quentel, F., Elléouet, C., et al. (2011). A silver-based metal–organic framework material as a ‘reservoir’of bactericidal metal ions. New J. Chem. 35, 1000–1003. doi: 10.1039/c1nj20202b
Bhanja, P., Mishra, S., Manna, K., Mallick, A., Das Saha, K., and Bhaumik, A. (2017). Covalent organic framework material bearing phloroglucinol building units as a potent anticancer agent. ACS Appl. Mater. Interfaces 9, 31411–31423. doi: 10.1021/acsami.7b07343
Boosari, S., Eskandari, S., Shakouri, A., and Fathizadeh, M. (2018). Effect of heating period and temperature on the synthesis of nano-beta zeolite assisted by microwaves. J. Membr. Sci. Technol. 8:180. doi: 10.4172/2155-9589.1000180
Brown, E. D., and Wright, G. D. (2016). Antibacterial drug discovery in the resistance era. Nature 529, 336–343. doi: 10.1038/nature17042
Cagetti, M., Mastroberardino, S., Milia, E., Cocco, F., Lingström, P., and Campus, G. (2013). The use of probiotic strains in caries prevention: a systematic review. Nutrients 5, 2530–2550. doi: 10.3390/nu5072530
Cai, W., Wang, J., Chu, C., Chen, W., Wu, C., and Liu, G. (2019). Metal–organic framework-based stimuli-responsive systems for drug delivery. Adv. Sci. 6:1801526. doi: 10.1002/advs.201801526
Cao, P., Wu, X., Zhang, W., Zhao, L., Sun, W., and Tang, Z. (2020). Killing oral bacteria using metal–organic frameworks. Ind. Eng. Chem. Res. 59, 1559–1567. doi: 10.1021/acs.iecr.9b05659
Carné, A., Carbonell, C., Imaz, I., and Maspoch, D. (2011). Nanoscale metal–organic materials. Chem. Soc. Rev. 40, 291–305. doi: 10.1039/c0cs00042f
Casemiro, L. A., Martins, C. H. G., Pires-de-Souza, F., de, C. P., and Panzeri, H. (2008). Antimicrobial and mechanical properties of acrylic resins with incorporated silver–zinc zeolite – part I. Gerodontology 25, 187–194. doi: 10.1111/j.1741-2358.2007.00198.x
Chen, J., Zhang, X., Huang, C., Cai, H., Hu, S., Wan, Q., et al. (2017). Osteogenic activity and antibacterial effect of porous titanium modified with metal-organic framework films. J. Biomed. Mater. Res. A 105, 834–846. doi: 10.1002/jbm.a.35960
Chen, S., Popovich, J., Iannuzo, N., Haydel, S. E., and Seo, D.-K. (2017). Silver-ion-exchanged nanostructured zeolite X as antibacterial agent with superior ion release kinetics and efficacy against methicillin-resistant Staphylococcus aureus. ACS Appl. Mater. Interfaces 9, 39271–39282. doi: 10.1021/acsami.7b15001
Chen, Y., Chen, X., Yu, H., Zhou, H., and Xu, S. (2019). Oral microbiota as promising diagnostic biomarkers for gastrointestinal cancer: a systematic review. Onco Targets Ther. 12, 11131–11144. doi: 10.2147/ott.s230262
Chiericatti, C., Basilico, J. C., Basilico, M. L. Z., and Zamaro, J. M. (2012). Novel application of HKUST-1 metal–organic framework as antifungal: biological tests and physicochemical characterizations. Microporous Mesoporous Mater. 162, 60–63. doi: 10.1016/j.micromeso.2012.06.012
Çınar, Ç., Ulusu, T., Özçelik, B., Karamüftüoğlu, N., and Yücel, H. (2009). Antibacterial effect of silver-zeolite containing root-canal filling material. J. Biomed. Mater. Res. B Appl. Biomater. 90, 592–595. doi: 10.1002/jbm.b.31321
Cui, W. G., Hu, T. L., and Bu, X. H. (2019). Metal–organic framework materials for the separation and purification of light hydrocarbons. Adv. Mater. 32:e1806445. doi: 10.1002/adma.201806445
Darveau, R. P. (2010). Periodontitis: a polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 8, 481–490. doi: 10.1038/nrmicro2337
Della Rocca, J., Liu, D., and Lin, W. (2011). Nanoscale metal–organic frameworks for biomedical imaging and drug delivery. Acc. Chem. Res. 44, 957–968. doi: 10.1021/ar200028a
Dizaj, S. M., Lotfipour, F., Barzegar-Jalali, M., Zarrintan, M. H., and Adibkia, K. (2014). Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 44, 278–284. doi: 10.1016/j.msec.2014.08.031
Dong, B., Belkhair, S., Zaarour, M., Fisher, L., Verran, J., Tosheva, L., et al. (2014). Silver confined within zeolite EMT nanoparticles: preparation and antibacterial properties. Nanoscale 6, 10859–10864. doi: 10.1039/c4nr03169e
Dreyer, H., Grischke, J., Tiede, C., Eberhard, J., Schweitzer, A., Toikkanen, S. E., et al. (2018). Epidemiology and risk factors of peri-implantitis: a systematic review. J. Periodontal Res. 53, 657–681. doi: 10.1111/jre.12562
Du, B. D., Phu, D. V., Quoc, L. A., and Hien, N. Q. (2017). Synthesis and investigation of antimicrobial activity of Cu2O nanoparticles/zeolite. J. Nanopart. 138, 2823–2828. doi: 10.1155/2017/7056864
