
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Bioeng. Biotechnol. , 17 December 2019
Sec. Nanobiotechnology
Volume 7 - 2019 | https://doi.org/10.3389/fbioe.2019.00406
 Cecilia Velino1
Cecilia Velino1 Francesca Carella1
Francesca Carella1 Alessio Adamiano1
Alessio Adamiano1 Maurizio Sanguinetti2,3
Maurizio Sanguinetti2,3 Alberto Vitali4
Alberto Vitali4 Daniele Catalucci5,6
Daniele Catalucci5,6 Francesca Bugli2,3*
Francesca Bugli2,3* Michele Iafisco1*
Michele Iafisco1*Cystic fibrosis (CF) is a genetic disease affecting today nearly 70,000 patients worldwide and characterized by a hypersecretion of thick mucus difficult to clear arising from the defective CFTR protein. The over-production of the mucus secreted in the lungs, along with its altered composition and consistency, results in airway obstruction that makes the lungs susceptible to recurrent and persistent bacterial infections and endobronchial chronic inflammation, which are considered the primary cause of bronchiectasis, respiratory failure, and consequent death of patients. Despite the difficulty of treating the continuous infections caused by pathogens in CF patients, various strategies focused on the symptomatic therapy have been developed during the last few decades, showing significant positive impact on prognosis. Moreover, nowadays, the discovery of CFTR modulators as well as the development of gene therapy have provided new opportunity to treat CF. However, the lack of effective methods for delivery and especially targeted delivery of therapeutics specifically to lung tissues and cells limits the efficiency of the treatments. Nanomedicine represents an extraordinary opportunity for the improvement of current therapies and for the development of innovative treatment options for CF previously considered hard or impossible to treat. Due to the peculiar environment in which the therapies have to operate characterized by several biological barriers (pulmonary tract, mucus, epithelia, bacterial biofilm) the use of nanotechnologies to improve and enhance drug delivery or gene therapies is an extremely promising way to be pursued. The aim of this review is to revise the currently used treatments and to outline the most recent progresses about the use of nanotechnology for the management of CF.
Cystic fibrosis (CF) is the most common autosomal recessive disease, affecting today nearly 70,000 patients worldwide (Savla and Minko, 2013; da Silva et al., 2017; Haque et al., 2018), while ~1,000 new cases are diagnosed every year (Cystic Fibrosis Foundation, 2018). The disease is caused by mutations in the gene located on chromosome 7, that encodes the cystic fibrosis transmembrane conductance regulator (CFTR) protein. CFTR protein works as a chloride channel on the apical membrane of epithelial cells (Turcios, 2005; Høiby, 2011; Robinson et al., 2018), and it is responsible for the regulation of chloride ions secretion and sodium ions reabsorption (Ibrahim et al., 2011b). Since the identification of CFTR gene in 1989, more than 1,500 mutations have been described, each leading to CF disease (Ratjen, 2009). Those mutations have been initially classified in five major classes according to their effect on CFTR function (Figure 1): (i) mutations interfering with protein synthesis, (ii) mutations affecting protein maturation, (iii) mutations altering channel regulation, (iv) mutations affecting chloride conductance, and (v) mutations reducing the level of normally functioning CFTR at the apical membrane (Fanen et al., 2014). This classification system has been updated by De Boeck and Amaral (2016), which introduced two more classes: class (vi) includes mutations which destabilize CFTR at the cell surface, while mutations of class (vii), called “unrescuable” have the same outcomes as class (i) (i.e., the absence of CFTR protein), but they cannot be rescued by corrective therapy (Amaral, 2015; De Boeck and Amaral, 2016) (Figure 1). Among all the mutations, the most common is named F508del, which, through the deletion of phenylalanine at position 508 of the CFTR protein (O'Sullivan and Freedman, 2009; Gaspar et al., 2013; McNeer et al., 2015), prevents the protein from trafficking to the cell surface, thus significantly reducing CFTR function. It was estimated that 90% of CF patients carry at least one copy of this mutated form (Cartiera et al., 2010).
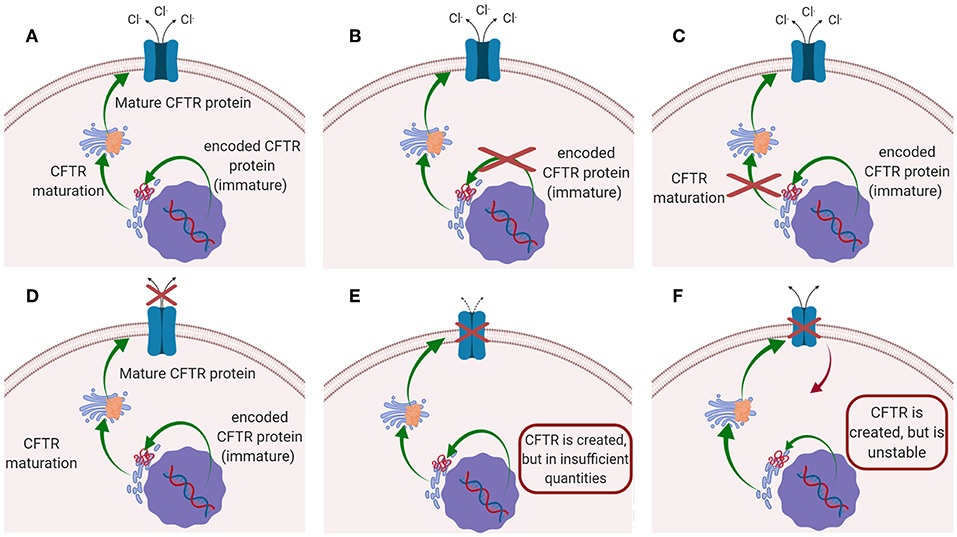
Figure 1. Schematic representation of the mutations on CFTR leading to CF disease: (A) CFTR works normally (no mutations); (B) Class I and VII mutation; (C) Class II mutations; (D) classes III and IV mutations; (E) Class V mutation; (F) class VI mutations.
The consequence of mutations on CFTR results in impaired chloride ions secretion and hyper-absorption of sodium ions across epithelia (Cristallini et al., 2019), causing a hypersecretion of thick mucus difficult to clear. The over-production of the mucus secreted in the lungs, along with its altered composition and consistency, results in airway obstruction that makes the lungs susceptible to recurrent and persistent bacterial infections (Bahadori and Mohammadi, 2012) and endobronchial chronic inflammation, which are considered the primary cause of bronchiectasis, respiratory failure, and consequent death of patients (Henke and Ratjen, 2007). The presence of abnormal thick and viscous mucus impairs the mucociliary clearance and favors the bacterial colonization. The microorganisms residing inside the immobile mucus find a perfect environment to settle and are shielded against immune response as well as against antimicrobial drugs. Although the most severe problems related to CF, in terms of symptoms and treatments, involve the lungs (Davies et al., 2007), patients affected by CF often suffer of several other diseases occurring within other epithelial-lined organs such as small intestine bacterial overgrowth, pancreatic exocrine insufficiency, cirrhosis of the liver and progressive hepatic dysfunction, and infertility (Ramsey, 1996).
The most common pathogen affecting the airways in CF patients is Pseudomonas Aeruginosa (PA), although other microorganisms may play an important role on the pathogenesis of lung function declines, such as Haemophilius influenzae, Staphilococcus aureus, Stenotrophomonas maltophilia, Burkholderia cepacian (Rowe et al., 2005; Döring et al., 2012; d'Angelo et al., 2014) and the filamentous fungus Aspergillus fumigatus which often co-colonizes the lungs with PA (Zhao et al., 2018). This set of pathogens are frequently acquired in an age dependent sequence (Figure 2): S. Aureus and H. Influenzae are the most prevalent bacterial pathogens in younger patients, while PA chronically infects 80% of CF patients by late adolescence. Other pathogens, such as B. Cepacia and S. Maltophilia are less frequent but particularly troublesome in CF patients due to their multi-drug resistant phenotypes. The frequency of infections caused by these species increases with patient age, resulting in a significant health risk to CF patients surviving to adulthood (Pompilio et al., 2018). The U.S. patent registry reported that in 2017 the median age at death was 30.7 years, while about 15% of deaths occurred before 20 years of age (Cystic Fibrosis Foundation, 2018).
PA belongs to the family of Gram-negative organisms (Alikhani et al., 2018). Due to its wide genetic diversity, it can persist in CF patients' lungs and, if established, remains very difficult to eradicate. The initial infection usually involves a “non-mucoid” strain of the pathogen that can be cleared by the host or eradicated with an aggressive antibiotic treatment. Over time, as the colonization advances, PA colonies alter their pattern of gene expression in the CF lung and start producing a mucoid coating made of alginate which protects the bacteria against antibiotics and phagocytosis (Turcios, 2005; O'Sullivan and Freedman, 2009; Gaspar et al., 2013; Bhagirath et al., 2016). In this mucoid form, PA is able to form a biofilm which makes the pathogen up to 100 times more tolerant to antimicrobial treatments (Al-Obaidi et al., 2018; Ernst et al., 2018; Kłodzinska et al., 2018; Lu et al., 2018). Besides, the ability of PA to establish drug resistant biofilms is thought to be facilitated by the hypersecretion of the thick and viscous mucus layer in the CF airway, which provides a low oxygen environment (Moreau-Marquis et al., 2008). Essentially, both the presence of thick bronchiolar mucus and bacterial biofilm contribute to poor lung penetration of antimicrobial agents, thus leading to clinical aggravation and inefficacy of the therapies.
Despite the difficulty of treating the continuous infections caused by PA and other pathogens in CF patients, various treatment strategies have been developed during the last few decades, showing significant positive impact on prognosis (Heijerman et al., 2009).
Together with other organs, such as skin and intestines, the lungs are in direct contact with the external environment (Yang et al., 2008). Systemic delivery of drugs via inhalation routes (oral and nasal) constitutes an effective alternative to parenteral drug delivery to treat pulmonary disease. There are several advantages in drug administration via pulmonary route, including rapid onset of action, non-invasive nature, high permeability of the lungs (Sung et al., 2007), rapid absorption (Paranjpe and Müller-Goymann, 2014) and improved patient compliance. Hence, this strategy has been used for local delivery of several types of drugs, from antibiotics to chemotherapeutics, from peptides to vaccines (Muralidharan et al., 2015). Nowadays all the most used antimicrobials for CF such as tobramycin, colistimethate sodium, and aztreonam lysine (Littlewood et al., 2012) have been developed as inhalation formulations, as reported below in more details.
For long time the research for CF treatment was mainly focused on the symptomatic therapy, aimed at attenuating disease progression and delaying the onset of lung damage through different types of drugs. However, most of these therapies only treat the symptoms of CF, without correct the underlying causes of the disease (Savla and Minko, 2013). Nowadays, the discovery of CFTR modulators that aim to increase or potentially restore the function of the disease-causing CFTR protein (Lopes-Pacheco, 2016) as well as the development of gene therapy have provided new opportunities to treat CF (Perry et al., 2017).
Currently, antibiotics and anti-inflammatory drugs are widely used for the symptomatic treatment of CF related infections and inflammations. In addition, bronchodilators, mucolytics and osmotic agents are administered to improve airway and sputum clearance. As the airways are a major therapeutic target in the treatment of CF, many drugs are delivered by inhalation by using nebulizers, pressurized metered dose inhalers (pMDIs) and dry powder inhalers (DPIs). Nebulizers were the first devices that appeared in the market followed by pMDIs and DPIs. Characteristics of these devices are reported in Table 1. The main benefit of topical inhalation is the delivery of relatively high doses of the drug directly to the target site, while minimizing the risk of ototoxicity and nephrotoxicity (Abramowsky and Swinehart, 1982; Ramsey, 1996; Geller, 2009; Agent and Parrott, 2015).
Tobramicyn is an aminoglycoside antibiotic that has been widely used to treat various types of bacterial infections, especially those caused by gram-negative bacteria, such as PA (Geller et al., 2003; Van Westreenen and Tiddens, 2010). Tobramycin acts as a bactericidal drug by irreversibly binding to the 30S bacterial ribosome, thus inhibiting protein synthesis (Heijerman et al., 2009). The first formulation of tobramycin was approved in 1998 as tobramycin inhalation solution (TIS) (TOBI®, Novartis AG, Switzerland; Bramitob®, Chiesi Farmaceutici S.p.A, Italy) (Geller et al., 2011b). Since then, new dry powder formulations have been developed such as the tobramycin inhalation powder (TIP) (TOBI® Podhaler®, Novartis AG, Switzerland) that is the first marketed dry powder inhaled antibiotic. This dry powder has been manufactured using a technology, termed PulmoSphere® (Novartis AG, Switzerland) (VanDevanter and Geller, 2011), and has been shown to be even more effective as compared to TIS (Smyth et al., 2014; Hamed et al., 2017). Despite the fact that tobramycin is usually well-tolerated, some of the patients treated with TIP experienced cough, bronchospasm, moderate tinnitus and dysphonia (Heijerman et al., 2009; Shteinberg and Elborn, 2015). The approved dose for TIS is 300 mg of antibiotic nebulised by a jet nebulizer twice a day every other month (Cheer et al., 2003; Somayaji and Parkins, 2015), while for TIP the treatment consists in 112 mg (four 28 mg capsules) inhaled twice a day in alternating cycles of 28-day on/off treatment (Döring et al., 2012). These capsules are composed of low-density porous particles, whose absorption is improved using a portable DPI, named Podhaler® that improves flow and dispersion of formulation. The administration by inhalation of dry powder is preferred to aerosolized solution because it is simpler and shorter and significantly improves the quality of patient's daily life. Moreover, the recommended dose of TIP has showed a significant improvement in lung function compared to TIS (Gaspar et al., 2013).
Aztreonam is an antibiotic belonging to the family of monocyclic β-lactams. It is active against Gram-negative bacteria by inhibiting the synthesis of their cell wall (Heijerman et al., 2009). The first formulation of intravenous aztreonam was developed in 1980s, and its excellent activity against PA and other pathogens made this antibiotic a promising drug for the treatment of chronic bacterial infections. However, several side-effects were registered in CF patients, such as rash, nausea, vomiting and diarrhea. Moreover, the intravenous formulation contained the arginine salt, which induced airway inflammation and caused bronchoconstriction. The substitution of arginine salt with lysine salt was subsequently made in order to develop a safer formulation of aztreonam for aerosolized use (Kirkby et al., 2011). Aztreonam lysine (AZLI) (Cayston®, Gilead Sciences Inc., USA) was approved in 2010 and the recommended dose is 75 mg inhaled thrice daily, in an alternate off/on cycle of 28 days. In several clinical studies it has been demonstrated that AZLI significantly improves clinical outcomes in CF, including lung function, quality of life, nutritional status and reduced bacterial density in sputum by efficaciously suppressing chronic PA infections (Döring et al., 2012; Heirali et al., 2019).
Colistin, also known as polymyxin E, is a cyclic polypeptide antibiotic derived from Bacillus polymyxa varietas colistinus. Among the five different polymyxins discovered in 1947, only two of them have been used for clinical purposes, polymyxin B (known as colomycin) and polymyxin E (colistin) (Storm et al., 1977). The bactericidal action of colistin against gram-negative bacteria is given by its cationic nature (Heijerman et al., 2009). The cationic polypeptides of colistin interact with the anionic lypopolysaccharides in the outer membrane of the pathogen, causing an imbalance within the cell membrane and a subsequent change in permeability which ultimately causes cell death (Schwarz, 2015). Colistin was first marketed in the 1950s. Historically, it was administered intravenously, but reports of nephrotoxicity and neurotoxicity discouraged its use, especially after the appearance of other less toxic antimicrobials, such as tobramycin. However, the lack of treatment options for multi-drug resistant pathogens has led to the recovery of colistin as an antimicrobial agent, especially in its inhaled formulation (Lim et al., 2010). Polymyxin E is commercially available in two forms: colistin sulfate (for topic use) or as colistimethate sodium salt, an inactive prodrug given via intravenous or inhalation (Van Westreenen and Tiddens, 2010). While colistimethate sodium salt is currently used to treat chronic PA infection and is generally well-tolerated by inhalation, colistin sulfate is not suitable for treating CF patients due to several side effects, such as throat irritation, cough and severe bronchoconstriction (Gaspar et al., 2013). Colistin dry inhalation powder (CDPI) formulation is commercially available as Colobreathe® (Forest laboratories Inc., USA), which contains a dose of 125 mg colistimethate sodium salt to be administrated through a hand-held inhaler named Turbospin® (Forest laboratories Inc., USA). The recommended dose is a 125 mg capsule twice daily (Schuster et al., 2013). CDPI shows several advantages over the nebulized solution form, including shorter administration (1 min) and an enhanced portability (Conole and Keating, 2014). Colistin is often used in combination with other antimicrobials, such as ciprofloxacin and tobramycin, in order to improve the patient's conditions.
