- 1Division of Trauma and Orthopaedics, Department of Surgery, University of Cambridge, Cambridge, United Kingdom
- 2School of Clinical Medicine, University of Cambridge, Cambridge, United Kingdom
Damage to joints through injury or disease can result in cartilage loss, which if left untreated can lead to inflammation and ultimately osteoarthritis. There is currently no cure for osteoarthritis and management focusses on symptom control. End-stage osteoarthritis can be debilitating and ultimately requires joint replacement in order to maintain function. Therefore, there is growing interest in innovative therapies for cartilage repair. In this systematic literature review, we sought to explore the in vivo evidence for the use of human Mesenchymal Stem Cell-derived Extracellular Vesicles (MSC-EVs) for treating cartilage damage. We conducted a systematic literature review in accordance with the PRISMA protocol on the evidence for the treatment of cartilage damage using human MSC-EVs. Studies examining in vivo models of cartilage damage were included. A risk of bias analysis of the studies was conducted using the SYRCLE tool. Ten case-control studies were identified in our review, including a total of 159 murine subjects. MSC-EVs were harvested from a variety of human tissues. Five studies induced osteoarthritis, including cartilage loss through surgical joint destabilization, two studies directly created osteochondral lesions and three studies used collagenase to cause cartilage loss. All studies in this review reported reduced cartilage loss following treatment with MSC-EVs, and without significant complications. We conclude that transplantation of MSC-derived EVs into damaged cartilage can effectively reduce cartilage loss in murine models of cartilage injury. Additional randomized studies in animal models that recapitulates human osteoarthritis will be necessary in order to establish findings that inform clinical safety in humans.
Introduction
Damage to joints through injury or disease can result in cartilage loss, which if left untreated can lead to inflammation and ultimately osteoarthritis (OA) (Davies-Tuck et al., 2008). OA affects up to three out of 10 people over the age of 60 years (Woolf and Pfleger, 2003), and this is projected to increase substantially (Turkiewicz et al., 2014). There is currently no cure for OA and management is focused on symptom control (Mcalindon et al., 2014). Furthermore, the search for Disease Modifying Osteoarthritis Drugs (DMOAD) has not been fruitful, and there are no approved DMOADs. End-stage OA can be severely debilitating and ultimately requires joint replacement in order to maintain function (Gillam et al., 2013). Joint replacement is costly and carries perioperative morbidity (Berstock et al., 2014) as well as unsatisfactory outcomes (Nilsdotter et al., 2003). Therefore, there is a need for innovative therapies to treat cartilage defects and in doing so, prevent OA.
The established treatment of microfracture for focal cartilage defects aims to encourage endogenous cells to repopulate areas of cartilage loss, but this has demonstrated limited effectiveness (Weber et al., 2018). A large number of studies have investigated tissue engineering and cellular regenerative approaches to treating cartilage defects (Negoro et al., 2018). Acellular biomaterial scaffolds are costly to develop and implantation of these scaffolds into cartilage defects exhibits a high failure rate (Vindas Bolaños et al., 2017). Certain cell-based approaches such as Autologous Chondrocyte Implantation (ACI) can be effective but cause donor-site morbidity (Reddy et al., 2007; Bexkens et al., 2017). Recently, there has been an increasing body of evidence to support the use of Mesenchymal Stem Cells (MSCs) in cartilage repair (Borakati et al., 2018).
MSCs are multipotent adult stromal cells that may be derived from a number of tissues including bone marrow, synovium, adipose, umbilical cord and dental pulp, and so are readily available for autologous harvest (Fernandes et al., 2018; Fabre et al., 2019). Ease of extraction and the potential for ex vivo expansion make MSCs an attractive option for tissue repair. To this end, studies have shown the therapeutic potential of MSC transplantation in promoting regeneration of tissues such as bone, cartilage, and nerve (Katagiri et al., 2017; Freitag et al., 2019; Masgutov et al., 2019). However, MSC transplantation is not without risks. Certain studies have revealed potential immunogenic complications related to repeated allogenic transplantation of MSCs (Cho et al., 2008) and others have reported possible tumorigenic properties (Beckermann et al., 2008). There is also in vivo evidence to suggest that, when transplanted in the presence of malignancy, MSCs may increase the risk of metastasis (Karnoub et al., 2007). Suboptimal engraftment and delocalization from the target site create difficulty in maintaining sustained benefit following transplantation, and suggest that observed long-term benefits may not result from MSC differentiation alone (Zwolanek et al., 2017).
Increasingly so, studies are focussing on the paracrine function of MSCs as the predominant mechanism of their regenerative effects (Linero and Chaparro, 2014; Xu et al., 2016). MSC-derived extracellular vesicles (MSC-EVs) are gaining interest as a cell-free therapeutic option for cartilage repair. As part of MSC secretome, EVs are nanovesicles ranging from 10 nm to several micrometers that contain various components including genetic material in the form of messenger RNA (mRNA), microRNA (miRNA), lipids and bioactive proteins (Di Vizio et al., 2012; Huang et al., 2017; Théry et al., 2018). MSC-EVs are characterized by cell-surface expression of generic EV markers such as CD9, CD81, CD82, TSG101, and Alix. Transplantation of MSC-EVs may carry certain advantages over cell-based therapies. Firstly, accurate quantification of number of transplanted MSCs may be difficult and their effects could also be less predictable than that of EVs within the recipient site, potentially making outcomes of clinical trials less reproducible. Production costs of EVs at a large scale could be lower than that of MSCs (Cha et al., 2018). Furthermore, EVs exert low potential for toxicity and immunogenicity with repeated transplantation (Zhu X. et al., 2017; Saleh et al., 2019), and therefore avoid the undesired immunogenic properties of MSCs (Gu et al., 2015). As a cell-free therapy, MSC-EVs can be stored by cryopreservation whereas MSCs cannot, and therefore have greater potential as an off-the-shelf treatment option (Vlassov et al., 2012).
Through mechanisms including direct receptor interaction, membrane fusion, and internalization, EVs are able to influence recipient cell behavior to promote a variety of effects relevant to cartilage repair. In vitro evidence shows that MSC-EVs exert anti-inflammatory effects through influencing IL-6 and TGF-β secretion by dendritic cells. Furthermore, MSC-EVs are found to contain miRNAs such as miR-21-5p which target the CCR7 gene for degradation (Reis et al., 2018) and non-coding RNA that mediate an anti-inflammatory response (Fatima et al., 2017). Co-culture of MSCs with chondrocytes is found to promote matrix production and chondrocyte chondrogenesis in vitro, and these effects appear to be EV-dependent (Kim et al., 2019). In vitro studies also suggest that chondrocytes take-up MSC-EVs that upregulate type II collagen production (Vonk et al., 2018). Finally, results of recent ex vivo studies have suggested that MSC-conditioned media is able to downregulate the expression of genes that promote extra-cellular matrix degradation such as MMP1, MMP13, and IL-1β in synovial explants (van Buul et al., 2012) facilitating cartilage repair, suggesting a role for EVs (Nawaz et al., 2018).
Recently, there has been increasing interest in the use of MSC-EVs in cartilage repair and some systematic reviews have examined the in vitro evidence for animal MSC-EVs. In this systematic literature review, we sought to explore the in vivo evidence for the use of human-derived MSC-EVs in murine models of cartilage repair.
