- Molecular Microbiology and Biotechnology, Institute of Biology Leiden, Leiden University, Leiden, Netherlands
Filamentous fungi are the most important microorganisms for the industrial production of plant polysaccharide degrading enzymes due to their unique ability to secrete these proteins efficiently. These carbohydrate active enzymes (CAZymes) are utilized industrially for the hydrolysis of plant biomass for the subsequent production of biofuels and high-value biochemicals. The expression of the genes encoding plant biomass degrading enzymes is tightly controlled. Naturally, large amounts of CAZymes are produced and secreted only in the presence of the plant polysaccharide they specifically act on. The signal to produce is conveyed via so-called inducer molecules which are di- or mono-saccharides (or derivatives thereof) released from the specific plant polysaccharides. The presence of the inducer results in the activation of a substrate-specific transcription factor (TF), which is required not only for the controlled expression of the genes encoding the CAZymes, but often also for the regulation of the expression of the genes encoding sugar transporters and catabolic pathway enzymes needed to utilize the released monosaccharide. Over the years, several substrate-specific TFs involved in the degradation of cellulose, hemicellulose, pectin, starch and inulin have been identified in several fungal species and systems biology approaches have made it possible to uncover the enzyme networks controlled by these TFs. The requirement for specific inducers for TF activation and subsequently the expression of particular enzyme networks determines the choice of feedstock to produce enzyme cocktails for industrial use. It also results in batch-to-batch variation in the composition and amounts of enzymes due to variations in sugar composition and polysaccharide decorations of the feedstock which hampers the use of cheap feedstocks for constant quality of enzyme cocktails. It is therefore of industrial interest to produce specific enzyme cocktails constitutively and independently of inducers. In this review, we focus on the methods to modulate TF activities for inducer-independent production of CAZymes and highlight various approaches that are used to construct strains displaying constitutive expression of plant biomass degrading enzyme networks. These approaches and combinations thereof are also used to construct strains displaying increased expression of CAZymes under inducing conditions, and make it possible to design strains in which different enzyme mixtures are simultaneously produced independently of the carbon source.
Introduction
Plant biomass is the most abundant renewable carbon source in the world and represents the major natural substrate for fungi (Kowalczyk et al., 2014). Plant biomass mainly consists of plant cell wall material, which contains the polysaccharides, i.e., cellulose, hemicellulose and pectin, lignin, and structural proteins (Loqué et al., 2015). Cellulose, the most abundant plant cell wall polysaccharide, is a linear chain of glucose (Kolpak and Blackwell, 1976). Hemicelluloses are complex heteropolysaccharides with xylan- (linear chain of xylose), glucan- (linear chain of glucose), glucomannan- (linear chain of glucose and mannose), or mannan- (linear chain of mannose) backbones, with several different types of monomers attached to the backbone to give e.g., arabinoxylan or xyloglucan (Scheller and Ulvskov, 2010). Pectins are other complex polysaccharides containing D-galacturonic acid (GA) as the main sugar acid in their backbones. Polygalacturonic acid (PGA) is a linear chain of GA and the most abundant pectin substructure. Other pectin substructures are rhamnogalacturonan I (linear chain of alternating GA and rhamnose residues), rhamnogalacturonan II and xylogalacturonan, and contain several different types of monomers or polymers attached to their backbones (Caffall and Mohnen, 2009). Plant biomass also contains the plant cell storage polysaccharides starch, a linear (amylose) or branched (amylopectin) polymer of glucose, and inulin, a polymer of fructose residues with a terminal glucose residue (Gidley, 2001; Ritsema and Smeekens, 2003).
Polysaccharides present in plant biomass are the target of the degrading enzymes secreted abundantly by filamentous fungi. Fungal carbohydrate active enzymes (CAZymes) are also utilized industrially for the hydrolysis of plant biomass for the subsequent production of mainly bioethanol, and high-value biochemicals (Kowalczyk et al., 2014; Gupta et al., 2016; Benocci et al., 2017). Plant biomass degrading enzymes are classified into families based on their sequence, such as glycoside hydrolases, polysaccharide lyases and carbohydrate esterases, abbreviated as GH, PL and CE, respectively (Carbohydrate Active Enzymes database, http://www.cazy.org/) (Lombard et al., 2014). Filamentous fungi secrete large amounts of CAZymes only in the presence of the plant polysaccharide they specifically act on. For a review on the substrate specificity of CAZymes we refer to Kowalczyk et al. (2014) and de Vries et al. (2017). The signal for tailor-made production of CAZymes is conveyed via so-called inducer molecules which are di- or mono-saccharides (or derivatives thereof) released from the specific plant polysaccharides. The presence of the inducer results in the activation of a substrate-specific transcription factor (TF), which is required for the controlled expression of the genes encoding not only the CAZymes, but usually also the transporters and catabolic pathway enzymes needed to utilize the released monosaccharide (Culleton et al., 2013; Benocci et al., 2017).
Several substrate-specific TFs involved in plant biomass degradation have been identified in filamentous fungal species. For a review on the TFs involved in plant biomass degradation we refer to Huberman et al. (2016) and Benocci et al. (2017). For example, it has been shown that induction of expression of the genes encoding cellulases when cellulose is present requires the presence of the transcriptional activators Clr-1 and Clr-2 in Neurospora crassa, ClrB in Aspergillus nidulans and Penicillium oxalicum, ManR in A. oryzae and Xyr1 in Trichoderma reesei (Stricker et al., 2006; Coradetti et al., 2012; Ogawa et al., 2013; Li et al., 2015). Clr-2, ClrB and ManR are orthologs (Kunitake and Kobayashi, 2017), while Xyr1 shows significant sequence similarity to the TFs involved in xylan degradation, XlnR in A. niger and Xlr-1 in N. crassa (Rauscher et al., 2006). The enzyme families controlled by orthologous transcription factors can vary. Although Xyr1 in T. reesei is essential for the expression of cellulases and xylanases, Xyr1 orthologs (named XlnR or Xlr-1 in other species) are essential only for the expression of xylanases in filamentous fungal species, such as A. oryzae (on xylose), A. niger (on xylan), A. nidulans (on xylan), Talaromyces cellulolyticus (on xylan), P. oxalicum (on cellulose), N. crassa (on xylan), Fusarium oxysporum (on xylan or wheat cell walls), and Myceliophthora thermophila (van Peij et al., 1998; Marui et al., 2002b; Stricker et al., 2006; Brunner et al., 2007; Tamayo et al., 2008; Sun et al., 2012; Fujii et al., 2014; Li et al., 2015; Wang et al., 2015). In A. oryzae, A. niger, T. cellulolyticus, and P. oxalicum, contribution of XlnR to the expression of cellulases during growth on cellulose and/or xylose/xylan has also been reported (Gielkens et al., 1999; Marui et al., 2002; Li et al., 2015; Okuda et al., 2016). Arabinan, present in the side chains of some form of hemicellulose, such as arabinogalactan, or some pectins, is degraded via CAZymes that are transcriptionally regulated by AraR in A. niger (Battaglia et al., 2011; Kowalczyk et al., 2017). Arabinan degradation in T. reesei is partially regulated via Xyr1 (Akel et al., 2009) and via Ara1 (Benocci et al., 2018). The TF GaaR, required for the expression of CAZymes degrading pectin, especially PGA, has been identified in both Botrytis cinerea and A. niger (Alazi et al., 2016; Zhang et al., 2016). Recently, Pdr-1 in N. crassa was described as a TF required for the expression of the genes encoding CAZymes that degrade several pectin substructures including PGA and rhamnogalacturonan I (Thieme et al., 2017). Transcriptional control of the genes involved in starch degradation is conducted by AmyR in A. niger and A. nidulans (Tani et al., 2001; vanKuyk et al., 2012).
The transcriptional activators involved in plant biomass degradation mentioned above were identified either via classical approaches, such as mutant complementation (xlnR in A. niger) and gene cloning (xlnR and amyR in A. nidulans and amyR A. niger), or via post-genomic approaches, such as yeast one-hybrid screening (gaaR in B. cinerea), screening TF deletion mutant collections (pdr-1 and clr-2 in N. crassa, manR in A. oryzae, and clrB and xlnR in P. oxalicum), and also based on sequence similarity (clrB in A. nidulans, araR and gaaR in A. niger, xyr1 in T. reesei and M. thermophila, and xlnR in F. oxysporum, T. cellulolyticus and A. oryzae) or their expression levels in the transcriptomics data (xlr-1 in N. crassa) (van Peij et al., 1998; Tani et al., 2001; Marui et al., 2002b; Rauscher et al., 2006; Brunner et al., 2007; Battaglia et al., 2011; Coradetti et al., 2012; Ogawa et al., 2012; Sun et al., 2012; vanKuyk et al., 2012; Fujii et al., 2014; Li et al., 2015; Wang et al., 2015; Alazi et al., 2016; Zhang et al., 2016; Thieme et al., 2017). Positively acting TFs involved in controlling expression of plant cell wall degrading enzymes belong to the Zn2Cys6 type family of TFs. TFs belonging to this family are found specifically in fungi (both yeasts and filamentous fungi) and contain a DNA-binding domain with six cysteine residues bound to two zinc atoms, usually close to their NH2-terminal end. Most of the Zn2Cys6 type TFs also contain a fungal-specific TF domain, known as the middle homology region, with a proposed role in regulating the activity of the TF (MacPherson et al., 2006). Apart from activators several repressor proteins involved in plant biomass degradation have been identified including wide domain repressors, such as the carbon catabolite repressor protein CreA/Cre1, or substrate specific repressors which are discussed in paragraphs 1.2 and 2.3, respectively.
