- Division of Applied Chemistry, Faculty of Engineering, Hokkaido University, Sapporo, Japan
Poly(2-hydroxybutyrate) [P(2HB)] is an artificial polyhydroxyalkanoate (PHA) synthesized using engineered 2-hydroxyalkanoate-polymerizing PHA synthase. In the present study, the effect of temperature on P(2HB) synthesis was investigated. Recombinant Escherichia coli harboring PHA synthetic genes were cultivated with 2HB and 3-hydroxybutyrate (3HB) supplementation at varied temperatures ranging from 24 to 36°C for the synthesis of P(2HB) and natural PHA P(3HB), respectively. P(2HB) production and its molecular weight increased considerably at a threshold temperature of 32–34°C. The trend was not observed during the synthesis of P(3HB). Notably, the threshold temperature was close to the glass transition temperature (Tg) of P(2HB) (30°C), while the Tg of P(3HB) (4°C) was much lower than the cultivation temperature. The results suggest that thermal motion of the polymer chains influenced the production and molecular weight of the obtained polymer. According to the results, the production and molecular weight of PHA drastically changes at the threshold temperature, which is linked to the Tg of the polymer. The hypothesis should be applicable to PHAs in general, and potentially explains the inability to biosynthesize high-molecular-weight polylactate homopolymer with a Tg of 60°C.
Introduction
Polyhydroxyalkanoates (PHAs) are bacterial storage polyesters that are applied as biobased, biodegradable and biocompatible thermoplastics (Sudesh et al., 2011; Zhang et al., 2018). PHAs exhibit a variety of contrasting physical properties, ranging from brittle to flexible and elastic, similar to petroleum-derived plastics (Muhammadi et al., 2015). The physical properties of polymer materials mainly depend on the structures of their monomeric constituents and their molecular weight. The variety of monomer constituents in a polymer is determined largely by the substrate specificity of a PHA synthase (Zou et al., 2017). In contrast, the factors jointly influence the molecular weight of PHA. For example, the concentrations of alcoholic compounds, which induce the chain-transfer reaction of PHA synthase, are negatively correlated with the molecular weight of a PHA (Tomizawa et al., 2010; Hiroe et al., 2015). Class I PHA synthases synthesize higher molecular weight polymers compared to class II PHA synthases. In addition, a high concentration of active PHA synthase protein in cells could decrease the molecular weight of a PHA (Sim et al., 1997). Despite the relatively extensive empirical knowledge above, the underlying mechanisms that influence the molecular weight of PHAs remain poorly understood.
Naturally occurring PHAs are typically composed of 3-hydroxyalkanoates, and the substrate specificity of the PHA synthases influence their carbon numbers (Zou et al., 2017). The biosynthesis of poly(2-hydroxypropionate) (polylactate or PLA), which is structurally similar to PHAs, has attracted the attention of researchers due to its superior properties. However, attempts to polymerize the corresponding monomer substrate, lactyl-coenzyme A (LA-CoA), using wild-type PHA synthases (Valentin and Steinbüchel, 1994) have been unsuccessful. The first lactate (LA)-polymerizing enzyme, which was an engineered PHA synthase from Pseudomonas sp. 61-3 with S325T/Q481K pairwise mutation (PhaC1PsSTQK), was identified in 2008 (Taguchi et al., 2008). The artificial PHA poly(LA-co-3-hydroxybutyrate) [P(LA-co-3HB)] copolymers synthesized using PhaC1PsSTQK exhibit semi-transparent and flexible properties (Ishii et al., 2017). A key finding was that PhaC1PsSTQK efficiently synthesized P(LA-co-3HB) copolymer, but not high-molecular-weight PLA homopolymer. Only a low amount (1 wt%) of PLA homopolymer-like polymer (~99 mol% LA) was obtained using PhaC1PsSTQK (Matsumoto and Taguchi, 2013). In addition, the molecular weight of the biosynthesized PLA-like polymer was low (103 g/mol in order of magnitude) compared to the molecular weight of typical PHAs (104-105 g/mol in order of magnitude) (Matsumoto and Taguchi, 2013; Ishii et al., 2017). Such correlation between monomer composition and molecular weight has not been reported in natural PHAs (Murugan et al., 2017). Therefore, the inability to biosynthesize PLA has attracted considerable attention among researchers.