Materials and Methods
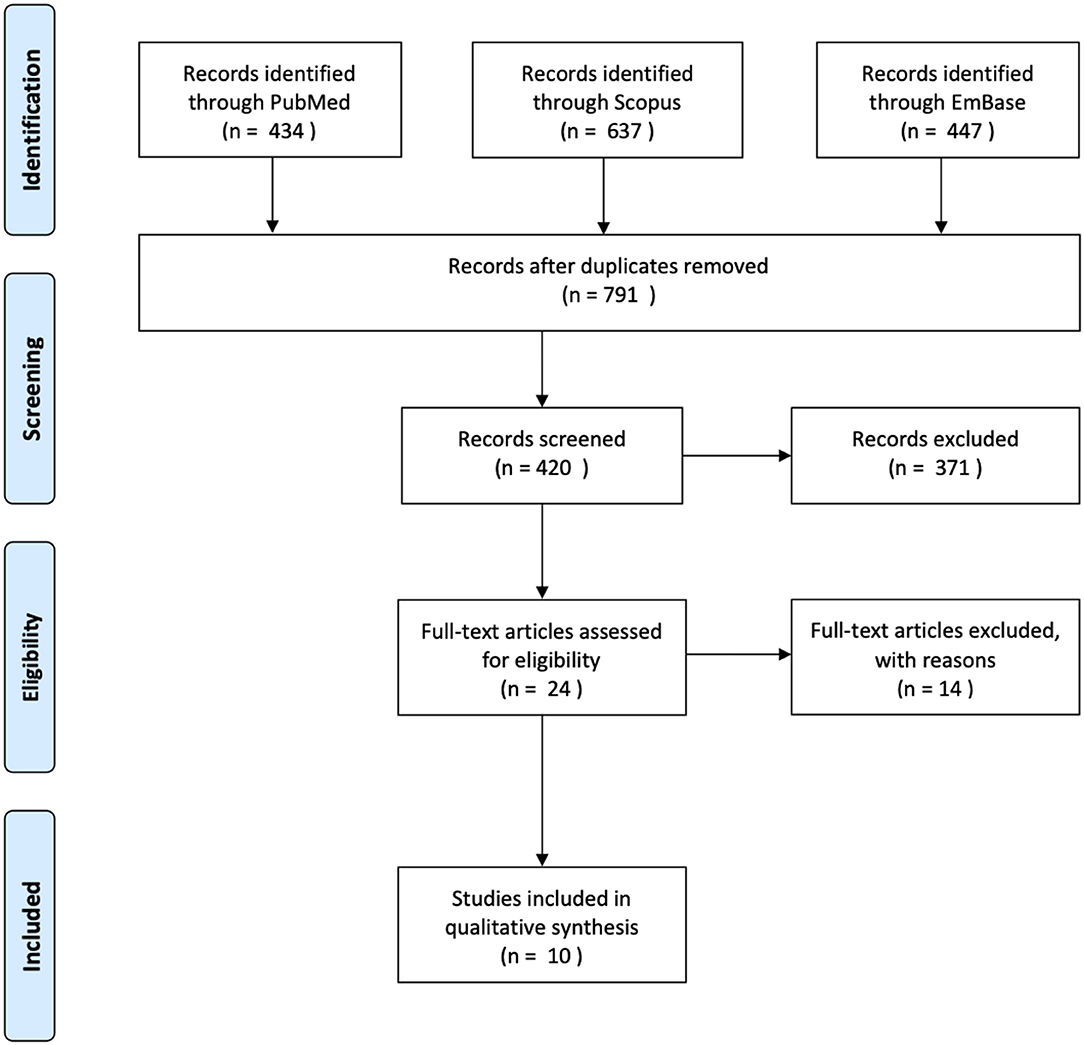
The methods used to conduct this review were according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement protocol (Moher et al., 2015). We carried out a literature search on PubMed, Scopus, and EmBase databases in January 2020, capturing articles starting from conception. The following search strategy was applied: (Mesenchymal stem cell OR MSC* OR Multipotent stromal cell OR Multipotent stem cell OR Mesenchymal stromal cell) AND (Extra-cellular vesicle OR extracellular vesicle OR EV* OR exosomal OR exosome) AND (osteoarthritis OR OA* OR osteochondral OR Cartilage). Following de-duplication, exclusion criteria was applied to studies not written in or translated into the English language. We excluded studies that only performed in vitro experiments. Studies that did not characterize or validate the cell populations as per the recommendations of the International Society for Cellular Therapy (ISCT) for MSC were excluded (Witwer et al., 2019). We included studies that examined the effects of human MSC-derived exosomes, studies examining animal MSC-derived exosomes were excluded. Studies that conducted characterization of EVs in accordance with The International Society for Extracellular Vesicles (ISEV) standards were included (Théry et al., 2018). We included case-control studies, randomized control trials, case series, and case reports with two or more subjects. After removing duplicates, a total of 727 studies underwent title screening (Figure 1). A total of 24 studies were examined in full text. Ten studies were included in our review.
Quality assessment was carried out independently KR and CM using the SYstematic Review Center for Laboratory animal Experimentation (SYRCLE) tool (Hooijmans et al., 2014), discrepancies in results were resolved by discussion.
Results
MSC Characteristics
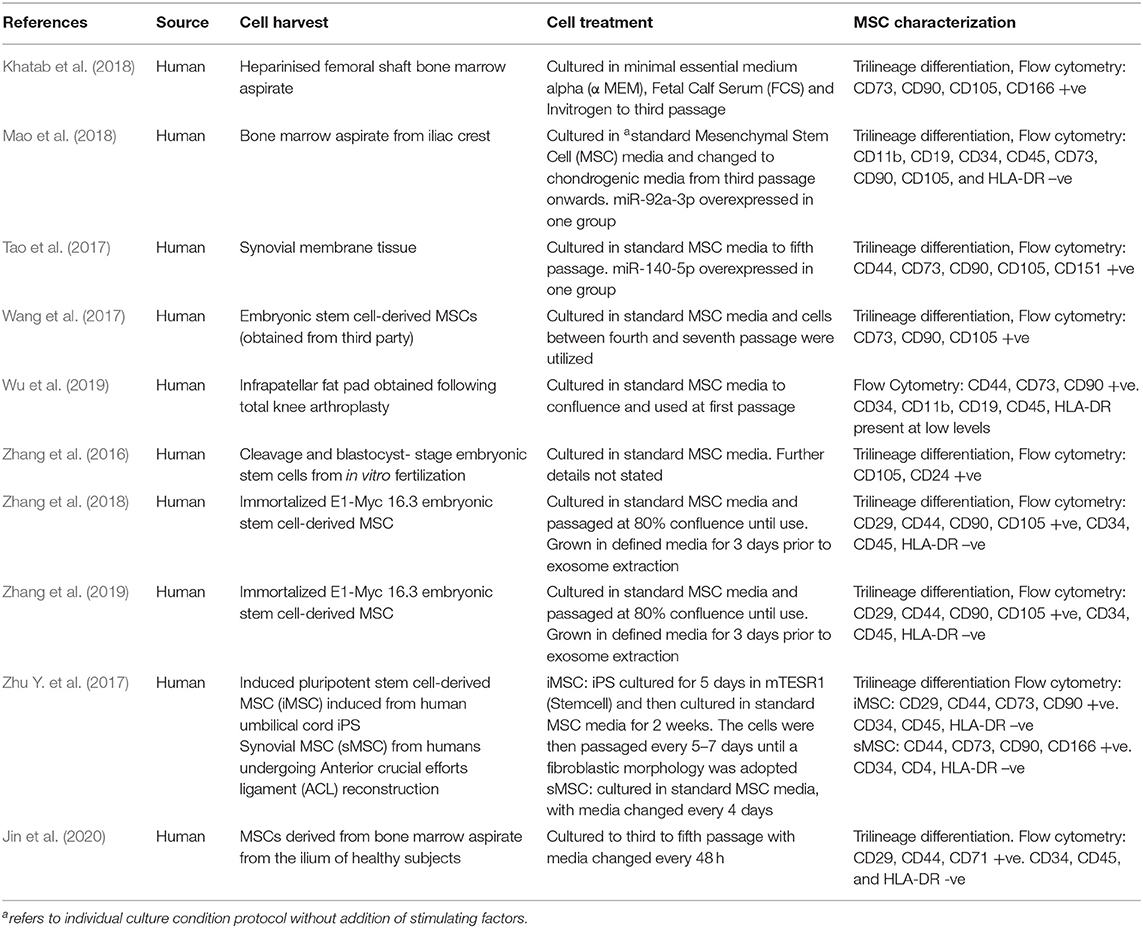
We identified 10 studies in our review, all of which were case-control studies with murine subjects. MSCs were derived from a variety of human tissue sources (Table 1). Four studies (Wang et al., 2017), three by the same group, used MSCs derived from embryonic stem cells (Zhang et al., 2016, 2018, 2019). Three studies obtained MSCs from bone marrow aspirate (Khatab et al., 2018; Mao et al., 2018; Jin et al., 2020), one from infrapatellar fat pad (Wu et al., 2019) and one from synovial tissue (Tao et al., 2017). One study compared EVs from Induced Pluripotent Stem Cell (iPS)-derived MSCs with synovium-derived MSCs (Zhu Y. et al., 2017). All MSCs were characterized using flow cytometry and trilineage differentiation. All MSCs expressed either CD44, CD90 or CD105, and most expressed low levels of HLA-DR.