Diversity in the Control of Transcription Factor Activities
The mechanisms by which the activity of TFs is regulated in response to stimuli can be diverse. Firstly, the protein level of the TFs might be controlled. This can be realized by regulation at the level of transcription, mRNA stability or protein stability resulting in low protein levels under non-inducing conditions and higher levels under inducing conditions. Secondly, the subcellular localization (nuclear import/export), DNA binding activity, and/or transcriptional activity of the TFs might be regulated by post-transcriptional modifications, such as phosphorylation, and/or via protein-protein or protein-metabolite interactions (MacPherson et al., 2006; Chang and Ehrlich, 2013; Tani et al., 2014). Finally, DNA site occupancy of TFs might depend on their cooperation or competition with other proteins and the chromatin accessibility (Granek and Clarke, 2005; Biggin, 2011).
Our knowledge about regulation of the activity of the TFs involved in biomass degradation is limited, yet growing. The amount of Clr-2 in N. crassa is regulated at the level of transcription by Clr-1, which gets activated and positively regulates clr-2 expression only under inducing conditions (on insoluble crystalline cellulose Avicel or cellobiose). The expression of the clr-2 ortholog in A. nidulans, clrB, on the other hand, is not drastically induced under inducing conditions (on Avicel) and the Clr-1 ortholog ClrA is not essential but only contributes to cellulase gene expression (Coradetti et al., 2012). This comparison indicates that activation mechanisms of even orthologous transcription factors differ between fungal species.
The expression of xyr1 in T. reesei is subject to Cre1-dependent carbon catabolite repression (CCR) (see below) and induced on carbon sources inducing cellulase expression (e.g., lactose, sophorose, or cellulose), but not on carbon sources inducing xylanase expression (e.g., xylose) (Mach-Aigner et al., 2008; Portnoy et al., 2011a; Lichius et al., 2014). Xyr1 was shown to accumulate in the nucleus during growth on an inducing carbon source (i.e., sophorose or low concentration of xylose), whereas it is degraded in the nucleus during growth on a repressing carbon source (i.e., glucose or a high concentration of xylose). The increased amount of nuclear Xyr1 correlates with the increased expression level of cellulase gene cbh1 on sophorose, and that of xylanase gene xyn2 on a low concentration of xylose (Lichius et al., 2014). Similar to T. reesei xyr1, the expression of xlnR in A. nidulans and P. oxalicum is also subject to CreA-dependent CCR (see below), whereas xlnR in A. niger is not regulated at the level of transcription, but constitutively transcribed at low levels (Tamayo et al., 2008; Mach-Aigner et al., 2012; Li et al., 2015). Moreover, XlnR was found to be localized in the nucleus regardless of the presence of inducer in A. niger (Hasper, 2004). In F. oxysporum, xlnR is transcriptionally regulated by both CCR and induction on xylose/xylan (Calero-Nieto et al., 2007). XlnR in A. oryzae is reversibly phosphorylated in response to xylose, which does not affect its protein stability and correlates with the expression of XlnR target genes (Noguchi et al., 2011). Recently, Kunitake and Kobayashi proposed that a conserved sequence in XlnR is involved in the xylose-mediated phosphorylation of XlnR in A. oryzae, implying a conserved mechanism regulating XlnR activity among Ascomycete fungi. Moreover, the observation that XlnR in A. oryzae is not phosphorylated in response to cellobiose, but required for cellulase expression, indicates that its activity is regulated by a different mechanism on cellobiose (Noguchi et al., 2011; Kunitake and Kobayashi, 2017).
The activity of the GA-responsive transcriptional activator GaaR in A. niger is regulated by a different mechanism. It is suggested to be inhibited by the repressor protein GaaX via protein-protein interaction under non-inducing conditions. Under inducing conditions (in the presence of GA), the inducer is proposed to bind to GaaX, resulting in a free and active form of GaaR (Niu et al., 2017). The amount of GaaR is not significantly regulated at the level of transcription (Alazi et al., 2016, 2018). In addition, GaaR-eGFP was shown to be constitutively localized in the nucleus (Alazi et al., 2018). Transcription of pdr-1 in N. crassa is induced under inducing conditions (on rhamnose) and repressed under repressing conditions. Moreover, the activity of Pdr-1 is suggested to be post-transcriptionally regulated, as its nuclear accumulation and transcriptional activity requires the presence of rhamnose in a strain overexpressing pdr-1 (Thieme et al., 2017).
The transcription of amyR is upregulated on inducing carbon sources (e.g., starch or maltose) and is subject to CreA-dependent CCR (see below) in A. nidulans (Tani et al., 2001). Further, AmyR was shown to localize in the nucleus in an inducer (i.e., isomaltose)-dependent manner and activate the expression of its target genes (Makita et al., 2009).
The Role of the Carbon Catabolite Repressor in the Production of Plant Biomass Degrading Enzymes
Apart from being upregulated under inducing conditions, the expression of CAZymes might also be controlled via CCR when a more energetically favorable carbon source, such as glucose, compared to plant biomass polysaccharides is available for fungi. The Cys2His2 type TF CreA/Cre1/Cre-1 (in Aspergillus species, T. reesei, and N. crassa, respectively) is the main transcriptional repressor contributing to CCR. Regulation of CreA activity has not been fully understood, but several studies have indicated that post-transcriptional modifications, such as ubiquitination and phosphorylation, affect CreA protein stability, subcellular localization and/or DNA binding activity (Cziferszky et al., 2002; Ries et al., 2016; see Adnan et al., 2017 for a recent review). CreA not only represses the expression of genes encoding CAZymes, but it might also repress the expression of some transcriptional activators, such as clrB and xlnR in P. oxalicum, xyr1/xlnR in T. reesei, A. nidulans and F. oxysporum, and amyR in A. nidulans, that are required for the expression of CAZymes (Tani et al., 2001; Calero-Nieto et al., 2007; Mach-Aigner et al., 2008; Tamayo et al., 2008; Li et al., 2015). Furthermore, CreA might repress the expression of CAZymes under high xylose concentrations, too, as was shown to be the case for XlnR and its target genes in A. nidulans, and for XlnR itself in A. oryzae (Tamayo et al., 2008; Ichinose et al., 2017). Elimination of CCR due to a lack of function of CreA and/or CreB, a ubiquitin-specific protease involved in CCR, or their orthologs has been reported to result in an increased expression of CAZymes degrading cellulose, hemicellulose, pectin or starch, under inducing and/or non-inducing conditions in filamentous fungi (Prathumpai et al., 2004; Mach-Aigner et al., 2008; Nakari-Setälä et al., 2009; Denton and Kelly, 2011; Sun and Glass, 2011; Fujii et al., 2013; Ichinose et al., 2014, 2017; Niu et al., 2015; Yang et al., 2015). However, even when CreA-dependent CCR is circumvented, the expression of CAZymes might require the presence of active transcriptional activators, which is normally achieved in the presence of inducing metabolites. For example, in a Cre1-negative T. reesei strain (Rut-C30), the full expression of Xyr1 target genes requires the presence of the inducer (i.e., xylose) (Mach-Aigner et al., 2008). Another study revealed that deletion of cre1 in T. reesei results in elevated production of cellulases and xylanases under inducing (on lactose) and, to a lesser extent, under non-inducing conditions (on glucose) (Nakari-Setälä et al., 2009). Genome-wide studies in T. reesei have also indicated that only a few of the cellulolytic genes were upregulated in a Δcre1 strain during growth on glucose (Portnoy et al., 2011b; Antoniêto et al., 2014). This indicates that the majority of these cellulolytic genes are not induced simply by derepression, but require additional inducing condition for expression. Similarly, although the expression of pectinases is subject to CreA-dependent CCR in A. niger, deletion of creA is not sufficient for an increased production of polygalacturonases under non-inducing conditions. Polygalacturonases are only produced in the presence of the inducer (i.e., GA) or when gaaX is deleted, showing that GA-responsive gene expression requires the presence of active GaaR relieved from GaaX inhibition even in a ΔcreA strain (Niu et al., 2015, 2017).
Approaches for Increased or Constitutive Expression of Plant Biomass Degrading Enzymes by Modulating Their Transcriptional Regulation
Plant biomass with varying sugar composition present in waste streams from agriculture, forestry and food industries represent a cheap, sustainable and renewable feedstock for the production of the CAZymes, and subsequently, valuable chemicals (Sweeney and Xu, 2012; Meyer et al., 2015). However, the composition of the enzyme cocktail will vary because of variation in the composition of the plant biomass. It is therefore of industrial interest to produce specific fungal enzyme cocktails constitutively, independently of inducers and the substrate used. Fungal production strains, such as A. niger CBS513.88, T. reesei Rut-C30, and P. oxalicum JU-A10-T, have been for a long time improved via multiple rounds of classical mutagenesis and screening approaches that can be time-consuming (Montenecourt and Eveleigh, 1979; Pel et al., 2007; Fang et al., 2010; van Hanh et al., 2010; Ho and Ho, 2015; Yao et al., 2015). However, emergence of the -omics era (genomics, transcriptomics etc.) and advances in recombinant technologies allow nowadays efficient strain improvement via genetic engineering approaches with minimal alterations in the genome (Meyer et al., 2010; Liu et al., 2013). In the remaining part of this review, we discuss the approaches to modulate transcriptional regulation in order to rationally design fungal strains with increased or constitutive production of plant biomass degrading enzymes, examples of which are given in Table 1. As will become clear in the following sections, the success of the approach used highly depends on the mechanism regulating the activity of the targeted TF.
