- 1Regenerative, Modular & Developmental Engineering Laboratory, National University of Ireland Galway, Galway, Ireland
- 2Science Foundation Ireland, Centre for Research in Medical Devices, National University of Ireland Galway, Galway, Ireland
Articular cartilage defects remain a clinical challenge. Articular cartilage defects progress to osteoarthritis, which negatively (e.g., remarkable pain, decreased mobility, distress) affects millions of people worldwide and is associated with excessive healthcare costs. Surgical procedures and cell-based therapies have failed to deliver a functional therapy. To this end, tissue engineering therapies provide a promise to deliver a functional cartilage substitute. Among the various scaffold fabrication technologies available, electrospinning is continuously gaining pace, as it can produce nano- to micro- fibrous scaffolds that imitate architectural features of native extracellular matrix supramolecular assemblies and can deliver variable cell populations and bioactive molecules. Herein, we comprehensively review advancements and shortfalls of various electrospun scaffolds in cartilage engineering.
Introduction
Adult articular cartilage is a relatively thin (2–4 mm), aneural, avascular, and alymphatic tissue that acts as cushion against physiological loads at joints. Once injured, it loses much of its carrying capacity, causing a susceptible environment for wearing and tearing between the joints (Correa and Lietman, 2017; Zhang et al., 2019). It has been reported that 60–66% of routine knee arthroscopies caused by articular cartilage defects. The breakdown molecules following injury cause an inflammation in the joints. This inflammation increases the level of synovial cytokines, alters the resident cell phenotypes and induces matrix-degrading enzymes. Thus, it causes a more conducive environment for tissue degradation, which finally ends up with osteoarthritis (OA) (Homandberg et al., 1993; Homandberg and Hui, 1996; Cecil et al., 2005; Kurz et al., 2005; Goldring et al., 2011; Camp et al., 2014). Conjecturally, up to 240 million people around the world suffer from OA. The observed symptoms (e.g., pain, stiffness, joint instability, and pain-related psychological distress) start approximately at the age of 55 and have devastating consequences in the quality of life of the patients (Hunter et al., 2008; Van Spil et al., 2019). In 2013, OA was the second most expensive health condition treated at US hospitals with $16.5 billion expenditure (Torio and Moore, 2013). Women have a higher age-related prevalence of arthritis than men, 10% men and 13% in women suffer from aged-related OA (aged 60 years or older) (Zhang and Jordan, 2010). This prevalence is projected to increase due to increasing aging population and obesity (Sun et al., 2015).
There are numerous treatments for articular cartilage defects, including extensive surgical interventions (e.g., osteotomy, distraction of joints), therapeutic interventions without active biologics (e.g., lavage, arthroscopy, debridement, shaving, laser chondroplasty, abrasion chondroplasty, pridie drilling, microfracture, and spongialization), therapeutic interventions with active biologics (e.g., perichondrial/periosteal grafts, osteochondral transplantation, allogenic osteochondral, and chondral grafting) and tissue engineering (a still elusive combination of scaffolds, cells, biologics). Cartilage engineering constitute the ultimate frontier, as all other interventions are nothing more than relieving the pain or delaying tissue degradation (Hunziker, 2002; Musumeci et al., 2014). Various scaffold fabrication technologies have been assessed over the years for cartilage engineering with variable degree of efficiency (Cheng et al., 2019; Li et al., 2019). Among them, electrospinning has emerged as a promising technique, due to its high versatility (e.g., ability to produce functionalised nanofibrous scaffolds with a variety of orientations, sizes, and mechanical properties) (Garg and Bowlin, 2011; Casanellas et al., 2018; Casanova et al., 2018; Li et al., 2018; Liu et al., 2018). Herein, we briefly describe the cellular and extracellular composition and architecture of cartilage, along with key modulators of chondrogenesis, and we comprehensively review advancements and shortfalls of electrospun scaffolds in cartilage engineering.
Cartilage
Cartilage Cellular Composition and Key Signaling Molecules in Chondrogenesis
Cartilage is a hypocellular tissue, with only 4% of its wet weight consisting of a highly differentiated cell population, called chondrocytes (Matzat et al., 2013). The morphology of chondrocytes varies in shape in each zone (see section Cartilage extracellular matrix composition and architecture). Chondrocytes together with the pericellular matrix (a basket-like network of fine fibrils of elaborate structure composed of laminin, fibronectin, biglycan, decorin, fibromodulin, matrilin 3, and cartilage oligo matrix protein) and the capsule (composed of collagen type VI, collagen type IX, and proteoglycans) surrounding the pericellular matrix form the chondron, which reduces the mechanical, osmotic and physicochemical changes induced by dynamic loading, maintain tissue homoeostasis and contribute to tissue regeneration (Muir, 1995; Alexopoulos et al., 2003; Youn et al., 2006; Vonk et al., 2014; Wilusz et al., 2014; Decker et al., 2015; Li and Xu, 2015). Alterations in the composition of the pericellular matrix is associated with OA (Wadhwa et al., 2005a,b; Hu et al., 2006; van der Weyden et al., 2006; Alexopoulos et al., 2009). Chondrocytes are responsible for synthesis of the articular cartilage extracellular matrix (ECM) (Bhosale and Richardson, 2008; Demoor et al., 2014) and its remodeling through secreted enzymes (e.g., matrix metalloproteinases, hyaluronidases, aggrecanases) (Buttle et al., 1997; Shlopov et al., 1997; Flannery et al., 1998; Demoor et al., 2014).
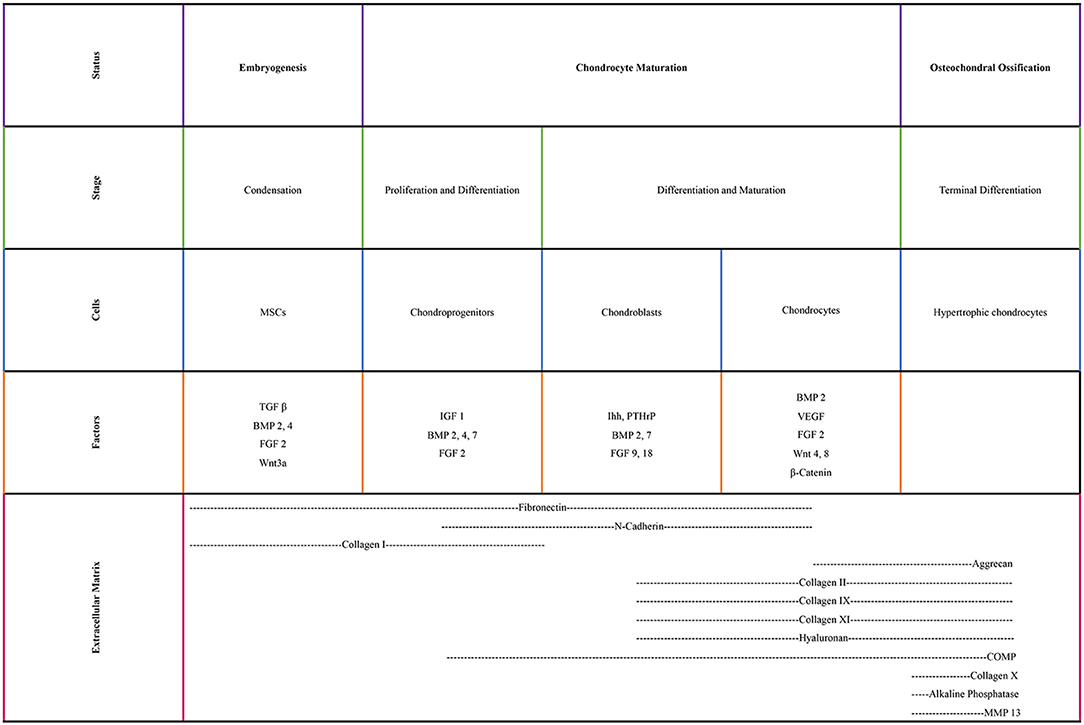
Chondrocytes are originated from mesenchymal stem cells (MSCs), found in the bone marrow of mature individuals. Condensation of MSCs and chondroprogenitor cell differentiation initiate cartilage formation. Expression of collagen type I and type II results in the onset of chondrogenesis (Archer and Francis-West, 2003; Demoor et al., 2014). Pre-chondrocytes start expressing cartilage-specific transcription factors (e.g., Sox9, Sox5, Sox6) and then they become mature chondrocytes by producing an ECM that has a great amount of proteoglycans (e.g., aggrecan) and collagens (e.g., collagen types II, IX, and XI) (Bi et al., 1999; Ikeda et al., 2004; Demoor et al., 2014). As the chondrocytes proliferate, they express collagen type VI and matrilin 1 under the control of the parathyroid hormone-related peptide/Indian hedgehog. Indian hedgehog is a secreted factor in hypertrophic chondrocytes, which is regulated by the activation of the cyclins. The cyclins regulate chondrocyte proliferation via formation of complexes with cyclin-dependent kinases. By secreting the cartilaginous matrix, MSCs differentiate to chondrocytes and they continue to divide during chondrogenesis. At the final step of their development, they become hypertrophic and secrete calcification proteins in the calcified zone (Temenoff and Mikos, 2000; Zelzer et al., 2001; Goldring, 2012).
Various transcription factors are crucial in chondrogenesis (Figure 1). Sox9, which is a master chondrogenic transcription factor during the chondrogenic differentiation, upregulates the transcriptional activity of collagen type II gene through interacting with the first intron-specific enhancer. Sox9 is crucial for articular cartilage formation and the hypertrophic maturation of chondrocytes. In the absence of Sox9, Sox5, and Sox6 induce the transcriptional activity of collagen type II gene, albeit slightly. These three members of the Sox family also regulate the gene expression of collagen type IX, collagen type XI and aggrecan (Lefebvre and Smits, 2005; Wuelling and Vortkamp, 2011; Demoor et al., 2014). Runx2 and Runx3 are expressed in pre-hypertrophic and hypertrophic chondrocytes. Deletion of Runx2 and Runx3 delays chondrocyte maturation. Hypertrophic chondrocytes cannot be formed when lacking these two transcription factors (Yoshida et al., 2004). c-Maf is a basic leucine zipper transcriptional activator and allows hypertrophic and terminal chondrocytes to terminally differentiate (MacLean et al., 2003; Lefebvre and Smits, 2005).
Growth factors also play key roles in chondrogenesis. Insulin-like growth factor1 induces collagen type II expression through increased binding activity of Sox trio (Seifarth et al., 2009; Renard et al., 2012; Legendre et al., 2013; Demoor et al., 2014). Transforming growth factor β1 initiates the condensation of MSCs to chondrocytes for the onset of chondrogenesis, increases the collagen type II gene expression levels during the early stage of chondrogenesis and inhibits the terminal differentiation of chondrocytes via increasing the expression of parathyroid hormone-related peptide (Li et al., 2005a; Demoor et al., 2014). Bone morphogenic protein 2 plays a pivotal role in the expression of the mature form of collagen type II (Rosen et al., 1994; Gouttenoire et al., 2010; Demoor et al., 2014).
WNT signaling is a well-studied pathway for differentiation and hypertrophy (Ripmeester et al., 2018). WNTs establish a large family of cysteine-rich morphogens that have an essential role in cartilage, bone and joint development. In vivo mice studies indicated that WNT signaling extended cell survival and inhibited the differentiation of chondrocytes toward hypertrophy (Zhu et al., 2008, 2009). WNT5a and WNT5b are important during differentiation of MSCs to chondrocytes (Church et al., 2002), chondrocyte proliferation and cartilage homeostasis (Sharma et al., 2013). However, overexpression of WNTs has been reported to lead to OA-like diseases (Lodewyckx and Lories, 2009).
The surface zone of articular cartilage contains a subpopulation called cartilage progenitor cells. These flat cells are responsible for the appositional growth of the cartilage tissue and express high level of stem cell surface marker (Hiraoka et al., 2006) and exhibit a significant degree of plasticity, in terms of differentiation toward chondrogenic, osteogenic, and adipogenic pathways (Morrison et al., 1997; Dowthwaite et al., 2004). Upon injury to a healthy cartilage, they migrate and emerged to the injury site. During OA progression, changes in the distribution of cartilage progenitors suggests that these cells may be responsible for communication between articular cartilage and subchondral bone (Jiang and Tuan, 2015).
Cartilage Extracellular Matrix Composition and Architecture
Cartilage is mainly comprised of collagens (types II, VI, IX, X, XI); collagen type II is the predominant collagen that forms the 90–95% of the fibril network of the matrix and 60–85% of the dry weight of cartilage (Buckwalter and Mankin, 1997; Mow et al., 1999; Poole et al., 2001; Pearle et al., 2005; Lim et al., 2014). Bound carbohydrate groups found in collagen type II allow to interact with water more than other types of collagen. Together with collagen type II, types IX and XI form a macro-fibrillar structure/fiber network, which provides tensile strength. Collagen type IX is cross-linked to the surface of the macro-fibrils, whereas collagen type XI located within and on the surface of the macro-fibrils. Collagen type VI forms microfibrils in pericellular sites. Collagen type X is only synthesized by hypertrophic chondrocytes, which takes place in calcified cartilage (Cohen et al., 1998; Temenoff and Mikos, 2000; Poole et al., 2001). Proteoglycans consist of a protein core and one or more glycosaminoglycan chains. Hyaluronic acid, chondroitin sulfate, keratan sulfate, dermatan sulfate, and heparan sulfate are some of the glycosaminoglycans found in articular cartilage. The predominant proteoglycan is the large chondroitin sulfate proteoglycan 1, called aggrecan, which forms a strong, porous-permeable, fiber-reinforced material together with collagen fibrils. Aggrecan, as the name implies, forms an aggregate structure that does not allow proteoglycans to diffuse out of the matrix throughout joint loading, thus plays an important role during compressive loading. Decorin, biglycan, and fibromodulin are present in minor quantities and do not significantly affect the physical properties of the tissue, unlike aggrecan (Buckwalter and Mankin, 1997; Cohen et al., 1998).