We recently measured the in vitro LA-CoA polymerization rate using PhaC1PsSTQK, and observed that polymerization halted when the molecular weight of the polymerized product, PLA, reached ~3,000 g/mol (Matsumoto et al., 2018b). Consequently, the inefficient PLA synthesis was attributed to the LA-CoA polymerization by PHA synthase. The halting of LA-CoA polymerization was potentially due to low thermal motion of the PLA chain at the reaction temperature (30°C) since the glass transition temperature (Tg) of PLA is 60°C, which suggested that temperature is a potential additional factor influencing PHA molecular weight.
Investigation of the effect of temperature on PLA biosynthesis, however, is a challenge, since PhaC1PsSTQK does not maintain the activity at 60°C. Therefore, we focused on the broad substrate specificity of PhaC1PsSTQK toward various 2-hydroxyalkanoates (2HAs), such as 2-hydroxybutyrate (2HB) (Matsumoto et al., 2013), glycolate (Matsumoto and Taguchi, 2013), and 2-hydroxy-4-methylvalerate (Mizuno et al., 2018). P(2HB) was selected as the target polymer because the Tg of the polymer (30°C) is in the cultivation temperature range of Escherichia coli. In addition, P(2HB) is transparent and exhibits stretchy properties that differ from those of natural PHAs (Matsumoto et al., 2013) and form homo- and hetero-stereocomplexes with isotactic 2HA polymers (Tsuji and Hayakawa, 2016). Therefore, the polymer potentially expands the applications of PHAs. The aim of the present study was to investigate the effect of temperature on the production and molecular weight of enzymatically synthesized P(2HB).
Methods
Plasmids and Culture Conditions
A plasmid, pBSphaC1STQKpct, bearing the propionyl-CoA transferase (pct) from Megasphaera elsdenii and phaC1PsSTQK genes has been constructed previously (Matsumoto et al., 2018a). E. coli JM109 harboring pBSphaC1STQKpct was cultured in 100 mL LB medium containing 2% glucose, 5 mg/mL sodium (R,S)-2HB, and 100 μg/mL ampicillin at 24, 26, 28, 30, 32, 34, and 36°C for 48 h. For P(3HB) production, 5 mg/mL sodium (R,S)-3HB was used instead of 2HB. The experiments were performed in biosafety level 1 laboratory and by researchers who had undergone biosafety training.
Polymer Analysis
The polymer was extracted from the lyophilized cells at 60°C using chloroform for 48 h. Chloroform was evaporated and the residual solid was rinsed with methanol to remove lipids. Polymer production and cellular polymer content were determined based on the weights of purified polymers. Molecular weight was determined using gel permeation chromatography as described previously (Taguchi et al., 2008). The polymer structure was confirmed using Proton Nuclear Magnetic Resonance (1H NMR) as described previously (data not shown) (Taguchi et al., 2008).
Results
P(2HB) were synthesized at varied temperatures in the engineered E. coli harboring the pct and phaC1PsSTQK. The P(2HB) production increased considerably at 34°C compared to the lower temperatures (Figure 1). In addition, the cellular polymer content also increased markedly from ~1 to 10 wt% at 34°C (Table 1). In contrast, in the 24–32°C range, P(2HB) production and cellular content did not change considerably. The results indicate that high temperature positively influenced P(2HB) production. The weight-averaged molecular weight (Mw) of P(2HB) increased more than 5-fold at 32°C and higher temperatures. The results demonstrate that P(2HB) had a specific threshold temperature, at which the extension of P(2HB) chains was enhanced considerably.