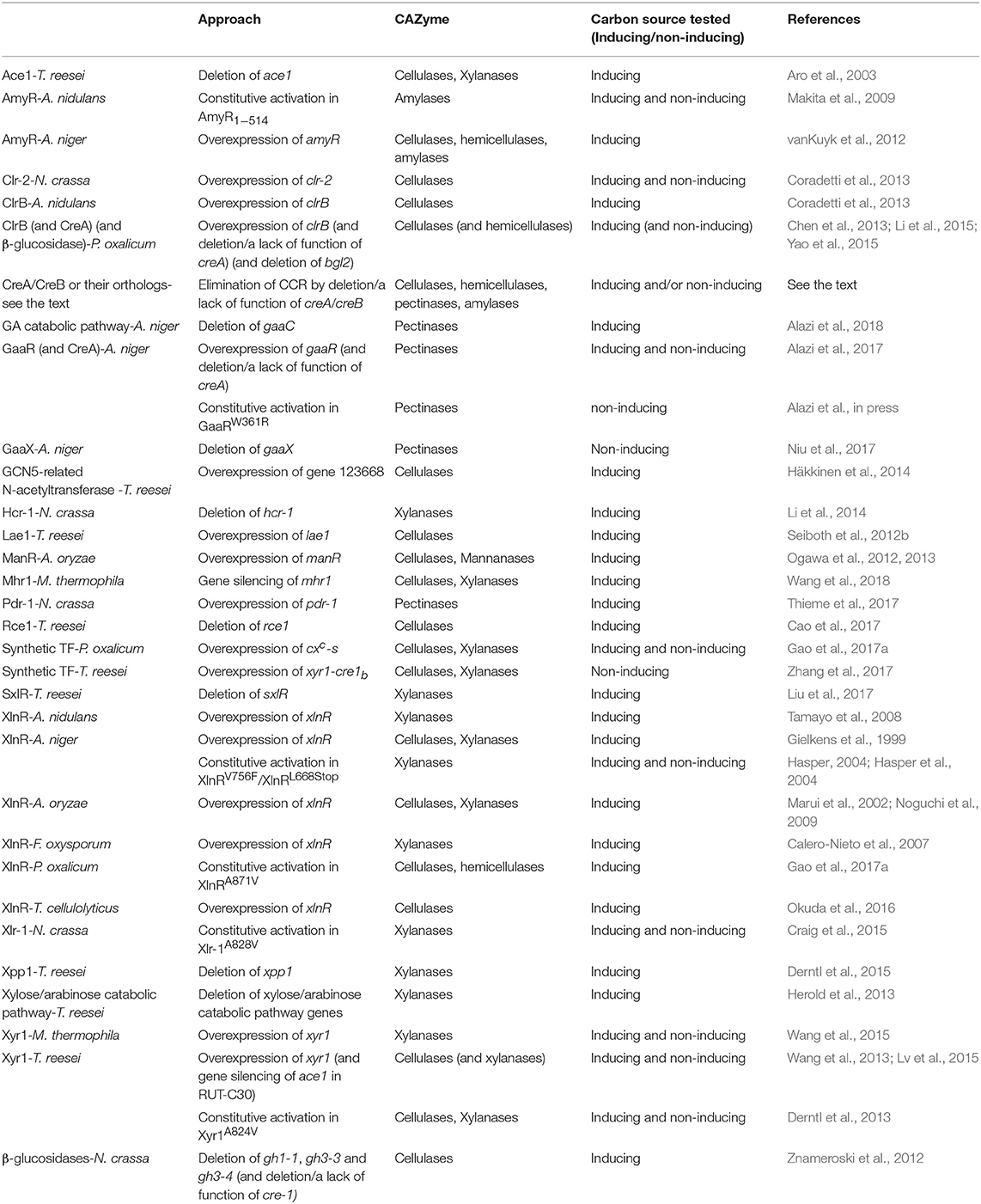
Table 1. Examples of rational design of industrial fungal strains with increased or constitutive production of CAZymes.
Overexpression of TFs
Increasing the amount of a TF at the level of transcription can be achieved by expressing multiple copies of the TF via its endogenous promoter, or (multiple copies of) the TF via a strong inducible/constitutive promoter. Overexpression of several TFs in Saccharomyces cerevisiae has been shown to result in an increased expression of the TF target genes, even under non-inducing conditions (Chua et al., 2006).
Inducer-independent production of cellulases in N. crassa was achieved by constitutively overexpressing clr-2 via the ccg-1 promoter (Coradetti et al., 2013). While deletion of clr-2 resulted in a drastic decrease in the expression of most of the genes encoding cellulases under inducing conditions [i.e., on insoluble crystalline cellulose (Avicel)], overexpression of clr-2 resulted in an increased expression of cellulases under inducing conditions and constitutive expression under non-inducing conditions. Expression of the genes encoding cellulases was higher under starvation (no carbon) conditions than on sucrose highlighting the effect of CCR on cellulase encoding genes under repressing conditions (i.e., on sucrose) even when clr-2 was overexpressed (Coradetti et al., 2013).
Unlike overexpression of clr-2 in N. crassa, overexpression of the clr-2 ortholog clrB in A. nidulans or in P. oxalicum, both via the constitutive A. nidulans gpdA promoter, did not result in an increased expression of CAZymes encoding genes under non-inducing conditions, but only under inducing conditions (on Avicel or cellulose, respectively) (Coradetti et al., 2013; Li et al., 2015). It was shown that deletion of creA in combination with clrB overexpression allows increased expression of cellulases under non-inducing conditions in P. oxalicum, indicating that the strong CreA-dependent repression on cellulase genes overrules ClrB-dependent induction (Li et al., 2015).
ManR in A. oryzae is the N. crassa clr-2 ortholog, regulating the expression of genes encoding cellulose and hemicellulose (including mannan, but not xylan) degrading enzymes in A. oryzae (Ogawa et al., 2012, 2013). Overexpression of ManR via the tef1 promoter resulted in an increased expression of cellulases and hemicellulases involved in mannan degradation under inducing conditions (i.e., on Avicel and mannan, respectively) (Ogawa et al., 2012, 2013). The effect of overexpression of ManR under non-inducing conditions has not been reported.
As mentioned before, in T. reesei, Xyr1 controls the transcriptional regulation of both cellulase encoding and xylanase encoding genes. Expression of xyr1 is subject to CCR and xyr1 expression is upregulated on carbon sources inducing cellulase production. Overexpression of xyr1 via the tcu1 promoter resulted in an inducer-independent production of cellulases, even under repressing conditions (i.e., on glucose) (Lv et al., 2015). All reported T. reesei Xyr1 orthologs in other filamentous fungal species regulate mainly the transcription of hemicellulase encoding genes and contribute less to the transcriptional regulation of cellulase encoding genes. Overexpression of xlnR via gpdA promoter in T. cellulolyticus resulted in an increased production of cellulases on cellulose, but not that of xylanases (Okuda et al., 2016). In A. niger, increased expression of cellulases and xylanases on xylose was observed in a strain carrying multiple copies of xlnR (Gielkens et al., 1999). Similarly, an A. oryzae strain overexpressing xlnR via the tef1 promoter showed an increased production of both cellulases and xylanases when grown on carbon sources known to induce cellulase (i.e., Avicel or cellobiose) or xylanases (i.e., xylose or xylan) production (Marui et al., 2002; Noguchi et al., 2009).
The studies mentioned above reported on the increased expression of xylanases in fungal strains overexpressing xlnR or its orthologs under inducing conditions. Only a few studies have reported the effect of overexpression of xlnR on the expression of xylanases under non-inducing conditions. For instance, xlnR overexpression via the constitutive gpdA promoter in A. nidulans or in F. oxysporum yields to increased production of xylanases encoding genes only in the presence of inducing carbon sources (e.g., xylose or xylan) (Calero-Nieto et al., 2007; Tamayo et al., 2008). It was also reported that when GFP-XlnR is overproduced in A. oryzae, it localizes constitutively in the nucleus, but its target genes are expressed only in the presence of xylose (Noguchi et al., 2011). These results indicate that XlnR in these organisms gets activated by an inducer-mediated post-transcriptional mechanism and that XlnR activation under non-inducing conditions cannot simply be achieved by overexpression. In M. thermophila, overexpression of xyr1 via the pdc promoter resulted in an increased expression of xylanases under both inducing (i.e., corncob) and non-inducing conditions (i.e., glucose) (Wang et al., 2015). This indicates that the mechanism of Xyr1 activation in M. thermophila differ from other filamentous fungi.
Another example of inducer-independent production of CAZymes by overexpression of TFs was given by a recent study in A. niger. Here it was shown that overexpression of gaaR in A. niger via the A. nidulans gpdA promoter leads to constitutive expression of the genes encoding pectinases, as well as GA transporters and GA catabolic pathway enzymes (Alazi et al., 2018). Deletion of creA further enhanced pectinase production under mildly repressing conditions (i.e., on fructose) indicating competing roles of GaaR and CreA to control the expression of GA-induced genes (Alazi et al., 2018). The effect of overexpression of gaaR on the expression of its target genes under non-inducing conditions is likely caused by its specific activation mechanism. Regulation of the activity of GaaR includes a specific repressor protein (GaaX) (Niu et al., 2017). It has been proposed that under non-inducing conditions the activity of GaaR is controlled through direct interaction by GaaX which prevents GaaR to be active (Niu et al., 2017). Modulating the amount of GaaR by overexpression affects the balance of GaaR-GaaX and results in the presence of uncomplexed active GaaR even under non-inducing conditions (Alazi et al., 2018).
The regulation of GA-induced gene expression shows some striking similarities with the regulation of genes involved in quinic acid catabolism. Quinic acid is present as an aromatic compound in the plant cell and can be released from tannins, which are water-soluble polyphenols, by the action of tannases known to be secreted by filamentous fungi (Wagh, 2010). Similar to regulation of GA utilization in A. niger, regulation of quinic acid utilization in A. nidulans involves a Zn2Cys6 type transcriptional activator (QutA) and a repressor (QutR), and strains expressing multiple copies of qutA displayed constitutive expression of the genes encoding quinic acid catabolic pathway enzymes (Lamb et al., 1996). The QutR and GaaX repressor proteins are both multidomain proteins with sequence similarity to the three C-terminal domains of AROM, indicating that these repressors share a common evolutionary origin (Niu et al., 2017).
On the other hand, overexpression of pdr-1, the pectin degradation regulator in N. crassa, via the gpd promoter of M. thermophila resulted in elevated expression of its target genes only under inducing conditions (on rhamnose). Therefore, it was proposed that Pdr-1 activity is regulated post-transcriptionally in a manner depending on the presence of the inducer but not on the amount of Pdr-1 (Thieme et al., 2017).