Fluoroquinolones belong to the family of quinolones, a group of molecules that is widely used as antibiotic because of their large spectrum of bactericidal activity, excellent bioavailability, rapid cellular uptake (Drusano, 1997; Wise and Honeybourne, 1999) and tolerance (Grillon et al., 2016). The bactericidal mechanism of action of fluoroquinolones consists in the inhibition of DNA replication and transcription by interacting with the DNA-gyrase complex (Young et al., 2013). This class of antibiotics are currently in a phase III of clinical trials (Maselli et al., 2017) as drugs to be administered during the “off” circle of TIS, in order to improve patient outcomes and to avoid drug resistance (Gaspar et al., 2013).
Ciprofloxacin and levofloxacin are the most investigated fluoroquinolones for the treatment of CF infections, due to their bactericidal activity against Gram-negative bacteria and their prolonged post-antibiotic effect (Andersson, 2003). Ciprofloxacin (first marketed as Cipro®, Bayer AG, Germany) is a second-generation fluoroquinolone. It is active against Gram-negative and Gram-positive bacteria, and it can be administered via oral or intravenous route, although inhalation formulations are under investigation and have reached the final phases of development. A phase II study on ciprofloxacin as dry powder for inhalation given at two doses levels (32.5 and 48.75 mg) twice daily for 28 days, has showed a significant decrease in PA density compared to placebo (Döring et al., 2012). The main side effects of ciprofloxacin are cartilage toxicity, sunlight sensitivity rash and emergence of resistance.
Levofloxacin is a third-generation fluoroquinolone with higher activity against Gram-positive pathogens compared to ciprofloxacin (Young et al., 2013). It can be administered either orally and intravenously, and recently also via inhalation with a novel liquid formulation (MP-376 or Aeroquin™, Mpex Pharmaceuticals, Inc., USA), that has reached the phase II study (Geller et al., 2011a). Aeroquin™ showed a reduced need for other antibiotics against PA for different doses given, which are 120 or 240 mg every day, and 240 mg twice a day for 28 days, respectively. Aeroquin™ exhibit a good degree of tolerance in patients treated with the antibiotic with respect to placebo, and is currently being studied in phase III of clinical trials (Ballmann et al., 2011; Döring et al., 2012).
Fosfomycin (first marketed as Monuril™, Zambon S.p.A, Italy) is a broad-spectrum antimicrobial active agent against Gram-negative and Gram-positive bacteria, and it is particularly effective against multidrug-resistant PA (Mirakhur et al., 2003; Spoletini et al., 2018). This drug exhibits bactericidal activity by inhibiting the initial step in cell wall synthesis, and it is commercially available in intravenous formulation as fosfomycin disodium, or in oral formulation as fosfomycin disodium and fosfomycin trometamol (Van Westreenen and Tiddens, 2010; Gaspar et al., 2013). Due to the easy development of drug resistance when the same antibiotic is administered repeatedly, fosfomycin is often used in combination with other antibiotics in order to prevent cross-resistance (Trapnell et al., 2012). Studies have shown that the intravenous administration of fosfomycin combined with other antimicrobials to patients colonized by multi-resistant PA resulted in a general improvement of the patients status with low side effects (Mirakhur et al., 2003). A combination of fosfomycin and tobramycin in a 4:1 ratio (FTI, Gilead Sciences Inc., USA) has reached phase III study, in which the safety and efficacy of FTI treatment followed by a 28 days course of AZLI has been evaluated. These studies demonstrated an improvement of clinical conditions in CF patients infected by PA. Moreover, the preliminary results obtained from this research suggest that fosfomycin could be used not only for the treatment of PA, but also for the treatment of other pathogens detected in the CF population (Ballmann et al., 2011; Döring et al., 2012).
CF patients often suffer from chronic airway inflammation, which ultimately leads to several physiological and metabolic changes, including weight loss, anorexia and metabolic breakdown. Hence, anti-inflammatory therapy represents an additional option to antibiotic treatments to avoid a decline in lung function (Heijerman et al., 2009). Several clinical trials have proven that anti-inflammatory therapies have positive outcomes; however, the related side effects have limited the use of these drugs for CF treatments (Konstan and Davis, 2002). The first anti-inflammatory drugs studied in CF were systemic corticosteroids and high-dose ibuprofen (Ibrahim et al., 2011b). Corticosteroids are used in CF to reduce endobronchial inflammation, but they also produce increases in the incidence of diabetes, cataracts, and growth failure. New inhalation formulations of these drugs have been developed in order to minimize systemic adverse side effects, although one potential disadvantage of inhaled corticosteroids is their difficulty in penetrating the sticky mucus within the CF patient's airways (Dinwiddie, 2005).
Compared to corticosteroids, the non-steroidal ibuprofen offered promising results in CF patients. Ibuprofen has been studied as a treatment for CF due to its ability to inhibit the migration and activation of neutrophils (Ramsey, 1996). These studies also demonstrated the effective benefit of ibuprofen therapy on CF patients, especially when the treatment is started before the development of severe inflammation and pathological changes in the lung (O'Sullivan and Freedman, 2009) and in children 5–13 years old with mild lung disease. The main disadvantage of the ibuprofen therapy is related to its narrow therapeutic window, which results in a particular carefulness in drug dosage; in fact, while low concentrations might not be sufficient to inhibit neutrophils migration in the lung, high dosage leads to an increased risk of gastrointestinal and renal toxicity (Turcios, 2005).
Other molecules primarily employed as antibiotics or mucolytics show ancillary anti-inflammatory activities with positive effects on CF treatment. As an example, studies carried out on oral administration of azithromycin, an antibiotic belonging to macrolides also employed as anti-inflammatory drug, have demonstrated an improvement of lung function (Friedlander and Albert, 2010). Leukotriene modifiers, oral N-Acetylcysteine, and inhaled glutathione are other kinds of anti-inflammatory drugs that were tested for CF. However, the CF Foundation has determined that there is not enough evidence to recommend for or against these drugs in patients with CF (Dasenbrook and Chmiel, 2017). Immunoglobulins and dornase alpha (DNase) (Sepe et al., 2019) are also used as anti-inflammatory agents in addition to their mucolytic activity.
As reported above, CF airways are characterized by the presence of a thick, viscous mucus. Hence, mucolytics and bronchodilators are often administered to improve airway clearance (Ong et al., 2019). Two main muco-active agents are administered via aerosol: N-acetylcysteine (NAC), which disrupts disulphide bonds in mucus, and DNase (Pulmozyme®, Genentech), which acts on enzymatically break down DNA in airway secretions. NAC lowers mucus viscosity and elasticity by substituting sulfhydryl groups for the disulphide bonds connecting mucin proteins. Differently, DNase mucolytic activity consists in its ability to reduce the size of DNA released in the sputum. In infected airways, in fact, the degeneration of neutrophils causes the release of DNA, which further increases the viscosity of secretions (Henke and Ratjen, 2007). In 2014 Charrier et al. developed a new cysteamine-based mucoactive agent with antimicrobial and antibiofilm features, marketed as Lynovex® (Novabiotics ltd., UK). This drug, which is already used for the treatment of cystinosis, has successfully completed a phase IIb clinical trial for oral formulation in acute infectious CF exacerbations, with positive top line data reported. Clinical studies are expected in 2019 (Charrier et al., 2014).
β-adrenergic agents, such as salmeterol, are the most commonly prescribed bronchodilators (Halfhide et al., 2016). They act by broadening the airways and relaxing airway muscles, then making easier to breathe. Other bronchodilators currently in use are anticholinergics.
Since the identification of the mutations in the gene coding the CFTR protein, several new drugs were developed to target the disease at the CFTR level by improving the defective or deficient activity of the mutated protein. Recently, a new and promising class of drugs called CFTR modulators has entered the CF therapeutic landscape. These drugs differ from prior therapies in that they aim to improve the production, intracellular processing, and/or function of the defective CFTR protein. Currently, five types of CFTR modulators have been developed: (i) read-through agents, which rescue protein synthesis; (ii) correctors, which enhance conformational stability of CFTR, thus rescuing the trafficking of the mature protein to the plasma membrane (PM); (iii) potentiators, which improve CFTR channel activity; (iv) stabilizers, which anchor CFTR channel at the PM and decrease protein degradation rate, thereby correcting the instability; (v) amplifiers, which work by increasing the amount of CFTR protein on the cell surface providing additional substrate for correctors and potentiators to act upon (Lopes-Pacheco, 2016). Several clinical trials are examining all these different classes to increase or restore the activity of the mutated CFTR protein in patients (Clancy et al., 2019).
Besides the general overlook of the main CFTR modulators reported in this section, many other therapeutic agents based on correctors and potentiators are undergoing various pre-clinical and clinical studies, and are extensively described by Rafeeq and Murad (2017).
CFTR amplifiers are novel mutation-independent compounds that work by increasing immature CFTR protein expression by stabilizing CFTR mRNA. These amplifiers, that act synergistically with correctors and potentiators to augment therapeutic benefits, can effectively overcome any “substrate” limitation. There are two types of amplifiers which have showed promising results in pre-clinical and clinical trials, PTI-428 and PTI-CH (Proteostasis Therapeutics Inc., USA), even though they are not yet available on the market (Kym et al., 2018; Haq et al., 2019). Read-through agents could benefit CF patients affected by class I mutations, since this mutation causes the presence of a premature stop codon which precludes the synthesis of full-length CFTR protein. Among read-through agents, Ataluren (also known as PTC124, PTC therapeutics Inc., USA) emerged as a mutation-based treatment for approximately 10% of patients bearing non-sense premature stop mutations that disrupt the production of full-length functional CFTR. Ataluren act by inducing premature termination codon (PTC) read-through and hence restores CFTR expression at the PM. In a phase II clinical trial Ataluren showed an improvement in the forced expiratory volume in 1 s (FEV1), although subsequent trials confirmed a slight improvement in FEV1 only in a subgroup of patients (Tosco et al., 2018). Other read-through agents have shown promising results in preclinical trials when combined to ivacaftor, including Ataluren derivatives and the FDA-approved natural herbal agent, escin (Mutyam et al., 2016).
Ivacaftor (Kalydeco™, Vertex Pharmaceuticals Ltd, UK), also known as VX-770, is a CFTR potentiator that increases CFTR channel opening on apical cell membrane (Thompson, 2016), allowing chloride ion to flow through the CFTR protein on the surface of epithelial cells. It has been used initially to restore CFTR function for G551D, the most common class III mutation, although its efficacy has been demonstrated for other less common mutations (such as G1244E, G1349D, G178R, G551S, S1251N, S1255P, S549N, and S549R) (Ponzano et al., 2018; Tosco et al., 2018). Being approved by FDA in 2012, Ivacaftor is the first commercially available treatment to target the basic defects in CF (Pettit, 2012). The efficacy and safety of Ivacaftor has been proved by several studies (Ramsey et al., 2011; De Boeck et al., 2014), where it was demonstrated a meaningful increase in FEV1 and a reduction in sweat chloride to a value below the usual diagnostic threshold for CF. Ivacaftor is generally well-tolerated by patients. The most occurring complication is represented by an elevation of liver enzymes, whose levels, however, return to baseline after drug discontinuation (Haq et al., 2019).
Lumacaftor (Vertex Pharmaceuticals Ltd, UK), also known as VX-809, has been approved by FDA in 2015 as a CFTR corrector for class II mutations, especially the F508del. This molecule improves CFTR maturation, thus allowing the protein to reach the cell membrane and transport chloride ions. In a large number of in vitro studies, Lumacaftor showed good efficacy and tolerability, although the treatment by itself showed a significant decrease only in sweat chloride levels and no improvements in FEV1 in a phase II trial with F508del-homozygous patients. In order to reach greater effects, accurate studies were carried out in combinations with correctors; compared to monotherapies, the combined therapy offers an enhancement of the CFTR rescuing in patients bearing F508del, as well as other II class mutations.
Due to the multi-domain structure and folding of CFTR, a single modulator is usually insufficient to act positively on different protein misfoldings. Despite an increased CFR modulators availability, ~40% of patients with CF are without effective therapy. To overcome this limitation, newer modulator-based therapies are made of a combination of drugs (Rafeeq and Murad, 2017). The first double formulation, consisting in a combination of Ivacaftor and Lumacaftor (Orkambi™, Vertex Pharmaceuticals Ltd, UK), have been approved in 2015 by FDA and EMA for patients who are homozygous for the F508del CFTR mutation. It has been found to be safe and effective, with a significant improvement in FEV1 and a reduction in exacerbations (Wainwright et al., 2015). The combination of Tezacaftor and Ivacaftor (Symdeko™ Vertex Pharmaceuticals Ltd, UK) is currently in phase III of clinical trials, and although it shows weak effectiveness in patients with F508del paired with another minimal function CFTR mutation, it has demonstrated to be more effective in patients homozygous for F508del or other heterozygous F508del individuals (Paranjape and Mogayzel, 2018).
Different combinations of CFTR modulators, along with the simultaneous administration of different drugs, are the key points of the precision medicine. This emerging paradigm, which combines preventive and therapeutic strategies, takes into account differences among individuals, and focuses on a patient-centered rather than mutation-centered approach (Maiuri et al., 2017). The newer and most promising research involves the use of first-generation potentiators and correctors in combination with a next-generation drugs, such as VX-152, VX-440, VX-445, and VX-659 (Vertex Pharmaceuticals Ltd, UK). This therapeutic approach, called triple therapy, has shown promising results for the restoration of the most common CFTR mutations. A phase II study of VX-440 in combination with Tezacaftor and Ivacaftor in CF patients having one F508del mutation and one minimal function mutation, as well as patients with two copies of the F508del mutation, reported an efficacy in the range of 9.6–12% improvement in FEV1 at the highest dosage in trials ranging from 2 to 4 weeks for both F508del homozygous and heterozygous patient populations (Chaudary, 2018; Haq et al., 2019). Following these positive responses, new formulations are currently under development. Recently, a new combination therapy has been approved by FDA as Trikafta™ (Vertex Pharmaceuticals Ltd, UK). It combines the action of two correctors (Elexacaftor and Tezacaftor) and a potentiator (Ivacaftor). This triple therapy showed increased therapeutic effects with respect to Orkambi™ or Symdeko™. In a randomized phase 3 study, Taylor-Cousar et al. developed a triple combination of next-generation correctors (VX-659 and VX-445) with Tezacaftor and Ivacaftor for patients with one or two F508del CFTR alleles. The results obtained showed an enhanced therapeutic activity that triple combinations provide in patients with F508del/F508del genotype (Taylor-Cousar et al., 2019).
Recent evidences indicate that CFTR does not act only as ion chloride channel, but is involved in the epithelial stress response, influences the proteostatic network (the cellular processes which regulate the fate of a protein inside the cell) and modulates autophagy. Autophagy is the most important mechanism for protein turnover and is essential in promoting cellular clearance from protein aggregates and ROS sources. Defective autophagy and impaired cellular proteostasis leads to a worsening of chronic CF lung disease. Thus, novel intervention strategies aim to correct the underlying proteostasis and autophagy impairments by rescuing F508del-CFTR to the PM. Lately, studies on proteostasis modulators and autophagy inducers reported encouraging results in preclinical phases (Bodas and Vij, 2019). Cysteamine, a proteostasis regulator FDA-approved 30 years ago for nephropathic cystinosis, has shown promising results in CF treatment (Charrier et al., 2014). Marketed in oral formulation as Cystagon™ (Recordati S.p.A, Italy) and Procysbi™ (Horizon Therapeutics plc, Ireland), it acts by targeting transglutaminase 2 (TG2, a multifunctional intermediary in cell autophagy). The TG2 inhibition increases the expression of CFTR and restores its function. A phase II trial of cystamine in combination with epigallocatechin gallate has been completed in homo and heterozygous CF patients with F508del (De Stefano et al., 2014; Tosco et al., 2016). The results showed decreased chloride concentrations, increased CFTR function, autophagy restoration and improvement in FEV1.
For many years, the main CF treatment strategies were focused on the downstream effects of CFTR dysfunction, such as airway obstruction, infection and inflammation. However, after the identification and cloning of the gene encoding the CFTR in 1989, along with an increased knowledge on how CFTR dysfunction causes lung disease, new targets and approaches for the CF treatment have been investigated (Ratjen, 2009). Hence, the novel strategies aim to restore or replace the CFTR gene rather than treating the pathologies associated with its defect (d'Angelo et al., 2014; Davies et al., 2019).
In general, gene therapy consists in delivering nucleic acids into a cell to replace or repress genes or biological functions. Due to its monogenic and autosomal nature, CF was one of the first diseases considered for this approach (Ibrahim et al., 2011b; Savla and Minko, 2013). In fact, in this pathology, the CFTR gene is the only gene affected, and the correction of only one of the two alleles is sufficient to revert the disease (Villate-Beitia et al., 2017). Furthermore, a small increase in CFTR expression may be beneficial to CF patients, since studies has shown that even in normal patients the protein is not expressed in high amounts (20–100 CFTR protein/cell). Several vectors have been tested and among these, viral systems are the most widely used, although non-viral vectors recently emerged as safer, easier and cheaper options with respect to viral vectors (Smith, 1997; Ratjen, 2009) (Figure 3). Finally, the CRISPR/Cas9 approach which is emerging as a powerful tool for engineering the genome in diverse organisms, is another interesting method for the gene theory of CF (Figure 3).