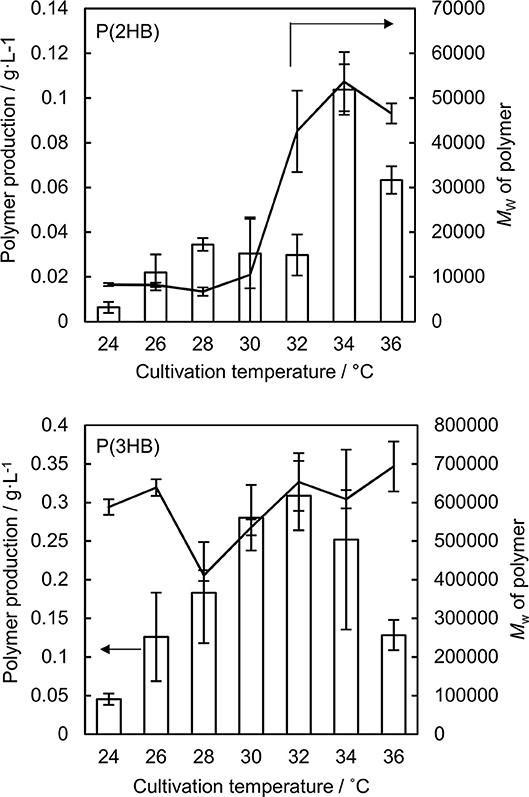
Figure 1. Production and weight-averaged molecular weight of P(2HB) and P(3HB) at a variety of cultivation temperature of engineered Escherichia coli. Line graph represents the weight-averaged molecular weight of the polymer. Bar graph represents the polymer production. Data are averages and standard deviations of at least three independent trials.
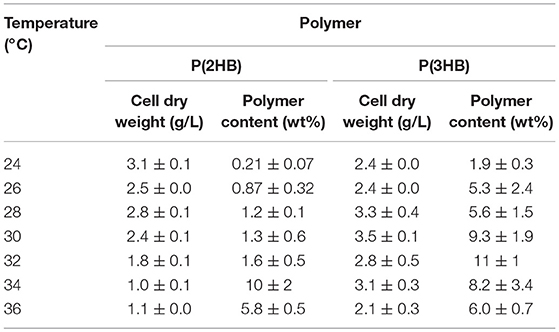
Table 1. Cellular polymer content of P(2HB) and P(3HB) in recombinant Escherichia coli cultivated under various temperatures.
The P(2HB) threshold temperature was in the 32–34°C range, which was close to the Tg of the polymer (30°C). However, reaction rate generally increases based on Arrhenius' equation, and therefore, the enhanced P(2HB) synthesis could have been due to elevated metabolic activity rather than the thermal properties of the polymer chain. Therefore, we tested the effect of temperature on P(3HB) production, which had a Tg of 4°C.
P(3HB) production and cellular content increased gradually with an increase in temperature up to 32°C, and then decreased at higher temperatures (Figure 1 and Table 1). Notably, P(3HB) molecular weight did not considerably change depending on the temperature (<1.4-fold). Therefore, temperature is a factor influencing PHA synthesis, with greater effects on P(2HB) synthesis compared to P(3HB) synthesis.
Discussion
The present study demonstrated that the molecular weight and the production of P(2HB) increased markedly at temperatures higher than the threshold temperature. We hypothesized that the phenomenon was due to an increase in the mobility of the polymer chain, which is essential for the progress of polymerization, at the threshold temperature. The threshold temperature is closely related to the Tg of a polymer. However, Tg is considered a macroscopic thermal property of the polymer linked to micro-Brownian motion, which is influenced by the intermolecular and intramolecular interactions among high-molecular-weight polymer chains. Conversely, in the initial stage of polymer synthesis by PHA synthase, the polymer chains interact with the enzyme, and potentially, with cytosolic molecules including water. The presence of solvents reportedly influences the Tg of a polymer (Picker and Hoag, 2002; Kikkawa et al., 2004; Yoshioka and Tashiro, 2004). Therefore, the P(2HB) synthesis threshold temperature was close but not necessarily equal to the Tg of the polymer.
The hypothesis accounted for the relatively low molecular weight of P(2HB) synthesized at 24–28°C (Figure 1). The Tg values of a polymer decrease with a decrease in the molecular weight below a certain range (Fox and Flory, 1950). Notably, the Tg of P(2HB) with a Mw of 27,000 g/mol was 30°C (Matsumoto et al., 2013), while the Tg of P(2HB) with a Mw of 13,000 g/mol was 27°C (Tsuji et al., 2010). P(2HB) with a Mw of <104 g/mol should exhibit relatively high molecular mobility, and therefore, could be obtained at low temperature conditions.