An A. niger strain carrying multiple copies of amyR displayed increased expression of AmyR target genes, such as CAZymes acting on starch as well as on cellulose and hemicellulose, under inducing conditions (i.e., on maltose, starch, and low concentration of glucose), but not under non-inducing condition (vanKuyk et al., 2012). This result is in line with the observation that in A. nidulans, nuclear localization of AmyR and thereby activation of its target genes is inducer-dependent (Makita et al., 2009).
In conclusion, we have seen that overexpression of TFs involved in plant biomass degradation can result in increased expression of their target genes under inducing conditions. However, in most cases overexpression of TFs, such as XlnR, Pdr-1 and AmyR, does not result in inducer-independent expression of their target genes. In these cases it is likely that an inducer molecule is required to activate the TF. The exact activation mechanism of XlnR, Pdr-1, and AmyR are currently unknown, and could involve direct interaction of the TF with its inducer, or could be related to post-translational modifications connected to the presence of inducers. Overexpression of TFs like GaaR and QutA results in inducer-independent expression of GaaR and QutA target genes most likely by titrating away the corresponding repressor proteins (GaaX and QutR, respectively). This illustrates that different mechanisms controlling TF activities result in different outcomes of overexpression of TFs under non-inducing conditions.
Constitutive Activation of TFs
The activity of a TF can be controlled in different ways, including post-transcriptional modifications affecting its localization, DNA binding or interaction with repressor protein(s). Over the past 15 years, mutations resulting in changes in amino acid sequences and thereby in constitutively active TFs have been identified via classical mutagenesis and screening approaches, or via pre-designed amino acid substitutions, domain removal or protein truncation analyses.
The first reported constitutively active form of XlnR (XlnRV756F) was identified in A. niger via a forward genetic screen. Expression of xlnRV756F resulted in a constitutive expression of xylanases even under repressing conditions (i.e., on fructose or glucose; Hasper, 2004; Hasper et al., 2004). Later, in T. reesei, a point mutation in Xyr1 (Xyr1A824V) introduced via UV mutagenesis was found to result in a constitutively active Xyr1 and constitutive expression of cellulases and xylanases even in the presence of a repressing carbon source (Derntl et al., 2013). In addition, overexpression of xyr1A824V in T. reesei was shown to be more effective in increasing cellulase production than overexpression of xyr1 under inducing conditions (e.g., Avicel) (Jiang et al., 2016). Both amino acids changes (V756F in XlnR and A824V in Xyr1) are located within the same predicted α-helix in the fungal-specific TF domain of XlnR/Xyr1 (Derntl et al., 2013). Although overexpression of xlr-1 in N. crassa via the ccg-1 promoter did not yield to constitutive expression of xylanases, overexpression of xlr-1A828V, which carries the homologous mutation as in xyr1A824V, resulted in a constitutive and increased production of xylanases under both inducing (on xylan) and non-inducing conditions (Craig et al., 2015). Similarly, overexpression of xlnR carrying the homologous point mutation (xlnRA871V) using the PDE_02864 promoter in a P. oxalicum strain that lacks creA and overexpresses clrB (see above), enabled even more increased production of cellulases and xylanases under inducing conditions (on wheat bran) (Gao et al., 2017a).
Recently, via a forward genetic screen, several different point mutations throughout AraR were found to give rise to a constitutively active AraR and constitutive expression of AraR target genes (abfA, abfB, and abfC). Unlike the mutations in XlnR that gave rise to inducer-independent and constitutive expression of its target genes, the mutations in AraR lead to constitutive expression of AraR target genes only under de-repressed conditions. Deletion of creA improved the production of CAZymes that degrade arabinan to a large extent, indicating the strong CCR on the genes encoding these CAZymes under repressing conditions on fructose (Reijngoud et al., manuscript in preparation).
We recently conducted a large forward genetic screen for mutants with constitutive expression of pectinases (Niu et al., 2017). Apart from identifying GaaX as a repressor for GA-induced genes expression, it also brought about the identification of a constitutive allele of GaaR (GaaRW361R) resulting in constitutive expression of pectinases under even repressing conditions (on glucose or fructose). Within GaaR, W361 is situated in the fungal-specific TF domain and is highly conserved among Aspergillus species (Alazi et al., in press).
Deletion of the C-terminal regions of several TFs involved in plant biomass degradation was shown to result in constitutive activation of the TFs indicating that these C-terminal parts contain an inhibitory domain. For example, in A. niger, truncation of XlnR from L668 resulted in a constitutive expression of xylanases (Hasper et al., 2004), and truncation of AraR from P646 was reported to cause constitutive activation of AraR (Jiang et al., 2016). Expression of the C-terminally truncated AmyR1−514 and AmyR1−511 resulted in constitutive localization of AmyR in the nucleus both in A. nidulans and A. oryzae, respectively. However, while constitutive amylase production was observed in A. nidulans (Makita et al., 2009), loss of expression was observed in A. oryzae (Suzuki et al., 2015), indicating that the effect of the truncation can also be species-specific.
Deletion or Down-Regulation of Specific Repressors
It has been shown that besides the general CCR, specific repressors might play a role in the transcriptional regulation of the genes encoding CAZymes. For instance, the Cys2His2 type TF Ace1 represses the expression of both cellulase and hemicellulase (i.e., xylanase) encoding genes, as well as the expression of xyr1 in T. reesei (Aro et al., 2003; Wang et al., 2013). Furthermore, Ace1 was shown to compete with Xyr1 to bind to the same sequence in the promoter of the xylanase gene xyn1 (Rauscher et al., 2006). Deletion of ace1 in the wild type background resulted in an increased expression of the genes encoding cellulases and xylanases only under inducing conditions (on cellulose), and gene silencing of ace1 led to an increase in constitutive expression of these genes when combined with the overexpression of xyr1 via the pdc promoter in a Cre1-negative background (Rut-C30) (Aro et al., 2003; Wang et al., 2013). Recently, another repressor, the Zn2Cys6 type TF Rce1, was identified in T. reesei that is involved in the repression of the genes encoding cellulases, but not xylanases. It was shown that Rce1 is constitutively localized in the nucleus and competes with Xyr1 to bind to the same sequence in the promoter of the cellulase gene cbh1 (Cao et al., 2017). Deletion of rce1 resulted in an increased production of cellulases under inducing conditions (on cellulose) (Cao et al., 2017). Deletion of a basic helix-loop-helix TF xpp1, xylanase promoter-binding protein 1, in T. reesei resulted in an increased expression of hemicellulase (i.e., xylanase) encoding genes, but not cellulase encoding genes, at late stages of cultivation under inducing conditions (on xylan) (Derntl et al., 2015). However, Xpp1 was later described as a general regulator of both primary and secondary metabolism and therefore not a factor specifically controlling xylanases expression (Derntl et al., 2017). In addition, the Zn2Cys6 type transcriptional repressor SxlR was found to bind to the promoters of specific (GH11 family) xylanase genes, and deletion of sxlR resulted in an increased expression of these xylanase genes under inducing conditions (on Avicel, xylan or lactose) (Liu et al., 2017).
Similarly, the Cys2His2 type TF Hcr-1 in N. crassa was shown to repress the expression of the genes encoding xylanases, but not the ones encoding cellulases. A Δhcr-1 strain exhibited an increased xylanase production under inducing conditions (on xylan or Avicel) (Li et al., 2014).
Recently a new TF, MhR1, was identified in M. thermophila as the regulator of cellulase and xylanase genes. Gene silencing of mhr1 resulted in an increased production of cellulases and xylanases, as well as increased expression of xyr1 and the genes encoding cellulases under inducing conditions (on wheat straw) (Wang et al., 2018).
As mentioned before, the activity of GaaR in A. niger is inhibited by the repressor protein GaaX, the amount of which is regulated at the level of transcription by induction on GA. GaaX was proposed to bind to- and inhibit GaaR under non-inducing conditions, and bind to the inducer molecule and release GaaR under inducing conditions. Deletion of gaaX resulted in a constitutive expression of pectinases, providing additional evidence for the proposed model of regulation of GA-responsive gene expression in A. niger (Niu et al., 2017). Similarly, deletion of QutR, the repressor protein involved in controlling the expression of quinic acid-responsive genes, also results in constitutive expression of at least eight genes involved in quinic acid uptake and metabolism (Levett et al., 2000).
Accumulation of Inducers
The intracellular accumulation of inducers is another effective method to boost the production of CAZymes by fungi. Cellulase production by N. crassa is greatly induced on insoluble, crystalline cellulose (Avicel). However, cellulase production is not observed on cellobiose, which is the soluble degradation product of cellulose, possibly due to rapid action of β-glucosidases converting cellobiose to glucose and subsequent glucose-mediated CCR. As first shown in N. crassa, deletion of three genes encoding the major β-glucosidases (intracellular enzyme Gh1-1, and extracellular enzymes Gh3-3 and Gh3-4) disrupted the hydrolysis of the inducer, cellobiose, and resulted in higher levels of expression of cellulases on cellobiose compared to the wild type strain grown on cellobiose, and similar to the wild type strain grown on Avicel. Moreover, deletion of cre-1 further increased cellulase production on cellobiose (Znameroski et al., 2012). Similarly, deletion of the major intracellular β-glucosidase bgl2 in a carbon catabolite de-repressed (ΔcreA) P. oxalicum strain that overexpresses clrB using the A. nidulans gpdA promoter, gave rise to higher levels of cellulase and hemicellulase (i.e., xylanase) production on cellulose compared to the wild type strain. These were similar to the levels of the industrial strain JU-A10-T grown on wheat bran (Yao et al., 2015). A similar effect of deleting bgl2 on cellulase and hemicellulase production was observed in P. oxalicum grown on cellulose (Chen et al., 2013).
Expression of hemicellulases, specifically xylanases, in T. reesei is induced both on xylose and arabinose. Although the catabolic pathways assimilating xylose and arabinose are interconnected, the physiological inducers triggering hemicellulase production appear to be different and were suggested to be xylose and L-arabitol, respectively. Expression of xylanases increased dramatically in single or double xylose/arabinose catabolic pathway enzyme deletion mutants, indicating the effect of accumulation of physiological inducers in these mutants (Seiboth et al., 2012a; Herold et al., 2013).