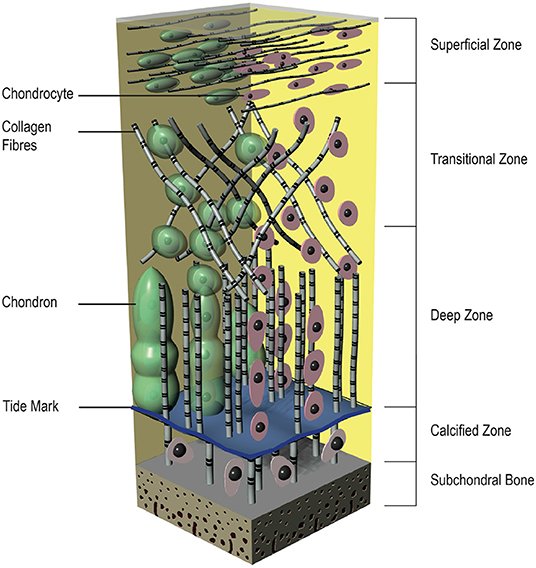
From top to bottom the articular cartilage can be divided into four distinct layers with different compositions, cell morphologies, and physiological characteristics (Figure 2). The superficial zone is the thinnest zone, constitutes 10–20% of the total cartilage volume and is responsible for tensile properties of the tissue. It includes a high density of ellipsoid chondrocytes (24,000 cell/mm3) with a parallel orientation to the surface. These ellipsoid chondrocytes synthesize high concentration of collagens [mainly type II and type IX collagen fibers with small diameter (20 nm) and parallel arrangement to the surface] and low concentration of proteoglycans; for this reason, this zone has the highest concentration of water. As a result of its construction, this zone protects deeper zones from shear, tensile, and compressive forces. Below the superficial zone, the transitional zone represents 40–60% of the total cartilage volume and has a lower cell density (10,300 cells/mm3). This middle zone shows more typical morphologic features of a hyaline cartilage, with more spherical cells, higher fiber diameter and higher aggrecan content (Temenoff and Mikos, 2000; Poole et al., 2001; Bhosale and Richardson, 2008; Sohier et al., 2008; Nazempour and Van Wie, 2016). Situated between the transitional zone and the calcified cartilage is the deep zone, which represents almost 30% of the total cartilage volume. It provides a great strength against compressive forces and contains the lowest cell density among all of the zones (7,700 cells/mm3). The cells in this zone are large and spherical and organized perpendicularly to the joint surface. Although the lowest cell density, the proteoglycan content and the fiber diameter (120 nm) are maximal in this zone. Between the deep zone and the subchondral bone, the calcified zone is located and constitutes an excellent interface that integrates with less resilient subchondral bone. There is a visible border between the deep and calcified zone, called tidemark. The calcified zone has a small volume of ellipsoid cells with an abundant calcified ECM, shows a very low metabolic activity. The chondrocytes in this zone exhibit a hypertrophic phenotype and, uniquely, they express collagen type X that can calcify surrounding ECM (Sohier et al., 2008; Sophia Fox et al., 2009).
Electrospinning
The History
Electrospinning is a highly versatile technique that produces ultrafine fibers with a diameter in the nano- to micro- meter range by using electrostatic fields. It has become popular in a wide range of biomedical and industrial applications, as it can produce fibrous mats with controlled orientations, sizes, porosity, mechanical properties, and with high surface area to volume ratio (Figure 3). In 1882, Lord Rayleigh first described electrospray, which inspired the idea of the electrospinning process. He investigated “The Rayleigh instability”; a highly charged droplet is unstable and would break down into smaller droplets when passes through a voltage gradient. After his initial work, the electrospraying of aqueous solutions achieved by the workmanship of Zeleny; his work made possible the current state of electrospinning. It is considered a direct extension of electrospraying, considering that continuous fibers are produced in electrospinning, whereas small droplets are produced in electrospraying. In 1934, Formhals achieved a feasible method to get fine fibers from a cellulose acetate solution and took out a variety of U.S. patents on this technology. In 1966, Simons observed that the use of more viscous solutions resulted in longer fibers. Later, Baumgarten discovered that the diameter of acrylic fibers could be controlled by the feed rate of the infusion pump. Despite these advances and patents in the field of electrospinning until the 1990s, there was no commercial interest in this technique. From the beginning of 1990's, as nanotechnology became a popular research area, the interest of electrospinning has increased (Li et al., 2007; Molnár and Vas, 2012; Braghirolli et al., 2014).
The Setup
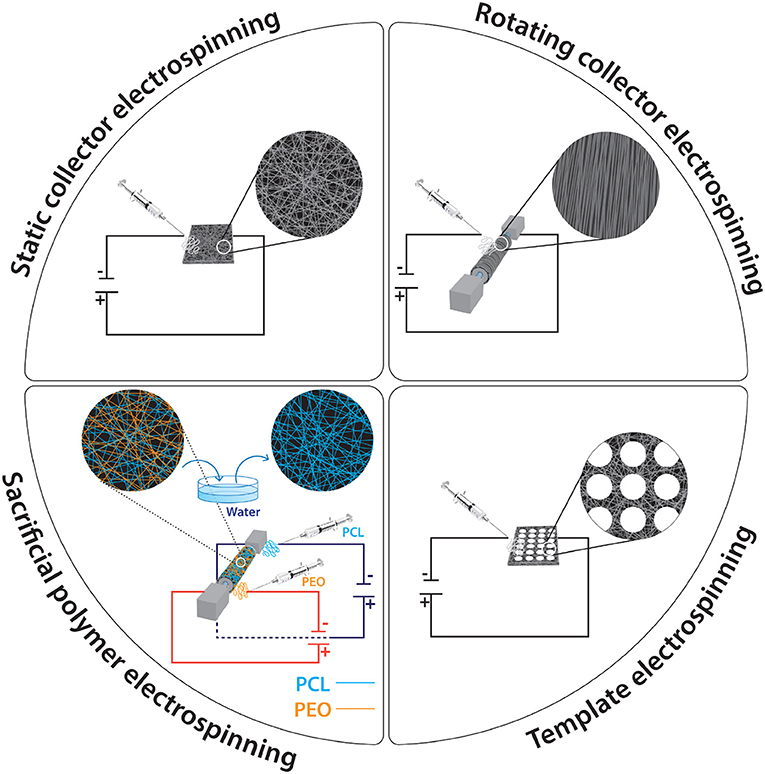
The electrospinning process is a simple, efficient spinning method that produces nanoscale to microscale fibers from polymer solutions or melts using electrostatic forces. It is a relatively easy to setup process, as it requires a syringe (polymer solution reservoir) with a small diameter needle (to charge the polymer solution), a flow control pump for reproducibility, a high voltage supply to produce a charged polymer jet and a collector. If the syringe is not set horizontally, the polymer flow can be driven by gravity. The voltage supply usually ranges from 10 to 50 kV (subject to solution viscosity, solvent volatility, etc.). The collector is usually a stationary plate, although advances in engineering have allowed the use of a rotating cylinder for the production of anisotropic fibers.
The Process
Electrospinning begins when the polymer solution emerges from the spinneret by the electrostatic forces. While it is extruded from the syringe, it forms a semi-spherical droplet at the end of the needle and due to the induction of charges on the polymer droplet, it causes instability within the polymer solution. When the reciprocal repulsion of the charges is enough to overcome the surface tension, a conical shape cone, known as Taylor cone, is formed via the elongation of polymer droplet. A liquid jet is the formed that flows through the direction of electric field. During this journey from the spinneret to the collector, the solvent in the liquid jet evaporates, increasing the surface charge on the jet. This increasing surface charge causes instability in the polymer jet and the polymer jet divides geometrically to compensate for the instability. First, it divides into two jets and, as the process continues, it divides into more and more jets. The action of the spinning force, which is caused by the electrostatic force on the continuously splitting polymer droplets, produces the non-woven nanofibers, which are deposited on the collector (Li and Tuan, 2009; Liu et al., 2012; Haider et al., 2015). Despite the electrospinning process seems quite simple, a number of process parameters should be adjusted in order to get desired morphology of nanofibers without droplets or beads (Pillay et al., 2013). These parameters can be divided 3 major group: solution parameters; process parameters; and ambient parameters.
Solution Parameters
All solution parameters (e.g., concentration, molecular weight, viscosity, surface tension, conductivity) are related to each other and affect the architectural features of the produced mat. The concentration of the solution is crucial for fiber formation to occur. Thus, an optimized solution concentration should be designated for each polymer. When the concentration is very low, the electrospinning process does not occur, instead of this, electrospraying is achieved via obtaining polymeric nano/micro particles, because of the low viscosity and high surface tensions of the solution. As the concentration goes a little higher, a mixture of beads and fibers occurs. A further increase in concentration changes the bead morphology from spherical to spindle-like. When the concentration reaches a suitable level, smooth fibers are obtained. Above this level, an increase in concentration results in an increase in fiber diameter. If the concentration is too high, instead of fibers, helix-shaped micro-ribbons are observed. The molecular weight of the polymer significantly affects the morphology of fibers, due to its effect on the entanglement of polymer chains in solution. When the concentration is fixed, decreasing the molecular weight causes bead, instead of fiber, formation. When increasing the molecular weight, the number of beads and droplets is decreased and smooth fibers are obtained. Considering that each of the solution parameters has an effect on each other, molecular weight reflects the entanglement of the polymer chains, thus it affects viscosity and variance in viscosity can cause different surface tension, which plays an important role in bead formation. The solution viscosity, which can be adjusted by the polymer concentration, is one of the key factors in terms of fiber morphology. Low viscosity prohibits continuous and smooth fiber production, whilst at a high viscosity, longer stress relaxation time occurs, which causes hard ejection of the jets from the polymer solution. At optimal viscosity, uniform in diameter fibers are produced.
Surface tension determines the boundaries of the electrospinning process. Higher surface tension causes instability of the jets and yields sprayed droplets. The solvent and the polymer used as well as the addition of ionizable salts determine the solution conductivity. It has been shown that an increased conductivity causes a decrease in the diameter of the electrospun fibers, whilst low conductivity produces fibers with beads. Natural polymers are polyelectrolytic in nature and the ions present increase the charge carrying capacity of the jet. Further, the addition of ionic salts, such as KH2PO4, NaH2PO4 and NaCl, affect fiber morphology and diameter and allow production of bead-less fibers with relatively smaller diameters (Bhardwaj and Kundu, 2010; Li and Wang, 2013).
Process Parameters
The process parameters (e.g., voltage, flow rate, distance between needle and collector, collector's features) are also playing a crucial role in the production of reproducible fibers. Obviously, the voltage is crucial in the electrospinning process. When the applied voltage overcomes the threshold voltage, fibrous scaffolds can be produced. When the other parameters are fixed, increasing voltage can cause the formation of beads and droplets. With respect to the influence of voltage on fiber diameter, two contradictory theories exist. High voltages are associated with more polymer ejections and thus larger in diameter fibers. On the other hand, increasing the applied voltage results in smaller in diameter fibers due to the electrostatic repulsive force on the fluid jet. Greater Columbic forces and stronger electric field, arising from high voltages, induce increased stretching of the solution, which results in reduction in the fiber diameter and rapid evaporation of the solvent. The flow rate influences the jet velocity and the material transfer rate. High flow rates produce large in diameter fibers, whilst lower flow rates are more desirable for reproducible fiber production, as they provide sufficient drying time to the jet to reach the collector (Subbiah et al., 2005; Pham et al., 2006; Bhardwaj and Kundu, 2010). The morphology of the fibers is also affected by the distance between the tip of the needle and the collector. Too long or too short distances cause beaded morphology; therefore, an optimal distance should be identified to allow the fiber to dry before reaching the collector (Ki et al., 2005; Hassiba et al., 2016). Having said that, a study has argued that of the other parameters are optimal, the distance has no crucial effect on fiber size and morphology (Pham et al., 2006). The collector acts as a conductive substrate, where the charged fibers are collected. Its conductivity affects the arrangement of the fibers, because of its influence on the charge of the deposited fibers; a low in conductivity collector causes the deposited fibers to detain some of their charges and this causes a repelling effect to the incoming fibers. Customarily, flat aluminum collectors are used, but they are often associated with detachment issues that affect morphology and mechanical properties (Stanger et al., 2009; Pillay et al., 2013). Alternative collector conformations include porous metals of variable porosity and pore shape (Fuller et al., 2016, 2019), wire mesh (Wang et al., 2005), pin (Sundaray et al., 2004), grids (Li et al., 2004), liquid bath (Ki et al., 2007), rotating rods or wheels and parallel or gridded bars for anisotropic fiber production (Xu et al., 2004). It is also worth noting that fibers have also been produced using a non-conductive collector and an AC high voltage electrospinning, instead of a normal DC high voltage (Kessick et al., 2004).