Adenoviral vectors (AV) were the first systems used for gene therapy clinical trials in 1993 by Zabner et al. (1993). The aim of this study was to evaluate the correction of the chloride transport defect, characteristic of CF-affected epithelia, by using an E1-deficient adenovirus, which expresses CFTR protein in airway epithelial cells. The target organ was the nasal epithelium, since the composition of this latter is similar to the lower airways but of much easier access. The results obtained by testing the therapy in three patients showed an effective correction of the chloride transport defects, without evidence of severe side effects. Even though the low number of treated subjects made difficult to establish the real efficacy of this approach, this study paved the way for subsequent investigations on AV for the treatment of CF. In fact, in the following years, more AV trials were carried out showing a partial efficacy of the therapy which was, however, lower than originally predicted by the pre-clinical trials (Griesenbach and Alton, 2009, 2013). Moreover, this transduction efficiency in most tissues was counterbalanced by adverse effects, such as inflammatory responses in the host organism. Hence, other gene delivery systems have been developed, such as adeno-associated viral vectors (AAVs).
AAVs exhibit several features suitable for gene delivery, such as high transduction efficiency, and broad serotype-dependent tissue tropism. Although a large number of serotypes have been identified, the most extensively investigated is the serotype AAV2. Several studies were carried out in which the vector was administered to the nose, sinuses and lungs of CF patients. Moss et al. (2004) confirmed the safety and tolerability of repeated doses of aerosolized AAV2 containing CFTR complementary DNA (cDNA). However, further studies on a larger number of patients showed a lack of improvement in lung function for repetitive administration (Moss et al., 2007). One of the possible reasons of these discouraging results may be attributed to the limited packaging of AAV, resulting in a low expression of the CFTR cDNA. Although AAVs present some disadvantages, arising mainly from the use of viruses as carriers, the ongoing research is trying to address and improve these limitations and develop novel and safer formulations (Griesenbach and Alton, 2012; Guggino and Cebotaru, 2017).
Non-viral vectors have the potential to overcome some limitations of viral vectors, due to their easy scale-up processing, safety and stability. They are constituted of two components, the nucleic acid (which, for CF, is a plasmid carrying the CFTR cDNA) and a carrier molecule which binds to the DNA. The recent advances in the field of material science and nanotechnology have prompted the development of nanomaterials which can be used as carriers for non-viral gene transfer formulations (Villate-Beitia et al., 2015, 2017). The two main classes of nanocarriers currently employed in non-viral vectors, cationic lipids and cationic polymers, act similarly by binding to negatively charged DNA. These classes of nanocarriers will be discussed in detail within section Nanotechnology for CF Treatment.
The CRISPR/Cas9 approach (Clustered Regularly Interspaced Short Palindromic Repeats) is a gene-editing strategy in which the specific mutated sequence of the defective CFTR gene is corrected by introducing changes in the DNA, allowing a permanent correction of genetic defects underlying disease (Pranke et al., 2019). This system is based on the bacterial CRISPR immune system, which confers resistance and adaptation. CRISPR are DNA sequences composing bacteria's genomes derived from DNA fragments of viruses that have infected the prokaryotic cell, which allow to prevent subsequent infections of the host organism (Doudna and Charpentier, 2014; Barrangou, 2015). Cas9 (CRISPR-associated protein 9) is an enzyme that uses CRISPR sequences as a guide to recognize and cleave specific strands of DNA complementary to the CRISPR sequence (Brouns et al., 1993; Ran et al., 2013). Engineered Cas9 cleaves the DNA in a sequence-specific mode and creates a specific double-stranded break, so the cell can fill the excised portion with the correct gene sequence. One of the most promising applications of CRISPR/Cas9 is given by its possible use as a technology for treating genetic disorder, including CF. This approach has been already tested in canine models for the treatment of Duchenne Muscular Dystrophy (Amoasii et al., 2018; Lim et al., 2018), and despite it has not been tested in animal models for the treatment of CF, it has been found effective in correcting CFTR defects (Hsu et al., 2014). The first studies on CRISPR/Cas9 on CF were carried out in 2013 by Schwank et al. (2013), in which they tested the recovery of functional CFTR in intestinal organoids obtained from CF patients, and proved the repairing of the mutation at the CFTR locus with CRISPR/Cas9 gene editing. More studies were carried out to investigate the potential of this gene editing approach, even though there are concerns about its applicability in vivo directly to the lungs: in fact, since Cas9 has bacterial origins (mainly Staphylococcus aureus) it is potentially immunogenic especially in CF patients whose lungs are colonized by this pathogen. Moreover, CRISPR/Cas9 can be destroyed by the cellular immune response, thus reducing its therapeutic effect (Miah et al., 2019).
The rapid progress of nanomedicine creates new perspectives to enhance the efficacy of inhalation treatment for lung diseases. In general, the application of nanotechnology to the design of drug delivery systems mainly allows a more effective delivery of therapeutics within the target site, thus preventing several adverse side effects of tissues and cells. Other important advantages include the achievement of enhanced therapeutic effects with lower drug doses, and the possibility to deliver hydrophobic molecules while ensuring the protection of the compounds from degradation (da Silva et al., 2017). Nano-sized structures, such as nanoparticles (NPs) and nanodevices, exhibit peculiar physical, chemical and biological properties (Zhang et al., 2010). In addition, nano-sized structures have dimensions comparable to those of biological molecules such as proteins and carbohydrates, hence they can easily interact with them inside and outside cells (Bahadori and Mohammadi, 2012). Drugs can be delivered to target sites either as entrapped or encapsulated within the NPs structure, or as adsorbed/attached on the NPs surface (Sung et al., 2007).
In the case of CF, the use of NPs could be a valid approach to overcome the thick mucus layer that is formed within the alveolar region as well as the bacterial biofilm (Ong et al., 2019). The CF mucus contains less water than normal mucus and presents mucin fibers (70–80%), DNA, actin and other macromolecules, which form a cross-linked network where the mesh size is reduced from above 500 nm (in healthy patients) to 300–100 nm in CF patients, depending on their conditions and on the disease stage (Ensign et al., 2012; Liu et al., 2014; Leal et al., 2018). This significant change in mucus structure and composition leads to an increased viscoelasticity (Ibrahim et al., 2011b; Ong et al., 2019), along with the formation of hydrophobic and electrostatic barriers that strongly inhibit NPs penetration. Small NPs are able to diffuse through the network of the mucus while larger ones are trapped in the meshes by physical size exclusion effect. However, penetration strongly depends also on NPs surface properties that influence the interaction with mucus such as superficial charge and hydrophobicity. It was demonstrated that the interaction between NPs and the mucus can be also decreased by modulating the particle surface charge, which needs to be as neutral as possible (with ζ-potential values in the ±10 mV range). Positively charged NPs tend to interact electrostatically with the mucins and the abundant amounts of free DNA and filamentous actin that are all negatively charged, while negatively charged NPs are repelled from the mucus by the hydrophobic domains on the mucins. Thus, neutral particles show good permeation properties due to lacking electrostatic interactions. Two common strategies to overcome the CF mucus barrier are the coating of NPs surface with a dense layer of the so-called “muco-inert” polymers [i.e., low-molecular-weight polyethylene glycol (PEG)] to eliminate both electrostatic and hydrophobic interactions or the use of mucolytic agents [i.e., N-acetylcysteine (NAC)] to reduce mucosal viscoelasticity (Savla and Minko, 2013; Ong et al., 2019).
Despite all the advantages of a NPs mediated therapy for the treatment of pulmonary disease, it is worth to highlight that the development of NPs formulations suitable for administration to the lungs by inhalation is not straightforward due to the complexity of lung anatomy and physiology and to NPs deposition mechanisms.
In order to be effective, when inhaled NPs must reach the deep airways and penetrate the mucus covering the alveoli. Hence, their dimension is a fundamental parameter to be considered in order to predict their deposition. The size of NPs must be small enough to penetrate the mucus and meanwhile big enough to avoid rapid exhalation. It has been proved that the optimal range that allows a good deposition in the alveolar region of the lungs is between 1 and 3 μm (Sung et al., 2007) (in which NPs deposit with gravitational sedimentation) and below 500 nm (in which NPs deposit in the deeper lung by a Brownian diffusion). Besides NPs size, however, there are other parameters that affect the deposition efficacy, such as particle morphology and the surface properties (Paranjpe and Müller-Goymann, 2014).
Nebulization can be used to overcome some NPs-to-lung delivery issues. Nebulizers generate aerosol from solutions or suspensions and create droplets with appropriate size for pulmonary delivery. However, the biggest limitation of this approach arises from the difficulty of developing long term stable nano-suspensions in order to avoid NPs aggregation and degradation. Moreover, most nebulizers show practical disadvantages from patient's point of view, such as limited portability, time-consuming application, low efficiency and high bacterial contamination. Hence, dry powder formulations are usually preferred (Newhouse et al., 2003). A well-known approach for the administration of NPs with DPI consists in the formation of nano-embedded microparticles (NEMs). These nano-in-micro systems [also called Trojan particles (Tsapis et al., 2002)] are able to transport and efficaciously release NPs within the lungs after the dissolution of the microparticle matrix, allowing NPs penetration within the mucus barrier. The most common technique used to convert NPs suspensions into stable, inhalable microparticles is the spray drying (Ruge et al., 2016). Recent studies have highlighted how the tuning of process parameters and the use of excipients can ensure a good re-dispersibility of NEM powders (Tomoda et al., 2008; Kho and Hadinoto, 2010). Several researches have focused on NEMs approach; the most widely used materials are biocompatible and biodegradable polymers as PLGA (Lebhardt et al., 2011; Ungaro et al., 2012b), chitosan (Grenha et al., 2005), gelatin (Sham et al., 2004), PCL (Kho et al., 2010), and polyacrylate (Hadinoto et al., 2007), usually coupled to mannitol, lactose or cyclodextrins as excipients.
The template-assisted technique is another method to prepare NEMs. In this approach, the cylindrical pores of a membrane serve as templates for the formation of elongated NPs, which can be released upon membrane's dissolution. Despite the template-assisted deposition is specific for conducting materials, which are usually insoluble in water and thus are difficult to be employed for drug delivery applications, it represents an innovative and promising approach to prepare NEMs with elongated shape, which are expected to better deposit in deeper lung regions than spherical NPs (Kohler et al., 2011; Tscheka et al., 2015).
Over the last decade, different types of NPs have been studied as nanocarriers for gene and drug delivery in CF treatment. Among these, liposomes, polymeric NPs, solid lipid NPs, and dendrimers (Zhang et al., 2010) represent the major classes of NPs for inhalation therapy that have been tested for CF treatment and that are described in the next paragraphs (Figure 4) and the listed in Table 2.
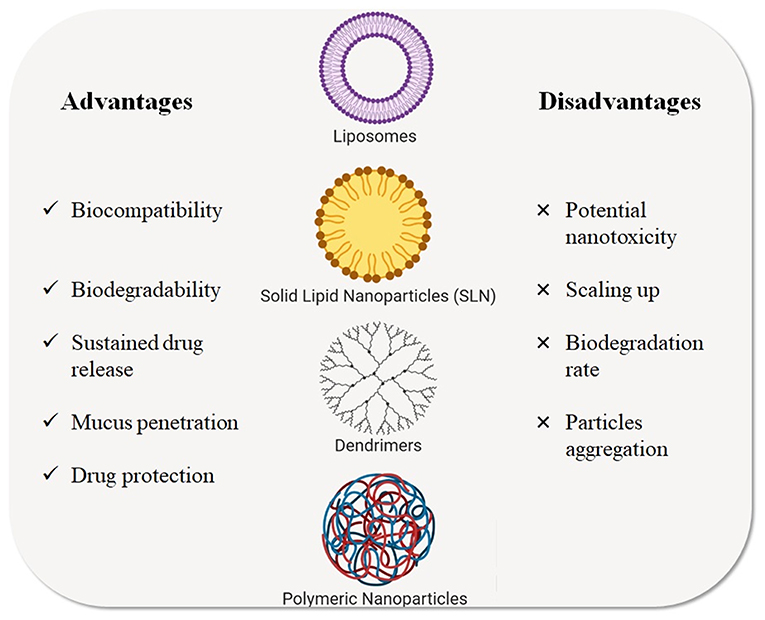
Figure 4. Types of nanotechnological platforms currently used for CF treatments. Advantages and disadvantages are highlighted.
Liposomes, whose structure was first described in 1965, are spherical lipid vesicles with size ranging from 20 nm to 20 μm, formed by a bi-layered membrane consisting of amphiphilic lipid molecules. Depending on their structure, liposomes can be classified in unilamellar vesicles (formed by a single bilayer membrane, size range from 25 nm to 1 μm) or multilamellar vesicles (constituted of several bilayer membranes, size range from 0.1 to 20 μm) (Rudokas et al., 2016). Due to their amphiphilic nature, lipid vesicles can entrap both lipophilic (within the bilayer) and hydrophilic drugs (within the aqueous core) (Moreno-Sastre et al., 2015). Liposomes have been extensively tested as inhalation delivery systems for many drugs, vitamins and nucleic acids for several diseases and pathological conditions (Kuzmov and Minko, 2015). One of the greatest advantages of these carriers is given by the design versatility that allows to obtain structures with the desired size and properties.
Antibiotic-loaded liposomes have been extensively studied to improve their pharmacokinetics and bioavailability, decrease their toxicity and achieve target selectivity (Drulis-Kawa and Dorotkiewicz-Jach, 2010). Liposomal antibiotics have the important advantage to overcome bacterial drug resistance of extracellular pathogens, along with their ability to protect the antibiotic molecules from enzymatic inactivation and degradation (Alipour et al., 2009). Lipid composition, size, charge, and drug/lipid ratio all influence the drug carrying capacity, the release rate and the lung deposition of liposomes (Cryan, 2005). Some phospholipids currently used as drug delivery systems are phosphatidylcholines (PC), phosphatidylglycerols (PG), phosphatidylethanolamines (PE), phosphatidylinositols (PI), dipalmitoyl-phosphatidylcholine (DPPC), distearoylphosphatidylcholine (DSPC), dimyristoyl-phosphatidylglycerol (DMPG), dimyristoyl-phosphatidylcholine (DMPC), and dioleoyl-phosphatidylethanolamine (DOPE) (Cryan, 2005; d'Angelo et al., 2014).
Liposomes can be prepared for inhalation both in liquid and in dry formulations, and several researches have proved their efficacy and safety when administered by inhalation. Some of the first studies on liposomal formulations were carried out in the 90s by Omri et al., who investigated the redemption (Omri et al., 1994), efficacy and toxicity (Beaulac et al., 1996, 1997) of liposome-encapsulated tobramycin in a comparative study on healthy and PA infected rats. The results showed a significant increase in drug residence time for both healthy and infected rats as compared to free tobramycin administration. The effects of liposomal composition on the drug release kinetics and efficacy were evaluated by the development of the so-called fluidosomes® [liposomes composed of DPPC: DMPG (10:1 to 15:1 ratio)] loaded with tobramycin. The administration of tobramycin-loaded fluidosomes in infected rats led to a complete eradication of the pathogen as compared to solid liposomes and free antibiotic. Furthermore, the detection of low levels of drug in the kidneys after fluidosomal administrations compared to free tobramicyn suggested a potential reduced nephrotoxicity. Drulis-Kawa et al. (2006) tested several liposomes loaded with meropenem (a β-lactame antibiotic) against PA strains, and demonstrated an improved efficacy of the drug loaded in two different cationic liposomes (PC/DOPE/SA 4:4:2 and PC/DOTAP/Chol 5:2:3). Subsequent studies confirmed that drugs encapsulated within cationic liposomes exhibits greater antibacterial efficacy, probably due to their ability to target bacteria biofilms through electrostatic interactions, thus allowing the drug release close to the pathogen. Messiaen et al. (2013) reached the same results by encapsulating tobramycin in neutral, anionic and cationic liposomes. The greater efficacy of drugs loaded within liposomes was confirmed by Mugabe et al. (2005), who encapsulated the antibiotic gentamicin within liposomes of different composition (DPMC, DPPC, and DSPC). Results showed an increased efficacy of drug activity against PA as compared to free gentamicin.
