
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Agron. , 06 October 2020
Sec. Plant-Soil Interactions
Volume 2 - 2020 | https://doi.org/10.3389/fagro.2020.580659
This article is part of the Research Topic Soil-Plant-Microbe Interactions: An Innovative Approach Towards Improving Soil Health and Plant Growth View all 26 articles
Kiwifruit early decline syndrome is a widespread disorder that affects worldwide kiwifruit orchards. During the last few years, the seriousness and diffusion of this disorder worsened; as a consequence, in several rural areas the economic sustainability of farms was seriously affected. The early symptoms involve leaves (epinasty, chlorosis, desiccation, and abscission), fruits (decrease of number, size, and sugar content), and roots (anomalous morphology and anatomy). After symptoms occurrence, in a short time plants collapse and die: frequently this happens in the same or in the following year. Even though several phytopathological or agronomical studies have been carried out, a clear and univocal explanation of the causes and the possible remedies are yet to be understood. A field trial was carried out in an experimental kiwifruit orchard, in which several soil management practices (ridging, amendment with compost, bioinoculation with selected rhizospheric microorganisms) were tested to evaluate their effect on early decline symptoms occurrence. The analysis of plant growth parameters, root morphology and anatomy, and symptoms onset were related to environmental data (air and soil temperature, soil water tension). The results pointed to a possible role and interaction between agronomic soil management and climatic conditions as the triggering factors of kiwifruit early decline syndrome.
Kiwifruit is an economically important fruit crop, totaling more than 4 million tons of fruits produced annually world-wide, 14% of which are produced in Italy (Ferguson, 2016; FAO, 2018). However, in the last decade, kiwifruit cultivation has been challenged by several threats. Serious damages to kiwifruit orchards arose from the bacteria Pseudomonas syringae pv. actinidiae (Scortichini et al., 2012) and from the insects Halyomorpha halys (Haye and Weber, 2017). More recently, a syndrome resulting in an early decline of the plant occurred, hereafter called kiwifruit early decline syndrome (KEDS). The plants affected by KEDS suddenly collapse and die (Montanaro et al., 2014; Tacconi et al., 2014; Tosi et al., 2015; Sorrenti et al., 2016; Nari and Vittone, 2017; Bardi, 2020; Spigaglia et al., 2020).
The plant of kiwifruit is frequently prone to physiological disorder symptoms that have been associated with anomalous root morphology and rotting of structural roots, attributed to soil-borne pathogens (Reid et al., 1991; Tacconi et al., 2015; Donati et al., 2019; Prencipe et al., 2020; Spigaglia et al., 2020) as well as to soil-borne abiotic stresses (Savé and Serrano, 1986; Smith et al., 1989; Reid et al., 1991). Among abiotic stresses, soil structure and texture (Reid et al., 1991), nutrients availability, organic matter content, heavy metal content, xenobiotics phytotoxicity, water availability, and temperature (Xyloyannis et al., 1986; Smith et al., 1989), as well as management practices, e.g., overpruning and overcropping (Black et al., 2012), have been considered as possible factors inducing KEDS (Bardi, 2020). None of these factors was clearly identified as the sole KEDS cause; however, almost all of them can be directly or indirectly related to the extreme sensitivity of kiwifruit to low oxygen availability in soil (Savé and Serrano, 1986; Smith et al., 1989; Reid et al., 1991). Therefore, soil management practices improving root aeration, limiting the risk of waterlogging and reducing the development of soil-borne diseases have been proposed to avoid KEDS occurrence (Reid et al., 1991; Sorrenti et al., 2016; Tacconi et al., 2019a). However, over the last few years these practices were not sufficient to prevent the worsening of KEDS occurrence in both young and adult orchards (Spigaglia et al., 2020). As a consequence, the kiwifruit area steadily and dramatically decreased across Northern and Central Italy. 12.6% Italian kiwifruit orchards were uprooted in 2018; the most affected region was Veneto where 70% kiwifruit orchard were uprooted, followed by Piedmont where the loss of the land area dedicated to kiwifruit production was 28% (Tacconi et al., 2019b; Spigaglia et al., 2020).