Ambient Parameters
Ambient parameters (e.g., humidity, temperature) also have an impact on the morphology of the fibers. Relative humidity can make the fibers thicker or thinner based on chemical nature of the polymer. In general, high humidity prohibits solvent evaporation and results in beaded or flat mats, as opposed to fibrous mats. High temperatures, due to reduction in surface tension and viscosity, yield small in diameter fibers (De Vrieze et al., 2009).
Electrospun Polymers in Cartilage Engineering
Overview of Polymers Used in Cartilage Engineering
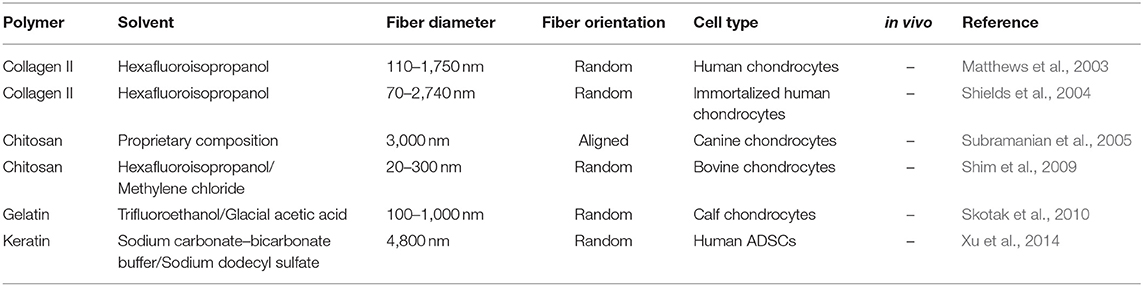
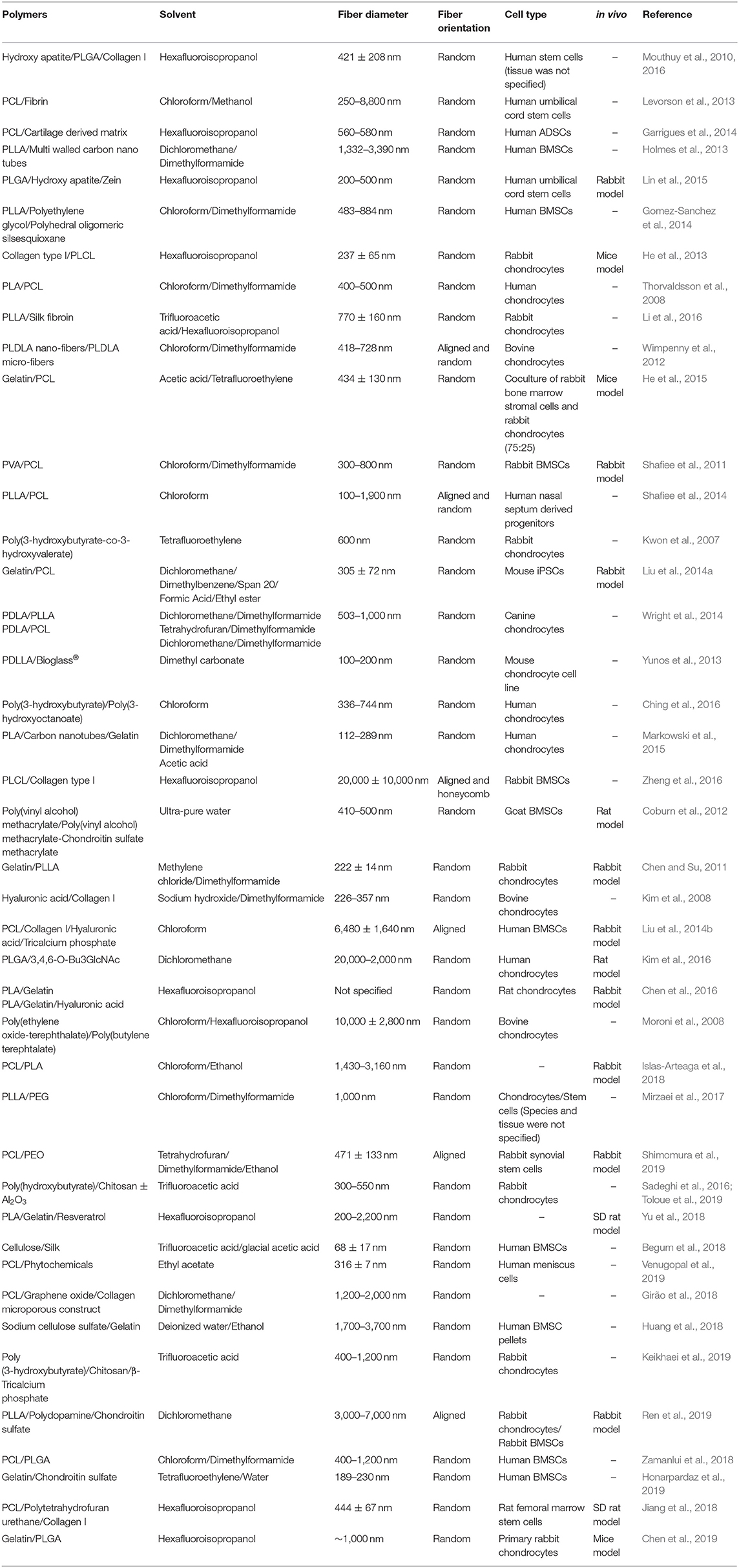
Up to now, numerous natural, synthetic and composite polymers have been electrospun and assessed for cartilage engineering; the critical issues for all of them are their compositional, structural, mechanical, degradation, and biocompatibility properties. The degradation products of natural polymers can be smoothly eliminated from the body and that is why they have been used extensively in cartilage repair and regeneration (Table 1). However, their degradation by the harsh solvents used in the electrospinning process (Yang et al., 2008; Zeugolis et al., 2008) requires heavy cross-linking to stabilize them, which frequently associated with cytotoxicity in vitro and foreign body response in vivo (Delgado et al., 2015), their fast degradation rate for a tissue that has slow recovery time and their potential immune responses and microbial/viral contaminants have restricted the use of natural polymers in the fabrication of electrospun scaffolds for cartilage engineering (Schmidt and Baier, 2000; Lavik and Langer, 2004). Synthetic polymers are in general stronger than natural polymers, can withheld the electrospinning process without any noticeable losses and offer controllable biodegradability (Cheung et al., 2007; Li et al., 2007; Zhang et al., 2009); for these reasons, synthetic (Table 2) and composites (Table 3) polymers are extensively used in cartilage engineering.
Poly(α-hydroxy esters) [e.g., poly(glycolic acid) (PGA), poly(lactic acid) (PLA), poly(ε-caprolactone) (PCL) and their copolymers] are used extensively for tissue engineering applications, as they are well-characterized and FDA approved for clinical use. The simplest linear aliphatic polyester is PGA. It is considered as a promising biomaterial due to the natural absorption of its degradation products; however, its rapid degradation rate makes it an inappropriate candidate for cartilage engineering. PLA is more hydrophobic than PGA with an addition of a methyl group; however, it is readily soluble in commonly used organic solvents. Based on the position of the methyl group, it has three isomers, which are poly(L-lactic acid) (PLLA), poly(D,L-lactic acid) (PDLLA), and poly(D-lactic acid) (PDLA). Compared to PGA, PLA degrades slowly (from 1 to over 2 years) because of the hydrophobic characteristics. However, the tensile strength and modulus of elasticity of PLA is lower than PGA. Although the use of PLA and PGA is limited for hard tissue regeneration, such as cartilage tissue, due to their relatively weak mechanical properties (Cheung et al., 2007), one study showed that bidirectionally aligned and layered PLA electrospun mats loaded with human meniscus cells in an ECM hydrogel displayed ~5-fold higher tensile modulus to the randomly aligned scaffolds; they had comparable tensile modulus to the human meniscus in the circumferential direction and they maintained physiological meniscus cells gene expression for COLA1A1, SOX9, and COMP (Baek et al., 2015).
In general, electrospun copolymers of poly(α-hydroxy esters) with tailored properties can be readily obtained and are extensively used in tissue engineering and regenerative medicine. When six commercially available poly(α-hydroxy esters) were incubated in physiological solutions, the PGA and PLGA50:50 scaffolds showed superior mechanical properties than the PLLA and PCL scaffolds; the PLLA and PCL scaffolds sustained their robust scaffold structure; and the PGA, PDLLA, PLGA50:50, and PLGA85:15 scaffolds exhibited a severe structural destruction due to polymer degradation. In terms of cell proliferation, PLLA scaffolds promoted the highest rate of proliferation between all polymers when seeded with chondrocytes and human BMSCs (Li et al., 2006a). A study that compared different PLGA ratios (75:25, 50:50) and a blend of 75:25 and 50:50 PLGA showed that the tensile modulus of the 75:25 and 50:50 PLGA scaffolds were similar to human skin and slightly lower than human cartilage, respectively (Shin et al., 2006). Due to its relatively cheap cost, high stability in ambient conditions, long degradation rate and the long regeneration time of cartilage tissue, PCL is favored in cartilage repair and regeneration, with numerous studies having demonstrated that electrospun PCL scaffolds promote cartilage cell proliferation, cartilage ECM synthesis and deposition and chondrogenic differentiation of various stem cell populations. Further, PCL nanofibrous scaffolds have shown higher chondrogenic differentiation, as judged by sGAG synthesis, of BMSCs than cell pellet cultures in TGF-β1 serum free media (Li et al., 2005b).
The Influence of Architectural Features on Cell Response
Over the years, numerous studies gave assessed the influence of architectural features (e.g., fiber orientation, fiber diameter, scaffold porosity) on cell fate. It has been shown that both aligned and random PLLA/PCL (Shafiee et al., 2014) and PCL/PLGA (Zamanlui et al., 2018) scaffolds support nasal septum-derived progenitor and human BMSCs, respectively, adhesion, proliferation and chondrogenesis. However, their proliferation was higher on the random scaffolds, whilst their differentiation was higher on the aligned scaffolds, rendering such conformation suitable for the superficial zone of the articular cartilage that exhibits an aligned orientation. Although both aligned nano- and micro- fibrous electrospun PCL scaffolds sustained growth of human BMSCs, the nano-fibrous scaffolds showed the highest chondrogenic activity, as judged by produced sGAG and collagen type II mRNA expression, suggesting that this combination may be suitable form for the superficial zone, which normally shows the highest level of collagen type II than the any other zone (Wise et al., 2009). Similar results were obtained with nano-fibrous, as opposed to micro-fibrous or smooth (film) PLLA (Li et al., 2006b) or PLDLA (Wimpenny et al., 2012) scaffolds; the nano-fibrous architecture maintained chondrocyte-like morphology and enhanced cartilage-specific mRNA expression and ECM synthesis. One should however note that not only the fiber size, but also the pore size has an important role in chondrogenesis. For example, micro-size PLLA fibers of 5 and 9 μm in diameter and with pore sizes of 27 and 29 μm respectively were more chondrogenic (e.g., aggrecan, chondroadherin, sox9, collagen type II) than nano-size PLLA fibers of 300 nm and 600 nm to 1,400 nm in diameter and with pore sizes of 2 and 3 μm respectively (Shanmugasundaram et al., 2011).
Electrospinning and Bioreactors
Considering that cells in vivo are subjected to numerous tissue-specific cues, modern molecular delivery (Pugliese et al., 2018) and tissue engineering (Calejo et al., 2019) employ multifactorial approaches to recapitulate the in vivo niche in vitro. To this end, electrospun fibers have joined forces with other in vitro microenvironment modulators to either maintain native chondrocyte phenotype or to direct stem cells toward chondrogenic lineage, especially now that it is clear that a stable chondrocyte phenotype is still elusive (Graceffa et al., 2018, 2019). For example, dynamic culture systems combined with electrospun scaffolds have shown beneficial effects in cartilage engineering (Martin et al., 2007; Janjanin et al., 2008; Khorshidi et al., 2016). A flow perfusion bioreactor, promoted chondrogenic differentiation of human BMSCs, as judged by increased expression of cartilage-associated genes (e.g., aggrecan, collagen type II, SOX9) and enhanced cell proliferation and ECM synthesis. However, there was no significant difference between bioreactor culture and static control culture, suggesting that the media fluid flow and the orientation of the electrospun meshes can also have an impact (Alves da Silva et al., 2010). Using a custom mold, PLLA electrospun scaffolds seeded with BMSCs and media supplemented with TGF-β1/IGF-1, after 42 days in a bioreactor system, the produced construct exhibited the highest (in comparison to TGF-β1 alone culture) Young's modulus values and collagen type II and aggrecan expression; a significant time-dependent increase in sGAG and hydroxyproline content was also reported (Janjanin et al., 2008).