Dzwigaj, S., and Che, M. (2006). Incorporation of Co (II) in dealuminated BEA zeolite at lattice tetrahedral sites evidenced by XRD, FTIR, diffuse reflectance UV- Vis, EPR, and TPR. J. Phys. Chem. B 110, 12490–12493. doi: 10.1021/jp0623387
Emam, H. E., Darwesh, O. M., and Abdelhameed, R. M. (2018). In-growth metal organic framework/synthetic hybrids as antimicrobial fabrics and its toxicity. Colloids Surf. B Biointerfaces 165, 219–228. doi: 10.1016/j.colsurfb.2018.02.028
Fakin, T., Ristić, A., Mavrodinova, V., and Zabukovec Logar, N. (2015). Highly crystalline binder-free ZSM-5 granules preparation. Microporous Mesoporous Mater. 213, 108–117. doi: 10.1016/j.micromeso.2015.04.010
Fang, F. C. (1997). Perspectives series: host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J. Clin. Invest. 99, 2818–2825. doi: 10.1172/jci119473
Fang, Q., Wang, J., Gu, S., Kaspar, R. B., Zhuang, Z., Zheng, J., et al. (2015). 3D porous crystalline polyimide covalent organic frameworks for drug delivery. J. Am. Chem. Soc. 137, 8352–8355. doi: 10.1021/jacs.5b04147
Ferreira, L., Fonseca, A. M., Botelho, G., Almeida-Aguiar, C., and Neves, I. C. (2012). Antimicrobial activity of faujasite zeolites doped with silver. Microporous Mesoporous Mater. 160, 126–132. doi: 10.1016/j.micromeso.2012.05.006
Ferreira, L., Guedes, J. F., Almeida-Aguiar, C., Fonseca, A. M., and Neves, I. C. (2016). Microbial growth inhibition caused by Zn/Ag-Y zeolite materials with different amounts of silver. Colloids Surf. B Biointerfaces 142, 141–147. doi: 10.1016/j.colsurfb.2016.02.042
Firouzjaei, M. D., Shamsabadi, A. A., Sharifian, G. M., Rahimpour, A., and Soroush, M. (2018). A novel nanocomposite with superior antibacterial activity: a silver-based metal organic framework embellished with graphene oxide. Adv. Mater. Interfaces 5:1701365. doi: 10.1002/admi.201701365
Gendreau, L., and Loewy, Z. G. (2011). Epidemiology and etiology of denture stomatitis. J. Prosthodont. 20, 251–260. doi: 10.1111/j.1532-849x.2011.00698.x
Ghivari, S. B., Bhattacharya, H., Bhat, K. G., and Pujar, M. A. (2017). Antimicrobial activity of root canal irrigants against biofilm forming pathogens-An in vitro study. J. Conserv. Dent. 20, 147–151. doi: 10.4103/jcd.jcd_38_16
Golmohamadpour, A., Bahramian, B., Khoobi, M., Pourhajibagher, M., Barikani, H. R., and Bahador, A. (2018). Antimicrobial photodynamic therapy assessment of three indocyanine green-loaded metal-organic frameworks against Enterococcus faecalis. Photodiagnosis Photodyn. Ther. 23, 331–338. doi: 10.1016/j.pdpdt.2018.08.004
González-Cabezas, C., Li, Y., Gregory, R. L., and Stookey, G. K. (1999). Distribution of three cariogenic bacteria in secondary carious lesions around amalgam restorations. Caries Res. 33, 357–365. doi: 10.1159/000016534
Guerra, W., Silva-Caldeira, P. P., Terenzi, H., and Pereira-Maia, E. C. (2016). Impact of metal coordination on the antibiotic and non-antibiotic activities of tetracycline-based drugs. Coord. Chem. Rev. 327, 188–199. doi: 10.1016/j.ccr.2016.04.009
Guo, Y.-F., Fang, W.-J., Fu, J.-R., Wu, Y., Zheng, J., Gao, G.-Q., et al. (2018). Facile synthesis of Ag@ZIF-8 core-shell heterostructure nanowires for improved antibacterial activities. Appl. Surf. Sci. 435, 149–155. doi: 10.1016/j.apsusc.2017.11.096
Guo, Y.-P., Long, T., Song, Z.-F., and Zhu, Z.-A. (2014). Hydrothermal fabrication of ZSM-5 zeolites: biocompatibility, drug delivery property, and bactericidal property. J. Biomed. Mater. Res. B Appl. Biomater. 102, 583–591. doi: 10.1002/jbm.b.33037
Hatamie, S., Ahadian, M. M., Soufi Zomorod, M., Torabi, S., Babaie, A., Hosseinzadeh, S., et al. (2019). Antibacterial properties of nanoporous graphene oxide/cobalt metal organic framework. Mater. Sci. Eng. C Mater. Biol. Appl. 104:109862. doi: 10.1016/j.msec.2019.109862
Hedström, A. (2001). Ion exchange of ammonium in zeolites: a literature review. J. Environ. Eng. 127, 673–681. doi: 10.1061/(asce)0733-93722001127:8(673)
Herrera, D., Matesanz, P., Bascones-Martínez, A., and Sanz, M. (2012). Local and systemic antimicrobial therapy in periodontics. J. Evid. Based Dent. Pract. 12, 50–60. doi: 10.1016/s1532-3382(12)70013-1
Hollanda, A. C. B., de Alencar, A. H. G., de Araújo Estrela, C. R., Bueno, M. R., and Estrela, C. (2008). Prevalence of endodontically treated teeth in a Brazilian adult population. Braz. Dent. J. 19, 313–317. doi: 10.1590/s0103-64402008000400005
Horcajada, P., Chalati, T., Serre, C., Gillet, B., Sebrie, C., Baati, T., et al. (2010). Porous metal–organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 9, 172–178. doi: 10.1038/nmat2608