An unusual “rapid apple decline,” with symptoms similar to KEDS, has become a major concern for apple growers, particularly in the northeastern United States (Singh et al., 2019). None of the several biotic and abiotic factors analyzed has been clearly identified as the cause of this decline, and it is speculated that differences in soil abiotic factors affecting moisture levels in roots, in combination with extreme weather conditions, might be the cause of rapid apple decline (Singh et al., 2019).
As a consequence of the global climate change, also in Italy a significant rise in temperature was observed over recent years, in particular during the summer season (RAN, 2010–2020). The role of climate, and in particular of high summer temperatures, on the onset of KEDS has not been studied yet. In order to verify how weather profiles can interfere with the kiwifruit orchard agronomical management and to look for possible solutions to KEDS occurrence, a trial was carried out in which different agronomical practices affecting the physical, chemical and biological characteristics of the soil were tested and weather data monitored, in particular the air and soil temperatures. Soil ridging was evaluated in comparison to traditional flat soil management, and the effect of soil biological fertility improvement by addition of compost or selected rhizospheric microorganisms was assessed. As the tested practices, in general, show a positive impact on root functionality (Diacono and Montemurro, 2011; Chaparro et al., 2012), an improvement of plant resilience against potential soil pathogenic or opportunistic microorganisms, as well as against abiotic stresses induced by extreme weather conditions, was hypothesized.
The trial was established in 2017 on a field (Saluzzo, Piedmont Region, North-West Italy, 44°38′49.8″N 7°31′52.6″E) located in a high risk area, i.e., characterized by high incidence of KEDS symptoms (Nari and Vittone, 2017). The field was a 30-years old kiwifruit orchards until 2016, when it was uprooted due to KEDS damages.
The soil was classified as loamy according to USDA classification (USDA, 1975) on both 0–30 and 30–60 cm depth. The major chemical and nutrient content characteristics of the 0–30 cm layer were: pH 6.8, 2.9% organic matter content, 87 ppm exchangeable K, 64 ppm soluble P and 2,409 ppm exchangeable Ca.
The trial was set up as a block design with 5 treatments and 4 replicates per treatment; 6 plants were analyzed for each replicate, with a total of 24 plants per treatment.
The soil was plowed up and tilled to assure suitable conditions for planting.
The following treatments were applied to the soil just before planting:
a) Soil without any additional tillage nor addition of compost (Control, C)
b) Soil tilled to form raised ridges of about 50–60 cm in rows (Ridged, R)
c) Soil without any additional tillage, with addition of compost (130 t ha−1, No-Ridged + Compost, CC)
d) Soil tilled to form raised ridges of about 50–60 cm in rows, with addition of compost (80 t·ha−1, Ridged + Compost, RC)
e) Soil tilled to form raised ridges of about 50–60 cm in rows, with addition of a microbial consortium (Micosat, CCS Aosta) composed of 40% mycorrhizal fungi (crude inoculum composed by Glomus spp. GB 67, G. mosseae GP 11, G. viscosum GC 4), 21.6% saprophytic fungi (Trichoderma harzianum TH 01, Trichoderma viride TV 03, Pochonia chlamydosporia PC 50) and 4,85 × 107 C.F.U. g−1 plant growth promoting bacteria (Bacillus amyloliquefaciens BA 41, Pseudomonas fluorescens PN 53, Pseudomonas spp. PT 65, Streptomyces spp. SA 51, SB 14 e ST 60) at a dose of 10 g·plant−1 (Ridged + Inoculum, RI). Each plant was supplemented with 40 ml of a mixture composed by 20 ml of an organic N fertilizer (4% N, 14% C—Eutrofit, AGM s.r.l.) and 20 ml of a humic extract (0.5% N, 1.5% C—Red radicale, Germina). The role of this supplement was to support the establishment of the microbial inoculum. The mixture was applied three times: at planting and twice again, with an interval of about a month between applications. In 2018 and 2019 the treatment was repeated with the same doses and timing.