One of the GA catabolic pathway intermediates, 2-keto-3-deoxy-L-galactonate, was recently shown to be the physiological inducer of the genes encoding pectinases in A. niger. It was demonstrated that deletion of gaaC, the gene encoding 2-keto-3-deoxy-L-galactonate aldolase, results in accumulation of 2-keto-3-deoxy-L-galactonate and thereby elevated expression levels of pectinase encoding genes (Alazi et al., 2018). Inducer molecules that are responsible to the activation of TFs represent therefore another interesting target to enhance expression of CAZymes by constructing strains in which the inducer accumulates either due to prevention of rapid hydrolysis of the inducer or due to inactivation of the metabolic genes that function downstream of the enzyme that forms the inducer.
Future Perspectives and Concluding Remarks
Increased expression of plant cell wall degrading enzymes can be achieved by overexpression of specific transcription factors or by identifying mutations in TFs leading to constitutive activation, and combinations thereof. It is also well established that CreA-dependent CCR has in many cases a negative effect on the production of enzymes and therefore CreA is an important target for improving enzyme production under both inducing and non-inducing conditions. Apart from the approaches described above in the previous paragraphs, modulating chromatin accessibility is yet an under-utilized approach to increase the expression of CAZyme encoding genes in filamentous fungi. Chromatin remodeling of the promoters of CAZyme encoding genes through the actions of the histone acetyltransferase Gcn5, the CCAAT-binding complex, and possibly the putative protein methyltransferase Lae1 is required for the full expression of these CAZymes in T. reesei (Zeilinger et al., 1998; Xin et al., 2013; Aghcheh and Kubicek, 2015; Li et al., 2016). Overexpression of a putative GCN5-related N-acetyltransferase (gene ID 123668) via the A. nidulans gpdA promoter or lae1 via the tef1 promoter resulted in an increased expression of cellulase encoding genes under inducing conditions (on lactose) (Seiboth et al., 2012b; Häkkinen et al., 2014). More recently, Cre1 was shown to be involved in chromatin accessibility of xyr1 promoter (Mello-de-Sousa et al., 2016).
As our knowledge about regulation of TF activities accumulates, various possibilities for rational design of industrial fungal strains emerge, such as combinations of different approaches as already mentioned above, or the use of synthetic TFs. Recently, inducer-independent production of CAZymes by filamentous fungi was achieved via synthetic TFs. For instance, overexpression via pdc1 promoter of a synthetic TF (xyr1-cre1b) consisting of Xyr1 DNA-binding and effector domains, and Cre1 DNA-binding domain, resulted in inducer-independent production of cellulases and hemicellulases (i.e., xylanases) in the Cre1-negative T. reesei strain Rut-C30 (Zhang et al., 2017). Another example of synthetic TFs (CXC-S) was shown in P. oxalicum, where the DNA-binding domain of the constitutively active XlnRA871V was replaced with that of ClrB. This synthetic TF was overexpressed using the A. nidulans gpdA promoter, yielding to inducer-independent, but still glucose-repressed, expression of cellulases and xylanases (Gao et al., 2017a,b).
To conclude, fungal strains with increased or constitutive production of plant biomass degrading enzymes can be rationally designed by tuning the transcriptional regulatory systems. Mutations that lead to constitutive expression of enzymes are difficult to predict and so far only identified via genetic screens. Once identified, these mutations can be successfully transferred to industrial strains or related species. In this review, we also highlighted that regulation of activities of orthologues TFs, or the set of genes regulated by orthologues TFs might be species-specific. It is therefore important that detailed studies on how TFs are activated are performed not only in a single species, which is subsequently used as a blue print to predict the activation mechanism in other fungal species, but performed in several representative fungal species across the filamentous fungi.
Author Contributions
EA and AR designed the content of the manuscript and collected literature. EA wrote the manuscript and AR critically commented on the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
EA was supported by a grant from BE-Basic (Flagship 10). We acknowledge the helpful comments of Dr. Jaap Visser and Prof. Dr. Peter J. Punt on the manuscript.
Abbreviations
CAZyme, carbohydrate active enzyme; GA, D-galacturonic acid; PGA, polygalacturonic acid; TF, transcription factor; CCR, carbon catabolite repression.
References
Adnan, M., Zheng, W., Islam, W., Arif, M., Abubakar, Y. S., Wang, Z., et al. (2017). Carbon catabolite repression in filamentous fungi. Int. J. Mol. Sci. 19:48. doi: 10.3390/ijms19010048
Aghcheh, R. K., and Kubicek, C. P. (2015). Epigenetics as an emerging tool for improvement of fungal strains used in biotechnology. Appl. Microbiol. Biotechnol. 99, 6167–6181. doi: 10.1007/s00253-015-6763-2
Akel, E., Benjamin, M., Seiboth, B., and Kubicek, C. P. (2009). Molecular regulation of Arabinan and L-Arabinose metabolism in Hypocrea jecorina (Trichoderma reesei). Eukaryot. Cell 8, 1837–1844. doi: 10.1128/EC.00162-09
Alazi, E., Khosravi, C., Homan, T. G., du Pré, S., Arentshorst, M., Di Falco, M., et al. (2017). The pathway intermediate 2-keto-3-deoxy-L-galactonate mediates the induction of genes involved in D-galacturonic acid utilization in Aspergillus niger. FEBS Lett. 591, 1408–1418. doi: 10.1002/1873-3468.12654
Alazi, E., Knetsch, T., Di Falco, M. D., Reid, I., Arentshorst, M., Visser, J., et al. (2018). Inducer-independent production of pectinases in Aspergillus niger by overexpression of the D-galacturonic acid responsive transcription factor gaaR. Appl. Microbiol. Biotechnol. 102, 2723–2736. doi: 10.1007/s00253-018-8753-7
Alazi, E., Niu, J., Kowalczyk, J. E., Peng, M., Aguilar Pontes, M. V., van Kan, J. A., et al. (2016). The transcriptional activator GaaR of Aspergillus niger is required for release and utilization of D-galacturonic acid from pectin. FEBS Lett. 590:12, 1804–1815. doi: 10.1002/1873-3468.12211
Alazi, E., Niu, J., Otto, S. B., Arentshorst, M., Pham, T. T. M., Tsang, A., et al. (in press). W361R mutation in GaaR, the regulator of D-galacturonic acid responsive genes, leads to constitutive production of pectinases in Aspergillus niger. Microbiology.
Antoniêto, A. C., dos Santos Castro, L., Silva-Rocha, R., Persinoti, G. F., and Silva, R. N. (2014). Defining the genome-wide role of CRE1 during carbon catabolite repression in Trichoderma reesei using RNA-Seq analysis. Fungal Genet Biol. 73:93–103. doi: 10.1016/j.fgb.2014.10.009
Aro, N., Ilmén, M., Saloheimo, A., and Penttilä, M. (2003). ACEI of Trichoderma reesei is a repressor of cellulase and xylanase expression. Appl. Environ. Microbiol. 69, 56–65. doi: 10.1128/AEM.69.1.56-65.2003
Battaglia, E., Visser, L., Nijssen, A., van Veluw, G. J., Wösten, H. A., and de Vries, R. P. (2011). Analysis of regulation of pentose utilisation in Aspergillus niger reveals evolutionary adaptations in Eurotiales. Stud. Mycol. 69, 31–38. doi: 10.3114/sim.2011.69.03
Benocci, T., Aguilar-Pontes, M. V., Kun, R. S., Seiboth, B., de Vries, R. P., and Daly, P. (2018). ARA1 regulates not only L-arabinose but also D-galactose catabolism in Trichoderma reesei. FEBS Lett. 592, 60–70. doi: 10.1002/1873-3468.12932
Benocci, T., Aguilar-Pontes, M. V., Zhou, M., Seiboth, B., and de Vries, R. P. (2017). Regulators of plant biomass degradation in ascomycetous fungi. Biotechnol. Biofuels 10, 1–25. doi: 10.1186/s13068-017-0841-x
Biggin, M. D. (2011). Animal transcription networks as highly connected, quantitative continua. Dev. Cell 21, 611–626. doi: 10.1016/j.devcel.2011.09.008
Brunner, K., Lichtenauer, A. M., Kratochwill, K., Delic, M., and Mach, R. L. (2007). Xyr1 regulates xylanase but not cellulase formation in the head blight fungus Fusarium graminearum. Curr. Genet. 52, 213–220. doi: 10.1007/s00294-007-0154-x
Caffall, K. H., and Mohnen, D. (2009). The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 344, 1879–1900. doi: 10.1016/j.carres.2009.05.021
Calero-Nieto, F., Di Pietro, A., Roncero, M. I., and Hera, C. (2007). Role of the transcriptional activator XlnR of Fusarium oxysporum in regulation of xylanase genes and virulence. Mol. Plant Microbe Interact. 20, 977–985. doi: 10.1094/MPMI-20-8-0977
Cao, Y., Zheng, F., Wang, L., Zhao, G., Chen, G., Zhang, W., et al. (2017). Rce1, a novel transcriptional repressor, regulates cellulase gene expression by antagonizing the trans-activator Xyr1 in Trichoderma reesei. Mol. Microbiol. 105, 65–83. doi: 10.1111/mmi.13685
Chang, P. K., and Ehrlich, K. C. (2013). Genome-wide analysis of the Zn(II)2Cys6 zinc cluster-encoding gene family in Aspergillus flavus. Appl. Microbiol. Biotechnol. 97, 4289–4300. doi: 10.1007/s00253-013-4865-2