Joshi and Misra (2001) carried out a study on liposomal (EPC-Chol) budesonide (an anti-inflammatory corticosteroid) in a dry powder formulation. This system was found to keep the desired drug levels in the lungs for a prolonged time, possibly allowing a better drug efficacy and lower adverse effect. Although positively charged liposomes showed better results on the increase of drug activity against several pathogens, they were found to be more toxic for human lung cells than uncharged or negatively charged systems (d'Angelo et al., 2014; Kuzmov and Minko, 2015); this evidence opened to the development of new strategies in order to design new liposomal formulations with less adverse effects on lung cells. Currently, two more antibiotics have been developed as liposomal formulations. The first is amikacin liposome inhalation suspension (Arikayce®, Insmed, Monmouth Junction, NJ, USA), which has completed the phase III of clinical trials and it has been approved by FDA in 2018. It consists of neutral liposomes (DPPC: Chol), with a mean size of 0,3 μm, provided as a sterile aqueous liposomal suspension administrated through an eFlow® Nebulizer. The second is liposomal ciprofloxacin (Pulmaquin® and Lipoquin®, Aradigm Corp., Hayward, CA, USA), which has completed phase II of clinical trials, showing a superior drug efficacy with respect to non-encapsulated ciprofloxacin (Paranjpe and Müller-Goymann, 2014; Moreno-Sastre et al., 2015). Due to the intrinsic structure of liposomes, a further step on the development of new liposomal formulations has been the encapsulation of more than one drug within the bi-layered structure (Van Rijt et al., 2014). Halwani et al. (2008) in 2008 tested the antimicrobial efficacy of a liposomal gentamicin formulation with Gallium metal (Lipo-Ga-GEN). Results indicated an improved efficacy of this multidrug formulation with respect to gentamicin alone in eradicating antibiotic-resistant PA.
As reported previously in this review, cationic liposomes are the most widely investigated systems to deliver DNA intracellularly. Manunta et al. (2017) developed a self-assembling nano-complex constituted of a DOTMA:DOPE liposome, an epithelial targeting peptide and siRNA (small interfering RNA, which represents an attractive approach for the treatment of several pulmonary diseases characterized by an over-expression of genes) against the α-subunit of epithelial sodium channel (ENaC), thus modulating sodium hyperabsorption and helping to restore the mucus hydration and the airway clearance. The authors showed the promising chance to use this approach as co-adjuvant therapy for CF.
Tagalakis et al. (2018) developed siRNA nanocomplexes that could mediate silencing of airway epithelial sodium channel (ENaC) with functional correction of epithelial ion transport. Receptor-targeted nanocomplexes (RTNs) are made of cationic liposomes and targeting peptide mixed with siRNA. In this study it was observed that NPs mediated delivery of siRNA offered an effective delivery by correcting mucociliary defects in human CF cells in vivo. A phase IIb clinical trials testing the efficacy of a non-viral gene therapy for CF has been carried out by Alton et al. (2015). In this study, the author complexed the plasmid DNA pGM169 with GL67A liposome and administered the complex to CF patients once a month for 1 year through a nebulizer. The results showed a statistically significant increase in FEV1, suggesting lung function stabilization and demonstrating the good potential of the pGM169/GL67A formulation as a safe, non-viral strategy for CF gene therapy (Trial ID: NCT00789867).
The main issues about liposomes as drug delivery for the treatment of pulmonary chronic diseases, such as CF, arise from the need to develop inhalable formulations which have to be delivered through nebulizers (d'Angelo et al., 2014). In fact, the nebulization process may affect liposomes stability and causes their aggregation. For this aim, several strategies are being investigated, such as lyophilisation (Chen et al., 2010), spray drying (Willis et al., 2012) and supercritical fluid technology (Misra et al., 2009), in order to stabilize liposomes and possibly achieve DPI liposomal formulations; some of these have shown promising potentials for pulmonary drug administration, but are still in an early development stage.
Solid lipid nanoparticles (SLNs) are lipophilic particulates (Yang et al., 2008) which have been studied as drug delivery systems since 1990s. In contrast to liposomes, they don't have a bi-layered structure, but are made of lipids which form a crystalline matrix, solid at room and body temperature, with mean diameters ranging from 40 to 1,000 nm (Weber et al., 2014). The most used solid lipids are fatty acids, triglycerides, steroids, phospholipids and waxes (Zhang et al., 2010; Paranjpe and Müller-Goymann, 2014). The most remarkable advantages of SLN are physical stability and low cytotoxicity, along with the possibility of easy, solvent free scaling-up processes (Mehnert and Mäder, 2012; Weber et al., 2014). However, their main limitation is represented by a low drug loading capacity, which is caused by an increased rigidity of the matrix during the storage (Müller et al., 2000).
The second generation of lipid nanoparticles, called lipid nanocarriers (NLC), consists of a solid lipid matrix made of more than one component, reducing NLC rigidity and allowing a higher drug loading. Even though these systems have been extensively studied for oral (Pinto and Müller, 1999; Müller et al., 2006), dermal (Wissing and Müller, 2001; Müller et al., 2002; Pardeike et al., 2009), parenteral (Joshi and Müller, 2009), and ocular (Araújo et al., 2010) administrations, pulmonary applications are still in early development. Various drugs have been encapsulated within SLNs for the treatment of CF and other pulmonary diseases, such as budesonide (Zhang et al., 2011) and ciprofloxacin (Jain and Banerjee, 2008). Amikacin loaded SLNs were developed by Varshosaz et al. (2010, 2013) and Ghaffari et al. (2011). After optimizing the particles morphology and size, they compared the efficacy of amikacin loaded SLNs with respect to the free drug. The results of these studies showed an increased efficacy of the loaded drug, which could be administered in lower doses (or longer intervals) when encapsulated in SLNs.
In 2018, Robinson et al. (2018) developed lipid-based NPs (LNPs) for packaging and delivery of chemically modified CFTR mRNA (cmCFTR) to restore chloride secretion. They demonstrated that cmRNA delivered by LNPs is effectively translated to a protein product that successfully reach cells membrane. Hence, LNP-cmRNA, whose mechanism of action (i.e., increase of CFTR channel opening on apical cell membrane) and efficacy are comparable with those observed for ivacaftor, represent a promising platform for the correction of CF. Finally, although studies on SLNs and NLCs as pulmonary carriers are in the first stages of research, they have shown good therapeutic potential.
Dendrimers are highly ordered, branched macromolecules that possess a tree-like architecture. Three regions can be distinguished in their structure: a core, several dendritic branches (called generations) and functional end groups located in the outer layer of the branches (Menjoge et al., 2010; Zhang et al., 2010; Mehta et al., 2019). This peculiar structure, which can be achieved either with divergent or convergent synthetic approaches, makes dendrimers suitable to a wide range of chemical modifications. Moreover, their highly branched nature provides high surface area to size ratio, which can be used to incorporate different chemical species within the structure in a host-guest approach. The first family of polyamidoamine dendrimers (PAMAM) were synthesized by Tomalia et al. (1985) and PAMAMs are now one of the most studied and used dendrimers. Since then, other types of dendrimers have been synthesized and studied for biomedical applications, such as polyetherhydroxylamine (PEHAM), polypropylenimine (PPI), and polyether dendrimers. Due to their unique physicochemical, biological and mechanical properties, these molecules have raised interest in the field of nanomedicine as possible nanoplatforms for antimicrobial and gene delivery. Drugs conjugated to dendrimers can be administered through different routes, such as oral, injection, ocular, nasal, and pulmonary ones. Compared to other nanocarriers commonly used in nanomedicine, dendrimers show some advantages. In fact, by tuning their structure, for example, it is possible to improve the drug functionality thanks to the multivalency effect. Moreover, from a pharmacological point of view, the high monodispersity of dendrimers allows a better uptake efficacy and bioavailability of drugs encapsulated within the structure, with an easier penetration across biological barriers by transcytosis (Menjoge et al., 2010; Mehta et al., 2019).
The main targets of pulmonary therapies using dendrimers as drug delivery systems are lung cancer, chronic obstructive pulmonary disease (COPD), asthma, tuberculosis. bronchiectasis and pneumonia (Rolland et al., 2009). Dendritic formulations to be administered via inhalation have been developed both as inhalable suspensions and dry powders. In 2017, Agnoletti et al. (2017) developed dendrimer-siRNA nanocomplexes for gene therapy, which is an attractive approach for the treatment of several pulmonary diseases characterized by an over-expression of genes. The nanocomplexes developed showed an efficient cellular uptake and a good gene silencing capability compared to other types of carriers. Brockman et al. (2017) evaluated a strategy to improve the bioavailability and targeted delivery of cysteamine, a FDA approved drug with anti-oxidant, anti-biofilm and mucolytic properties. They developed a PAMAM dendrimer whose terminal groups were modified to obtain a cysteamine-like structure (PAMAM-DENCYS) and demonstrated its efficacy in reducing PA infection and growth, along with its ability to rescue the misfolded F508del-CFTR from aggresome-bodies by inducing its trafficking to the plasma membrane. CFTR modulators were also embedded within dendritic structures, as reported in the work by Faraj et al., who standardized the therapeutic efficacy of the novel dendrimer-based autophagy-inducing agent, cysteamine. In their study, they synthesized and tested a novel nano-molecule consisting of cysteamine-core PAMAM dendrimer (G4 90/10 PAMAM DEN[CYS]) and demonstrated its efficacy in the treatment of patients with CF, since the nanostructure preserve cysteamine activity while increasing its bioavailability compared to other means of administration (Faraj et al., 2019). Several strategies have been developed to achieve a more effective cellular uptake of drugs-loaded dendrimers, avoiding their inactivation and elimination by immune response and macrophages activity. Among these, the coating with hydrophilic and biocompatible polymers is the most common strategy. PEG (polyethylene glycol) is the most frequently used polymer to coat and protect a variety of nanocarriers due to its ability to offer stealth properties to them and to drug molecules (Suk et al., 2009, 2011; Kaminskas et al., 2014; Taghavi Pourianazar et al., 2014; Zhong et al., 2016).
With the rapid development of nanotechnology in the field of nanomedicine, polymeric NPs have gained much importance, as they represent a major class of nanotherapeutics. Polymeric nanocarriers are widely used as drug delivery systems and administered via different routes, including inhalation (Kuzmov and Minko, 2015). The most relevant features of polymeric systems are their ability to perform a prolonged and controlled drug release, stabilize the drugs encapsulated and promote cellular uptake of pharmaceuticals. Moreover, polymeric NPs show great versatility: their morphological and surface properties, such as size, shape and zeta potential can be easily tuned by choosing different polymers length and kinds of surfactants and solvents for the synthesis. Moreover, their properties can be tailored by adding specific functional groups, drug moieties and target ligands on their surface. Among synthetic polymers, poly-lactic acid (PLA) and poly-lactic-co-glycolic acid (PLGA), both FDA approved, are the most widely used (Cryan, 2005) due to their great biocompatibility and biodegradability. In particular, PLGA offers the possibility to tailor its biodegradation rate by modifying its compositional ratio.
Polymeric NPs have been largely used to avoid side effects and prevent the early degradation of drug molecules, and also to improve the pharmacokinetic of drugs with scarce bioavailability. As an example, curcumin has been found to be a non-toxic, low-affinity sarcoendoplasmic reticulum calcium ATPase (SERCA) pump inhibitor which allows F508del CFTR to escape from the endoplasmic reticulum and reach cell membrane (Egan et al., 2004; Zeitlin, 2004). However, previous studies showed a low efficacy of this compound due to its poor absorption and rapid metabolism. In their research, Cartiera et al. (2010) encapsulated curcumin in PLGA NPs in order to increase its absorption and improve its bioavailability. The in vivo tests showed that curcumin loaded NPs enhanced the effects of the compound in CF mice compared to free drug.
Vij et al. (2010) developed PEGylated PLGA NPs encapsulating PS-341, a FDA approved proteasome inhibitor, which has been firstly employed for cancer therapy, even though recent studies have proved the importance of this class of drugs in pharmaco-gene therapy of CF. The authors of the study obtained a controlled and sustained delivery of drug, increasing its efficacy against PA infections.
Ciprofloxacin was successfully encapsulated in PLGA NPs by Günday Türeli et al. (2017), who evaluated the efficacy of the nanocomplex against PA strains, along with its ability to cross the mucus. Embedding the drug within a polymeric matrix allowed a high percentage of loading, while the nanometric size and surface properties permitted a good penetration of the bioactive payload within the mucus. Additionally, an enhanced antimicrobial activity of nano-formulated ciprofloxacin at lower drug doses was reported, indicating its promising potential as drug delivery system for CF antimicrobial treatment. Similar results were reported by Ernst et al. (2018) who encapsulated tobramycin within PEG-coated PLGA nano (≈230 nm) and micro (≈900 nm) particles and observed an enhance antimicrobial activity, along with a good penetration within mucus and bacterial biofilm in dynamic physiological conditions.
As reported above in this section, NPs have been tested as carriers not only for antimicrobial treatments but also for gene therapy. Haque et al. (2018) studied the efficacy of cmRNAhCFTR encapsulated in chitosan-coated PLGA NP's delivered to the lungs of a CFTR deficient mice by intra-venous and intratracheal administration. In this study, they observed a reduced chloride secretion and a general restore of critical lung function parameters, including a noticeable increase in FEV1, suggesting that NP-cmRNAhCFTR could be promising therapeutic option for CF patients. Another strategy used to overcome the issues of storing and to improve dispersion and deep lung deposition of NPs consists in the use of NEMs formulations (Muralidharan et al., 2015). In these preparations, NPs can be: (i) encapsulated in polymer-based carriers, (ii) loaded on the polymer surface or (iii) dispersed in a polymer matrix. In all these cases, the polymeric part dissolves upon exposure to the lung environment and releases NPs (Sung et al., 2007).
An attractive new class of non-viral gene delivery vectors is represented by poloxamine-based block copolymers (Richard-Fiardo et al., 2015). Poloxamines are x-shaped block copolymers constituted of poly(ethylen oxide)/poly(propylen oxide) (PEO/PPO) blocks bonded to a central ethylenediamine moiety. Poloxamines are commercially available in a wide range of PEO/PPO ratios and molecular weights under the tradename Tetronic®. Their peculiar structure confers several features, such as temperature and pH sensitiveness and ease of core modification. Due to their hydrophobic core, these nanocarriers are used to solubilize and stabilize poorly water-soluble drugs (Alvarez-Lorenzo et al., 2010). Despite their potential as non-viral vectors for gene therapy, poloxamines alone are not able to overcome some barriers posed by the in vitro transfection, while in vivo they exhibit a lower efficiency than viral vectors. Guan et al. (2019) developed a platform of synthetic peptides able to self-assemble to poloxamines and nucleic acids to form compacted NPs. The author demonstrated that the use of these NPs led to the enhancement of both mRNA and plasmid DNA expression both in vitro and in the lungs of CF mice, without showing any toxicity, thus providing a new strategy for the development of non-viral gene delivery.
Nanomedicine represents an extraordinary opportunity for the improvement of current therapies and for the development of innovative treatment options for CF previously considered hard or impossible to treat. The huge amount of research on NPs development as nano-carriers for gene and drug delivery directly to the lungs by inhalation has the opportunity to strongly modify symptomatic treatments for CF patients, as well as those based on CFTR modulators and gene therapies. Due to the peculiar environment in which the therapies have to operate, characterized by several biological barriers (pulmonary tract, mucus, epithelia, bacterial biofilm), the use of nanotechnologies to improve and enhance drug delivery or gene therapies seems to be an extremely promising way to be pursued. However, the road to a definitive realization is still long, although several nano-systems were already tested with successful results with FDA-approved drugs. A special attention should be paid in the use of biocompatible and biodegradable inhalable NPs, that must be well-tolerated in vivo as in the case of other disease treatment (Di Mauro et al., 2016; Miragoli et al., 2018). In vitro/in vivo studies represent a critical step before the selection of the best formulations to candidate for use in humans. Therefore, there is an impelling need of in vitro/in vivo CF models to acquire knowledge on the safety of the nano-carriers in the lungs. Not only traditional tests, but also newer models should be used to shed light on the possible mechanisms of NPs toxicity. In particular, the shortage of chronic toxicological data on NPs is one of the big gaps to be closed. Once these challenges will be addressed, sustained-release nanocarriers would represent a real benefit for both pharmaceutical companies working to develop novel inhaled products and patients suffering from CF.
The studies on genetic therapies as well as those based on CTFR modulators are already at an advanced stage, but not yet optimal and in some case with results not yet inclusive for a large part of CF patients. Therefore, it should also be considered that symptomatic therapies have not finished their function, indeed they need to be open to new solutions, because we believe that, unfortunately for a long time to come, they will be indispensable for many patients. In this domain, bacterial and fungal infections can be fought by the use of appropriate delivery of antibiotics mediated by NPs while inflammations, due to the peculiar physiological state of the patients, can be circumvented with the same approach by the use of anti-inflammatory drugs.
The genetic defects of CFTR channel may be restored by using highly specific drugs (i.e., modulators) targeting the defective channel protein restore, at least in part CFTR functions. In this latter case, patients treated with these drugs experienced severe systemic side effects and to boost their efficacy the drugs concentration and their permanence at the lungs, the major site of disease, should be improved. The pulmonary administration of CFTR modulators based on mucus penetrating NPs could be an effective strategy, potentially increasing higher local drug concentrations and minimizing side effects when compared to the oral route of administration (Porsio et al., 2018).