The compost applied in treatments CC and RC contained 23% organic C, 2.55% N (93% in organic forms), 1.6% P2O5 and 1.2% K2O; the pH was 8.2.
Potted kiwifruit plants cv Hayward derived from micropropagation were planted at the beginning of May. The orchard was managed according to usual agronomical practices for 3 years.
The environmental parameters were monitored throughout the 3 years. The watering management was conducted by controlled localized irrigation under the monitoring of soil water potential, in order to avoid excess moisture as a possible cause of roots hypoxic stress. On 2019 (third growing seasons) plants were analyzed for growth, KEDS symptoms and roots morphology and anatomy.
The primary role of soil borne pathogens on KEDS onset was excluded by assays carried out on randomly selected samples of soils and roots before (soils) and after (soils and roots) the experiment (unshown data).
Plant growth was evaluated five times during the vegetative season. The collar diameter was measured with a caliber. The diameter increase at each stage was calculated, and daily growth rate was determined as the diameter increase divided by the number of days of the period between the two consecutive measures. Results are shown referred to the day of mid bloom (DAMB = days after mid bloom), which occurred on May 31 (bloom started on 26 May and finished on 2 June).
KEDS symptoms were assessed three times during the vegetative season at 53, 82, and 123 DAMB in all plants of the orchard. All the plants of each treatment were classified by a qualitative scoring in three categories: (1) plants without KEDS symptoms, (2) plants showing KEDS symptoms, (3) dead plants.
Nutrient status of plants was assessed by measuring the chlorophyll index using a SPAD-502 m (Ling et al., 2011). Thirty leaves per plant were measured and the measure repeated on 4 plants per repetition.
At the end of the season, a plant representing the average growth expressed by the plants in each treatment (according to collar growth data) was uprooted carefully to assess the overall plant biomass (roots, shoots, and fruits).
Root segments obtained from the uprooted plants were cut from roots having about 1 cm diameter with a steel razor blade. The so prepared, fresh, cross sections of each cut piece were placed on an aluminum stub and coated with few nanometers of gold (about 10–15 nm) in order to prevent any charging effect during the analysis. A Zeiss EVO 50 XVP scanning electron microscope (SEM) equipped with LaB6 source was used for the analysis. The microscope is endowed with detectors for secondary and backscattered electrons for image acquisition and Energy Dispersion Spectroscopy (EDS) for elemental analysis. All the samples were observed under a voltage of 5 kV and an intensity of 50pA with the purpose to enhance the surface topography. For the same reason, secondary electron detector was used for the micrographs acquisition. Indeed, it is well-known that the backscattered electrons provide information on a higher mean depth of the sample, so that details on the surface topography can be lost. In addition, these signals are mainly useful for materials containing elements with significant differences in atomic number (indeed the images acquired in backscattered mode are also called Z-contrast images). As for the elemental analysis, the intensity was increased up to 100 pA based on the need to obtain a good signal/noise ratio.
Weather data were recorded throughout the 3 years (2017–2019) with a station (METOS®, Pessl Instruments GmbH) positioned in the orchard. Soil temperature was monitored using a temperature probe positioned at 20 cm depth. Soil water potential was measured using tensiometers (Watermark, Irrometer Company Inc.) positioned at 10, 20, 30, and 40 cm depth in the Control and Ridged plots. Daily mean values were calculated from hourly measures.
ANOVA and least significant difference (LSD) analyses were carried out using PRISM (GraphPad Software, 2016) and Excel (MS Software, 2016) software assuming a p-value threshold ≤ 0.05.
The onset of KEDS symptoms (epinasty, chlorosis, desiccation, and abscission of leaves) appeared at second year of plant growth (2018). On the third year (2019), at mid July (53 DAMB) initial symptoms of KEDS were visible only on 8.3% of plants grown on ridged plots with (RI) or without (R) inoculation of microorganisms, while no symptoms were observed in plants grown on ridged plots with the compost addition (RC) (Figure 1). A striking symptoms occurrence was instead recorded in non-ridged plants (C and CC), where about 83 and 50% of plants were affected respectively.