Consistent results have been observed for poly(LA-co-3HB) with various monomer fractions (Ishii et al., 2017). Figure 2 summaries the correlation between LA fraction, molecular weight and Tg of P(LA-co-3HB)s synthesized at 30°C. Since P(LA-co-3HB) is PLA (Tg = 60°C) and P(3HB) (Tg = 4°C) copolymer, the copolymer Tg should increase with an increase in LA fraction. However, the Tg values observed for P(LA-co-3HB)s reached a plateau below 30°C. The plateaued Tg were due to a decreasing trend in the polymer molecular weight with an increase in LA fraction. The result indicated that polymer synthesis is limited when the Tg is higher than the cultivation temperature.
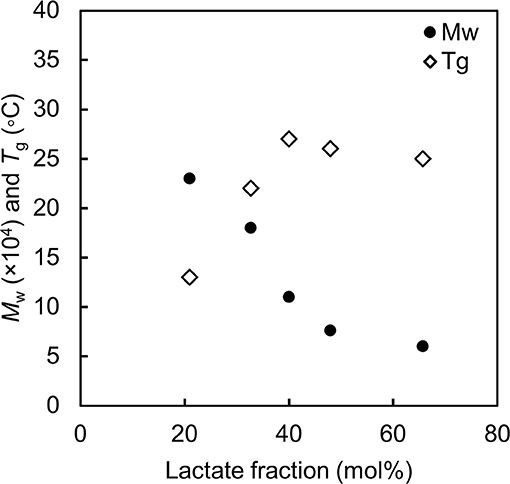
Figure 2. Molecular weight and glass transition temperature of poly(lactate-co-3HB) with various lactate fractions. Black circle: weight-averaged molecular weight. White diamond: glass transition temperature of the polymer. The data are from reference by Ishii et al. (2017).
The P(2HB) molecular weight reached a plateau at 32°C and did not increase further with an increase in temperature (Figure 1), which could be because the threshold temperature of P(2HB) represents a type of phase transition similar to glass transition. In general, the phase transition phenomenon refers to a rapid change in the physical properties and structure of molecules under certain conditions. Therefore, based on the hypothesis, polymer molecular weight would not be linearly correlated with temperature. In addition, a reduction in P(2HB) and P(3HB) production at 36°C was potentially due to the thermal instability of PhaC1PsSTQK. The enzyme was originally derived from Pseudomonas sp. 61-3, which has an optimal growth temperature of 30°C (Abe et al., 1994).
As mentioned above, the lower P(2HB) molecular weight observed at 30°C compared to the molecular weights at higher temperatures were considered due to the limited extension of the polymer chain. However, temperature could also influence the metabolic fluxes involved in monomer supplies. Therefore, the monomer supply rate could influence the polymer molecular weight. According to previously determined kinetic data, PCT exhibits 37.5-fold higher activity in the generation of 2HB-CoA than 3HB-CoA at 30°C (Matsumoto et al., 2018a). In contrast, PhaC1PsSTQK activity toward 2HB-CoA was 0.53-fold lower than toward 3HB-CoA (Matsumoto et al., 2018a). Therefore, in comparison with the moderate effect of temperature on P(3HB) synthesis, the 2HB-CoA supply rate was unlikely a P(2HB) synthesis limiting factor at 30°C. Further studies would be required to elucidate the problem.
In conclusion, the results of the present study demonstrated that temperature is a key factor influencing the molecular weight of PHAs. The threshold temperature of the enzymatic polymer synthesis should be close to the Tg of the polymer. The finding is consistent with our hypothesis that thermal motion of the polymer chain is required for its extension via polymerization. The biosynthesis of some artificial PHAs such as high-molecular-weight PLA, therefore, is limited because the Tg value of PLA is much higher than the cultivation temperatures. This could be a reason why PLA is not a storage compound in nature, and P(3HB) with a the Tg much lower than the temperatures appropriate for the growth and development of numerous bacteria is produced by a broad range of bacteria. Other reports have described the biosynthesis of PLA with a molecular weight of 3 × 104 (Jung et al., 2010). The inconsistent results will be discussed elsewhere.
Data Availability
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Author Contributions
KM designed experiments and wrote paper. YK performed experiments.
Funding
This work was supported by Advanced Low Carbon Technology Research and Development Program of Japan Science and Technology Agency (ALCA-JST) to KM (JPMJAL1509), and partly supported by JSPS Kakenhi Grant 17H01902 to KM.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Enago (www.enago.jp) for the English language review.