Chen, M., Qin, Y., Cao, Q., Liu, G., Li, J., Li, Z., et al. (2013). Promotion of extracellular lignocellulolytic enzymes production by restraining the intracellular β-glucosidase in Penicillium decumbens. Bioresour. Technol. 137, 33–40. doi: 10.1016/j.biortech.2013.03.099
Chua, G., Morris, Q. D., Sopko, R., Robinson, M. D., Ryan, O., Chan, E. T., et al. (2006). Identifying transcription factor functions and targets by phenotypic activation. Proc. Natl. Acad. Sci. U.S.A. 103, 12045–12050. doi: 10.1073/pnas.0605140103
Coradetti, S. T., Craig, J. P., Xiong, Y., Shock, T., Tian, C., and Glass, N. L. (2012). Conserved and essential transcription factors for cellulase gene expression in ascomycete fungi. Proc. Natl. Acad. Sci. U.S.A. 109, 7397–7402. doi: 10.1073/pnas.1200785109
Coradetti, S. T., Xiong, Y., and Glass, N. L. (2013). Analysis of a conserved cellulase transcriptional regulator reveals inducer-independent production of cellulolytic enzymes in Neurospora crassa. Microbiol. Open 2, 595–609. doi: 10.1002/mbo3.94
Craig, J. P., Coradetti, S. T., Starr, T. L., and Glass, N. L. (2015). Direct target network of the Neurospora crassa plant cell wall deconstruction regulators CLR-1, CLR-2, and XLR-1. Mbio 6, 1–11. doi: 10.1128/mBio.01452-15
Culleton, H., Mckie, V., and de Vries, R. P. (2013). Physiological and molecular aspects of degradation of plant polysaccharides by fungi: what have we learned from Aspergillus?. Biotechnol. J. 8, 884–894. doi: 10.1002/biot.201200382
Cziferszky, A., Mach, R. L., and Kubicek, C. P. (2002). Phosphorylation positively regulates DNA binding of the carbon catabolite repressor Cre1 of Hypocrea jecorina (Trichoderma reesei). J. Biol. Chem. 277, 14688–14694. doi: 10.1074/jbc.M200744200
de Vries, R. P., Riley, R., Wiebenga, A., Aguilar-Osorio, G., Amillis, S., Uchima, C. A., et al. (2017). Comparative genomics reveals high biological diversity and specific adaptations in the industrially and medically important fungal genus Aspergillus. Genome Biol. 18:28. doi: 10.1186/s13059-017-1151-0
Denton, J. A., and Kelly, J. M. (2011). Disruption of Trichoderma reesei cre2, encoding an ubiquitin C-terminal hydrolase, results in increased cellulase activity. BMC Biotechnol. 11, 1–10. doi: 10.1186/1472-6750-11-103
Derntl, C., Gudynaite-Savitch, L., Calixte, S., White, T., Mach, R. L., and Mach-Aigner, A. R. (2013). Mutation of the Xylanase regulator 1 causes a glucose blind hydrolase expressing phenotype in industrially used Trichoderma strains. Biotechnol. Biofuels 6, 1–11. doi: 10.1186/1754-6834-6-62
Derntl, C., Kluger, B., Bueschl, C., Schuhmacher, R., Mach, R. L., and Mach-Aigner, A. R. (2017). Transcription factor Xpp1 is a switch between primary and secondary fungal metabolism. Proc. Natl. Acad. Sci. U.S.A. 114, E560–E569. doi: 10.1073/pnas.1609348114
Derntl, C., Rassinger, A., Srebotnik, E., Mach, R. L., and Mach-Aigner, A. R. (2015). Xpp1 regulates the expression of xylanases, but not of cellulases in Trichoderma reesei. Biotechnol. Biofuels 8, 1–11. doi: 10.1186/s13068-015-0298-8
Fang, X., Shen, Y., Zhao, J., Bao, X., and Qu, Y. (2010). Status and prospect of lignocellulosic bioethanol production in China. Bioresour. Technol. 101, 4814–4819. doi: 10.1016/j.biortech.2009.11.050
Fujii, T., Inoue, H., and Ishikawa, K. (2013). Enhancing cellulase and hemicellulase production by genetic modification of the carbon catabolite repressor gene, creA, in Acremonium cellulolyticus. AMB Express 3, 1–9. doi: 10.1186/2191-0855-3-73
Fujii, T., Inoue, H., and Ishikawa, K. (2014). Characterization of the xylanase regulator protein gene, xlnR, in Talaromyces cellulolyticus (formerly known as Acremonium cellulolyticus). Biosci. Biotechnol. Biochem. 78, 1564–1567. doi: 10.1080/09168451.2014.923298
Gao, L., Li, Z., Xia, C., Qu, Y., Liu, M., Yang, P., et al. (2017a). Combining manipulation of transcription factors and overexpression of the target genes to enhance lignocellulolytic enzyme production in Penicillium oxalicum. Biotechnol. Biofuels 10, 1–16. doi: 10.1186/s13068-017-0783-3
Gao, L., Xia, C., Xu, J., Li, Z., Yu, L., Liu, G., et al. (2017b). Constitutive expression of chimeric transcription factors enables cellulase synthesis under non-inducing conditions in Penicillium oxalicum. Biotechnol. J. 12, 1–5. doi: 10.1002/biot.201700119
Gidley, M. J. (2001). “Starch structure/ function relationships: achievements and challenges,” in Starch: Advances in structure and function, eds T. L. Barsby, A. M. Donald, and P. J. Frazier (Cambridge: The Royal Society of Chemistry), 1–7.
Gielkens, M. M., Dekkers, E., Visser, J., and de Graaff, L. H. (1999). Two cellobiohydrolase-encoding genes from Aspergillus niger require D -Xylose and the xylanolytic transcriptional activator XlnR for their expression. Appl. Environ. Microbiol. 65, 4340–4345.
Granek, J. A., and Clarke, N. D. (2005). Explicit equilibrium modeling of transcription-factor binding and gene regulation. Genome. Biol. 6:r87. doi: 10.1186/gb-2005-6-10-r87
Gupta, V. K., Kubicek, C. P., Berrin, J. G., Wilson, D. W., Couturier, M., Berlin, A., et al. (2016). Fungal enzymes for bio-products from sustainable and waste biomass. Trends Biochem. Sci. 41, 633–645. doi: 10.1016/j.tibs.2016.04.006
Häkkinen, M., Valkonen, M. J., Westerholm-Parvinen, A., Aro, N., Arvas, M., Vitikainen, M., et al. (2014). Screening of candidate regulators for cellulase and hemicellulase production in Trichoderma reesei and identification of a factor essential for cellulase production. Biotechnol. Biofuels 7:14. doi: 10.1186/1754-6834-7-14
Hasper, A. A. (2004). Function and Mode of Regulation of the Transcriptional Activator XlnR From Aspergillus. Dissertation, Wageningen University, Wageningen.
Hasper, A. A., Trindade, L. M., van der Veen, D., van Ooyen, A. J., and de Graaff, L. H. (2004). Functional analysis of the transcriptional activator XlnR from Aspergillus niger. Microbiology 150, 1367–1375. doi: 10.1099/mic.0.26557-0
Herold, S., Bischof, R., Metz, B., Seiboth, B., and Kubicek, C. P. (2013). Xylanase gene transcription in Trichoderma reesei is triggered by different inducers representing different hemicellulosic pentose polymers. Eukaryot. Cell 12, 390–398. doi: 10.1128/EC.00182-12
Ho, H., and Ho, K. (2015). Fungal strain improvement of Aspergillus brasiliensis for overproduction of xylanase in submerged fermentation through UV irradiation and chemicals mutagenesis. J. Adv. Biol. Biotechnol. 3, 117–131. doi: 10.9734/JABB/2015/17274
Huberman, L. B., Liu, J., Qin, L., and Glass, N. L. (2016). Regulation of the lignocellulolytic response in filamentous fungi. Fungal Biol. Rev. 30, 101–111. doi: 10.1016/j.fbr.2016.06.001
Ichinose, S., Tanaka, M., Shintani, T., and Gomi, K. (2014). Improved α-amylase production by Aspergillus oryzae after a double deletion of genes involved in carbon catabolite repression. Appl. Microbiol. Biotechnol. 98, 335–343. doi: 10.1007/s00253-013-5353-4
Ichinose, S., Tanaka, M., Shintani, T., and Gomi, K. (2017). Increased production of biomass-degrading enzymes by double deletion of creA and creB genes involved in carbon catabolite repression in Aspergillus oryzae. J. Biosci. Bioeng. 125, 141–147. doi: 10.1016/j.jbiosc.2017.08.019
Jiang, Y., Duarte, A. V., van den Brink, J., Wiebenga, A., Zou, G., Wang, C., et al. (2016). Enhancing saccharification of wheat straw by mixing enzymes from genetically-modified Trichoderma reesei and Aspergillus niger. Biotechnol. Lett. 38, 65–70. doi: 10.1007/s10529-015-1951-9
Kolpak, F. J., and Blackwell, J. (1976). Determination of the structure of cellulose II. Macromolecules 9, 273–278. doi: 10.1021/ma60050a019
Kowalczyk, J. E., Benoit, I., and de Vries, R. P. (2014). Regulation of plant biomass utilization in Aspergillus. Adv. Appl. Microbiol. 88, 31–56. doi: 10.1016/B978-0-12-800260-5.00002-4
Kowalczyk, J. E., Lubbers, R. J. M., Peng, M., Battaglia, E., Visser, J., and de Vries, R. P. (2017). Combinatorial control of gene expression in Aspergillus niger grown on sugar beet pectin. Sci. Rep. 7, 1–12. doi: 10.1038/s41598-017-12362-y
Kunitake, E., and Kobayashi, T. (2017). Conservation and diversity of the regulators of cellulolytic enzyme genes in Ascomycete fungi. Curr. Genet. 63, 951–958. doi: 10.1007/s00294-017-0695-6
Lamb, H. K., Newton, G. H., Levett, L. J., Cairns, E., Roberts, C. F., and Hawkins, A. R. (1996). The QUTA activator and QUTR repressor proteins of Aspergillus nidulans interact to regulate transcription of the quinate utilization pathway genes. Microbiology 142, 1477–1490. doi: 10.1099/13500872-142-6-1477
Levett, L. J., Si-Hoe, S. M., Liddle, S., Wheeler, K., Smith, D., Lamb, H. K., et al. (2000). Identification of domains responsible for signal recognition and transduction within the QUTR transcription repressor protein. Biochem. J. 350, 189–197. doi: 10.1042/bj3500189
Li, J., Lin, L., Li, H., Tian, C., and Ma, Y. (2014). Transcriptional comparison of the filamentous fungus Neurospora crassa growing on three major monosaccharides D-glucose, D-xylose and L-arabinose. Biotechnol. Biofuels 7, 1–15. doi: 10.1186/1754-6834-7-31