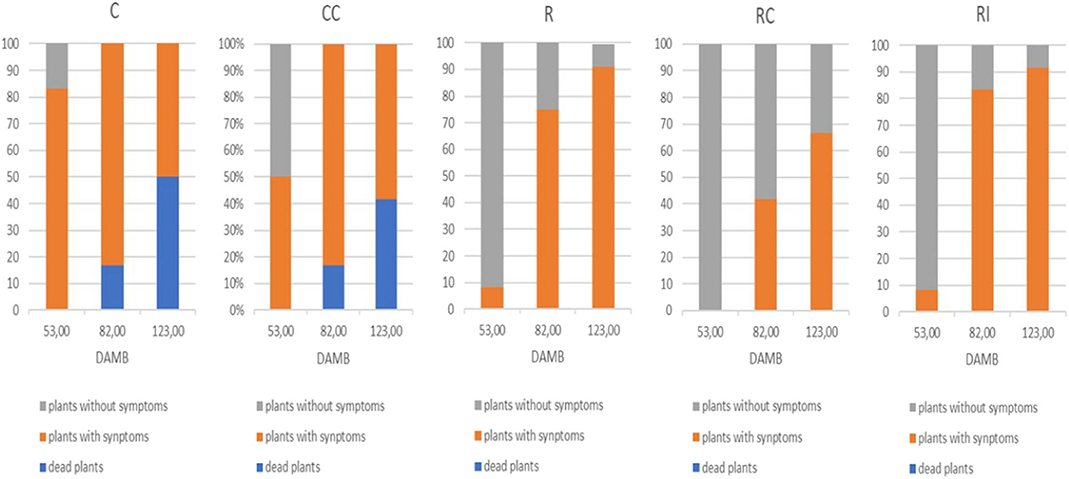
Figure 1. Qualitative scoring of KEDS symptoms in kiwifruit plants at different days after mid bloom (DAMB). C, Control; CC, Control + Compost; R, Ridged; RC, Ridged + Compost; RI, Ridged + Inoculum.
A general worsening of symptoms was observed throughout the season, and collapsed plants also appeared: in the not ridged plots (C and CC), dead plants (16.7%) were observed already in August (82 DAMB), and after a month (123 DAMB) they were more than 40%. In ridged plots, dead plants were not recorded in any assessment date. The addition of compost (RC) reduced the gravity of KEDS symptoms in comparison to the other two treatments (R and RI) (Figure 1).
The growth of the plants, measured with the increase of the trunk collar diameter, was significantly higher in ridged soils in comparison to not-ridged treatments (Figure 2A). The growth rate peaked around mid-July, 48 DAMB (Figure 2B), but a strong reduction of growth rate induced by the addition of the compost was observed in ridged plots; a similar growth reduction, even if not statistically significant, was observed also 48 DAMB in not-ridged treatment (CC). At the end of the season, the plants in ridged plots appeared more developed. One plant was chosen as representative for each treatment and uprooted, and the analysis of biomass was performed: it revealed that the plants from ridged plots produced a higher root biomass, and fruits were produced only in treatments in which soil biological fertility was improved with addition of compost or bioinocula (CC, RC, and RI) (Table 1).
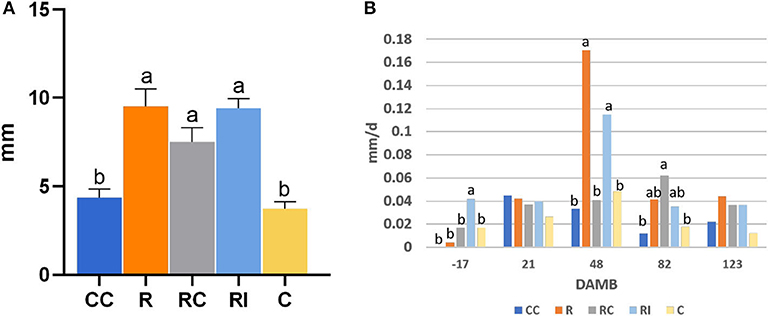
Figure 2. Effect of soil management practices on trunk collar diameter growth of kiwifruit plants measured at different days after mid bloom (DAMB). (A) Total diameter increase; (B) average daily diameter growth rate. C, Control; CC, Control + Compost; R, Ridged; RC, Ridged + Compost; RI, Ridged + Inoculum. Different letters mean significant differences (p < 0.05).