References
Abe, H., Doi, Y., Fukushima, T., and Eya, H. (1994). Biosynthesis from gluconate of a random copolyester consisting of 3-hydroxybutyrate and medium-chain-length 3-hydroxyalkanoates by Pseudomonas sp. 61-3. Int. J. Biol. Macromol. 16, 115–119. doi: 10.1016/0141-8130(94)90036-1
Fox, T. G., and Flory, P. J. (1950). 2nd-order transition temperatures and related properties of polystyrene.1. Influence of molecular weight. J. Appl. Phys. 21, 581–591. doi: 10.1063/1.1699711
Hiroe, A., Shiraishi, M., Mizuno, K., and Tsuge, T. (2015). Behavior of different polyhydroxyalkanoate synthases in response to the ethanol level in Escherichia coli cultures. Polym. J. 47, 767–770. doi: 10.1038/pj.2015.53
Ishii, D., Takisawa, K., Matsumoto, K., Ooi, T., Hikima, T., Takata, M., et al. (2017). Effect of monomeric composition on the thermal, mechanical and crystalline properties of poly[(R)-lactate-co-(R)-3-hydroxybutyrate]. Polymer 122, 169–173. doi: 10.1016/j.polymer.2017.06.039
Jung, Y. K., Kim, T. Y., Park, S. J., and Lee, S. Y. (2010). Metabolic engineering of Escherichia coli for the production of polylactic acid and its copolymers. Biotechnol. Bioeng. 105, 161–171. doi: 10.1002/bit.22548
Kikkawa, Y., Fujita, M., Abe, H., and Doi, Y. (2004). Effect of water on the surface molecular mobility of poly(lactide) thin films: an atomic force microscopy study. Biomacromolecules 5, 1187–1193. doi: 10.1021/bm0345007
Matsumoto, K., Hori, C., Fujii, R., Takaya, M., Ooba, T., Ooi, T., et al. (2018a). Dynamic changes of intracellular monomer levels regulate block sequence of polyhydroxyalkanoates in engineered Escherichia coli. Biomacromolecules 19, 662–671. doi: 10.1021/acs.biomac.7b01768
Matsumoto, K., Iijima, M., Hori, C., Utsunomia, C., Ooi, T., and Taguchi, S. (2018b). In vitro analysis of D-lactyl-CoA-polymerizing polyhydroxyalkanoate synthase in polylactate and poly(lactate-co-3-hydroxybutyrate) syntheses. Biomacromolecules. 19, 2889–2895. doi: 10.1021/acs.biomac.8b00454
Matsumoto, K., and Taguchi, S. (2013). Biosynthetic polyesters consisting of 2-hydroxyalkanoic acids: current challenges and unresolved questions. Appl. Microbiol. Biotechnol. 97, 8011–8021. doi: 10.1007/s00253-013-5120-6
Matsumoto, K., Terai, S., Ishiyama, A., Sun, J., Kabe, T., Song, Y., et al. (2013). One-pot microbial production, mechanical properties and enzymatic degradation of isotactic P[(R)-2-hydroxybutyrate] and its copolymer with (R)-lactate. Biomacromolecules 14, 1913–1918. doi: 10.1021/bm400278j
Mizuno, S., Enda, Y., Saika, A., Hiroe, A., and Tsuge, T. (2018). Biosynthesis of polyhydroxyalkanoates containing 2-hydroxy-4-methylvalerate and 2-hydroxy-3-phenylpropionate units from a related or unrelated carbon source. J. Biosci. Bioeng. 125, 295–300. doi: 10.1016/j.jbiosc.2017.10.010
Muhammadi, S., Afzal, M., and Hameed, S. (2015). Bacterial polyhydroxyalkanoates-eco-friendly next generation plastic: production, biocompatibility, biodegradation, physical properties and applications. Green Chem. Lett. Rev. 8, 56–77. doi: 10.1080/17518253.2015.1109715
Murugan, P., Gan, C. Y., and Sudesh, K. (2017). Biosynthesis of P(3HB-co-3HHx) with improved molecular weights from a mixture of palm olein and fructose by Cupriavidus necator Re2058/pCB113. Int. J. Biol. Macromol. 102, 1112–1119. doi: 10.1016/j.ijbiomac.2017.05.006
Picker, K. M., and Hoag, S. W. (2002). Characterization of the thermal properties of microcrystalline cellulose by modulated temperature differential scanning calorimetry. J. Pharm. Sci. 91, 342–349. doi: 10.1002/jps.10018