Li, Y., Zheng, X., Zhang, X., Bao, L., Zhu, Y., Qu, Y., et al. (2016). The different roles of Penicillium oxalicum LaeA in the production of extracellular cellulase and ß-xylosidase. Front. Microbiol. 7:2091. doi: 10.3389/fmicb.2016.02091
Li, Z., Yao, G., Wu, R., Gao, L., Kan, Q., Liu, M., et al. (2015). Synergistic and dose-controlled regulation of cellulase gene expression in Penicillium oxalicum. PLoS Genet. 11, 1–45. doi: 10.1371/journal.pgen.1005509
Lichius, A., Seidl-Seiboth, V., Seiboth, B., and Kubicek, C. P. (2014). Nucleo-cytoplasmic shuttling dynamics of the transcriptional regulators XYR1 and CRE1 under conditions of cellulase and xylanase gene expression in Trichoderma reesei. Mol. Microbiol. 94, 1162–1178. doi: 10.1111/mmi.12824
Liu, G., Zhang, L., Qin, Y., Zou, G., Li, Z., Yan, X., et al. (2013). Long-term strain improvements accumulate mutations in regulatory elements responsible for hyper-production of cellulolytic enzymes. Sci. Rep. 3, 1–7. doi: 10.1038/srep01569
Liu, R., Chen, L., Jiang, Y., Zou, G., and Zhou, Z. (2017). A novel transcription factor specifically regulates GH11 xylanase genes in Trichoderma reesei. Biotechnol. Biofuels 10:194. doi: 10.1186/s13068-017-0878-x
Lombard, V., Golaconda Ramulu, H., Drula, E., Coutinho, P. M., and Henrissat, B. (2014). The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 42, 490–495. doi: 10.1093/nar/gkt1178
Loqué, D., Scheller, H. V., and Pauly, M. (2015). Engineering of plant cell walls for enhanced biofuel production. Curr. Opin. Plant Biol. 25, 151–161. doi: 10.1016/j.pbi.2015.05.018
Lv, X., Zheng, F., Li, C., Zhang, W., Chen, G., and Liu, W. (2015). Characterization of a copper responsive promoter and its mediated overexpression of the xylanase regulator 1 results in an induction-independent production of cellulases in Trichoderma reesei. Biotechnol. Biofuels 8, 1–14. doi: 10.1186/s13068-015-0249-4
Mach-Aigner, A. R., Omony, J., Jovanovic, B., van Boxtel, A. J., and de Graaff, L. H. (2012). D-Xylose concentration-dependent hydrolase expression profiles and the function of CreA and XlnR in Aspergillus niger. Appl. Environ. Microbiol. 78, 3145–3155. doi: 10.1128/AEM.07772-11
Mach-Aigner, A. R., Pucher, M. E., Steiger, M. G., Bauer, G. E., Preis, S. J., and Mach, R. L. (2008). Transcriptional regulation of xyr1, encoding the main regulator of the xylanolytic and cellulolytic enzyme system in Hypocrea jecorina. Appl. Environ. Microbiol. 74, 6554–6562. doi: 10.1128/AEM.01143-08
MacPherson, S., Larochelle, M., and Turcotte, B. (2006). A fungal family of transcriptional regulators: the zinc cluster proteins. Microbiol. Mol. Biol. Rev. 70, 583–604. doi: 10.1128/MMBR.00015-06
Makita, T., Katsuyama, Y., Tani, S., Suzuki, H., Kato, N., Todd, R. B., et al. (2009). Inducer-dependent nuclear localization of a Zn(II)2Cys6 transcriptional activator, AmyR, in Aspergillus nidulans. Biosci. Biotechnol. Biochem. 73, 391–399. doi: 10.1271/bbb.80654
Marui, J., Kitamoto, N., Kato, M., Kobayashi, T., and Tsukagoshi, N. (2002). Transcriptional activator, AoXlnR, mediates cellulose-inductive expression of the xylanolytic and cellulolytic genes in Aspergillus oryzae. FEBS Lett. 528, 279–282. doi: 10.1016/S0014-5793(02)03328-8
Marui, J., Tanaka, A., Mimura, S., de Graaff, L. H., Visser, J., Kitamoto, N., et al. (2002b). A transcriptional activator, AoXlnR, controls the expression of genes encoding xylanolytic enzymes in Aspergillus oryzae. Fungal Genet. Biol. 35, 157–169. doi: 10.1006/fgbi.2001.1321
Mello-de-Sousa, T. M., Rassinger, A., Derntl, C., Poças-Fonseca, M. J., Mach, R. L., and Mach-Aigner, A. R. (2016). The relation between promoter chromatin status, Xyr1 and cellulase ex-pression in Trichoderma reesei. Curr. Genomics 17, 145–152. doi: 10.2174/1389202917666151116211812
Meyer, V., Fiedler, M., Nitsche, B., and King, R. (2015). The cell factory Aspergillus enters the big data era : opportunities and challenges for optimising product formation. Adv. Biochem. Eng. Biotechnol. 149, 91–132. doi: 10.1007/10_2014_297
Meyer, V., Ram, A. F. J., and Punt, P. J. (2010). “Genetics, genetic manipulation, and approaches to strain improvement of filamentous fungi,” in Manual of Industrial Microbiology and Biotechnology, eds R. Baltz, A. Demain, J. Davies, A. Bull, B. Junker, L. Katz et al. (Washington, DC: ASM Press), 318–329.
Montenecourt, B., and Eveleigh, D. (1979). “Selective screening methods for the isolation of high yielding cellulase mutants of Trichoderma reesei,” in Hydrolysis of Cellulose: Mechanisms of Enzymatic and Acid Catalysis. eds R. Brown, L. Jurasek (Washington, DC: American Chemical Society), 289–301.
Nakari-Setälä, T., Paloheimo, M., Kallio, J., Vehmaanperä, J., Penttilä, M., and Saloheimo, M. (2009). Genetic modification of carbon catabolite repression in Trichoderma reesei for improved protein production. Appl. Environ. Microbiol. 75, 4853–4860. doi: 10.1128/AEM.00282-09
Niu, J., Alazi, E., Reid, I. D., Arentshorst, M., Punt, P. J., Visser, J., et al. (2017). An evolutionarily conserved transcriptional activator-repressor module controls expression of genes for D-galacturonic acid utilization in Aspergillus niger. Genetics 205, 169–183. doi: 10.1534/genetics.116.194050
Niu, J., Homan, T. G., Arentshorst, M., de Vries, R. P., Visser, J., and Ram, A. F. (2015). The interaction of induction and repression mechanisms in the regulation of galacturonic acid-induced genes in Aspergillus niger. Fungal Genet. Biol. 82, 32–42. doi: 10.1016/j.fgb.2015.06.006
Noguchi, Y., Sano, M., Kanamaru, K., Ko, T., Takeuchi, M., Kato, M., et al. (2009). Genes regulated by AoXlnR, the xylanolytic and cellulolytic transcriptional regulator, in Aspergillus oryzae. Appl. Microbiol. Biotechnol. 85, 141–154. doi: 10.1007/s00253-009-2236-9
Noguchi, Y., Tanaka, H., Kanamaru, K., Kato, M., and Kobayashi, T. (2011). Xylose triggers reversible phosphorylation of XlnR, the fungal transcriptional activator of xylanolytic and cellulolytic genes in Aspergillus oryzae. Biosci. Biotechnol. Biochem. 75, 953–959. doi: 10.1271/bbb.100923
Ogawa, M., Kobayashi, T., and Koyama, Y. (2012). ManR, a novel Zn(II)2Cys6 transcriptional activator, controls the β-mannan utilization system in Aspergillus oryzae. Fungal Genet. Biol. 49, 987–995. doi: 10.1016/j.fgb.2012.09.006
Ogawa, M., Kobayashi, T., and Koyama, Y. (2013). ManR, a transcriptional regulator of the β-mannan utilization system, controls the cellulose utilization system in Aspergillus oryzae. Biosci. Biotechnol. Biochem. 77, 426–429. doi: 10.1271/bbb.120795
Okuda, N., Fujii, T., Inoue, H., Ishikawa, K., and Hoshino, T. (2016). Enhancing cellulase production by overexpression of xylanase regulator protein gene, xlnR, in Talaromyces cellulolyticus cellulase hyperproducing mutant strain. Biosci. Biotechnol. Biochem. 80, 2065–2068. doi: 10.1080/09168451.2016.1189315
Pel, H. J., de Winde, J. H., Archer, D. B., Dyer, P. S., Hofmann, G., Schaap, P. J., et al. (2007). Genome sequencing and analysis of the versatile cell factory Aspergillus niger CBS 513.88. Nat. Biotechnol. 25, 221–231. doi: 10.1038/nbt1282
Portnoy, T., Margeot, A., Linke, R., Atanasova, L., Fekete, E., Sándor, E., Hartl, L., et al. (2011b). The CRE1 carbon catabolite repressor of the fungus Trichoderma reesei: a master regulator of carbon assimilation BMC Genomics 12:269. doi: 10.1186/1471-2164-12-269
Portnoy, T., Margeot, A., Seidl-Seiboth, V., Le Crom, S., Chaabane, F. B., Linke, R., et al. (2011a). Differential regulation of the cellulase transcription factors XYR1, ACE2, and ACE1 in Trichoderma reesei strains producing high and low levels of cellulase. Eukaryot. Cell 10, 262–271. doi: 10.1128/EC.00208-10
Prathumpai, W., McIntyre, M., and Nielsen, J. (2004). The effect of CreA in glucose and xylose catabolism in Aspergillus nidulans. Appl. Microbiol. Biotechnol. 63, 748–753. doi: 10.1007/s00253-003-1409-1
Rauscher, R., Würleitner, E., Wacenovsky, C., Aro, N., Stricker, A. R., Zeilinger, S., et al. (2006). Transcriptional regulation of xyn1, encoding xylanase I, in Hypocrea jecorina. Eukaryot. Cell 5:447–456. doi: 10.1128/EC.5.3.447-456.2006
Ries, L. N., Beattie, S. R., Espeso, E. A., Cramer, R. A., and Goldman, G. H. (2016). Diverse regulation of the CreA carbon catabolite repressor in Aspergillus nidulans. Genetics 203, 335–352. doi: 10.1534/genetics.116.187872
Ritsema, T., and Smeekens, S. (2003). Fructans: beneficial for plants and humans. Curr. Opin. Plant Biol. 6, 223–230. doi: 10.1016/S1369-5266(03)00034-7
Scheller, H. V., and Ulvskov, P. (2010). Hemicelluloses. Annu. Rev. Plant Biol. 61, 263–289. doi: 10.1146/annurev-arplant-042809-112315
Seiboth, B., Herold, S., and Kubicek, C. P. (2012a). “Metabolic engineering of inducer formation for cellulase and hemicellulase gene expression in Trichoderma reesei,” in Reprogramming Microbial Metabolic Pathways, Volume 64 of Subcellular Biochemistry, eds X. Wang, J. Chen, and P. J. Quinn (Dordrecht: Springer), 367–390.