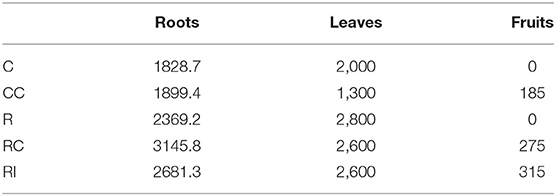
Table 1. Biomass (total roots, leaves and fruits; g, fresh weight) produced by a single plant, chosen as representative for each treatment, uprooted at the end of the third growth season.
The value of the chlorophyll index was similar in all treatments at the beginning of the vegetative growth (0 and 20 DAMB) (Figure 3). At mid-summer (53 DAMB), the chlorophyll index significantly increased in all treatments in which soil biological fertility was improved with addition of compost or bioinocula (CC, RC, and RI) (Figure 3).
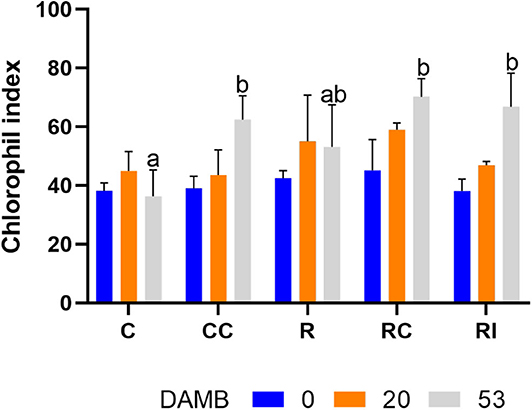
Figure 3. Effect of the soil management method on the chlorophyll index determined by a SPAD-502 m on kiwifruit plants at different days after mid bloom (DAMB). C, Control; CC, Control + Compost; R, Ridged; RC, Ridged + Compost; RI, Ridged + Inoculum. Different letters mean significant differences (p < 0.05).
KEDS symptoms and differential shoots' growth were paralleled by a contrasting architecture and development of the root system in plants growing under the different trial treatments (Supplementary Picture 1). The plants growing on not-ridged soil showed typical symptoms of KEDS: a strongly reduced development of the root system, with a very limited occurrence of fine roots, fibrous roots with cortical decay, loss of rigidity and detachment of the cortex from the central stele. Moreover, the roots surface appeared covered by a viscous mucilage that entrapped rhizospheric soil (Supplementary Picture 2A) and formed clumps mixed to rod-shape crystals (Supplementary Picture 2B). Contrastingly, the root system of plants grown on ridged plots appeared better developed, with production of fine roots, particularly when compost or the microbial consortium were added.
The SEM analysis of roots segments showed an anomalous root anatomy structure, particularly evident in plants from flatted soils treatment (C) (Figure 4). A detachment of tissue layers was observed in correspondence of the root meristems: xylem was detached from phloem and phloem was detached from cortex (Figure 4A). The layers detachment was less pronounced in the roots of plants from ridged plots (Figures 4B–D). The xylem vessels in the roots of the C plant (Figure 4A) were characterized by a reduced diameter in comparison to R, RC and RI (Figures 4B–D). Phloem of C roots appeared destructured, with wide collapsed areas in which the cells appeared distorted or dissolved. In this layer, abundant bundles of crystals were present (Figure 5A), identifiable as raphides, i.e., crystals of calcium oxalate. EDS analysis confirmed the presence of calcium, oxygen and carbon in the crystals. Although a correct stoichiometric assessment cannot be performed with this semi-quantitative method, the presence of these elements, particularly calcium, not observed in other parts of the roots, indicated that the crystals were composed of calcium oxalate (Figure 5B). SEM analysis allowed to observe that raphides boundles were frequently associated to collapsed areas of phloem: in roots from the most symptomatic plants, empty areas were observed in shape of canals, likely formed from cells dissolution; in the inner lumen of these canals, raphides were abundant (Figure 5A) and sometimes associated to mucilage (Figure 5B). The phloem parenchyma of roots from ridged plots appeared loaded with starch granules (Figure 6A), while in control plants starch granules were present in a lower amount and were absent in proximity of the detachment layers (Figure 6B).