Horcajada, P., Gref, R., Baati, T., Allan, P. K., Maurin, G., Couvreur, P., et al. (2012). Metal–organic frameworks in biomedicine. Chem. Rev. 112, 1232–1268. doi: 10.1021/cr200256v
Horike, S., Kadota, K., Itakura, T., Inukai, M., and Kitagawa, S. (2015). Synthesis of magnesium ZIF-8 from Mg (BH4)2. Dalton Trans. 44, 15107–15110. doi: 10.1039/c5dt01183c
Huang, N., Wang, P., and Jiang, D. (2016). Covalent organic frameworks: a materials platform for structural and functional designs. Nat. Rev. Mater. 1:16068. doi: 10.1038/natrevmats.2016.68
Huang, X., Li, L.-D., Lyu, G.-M., Shen, B.-Y., Han, Y.-F., Shi, J.-L., et al. (2018). Chitosan-coated cerium oxide nanocubes accelerate cutaneous wound healing by curtailing persistent inflammation. Inorg. Chem. Front. 5, 386–393. doi: 10.1039/c7qi00707h
Hughes, D. (2003). Exploiting genomics, genetics and chemistry to combat antibiotic resistance. Nat. Rev. Genet. 4, 432–441. doi: 10.1038/nrg1084
Huxford, R. C., Rocca, J. D., and Lin, W. (2010). Metal-organic frameworks as potential drug carriers. Curr. Opin. Chem. Biol. 14, 262–268. doi: 10.1016/j.cbpa.2009.12.012
Hynek, J., Zelenka, J., Rathouskyì, J., Kubaìt, P., Ruml, T., Demel, J., et al. (2018). Designing porphyrinic covalent organic frameworks for the photodynamic inactivation of bacteria. ACS Appl. Mater. Interfaces 10, 8527–8535. doi: 10.1021/acsami.7b19835
Izumi, Y., and Nakazawa, T. (1986). Development of zeolite for non-phosphated detergents in Japan. Pure Appl. Chem. 58, 1397–1404. doi: 10.1351/pac198658101397
Jokstad, A. (2016). Secondary caries and microleakage. Dent. Mater. 32, 11–25. doi: 10.1016/j.dental.2015.09.006
Karakeçili, A., Topuz, B., Korpayev, S., and Erdek, M. (2019). Metal-organic frameworks for on-demand pH controlled delivery of vancomycin from chitosan scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 105:110098. doi: 10.1016/j.msec.2019.110098
Kassebaum, N. J., Smith, A. G. C., Bernabé, E., Fleming, T. D., Reynolds, A. E., Vos, T., et al. (2017). Global, regional, and national prevalence, incidence, and disability-adjusted life years for oral conditions for 195 countries, 1990–2015: a systematic analysis for the global burden of diseases, injuries, and risk factors. J. Dent. Res. 96, 380–387. doi: 10.1177/0022034517693566
Kavanagh, N., Ryan, E. J., Widaa, A., Sexton, G., Fennell, J., O’Rourke, S., et al. (2018). Staphylococcal osteomyelitis: disease progression, treatment challenges, and future directions. Clin. Microbiol. Rev. 31:e00084-17. doi: 10.1128/cmr.00084-17
Kawahara, K., Tsuruda, K., Morishita, M., and Uchida, M. (2000). Antibacterial effect of silver-zeolite on oral bacteria under anaerobic conditions. Dent. Mater. 16, 452–455. doi: 10.1016/S0109-5641(00)00050-6
Khademi, S., Roozbehani, B., Hamadanian, M., and Khademi, N. (2020). Synthesis and characterization of high flux and antibacterial film nanocomposite based on epoxy-zeolite NaA. J. Nanostruct. 10, 177–184. doi: 10.22052/jns.2020.01.019
Kihara, T., Zhang, Y., Hu, Y., Mao, Q., Tang, Y., and Miyake, J. (2011). Effect of composition, morphology and size of nanozeolite on its in vitro cytotoxicity. J. Biosci. Bioeng. 111, 725–730. doi: 10.1016/j.jbiosc.2011.01.017
Kotchey, G. P., Allen, B. L., Vedala, H., Yanamala, N., Kapralov, A. A., Tyurina, Y. Y., et al. (2011). The enzymatic oxidation of graphene oxide. ACS Nano 5, 2098–2108. doi: 10.1021/nn103265h
Krishna, R. (2018). Methodologies for screening and selection of crystalline microporous materials in mixture separations. Sep. Purif. Technol. 194, 281–300. doi: 10.1016/j.seppur.2017.11.056
Leão, J. C., Gomes, V. B., and Porter, S. (2007). Ulcerative lesions of the mouth: an update for the general medical practitioner. Clinics 62, 769–780. doi: 10.1590/S1807-59322007000600018
Levi, M. E., and Eusterman, V. D. (2011). Oral infections and antibiotic therapy. Otolaryngol. Clin. North Am. 44, 57–78. doi: 10.1016/j.otc.2010.10.003
Li, H., Eddaoudi, M., O’Keeffe, M., and Yaghi, O. M. (1999). Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 402, 276–279. doi: 10.1038/46248
Li, J., Gopal, A., Karaosmanoglu, S., Lin, J., Munshi, T., Zhang, W., et al. (2020). Photosensitizer doped zeolitic imidazolate framework-8 nanocomposites for combined antibacterial therapy to overcome methicillin-resistant Staphylococcus aureus (MRSA). Colloids Surf. B Biointerfaces 190:110900. doi: 10.1016/j.colsurfb.2020.110900