Improving Cell Infiltration and Nutrient/Waste Transport
Highly dense/small porosity electrospun scaffolds often cause low cell infiltration and limited nutrient access to the deeper sides of the cartilage tissue (Nam et al., 2007; Skotak et al., 2011; Coburn et al., 2012). To enhance cellular infiltration and nutrient/excrete transport, various ingenious engineering approaches have been assessed over the years, including combination of nano-micro fibrous scaffolds (Kim et al., 2008; Thorvaldsson et al., 2008; Levorson et al., 2013), salt leaching techniques (Wright et al., 2014), controlled fiber density (Coburn et al., 2012), electrospinning in liquids (Thorvaldsson et al., 2008), and sacrificial fibers (Baker et al., 2008; Whited et al., 2011), with remarkable results. For example, an electrospun scaffold comprised of PCL microfibers and fibrin nanofibers resulted in higher human umbilical cord blood MSCs infiltration and GAG synthesis than PCL microfibres and PCL micro- and nano-fibers (Levorson et al., 2013). Electrospinning of PVA/methacrylate/chondroitin sulfate in ethanol bath enhanced goat BMSCs infiltration, proliferation and chondrogenesis in vitro and cartilage regeneration in vivo, even without cells or any other exogenous factor (Thorvaldsson et al., 2008). Salt leaching of chitosan hydrogels reinforced with either PDLA/PLLA or PDLA/PCL has been shown to increase porosity; however, the PDLA/PLLA-based scaffolds provided a favorable elastic modulus for articular cartilage, whilst the PDLA/PCL-based scaffolds exhibited better biological response (Slivka et al., 2001; Wright et al., 2014).
From Two-Dimensional to Three-Dimensional Constructs
To more closely imitate the native three-dimensional cartilage architecture, multi-layer horizontally, randomly, and vertically aligned fibers PCL fibers in a graphene-oxide-collagen microporous network have been developed (Girão et al., 2018). To imitate the three-dimensional cartilage architecture and composition, PCL/cartilage-derived matrix electrospun fibers were produced in single- and multi- layered conformations; the resultant multi-layered scaffolds enhanced chondrogenesis of human ADSCs, as judged by increased sGAG synthesis and increased gene expression of collagen type X, but had lower elastic modulus to PCL-alone scaffolds (Garrigues et al., 2014). Despite these significant advancements, scalability of such constructs is of concern. For this reason, electrospinning has been combined with other fabrication technologies for the development of three-dimensional constructs that closely imitate native cartilage architectural features. For example, electrospinning with rapid prototyping resulted in scaffolds with acceptable mechanical properties that supported bovine chondrocyte growth and cartilage-ECM synthesis for 4 weeks in vitro (Moroni et al., 2008). Electrospinning combined with freeze-drying has been shown to yield scaffolds that supported rabbit BMSC growth in vitro (Zheng et al., 2016) and to successfully regenerate osteochondral defects in a rabbit model (Liu et al., 2014b). More complex scaffolds have also been prepared and demonstrated efficacy in a mice model using electrospinning, three-dimensional printing and freeze drying (Chen et al., 2019).
Preclinical Data
It is worth noting that all small animal in vivo data have shown promising results. For example, in nude mice, layer-by-layer sandwich constructs of collagen/PLCL seeded with rabbit auricular chondrocytes reached 83% Young's modulus of native auricular cartilage after 12 weeks of implantation (He et al., 2013). PVA with chondroitin sulfate electrospun fibers in a rat osteochondral defect model resulted in enhanced chondrogenesis, compared to the empty control group (Coburn et al., 2012). Resveratrol-PLA-gelatin scaffolds resulted in faster healing than PLA-gelatin scaffolds in a rat articular cartilage defect model 12 weeks post-implantation (Yu et al., 2018). In a rabbit cartilage defect model, aligned PLLA-polydopamine-chondroitin sulfate fibers facilitated the filling of defects and the regeneration of hyaline cartilage-like tissue (Ren et al., 2019). In rabbit meniscal defects, aligned PCL fibers (produced with sacrificial PEO fibers) combined with a tissue engineered construct derived from synovial mesenchymal stem cells significantly contributed to the prevention of meniscal extrusion, exerted a chondroprotective effect and meniscal defects were repaired with a fibrocartilaginous tissue (Shimomura et al., 2019). In the only large animal model in vivo work (7 mm full thickness cartilage defect swine model), PCL fibers loaded with human BMSCs showed the most complete repair, generated hyaline cartilage-like tissue and had the highest equilibrium compressive stress of 1.5 MPa in the regenerated cartilage after 6 months of implantation, in comparison to PCL scaffolds alone and PCL/allogenic chondrocytes constructs (Li et al., 2009). Despite these profound preclinical data, no clinical studies are available to-date in cartilage engineering.
Critical Analysis and Outlook
Electrospinning has been adopted in tissue engineering and regenerative medicine since the 1990's. Since then, a substantial amount of work has been conducted, as evidenced by the wealth of scientific publications available (e.g., 8,103 papers in PubMed; term searched “electrospinning” in all fields). In cartilage space, the electrospinning technology is still at its infancy, which can be substantiated by the low number of scientific publications available (e.g., 155 papers in PubMed; terms searched “electrospinning” and “cartilage” in all fields). Nonetheless, significant strides (e.g., development of three-dimensional tissue equivalents that, to a certain extent, replicate the complex cartilage architecture and composition and have resulted in promising in vivo data in small animal preclinical models) have been achieved. However, it is also apparent that large animal experimentation and clinical translation are lagging behind for cartilage and also other clinical indications (e.g., only 5 clinical studies appear at clinicaltrials.gov, term searched “electrospinning” in all studies). This limited technology transfer from benchtop to large animal models and to clinical setting may be attributed to scalability and infrastructure costs required to produce reproducible fibers (e.g., controlled temperature/humidity chambers, automated systems, variable collectors, multi-syringe systems). Considering though that electrospun scaffolds have started becoming commercially and clinically available (Ryan et al., 2015), we believe that in the years to come they will also be assessed in cartilage engineering.
We also believe that in the years to come electrospun scaffolds together with other in vitro microenvironment modulators will play a crucial role in the development of functional cell therapies for cartilage engineering. For example, the positive impact of bioreactors in musculoskeletal tissue engineering has been well-established (Peroglio et al., 2018) and electrospun scaffolds coupled with bioreactors have shown promise to-date, even for complex structures, such as the cartilage-bone interface (Baumgartner et al., 2019). Further, considering that extracellular matrix is key modulator of cell fate through provision of biophysical, biochemical, and biological signals (Guilak et al., 2009; Watt and Huck, 2013; Kumar et al., 2017; Muncie and Weaver, 2018; Smith et al., 2018; Novoseletskaya et al., 2019), strategies that enhance and accelerate native extracellular matrix synthesis [e.g., hypoxia (Taheem et al., 2019)] and deposition [e.g., macromolecular crowding (Graceffa and Zeugolis, 2019)] coupled with electrospinning are likely to lead to more biomimetic three-dimensional cartilage equivalents. It is also worth noting, that although the cell-sheet/scaffold-free technology has shown promise in human cartilage engineering (Sato et al., 2019), only thin layers of tissue can be developed, which imposes the need of either multi-layered approaches that are often associated with delamination and cell death in the middle layers due to poor nutrient/waste transport (Sekine et al., 2011) or multiple surgeries (Shimizu et al., 2006; Komae et al., 2017). Considering that advances in engineering are now allowing the development of porous electrospun scaffolds (Ameer et al., 2019), we believe that temperature-responsive electrospun scaffolds will play a key role in the development of scaffold-free three-dimensional tissue-like surrogates in the years to come.
Conclusions
Electrospinning can produce nano- to micro-range fibrous constructs that closely imitate the architecture of native tissues. Further, has the capacity to deliver cells and therapeutic molecules at the side of injury. Advancements in fabrication methods have addressed scalability issues and have allowed the development of porous structures than enable cell infiltration and growth for prolonged periods of times. Despite all these advantages, electrospun scaffolds have yet to be assessed comprehensively in preclinical models and clinical setting, which has compromised wide acceptance of this pioneering technology in biomedicine.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This work was supported by Science Foundation Ireland, Career Development Award (Grant No. 15/CDA/3629) and Science Foundation Ireland/European Regional Development Fund (Grant No. 13/RC/2073).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Alexopoulos, L. G., Haider, M. A., Vail, T. P., and Guilak, F. (2003). Alterations in the mechanical properties of the human chondrocyte pericellular matrix with osteoarthritis. J. Biomech. Eng. 125, 323–333. doi: 10.1115/1.1579047
Alexopoulos, L. G., Youn, I., Bonaldo, P., and Guilak, F. (2009). Developmental and osteoarthritic changes in Col6a1-knockout mice: Biomechanics of type VI collagen in the cartilage pericellular matrix. Arthritis Rheum. 60, 771–779. doi: 10.1002/art.24293
Alves da Silva, M., Martins, A., Costa-Pinto, A. R., Monteiro, N., Faria, S., Reis, R. L., et al. (2017). Electrospun nanofibrous meshes cultured with Wharton's jelly stem cell: an alternative for cartilage regeneration, without the need of growth factors. Biotechnol. J. 12:1700073. doi: 10.1002/biot.201700073
Alves da Silva, M. L., Martins, A., Costa-Pinto, A. R., Costa, P., Faria, S., Gomes, M., et al. (2010). Cartilage tissue engineering using electrospun PCL nanofiber meshes and MSCs. Biomacromolecules 11, 3228–3236. doi: 10.1021/bm100476r
Ameer, J., Pr, A., and Kasoju, N. (2019). Strategies to tune electrospun scaffold porosity for effective cell response in tissue engineering, J. Funct. Biomater. 10:E30. doi: 10.3390/jfb10030030
Archer, C. W., and Francis-West, P. (2003). The chondrocyte, Int. J. Biochem. Cell Biol. 35, 401–404. doi: 10.1016/S1357-2725(02)00301-1
Baek, J., Chen, X., Sovani, S., Jin, S., Grogan, S. P., and D'Lima, D. D. (2015). Meniscus tissue engineering using a novel combination of electrospun scaffolds and human meniscus cells embedded within an extracellular matrix hydrogel. J. Orthop. Res. 33, 572–583. doi: 10.1002/jor.22802
Baker, B. M., Gee, A. O., Metter, R. B., Nathan, A. S., Marklein, R. A., Burdick, J. A., et al. (2008). The potential to improve cell infiltration in composite fiber-aligned electrospun scaffolds by the selective removal of sacrificial fibers. Biomaterials 29, 2348–2358. doi: 10.1016/j.biomaterials.2008.01.032
Baumgartner, W., Otto, L., Hess, S., Stark, W., Märsmann, S., Bürgisser, G., et al. (2019). Cartilage/bone interface fabricated under perfusion: spatially organized commitment of adipose-derived stem cells without medium supplementation. J. Biomed. Mater Res. B. 107, 1833–1843. doi: 10.1002/jbm.b.34276
Begum, R., Su, B., Perriman, A., Scarpa, F., and Kafienah, W. (2018). Electrospun cellulose-silk composite nanofibres direct mesenchymal stem cell chondrogenesis in the absence of biological stimulation. bioRxiv 434316. doi: 10.1101/434316
Bhardwaj, N., and Kundu, S. C. (2010). Electrospinning: a fascinating fiber fabrication technique. Biotechnol. Adv. 28, 325–347. doi: 10.1016/j.biotechadv.2010.01.004
Bhosale, A. M., and Richardson, J. B. (2008). Articular cartilage: Structure, injuries and review of management. Br. Med. Bull. 87, 77–95. doi: 10.1093/bmb/ldn025
Bi, W., Deng, J. M., Zhang, Z., Behringer, R. R., and de Crombrugghe, B. (1999). Sox9 is required for cartilage formation. Nat. Genet. 22:85. doi: 10.1038/8792
Braghirolli, D. I., Steffens, D., and Pranke, P. (2014). Electrospinning for regenerative medicine: a review of the main topics. Drug Discov. Today 19, 743–753. doi: 10.1016/j.drudis.2014.03.024
Buckwalter, J., and Mankin, H. (1997). Articular cartilage: tissue design and chondrocyte-matrix interactions. Instr. Course. Lect. 47, 477–486.