Sim, S. J., Snell, K. D., Hogan, S. A., Stubbe, J., Rha, C., and Sinskey, A. J. (1997). PHA synthase activity controls the molecular weight and polydispersity of polyhydroxybutyrate in vivo. Nat. Biotechnol. 15, 63–67. doi: 10.1038/nbt0197-63
Sudesh, K., Bhubalan, K., Chuah, J. A., Kek, Y. K., Kamilah, H., Sridewi, N., et al. (2011). Synthesis of polyhydroxyalkanoate from palm oil and some new applications. Appl. Microbiol. Biotechnol. 89, 1373–1386. doi: 10.1007/s00253-011-3098-5
Taguchi, S., Yamada, M., Matsumoto, K., Tajima, K., Satoh, Y., Munekata, M., et al. (2008). A microbial factory for lactate-based polyesters using a lactate-polymerizing enzyme. Proc. Natl. Acad. Sci. U.S.A. 105, 17323–17327. doi: 10.1073/pnas.0805653105
Tomizawa, S., Saito, Y., Hyakutake, M., Nakamura, Y., Abe, H., and Tsuge, T. (2010). Chain transfer reaction catalyzed by various polyhydroxyalkanoate synthases with poly(ethylene glycol) as an exogenous chain transfer agent. Appl. Microbiol. Biotechnol. 87, 1427–1435. doi: 10.1007/s00253-010-2601-8
Tsuji, H., and Hayakawa, T. (2016). Heterostereocomplex- and homocrystallization and thermal properties and degradation of substituted poly(lactic acid)s, poly(L-2-hydroxybutanoic acid) and poly(D-2-hydroxy-3-methylbutanoic acid). Macromol. Chem. Phys. 217, 2483–2493. doi: 10.1002/macp.201600359
Tsuji, H., Yamamoto, S., Okumura, A., and Sugiura, Y. (2010). Heterostereocomplexation between biodegradable and optically active polyesters as a versatile preparation method for biodegradable materials. Biomacromolecules 11, 252–258. doi: 10.1021/bm901113v
Valentin, H. E., and Steinbüchel, A. (1994). Application of enzymatically synthesized short-chain-length hydroxy fatty acid coenzyme A thioesters for assay of polyhydroxyalkanoic acid synthases. Appl. Microbiol. Biotechnol. 40, 699–709. doi: 10.1007/BF00173332
Yoshioka, A., and Tashiro, K. (2004). Solvent effect on the glass transition temperature of syndiotactic polystyrene viewed from time-resolved measurements of infrared spectra at the various temperatures and its simulation by molecular dynamics calculation. Macromolecules 37, 467–472. doi: 10.1021/ma035505z
Zhang, J., Shishatskaya, E. I., Volova, T. G., Da Silva, L. F., and Chen, G. Q. (2018). Polyhydroxyalkanoates (PHA) for therapeutic applications. Mater. Sci. Eng. C Mater. Biol. Appl. 86, 144–150. doi: 10.1016/j.msec.2017.12.035
Keywords: biobased plastic, polyester, enzymatic synthesis, lactate-polymerizing enzyme, artificial biopolymer
Citation: Matsumoto K and Kageyama Y (2019) Increased Production and Molecular Weight of Artificial Polyhydroxyalkanoate Poly(2-hydroxybutyrate) Above the Glass Transition Temperature Threshold. Front. Bioeng. Biotechnol. 7:177. doi: 10.3389/fbioe.2019.00177
Received: 25 April 2019; Accepted: 09 July 2019;
Published: 24 July 2019.
Edited by:
Masoud Mozafari, Materials and Energy Research Center, IranReviewed by:
Narendra Pal Singh Chauhan, Bhupal Nobles University, IndiaChristopher John Brigham, Wentworth Institute of Technology, United States
S. Venkata Mohan, Indian Institute of Chemical Technology (CSIR), India
Si Jae Park, Ewha Womans University, South Korea
Copyright © 2019 Matsumoto and Kageyama. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ken'ichiro Matsumoto, mken@eng.hokudai.ac.jp
 Ken'ichiro Matsumoto
Ken'ichiro Matsumoto Yuki Kageyama
Yuki Kageyama