Li, P., Li, J., Feng, X., Li, J., Hao, Y., Zhang, J., et al. (2019). Metal-organic frameworks with photocatalytic bactericidal activity for integrated air cleaning. Nat. Commun. 10:2177. doi: 10.1038/s41467-019-10218-9
Li, W., Qi, M., Sun, X., Chi, M., Wan, Y., Zheng, X., et al. (2020). Novel dental adhesive containing silver exchanged EMT zeolites against cariogenic biofilms to combat dental caries. Microporous Mesoporous Mater. 299:110113. doi: 10.1016/j.micromeso.2020.110113
Li, S., Dong, S., Xu, W., Tu, S., Yan, L., Zhao, C., et al. (2018). Antibacterial hydrogels. Adv. Sci. 5:1700527. doi: 10.1002/advs.201700527
Li, X., Qi, M., Li, C., Dong, B., Wang, J., Weir, M. D., et al. (2019). Novel nanoparticles of cerium-doped zeolitic imidazolate frameworks with dual benefits of antibacterial and anti-inflammatory functions against periodontitis. J. Mater. Chem. B 7, 6955–6971. doi: 10.1039/c9tb01743g
Li, X., and Wang, Q. (2015). Effects of different root canal preparation methods on root fracture resistance: a systematic review of the literature. World J. Stomatol. 4, 108–114. doi: 10.5321/wjs.v4.i2.108
Li, Z., Hüve, J., Krampe, C., Luppi, G., Tsotsalas, M., Klingauf, J., et al. (2013). Internalization pathways of anisotropic disc-shaped Zeolite L Nanocrystals with different surface properties in HeLa cancer cells. Small 9, 1809–1820. doi: 10.1002/smll.201201702
Li, Z., and Yang, Y.-W. (2017). Creation and bioapplications of porous organic polymer materials. J. Mater. Chem. B 5, 9278–9290. doi: 10.1039/c7tb02647a
Li, Z.-Q., Qiu, L.-G., Xu, T., Wu, Y., Wang, W., Wu, Z.-Y., et al. (2009). Ultrasonic synthesis of the microporous metal–organic framework Cu3(BTC)2 at ambient temperature and pressure: an efficient and environmentally friendly method. Mater. Lett. 63, 78–80. doi: 10.1016/j.matlet.2008.09.010
Lima, E., Guerra, R., Lara, V., and Guzmán, A. (2013). Gold nanoparticles as efficient antimicrobial agents for Escherichia coli and Salmonella typhi. Chem. Cent. J. 7:11. doi: 10.1186/1752-153x-7-11
Lin, J., Ding, J., Dai, Y., Wang, X., Wei, J., and Chen, Y. (2017). Antibacterial zinc oxide hybrid with gelatin coating. Mater. Sci. Eng. C Mater. Biol. Appl. 81, 321–326. doi: 10.1016/j.msec.2017.08.009
Lin, S., Liu, X., Tan, L., Cui, Z., Yang, X., Yeung, K. W., et al. (2017). Porous iron-carboxylate metal–organic framework: a novel bioplatform with sustained antibacterial efficacy and nontoxicity. ACS Appl. Mater. Interfaces 9, 19248–19257. doi: 10.1021/acsami.7b04810
Liu, T., Hu, X., Wang, Y., Meng, L., Zhou, Y., Zhang, J., et al. (2017). Triazine-based covalent organic frameworks for photodynamic inactivation of bacteria as type-II photosensitizers. J. Photochem. Photobiol. B 175, 156–162. doi: 10.1016/j.jphotobiol.2017.07.013
Liu, X., Yan, Z., Zhang, Y., Liu, Z., Sun, Y., Ren, J., et al. (2019). Two-dimensional metal–organic framework/enzyme hybrid nanocatalyst as a benign and self-activated cascade reagent for in vivo wound healing. ACS Nano 13, 5222–5230. doi: 10.1021/acsnano.8b09501
Liu, Y., Gong, C. S., Dai, Y., Yang, Z., Yu, G., Liu, Y., et al. (2019). In situ polymerization on nanoscale metal-organic frameworks for enhanced physiological stability and stimulus-responsive intracellular drug delivery. Biomaterials 218:119365. doi: 10.1016/j.biomaterials.2019.119365
Liu, Z., Tan, L., Liu, X., Liang, Y., Zheng, Y., Yeung, K. W. K., et al. (2020). Zn2+-assisted photothermal therapy for rapid bacteria-killing using biodegradable humic acid encapsulated MOFs. Colloids Surf. B Biointerfaces 188:110781. doi: 10.1016/j.colsurfb.2020.110781
Lu, X., Ye, J., Zhang, D., Xie, R., Bogale, R. F., Sun, Y., et al. (2014). Silver carboxylate metal–organic frameworks with highly antibacterial activity and biocompatibility. J. Inorg. Biochem. 138, 114–121. doi: 10.1016/j.jinorgbio.2014.05.005
Luo, D., Wang, C., Tong, Y., Liu, C., Xiao, Y., Zhu, Z., et al. (2020). An NIF-doped ZIF-8 hybrid membrane for continuous antimicrobial treatment. RSC Adv. 10, 7360–7367. doi: 10.1039/d0ra00108b
Manger, D., Walshaw, M., Fitzgerald, R., Doughty, J., Wanyonyi, K., White, S., et al. (2017). Evidence summary: the relationship between oral health and pulmonary disease. Br. Dent. J. 222, 527–533. doi: 10.1038/sj.bdj.2017.315
Massoudinejad, M., Shahsavani, A., Kamarehie, B., Jafari, A., Ghaderpoori, M., Amini, M. M., et al. (2018). Highly efficient adsorption of fluoride from aqueous solutions by metal organic frameworks: modeling, isotherms, and kinetics. Fluoride 51, 355–365.