EV Characteristics
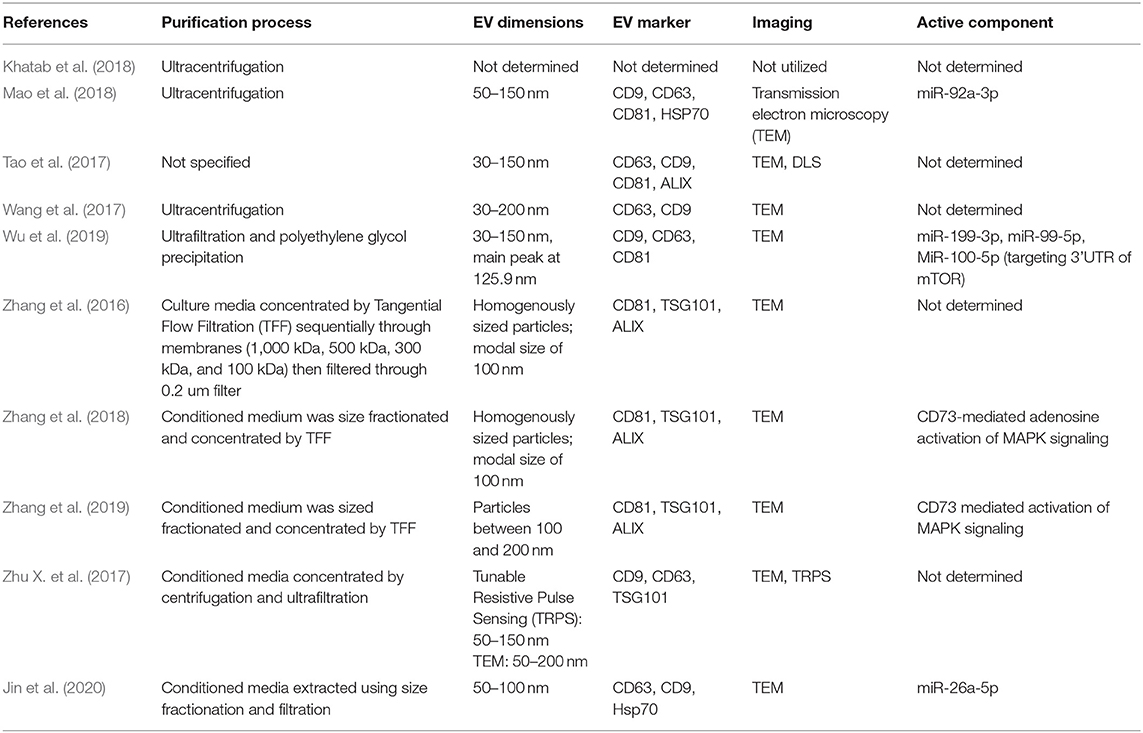
Ultracentrifugation and ultrafiltration were the two most common methods for isolation of exosomes (Table 2). One study used polyethylene glycol precipitation as part of the purification process (Wu et al., 2019), and several used tangential flow filtration (TFF). EV dimensions were determined using Transmission Electron Microscopy (TEM) in all but one study (Khatab et al., 2018). EV size ranged from 30 to 200 nm, with a modal mean size of around 100 nm. Flow cytometry and western blotting were standard methods for characterizing EVs. CD9, CD63, CD81, and ALIX were the most common EV markers identified. Five out of 10 studies that determined the bioactive component of the EVs used with Reverse Transcriptase quantitative Polymerase Chain Reaction (RT-qPCR) or Western blot analysis. Three studies attributed the effects of EVs to various miRNA. Two studies determined that CD73-mediated protein kinase activation was responsible for the in vivo effects of EV transplantation (Zhang et al., 2018, 2019).
Animal Models
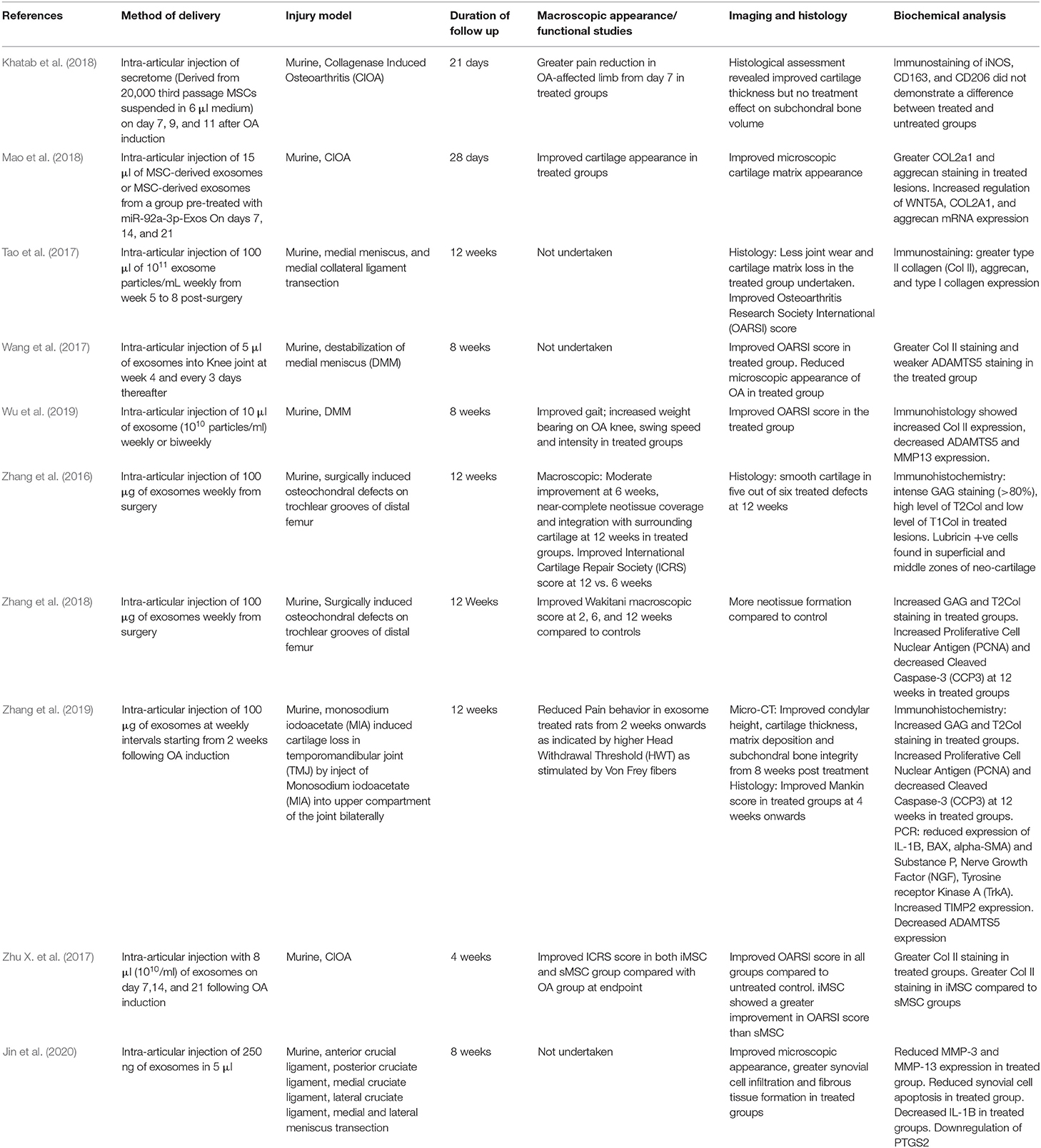
All studies used murine models; five studies induced osteoarthritis, including cartilage loss through surgical joint destabilization, two studies directly created osteochondral lesions and three studies used collagenase to cause cartilage loss. All but one study, which examined the TMJ, studied the knee joint. Follow-up duration ranged from 3 to 12 weeks after induction of cartilage injury. All studies delivered EVs via intra-articular injection. No significant side-effects were reported in any subjects. Varying amounts of EVs were used between studies, and the amount was quantified using different measures (Table 3). Some studies injected a given volume with a known concentration of EVs, whereas others determined the mass of EVs delivered.