Buttle, D. J., Fowles, A., Ilic, M. Z., and Handley, C. J. (1997). “Aggrecanase” activity is implicated in tumour necrosis factor alpha mediated cartilage aggrecan breakdown but is not detected by an in vitro assay. Mol. Pathol. 50, 153–159. doi: 10.1136/mp.50.3.153
Calejo, I., Costa-Almeida, R., Reis, R., and Gomes, M. (2019). A Physiology-Inspired Multifactorial Toolbox in Soft-to-Hard Musculoskeletal Interface Tissue Engineering. Trends Biotechnol. Available online at: https://www.sciencedirect.com/science/article/pii/S0167779919301520
Camp, C. L., Stuart, M. J., and Krych, A. J. (2014). Current concepts of articular cartilage restoration techniques in the knee. Sports Health. 6, 265–273. doi: 10.1177/1941738113508917
Casanellas, I., García-Lizarribar, A., Lagunas, A., and Samitier, J. (2018). Producing 3D biomimetic nanomaterials for musculoskeletal system regeneration. Front. Bioeng. Biotechnol. 6:128. doi: 10.3389/fbioe.2018.00128
Casanova, M., Reis, R., Martins, A., and Neves, N. (2018). The use of electrospinning technique on osteochondral tissue engineering. Adv. Exp. Med. Biol. 1058, 247–263. doi: 10.1007/978-3-319-76711-6_11
Cecil, D. L., Johnson, K., Rediske, J., Lotz, M., Schmidt, A. M., and Terkeltaub, R. (2005). Inflammation-induced chondrocyte hypertrophy is driven by receptor for advanced glycation end products. J. Immunol. 175, 8296–8302. doi: 10.4049/jimmunol.175.12.8296
Chen, J.-P., and Su, C.-H. (2011). Surface modification of electrospun PLLA nanofibers by plasma treatment and cationized gelatin immobilization for cartilage tissue engineering. Acta Biomater. 7, 234–243. doi: 10.1016/j.actbio.2010.08.015
Chen, W., Chen, S., Morsi, Y., El-Hamshary, H., El-Newhy, M., Fan, C., et al. (2016). Superabsorbent 3D scaffold based on electrospun nanofibers for cartilage tissue engineering. ACS Appl. Mater Interfaces. 8, 24415–24425. doi: 10.1021/acsami.6b06825
Chen, W., Xu, Y., Liu, Y., Wang, Z., Li, Y., Jiang, G., et al. (2019). Three-dimensional printed electrospun fiber-based scaffold for cartilage regeneration. Materi Des. 179:107886. doi: 10.1016/j.matdes.2019.107886
Cheng, A., Schwartz, Z., Kahn, A., Li, X., Shao, Z., Sun, M., et al. (2019). Advances in porous scaffold design for bone and cartilage tissue engineering and regeneration. Tissue Eng. Part B. 25, 14–29. doi: 10.1089/ten.teb.2018.0119
Cheung, H.-Y., Lau, K.-T., Lu, T.-P., and Hui, D. (2007). A critical review on polymer-based bio-engineered materials for scaffold development. Composites B. 38, 291–300. doi: 10.1016/j.compositesb.2006.06.014
Ching, K. Y., Andriotis, O. G., Li, S., Basnett, P., Su, B., Roy, I., et al. (2016). Nanofibrous poly(3-hydroxybutyrate)/poly(3-hydroxyoctanoate) scaffolds provide a functional microenvironment for cartilage repair. J Biomater. Appl. 31, 77–91. doi: 10.1177/0885328216639749
Church, V., Nohno, T., Linker, C., Marcelle, C., and Francis-West, P. (2002). Wnt regulation of chondrocyte differentiation. J. Cell Sci. 115, 4809–4818. doi: 10.1242/jcs.00152
Coburn, J. M., Gibson, M., Monagle, S., Patterson, Z., and Elisseeff, J. H. (2012). Bioinspired nanofibers support chondrogenesis for articular cartilage repair. Proc. Natl. Acad. Sci. U.S.A. 109, 10012–10017. doi: 10.1073/pnas.1121605109
Cohen, N. P., Foster, R. J., and Mow, V. C. (1998). Composition and dynamics of articular cartilage: structure, function, and maintaining healthy state. J. Orthop. Sports Phys. Ther. 28, 203–215. doi: 10.2519/jospt.1998.28.4.203
Correa, D., and Lietman, S. A. (2017). Articular cartilage repair: Current needs, methods and research directions. Semin Cell Dev. Biol. 62, 67–77. doi: 10.1016/j.semcdb.2016.07.013
De Vrieze, S., Van Camp, T., Nelvig, A., Hagström, B., Westbroek, P., and De Clerck, K. (2009). The effect of temperature and humidity on electrospinning. J. Mater Sci. 44, 1357–1362. doi: 10.1007/s10853-008-3010-6
Decker, R. S., Koyama, E., and Pacifici, M. (2015). Articular cartilage: Structural and developmental intricacies and questions. Curr. Osteoporos. Rep. 13, 407–414. doi: 10.1007/s11914-015-0290-z
Delgado, L., Bayon, Y., Pandit, A., and Zeugolis, D. (2015). To cross-link or not to cross-link? Cross-linking associated foreign body response of collagen-based devices. Tissue Eng. Part B. 21, 298–313. doi: 10.1089/ten.teb.2014.0290
Demoor, M., Ollitrault, D., Gomez-Leduc, T., Bouyoucef, M., Hervieu, M., Fabre, H., et al. (2014). Cartilage tissue engineering: molecular control of chondrocyte differentiation for proper cartilage matrix reconstruction. Biochim. Biophys. Acta. 1840, 2414–2440. doi: 10.1016/j.bbagen.2014.02.030
Dowthwaite, G., Bishop, J., Redman, S., Khan, I., Rooney, P., Evans, D., et al. (2004). The surface of articular cartilage contains a progenitor cell population. J. Cell Sc. 117, 889–897. doi: 10.1242/jcs.00912
Flannery, C. R., Little, C. B., Hughes, C. E., and Caterson, B. (1998). Expression and activity of articular cartilage hyaluronidases. Biochem. Biophys. Res. Commun. 251, 824–829. doi: 10.1006/bbrc.1998.9561
Fuller, K., Gaspar, D., Delgado, L., Pandit, A., and Zeugolis, D. (2016). Influence of porosity and pore shape on structural, mechanical and biological properties of poly ϵ-caprolactone electro-spun fibrous scaffolds. Nanomedicine 11, 1031–1040. doi: 10.2217/nnm.16.21
Fuller, K., Gaspar, D., Delgado, L., and Zeugolis, D. (2019). Development macro-porous electro-spun meshes with clinically relevant mechanical properties - A technical note. Biomed. Mater. 14:024103. doi: 10.1088/1748-605X/aaf929
Garg, K., and Bowlin, G. L. (2011). Electrospinning jets and nanofibrous structures. Biomicrofluidics 5:13403. doi: 10.1063/1.3567097
Garrigues, N. W., Little, D., Sanchez-Adams, J., Ruch, D. S., and Guilak, F. (2014). Electrospun cartilage-derived matrix scaffolds for cartilage tissue engineering. J. Biomed. Mater. Res. A. 102, 3998–4008. doi: 10.1002/jbm.a.35068
Girão, A. F., Semitela, Â., Ramalho, G., Completo, A., and Marques, P. A. (2018). Mimicking nature: Fabrication of 3D anisotropic electrospun polycaprolactone scaffolds for cartilage tissue engineering applications. Composites B 154, 99–107. doi: 10.1016/j.compositesb.2018.08.001
Goldring, M. B. (2012). Chondrogenesis, chondrocyte differentiation, and articular cartilage metabolism in health and osteoarthritis. Ther. Adv. Musculoskelet Dis. 4, 269–285. doi: 10.1177/1759720X12448454
Goldring, M. B., Otero, M., Plumb, D. A., Dragomir, C., Favero, M., El Hachem, K., et al. (2011). Roles of inflammatory and anabolic cytokines in cartilage metabolism: signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur. Cell Mater. 21, 202–220. doi: 10.22203/eCM.v021a16
Gomez-Sanchez, C., Kowalczyk, T., Ruiz De Eguino, G., Lopez-Arraiza, A., Infante, A., Rodriguez, C. I., et al. (2014). Electrospinning of poly(lactic acid)/polyhedral oligomeric silsesquioxane nanocomposites and their potential in chondrogenic tissue regeneration. J. Biomater. Sci. Polym. Ed. 25, 802–825. doi: 10.1080/09205063.2014.910151
Gouttenoire, J., Bougault, C., Aubert-Foucher, E., Perrier, E., Ronzière, M.-C., Sandell, L., et al. (2010). BMP-2 and TGF-β1 differentially control expression of type II procollagen and α10 and α11 integrins in mouse chondrocytes. Eur. J. Cell Biol. 89, 307–314. doi: 10.1016/j.ejcb.2009.10.018
Graceffa, V., Vinatier, C., Guicheux, J., Evans, C., Stoddart, M., Alini, M., et al. (2018). State of art and limitations in genetic engineering to induce stable chondrogenic phenotype. Biotechnol. Adv. 36, 1855–1869. doi: 10.1016/j.biotechadv.2018.07.004
Graceffa, V., Vinatier, C., Guicheux, J., Stoddart, M., Alini, M., and Zeugolis, D. (2019). Chasing chimeras - The elusive stable chondrogenic phenotype. Biomaterials 192, 199–225. doi: 10.1016/j.biomaterials.2018.11.014
Graceffa, V., and Zeugolis, D. (2019). Carrageenan enhances chondrogenesis and osteogenesis in human bone marrow stem cell culture. Eur. Cell Mater. 37, 310–332. doi: 10.22203/eCM.v037a19
Guilak, F., Cohen, D., Estes, B., Gimble, J., Liedtke, W., and Chen, C. (2009). Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 5, 17–26. doi: 10.1016/j.stem.2009.06.016
Guimarães, A., Martins, A., Pinho, E. D., Faria, S., Reis, R. L., and Neves, N. M. (2010). Solving cell infiltration limitations of electrospun nanofiber meshes for tissue engineering applications. Nanomedicine 5, 539–554. doi: 10.2217/nnm.10.31
Haider, A., Haider, S., and Kang, I.-K. (2015). A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 11, 1165–1188. doi: 10.1016/j.arabjc.2015.11.015
Hassiba, A. J., El Zowalaty, M. E., Nasrallah, G. K., Webster, T. J., Luyt, A. S., Abdullah, A. M., et al. (2016). Review of recent research on biomedical applications of electrospun polymer nanofibers for improved wound healing. Nanomedicine11, 715–737. doi: 10.2217/nnm.15.211
He, X., Feng, B., Huang, C., Wang, H., Ge, Y., Hu, R., et al. (2015). Electrospun gelatin/polycaprolactone nanofibrous membranes combined with a coculture of bone marrow stromal cells and chondrocytes for cartilage engineering. Int. J. Nanomedicine 10, 2089–2099. doi: 10.2147/IJN.S79461
He, X., Fu, W., Feng, B., Wang, H., Liu, Z., Yin, M., et al. (2013). Electrospun collagen/poly(L-lactic acid-co-epsilon-caprolactone) hybrid nanofibrous membranes combining with sandwich construction model for cartilage tissue engineering. J. Nanosci. Nanotechnol. 13, 3818–3825. doi: 10.1166/jnn.2013.7436
Hiraoka, K., Grogan, S., Olee, T., and Lotz, M. (2006). Mesenchymal progenitor cells in adult human articular cartilage. Biorheology 43, 447–454. doi: 10.1002/art.20269
Holmes, B., Castro, N. J., Li, J., Keidar, M., and Zhang, L. G. (2013). Enhanced human bone marrow mesenchymal stem cell functions in novel 3D cartilage scaffolds with hydrogen treated multi-walled carbon nanotubes. Nanotechnology 24:365102. doi: 10.1088/0957-4484/24/36/365102
Homandberg, G., Meyers, R., and Williams, J. (1993). Intraarticular injection of fibronectin fragments causes severe depletion of cartilage proteoglycans in vivo. J. Rheumatol. 20, 1378–1382.