Matsumura, Y., Yoshikata, K., Kunisaki, S., and Tsuchido, T. (2003). Mode of bactericidal action of silver zeolite and its comparison with that of silver nitrate. Appl. Environ. Microbiol. 69, 4278–4281. doi: 10.1128/AEM.69.7.4278-4281.2003
McCusker, L. B., and Baerlocher, C. (2005). Zeolite structures. Stud. Surf. Sci. Catal. 157, 41–64. doi: 10.1016/S0167-2991(05)80005-9
Miao, W., Wang, J., Liu, J., and Zhang, Y. (2018). Self-cleaning and antibacterial zeolitic imidazolate framework coatings. Adv. Mater. Interfaces 5:1800167. doi: 10.1002/admi.201800167
Mima, E. G., Vergani, C. E., Machado, A. L., Massucato, E. M. S., Colombo, A. L., Bagnato, V. S., et al. (2012). Comparison of photodynamic therapy versus conventional antifungal therapy for the treatment of denture stomatitis: a randomized clinical trial. Clin. Microbiol. Infect. 18, E380–E388. doi: 10.1111/j.1469-0691.2012.03933.x
Mitra, S., Kandambeth, S., Biswal, B. P., Khayum, M. A., Choudhury, C. K., et al. (2016). Self-exfoliated guanidinium-based ionic covalent organic nanosheets (iCONs). J. Am. Chem. Soc. 138, 2823–2828. doi: 10.1021/jacs.5b13533
Mitra, S., Sasmal, H. S., Kundu, T., Kandambeth, S., Illath, K., Diìaz, D., et al. (2017). Targeted drug delivery in covalent organic nanosheets (CONs) via sequential postsynthetic modification. J. Am. Chem. Soc. 139, 4513–4520. doi: 10.1021/jacs.7b00925
Mohammadi, Z., Soltani, M. K., and Shalavi, S. (2014). An update on the management of endodontic biofilms using root canal irrigants and medicaments. Iran. Endod. J. 9, 89–97.
Mori, W., Takamizawa, S., Kato, C. N., Ohmura, T., and Sato, T. (2004). Molecular-level design of efficient microporous materials containing metal carboxylates: inclusion complex formation with organic polymer, gas-occlusion properties, and catalytic activities for hydrogenation of olefins. Microporous Mesoporous Mater. 73, 31–46. doi: 10.1002/chin.200505273
Nabipour, H., Hossaini Sadr, M., and Rezanejade Bardajee, G. (2017). Release behavior, kinetic and antimicrobial study of nalidixic acid from [Zn2(bdc)2(dabco)] metal-organic frameworks. J. Coord. Chem. 70, 2771–2784. doi: 10.1080/00958972.2017.1363391
Naseri, H., Sharifi, A., Ghaedi, M., Dashtian, K., Khoramrooz, S. S., Manzouri, L., et al. (2018). Sonochemical incorporated of cytosine in Cu-H2bpdc as an antibacterial agent against standard and clinical strains of Proteus mirabilis with rsbA gene. Ultrason. Sonochem. 44, 223–230. doi: 10.1016/j.ultsonch.2018.02.031
Nasrabadi, M., Ghasemzadeh, M. A., and Monfared, M. R. Z. (2019). The preparation and characterization of UiO-66 metal–organic frameworks for the delivery of the drug ciprofloxacin and an evaluation of their antibacterial activities. New J. Chem. 43, 16033–16040. doi: 10.1039/c9nj03216a
Nedeljkovic, I., Teughels, W., De Munck, J., Van Meerbeek, B., and Van Landuyt, K. L. (2015). Is secondary caries with composites a material-based problem? Dent. Mater. 31, e247–e277. doi: 10.1016/j.dental.2015.09.001
Nikawa, H., Hayashi, S., Nikawa, Y., Hamada, T., and Samaranayake, L. P. (1993). Interactions between denture lining material, protein pellicles and Candida albicans. Arch. Oral. Biol. 38, 631–634. doi: 10.1016/0003-9969(93)90132-6
Nikawa, H., Yamamoto, T., Hamada, T., Rahardjo, M., Murata, H., and Nakanoda, S. (1997). Antifungal effect of zeolite-incorporated tissue conditioner against Candida albicans growth and/or acid production. J. Oral Rehabil. 24, 350–357. doi: 10.1046/j.1365-2842.1997.d01-297.x
Nosrati, R., Olad, A., and Nofouzi, K. (2015). A self-cleaning coating based on commercial grade polyacrylic latex modified by TiO2/Ag-exchanged-zeolite-A nanocomposite. Appl. Surf. Sci. 346, 543–553. doi: 10.1016/j.apsusc.2015.04.056
Odabaş, M. E., Çinar, Ç., Akça, G., Araz, Ý., Ulusu, T., and Yücel, H. (2011). Short-term antimicrobial properties of mineral trioxide aggregate with incorporated silver-zeolite. Dent. Traumatol. 27, 189–194. doi: 10.1111/j.1600-9657.2011.00986.x
Omar, G. S., Wilson, M., and Nair, S. P. (2008). Lethal photosensitization of wound-associated microbes using indocyanine green and near-infrared light. BMC Microbiol. 8:111. doi: 10.1186/1471-2180-8-111
Papapanou, P. N. (1996). Periodontal diseases: epidemiology. Ann. Periodontol. 1, 1–36. doi: 10.1902/annals.1996.1.1.1
Papapanou, P. N., and Susin, C. (2017). Periodontitis epidemiology: is periodontitis under-recognized, over-diagnosed, or both? Periodontology 2000, 45–51. doi: 10.1111/prd.12200
Pihlstrom, B. L., Michalowicz, B. S., and Johnson, N. W. (2005). Periodontal diseases. Lancet 366, 1809–1820. doi: 10.1016/S0140-6736(05)67728-8