Seiboth, B., Karimi, R. A., Phatale, P. A., Linke, R., Hartl, L., Sauer, D. G., et al. (2012b). The putative protein methyltransferase LAE1 controls cellulase gene expression in Trichoderma reesei. Mol. Microbiol. 84, 1150–1164. doi: 10.1111/j.1365-2958.2012.08083.x
Stricker, A. R., Grosstessner-Hain, K., Würleitner, E., and Mach, R. L. (2006). Xyr1 (Xylanase Regulator 1) regulates both the hydrolytic enzyme system and D-xylose metabolism in Hypocrea jecorina. Eukaryot. Cell 5, 2128–2137. doi: 10.1128/EC.00211-06
Sun, J., and Glass, N. L. (2011). Identification of the CRE-1 cellulolytic regulon in Neurospora crassa. PLoS ONE 6:e25654. doi: 10.1371/journal.pone.0025654
Sun, J., Tian, C., Diamond, S., and Glass, N. L. (2012). Deciphering transcriptional regulatory mechanisms associated with hemicellulose degradation in Neurospora crassa. Eukaryot. Cell 11, 482–493. doi: 10.1128/EC.05327-11
Suzuki, K., Tanaka, M., Konno, Y., Ichikawa, T., Ichinose, S., Hasegawa-Shiro, S., et al. (2015). Distinct mechanism of activation of two transcription factors, AmyR and MalR, involved in amylolytic enzyme production in Aspergillus oryzae. Appl. Microbiol. Biotechnol. 99, 1805–1815. doi: 10.1007/s00253-014-6264-8
Sweeney, M. D., and Xu, F. (2012). Biomass converting enzymes as industrial biocatalysts for fuels and chemicals: recent developments. Catalysts 2, 244–263. doi: 10.3390/catal2020244
Tamayo, E. N., Villanueva, A., Hasper, A. A., de Graaff, L. H., Ramón, D., and Orejas, M. (2008). CreA mediates repression of the regulatory gene xlnR which controls the production of xylanolytic enzymes in Aspergillus nidulans. Fungal Genet. Biol. 45, 984–993. doi: 10.1016/j.fgb.2008.03.002
Tani, S., Katsuyama, Y., Hayashi, T., Suzuki, H., Kato, M., Gomi, K., et al. (2001). Characterization of the amyR gene encoding a transcriptional activator for the amylase genes in Aspergillus nidulans. Curr. Genet. 39, 10–15. doi: 10.1007/s002940000175
Tani, S., Kawaguchi, T., and Kobayashi, T. (2014). Complex regulation of hydrolytic enzyme genes for cellulosic biomass degradation in filamentous fungi. Appl. Microbiol. Biotechnol. 98, 4829–4837. doi: 10.1007/s00253-014-5707-6
Thieme, N., Wu, V. W., Dietschmann, A., Salamov, A. A., Wang, M., Johnson, J., et al. (2017). The transcription factor PDR-1 is a multi-functional regulator and key component of pectin deconstruction and catabolism in Neurospora crassa. Biotechnol. Biofuels 10, 1–21. doi: 10.1186/s13068-017-0807-z
van Hanh, V., Pham, T. A., and Kim, K. (2010). Improvement of a fungal strain by repeated and sequential mutagenesis and optimization of solid-state fermentation for the hyper-production of raw-starch-digesting enzyme. J. Microbiol. Biotechnol. 20, 718–726. doi: 10.4014/jmb.0908.08016
van Peij, N. N., Visser, J., and de Graaff, L. H. (1998). Isolation and analysis of xlnR, encoding a transcriptional activator co-ordinating xylanolytic expression in Aspergillus niger. Mol. Microbiol. 27, 131–142. doi: 10.1046/j.1365-2958.1998.00666.x
vanKuyk, P. A., Benen, J. A., Wösten, H. A. B., Visser, J., and de Vries, R. P. (2012). A broader role for AmyR in Aspergillus niger: regulation of the utilisation of D-glucose or D-galactose containing oligo- and polysaccharides. Appl. Microbiol. Biotechnol. 93, 285–293. doi: 10.1007/s00253-011-3550-6
Wagh, S. A. (2010). Bioconversion of Tannic Acid to Gallic Acid by Using Fungal Tannase. Dissertation, National Chemical Laboratory, Pune.
Wang, J., Gong, Y., Zhao, S., and Liu, G. (2018). A new regulator of cellulase and xylanase in the thermophilic fungus Myceliophthora thermophila strain ATCC 42464. 3 Biotech 8:160. doi: 10.1007/s13205-017-1069-y
Wang, J., Wu, Y., Gong, Y., Yu, S., and Liu, G. (2015). Enhancing xylanase production in the thermophilic fungus Myceliophthora thermophila by homologous overexpression of Mtxyr1. J. Ind. Microbiol. Biotechnol. 42, 1233–1241. doi: 10.1007/s10295-015-1628-3
Wang, S., Liu, G., Wang, J., Yu, J., Huang, B., and Xing, M. (2013). Enhancing cellulase production in Trichoderma reesei RUT C30 through combined manipulation of activating and repressing genes. J. Ind. Microbiol. Biotechnol. 40, 633–641. doi: 10.1007/s10295-013-1253-y
Xin, Q., Gong, Y., Lv, X., Chen, G., and Liu, W. (2013). Trichoderma reesei histone acetyltransferase Gcn5 regulates fungal growth, conidiation, and cellulase gene expression. Curr. Microbiol. 67, 580–589. doi: 10.1007/s00284-013-0396-4
Yang, F., Gong, Y., Liu, G., Zhao, S. M., and Wang, J. (2015). Enhancing cellulase production in thermophilic fungus Myceliophthora thermophila ATCC42464 by RNA interference of cre1 gene expression. J. Microbiol. Biotechnol. 25, 1101–1107. doi: 10.4014/jmb.1501.01049
Yao, G., Li, Z., Gao, L., Wu, R., Kan, Q., Liu, G., et al. (2015). Redesigning the regulatory pathway to enhance cellulase production in Penicillium oxalicum. Biotechnol. Biofuels 8:71. doi: 10.1186/s13068-015-0253-8
Zeilinger, S., Mach, R. L., and Kubicek, C. P. (1998). Two adjacent protein binding motifs in the cbh2 (Cellobiohydrolase II-encoding) promoter of the fungus Hypocrea jecorina (Trichoderma reesei) cooperate in the induction by cellulose. J. Biol. Chem. 273, 34463–34471. doi: 10.1074/jbc.273.51.34463
Zhang, L., Lubbers, R. J. M., Simon, A., Stassen, J. H. M., Vargas Ribera, P. R., Viaud, M., et al. (2016). A novel Zn2Cys6 transcription factor BcGaaR regulates D-galacturonic acid utilization in Botrytis cinerea. Mol. Microbiol. 100, 247–262. doi: 10.1111/mmi.13314
Zhang, X., Li, Y., Zhao, X., and Bai, F. (2017). Constitutive cellulase production from glucose using the recombinant Trichoderma reesei strain overexpressing an artificial transcription activator. Bioresour. Technol. 223, 317–322. doi: 10.1016/j.biortech.2016.10.083
Keywords: CAZyme, plant biomass degradation, strain design, industrial fungi, transcriptional regulation, overexpression of transcription factors, constitutively active transcription factors, inducer accumulation
Citation: Alazi E and Ram AFJ (2018) Modulating Transcriptional Regulation of Plant Biomass Degrading Enzyme Networks for Rational Design of Industrial Fungal Strains. Front. Bioeng. Biotechnol. 6:133. doi: 10.3389/fbioe.2018.00133
Received: 28 March 2018; Accepted: 05 September 2018;
Published: 25 September 2018.
Edited by:
Gustavo Henrique Goldman, Universidade de São Paulo, BrazilReviewed by:
Ramón Alberto Batista-García, Universidad Autónoma del Estado de Morelos, MexicoBernhard Seiboth, Technische Universität Wien, Austria
Copyright © 2018 Alazi and Ram. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arthur F. J. Ram, YS5mLmoucmFtQGJpb2xvZ3kubGVpZGVudW5pdi5ubA==
 Ebru Alazi
Ebru Alazi Arthur F. J. Ram
Arthur F. J. Ram