Together with these strategies, various different advanced gene therapies seem to be the most promising to eradicate CF from patients in a definitive way, especially if these therapies will be enhanced by the use of nanotechnology in an appropriate way. Overall, genetic editing tools such as CRISPR/Cas9 look more convincing in producing an effective and definitive resolution of CF disease. The major problem to be addressed, is the possibility that Cas9 recognizes sequences different from the target one and therefore introduces unpredicted changes. In fact, the RNA fragment that guides Cas9 admits some incorrect pairing, making Cas9 able to cut even regions of DNA other than the target. This issue can currently be very limited by identifying very selective RNA guide and using alternative or modified Cas9 that allow greater specificity, such as the one developed by Casini et al. (2018). The same authors and collaborators (Maule et al., 2019) developed a genome editing strategy to repair 3272-26A>G and 3849+10kbC>T CFTR mutations. Both mutations alter the correct splicing of the CFTR gene leading to a premature termination codon and consequent expression of a truncated non-functional CFTR protein. In this study, the AsCas12a nuclease (from Acidaminococcus sp. BV3L6) and a single CRISPR RNA were used for gene correction in intestinal organoids and airway epithelial cells derived from CF patients carrying the 3272–26A>G or 3849+10kbC>T mutations. The results obtained shown highly precise genetic repair with a complete absence of detectable off-targets. Even more recently, Ricciardi et al. (2018) applied gene editing in the fetuses of pregnant mice to correct the most common F508del mutation in the CFTR gene. They used donor DNA and synthetic molecules that mimic DNA, called peptide nucleic acids, delivered by biodegradable polymeric NPs to target the CFTR gene. Cell's own DNA repair pathways were activated by the triple helix formed by the NPs linked to the target gene in order to fix the mutation in fetal cells. The lungs of mice born with the repaired mutations, appeared normal without obvious signs of inflammation. The team will continue to study mice organ health. Despite clinical trials are years away, Ricciardi theorized that the therapy in humans would be safely deliver at around 18 weeks of gestation.
We must obviously be extremely careful, considering the enormous quantity of preclinical studies in animal models to be carried out before a possible application in a human environment, but it is inevitable to think that in utero DNA editing could constitute the new frontier of medicine and project us far ahead in the treatment of diseases like CF. Today, thanks to amniocentesis and non-invasive prenatal tests performed on blood samples, we can identify serious and dangerous genetic diseases in great advance. Tomorrow, thanks to genetic editing protocols, we hope to be able to defeat them definitely.
CV and MI have designed and written the manuscript. AA, FC, FB, AV, DC, and MS have proofread and given comments as well as suggestions. MI has supervised and finalized the manuscript.
This study was supported by Fondazione per la ricerca sulla Fibrosi Cistica - Onlus (Project FFC#20/2018).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank the Fondazione per la ricerca sulla Fibrosi Cistica - Onlus for its grant (FFC#20/2018).
Abramowsky, C. R., and Swinehart, G. L. (1982). The nephropathy of cystic fibrosis: a human model of chronic nephrotoxicity. Hum. Pathol. 13, 934–939. doi: 10.1016/S0046-8177(82)80056-7
Agent, P., and Parrott, H. (2015). Inhaled therapy in cystic fibrosis: agents, devices and regimens. Breathe 11, 111–118. doi: 10.1183/20734735.021014
Agnoletti, M., Bohr, A., Thanki, K., Wan, F., Zeng, X., Boetker, J. P., et al. (2017). Inhalable siRNA-loaded nano-embedded microparticles engineered using microfluidics and spray drying. Eur. J. Pharm. Biopharm. 120, 9–21. doi: 10.1016/j.ejpb.2017.08.001
Alikhani, Z., Salouti, M., and Ardestani, M. S. (2018). Synthesis and immunological evaluation of a nanovaccine based on PLGA nanoparticles and alginate antigen against infections caused by Pseudomonas aeruginosa. Biomed. Phys. Eng. Express 4, 1–9. doi: 10.1088/2057-1976/aabfac
Alipour, M., Halwani, M., Omri, A., and Suntres, Z. E. (2008). Antimicrobial effectiveness of liposomal polymyxin B against resistant Gram-negative bacterial strains. Int. J. Pharm. 355, 293–298. doi: 10.1016/j.ijpharm.2007.11.035
Alipour, M., Suntres, Z. E., Halwani, M., Azghani, A. O., and Omri, A. (2009). Activity and interactions of liposomal antibiotics in presence of polyanions and sputum of patients with cystic fibrosis. PLoS ONE 4:e5724. doi: 10.1371/journal.pone.0005724
Al-Obaidi, H., Kalgudi, R., and Zariwala, M. G. (2018). Fabrication of inhaled hybrid silver/ciprofloxacin nanoparticles with synergetic effect against Pseudomonas aeruginosa. Eur. J. Pharm. Biopharm. 128, 27–35. doi: 10.1016/j.ejpb.2018.04.006
Alton, E. W. F. W., Armstrong, D. K., Ashby, D., Bayfield, K. J., Bilton, D., Bloomfield, E. V., et al. (2015). Repeated nebulisation of non-viral CFTR gene therapy in patients with cystic fibrosis: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir. Med. 3, 684–691. doi: 10.1016/S2213-2600(15)00245-3
Alton, E. W. F. W., Middleton, P. G., Caplen, N. J., Smith, S. N., Steel, D. M., Munkonge, F. M., et al. (1993). Non invasive liposome mediated gene delivery can correct the ion transport defect in cystic fibrosis mutant mice. Nat. Genet. 5, 135–142. doi: 10.1038/ng1093-135
Alvarez-Lorenzo, C., Rey-Rico, A., Sosnik, A., Taboada, P., and Concheiro, A. (2010). Poloxamine-based nanomaterials for drug delivery. Front. Biosci. 2 E, 424–440. doi: 10.2741/e102
Amaral, M. D. (2015). Novel personalized therapies for cystic fibrosis: treating the basic defect in all patients. J. Intern. Med. 277, 155–166. doi: 10.1111/joim.12314
Amoasii, L., Hildyard, J. C. W., Li, H., Sanchez-Ortiz, E., Mireault, A., Caballero, D., et al. (2018). Gene editing restores dystrophin expression in a canine model of Duchenne muscular dystrophy. Science 362, 86–91. doi: 10.1126/science.aau1549
Andersson, M. I. (2003). Development of the quinolones. J. Antimicrob. Chemother. 51, 1–11. doi: 10.1093/jac/dkg212
Araújo, J., Gonzalez-Mira, E., Egea, M. A., Garcia, M. L., and Souto, E. B. (2010). Optimization and physicochemical characterization of a triamcinolone acetonide-loaded NLC for ocular antiangiogenic applications. Int. J. Pharm. 393, 168–176. doi: 10.1016/j.ijpharm.2010.03.034
Ballmann, M., Smyth, A., and Geller, D. E. (2011). Therapeutic approaches to chronic cystic fibrosis respiratory infections with available, emerging aerosolized antibiotics. Respir. Med. 105(Suppl 2), S2–S8. doi: 10.1016/S0954-6111(11)70021-X
Barrangou, R. (2015). The roles of CRISPR-Cas systems in adaptive immunity and beyond. Curr. Opin. Immunol. 32, 36–41. doi: 10.1016/j.coi.2014.12.008
Beaulac, C., Clément-Major, S., Hawari, J., and Lagacé, J. (1996). Eradication of mucoid Pseudomonas aeruginosa with fluid liposome-encapsulated tobramycin in an animal model of chronic pulmonary infection. Antimicrob. Agents Chemother. 40, 665–669. doi: 10.1128/AAC.40.3.665
Beaulac, C., Clement-Major, S., Hawari, J., and Lagace, J. (1997). In vitro kinetics of drug release and pulmonary retention of microencapsulated antibiotic in liposomal formulations in relation to the lipid composition. J. Microencapsul. 14, 335–348. doi: 10.3109/02652049709051137
Bhagirath, A. Y., Li, Y., Somayajula, D., Dadashi, M., Badr, S., and Duan, K. (2016). Cystic fibrosis lung environment and Pseudomonas aeruginosa infection. BMC Pulm. Med. 16:174. doi: 10.1186/s12890-016-0339-5
Bielski, E., Zhong, Q., Mirza, H., Brown, M., Molla, A., Carvajal, T., et al. (2017). TPP-dendrimer nanocarriers for siRNA delivery to the pulmonary epithelium and their dry powder and metered-dose inhaler formulations. Int. J. Pharm. 527, 171–183. doi: 10.1016/j.ijpharm.2017.05.046
Bodas, M., and Vij, N. (2019). Adapting proteostasis and autophagy for controlling the pathogenesis of cystic fibrosis lung disease. Front. Pharmacol. 10:20. doi: 10.3389/fphar.2019.00020
Brockman, S. M., Bodas, M., Silverberg, D., Sharma, A., and Vij, N. (2017). Dendrimer-based selective autophagy-induction rescues δF508-CFTR and inhibits Pseudomonas aeruginosa infection in cystic fibrosis. PLoS ONE 12:e0184793. doi: 10.1371/journal.pone.0184793
Brouns, S. J. J., Jore, M. M., Lundgren, M., Westra, E. R., Slijkhuis, R. J. H., Snijders, A. P. L., et al. (1993). Small Crispr Rnas guide antiviral defense in prokaryotes. Cancer Epidemiol. Biomarkers Prev. 2, 531–535.
Caretti, A., Bragonzi, A., Facchini, M., De Fino, I., Riva, C., Gasco, P., et al. (2014). Anti-inflammatory action of lipid nanocarrier-delivered myriocin: therapeutic potential in cystic fibrosis. Biochim. Biophys. Acta Gen. Subj. 1840, 586–594. doi: 10.1016/j.bbagen.2013.10.018
Cartiera, M. S., Ferreira, E. C., Caputo, C., Egan, M. E., Caplan, M. J., and Saltzman, W. M. (2010). Partial correction of cystic fibrosis defects with PLGA nanoparticles encapsulating curcumin. Mol. Pharm. 7, 86–93. doi: 10.1021/mp900138a
Casini, A., Olivieri, M., Petris, G., Montagna, C., Reginato, G., Maule, G., et al. (2018). A highly specific SpCas9 variant is identified by in vivo screening in yeast. Nat. Biotechnol. 36, 265–271. doi: 10.1038/nbt.4066
Charrier, C., Rodger, C., Robertson, J., Kowalczuk, A., Shand, N., Fraser-Pitt, D., et al. (2014). Cysteamine (Lynovex®), a novel mucoactive antimicrobial and antibiofilm agent for the treatment of cystic fibrosis. Orphanet J. Rare Dis. 9:189. doi: 10.1186/s13023-014-0189-2
Chaudary, N. (2018). Triplet CFTR modulators: future prospects for treatment of cystic fibrosis. Ther. Clin. Risk Manag. 14, 2375–2383. doi: 10.2147/TCRM.S147164
Cheer, S. M., Waugh, J., and Noble, S. (2003). Inhaled Tobramycin (TOBI®): a review of its use in the management of Pseudomonas aeruginosa infections in patients with cystic fibrosis. Drugs 63, 2501–2520. doi: 10.2165/00003495-200363220-00015
Chen, C., Han, D., Cai, C., and Tang, X. (2010). An overview of liposome lyophilization and its future potential. J. Control. Release 142, 299–311. doi: 10.1016/j.jconrel.2009.10.024
Clancy, J. P., Cotton, C. U., Donaldson, S. H., Solomon, G. M., VanDevanter, D. R., Boyle, M. P., et al. (2019). CFTR modulator theratyping: current status, gaps and future directions. J. Cyst. Fibros. 18, 22–34. doi: 10.1016/j.jcf.2018.05.004
Conole, D., and Keating, G. M. (2014). Colistimethate sodium dry powder for inhalation: a review of its use in the treatment of chronic Pseudomonas aeruginosa infection in patients with cystic fibrosis. Drugs 74, 377–387. doi: 10.1007/s40265-014-0181-0
Conti, D. S., Brewer, D., Grashik, J., Avasarala, S., and Da Rocha, S. R. P. (2014). Poly(amidoamine) dendrimer nanocarriers and their aerosol formulations for siRNA delivery to the lung epithelium. Mol. Pharm. 11, 1808–1822. doi: 10.1021/mp4006358
Cristallini, C., Barbani, N., Ventrelli, L., Summa, C., Filippi, S., Capelôa, T., et al. (2019). Biodegradable microparticles designed to efficiently reach and act on cystic fibrosis mucus barrier. Mater. Sci. Eng. C 95, 19–28. doi: 10.1016/j.msec.2018.10.064
Cryan, S. A. (2005). Carrier-based strategies for targeting protein and peptide drugs to the lungs. AAPS J. 7:E20–E41. doi: 10.1208/aapsj070104
Cystic Fibrosis Foundation (2018). 2017 Patient Registry: Annual Data Report. Cyst. Fibros. Found. 77. Available online at: https://www.cff.org/Research/Researcher-Resources/Patient-Registry/2017-Patient-Registry-Annual-Data-Report.pdf (accessed September 2019).
da Silva, A. L., Cruz, F. F., Rocco, P. R. M., and Morales, M. M. (2017). New perspectives in nanotherapeutics for chronic respiratory diseases. Biophys. Rev. 9, 793–803. doi: 10.1007/s12551-017-0319-x
d'Angelo, I., Conte, C., La Rotonda, M. I., Miro, A., Quaglia, F., and Ungaro, F. (2014). Improving the efficacy of inhaled drugs in cystic fibrosis: challenges and emerging drug delivery strategies. Adv. Drug Deliv. Rev. 75, 92–111. doi: 10.1016/j.addr.2014.05.008
Dasenbrook, E. C., and Chmiel, J. F. (2017). “Anti-inflammatory therapies for cystic fibrosis,” in Treatment of Cystic Fibrosis and Other Rare Lung Diseases, eds A. Azuma and M. S. Schechter (Basel: Springer Basel), 139–151. doi: 10.1007/978-3-0348-0977-1_8
Davies, G., Griesenbach, U., Alton, E., and Davies, J. C. (2019). “Chapter 53: Molecular therapies for cystic fibrosis,” in Kendig's Disorders of the Respiratory Tract in Children, 9th Edn, eds R. W. Wilmott, R. Deterding, A. Li, F. Ratjen, P. Sly, H. J. Zar, and A. Bush (Philadelphia, PA), 800–811. doi: 10.1016/B978-0-323-44887-1.00053-5
Davies, J. C., Alton, E. W. F. W., and Bush, A. (2007). Cystic fibrosis. Br. Med. J. 335, 1255–1259. doi: 10.1136/bmj.39391.713229.AD
De Boeck, K., and Amaral, M. D. (2016). Progress in therapies for cystic fibrosis. Lancet Respir. Med. 4, 662–674. doi: 10.1016/S2213-2600(16)00023-0
De Boeck, K., Munck, A., Walker, S., Faro, A., Hiatt, P., Gilmartin, G., et al. (2014). Efficacy and safety of ivacaftor in patients with cystic fibrosis and a non-G551D gating mutation. J. Cyst. Fibros. 13, 674–680. doi: 10.1016/j.jcf.2014.09.005
De Stefano, D., Coletta, C., D'Emmanuele Di Villa Bianca, R., Falcone, L., D'Angelo, I., Ungaro, F., et al. (2013). A decoy oligonucleotide to NF-κB delivered through inhalable particles prevents LPS-induced rat airway inflammation. Am. J. Respir. Cell Mol. Biol. 49, 288–295. doi: 10.1165/rcmb.2012-0473OC
De Stefano, D., Ungaro, F., Giovino, C., Polimeno, A., Quaglia, F., and Carnuccio, R. (2011). Sustained inhibition of IL-6 and IL-8 expression by decoy ODN to NF-κB delivered through respirable large porous particles in LPS-stimulated cystic fibrosis bronchial cells. J. Gene Med. 13, 200–208. doi: 10.1002/jgm.1546
De Stefano, D., Villella, V. R., Esposito, S., Tosco, A., Sepe, A., De Gregorio, F., et al. (2014). Restoration of CFTR function in patients with cystic fibrosis carrying the F508del-CFTR mutation. Autophagy 10, 2053–2074. doi: 10.4161/15548627.2014.973737
Di Mauro, V., Iafisco, M., Salvarani, N., Vacchiano, M., Carullo, P., Ramírez-Rodríguez, G. B., et al. (2016). Bioinspired negatively charged calcium phosphate nanocarriers for cardiac delivery of MicroRNAs. Nanomedicine 11, 891–906. doi: 10.2217/nnm.16.26
Dinwiddie, R. (2005). Anti-inflammatory therapy in cystic fibrosis. J. Cyst. Fibros. 4, 45–48. doi: 10.1016/j.jcf.2005.05.010
Döring, G., Flume, P., Heijerman, H., and Elborn, J. S. (2012). Treatment of lung infection in patients with cystic fibrosis: current and future strategies. J. Cyst. Fibros. 11, 461–479. doi: 10.1016/j.jcf.2012.10.004
Doudna, J. A., and Charpentier, E. (2014). The new frontier of genome engineering with CRISPR-Cas9. Science 346:1258096. doi: 10.1126/science.1258096
Drulis-Kawa, Z., and Dorotkiewicz-Jach, A. (2010). Liposomes as delivery systems for antibiotics. Int. J. Pharm. 387, 187–198. doi: 10.1016/j.ijpharm.2009.11.033
Drulis-Kawa, Z., Gubernator, J., Dorotkiewicz-Jach, A., Doroszkiewicz, W., and Kozubek, A. (2006). In vitro antimicrobial activity of liposomal meropenem against Pseudomonas aeruginosa strains. Int. J. Pharm. 315, 59–66. doi: 10.1016/j.ijpharm.2006.02.017
Drusano, G. L. (1997). Pharmacokinetics and Pharmacodynamics of Fluoroquinolones and other Antimicrobial Agents. Jap. J. Chemother. 45, 29–54.