Homandberg, G. A., and Hui, F. (1996). Association of proteoglycan degradation with catabolic cytokine and stromelysin release from cartilage cultured with fibronectin fragments. Arch. Biochem. Biophys. 334, 325–331. doi: 10.1006/abbi.1996.0461
Honarpardaz, A., Irani, S., Pezeshki-Modaress, M., Zandi, M., and Sadeghi, A. (2019). Enhanced chondrogenic differentiation of bone marrow mesenchymal stem cells on gelatin/glycosaminoglycan electrospun nanofibers with different amount of glycosaminoglycan. J. Biomed. Mater Res. A. 107, 38–48. doi: 10.1002/jbm.a.36501
Hu, K., Xu, L., Cao, L., Flahiff, C. M., Brussiau, J., Ho, K., et al. (2006). Pathogenesis of osteoarthritis-like changes in the joints of mice deficient in type IX collagen. Arthritis. Rheum. 54, 2891–2900. doi: 10.1002/art.22040
Huang, G. P., Molina, A., Tran, N., Collins, G., and Arinzeh, T. L. (2018). Investigating cellulose derived glycosaminoglycan mimetic scaffolds for cartilage tissue engineering applications. J. Tissue Eng. Regen. Med. 12, e592–e603. doi: 10.1002/term.2331
Hunter, D. J., McDougall, J. J., and Keefe, F. J. (2008). The symptoms of osteoarthritis and the genesis of pain. Rheum. Dis. Clin. North Am. 34, 623–643. doi: 10.1016/j.rdc.2008.05.004
Hunziker, E. B. (2002). Articular cartilage repair: Basic science and clinical progress. A review of the current status and prospects. Osteoarthr. Cartilage 10, 432–463. doi: 10.1053/joca.2002.0801
Ikeda, T., Kamekura, S., Mabuchi, A., Kou, I., Seki, S., Takato, T., et al. (2004). The combination of SOX5, SOX6, and SOX9 (the SOX trio) provides signals sufficient for induction of permanent cartilage. Arthritis. Rheum. 50, 3561–3573. doi: 10.1002/art.20611
Islas-Arteaga, N. C., Raya Rivera, A., Esquiliano Rendon, D. R., Morales-Corona, J., Ontiveros-Nevares, P. G., Flores Sánchez, M. G., et al. (2018). Electrospun scaffolds with surfaces modified by plasma for regeneration of articular cartilage tissue: a pilot study in rabbit. Int. J. Polym. Mater Pol. Biomat. 68, 1–10. doi: 10.1080/00914037.2018.1534109
Janjanin, S., Li, W. J., Morgan, M. T., Shanti, R. M., and Tuan, R. S. (2008). Mold-shaped, nanofiber scaffold-based cartilage engineering using human mesenchymal stem cells and bioreactor. J. Surg. Res. 149, 47–56. doi: 10.1016/j.jss.2007.12.788
Jiang, T., Kai, D., Liu, S., Huang, X., Heng, S., Zhao, J., et al. (2018). Mechanically cartilage-mimicking poly (PCL-PTHF urethane)/collagen nanofibers induce chondrogenesis by blocking NF–kappa B signaling pathway. Biomaterials 178, 281–292. doi: 10.1016/j.biomaterials.2018.06.023
Jiang, Y., and Tuan, R. (2015). Origin and function of cartilage stem/progenitor cells in osteoarthritis. Nat. Rev. Rheumatol. 11, 206–212. doi: 10.1038/nrrheum.2014.200
Keikhaei, S., Mohammadalizadeh, Z., Karbasi, S., and Salimi, A. (2019). Evaluation of the effects of β-tricalcium phosphate on physical, mechanical and biological properties of Poly (3-hydroxybutyrate)/chitosan electrospun scaffold for cartilage tissue engineering applications. Mater. Technol. 34, 615–625. doi: 10.1080/10667857.2019.1611053
Kessick, R., Fenn, J., and Tepper, G. (2004). The use of AC potentials in electrospraying and electrospinning processes. Polymer 45, 2981–2984. doi: 10.1016/j.polymer.2004.02.056
Khorshidi, S., Solouk, A., Mirzadeh, H., Mazinani, S., Lagaron, J. M., Sharifi, S., et al. (2016). A review of key challenges of electrospun scaffolds for tissue-engineering applications. J. Tissue Eng. Regen. Med. 10, 715–738. doi: 10.1002/term.1978
Ki, C. S., Baek, D. H., Gang, K. D., Lee, K. H., Um, I. C., and Park, Y. H. (2005). Characterization of gelatin nanofiber prepared from gelatin–formic acid solution. Polymer 46, 5094–5102. doi: 10.1016/j.polymer.2005.04.040
Ki, C. S., Kim, J. W., Hyun, J. H., Lee, K. H., Hattori, M., Rah, D. K., et al. (2007). Electrospun three-dimensional silk fibroin nanofibrous scaffold. J. Appl. Polym. 106, 3922–3928. doi: 10.1002/app.26914
Kim, C., Shores, L., Guo, Q., Aly, A., Jeon, O. H., Kim do, H., et al. (2016). Electrospun microfiber scaffolds with anti-inflammatory tributanoylated N-acetyl-d-glucosamine promote cartilage regeneration. Tissue Eng. Part A. 22, 689–697. doi: 10.1089/ten.tea.2015.0469
Kim, T. G., Chung, H. J., and Park, T. G. (2008). Macroporous and nanofibrous hyaluronic acid/collagen hybrid scaffold fabricated by concurrent electrospinning and deposition/leaching of salt particles. Acta Biomater. 4, 1611–1619. doi: 10.1016/j.actbio.2008.06.008
Komae, H., Sekine, H., Dobashi, I., Matsuura, K., Ono, M., Okano, T., et al. (2017). Three-dimensional functional human myocardial tissues fabricated from induced pluripotent stem cells. J. Tissue Eng. Regen. Med. 11, 926–935. doi: 10.1002/term.1995
Kumar, A., Placone, J., and Engler, A. (2017). Understanding the extracellular forces that determine cell fate and maintenance. Development 144, 4261–4270. doi: 10.1242/dev.158469
Kuo, Y. C., Hung, S. C., and Hsu, S. H. (2014). The effect of elastic biodegradable polyurethane electrospun nanofibers on the differentiation of mesenchymal stem cells. Colloids Surf. B. 122, 414–422. doi: 10.1016/j.colsurfb.2014.07.017
Kurz, B., Lemke, A. K., Fay, J., Pufe, T., Grodzinsky, A. J., and Schünke, M. (2005). Pathomechanisms of cartilage destruction by mechanical injury. Ann. Anat. 187, 473–485. doi: 10.1016/j.aanat.2005.07.003
Kwon, O. H., Lee, I. S., Ko, Y. G., Meng, W., Jung, K. H., Kang, I. K., et al. (2007). Electrospinning of microbial polyester for cell culture. Biomed. Mater. 2, S52–S58. doi: 10.1088/1748-6041/2/1/S08
Lavik, E., and Langer, R. (2004). Tissue engineering: current state and perspectives. Appl. Microbiol. Biotechnol. 65, 1–8. doi: 10.1007/s00253-004-1580-z
Lefebvre, V., and Smits, P. (2005). Transcriptional control of chondrocyte fate and differentiation. Birth. Defects Res. C. 75, 200–212. doi: 10.1002/bdrc.20048
Legendre, F., Ollitrault, D., Hervieu, M., Bauge, C., Maneix, L., Goux, D., et al. (2013). Enhanced hyaline cartilage matrix synthesis in collagen sponge scaffolds by using siRNA to stabilize chondrocytes phenotype cultured with bone morphogenetic protein-2 under hypoxia. Tissue Eng. Part C. 19, 550–567. doi: 10.1089/ten.tec.2012.0508
Levorson, E. J., Raman Sreerekha, P., Chennazhi, K. P., Kasper, F. K., Nair, S. V., and Mikos, A. G. (2013). Fabrication and characterization of multiscale electrospun scaffolds for cartilage regeneration, Biomed Mater. 8:014103. doi: 10.1088/1748-6041/8/1/014103
Li, D., Wang, Y., and Xia, Y. (2004). Electrospinning nanofibers as uniaxially aligned arrays and layer-by-layer stacked films. Adv. Mater. 16, 361–366. doi: 10.1002/adma.200306226
Li, G., Shi, S., Lin, S., Zhou, T., Shao, X., Huang, Q., et al. (2018). Electrospun fibers for cartilage tissue regeneration. Curr. Stem Cell Res. Ther. 13, 591–599. doi: 10.2174/1574888X13666180417120508
Li, J., Chen, G., Xu, X., Abdou, P., Jiang, Q., Shi, D., et al. (2019). Advances of injectable hydrogel-based scaffolds for cartilage regeneration. Regen Biomater. 6, 129–140. doi: 10.1093/rb/rbz022
Li, T.-F., O'Keefe, R. J., and Chen, D. (2005a). TGF-β signaling in chondrocytes. Front. Biosci. 10, 681–688. doi: 10.2741/1563
Li, W. J., Chiang, H., Kuo, T. F., Lee, H. S., Jiang, C. C., and Tuan, R. S. (2009). Evaluation of articular cartilage repair using biodegradable nanofibrous scaffolds in a swine model: a pilot study. J. Tissue Eng. Regen Med. 3, 1–10. doi: 10.1002/term.127
Li, W. J., Cooper, J. A. Jr., Mauck, R. L., and Tuan, R. S. (2006a). Fabrication and characterization of six electrospun poly(alpha-hydroxy ester)-based fibrous scaffolds for tissue engineering applications. Acta Biomater. 2, 377–385. doi: 10.1016/j.actbio.2006.02.005
Li, W. J., Danielson, K. G., Alexander, P. G., and Tuan, R. S. (2003). Biological response of chondrocytes cultured in three-dimensional nanofibrous poly(epsilon-caprolactone) scaffolds. J. Biomed. Mater. Res. A. 67, 1105–1114. doi: 10.1002/jbm.a.10101
Li, W. J., Jiang, Y. J., and Tuan, R. S. (2006b). Chondrocyte phenotype in engineered fibrous matrix is regulated by fiber size. Tissue Eng. 12, 1775–1785. doi: 10.1089/ten.2006.12.1775
Li, W. J., Shanti, R. M., and Tuan, R. S. (2007). Electrospinning Technology for Nanofibrous Scaffolds in Tissue Engineering. Nanotechnologies for the life sciences. doi: 10.1002/9783527610419.ntls0097
Li, W. J., and Tuan, R. S. (2009). Fabrication and application of nanofibrous scaffolds in tissue engineering. Curr. Protocols Cell Biol. 42, 25.22. 21–25.22.12. doi: 10.1002/0471143030.cb2502s42
Li, W. J., Tuli, R., Okafor, C., Derfoul, A., Danielson, K. G., Hall, D. J., et al. (2005b). A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials 26, 599–609. doi: 10.1016/j.biomaterials.2004.03.005
Li, Y., and Xu, L. (2015). Advances in understanding cartilage remodeling. F1000Res. 4:642. doi: 10.12688/f1000research.6514.1
Li, Z., Liu, P., Yang, T., Sun, Y., You, Q., Li, J., et al. (2016). Composite poly(l-lactic-acid)/silk fibroin scaffold prepared by electrospinning promotes chondrogenesis for cartilage tissue engineering. J. Biomater. Appl. 30, 1552–1565. doi: 10.1177/0885328216638587
Li, Z., and Wang, C. (eds.). (2013). “Effects of working parameters on electrospinning,” in One-Dimensional Nanostructures. SpringerBriefs in Materials (Berlin, Heidelberg: Springer), 15–28. doi: 10.1007/978-3-642-36427-3_2
Lim, E.-H., Sardinha, J. P., and Myers, S. (2014). Nanotechnology biomimetic cartilage regenerative scaffolds. Arch. Plast Surg. 41, 231–240. doi: 10.5999/aps.2014.41.3.231
Lin, Y. X., Ding, Z. Y., Zhou, X. B., Li, S. T., Xie de, M., Li, Z. Z., et al. (2015). In vitro and in vivo evaluation of the developed PLGA/HAp/Zein scaffolds for bone-cartilage interface regeneration. Biomed. Environ. Sci. 28, 1–12. doi: 10.3967/bes2015.001
Liu, J., Nie, H., Xu, Z., Niu, X., Guo, S., Yin, J., et al. (2014a). The effect of 3D nanofibrous scaffolds on the chondrogenesis of induced pluripotent stem cells and their application in restoration of cartilage defects. PLoS ONE 9:e111566. doi: 10.1371/journal.pone.0111566
Liu, W., Thomopoulos, S., and Xia, Y. (2012). Electrospun nanofibers for regenerative medicine. Adv. Healthc Mater. 1, 10–25. doi: 10.1002/adhm.201100021
Liu, X., Liu, S., Liu, S., and Cui, W. (2014b). Evaluation of oriented electrospun fibers for periosteal flap regeneration in biomimetic triphasic osteochondral implant. J. Biomed. Mater Res B. 102, 1407–1414. doi: 10.1002/jbm.b.33119
Liu, Y., Liu, L., Wang, Z., Zheng, G., Chen, Q., and Luo, E. (2018). Application of electrospinning strategy on cartilage tissue engineering. Curr. Stem Cell Res. Ther. 13, 526–532. doi: 10.2174/1574888X13666180628163515
Lodewyckx, L., and Lories, R. J. (2009). WNT Signaling in osteoarthritis and osteoporosis: What is the biological significance for the clinician? Curr. Rheumatol. Rep. 11, 23–30. doi: 10.1007/s11926-009-0004-6
MacLean, H. E., Kim, J. I., Glimcher, M. J., Wang, J., Kronenberg, H. M., and Glimcher, L. H. (2003). Absence of transcription factor c-maf causes abnormal terminal differentiation of hypertrophic chondrocytes during endochondral bone development. Dev. Biol. 262, 51–63. doi: 10.1016/S0012-1606(03)00324-5
Markowski, J., Magiera, A., Lesiak, M., Sieron, A. L., Pilch, J., and Blazewicz, S. (2015). Preparation and characterization of nanofibrous polymer scaffolds for cartilage tissue engineering. J. Nanomat. 2015:564087. doi: 10.1155/2015/564087
Martin, I., Miot, S., Barbero, A., Jakob, M., and Wendt, D. (2007). Osteochondral tissue engineering, J Biomech. 40, 750–765. doi: 10.1016/j.jbiomech.2006.03.008
Matthews, J. A., Boland, E. D., Wnek, G. E., Simpson, D. G., and Bowlin, G. L. (2003). Electrospinning of collagen type II: a feasibility study. J. Bioact. Compat. Pol. 18, 125–134. doi: 10.1177/0883911503018002003
Matzat, S. J., van Tiel, J., Gold, G. E., and Oei, E. H. (2013). Quantitative MRI techniques of cartilage composition. Quant. Imaging Med. Surg. 3, 162–174. doi: 10.3978/j.issn.2223-4292.2013.06.04
McCullen, S. D., Autefage, H., Callanan, A., Gentleman, E., and Stevens, M. M. (2012). Anisotropic fibrous scaffolds for articular cartilage regeneration. Tissue Eng. Part A. 18, 2073–2083. doi: 10.1089/ten.tea.2011.0606
Mirzaei, S., Karkhaneh, A., Soleimani, M., Ardeshirylajimi, A., Seyyed Zonouzi, H., and Hanaee-Ahvaz, H. (2017). Enhanced chondrogenic differentiation of stem cells using an optimized electrospun nanofibrous PLLA/PEG scaffolds loaded with glucosamine. J. Biomed. Mater. Res. A. 105, 2461–2474. doi: 10.1002/jbm.a.36104
Molnár, K., and Vas, L. (2012). “Electrospun composite nanofibers and polymer composites,” in Synthetic Polymer-Polymer Composites, eds D. Bhattacharyya and S. Fakirov (Munchen: Carl Hanser Verlag GmbH & Co. KG), 301–349. doi: 10.3139/9781569905258.010
Moroni, L., Schotel, R., Hamann, D., de Wijn, J. R., and van Blitterswijk, C. A. (2008). 3D fiber-deposited electrospun integrated scaffolds enhance cartilage tissue formation. Adv. Funct. Mater. 18, 53–60. doi: 10.1002/adfm.200601158