In vivo Findings
Two studies measured pain scores and one conducted gait analysis following treatment with EVs, and all three showed improved functional scores. Zhang et al. (2019) used Micro-Computed Tomography (micro-CT) to assess cartilage morphology and found improved bone integrity from 8 weeks onwards. Gene-expression analysis undertaken in several studies. Mao et al. reported increased chondrogenic gene regulation after EV treatment. Using PCR, Zhang et al. (2019) and Jin et al. detected reduced regulation of pro-inflammatory cytokines in their studies.
Joint appearance was interrogated macroscopically, microscopically or using both approaches. All studies reported a reduction in cartilage loss after treatment with EVs. Microscopic histological analysis focused on assessing cartilage thickness and cartilage matrix appearance. Four studies conducted subjective quantification of appearance using the OARSI score. All studies found improved cartilage appearance or OARSI score in the treated groups, although Khatab et al. reported no treatment effect on subchondral bone volume at 3 weeks. Immunohistology was utilized in all studies and all reported increased collagen type II staining with several groups reporting reduced MMP3 immunostaining. Three studies reported reduced staining of apoptotic markers.
Quality of Studies
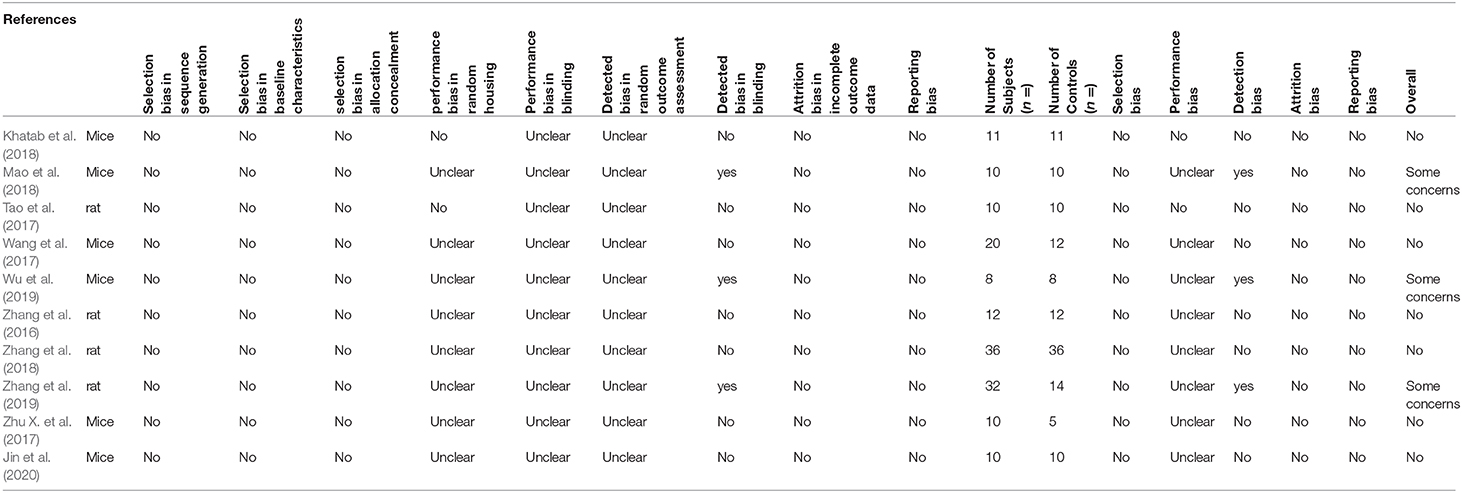
The SYRCLE tool was used to grade each study using 15 different parameters. Seven out of the 10 studies had a low level of concern overall and three studies had some concern toward risk of bias (Table 4). Blinding and detection bias constituted the main contributors to bias in the studies. There was little selection and reporting bias among the studies, but randomization of subjects were not mentioned in most studies. Overall, the studies included in this review were of high quality and low risk of bias.
Discussion
All 10 studies in this review reported reduced cartilage loss following treatment with MSC-EVs. A variety of outcome measures were employed in each study to examine the impact of EVs on cartilage loss, but not all studies found improvements in every parameter measured. A total of 159 subjects were treated with MSC-EVs without significant or immunogenic complications. While all the MSC-EVs were derived from human MSCs, the subjects were all animal models of cartilage injury, and therefore only indirectly inform safety and effectiveness in human subjects.
The capacity for MSCs to expand ex vivo varies with cell source (Fazzina et al., 2016) and anatomical site (Davies et al., 2017). The ability of MSCs to undergo chondrogenic differentiation also appears to differ between cell source (Bernardo et al., 2007). The source of EVs varied significantly between studies as the MSCs were harvested from different tissues and anatomical donor sites. The influence of MSC source on the chondrogenic potential of EVs remains unclear, with some studies suggesting that certain MSC-EVs reduce type I and III collagen production (Li et al., 2013). There is evidence that the biological properties of EVs are dependent on MSC source (Kehl et al., 2019) and it is also apparent that certain MSCs, for example those derived from amniotic fluid, may produce a greater number of EVs than bone marrow-derived MSCs when controlled for cell number (Tracy et al., 2019). In this review, two studies used MSCs from immortalized cell lines (Zhang et al., 2018, 2019) this provides an advantage over autologous harvest as it is not invasive. Zhu et al. compared iPS-derived MSC-EVs with sMSC-derived EVs and found the former to be superior in cartilage repair. Relative cost and convenience of production will dictate which of these is favorable. These are important considerations in tissue engineering as the optimal source cell should achieve a balance between ease of harvest and acceptable EV production. MSC-EV bioactivity also appear to depend on cell-culture conditions, for example, the anti-apoptotic effects of adipose-derived MSC secretome can be affected by oxygen tension (An et al., 2015). Therefore, future studies will be required to delineate the relationship between MSC cell source and secretome in order to select the best source for optimal large-scale EV production.
Most studies in the literature examining EV function, in line with our findings, use ultracentrifugation as the main component of the isolation procedure (Gardiner et al., 2016). There is evidence that suggests that ultracentrifugation could increase the amount of contamination by macromolecules within the MSC culture media (Webber and Clayton, 2013). This may be of relevance in our interpretation as MSC-conditioned media is known to contain non-vesicular bioactive components that may promote chondrogenesis (Chen et al., 2018) and lead to an overestimation of EV effectiveness in cartilage repair. While this may make comparisons difficult, the augmented repair is not necessarily an undesired effect. Furthermore, while the effect of multiple washing stages that form part of the centrifugation process improves purity, it may decrease the total number of EVs obtained (Webber and Clayton, 2013) TFF was the next most commonly used method of concentrating EVs. Compared to ultracentrifugation, TFF achieves a greater EV yield, with a reduced amount of non-vesicular macromolecules contamination (Busatto et al., 2018). Other forms of flow-based purification such as Cross-flow isolation are also favorable over ultracentrifugation in terms of rate of production at large scale (McNamara et al., 2018). Ultimately, robust cost-effectiveness studies may be required to determine the optimal method of purification. The transferability of this review may also be limited by the inconsistent methodologies used by the studies to characterize the EVs used. Apart from one study that did not report on any EV markers (Khatab et al., 2018), all other studies identified markers recognized by the Minimal Information for Studies of Extracellular Vesicles (MISEV) criteria (Théry et al., 2018). EV dimensions were found to range from 30 to 200 nm. The range of 50–150 nm was most commonly reported and may reflect the bias of measurement devices. Future studies should attempt to ascertain the main peak value within the detected range as Wu et al. (2019) did in their study.