Egan, M. E., Pearson, M., Weiner, S. A., Rajendran, V., Rubin, D., Glöckner-Pagel, J., et al. (2004). Curcumin, a major constituent of turmeric, corrects cystic fibrosis defects. Science 304:600–602. doi: 10.1126/science.1093941
Ensign, L. M., Schneider, C., Suk, J. S., Cone, R., and Hanes, J. (2012). Mucus penetrating nanoparticles: biophysical tool and method of drug and gene delivery. Adv. Mater. 24, 3887–3894. doi: 10.1002/adma.201201800
Ernst, J., Klinger-Strobel, M., Arnold, K., Thamm, J., Hartung, A., Pletz, M. W., et al. (2018). Polyester-based particles to overcome the obstacles of mucus and biofilms in the lung for tobramycin application under static and dynamic fluidic conditions. Eur. J. Pharm. Biopharm. 131, 120–129. doi: 10.1016/j.ejpb.2018.07.025
Fanen, P., Wohlhuter-Haddad, A., and Hinzpeter, A. (2014). Genetics of cystic fibrosis: CFTR mutation classifications toward genotype-based CF therapies. Int. J. Biochem. Cell Biol. 52, 94–102. doi: 10.1016/j.biocel.2014.02.023
Faraj, J., Bodas, M., Pehote, G., Swanson, D., Sharma, A., and Vij, N. (2019). Novel cystamine-core dendrimer-formulation rescues ΔF508-CFTR and inhibits Pseudomonas aeruginosa infection by augmenting autophagy. Expert Opin. Drug Deliv. 16, 177–186. doi: 10.1080/17425247.2019.1575807
Fielding, R. M., Lewis, R. O., and Moon-McDermott, L. (1998). Altered tissue distribution and elimination of amikacin encapsulated in unilamellar, low clearance liposomes (MiKasome®). Pharm. Res. 15, 1775–1781. doi: 10.1023/A:1011925132473
Friedlander, A. L., and Albert, R. K. (2010). Chronic macrolide therapy in inflammatory airways diseases. Chest 138, 1202–1212. doi: 10.1378/chest.10-0196
Gaspar, M. C., Couet, W., Olivier, J. C., Pais, A. A. C. C., and Sousa, J. J. S. (2013). Pseudomonas aeruginosa infection in cystic fibrosis lung disease and new perspectives of treatment: a review. Eur. J. Clin. Microbiol. Infect. Dis. 32, 1231–1252. doi: 10.1007/s10096-013-1876-y
Geller, D. E. (2009). Aerosol antibiotics in cystic fibrosis. Respir. Care 54, 658–670. doi: 10.4187/aarc0537
Geller, D. E., Flume, P. A., Staab, D., Fischer, R., Loutit, J. S., and Conrad, D. J. (2011a). Levofloxacin inhalation solution (MP-376) in patients with cystic fibrosis with Pseudomonas aeruginosa. Am. J. Respir. Crit. Care Med. 183, 1510–1516. doi: 10.1164/rccm.201008-1293OC
Geller, D. E., Rosenfeld, M., Waltz, D. A., and Wilmott, R. W. (2003). Efficiency of pulmonary administration of tobramycin solution for inhalation in cystic fibrosis using an improved drug delivery system. Chest 123, 28–36. doi: 10.1378/chest.123.1.28
Geller, D. E., Weers, J., and Heuerding, S. (2011b). Development of an inhaled dry-powder formulation of tobramycin using pulmosphereTM technology. J. Aerosol Med. Pulm. Drug Deliv. 24, 175–182. doi: 10.1089/jamp.2010.0855
Ghaffari, S., Varshosaz, J., Saadat, A., and Atyabi, F. (2011). Stability and antimicrobial effect of amikacin-loaded solid lipid nanoparticles. Int. J. Nanomed. 6, 35–43. doi: 10.2147/IJN.S13671
Grenha, A., Seijo, B., and Remuñán-López, C. (2005). Microencapsulated chitosan nanoparticles for lung protein delivery. Eur. J. Pharm. Sci. 25, 427–437. doi: 10.1016/j.ejps.2005.04.009
Griesenbach, U., and Alton, E. W. (2009). Gene transfer to the lung: lessons learned from more than 2 decades of CF gene therapy. Adv. Drug Deliv. Rev. 61, 128–139. doi: 10.1016/j.addr.2008.09.010
Griesenbach, U., and Alton, E. W. (2012). Progress in gene and cell therapy for cystic fibrosis lung disease. Curr. Pharm. Des. 18, 642–662. doi: 10.2174/138161212799315993
Griesenbach, U., and Alton, E. W. (2013). Moving forward: cystic fibrosis gene therapy. Hum. Mol. Genet. 22, R52–R58. doi: 10.1093/hmg/ddt372
Grillon, A., Schramm, F., Kleinberg, M., and Jehl, F. (2016). Comparative activity of ciprofloxacin, levofloxacin and moxifloxacin against Klebsiella pneumoniae, Pseudomonas aeruginosa and Stenotrophomonas maltophilia assessed by minimum inhibitory concentrations and time-kill studies. PLoS ONE 11:e0156690. doi: 10.1371/journal.pone.0156690
Guan, S., Munder, A., Hedtfeld, S., Braubach, P., Glage, S., Zhang, L., et al. (2019). Self-assembled peptide–poloxamine nanoparticles enable in vitro and in vivo genome restoration for cystic fibrosis. Nat. Nanotechnol. 14, 287–297. doi: 10.1038/s41565-018-0358-x
Gubernator, J., Drulis-Kawa, Z., Dorotkiewicz-Jach, A., Doroszkiewicz, W., and Kozubek, A. (2008). In vitro antimicrobial activity of liposomes containing ciprofloxacin,meropenem and gentamicin against gram-negative clinical bacterial strains. Lett. Drug Des. Discov. 4, 297–304. doi: 10.2174/157018007784620040
Guggino, W. B., and Cebotaru, L. (2017). Adeno-associated virus (AAV) gene therapy for cystic fibrosis: current barriers and recent developments. Expert Opin. Biol. Ther. 17, 1265–1273. doi: 10.1080/14712598.2017.1347630
Günday Türeli, N., Torge, A., Juntke, J., Schwarz, B. C., Schneider-Daum, N., Türeli, A. E., et al. (2017). Ciprofloxacin-loaded PLGA nanoparticles against cystic fibrosis P. aeruginosa lung infections. Eur. J. Pharm. Biopharm. 117, 363–371. doi: 10.1016/j.ejpb.2017.04.032
Hadinoto, K., Phanapavudhikul, P., Kewu, Z., and Tan, R. B. H. (2007). Dry powder aerosol delivery of large hollow nanoparticulate aggregates as prospective carriers of nanoparticulate drugs: effects of phospholipids. Int. J. Pharm. 333, 187–198. doi: 10.1016/j.ijpharm.2006.10.009
Halfhide, C., Evans, H. J., and Couriel, J. (2016). Inhaled bronchodilators for cystic fibrosis. Cochrane Database Syst. Rev. 2016:CD003428. doi: 10.1002/14651858.CD003428.pub3
Halwani, M., Mugabe, C., Azghani, A. O., Lafrenie, R. M., Kumar, A., and Omri, A. (2007). Bactericidal efficacy of liposomal aminoglycosides against Burkholderia cenocepacia. J. Antimicrob. Chemother. 60, 760–769. doi: 10.1093/jac/dkm289
Halwani, M., Yebio, B., Suntres, Z. E., Alipour, M., Azghani, A. O., and Omri, A. (2008). Co-encapsulation of gallium with gentamicin in liposomes enhances antimicrobial activity of gentamicin against Pseudomonas aeruginosa. J. Antimicrob. Chemother. 62, 1291–1297. doi: 10.1093/jac/dkn422
Hamed, K., Conti, V., Tian, H., and Loefroth, E. (2017). Adherence to tobramycin inhaled powder vs inhaled solution in patients with cystic fibrosis: analysis of US insurance claims data. Patient Prefer. Adherence 11, 831–838. doi: 10.2147/PPA.S134759
Haq, I. J., Parameswaran, M. C., Abidin, N. Z., Socas, A., Gonzalez-Ciscar, A., Gardner, A. I., et al. (2019). Modulator therapies for cystic fibrosis. Paediatr. Child Heal. 29, 151–157. doi: 10.1016/j.paed.2019.01.011
Haque, A. K. M. A., Dewerth, A., Antony, J. S., Riethmüller, J., Schweizer, G. R., Weinmann, P., et al. (2018). Chemically modified hCFTR mRNAs recuperate lung function in a mouse model of cystic fibrosis. Sci. Rep. 8:16776. doi: 10.1038/s41598-018-34960-0
Heijerman, H., Westerman, E., Conway, S., and Touw, D. (2009). Inhaled medication and inhalation devices for lung disease in patients with cystic fibrosis: a European consensus. J. Cyst. Fibros. 8, 295–315. doi: 10.1016/j.jcf.2009.04.005
Heirali, A. A., Acosta, N., Storey, D. G., Workentine, M. L., Somayaji, R., Laforest-Lapointe, I., et al. (2019). The effects of cycled inhaled aztreonam on the cystic fibrosis (CF) lung microbiome. J. Cyst. Fibros. 18, 829–837. doi: 10.1016/j.jcf.2019.02.010
Henke, M. O., and Ratjen, F. (2007). Mucolytics in cystic fibrosis. Paediatr. Respir. Rev. 8, 24–29. doi: 10.1016/j.prrv.2007.02.009
Høiby, N. (2011). Recent advances in the treatment of Pseudomonas aeruginosa infections in cystic fibrosis. BMC Med. 9:32. doi: 10.1186/1741-7015-9-32
Hsu, P. D., Lander, E. S., and Zhang, F. (2014). Development and applications of CRISPR-Cas9 for genome engineering. Cell 157, 1262–1278. doi: 10.1016/j.cell.2014.05.010
Ibrahim, B. M., Park, S., Han, B., and Yeo, Y. (2011a). A strategy to deliver genes to cystic fibrosis lungs: a battle with environment. J. Control. Rel. 155, 289–295. doi: 10.1016/j.jconrel.2011.07.039
Ibrahim, B. M., Tsifansky, M. D., Yang, Y., and Yeo, Y. (2011b). Challenges and advances in the development of inhalable drug formulations for cystic fibrosis lung disease. Expert Opin. Drug Deliv. 8, 451–466. doi: 10.1517/17425247.2011.561310
Jain, D., and Banerjee, R. (2008). Comparison of ciprofloxacin hydrochloride-loaded protein, lipid, and chitosan nanoparticles for drug delivery. J. Biomed. Mater. Res. B Appl. Biomater. 86, 105–112. doi: 10.1002/jbm.b.30994
Joshi, M., and Misra, A. N. (2001). Pulmonary disposition of budesonide from liposomal dry powder inhaler. Methods Find. Exp. Clin. Pharmacol. 23, 531–536. doi: 10.1358/mf.2001.23.10.677118
Joshi, M. D., and Müller, R. H. (2009). Lipid nanoparticles for parenteral delivery of actives. Eur. J. Pharm. Biopharm. 71, 161–172. doi: 10.1016/j.ejpb.2008.09.003
Kaminskas, L. M., McLeod, V. M., Ryan, G. M., Kelly, B. D., Haynes, J. M., Williamson, M., et al. (2014). Pulmonary administration of a doxorubicin-conjugated dendrimer enhances drug exposure to lung metastases and improves cancer therapy. J. Control. Release 183, 18–26. doi: 10.1016/j.jconrel.2014.03.012
Kho, K., Cheow, W. S., Lie, R. H., and Hadinoto, K. (2010). Aqueous re-dispersibility of spray-dried antibiotic-loaded polycaprolactone nanoparticle aggregates for inhaled anti-biofilm therapy. Powder Technol. 203, 432–439. doi: 10.1016/j.powtec.2010.06.003
Kho, K., and Hadinoto, K. (2010). Aqueous re-dispersibility characterization of spray-dried hollow spherical silica nano-aggregates. Powder Technol. 198, 354–363. doi: 10.1016/j.powtec.2009.11.031
Kirkby, S., Novak, K., and McCoy, K. (2011). Aztreonam (for inhalation solution) for the treatment of chronic lung infections in patients with cystic fibrosis: an evidence-based review. Core Evid. 6, 59–66. doi: 10.2147/CE.S11181
Kłodzinska, S. N., Molchanova, N., Franzyk, H., Hansen, P. R., Damborg, P., and Nielsen, H. M. (2018). Biopolymer nanogels improve antibacterial activity and safety profile of a novel lysine-based α-peptide/β-peptoid peptidomimetic. Eur. J. Pharm. Biopharm. 128, 1–9. doi: 10.1016/j.ejpb.2018.03.012
Kohler, D., Schneider, M., Krüger, M., Lehr, C. M., Möhwald, H., and Wang, D. (2011). Template-assisted polyelectrolyte encapsulation of nanoparticles into dispersible, hierarchically nanostructured microfibers. Adv. Mater. 23, 1376–1379. doi: 10.1002/adma.201004048
Konstan, M. W., and Davis, P. B. (2002). Pharmacological approaches for the discovery and development of new anti-inflammatory agents for the treatment of cystic fibrosis. Adv. Drug Deliv. Rev. 54, 1409–1423. doi: 10.1016/S0169-409X(02)00146-1
Kuzmov, A., and Minko, T. (2015). Nanotechnology approaches for inhalation treatment of lung diseases. J. Control. Release 219, 500–518. doi: 10.1016/j.jconrel.2015.07.024
Kym, P. R., Wang, X., Pizzonero, M., and Van der Plas, S. E. (2018). Recent progress in the discovery and development of small-molecule modulators of CFTR. Prog. Med. Chem. 57, 235–276. doi: 10.1016/bs.pmch.2018.01.001
Leal, J., Dong, T., Taylor, A., Siegrist, E., Gao, F., Smyth, H. D. C., et al. (2018). Mucus-penetrating phage-displayed peptides for improved transport across a mucus-like model. Int. J. Pharm. 553, 57–64. doi: 10.1016/j.ijpharm.2018.09.055
Lebhardt, T., Roesler, S., Uusitalo, H. P., and Kissel, T. (2011). Surfactant-free redispersible nanoparticles in fast-dissolving composite microcarriers for dry-powder inhalation. Eur. J. Pharm. Biopharm. 78, 90–96. doi: 10.1016/j.ejpb.2010.12.002
Lim, K. R. Q., Yoon, C., and Yokota, T. (2018). Applications of CRISPR/Cas9 for the treatment of duchenne muscular dystrophy. J. Pers. Med. 8:E38. doi: 10.20944/preprints201811.0018.v1
Lim, L. M., Ly, N., Anderson, D., Yang, J. C., Macander, L., Jarkowski, A., et al. (2010). Resurgence of colistin: a review of resistance, toxicity, pharmacodynamics, and dosing. Pharmacotherapy 30, 1279–1291. doi: 10.1592/phco.30.12.1279
Littlewood, K. J., Higashi, K., Jansen, J. P., Capkun-Niggli, G., Balp, M. M., Doering, G., et al. (2012). A network meta-analysis of the efficacy of inhaled antibiotics for chronic Pseudomonas infections in cystic fibrosis. J. Cyst. Fibros. 11, 419–426. doi: 10.1016/j.jcf.2012.03.010
Liu, M., Zhang, J., Shan, W., and Huang, Y. (2014). Developments of mucus penetrating nanoparticles. Asian J. Pharm. Sci. 10, 275–282. doi: 10.1016/j.ajps.2014.12.007
Lopes-Pacheco, M. (2016). CFTR modulators: Shedding light on precision medicine for cystic fibrosis. Front. Pharmacol. 7:275. doi: 10.3389/fphar.2016.00275
Lu, H. D., Pearson, E., Ristroph, K. D., Duncan, G. A., Ensign, L. M., Suk, J. S., et al. (2018). Pseudomonas aeruginosa pyocyanin production reduced by quorum-sensing inhibiting nanocarriers. Int. J. Pharm. 544, 75–82. doi: 10.1016/j.ijpharm.2018.03.058
Maiuri, L., Raia, V., and Kroemer, G. (2017). Strategies for the etiological therapy of cystic fibrosis. Cell Death Differ. 24, 1825–1844. doi: 10.1038/cdd.2017.126
Manunta, M. D. I., Tagalakis, A. D., Attwood, M., Aldossary, A. M., Barnes, J. L., Munye, M. M., et al. (2017). Delivery of ENaC siRNA to epithelial cells mediated by a targeted nanocomplex: a therapeutic strategy for cystic fibrosis. Sci. Rep. 7:700. doi: 10.1038/s41598-017-00662-2
Maselli, D. J., Keyt, H., and Restrepo, M. I. (2017). Inhaled antibiotic therapy in chronic respiratory diseases. Int. J. Mol. Sci. 18:E1062. doi: 10.3390/ijms18051062
Maule, G., Casini, A., Montagna, C., Ramalho, A. S., De Boeck, K., Debyser, Z., et al. (2019). Allele specific repair of splicing mutations in cystic fibrosis through AsCas12a genome editing. Nat. Commun. 10, 1–11. doi: 10.1038/s41467-019-11454-9
McKiernan, P. J., Cunningham, O., Greene, C. M., and Cryan, S. A. (2013). Targeting miRNA-based medicines to cystic fibrosis airway epithelial cells using nanotechnology. Int. J. Nanomed. 8, 3907–3915. doi: 10.2147/IJN.S47551