Popović, Z., Otter, M., Calzaferri, G., and De Cola, L. (2007). Selbstorganisation lebender systeme mit funktionalen nanomaterialien. Angew. Chem. 119, 6301–6304. doi: 10.1002/ange.200701019
Popovych, N., Kyriienko, P., Soloviev, S., Baran, R., Millot, Y., and Dzwigaj, S. (2016). Identification of the silver state in the framework of Ag-containing zeolite by XRD, FTIR, photoluminescence, 109Ag NMR, EPR, DR UV-vis, TEM and XPS investigations. Phys. Chem. Chem. Phys. 18, 29458–29465. doi: 10.1039/c6cp05263k
Prasetya, N., Himma, N. F., Sutrisna, P. D., Wenten, I., and Ladewig, B. P. (2019). A review on emerging organic-containing microporous material membranes for carbon capture and separation. Chem. Eng. J. 2019:123575. doi: 10.1016/j.cej.2019.123575
Qi, M., Chi, M., Sun, X., Xie, X., Weir, M. D., Oates, T. W., et al. (2019). Novel nanomaterial-based antibacterial photodynamic therapies to combat oral bacterial biofilms and infectious diseases. Int. J. Nanomed. 14:6937. doi: 10.2147/ijn.s212807
Rai, M., Yadav, A., and Gade, A. (2009). Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 27, 76–83. doi: 10.1016/j.biotechadv.2008.09.002
Rainey, K., Michalek, S. M., Wen, Z. T., and Wu, H. (2018). Glycosyltransferase-mediated biofilm matrix dynamics and virulence of Streptococcus mutans. Appl. Environ. Microbiol. 85:e02247-18. doi: 10.1128/AEM.02247-18
Ramage, G., Tomsett, K., Wickes, B. L., López-Ribot, J. L., and Redding, S. W. (2004). Denture stomatitis: a role for Candida biofilms. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 98, 53–59. doi: 10.1016/j.tripleo.2003.04.002
Redfern, J., Geerts, L., Seo, J. W., Verran, J., Tosheva, L., and Wee, L. H. (2018). Toxicity and antimicrobial properties of ZnO@ZIF-8 embedded silicone against planktonic and biofilm catheter-associated pathogens. ACS Appl. Nano Mater. 1, 1657–1665. doi: 10.1021/acsanm.8b00140
Restrepo, J., Serroukh, Z., Santiago-Morales, J., Aguado, S., Gómez-Sal, P., Mosquera, M. E. G., et al. (2017). An antibacterial Zn–MOF with hydrazinebenzoate linkers. Eur. J. Inorg. Chem. 2017, 574–580. doi: 10.1002/ejic.201601185
Righolt, A., Jevdjevic, M., Marcenes, W., and Listl, S. (2018). Global-, regional-, and country-level economic impacts of dental diseases in 2015. J. Dent. Res. 97, 501–507. doi: 10.1177/0022034517750572
Rimoli, M. G., Rabaioli, M. R., Melisi, D., Curcio, A., Mondello, S., Mirabelli, R., et al. (2008). Synthetic zeolites as a new tool for drug delivery. J. Biomed. Mater. Res. A 87, 156–164. doi: 10.1002/jbm.a.31763
Ruparelia, J. P., Chatterjee, A. K., Duttagupta, S. P., and Mukherji, S. (2008). Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 4, 707–716. doi: 10.1016/j.actbio.2007.11.006
Saint-Cricq, P., Kamimura, Y., Itabashi, K., Sugawara-Narutaki, A., Shimojima, A., and Okubo, T. (2012). Antibacterial activity of silver-loaded “green zeolites”. Eur. J. Inorg. Chem. 2012, 3398–3402. doi: 10.1002/ejic.201200476
Salehi, H., Mehrasa, M., Nasri-Nasrabadi, B., Doostmohammadi, M., Seyedebrahimi, R., Davari, N., et al. (2017). Effects of nanozeolite/starch thermoplastic hydrogels on wound healing. J. Res. Med. Sci. 22:110. doi: 10.4103/jrms.jrms_1037_16
Santo, C. E., Quaranta, D., and Grass, G. (2012). Antimicrobial metallic copper surfaces kill Staphylococcus haemolyticus via membrane damage. Microbiologyopen 1, 46–52. doi: 10.1002/mbo3.2
Sanz, M., del Castillo, A. M., Jepsen, S., Gonzalez-Juanatey, J. R., D’Aiuto, F., Bouchard, P., et al. (2020). Periodontitis and cardiovascular diseases: consensus report. J. Clin. Periodontol. 47, 268–288. doi: 10.1111/jcpe.13189
Schejn, A., Aboulaich, A., Balan, L., Falk, V., Lalevée, J., Medjahdi, G., et al. (2015). Cu2+-doped zeolitic imidazolate frameworks (ZIF-8): efficient and stable catalysts for cycloadditions and condensation reactions. Catal. Sci. Technol. 5, 1829–1839. doi: 10.1039/c4cy01505c
Schneid, T. R. (1992). An in vitro analysis of a sustained release system for the treatment of denture stomatitis. Spec. Care Dentist. 12, 245–250. doi: 10.1111/j.1754-4505.1992.tb00458.x
Selwitz, R. H., Ismail, A. I., and Pitts, N. B. (2007). Dental caries. Lancet 369, 51–59. doi: 10.1016/S0140-6736(07)60031-2
Shearier, E., Cheng, P., Zhu, Z., Bao, J., Hu, Y. H., and Zhao, F. (2016). Surface defection reduces cytotoxicity of Zn(2-methylimidazole)2(ZIF-8) without compromising its drug delivery capacity. RSC Adv. 6, 4128–4135. doi: 10.1039/C5RA24336J