Whilst all studies directly injected EVs intra-articularly, the dose delivered was highly varied. Although EV transplantation is yet to be tested in clinical studies, proof-of-concept human clinical trials of intra-articular injection of MSCs to treat cartilage lesions demonstrate a dose-dependent effect without increased risk of adverse effects (Jo et al., 2014). Similar studies will be required to establish such a relationship for EV transplantation. In MSC treatment of cartilage lesions, intra-articular injection appear to promote good engraftment rates with minimal off-site engraftment (Satué et al., 2019). In in vivo studies of anterior cruciate ligament repair, intravenous injection of MSCs concomitantly with intra-articular injection produced improved outcomes (Muir et al., 2016). Likewise, intravenous EV injection appear to produce a dose-dependent immunosuppressive effect that may be beneficial for treating arthritis (Cosenza et al., 2018), but the effect on cartilage repair remains unknown, and the potential for off-site engraftment of EVs is not yet characterized.
All animal studies to date on human-derived MSC-EVs have focussed on murine models. Articular cartilage repair can be studied in murine models in several ways. Firstly, the joint may be surgically destabilized such as in destabilization of medial meniscus (DMM) models, leading to altered weight-bearing and subsequently generalized OA changes, which includes cartilage loss. These models are reliable, reproducible, and have high disease penetrance. The time-course over which cartilage changes develop is delayed and therefore recapitulates the nature of human disease (Glasson et al., 2007). Cartilage can also be excised surgically through induction of an osteochondral defect. These defects may progress at different rates depending on the operative site (Haase et al., 2019), and so makes for difficulty in determining the optimal timing of EV treatment. In contrast, DMM models may progress to display cartilage loss at time points later than direct osteochondral injury and therefore may not benefit from EV treatment in the acute post-injury phase. The amount of lesion healing however appeared to be similar when comparing findings from Zhang et al. (2016) and Zhang et al. (2018), where the same treatment was given after DMM and direct osteochondral injury, respectively. Cartilage loss may also be induced using enzymes or chemicals that degrade the cartilage. Three studies induced chondral injury in murine models using collagenase and one study used monosodium iodoacetate (MIA). Chemical induction is less predictable and may also attenuate the effects of cell-based therapies mimicking its effects on tissue-native cells (Taghizadeh et al., 2018). One study focused on the temporomandibular joint (TMJ). As the pathoaetiology and epidemiology of these non-weight bearing joints typically differ, with radiographic TMJ cartilage loss often being asymptomatic (Schmitter et al., 2010), it may not be relevant to compare functional outcomes following treatment.
It is important to determine standardized methods of assessing outcomes relevant to cartilage repair. Several studies reported improvements in the macroscopic appearance of cartilage; while macroscopic appearance scoring is predictive of histological scoring (Goebel et al., 2017), it is unclear how this correlates with functional improvements. The three studies that used collagenase to induce cartilage loss, often termed collagenase induced OA (CIOA) models, assessed the highly clinically relevant outcomes of pain behavior, with both reporting reduction from early stages. It is difficult to draw conclusions from these results as cartilage loss and eventual OA in the murine model is typically late in onset and appears over 10 weeks following injury (Inglis et al., 2008). It is encouraging however, that all studies reported improved microscopic cartilage repair, with several studies employing various subjective quantitative scoring systems (Tao et al., 2017; Wang et al., 2017; Zhu Y. et al., 2017; Wu et al., 2019). It may be informative to conduct a pooled analysis of such outcomes, but it is unlikely to be meaningful in this study owing to the heterogeneity in scores used. Not all studies reported favorable outcomes when assessing markers of cartilage repair. Khatab et al. conducted an immunohistochemical analysis of iNOS and CD206 expression which are markers of inflammation and M2 macrophage, respectively (Fahy et al., 2014), and found no difference between the groups at the end of the study. Inflammation probably attenuates cartilage repair by interfering with chondrocyte activity and so could be of greater relevance in the acute stage (Tung et al., 2002). The majority of studies showed improved collagen immunostaining, and greater expression of chondrogenic genes in tissue samples. Several groups evaluated cell apoptosis as an outcome and found decreased expression of markers of apoptosis following treatment (Zhang et al., 2018; Jin et al., 2020). Indeed, chondrocyte apoptosis may be reflective of matrix depletion in the cartilage (Kim et al., 2001).
In addition to promoting chondrogenesis in chondrocytes, it is likely that EVs contribute to cartilage repair via effects on other cell types. Macrophages are postulated to be a key target for MSC-EVs, with evidence suggesting that EVs cause an M2 phenotype polarization that promotes resolution of inflammation and so promotes cartilage repair (Chen et al., 2019). This notion is supported by the fact that macrophages pre-conditioned with MSC-EVs, termed EV-educated macrophages (EEMs) support tendon healing in vivo to a greater extent than treatment with MSC-EVs alone (Chamberlain et al., 2019). Others suggest that MSC-EV secretome actually augments the immunomodulatory effects of MSCs via autocrine action. It appears that IL-1β-pretreated MSCs induce macrophages into an anti-inflammatory phenotype only when in the presence of EVs containing miR-146a (Song et al., 2017). Transplantation of circulating EVs from septic mice however, appear to encourage neutrophil migration and macrophage inflammation; this is attributed to certain miRNAs including miR-126-3p, miR-222-3p, and miR-181a-5p, suggesting that EV-dependent modulation of inflammation is content and context dependent (Xu et al., 2018). Further to this, caution should be exercised with MSC-EV transplantation in certain patient cohorts, as MSC-EV have been shown to attenuate the ability of macrophages to suppress cancer cells, and in doing so promotes tumorigenicity (Ren et al., 2019).
Conclusion
Due to the plethora of pathways through which MSC-EVs can promote cartilage repair, a key step to studying their effects in animal models is to establish the roles of the different bioactive components within EVs. Similarly, outcome measures utilized in studies should complement this. We recommend that cartilage appearance and chondrogenic gene expression should be primary outcomes in addition to quantifiable and clinically relevant functional outcomes such as pain reduction or animal gait analysis. Likewise, it would be beneficial to establish a link between functional and histological outcomes, as the value of assessing histology may otherwise be minimal. In order to establish the optimal way to deliver clinical benefit using MSC-EVs, the most efficient MSC cell source, methodology of cell culture and EV purification should be investigated for the purposes of cartilage repair. Finally, randomized studies in animal models that recapitulates the human disease will be necessary in order to establish a dose-response relationship and therefore clinical safety before we proceed to human trials.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author/s.
Author Contributions
KT and WK conceptualized the review manuscript. KT wrote the manuscript under the supervision of FH and WK. KT and AK assembled study data and conducted literature searches. KR and CM conducted data analysis and risk of bias analysis. All authors contributed to editing and approving the manuscript.