McNeer, N. A., Anandalingam, K., Fields, R. J., Caputo, C., Kopic, S., Gupta, A., et al. (2015). Nanoparticles that deliver triplex-forming peptide nucleic acid molecules correct F508del CFTR in airway epithelium. Nat. Commun. 6:6952. doi: 10.1038/ncomms7952
Meers, P., Neville, M., Malinin, V., Scotto, A. W., Sardaryan, G., Kurumunda, R., et al. (2008). Biofilm penetration, triggered release and in vivo activity of inhaled liposomal amikacin in chronic Pseudomonas aeruginosa lung infections. J. Antimicrob. Chemother. 61, 859–868. doi: 10.1093/jac/dkn059
Mehnert, W., and Mäder, K. (2012). Solid lipid nanoparticles: production, characterization and applications. Adv. Drug Deliv. Rev. 64, 83–101. doi: 10.1016/j.addr.2012.09.021
Mehta, P., Kadam, S., Pawar, A., and Bothiraja, C. (2019). Dendrimers for pulmonary delivery: current perspectives and future challenges. New J. Chem. 43, 8396–8409. doi: 10.1039/C9NJ01591D
Menjoge, A. R., Kannan, R. M., and Tomalia, D. A. (2010). Dendrimer-based drug and imaging conjugates: design considerations for nanomedical applications. Drug Discov. Today 15, 171–185. doi: 10.1016/j.drudis.2010.01.009
Messiaen, A. S., Forier, K., Nelis, H., Braeckmans, K., and Coenye, T. (2013). Transport of nanoparticles and tobramycin-loaded liposomes in Burkholderia cepacia complex biofilms. PLoS ONE 8:e79220. doi: 10.1371/journal.pone.0079220
Miah, K. M., Hyde, S. C., and Gill, D. R. (2019). Emerging gene therapies for cystic fibrosis. Expert Rev. Respir. Med. 13, 709–725. doi: 10.1080/17476348.2019.1634547
Middleton, P. G., Caplen, N. J., Gao, X., Huang, L., Gaya, H., Geddes, D. M., et al. (1994). Nasal application of the cationic liposome DC-Chol:DOPE does not alter ion transport, lung function or bacterial growth. Eur. Respir. J. 7, 442–445. doi: 10.1183/09031936.94.07030442
Miragoli, M., Ceriotti, P., Iafisco, M., Vacchiano, M., Salvarani, N., Alogna, A., et al. (2018). Inhalation of peptide-loaded nanoparticles improves heart failure. Sci. Transl. Med. 10:eaan6205. doi: 10.1126/scitranslmed.aan6205
Mirakhur, A., Gallagher, M. J., Ledson, M. J., Hart, C. A., and Walshaw, M. J. (2003). Fosfomycin therapy for multiresistant Pseudomonas aeruginosa in cystic fibrosis. J. Cyst. Fibros 2, 19–24. doi: 10.1016/S1569-1993(02)00143-1
Misra, A., Jinturkar, K., Patel, D., Lalani, J., and Chougule, M. (2009). Recent advances in liposomal dry powder formulations: Preparation and evaluation. Expert Opin. Drug Deliv. 6, 71–89. doi: 10.1517/17425240802652309
Moreau-Marquis, S., Stanton, B. A., and O'Toole, G. A. (2008). Pseudomonas aeruginosa biofilm formation in the cystic fibrosis airway. Pulm. Pharmacol. Ther. 21, 595–599. doi: 10.1016/j.pupt.2007.12.001
Moreno-Sastre, M., Pastor, M., Salomon, C. J., Esquisabel, A., and Pedraz, J. L. (2015). Pulmonary drug delivery: a review on nanocarriers for antibacterial chemotherapy. J. Antimicrob. Chemother. 70, 2945–2955. doi: 10.1093/jac/dkv192
Moss, R. B., Milla, C., Colombo, J., Accurso, F., Zeitlin, P. L., Clancy, J. P., et al. (2007). Repeated aerosolized AAV-CFTR for treatment of cystic fibrosis: a randomized placebo-controlled phase 2B trial. Hum. Gene Ther. 18, 726–732. doi: 10.1089/hum.2007.022
Moss, R. B., Rodman, D., Spencer, L. T., Aitken, M. L., Zeitlin, P. L., Waltz, D., et al. (2004). Repeated adeno-associated virus serotype 2 aerosol-mediated cystic fibrosis transmembrane regulator gene transfer to the lungs of patients with cystic fibrosis: a multicenter, double-blind, placebo-controlled trial. Chest 125, 509–521. doi: 10.1378/chest.125.2.509
Mugabe, C., Azghani, A. O., and Omri, A. (2005). Liposome-mediated gentamicin delivery: development and activity against resistant strains of Pseudomonas aeruginosa isolated from cystic fibrosis patients. J. Antimicrob. Chemother. 55, 269–271. doi: 10.1093/jac/dkh518
Mugabe, C., Halwani, M., Azghani, A. O., Lafrenie, R. M., and Omri, A. (2006). Mechanism of enhanced activity of liposome-entrapped aminoglycosides against resistant strains of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 50, 2016–2022. doi: 10.1128/AAC.01547-05
Müller, R. H., Mäder, K., and Gohla, S. (2000). Solid lipid nanoparticles (SLN) for controlled drug delivery - a review of the state of the art. Eur. J. Pharm. Biopharm. 50, 161–177. doi: 10.1016/S0939-6411(00)00087-4
Müller, R. H., Radtke, M., and Wissing, S. A. (2002). Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 54(Suppl 1):S131–155. doi: 10.1016/S0169-409X(02)00118-7
Müller, R. H., Runge, S., Ravelli, V., Mehnert, W., Thünemann, A. F., and Souto, E. B. (2006). Oral bioavailability of cyclosporine: solid lipid nanoparticles (SLN®) versus drug nanocrystals. Int. J. Pharm. 317, 82–89. doi: 10.1016/j.ijpharm.2006.02.045
Muralidharan, P., Malapit, M., Mallory, E., Hayes, D., and Mansour, H. M. (2015). Inhalable nanoparticulate powders for respiratory delivery. Nanomed. Nanotechnol. Biol. Med. 11, 1189–1199. doi: 10.1016/j.nano.2015.01.007
Mutyam, V., Du, M., Xue, X., Keeling, K. M., Lucile White, E., Robert Bostwick, J., et al. (2016). Discovery of clinically approved agents that promote suppression of cystic fibrosis transmembrane conductance regulator nonsense mutations. Am. J. Respir. Crit. Care Med. 194, 1092–1103. doi: 10.1164/rccm.201601-0154OC
Newhouse, M. T., Hirst, P. H., Duddu, S. P., Walter, Y. H., Tarara, T. E., Clark, A. R., et al. (2003). Inhalation of a dry powder tobramycin pulmosphere formulation in healthy volunteers. Chest 124, 360–366. doi: 10.1378/chest.124.1.360
Okusanya, Ó. O., Bhavnani, S. M., Hammel, J., Minic, P., Dupont, L. J., Forrest, A., et al. (2009). Pharmacokinetic and pharmacodynamic evaluation of liposomal amikacin for inhalation in cystic fibrosis patients with chronic pseudomonal infection. Antimicrob. Agents Chemother. 53, 3847–3854. doi: 10.1128/AAC.00872-08
Omri, A., Beaulac, C., Bouhajib, M., Montplaisir, S., Sharkawi, M., and Lagace, J. (1994). Pulmonary retention of free and liposome-encapsulated tobramycin after intratracheal administration in uninfected rats and rats infected with Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 38, 1090–1095. doi: 10.1128/AAC.38.5.1090
Omri, A., Suntres, Z. E., and Shek, P. N. (2002). Enhanced activity of liposomal polymyxin B against Pseudomonas aeruginosa in a rat model of lung infection. Biochem. Pharmacol. 64, 1407–1413. doi: 10.1016/S0006-2952(02)01346-1
Ong, V., Mei, V., Cao, L., Lee, K., and Chung, E. J. (2019). Nanomedicine for Cystic Fibrosis. SLAS Technol. 24, 169–180. doi: 10.1177/2472630318824334
Osman, R., Al Jamal, K. T., Kan, P. L., Awad, G., Mortada, N., EL-Shamy, A. E., et al. (2013). Inhalable DNase I microparticles engineered with biologically active excipients. Pulm. Pharmacol. Ther. 26, 700–709. doi: 10.1016/j.pupt.2013.07.010
O'Sullivan, B. P., and Freedman, S. D. (2009). Cystic fibrosis. Lancet 373, 1891–1904. doi: 10.1016/S0140-6736(09)60327-5
Paranjape, S. M., and Mogayzel, P. J. (2018). Cystic fibrosis in the era of precision medicine. Paediatr. Respir. Rev. 25, 64–72. doi: 10.1016/j.prrv.2017.03.001
Paranjpe, M., and Müller-Goymann, C. C. (2014). Nanoparticle-mediated pulmonary drug delivery: a review. Int. J. Mol. Sci. 15, 5852–5873. doi: 10.3390/ijms15045852
Pardeike, J., Hommoss, A., and Müller, R. H. (2009). Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm. 366, 170–184. doi: 10.1016/j.ijpharm.2008.10.003
Perry, L. A., Penny-Dimri, J. C., Aslam, A. A., Lee, T. W., and Southern, K. W. (2017). Topical cystic fibrosis transmembrane conductance regulator gene replacement for cystic fibrosis-related lung disease. Paediatr. Respir. Rev. 22, 47–49. doi: 10.1016/j.prrv.2016.10.005
Pettit, R. S. (2012). Los medicamentos modificadores de la regulación de la conducción de la fibrosis quística a través de la membrana (CFTR): el futuro del tratamiento de la fibrosis quística. Ann. Pharmacother. 46, 1065–1075. doi: 10.1345/aph.1R076
Pilcer, G., Goole, J., Van Gansbeke, B., Blocklet, D., Knoop, C., Vanderbist, F., et al. (2008). Pharmacoscintigraphic and pharmacokinetic evaluation of tobramycin DPI formulations in cystic fibrosis patients. Eur. J. Pharm. Biopharm. 68, 413–421. doi: 10.1016/j.ejpb.2007.05.005
Pilcer, G., Sebti, T., and Amighi, K. (2006). Formulation and characterization of lipid-coated tobramycin particles for dry powder inhalation. Pharm. Res. 23, 931–940. doi: 10.1007/s11095-006-9789-4
Pinto, J. F., and Müller, R. H. (1999). Pellets as carriers of solid lipid nanoparticles (SLN) for oral administration of drugs. Pharmazie 54, 506–509.
Pompilio, A., Geminiani, C., Bosco, D., Rana, R., Aceto, A., Bucciarelli, T., et al. (2018). Electrochemically synthesized silver nanoparticles are active against planktonic and biofilm cells of Pseudomonas aeruginosa and other cystic fibrosis-associated bacterial pathogens. Front. Microbiol. 9:1349. doi: 10.3389/fmicb.2018.01349
Ponzano, S., Nigrelli, G., Fregonese, L., Eichler, I., Bertozzi, F., Bandiera, T., et al. (2018). A European regulatory perspective on cystic fibrosis: current treatments, trends in drug development and translational challenges for CFTR modulators. Eur. Respir. Rev. 27:170124. doi: 10.1183/16000617.0124-2017
Porsio, B., Craparo, E. F., Mauro, N., Giammona, G., and Cavallaro, G. (2018). Mucus and cell-penetrating nanoparticles embedded in nano-into-micro formulations for pulmonary delivery of ivacaftor in patients with cystic fibrosis. ACS Appl. Mater. Interfaces 10, 165–181. doi: 10.1021/acsami.7b14992
Porteous, D. J., Dorin, J. R., McLachlan, G., Davidson-Smith, H., Davidson, H., Stevenson, B. J., et al. (1997). Evidence for safety and efficacy of DOTAP cationic liposome mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis. Gene Ther. 4, 210–218. doi: 10.1038/sj.gt.3300390
Pranke, I., Golec, A., Hinzpeter, A., Edelman, A., and Sermet-Gaudelus, I. (2019). Emerging therapeutic approaches for cystic fibrosis. From gene editing to personalized medicine. Front. Pharmacol. 10:121. doi: 10.3389/fphar.2019.00121
Rafeeq, M. M., and Murad, H. A. S. (2017). Cystic fibrosis: current therapeutic targets and future approaches. J. Transl. Med. 15:84. doi: 10.1186/s12967-017-1193-9
Ramsey, B. W. (1996). Management of pulmonary disease in patients with cystic fibrosis. N. Engl. J. Med. 335, 179–188. doi: 10.1056/NEJM199607183350307
Ramsey, B. W., Davies, J., McElvaney, N. G., Tullis, E., Bell, S. C., Drevínek, P., et al. (2011). A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N. Engl. J. Med. 365, 1663–1672. doi: 10.1056/NEJMoa1105185
Ran, F. A., Hsu, P. D., Wright, J., Agarwala, V., Scott, D. A., and Zhang, F. (2013). Genome engineering using the CRISPR-Cas9 system HHS Public Access. Nat Protoc. 8, 2281–2308. doi: 10.1038/nprot.2013.143
Ratjen, F. A. (2009). Cystic fibrosis: pathogenesis and future treatment strategies. Respir. Care 54, 595–560. doi: 10.4187/aarc0427
Ricciardi, A. S., Bahal, R., Farrelly, J. S., Quijano, E., Bianchi, A. H., Luks, V. L., et al. (2018). In utero nanoparticle delivery for site-specific genome editing. Nat. Commun. 9:2481. doi: 10.1038/s41467-018-04894-2
Richard-Fiardo, P., Hervouet, C., Marsault, R., Franken, P. R., Cambien, B., Guglielmi, J., et al. (2015). Evaluation of tetrafunctional block copolymers as synthetic vectors for lung gene transfer. Biomaterials 45, 10–17. doi: 10.1016/j.biomaterials.2014.12.051
Robinson, E., MacDonald, K. D., Slaughter, K., McKinney, M., Patel, S., Sun, C., et al. (2018). Lipid nanoparticle-delivered chemically modified mRNA restores chloride secretion in cystic fibrosis. Mol. Ther. 26, 2034–2046. doi: 10.1016/j.ymthe.2018.05.014
Rolland, O., Turrin, C. O., Caminade, A. M., and Majoral, J. P. (2009). Dendrimers and nanomedicine: multivalency in action. New J. Chem. 33, 1809–1824. doi: 10.1039/b901054h
Rowe, S. M., Miller, S., and Sorscher, E. J. (2005). Mechanisms of disease cystic fibrosis. N. Engl. J. Med. 352, 1992–2001. doi: 10.1056/NEJMra043184
Rudokas, M., Najlah, M., Alhnan, M. A., and Elhissi, A. (2016). Liposome delivery systems for inhalation: a critical review highlighting formulation issues and anticancer applications. Med. Princip. Pract. 25(Suppl. 2), 60–72. doi: 10.1159/000445116
Ruge, C. A., Bohr, A., Beck-Broichsitter, M., Nicolas, V., Tsapis, N., and Fattal, E. (2016). Disintegration of nano-embedded microparticles after deposition on mucus: a mechanistic study. Colloids Surfaces B Biointerfaces 139, 219–227. doi: 10.1016/j.colsurfb.2015.12.017
Rukholm, G., Mugabe, C., Azghani, A. O., and Omri, A. (2006). Antibacterial activity of liposomal gentamicin against Pseudomonas aeruginosa: a time-kill study. Int. J. Antimicrob. Agents 27, 247–252. doi: 10.1016/j.ijantimicag.2005.10.021
Sachetelli, S., Beaulac, C., Riffon, R., and Lagacé, J. (1999). Evaluation of the pulmonary and systemic immunogenicity of Fluidosomes, a fluid liposomal-tobramycin formulation for the treatment of chronic infections in lungs. Biochim. Biophys. Acta 1428, 334–340. doi: 10.1016/S0304-4165(99)00078-1
Sachetelli, S., Khalil, H., Chen, T., Beaulac, C., Sénéchal, S., and Lagacé, J. (2000). Demonstration of a fusion mechanism between a fluid bactericidal liposomal formulation and bacterial cells. Biochim. Biophys. Acta 1463, 254–266. doi: 10.1016/S0005-2736(99)00217-5
Savla, R., and Minko, T. (2013). Nanotechnology approaches for inhalation treatment of fibrosis. J. Drug Target 21, 914–925. doi: 10.3109/1061186X.2013.829078
Schiffelers, R. M., Storm, G., Ten Kate, M. T., Stearne-Cullen, L. E. T., Den Hollander, J. G., Verbrugh, H. A., et al. (2001). In vivo synergistic interaction of liposome-coencapsulated gentamicin and ceftazidime. J. Pharmacol. Exp. Ther. 298, 369–375.