Shen, X., Zhang, Y., Ma, P., Sutrisno, L., Luo, Z., Hu, Y., et al. (2019). Fabrication of magnesium/zinc-metal organic framework on titanium implants to inhibit bacterial infection and promote bone regeneration. Biomaterials 212, 1–16. doi: 10.1016/j.biomaterials.2019.05.008
Simon-Yarza, T., Mielcarek, A., Couvreur, P., and Serre, C. (2018). Nanoparticles of metal-organic frameworks: on the road to in vivo efficacy in biomedicine. Adv. Mater. 30:1707365. doi: 10.1002/adma.201707365
Siqueira, J. F. (2002). Endodontic infections: concepts, paradigms, and perspectives. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 94, 281–293. doi: 10.1067/moe.2002.126163
Siqueira, J. F., and Rôças, I. N. (2004). Polymerase chain reaction–based analysis of microorganisms associated with failed endodontic treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 97, 85–94. doi: 10.1016/s1079-2104(03)00353-6
Smeets, R., Henningsen, A., Jung, O., Heiland, M., Hammächer, C., and Stein, J. M. (2014). Definition, etiology, prevention and treatment of peri-implantitis-a review. Head Face Med. 10:34. doi: 10.1186/1746-160x-10-34
Song, Z., Wu, Y., Cao, Q., Wang, H., Wang, X., and Han, H. (2018). pH-responsive, light-triggered on-demand antibiotic release from functional metal–organic framework for bacterial infection combination therapy. Adv. Funct. Mater. 28:1800011. doi: 10.1002/adfm.201800011
Strassert, C. A., Otter, M., Albuquerque, R. Q., Höne, A., Vida, Y., Maier, B., et al. (2009). Photoactive hybrid nanomaterial for targeting, labeling, and killing antibiotic-resistant bacteria. Angew. Chem. Int. Ed. Engl. 48, 7928–7931. doi: 10.1002/anie.200902837
Taherzade, S. D., Soleimannejad, J., and Tarlani, A. (2017). Application of metal-organic framework Nano-MIL-100 (Fe) for sustainable release of doxycycline and tetracycline. Nanomaterials 7:215. doi: 10.3390/nano7080215
Tamames-Tabar, C., Cunha, D., Imbuluzqueta, E., Ragon, F., Serre, C., Blanco-Prieto, M. J., et al. (2014). Cytotoxicity of nanoscaled metal–organic frameworks. J. Mater. Chem. B 2, 262–271. doi: 10.1039/c3tb20832j
Tamames-Tabar, C., Imbuluzqueta, E., Guillou, N., Serre, C., Miller, S. R., Elkaïm, E., et al. (2015). A Zn azelate MOF: combining antibacterial effect. CrystEngComm 17, 456–462. doi: 10.1039/c4ce00885e
Thakare, S. R., and Ramteke, S. M. (2017). Fast and regenerative photocatalyst material for the disinfection of E. coli from water: silver nano particle anchor on MOF-5. Catal. Commun. 102, 21–25. doi: 10.1016/j.catcom.2017.06.008
Tonetti, M. S., Jepsen, S., Jin, L., and Otomo-Corgel, J. (2017). Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: a call for global action. J. Clin. Periodontol. 44, 456–462. doi: 10.1111/jcpe.12732
Torres-Giner, S., Torres, A., Ferrándiz, M., Fombuena, V., and Balart, R. (2017). Antimicrobial activity of metal cation-exchanged zeolites and their evaluation on injection-molded pieces of bio-based high-density polyethylene. J. Food Saf. 37:e12348. doi: 10.1111/jfs.12348
Tosheva, L., Belkhair, S., Gackowski, M., Malic, S., Al-Shanti, N., and Verran, J. (2017). Rapid screening of the antimicrobial efficacy of Ag zeolites. Colloids Surf. B Biointerfaces 157, 254–260. doi: 10.1016/j.colsurfb.2017.06.001
Unamuno, X., Imbuluzqueta, E., Salles, F., Horcajada, P., and Blanco-Prieto, M. J. (2018). Biocompatible porous metal-organic framework nanoparticles based on Fe or Zr for gentamicin vectorization. Eur. J. Pharm. Biopharm. 132, 11–18. doi: 10.1016/j.ejpb.2018.08.013
Valtchev, V., Majano, G., Mintova, S., and Pérez-Ramírez, J. (2013). Tailored crystalline microporous materials by post-synthesis modification. Chem. Soc. Rev. 42, 263–290. doi: 10.1039/c2cs35196j
Vos, T., Abajobir, A. A., Abate, K. H., Abbafati, C., Abbas, K., Abdallah, F., et al. (2017). Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390, 1211–1259. doi: 10.1016/s0140-6736(17)32154-2
Vyas, V. S., Vishwakarma, M., Moudrakovski, I., Haase, F., Savasci, G., Ochsenfeld, C., et al. (2016). Exploiting noncovalent interactions in an imine-based covalent organic framework for quercetin delivery. Adv. Mater. 28, 8749–8754. doi: 10.1002/adma.201603006
Wan, S., Guo, J., Kim, J., Ihee, H., and Jiang, D. (2008). A belt-shaped, blue luminescent, and semiconducting covalent organic framework. Angew. Chem. Int. Ed. Engl. 47, 8826–8830. doi: 10.1002/anie.200803826
Wan, S., Guo, J., Kim, J., Ihee, H., and Jiang, D. (2009). A photoconductive covalent organic framework: self-condensed arene cubes composed of eclipsed 2D polypyrene sheets for photocurrent generation. Angew. Chem. Int. Ed. Engl. 48, 5439–5442. doi: 10.1002/anie.200900881