Funding
The authors gratefully acknowledge the financial support of Versus Arthritis (Formerly Arthritis Research UK) through Versus Arthritis Tissue Engineering & Regenerative Therapies Center (Grant 21156).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
An, H. Y., Shin, H. S., Choi, J. S., Kim, H. J., Lim, J. Y., and Kim, Y. M. (2015). Adipose mesenchymal stem cell secretome modulated in hypoxia for remodeling of radiation-induced salivary gland damage. PLoS ONE 10:e0141862. doi: 10.1371/journal.pone.0141862
Beckermann, B. M., Kallifatidis, G., Groth, A., Frommhold, D., Apel, A., Mattern, J., et al. (2008). VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma. Br. Cancer J. 99, 622–631. doi: 10.1038/sj.bjc.6604508
Bernardo, M. E., Emons, J. A. M., Karperien, M., Nauta, A. J., Willemze, R., Roelofs, H., et al. (2007). Human mesenchymal stem cells derived from bone marrow display a better chondrogenic differentiation compared with other sources. Connect. Tissue Res. 48, 132–140. doi: 10.1080/03008200701228464
Berstock, J. R., Beswick, A. D., Lenguerrand, E., Whitehouse, M. R., and Blom, A. W. (2014). Mortality after total hip replacement surgery: a systematic review. Bone Joint Res. 3, 175–182. doi: 10.1302/2046-3758.36.2000239
Bexkens, R., Ogink, P. T., Doornberg, J. N., Kerkhoffs, G. M. M. J., Eygendaal, D., Oh, L. S., et al. (2017). Donor-site morbidity after osteochondral autologous transplantation for osteochondritis dissecans of the capitellum: a systematic review and meta-analysis. Knee Surg. Sports Traumatol. Arthrosc. 25, 2237–2246. doi: 10.1007/s00167-017-4516-8
Borakati, A., Mafi, R., Mafi, P., and Khan, W. S. (2018). A systematic review and meta-analysis of clinical trials of mesenchymal stem cell therapy for cartilage repair. Curr. Stem Cell Res. Ther. 13, 215–225. doi: 10.2174/1574888X12666170915120620
Busatto, S., Vilanilam, G., Ticer, T., Lin, W.-L., Dickson, D., Shapiro, S., et al. (2018). Tangential flow filtration for highly efficient concentration of extracellular vesicles from large volumes of fluid. Cells 7:273. doi: 10.3390/cells7120273
Cha, J. M., Shin, E. K., Sung, J. H., Moon, G. J., Kim, E. H., Cho, Y. H., et al. (2018). Efficient scalable production of therapeutic microvesicles derived from human mesenchymal stem cells. Sci. Rep. 8:1171. doi: 10.1038/s41598-018-19211-6
Chamberlain, C. S., Clements, A. E. B., Kink, J. A., Choi, U., Baer, G. S., Halanski, M. A., et al. (2019). Extracellular vesicle-educated macrophages promote early achilles tendon healing. Stem Cells 37, 652–662. doi: 10.1002/stem.2988
Chen, P., Zheng, L., Wang, Y., Tao, M., Xie, Z., Xia, C., et al. (2019). Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics 9, 2439–2459. doi: 10.7150/thno.31017
Chen, Y. C., Chang, Y. W., Tan, K. P., Shen, Y. S., Wang, Y. H., and Chang, C. H. (2018). Can mesenchymal stem cells and their conditioned medium assist inflammatory chondrocytes recovery? PLoS ONE 13:e0205563. doi: 10.1371/journal.pone.0205563
Cho, P. S., Messina, D. J., Hirsh, E. L., Chi, N., Goldman, S. N., Lo, D. P., et al. (2008). Immunogenicity of umbilical cord tissue-derived cells. Blood 111, 430–438. doi: 10.1182/blood-2007-03-078774
Cosenza, S., Toupet, K., Maumus, M., Luz-Crawford, P., Blanc-Brude, O., Jorgensen, C., et al. (2018). Mesenchymal stem cells-derived exosomes are more immunosuppressive than microparticles in inflammatory arthritis. Theranostics 8, 1399–1410. doi: 10.7150/thno.21072
Davies, B. M., Snelling, S. J. B., Quek, L., Hakimi, O., Ye, H., Carr, A., et al. (2017). Identifying the optimum source of mesenchymal stem cells for use in knee surgery. J. Orthop. Res. 35, 1868–1875. doi: 10.1002/jor.23501
Davies-Tuck, M. L., Wluka, A. E., Wang, Y., Teichtahl, A. J., Jones, G., Ding, C., et al. (2008). The natural history of cartilage defects in people with knee osteoarthritis. Osteoarthr. Cartil. 16, 337–342. doi: 10.1016/j.joca.2007.07.005
Di Vizio, D., Morello, M., Dudley, A. C., Schow, P. W., Adam, R. M., Morley, S., et al. (2012). Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. Am. J. Pathol. 181, 1573–1584. doi: 10.1016/j.ajpath.2012.07.030
Fabre, H., Ducret, M., Degoul, O., Rodriguez, J., Perrier-Groult, E., Aubert-Foucher, E., et al. (2019). Characterization of different sources of human MSCs expanded in serum-free conditions with quantification of chondrogenic induction in 3D. Stem Cells Int. 2019:2186728. doi: 10.1155/2019/2186728
Fahy, N., de Vries-van Melle, M. L., Lehmann, J., Wei, W., Grotenhuis, N., Farrell, E., et al. (2014). Human osteoarthritic synovium impacts chondrogenic differentiation of mesenchymal stem cells via macrophage polarisation state. Osteoarthr. Cartil. 22, 1167–1175. doi: 10.1016/j.joca.2014.05.021
Fatima, F., Ekstrom, K., Nazarenko, I., Maugeri, M., Valadi, H., Hill, A. F., et al. (2017). Non-coding RNAs in mesenchymal stem cell-derived extracellular vesicles: deciphering regulatory roles in stem cell potency, inflammatory resolve, and tissue regeneration. Front. Genet. 8:161. doi: 10.3389/fgene.2017.00161
Fazzina, R., Iudicone, P., Fioravanti, D., Bonanno, G., Totta, P., Zizzari, I. G., et al. (2016). Potency testing of mesenchymal stromal cell growth expanded in human platelet lysate from different human tissues. Stem Cell Res. Ther. 7:122. doi: 10.1186/s13287-016-0383-3
Fernandes, T. L., Kimura, H. A., Pinheiro, C. C. G., Shimomura, K., Nakamura, N., Ferreira, J. R., et al. (2018). Human synovial mesenchymal stem cells good manufacturing practices for articular cartilage regeneration. Tissue Eng. Part C Methods 24, 709–716. doi: 10.1089/ten.tec.2018.0219
Freitag, J., Bates, D., Wickham, J., Shah, K., Huguenin, L., Tenen, A., et al. (2019). Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: a randomized controlled trial. Regen. Med. 14, 213–230. doi: 10.2217/rme-2018-0161
Gardiner, C., Di Vizio, D., Sahoo, S., Théry, C., Witwer, K. W., Wauben, M., et al. (2016). Techniques used for the isolation and characterization of extracellular vesicles: results of a worldwide survey. J. Extracell. Vesicles 5:32945. doi: 10.3402/jev.v5.32945
Gillam, M. H., Lie, S. A., Salter, A., Furnes, O., Graves, S. E., Havelin, L. I., et al. (2013). The progression of end-stage osteoarthritis: analysis of data from the Australian and Norwegian joint replacement registries using a multi-state model. Osteoarthr. Cartil. 21, 405–412. doi: 10.1016/j.joca.2012.12.008
Glasson, S. S., Blanchet, T. J., and Morris, E. A. (2007). The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthr. Cartil. 15, 1061–1069. doi: 10.1016/j.joca.2007.03.006
Goebel, L., Orth, P., Cucchiarini, M., Pape, D., and Madry, H. (2017). Macroscopic cartilage repair scoring of defect fill, integration and total points correlate with corresponding items in histological scoring systems – a study in adult sheep. Osteoarthr. Cartil. 25, 581–588. doi: 10.1016/j.joca.2016.10.014