Morrison, S., Shah, N., and Anderson, D. (1997). Regulatory mechanisms in stem cell biology. Cell 88, 287–298. doi: 10.1016/S0092-8674(00)81867-X
Mouthuy, P. A., El-Sherbini, Y., Cui, Z., and Ye, H. (2016). Layering PLGA-based electrospun membranes and cell sheets for engineering cartilage-bone transition. J. Tissue Eng. Regen. Med. 10, E263–E274. doi: 10.1002/term.1765
Mouthuy, P. A., Ye, H., Triffitt, J., Oommen, G., and Cui, Z. (2010). Physico-chemical characterization of functional electrospun scaffolds for bone and cartilage tissue engineering. Proc. Inst. Mech. Eng. H. 224, 1401–1414. doi: 10.1243/09544119JEIM824
Mow, V. C., Wang, C. C., and Hung, C. T. (1999). The extracellular matrix, interstitial fluid and ions as a mechanical signal transducer in articular cartilage. Osteoarthr. Cartilage 7, 41–58. doi: 10.1053/joca.1998.0161
Muir, H. (1995). The chondrocyte, architect of cartilage. Biomechanics, structure, function and molecular biology of cartilage matrix macromolecules. Bioessays 17, 1039–1048. doi: 10.1002/bies.950171208
Muncie, J., and Weaver, V. (2018). The physical and biochemical properties of the extracellular matrix regulate cell fate. Curr. Top. Dev. Biol. 130, 1–37. doi: 10.1016/bs.ctdb.2018.02.002
Munir, N., McDonald, A., and Callanan, A. (2019). A combinatorial approach: cryo-printing and electrospinning hybrid scaffolds for cartilage tissue engineering. Bioprinting 16:e00056. doi: 10.1016/j.bprint.2019.e00056
Musumeci, G., Castrogiovanni, P., Leonardi, R., Trovato, F. M., Szychlinska, M. A., Di Giunta, A., et al. (2014). New perspectives for articular cartilage repair treatment through tissue engineering: a contemporary review. World J. Orthop. 5:80. doi: 10.5312/wjo.v5.i2.80
Nam, J., Huang, Y., Agarwal, S., and Lannutti, J. (2007). Improved cellular infiltration in electrospun fiber via engineered porosity. Tissue Eng. 13, 2249–2257. doi: 10.1089/ten.2006.0306
Nazempour, A., and Van Wie, B. J. (2016). Chondrocytes, mesenchymal stem cells, and their combination in articular cartilage regenerative medicine. Ann. Biomed. Eng. 44, 1325–1354. doi: 10.1007/s10439-016-1575-9
Novoseletskaya, E., Grigorieva, O., Efimenko, A., and Kalinina, N. (2019). Extracellular matrix in the regulation of stem cell differentiation. Biochemistry. 84, 232–240. doi: 10.1134/S0006297919030052
Pearle, A. D., Warren, R. F., and Rodeo, S. A. (2005). Basic science of articular cartilage and osteoarthritis. Clin. Sports Med. 24, 1–12. doi: 10.1016/j.csm.2004.08.007
Peroglio, M., Gaspar, D., Zeugolis, D., and Alini, M. (2018). Relevance of bioreactors and whole tissue cultures for the translation of new therapies to humans. J. Orthop. Res. 36, 10–21. doi: 10.1002/jor.23655
Pham, Q. P., Sharma, U., and Mikos, A. G. (2006). Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng. 12, 1197–1211. doi: 10.1089/ten.2006.12.1197
Pillay, V., Dott, C., Choonara, Y. E., Tyagi, C., Tomar, L., Kumar, P., et al. (2013). A review of the effect of processing variables on the fabrication of electrospun nanofibers for drug delivery applications. J. Nanomat. 2013:22. doi: 10.1155/2013/789289
Poole, A. R., Kojima, T., Yasuda, T., Mwale, F., Kobayashi, M., and Laverty, S. (2001). Composition and structure of articular cartilage: a template for tissue repair: Clin. Orthop. Relat. Res. 391, S26–S33. doi: 10.1097/00003086-200110001-00004
Pugliese, E., Coentro, J., and Zeugolis, D. (2018). Advancements and challenges in multidomain multicargo delivery vehicles. Adv. Mater. 30:e1704324. doi: 10.1002/adma.201704324
Ren, X., Li, J., Li, J., Jiang, Y., Li, L., Yao, Q., et al. (2019). Aligned porous fibrous membrane with a biomimetic surface to accelerate cartilage regeneration. Chem. Eng. J. 370, 1027–1038. doi: 10.1016/j.cej.2019.03.271
Renard, E., Porée, B., Chadjichristos, C., Kypriotou, M., Maneix, L., Bigot, N., et al. (2012). Sox9/Sox6 and Sp1 are involved in the insulin-like growth factor-I-mediated upregulation of human type II collagen gene expression in articular chondrocytes. J. Mol. Med. 90, 649–666. doi: 10.1007/s00109-011-0842-3
Ripmeester, E., Timur, U., Caron, M., and Welting, T. (2018). Recent insights into the contribution of the changing hypertrophic chondrocyte phenotype in the development and progression of osteoarthritis. Front. Bioeng. Biotechnol. 6:18. doi: 10.3389/fbioe.2018.00018
Rosen, V., Nove, J., Song, J. J., Thies, R. S., Cox, K., and Wozney, J. M. (1994). Responsiveness of clonal limb bud cell lines to bone morphogenetic protein 2 reveals a sequential relationship between cartilage and bone cell phenotypes. J. Bone Miner Res. 9, 1759–1768. doi: 10.1002/jbmr.5650091113
Rowland, D. C., Aquilina, T., Klein, A., Hakimi, O., Alexis-Mouthuy, P., Carr, A. J., et al. (2016). A comparative evaluation of the effect of polymer chemistry and fiber orientation on mesenchymal stem cell differentiation. J. Biomed. Mater Res. A. 104, 2843–2853. doi: 10.1002/jbm.a.35829
Ryan, C., Fuller, K., Larrañaga, A., Biggs, M., Bayon, Y., Sarasua, J., et al. (2015). An academic, clinical and industrial update on electrospun, additive manufactured and imprinted medical devices. Expert. Rev. Med. Devices. 12, 601–612. doi: 10.1586/17434440.2015.1062364
Sadeghi, D., Karbasi, S., Razavi, S., Mohammadi, S., Shokrgozar, M. A., and Bonakdar, S. (2016). Electrospun poly(hydroxybutyrate)/chitosan blend fibrous scaffolds for cartilage tissue engineering. J. Appl. Polym. 133, 171. doi: 10.1002/app.44171
Sato, M., Yamato, M., Mitani, G., Takagaki, T., Hamahashi, K., Nakamura, Y., et al. (2019). Combined surgery and chondrocyte cell-sheet transplantation improves clinical and structural outcomes in knee osteoarthritis. NPJ. Regen. Med. 4:4. doi: 10.1038/s41536-019-0069-4
Schmidt, C. E., and Baier, J. M. (2000). Acellular vascular tissues: natural biomaterials for tissue repair and tissue engineering. Biomaterials 21, 2215–2231. doi: 10.1016/S0142-9612(00)00148-4
Schneider, T., Kohl, B., Sauter, T., Kratz, K., Lendlein, A., Ertel, W., et al. (2012). Influence of fiber orientation in electrospun polymer scaffolds on viability, adhesion and differentiation of articular chondrocytes. Clin. Hemorheol. Microcirc. 52, 325–336. doi: 10.3233/CH-2012-1608
Seifarth, C., Csaki, C., and Shakibaei, M. (2009). Anabolic actions of IGF-I and TGF-ß1 on interleukin-1ß-treated human articular chondrocytes: evaluation in two and three dimensional cultures. Histol. Histopathol. 24, 1245–1262. doi: 10.14670/HH-24.1245
Sekine, W., Haraguchi, Y., Shimizu, T., Umezawa, A., and Okano, T. (2011). Thickness limitation and cell viability of multi-layered cell sheets and overcoming the diffusion limit by a porous-membrane culture insert. J. Biochip. Tissue Chip. S1:007. doi: 10.4172/2153-0777.S1-007
Shafiee, A., Seyedjafari, E., Sadat Taherzadeh, E., Dinarvand, P., Soleimani, M., and Ai, J. (2014). Enhanced chondrogenesis of human nasal septum derived progenitors on nanofibrous scaffolds. Mater. Sci. Eng. C 40, 445–454. doi: 10.1016/j.msec.2014.04.027
Shafiee, A., Soleimani, M., Chamheidari, G. A., Seyedjafari, E., Dodel, M., Atashi, A., et al. (2011). Electrospun nanofiber-based regeneration of cartilage enhanced by mesenchymal stem cells. J. Biomed. Mater. Res. A 99, 467–478. doi: 10.1002/jbm.a.33206
Shanmugasundaram, S., Chaudhry, H., and Arinzeh, T. L. (2011). Microscale versus nanoscale scaffold architecture for mesenchymal stem cell chondrogenesis. Tissue Eng. Part A 17, 831–840. doi: 10.1089/ten.tea.2010.0409
Sharma, A. R., Jagga, S., Lee, S.-S., and Nam, J.-S. (2013). Interplay between cartilage and subchondral bone contributing to pathogenesis of osteoarthritis. Int. J. Mol. Sci. 14, 19805–19830. doi: 10.3390/ijms141019805
Shields, K. J., Beckman, M. J., Bowlin, G. L., and Wayne, J. S. (2004). Mechanical properties and cellular proliferation of electrospun collagen type II. Tissue Eng. 10, 1510–1517. doi: 10.1089/1076327042500373
Shim, I. K., Suh, W. H., Lee, S. Y., Lee, S. H., Heo, S. J., Lee, M. C., et al. (2009). Chitosan nano-/microfibrous double-layered membrane with rolled-up three-dimensional structures for chondrocyte cultivation. J. Biomed. Mater. Res. A 90, 595–602. doi: 10.1002/jbm.a.32109
Shimizu, T., Sekine, H., Yang, J., Isoi, Y., Yamato, M., Kikuchi, A., et al. (2006). Polysurgery of cell sheet grafts overcomes diffusion limits to produce thick, vascularized myocardial tissues. FASEB J. 20, 708–710. doi: 10.1096/fj.05-4715fje
Shimomura, K., Rothrauff, B. B., Hart, D. A., Hamamoto, S., Kobayashi, M., Yoshikawa, H., et al. (2019). Enhanced repair of meniscal hoop structure injuries using an aligned electrospun nanofibrous scaffold combined with a mesenchymal stem cell-derived tissue engineered construct. Biomaterials 192, 346–354. doi: 10.1016/j.biomaterials.2018.11.009
Shin, H. J., Lee, C. H., Cho, I. H., Kim, Y. J., Lee, Y. J., Kim, I. A., et al. (2006). Electrospun PLGA nanofiber scaffolds for articular cartilage reconstruction: mechanical stability, degradation and cellular responses under mechanical stimulation in vitro. J. Biomater. Sci. Polym. Ed. 17, 103–119. doi: 10.1163/156856206774879126
Shlopov, B. V., Lie, W.-R., Mainardi, C. L., Cole, A. A., Chubinskaya, S., and Hasty, K. A. (1997). Osteoarthritic lesions. Involvement of three different collagenases. Arthritis Rheum. 40, 2065–2074. doi: 10.1002/art.1780401120
Skotak, M., Noriega, S., Larsen, G., and Subramanian, A. (2010). Electrospun cross-linked gelatin fibers with controlled diameter: the effect of matrix stiffness on proliferative and biosynthetic activity of chondrocytes cultured in vitro. J. Biomed. Mater Res. A 95, 828–836. doi: 10.1002/jbm.a.32850
Skotak, M., Ragusa, J., Gonzalez, D., and Subramanian, A. (2011). Improved cellular infiltration into nanofibrous electrospun cross-linked gelatin scaffolds templated with micrometer-sized polyethylene glycol fibers. Biomed. Mater. 6:055012. doi: 10.1088/1748-6041/6/5/055012
Slivka, M. A., Leatherbury, N. C., Kieswetter, K., and Niederauer, G. G. (2001). Porous, resorbable, fiber-reinforced scaffolds tailored for articular cartilage repair. Tissue Eng. 7, 767–780. doi: 10.1089/107632701753337717
Smith, L., Cho, S., and Discher, D. (2018). Stem cell differentiation is regulated by extracellular matrix mechanics. Physiology 33, 16–25. doi: 10.1152/physiol.00026.2017
Sohier, J., Moroni, L., van Blitterswijk, C., de Groot, K., and Bezemer, J. M. (2008). Critical factors in the design of growth factor releasing scaffolds for cartilage tissue engineering. Expert Opin. Drug Deliv. 5, 543–566. doi: 10.1517/17425247.5.5.543
Sophia Fox, A. J., Bedi, A., and Rodeo, S. A. (2009). The basic science of articular cartilage: structure, composition, and function. Sports Health. 1, 461–468. doi: 10.1177/1941738109350438
Stanger, J., Tucker, N., Wallace, A., Larsen, N., Staiger, M., and Reeves, R. (2009). The effect of electrode configuration and substrate material on the mass deposition rate of electrospinning. J. Appl. Polym. 112, 1729–1737. doi: 10.1002/app.29663
Subbiah, T., Bhat, G., Tock, R., Parameswaran, S., and Ramkumar, S. (2005). Electrospinning of nanofibers. J. Appl. Polym. 96, 557–569. doi: 10.1002/app.21481
Subramanian, A., Vu, D., Larsen, G. F., and Lin, H.-Y. (2005). Preparation and evaluation of the electrospun chitosan/PEO fibers for potential applications in cartilage tissue engineering. J. Biomater. Sci. Polym Ed. 16, 861–873. doi: 10.1163/1568562054255682
Sun, A., Lin, H., Beck, A., Kilroy, E., and Tuan, R. (2015). Projection stereolithographic fabrication of human adipose stem cell-incorporated biodegradable scaffolds for cartilage tissue engineering. Front. Bioeng. Biotechnol. 3:115. doi: 10.3389/fbioe.2015.00115
Sundaray, B., Subramanian, V., Natarajan, T., Xiang, R.-Z., Chang, C.-C., and Fann, W.-S. (2004). Electrospinning of continuous aligned polymer fibers. Appl. Phys. Lett. 84, 1222–1224. doi: 10.1063/1.1647685
Taheem, D., Jell, G., and Gentleman, E. (2019). Hypoxia inducible factor-1α in osteochondral tissue engineering. Tissue Eng Part B. https://www.liebertpub.com/doi/full/10.1089/ten.teb.2019.0283
Temenoff, J. S., and Mikos, A. G. (2000). Review: tissue engineering for regeneration of articular cartilage. Biomaterials 21, 431–440. doi: 10.1016/S0142-9612(99)00213-6
Thorvaldsson, A., Stenhamre, H., Gatenholm, P., and Walkenstrom, P. (2008). Electrospinning of highly porous scaffolds for cartilage regeneration. Biomacromolecules 9, 1044–1049. doi: 10.1021/bm701225a
Toloue, E. B., Karbasi, S., Salehi, H., and Rafienia, M. (2019). Evaluation of mechanical properties and cell viability of poly (3-hydroxybutyrate)-chitosan/Al2O3nanocomposite scaffold for cartilage tissue engineering. J. Med. Signals Sens. 9, 111–116. doi: 10.4103/jmss.JMSS_56_18
Torio, C., and Moore, B. (2013). National Inpatient Hospital Costs: The Most Expensive Conditions by Payer. Statistical Brief.