Wang, J., Wang, Z., Guo, S., Zhang, J., Song, Y., Dong, X., et al. (2011). Antibacterial and anti-adhesive zeolite coatings on titanium alloy surface. Microporous Mesoporous Mater. 146, 216–222. doi: 10.1016/j.micromeso.2011.04.005
Wojciechowska, A., Gągor, A., Zierkiewicz, W., Jarząb, A., Dylong, A., and Duczmal, M. (2015). Metal–organic framework in an l-arginine copper (ii) ion polymer: structure, properties, theoretical studies and microbiological activity. RSC Adv. 5, 36295–36306. doi: 10.1039/c5ra02790j
Wuttke, S., Zimpel, A., Bein, T., Braig, S., Stoiber, K., Vollmar, A., et al. (2017). Validating metal-organic framework nanoparticles for their nanosafety in diverse biomedical applications. Adv. Healthc. Mater. 6:1600818. doi: 10.1002/adhm.201600818
Wyszogrodzka, G., Marszałek, B., Gil, B., and Dorożyński, P. (2016). Metal-organic frameworks: mechanisms of antibacterial action and potential applications. Drug Discov. Today 21, 1009–1018. doi: 10.1016/j.drudis.2016.04.009
Ximing, G., Bin, G., Yuanlin, W., and Shuanghong, G. (2017). Preparation of spherical metal–organic frameworks encapsulating Ag nanoparticles and study on its antibacterial activity. Mater. Sci. Eng. C Mater. Biol. Appl. 80, 698–707. doi: 10.1016/j.msec.2017.07.027
Xue, M., Li, B., Qiu, S., and Chen, B. (2016). Emerging functional chiral microporous materials: synthetic strategies and enantioselective separations. Mater. Today 19, 503–515. doi: 10.1016/j.mattod.2016.03.003
Yang, F., Dong, W., He, F., Wang, X., Zhao, S., and Yang, G. (2012). Osteoblast response to porous titanium surfaces coated with zinc-substituted hydroxyapatite. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 113, 313–318. doi: 10.1016/j.tripleo.2011.02.049
Yuan, X., Smith, R. J. Jr., Guan, H., Ionita, C. N., Khobragade, P., Dziak, R., et al. (2016). Hybrid biomaterial with conjugated growth factors and mesenchymal stem cells for ectopic bone formation. Tissue Eng. Part A 22, 928–939. doi: 10.1089/ten.TEA.2016.0052
Zhang, W., Du, L., Bi, F., and He, H. (2015). A novel SrTiO3/HZSM-5 photocatalyst prepared by sol–gel method. Mater. Lett. 157, 103–105. doi: 10.1016/j.matlet.2015.05.056
Zhang, X., Liu, L., Huang, L., Zhang, W., Wang, R., Yue, T., et al. (2019). The highly efficient elimination of intracellular bacteria via a metal organic framework (MOF)-based three-in-one delivery system. Nanoscale 11, 9468–9477. doi: 10.1039/c9nr01284b
Zhang, X., Sun, J., Liu, J., Xu, H., Dong, B., Sun, X., et al. (2018). Label-free electrochemical immunosensor based on conductive Ag contained EMT-style nano-zeolites and the application for α-fetoprotein detection. Sens. Actuators B 255, 2919–2926. doi: 10.1016/j.snb.2017.09.112
Zhang, Y., Zhang, X., Song, J., Jin, L., Wang, X., and Quan, C. (2019). Ag/H-ZIF-8 nanocomposite as an effective antibacterial agent against pathogenic bacteria. Nanomaterials 9:1579. doi: 10.3390/nano9111579
Zhao, F., Liu, H., Mathe, S. D., Dong, A., and Zhang, J. (2018). Covalent organic frameworks: from materials design to biomedical application. Nanomaterials 8:15. doi: 10.3390/nano8010015
Zhu, L., and Zhang, Y.-B. (2017). Crystallization of covalent organic frameworks for gas storage applications. Molecules 22:1149. doi: 10.3390/molecules22071149
Zhu, W., Liu, P., Xiao, S., Wang, W., Zhang, D., and Li, H. (2015). Microwave-assisted synthesis of Ag-doped MOFs-like organotitanium polymer with high activity in visible-light driven photocatalytic NO oxidization. Appl. Catal. B 17, 46–51. doi: 10.1016/j.apcatb.2015.02.003
Keywords: microporous frameworks, nanomaterials, drug delivery, antibacterial, oral infections
Citation: Wan Y, Xu W, Ren X, Wang Y, Dong B and Wang L (2020) Microporous Frameworks as Promising Platforms for Antibacterial Strategies Against Oral Diseases. Front. Bioeng. Biotechnol. 8:628. doi: 10.3389/fbioe.2020.00628
Received: 03 May 2020; Accepted: 22 May 2020;
Published: 12 June 2020.
Edited by:
Jianxun Ding, Changchun Institute of Applied Chemistry (CAS), ChinaReviewed by:
Huakun Xu, University of Maryland, Baltimore, United StatesLei Cheng, Sichuan University, China
Ana Palcic, Rudjer Boskovic Institute, Croatia
Kok Giap Haw, National University of Singapore, Singapore
Copyright © 2020 Wan, Xu, Ren, Wang, Dong and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Biao Dong, ZG9uZ2JAamx1LmVkdS5jbg==; Lin Wang, d2FuZ2xpbjE5ODJAamx1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Yao Wan
Yao Wan Wenzhou Xu
Wenzhou Xu Xuan Ren
Xuan Ren Yu Wang
Yu Wang Biao Dong
Biao Dong Lin Wang
Lin Wang