Gu, L.-H., Zhang, T.-T., Li, Y., Yan, H.-J., Qi, H., and Li, F.-R. (2015). Immunogenicity of allogeneic mesenchymal stem cells transplanted via different routes in diabetic rats. Cell. Mol. Immunol. 12, 444–455. doi: 10.1038/cmi.2014.70
Haase, T., Sunkara, V., Kohl, B., Meier, C., Bußmann, P., Becker, J., et al. (2019). Discerning the spatio-temporal disease patterns of surgically induced OA mouse models. PLoS ONE 14:e0213734. doi: 10.1371/journal.pone.0213734
Hooijmans, C. R., Rovers, M. M., De Vries, R. B. M., Leenaars, M., Ritskes-Hoitinga, M., and Langendam, M. W. (2014). SYRCLE's risk of bias tool for animal studies. BMC Med. Res. Methodol. 14:43. doi: 10.1186/1471-2288-14-43
Huang, C., Quinn, D., Sadovsky, Y., Suresh, S., and Hsia, K. J. (2017). Formation and size distribution of self-assembled vesicles. Proc. Natl. Acad. Sci. U.S.A. 114, 2910–2915. doi: 10.1073/pnas.1702065114
Inglis, J. J., McNamee, K. E., Chia, S. L., Essex, D., Feldmann, M., Williams, R. O., et al. (2008). Regulation of pain sensitivity in experimental osteoarthritis by the endogenous peripheral opioid system. Arthritis Rheum. 58, 3110–3119. doi: 10.1002/art.23870
Jin, Z., Ren, J., and Qi, S. (2020). Human bone mesenchymal stem cells-derived exosomes overexpressing microRNA-26a-5p alleviate osteoarthritis via down-regulation of PTGS2. Int. Immunopharmacol. 78:105946. doi: 10.1016/j.intimp.2019.105946
Jo, C. H., Lee, Y. G., Shin, W. H., Kim, H., Chai, J. W., Jeong, E. C., et al. (2014). Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells 32, 1254–1266. doi: 10.1002/stem.1634
Karnoub, A. E., Dash, A. B., Vo, A. P., Sullivan, A., Brooks, M. W., Bell, G. W., et al. (2007). Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449, 557–563. doi: 10.1038/nature06188
Katagiri, W., Watanabe, J., Toyama, N., Osugi, M., Sakaguchi, K., and Hibi, H. (2017). Clinical study of bone regeneration by conditioned medium from mesenchymal stem cells after maxillary sinus floor elevation. Implant Dent. 26, 607–612. doi: 10.1097/ID.0000000000000618
Kehl, D., Generali, M., Mallone, A., Heller, M., Uldry, A.-C., Cheng, P., et al. (2019). Proteomic analysis of human mesenchymal stromal cell secretomes: a systematic comparison of the angiogenic potential. NPJ Regen. Med. 4, 1–13. doi: 10.1038/s41536-019-0070-y
Khatab, S., van Osch, G. J. V. M., Kops, N., Bastiaansen-Jenniskens, Y. M., Bos, P. K., Verhaar, J. A. N., et al. (2018). Mesenchymal stem cell secretome reduces pain and prevents carti lage damage in a muri ne osteoarthri ti s model. Eur. Cells Mater. 36, 218–230. doi: 10.22203/eCM.v036a16
Kim, H. A., Suh, D. I., and Song, Y. W. (2001). Relationship between chondrocyte apoptosis and matrix depletion in human articular cartilage. J. Rheumatol. 28, 2038–2045.
Kim, M., Steinberg, D. R., Burdick, J. A., and Mauck, R. L. (2019). Extracellular vesicles mediate improved functional outcomes in engineered cartilage produced from MSC/chondrocyte cocultures. Proc. Natl. Acad. Sci. U.S.A. 116, 1569–1578. doi: 10.1073/pnas.1815447116
Li, T., Yan, Y., Wang, B., Qian, H., Zhang, X., Shen, L., et al. (2013). Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 22, 845–854. doi: 10.1089/scd.2012.0395
Linero, I., and Chaparro, O. (2014). Paracrine effect of mesenchymal stem cells derived from human adipose tissue in bone regeneration. PLoS ONE 9:e107001. doi: 10.1371/journal.pone.0107001
Mao, G., Zhang, Z., Hu, S., Zhang, Z., Chang, Z., Huang, Z., et al. (2018). Exosomes derived from miR-92a-3poverexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res. Ther. 9:247. doi: 10.1186/s13287-018-1004-0
Masgutov, R., Masgutova, G., Mullakhmetova, A., Zhuravleva, M., Shulman, A., Rogozhin, A., et al. (2019). Adipose-derived mesenchymal stem cells applied in fibrin glue stimulate peripheral nerve regeneration. Front. Med. 6:68. doi: 10.3389/fmed.2019.00068
Mcalindon, T. E., Bannuru, R. R., Sullivan, M. C., Arden, N. K., Berenbaum, F., Bierma-Zeinstra, S. M., et al. (2014). OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr. Cartil. 22, 363–388. doi: 10.1016/j.joca.2014.01.003
McNamara, R. P., Caro-Vegas, C. P., Costantini, L. M., Landis, J. T., Griffith, J. D., Damania, B. A., et al. (2018). Large-scale, cross-flow based isolation of highly pure and endocytosis-competent extracellular vesicles. J. Extracell. Vesicles 7:1541396. doi: 10.1080/20013078.2018.1541396
Moher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., et al. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 4:1. doi: 10.1186/2046-4053-4-1
Muir, P., Hans, E. C., Racette, M., Volstad, N., Sample, S. J., Heaton, C., et al. (2016). Autologous bone marrow-derived mesenchymal stem cells modulate molecular markers of inflammation in dogs with cruciate ligament rupture. PLoS ONE 11:e0159095. doi: 10.1371/journal.pone.0159095
Nawaz, M., Shah, N., Zanetti, B., Maugeri, M., Silvestre, R., Fatima, F., et al. (2018). Extracellular vesicles and matrix remodeling enzymes: the emerging roles in extracellular matrix remodeling, progression of diseases and tissue repair. Cells 7:167. doi: 10.3390/cells7100167
Negoro, T., Takagaki, Y., Okura, H., and Matsuyama, A. (2018). Trends in clinical trials for articular cartilage repair by cell therapy. NPJ Regen. Med. 3:17. doi: 10.1038/s41536-018-0055-2
Nilsdotter, A. K., Petersson, I. F., Roos, E. M., and Lohmander, L. S. (2003). Predictors of patient relevant outcome after total hip replacement for osteoarthritis: a prospective study. Ann. Rheum. Dis. 62, 923–930. doi: 10.1136/ard.62.10.923
Reddy, S., Pedowitz, D. I., Parekh, S. G., Sennett, B. J., and Okereke, E. (2007). The morbidity associated with osteochondral harvest from asymptomatic knees for the treatment of osteochondral lesions of the talus. Am. J. Sports Med. 35, 80–85. doi: 10.1177/0363546506290986
Reis, M., Mavin, E., Nicholson, L., Green, K., Dickinson, A. M., and Wang, X. (2018). Mesenchymal stromal cell-derived extracellular vesicles attenuate dendritic cell maturation and function. Front. Immunol. 9:2538. doi: 10.3389/fimmu.2018.02538
Ren, W., Hou, J., Yang, C., Wang, H., Wu, S., Wu, Y., et al. (2019). Extracellular vesicles secreted by hypoxia pre-challenged mesenchymal stem cells promote non-small cell lung cancer cell growth and mobility as well as macrophage M2 polarization via miR-21-5p delivery. J. Exp. Clin. Cancer Res. 38:62. doi: 10.1186/s13046-019-1027-0
Saleh, A. F., Lázaro-Ibáñez, E., Forsgard, M. A. M., Shatnyeva, O., Osteikoetxea, X., Karlsson, F., et al. (2019). Extracellular vesicles induce minimal hepatotoxicity and immunogenicity. Nanoscale 11, 6990–7001. doi: 10.1039/C8NR08720B