Toyokawa, N., Fujioka, H., Kokubu, T., Nagura, I., Inui, A., Sakata, R., et al. (2010). Electrospun synthetic polymer scaffold for cartilage repair without cultured cells in an animal model. Arthroscopy 26, 375–383. doi: 10.1016/j.arthro.2009.08.006
van der Weyden, L., Wei, L., Luo, J., Yang, X., Birk, D. E., Adams, D. J., et al. (2006). Functional knockout of the matrilin-3 gene causes premature chondrocyte maturation to hypertrophy and increases bone mineral density and osteoarthritis. Am. J. Pathol. 169, 515–527. doi: 10.2353/ajpath.2006.050981
Van Spil, W. E., Kubassova, O., Boesen, M., Bay-Jensen, A.-C., and Mobasheri, A. (2019). Osteoarthritis phenotypes and novel therapeutic targets. Biochem. Pharmacol. 165, 41–48. doi: 10.1016/j.bcp.2019.02.037
Venugopal, E., Sahanand, K. S., Bhattacharyya, A., and Rajendran, S. (2019). Electrospun PCL nanofibers blended with Wattakaka volubilis active phytochemicals for bone and cartilage tissue engineering. Nanomedicine 21:102044. doi: 10.1016/j.nano.2019.102044
Vonk, L. A., de Windt, T. S., Kragten, A. H., Beekhuizen, M., Mastbergen, S. C., Dhert, W. J., et al. (2014). Enhanced cell-induced articular cartilage regeneration by chondrons: the influence of joint damage and harvest site. Osteoarthr. Cartilage 22, 1910–1917. doi: 10.1016/j.joca.2014.08.005
Wadhwa, S., Embree, M., Ameye, L., and Young, M. F. (2005a). Mice deficient in biglycan and fibromodulin as a model for temporomandibular joint osteoarthritis. Cells Tissues Organs. 181, 136–143. doi: 10.1159/000091375
Wadhwa, S., Embree, M. C., Kilts, T., Young, M. F., and Ameye, L. G. (2005b). Accelerated osteoarthritis in the temporomandibular joint of biglycan/fibromodulin double-deficient mice. Osteoarthr. Cartilage 13, 817–827. doi: 10.1016/j.joca.2005.04.016
Wang, X., Um, I. C., Fang, D., Okamoto, A., Hsiao, B. S., and Chu, B. (2005). Formation of water-resistant hyaluronic acid nanofibers by blowing-assisted electro-spinning and non-toxic post treatments. Polymer 46, 4853–4867. doi: 10.1016/j.polymer.2005.03.058
Watt, F., and Huck, W. (2013). Role of the extracellular matrix in regulating stem cell fate. Nat. Rev. Mol. Cell. Biol. 14, 467–473. doi: 10.1038/nrm3620
Whited, B. M., Whitney, J. R., Hofmann, M. C., Xu, Y., and Rylander, M. N. (2011). Pre-osteoblast infiltration and differentiation in highly porous apatite-coated PLLA electrospun scaffolds. Biomaterials 32, 2294–2304. doi: 10.1016/j.biomaterials.2010.12.003
Wilusz, R. E., Sanchez-Adams, J., and Guilak, F. (2014). The structure and function of the pericellular matrix of articular cartilage Matrix Biol. 39, 25–32. doi: 10.1016/j.matbio.2014.08.009
Wimpenny, I., Ashammakhi, N., and Yang, Y. (2012). Chondrogenic potential of electrospun nanofibres for cartilage tissue engineering. J. Tissue Eng. Regen. Med. 6, 536–549. doi: 10.1002/term.459
Wise, J. K., Yarin, A. L., Megaridis, C. M., and Cho, M. (2009). Chondrogenic differentiation of human mesenchymal stem cells on oriented nanofibrous scaffolds: engineering the superficial zone of articular cartilage, Tissue Eng. Part A. 15, 913–921. doi: 10.1089/ten.tea.2008.0109
Wright, L. D., McKeon-Fischer, K. D., Cui, Z., Nair, L. S., and Freeman, J. W. (2014). PDLA/PLLA and PDLA/PCL nanofibers with a chitosan-based hydrogel in composite scaffolds for tissue engineered cartilage. J. Tissue Eng. Regen Med. 8, 946–954. doi: 10.1002/term.1591
Wuelling, M., and Vortkamp, A. (2011). “Chondrocyte proliferation and differentiation,” in Cartilage and Bone Development and Its Disorders (Karger Publishers), 1–11. doi: 10.1159/000328081
Xu, C. Y., Inai, R., Kotaki, M., and Ramakrishna, S. (2004). Aligned biodegradable nanofibrous structure: a potential scaffold for blood vessel engineering. Biomaterials 25, 877–886. doi: 10.1016/S0142-9612(03)00593-3
Xu, H., Cai, S., Xu, L., and Yang, Y. (2014). Water-stable three-dimensional ultrafine fibrous scaffolds from keratin for cartilage tissue engineering. Langmuir 30, 8461–8470. doi: 10.1021/la500768b
Yang, L., Fiti,é, C., van der Werf, K., Bennink, M., Dijkstra, P., and Feijen, J. (2008). Mechanical properties of single electrospun collagen type I fibers. Biomaterials 29, 955–962. doi: 10.1016/j.biomaterials.2007.10.058
Yoshida, C. A., Yamamoto, H., Fujita, T., Furuichi, T., Ito, K., Inoue, K.-,i., et al. (2004). Runx2 and Runx3 are essential for chondrocyte maturation, and Runx2 regulates limb growth through induction of Indian hedgehog. Genes Dev. 18, 952–963. doi: 10.1101/gad.1174704
Youn, I., Choi, J. B., Cao, L., Setton, L. A., and Guilak, F. (2006). Zonal variations in the three-dimensional morphology of the chondron measured in situ using confocal microscopy. Osteoarthr. Cartilage 14, 889–897. doi: 10.1016/j.joca.2006.02.017
Yu, F., Li, M., Yuan, Z., Rao, F., Fang, X., Jiang, B., et al. (2018). Mechanism research on a bioactive resveratrol–PLA–gelatin porous nano-scaffold in promoting the repair of cartilage defect. Int. J. Nanomedicine 13:7845. doi: 10.2147/IJN.S181855
Yunos, D. M., Ahmad, Z., Salih, V., and Boccaccini, A. R. (2013). Stratified scaffolds for osteochondral tissue engineering applications: electrospun PDLLA nanofibre coated Bioglass(R)-derived foams. J. Biomater. Appl. 27, 537–551. doi: 10.1177/0885328211414941
Zamanlui, S., Mahmoudifard, M., Soleimani, M., Bakhshandeh, B., Vasei, M., and Faghihi, S. (2018). Enhanced chondrogenic differentiation of human bone marrow mesenchymal stem cells on PCL/PLGA electrospun with different alignments and compositions. Int. J. Polym. Mater. Pol. Biomat. 67, 50–60. doi: 10.1080/00914037.2017.1297941
Zelzer, E., Glotzer, D. J., Hartmann, C., Thomas, D., Fukai, N., Soker, S., et al. (2001). Tissue specific regulation of VEGF expression during bone development requires Cbfa1/Runx2. Mech. Dev. 106, 97–106. doi: 10.1016/S0925-4773(01)00428-2
Zeugolis, D., Khew, S., Yew, E., Ekaputra, A., Tong, Y., Yung, L., et al. (2008). Electro-spinning of pure collagen nano-fibres - Just an expensive way to make gelatin? Biomaterials 29, 2293–2305. doi: 10.1016/j.biomaterials.2008.02.009
Zhang, L., Hu, J., and Athanasiou, K. A. (2009). The role of tissue engineering in articular cartilage repair and regeneration. Crit. Rev. Biomed. Eng. 37, 1–57. doi: 10.1615/CritRevBiomedEng.v37.i1-2.10
Zhang, Y., and Jordan, J. (2010). Epidemiology of osteoarthritis. Clin Geriatr. Med. 26, 355–369. doi: 10.1016/j.cger.2010.03.001
Zhang, Y., Liu, X., Zeng, L., Zhang, J., Zuo, J., Zou, J., et al. (2019). Polymer fiber scaffolds for bone and cartilage tissue engineering. Adv. Funct. Mater. 29:1903279. doi: 10.1002/adfm.201903279
Zheng, X., Wang, W., Liu, S., Wu, J., Li, F., Cao, L., et al. (2016). Enhancement of chondrogenic differentiation of rabbit mesenchymal stem cells by oriented nanofiber yarn-collagen type I/hyaluronate hybrid. Mater. Sci. Eng. C. 58, 1071–1076. doi: 10.1016/j.msec.2015.07.066
Zhu, M., Chen, M., Zuscik, M., Wu, Q., Wang, Y. J., Rosier, R. N., et al. (2008). Inhibition of beta-catenin signaling in articular chondrocytes results in articular cartilage destruction. Arthritis. Rheum. 58, 2053–2064. doi: 10.1002/art.23614
Keywords: electrospinning, fibrous scaffolds, cartilage engineering, functionalised scaffolds, in vivo models
Citation: Yilmaz EN and Zeugolis DI (2020) Electrospun Polymers in Cartilage Engineering—State of Play. Front. Bioeng. Biotechnol. 8:77. doi: 10.3389/fbioe.2020.00077
Received: 11 November 2019; Accepted: 29 January 2020;
Published: 18 February 2020.
Edited by:
Lorenzo Moroni, Maastricht University, NetherlandsReviewed by:
Fang Yang, Radboud University Nijmegen Medical Centre, NetherlandsRui Cruz Pereira, Fondazione Istituto Italiano di Technologia, Italy
Copyright © 2020 Yilmaz and Zeugolis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dimitrios I. Zeugolis, ZGltaXRyaW9zLnpldWdvbGlzJiN4MDAwNDA7bnVpZ2Fsd2F5Lmll
 Elif Nur Yilmaz
Elif Nur Yilmaz Dimitrios I. Zeugolis
Dimitrios I. Zeugolis