Schuster, A., Haliburn, C., Döring, G., and Goldman, M. H. (2013). Safety, efficacy and convenience of colistimethate sodium dry powder for inhalation (Colobreathe DPI) in patients with cystic fibrosis: a randomised study. Thorax 68, 344–350. doi: 10.1136/thoraxjnl-2012-202059
Schwank, G., Koo, B. K., Sasselli, V., Dekkers, J. F., Heo, I., Demircan, T., et al. (2013). Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell 13, 653–658. doi: 10.1016/j.stem.2013.11.002
Schwarz, C. (2015). Colobreathe® for the treatment of cystic fibrosis-associated pulmonary infections. Pulm. Ther. 1, 19–30. doi: 10.1007/s41030-015-0007-6
Sepe, A., Villella, V. R., Cimbalo, C., Castaldo, A., Nunziata, F., Corcione, A., et al. (2019). Inhaled medications in cystic fibrosis beyond antibiotics. Minerva Pediatr. 71, 371–375. doi: 10.23736/S0026-4946.19.05509-9
Sham, J. O. H., Zhang, Y., Finlay, W. H., Roa, W. H., and Löbenberg, R. (2004). Formulation and characterization of spray-dried powders containing nanoparticles for aerosol delivery to the lung. Int. J. Pharm. 269, 457–467. doi: 10.1016/j.ijpharm.2003.09.041
Shteinberg, M., and Elborn, J. S. (2015). Use of inhaled tobramycin in cystic fibrosis. Adv. Ther. 32, 1–9. doi: 10.1007/s12325-015-0179-3
Smith, P. L. (1997). Peptide delivery via the pulmonary route: a valid approach to local and systemic delivery. J. Control. Release 46, 99–106. doi: 10.1016/S0168-3659(96)01579-9
Smyth, A. R., Bell, S. C., Bojcin, S., Bryon, M., Duff, A., Flume, P., et al. (2014). European cystic fibrosis society standards of care: best practice guidelines. J. Cyst. Fibros. 13, S23–S42. doi: 10.1016/j.jcf.2014.03.010
Somayaji, R., and Parkins, M. D. (2015). Tobramycin inhalation powder: an efficient and efficacious therapy for the treatment of Pseudomonas aeruginosa infection in cystic fibrosis. Ther. Deliv. 6, 121–137. doi: 10.4155/tde.14.94
Spoletini, G., Kennedy, M., Flint, L., Graham, T., Etherington, C., Shaw, N., et al. (2018). Intravenous fosfomycin for pulmonary exacerbation of cystic fibrosis: real life experience of a large adult CF centre. Pulm. Pharmacol. Ther. 50, 82–87. doi: 10.1016/j.pupt.2018.04.007
Stass, H., Nagelschmitz, J., Willmann, S., Delesen, H., Gupta, A., and Baumann, S. (2013a). Inhalation of a dry powder ciprofloxacin formulation in healthy subjects: a phase i study. Clin. Drug Investig. 33, 419–427. doi: 10.1007/s40261-013-0082-0
Stass, H., Weimann, B., Nagelschmitz, J., Rolinck-Werninghaus, C., and Staab, D. (2013b). Tolerability and pharmacokinetic properties of ciprofloxacin dry powder for inhalation in patients with cystic fibrosis: a phase I, randomized, dose-escalation study. Clin. Ther. 35, 1571–1581. doi: 10.1016/j.clinthera.2013.08.003
Stern, M., Ulrich, K., Geddes, D. M., and Alton, E. W. F. W. (2003). Poly (D, L-lactide-co-glycolide)/DNA microspheres to facilitate prolonged transgene expression in airway epithelium in vitro, ex vivo and in vivo. Gene Ther. 10, 1282–1288. doi: 10.1038/sj.gt.3301994
Storm, D. R., Rosenthal, K. S., and Swanson, P. E. (1977). Polymyxin and related peptide antibiotics. Annu. Rev. Biochem. 46, 723–763. doi: 10.1146/annurev.bi.46.070177.003451
Suk, J. S., Kim, A. J., Trehan, K., Schneider, C. S., Cebotaru, L., Woodward, O. M., et al. (2014). Lung gene therapy with highly compacted DNA nanoparticles that overcome the mucus barrier. J. Control. Release 178, 8–17. doi: 10.1016/j.jconrel.2014.01.007
Suk, J. S., Lai, S. K., Boylan, N. J., Dawson, M. R., Boyle, M. P., and Hanes, J. (2011). Rapid transport of muco-inert nanoparticles in cystic fibrosis sputum treated with N-acetyl cysteine. Nanomedicine 6, 365–375. doi: 10.2217/nnm.10.123
Suk, J. S., Lai, S. K., Wang, Y. Y., Ensign, L. M., Zeitlin, P. L., Boyle, M. P., et al. (2009). The penetration of fresh undiluted sputum expectorated by cystic fibrosis patients by non-adhesive polymer nanoparticles. Biomaterials 30, 2591–2597. doi: 10.1016/j.biomaterials.2008.12.076
Sung, J. C., Pulliam, B. L., and Edwards, D. A. (2007). Nanoparticles for drug delivery to the lungs. Trends Biotechnol. 25, 563–570. doi: 10.1016/j.tibtech.2007.09.005
Tagalakis, A. D., Munye, M. M., Ivanova, R., Chen, H., Smith, C. M., Aldossary, A. M., et al. (2018). Effective silencing of ENaC by siRNA delivered with epithelial-targeted nanocomplexes in human cystic fibrosis cells and in mouse lung. Thorax 73, 847–856. doi: 10.1136/thoraxjnl-2017-210670
Taghavi Pourianazar, N., Mutlu, P., and Gunduz, U. (2014). Bioapplications of poly(amidoamine) (PAMAM) dendrimers in nanomedicine. J. Nanoparticle Res. 16:2342. doi: 10.1007/s11051-014-2342-1
Taylor-Cousar, J. L., Mall, M. A., Ramsey, B. W., McKone, E. F., Tullis, E., Marigowda, G., et al. (2019). Clinical development of triple-combination CFTR modulators for cystic fibrosis patients with one or two F508del alleles. ERJ Open Res. 5, 00082–02019. doi: 10.1183/23120541.00082-2019
Thompson, K. (2016). Conventional therapies and emergent precision medicines for cystic fibrosis: challenges and opportunities. Clin. Pharm. 8, 1–20. doi: 10.1211/CP.2016.20201932
Tomalia, D. A., Baker, H., Dewald, J., Hall, M., Kallos, G., Martin, S., et al. (1985). A new class of polymers: starburst-dendritic macromolecules. Polym. J. 17, 117–132. doi: 10.1295/polymj.17.117
Tomoda, K., Ohkoshi, T., Kawai, Y., Nishiwaki, M., Nakajima, T., and Makino, K. (2008). Preparation and properties of inhalable nanocomposite particles: effects of the temperature at a spray-dryer inlet upon the properties of particles. Colloids Surf. B Biointerfaces 61, 138–144. doi: 10.1016/j.colsurfb.2007.07.016
Tosco, A., De Gregorio, F., Esposito, S., De Stefano, D., Sana, I., Ferrari, E., et al. (2016). A novel treatment of cystic fibrosis acting on-target: cysteamine plus epigallocatechin gallate for the autophagy-dependent rescue of class II-mutated CFTR. Cell Death Differ. 23, 1380–1393. doi: 10.1038/cdd.2016.22
Tosco, A., Villella, V. R., Castaldo, A., Kroemer, G., Maiuri, L., and Raia, V. (2018). Repurposing therapies for the personalised treatment of cystic fibrosis. Expert Opin. Orphan Drugs 6, 361–373. doi: 10.1080/21678707.2018.1483231
Trapnell, B. C., McColley, S. A., Kissner, D. G., Rolfe, M. W., Rosen, J. M., McKevitt, M., et al. (2012). Fosfomycin/tobramycin for inhalation in patients with cystic fibrosis with Pseudomonas airway infection. Am. J. Respir. Crit. Care Med. 185, 171–178. doi: 10.1164/rccm.201105-0924OC
Tsapis, N., Bennett, D., Jackson, B., Weitz, D. A., and Edwards, D. A. (2002). Trojan particles: Large porous carriers of nanoparticles for drug delivery. Proc. Natl. Acad. Sci. U.S.A. 99, 12001–12005. doi: 10.1073/pnas.182233999
Tscheka, C., Hittinger, M., Lehr, C. M., Schneider-Daum, N., and Schneider, M. (2015). Macrophage uptake of cylindrical microparticles investigated with correlative microscopy. Eur. J. Pharm. Biopharm. 95, 151–155. doi: 10.1016/j.ejpb.2015.03.010
Turcios, N. L. (2005). Cystic fibrosis: an overview. J. Clin. Gastroenterol. 39, 307–317. doi: 10.1097/01.mcg.0000155140.63510.cd
Ungaro, F., D'Angelo, I., Coletta, C., D'Emmanuele Di Villa Bianca, R., Sorrentino, R., Perfetto, B., et al. (2012a). Dry powders based on PLGA nanoparticles for pulmonary delivery of antibiotics: modulation of encapsulation efficiency, release rate and lung deposition pattern by hydrophilic polymers. J. Control. Release 157, 149–159. doi: 10.1016/j.jconrel.2011.08.010
Ungaro, F., D'Angelo, I., Miro, A., La Rotonda, M. I., and Quaglia, F. (2012b). Engineered PLGA nano- and micro-carriers for pulmonary delivery: challenges and promises. J. Pharm. Pharmacol. 64, 1217–1235. doi: 10.1111/j.2042-7158.2012.01486.x
Van Rijt, S. H., Bein, T., and Meiners, S. (2014). Medical nanoparticles for next generation drug delivery to the lungs. Eur. Respir. J. 44, 765–774. doi: 10.1183/09031936.00212813
Van Westreenen, M., and Tiddens, H. A. W. M. (2010). New antimicrobial strategies in cystic fibrosis. Pediatr. Drugs 12, 343–352. doi: 10.2165/11316240-000000000-00000
VanDevanter, D. R., and Geller, D. E. (2011). Tobramycin administered by the TOBI® Podhaler® for persons with cystic fibrosis: a review. Med. Devices Evid. Res. 4, 179–188. doi: 10.2147/MDER.S16360
Varshosaz, J., Ghaffari, S., Khoshayand, M. R., Atyabi, F., Azarmi, S., and Kobarfard, F. (2010). Development and optimization of solid lipid nanoparticles of amikacin by central composite design. J. Liposome Res. 20, 97–104. doi: 10.3109/08982100903103904
Varshosaz, J., Ghaffari, S., Mirshojaei, S. F., Jafarian, A., Atyabi, F., Kobarfard, F., et al. (2013). Biodistribution of amikacin solid lipid nanoparticles after pulmonary delivery. Biomed Res. Int. 2013:136859. doi: 10.1155/2013/136859
Vij, N., Min, T., Marasigan, R., Belcher, C. N., Mazur, S., Ding, H., et al. (2010). Development of PEGylated PLGA nanoparticle for controlled and sustained drug delivery in cystic fibrosis. J. Nanobiotechnol. 8:22. doi: 10.1186/1477-3155-8-22
Villate-Beitia, I., Puras, G., Zarate, J., Agirre, M., Ojeda, E., and Pedraz, J. L. (2015). “First insights into non-invasive administration routes for non-viral gene therapy,” in Gene Therapy – Principles and Challenges, ed D. Hashad (Croatia: InTech), 145–177.
Villate-Beitia, I., Zarate, J., Puras, G., and Pedraz, J. L. (2017). Gene delivery to the lungs: pulmonary gene therapy for cystic fibrosis. Drug Dev. Ind. Pharm. 43, 1071–1081. doi: 10.1080/03639045.2017.1298122
Wainwright, C. E., Elborn, J. S., Ramsey, B. W., Marigowda, G., Huang, X., Cipolli, M., et al. (2015). Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for phe508del CFTR. N. Engl. J. Med. 373, 220–231. doi: 10.1056/NEJMoa1409547
Wallace, S. J., Li, J., Nation, R. L., Prankerd, R. J., and Boyd, B. J. (2012). Interaction of colistin and colistin methanesulfonate with liposomes: colloidal aspects and implications for formulation. J. Pharm. Sci. 101, 3347–3359. doi: 10.1002/jps.23203
Weber, S., Zimmer, A., and Pardeike, J. (2014). Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) for pulmonary application: a review of the state of the art. Eur. J. Pharm. Biopharm. 86, 7–22. doi: 10.1016/j.ejpb.2013.08.013
Willis, L., Hayes, D., and Mansour, H. M. (2012). Therapeutic liposomal dry powder inhalation aerosols for targeted lung delivery. Lung 190, 251–262. doi: 10.1007/s00408-011-9360-x
Wise, R., and Honeybourne, D. (1999). Pharmacokinetics and pharmacodynamics of fluoroquinolones in the respiratory tract. Eur. Respir. J. 14, 221–229. doi: 10.1034/j.1399-3003.1999.14a38.x
Wissing, S. A., and Müller, R. H. (2001). Solid lipid nanoparticles (SLN) - A novel carrier for UV blockers. Pharmazie 56, 783–786.
Yang, W., Peters, J. I., and Williams, R. O. (2008). Inhaled nanoparticles-a current review. Int. J. Pharm. 356, 239–247. doi: 10.1016/j.ijpharm.2008.02.011
Young, D. C., Zobell, J. T., Stockmann, C., Waters, C. D., Ampofo, K., Sherwin, C. M. T., et al. (2013). Optimization of anti-pseudomonal antibiotics for cystic fibrosis pulmonary exacerbations: V. Aminoglycosides. Pediatr. Pulmonol. 48, 1047–1061. doi: 10.1002/ppul.22813
Zabner, J., Couture, L. A., Gregory, R. J., Graham, S. M., Smith, A. E., and Welsh, M. J. (1993). Adenovirus-mediated gene transfer transiently corrects the chloride transport defect in nasal epithelia of patients with cystic fibrosis. Cell 75, 207–216. doi: 10.1016/0092-8674(93)80063-K
Zeitlin, P. (2004). Can curcumin cure cystic fibrosis? N. Engl. J. Med. 351, 606–608. doi: 10.1056/NEJMcibr041584
Zhang, L., Pornpattananangkul, D., Hu, C.-M., and Huang, C.-M. (2010). Development of nanoparticles for antimicrobial drug delivery. Curr. Med. Chem. 17, 585–594. doi: 10.2174/092986710790416290
Zhang, P. R., Tu, Y. F., Wang, S., Wang, Y. H., Xie, Y., Li, M., et al. (2011). Preparation and characterization of budesonideloaded solid lipid nanoparticles for pulmonary delivery. J. Chin. Pharm. Sci. 20, 390–396. doi: 10.5246/jcps.2011.04.049
Zhao, J., Cheng, W., He, X., and Liu, Y. (2018). The co-colonization prevalence of Pseudomonas aeruginosa and Aspergillus fumigatus in cystic fibrosis: a systematic review and meta-analysis. Microb. Pathog. 125, 122–128. doi: 10.1016/j.micpath.2018.09.010
Keywords: cystic fibrosis, nanoparticles, drug delivery, gene therapy, lung pathology
Citation: Velino C, Carella F, Adamiano A, Sanguinetti M, Vitali A, Catalucci D, Bugli F and Iafisco M (2019) Nanomedicine Approaches for the Pulmonary Treatment of Cystic Fibrosis. Front. Bioeng. Biotechnol. 7:406. doi: 10.3389/fbioe.2019.00406
Received: 14 October 2019; Accepted: 27 November 2019;
Published: 17 December 2019.
Edited by:
Valerio Voliani, Italian Institute of Technology (IIT), ItalyReviewed by:
Domenico Cassano, Azienda Sanitaria Locale Salerno, ItalyCopyright © 2019 Velino, Carella, Adamiano, Sanguinetti, Vitali, Catalucci, Bugli and Iafisco. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesca Bugli, ZnJhbmNlc2NhLmJ1Z2xpQHVuaWNhdHQuaXQ=; Michele Iafisco, bWljaGVsZS5pYWZpc2NvQGlzdGVjLmNuci5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.