Satué, M., Schüler, C., Ginner, N., and Erben, R. G. (2019). Intra-articularly injected mesenchymal stem cells promote cartilage regeneration, but do not permanently engraft in distant organs. Sci. Rep. 9:10153. doi: 10.1038/s41598-019-46554-5
Schmitter, M., Essig, M., Seneadza, V., Balke, Z., Schröder, J., and Rammelsberg, P. (2010). Prevalence of clinical and radiographic signs of osteoarthrosis of the temporomandibular joint in an older persons community. Dentomaxillofac. Radiol. 39, 231–234. doi: 10.1259/dmfr/16270943
Song, Y., Dou, H., Li, X., Zhao, X., Li, Y., Liu, D., et al. (2017). Exosomal miR-146a contributes to the enhanced therapeutic efficacy of interleukin-1β-primed mesenchymal stem cells against sepsis. Stem Cells 35, 1208–1221. doi: 10.1002/stem.2564
Taghizadeh, R. R., Cetrulo, K. J., and Cetrulo, C. L. (2018). Collagenase impacts the quantity and quality of native mesenchymal stem/stromal cells derived during processing of umbilical cord tissue. Cell Transplant. 27, 181–193. doi: 10.1177/0963689717744787
Tao, S.-C., Yuan, T., Zhang, Y.-L., Yin, W.-J., Guo, S.-C., and Zhang, C.-Q. (2017). Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics 7, 180–195. doi: 10.7150/thno.17133
Théry, C., Witwer, K. W., Aikawa, E., Alcaraz, M. J., Anderson, J. D., Andriantsitohaina, R., et al. (2018). Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 7:1535750. doi: 10.1080/20013078.2018.1535750
Tracy, S. A., Ahmed, A., Tigges, J. C., Ericsson, M., Pal, A. K., Zurakowski, D., et al. (2019). A comparison of clinically relevant sources of mesenchymal stem cell-derived exosomes: bone marrow and amniotic fluid. J. Pediatr. Surg. 54, 86–90. doi: 10.1016/j.jpedsurg.2018.10.020
Tung, J. T., Arnold, C. E., Alexander, L. H., Yuzbasiyan-Gurkan, V., Venta, P. J., Richardson, D. W., et al. (2002). Evaluation of the influence of prostaglandin E2 on recombinant equine interleukin-1 β-stimulated matrix metalloproteinases 1, 3, and 13 and tissue inhibitor of matrix metalloproteinase 1 expression in equine chondrocyte cultures. Am. J. Vet. Res. 63, 987–993. doi: 10.2460/ajvr.2002.63.987
Turkiewicz, A., Petersson, I. F., Björk, J., Hawker, G., Dahlberg, L. E., Lohmander, L. S., et al. (2014). Current and future impact of osteoarthritis on health care: a population-based study with projections to year 2032. Osteoarthr. Cartil. 22, 1826–1832. doi: 10.1016/j.joca.2014.07.015
van Buul, G. M., Villafuertes, E., Bos, P. K., Waarsing, J. H., Kops, N., Narcisi, R., et al. (2012). Mesenchymal stem cells secrete factors that inhibit inflammatory processes in short-term osteoarthritic synovium and cartilage explant culture. Osteoarthr. Cartil. 20, 1186–1196. doi: 10.1016/j.joca.2012.06.003
Vindas Bolaños, R. A., Cokelaere, S. M., Estrada McDermott, J. M., Benders, K. E. M., Gbureck, U., Plomp, S. G. M., et al. (2017). The use of a cartilage decellularized matrix scaffold for the repair of osteochondral defects: the importance of long-term studies in a large animal model. Osteoarthr. Cartil. 25, 413–420. doi: 10.1016/j.joca.2016.08.005
Vlassov, A. V., Magdaleno, S., Setterquist, R., and Conrad, R. (2012). Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochimica Biophys. Acta 1820, 940–948. doi: 10.1016/j.bbagen.2012.03.017
Vonk, L. A., van Dooremalen, S. F. J., Liv, N., Klumperman, J., Coffer, P. J., Saris, D. B. F., et al. (2018). Mesenchymal stromal/stem cell-derived extracellular vesicles promote human cartilage regeneration in vitro. Theranostics 8, 906–920. doi: 10.7150/thno.20746
Wang, Y., Yu, D., Liu, Z., Zhou, F., Dai, J., Wu, B., et al. (2017). Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res. Ther. 8:189. doi: 10.1186/s13287-017-0632-0
Webber, J., and Clayton, A. (2013). How pure are your vesicles? J. Extracell. Vesicles 2:19861. doi: 10.3402/jev.v2i0.19861
Weber, A. E., Locker, P. H., Mayer, E. N., Cvetanovich, G. L., Tilton, A. K., Erickson, B. J., et al. (2018). Clinical outcomes after microfracture of the knee: midterm follow-up. Orthop. J. Sport. Med. 6:2325967117753572. doi: 10.1177/2325967117753572
Witwer, K. W., Van Balkom, B. W. M., Bruno, S., Choo, A., Dominici, M., Gimona, M., et al. (2019). Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J. Extracell. Vesicles 8:1609206. doi: 10.1080/20013078.2019.1609206
Woolf, A. D., and Pfleger, B. (2003). Burden of major musculoskeletal conditions. Bull. World Health Organ. 81, 646–656.
Wu, J., Kuang, L., Chen, C., Yang, J., Zeng, W. N., Li, T., et al. (2019). miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials 206, 87–100. doi: 10.1016/j.biomaterials.2019.03.022
Xu, J., Feng, Y., Jeyaram, A., Jay, S. M., Zou, L., and Chao, W. (2018). Circulating plasma extracellular vesicles from septic mice induce inflammation via MicroRNA- and TLR7-dependent mechanisms. J. Immunol. 201, 3392–3400. doi: 10.4049/jimmunol.1801008
Xu, L., Wu, Y., Xiong, Z., Zhou, Y., Ye, Z., and Tan, W. S. (2016). Mesenchymal stem cells reshape and provoke proliferation of articular chondrocytes by paracrine secretion. Sci. Rep. 6:32705. doi: 10.1038/srep32705
Zhang, S., Chu, W. C., Lai, R. C., Lim, S. K., Hui, J. H. P., and Toh, W. S. (2016). Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthr. Cartil. 24, 2135–2140. doi: 10.1016/j.joca.2016.06.022
Zhang, S., Chuah, S. J., Lai, R. C., Hui, J. H. P., Lim, S. K., and Toh, W. S. (2018). MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials 156, 16–27. doi: 10.1016/j.biomaterials.2017.11.028
Zhang, S., Teo, K. Y. W., Chuah, S. J., Lai, R. C., Lim, S. K., and Toh, W. S. (2019). MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials 200, 35–47. doi: 10.1016/j.biomaterials.2019.02.006
Zhu, X., Badawi, M., Pomeroy, S., Sutaria, D. S., Xie, Z., Baek, A., et al. (2017). Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. J. Extracell. Vesicles 6:1324730. doi: 10.1080/20013078.2017.1324730
Zhu, Y., Wang, Y., Zhao, B., Niu, X., Hu, B., Li, Q., et al. (2017). Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res. Ther. 8:64. doi: 10.1186/s13287-017-0510-9
Keywords: extracellular vesicle, mesenchymal stem cell, cartilage, tissue engineering, osteoarthritis
Citation: To K, Romain K, Mak C, Kamaraj A, Henson F and Khan W (2020) The Treatment of Cartilage Damage Using Human Mesenchymal Stem Cell-Derived Extracellular Vesicles: A Systematic Review of in vivo Studies. Front. Bioeng. Biotechnol. 8:580. doi: 10.3389/fbioe.2020.00580
Received: 08 April 2020; Accepted: 13 May 2020;
Published: 11 June 2020.
Edited by:
Tong-Chuan He, University of Chicago Medicine, United StatesReviewed by:
Muhammad Nawaz, University of Gothenburg, SwedenMing Pei, West Virginia University, United States
Copyright © 2020 To, Romain, Mak, Kamaraj, Henson and Khan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wasim Khan, d2FzaW1raGFuQGRvY3RvcnMub3JnLnVr
 Kendrick To
Kendrick To Karl Romain2
Karl Romain2 Wasim Khan
Wasim Khan