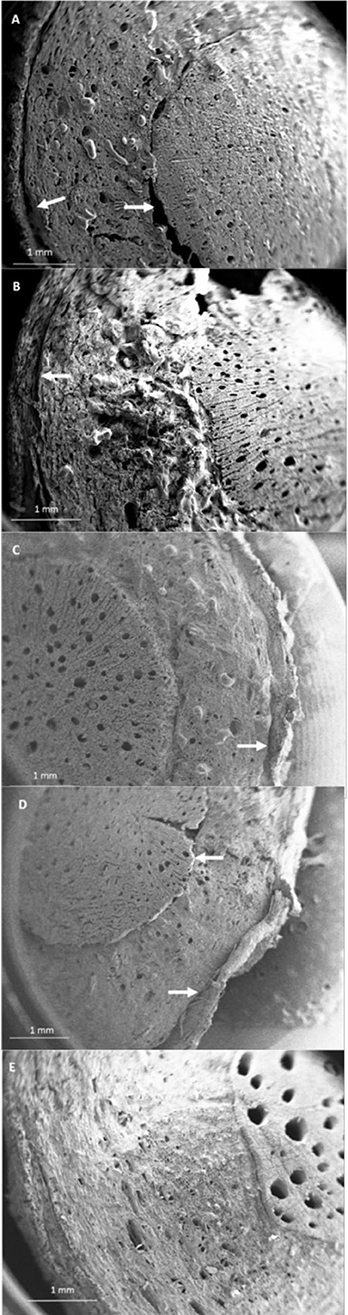
Figure 4. SEM micrographs showing modifications of the root anatomy in kiwifruit plants grown under different soil management conditions. Arrows are showing detachment in correspondence of the meristematic layers between xylem-phloem and phloem-cortex. (A) Control (C), (B) Ridged + Compost (RC), (C) Ridged + Inoculum (RI), (D) Ridged (R), (E) root of a healthy plant from a private garden.
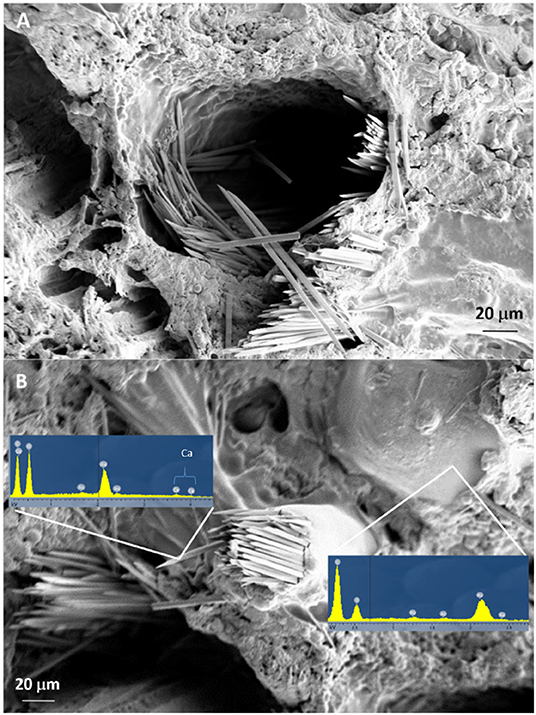
Figure 5. SEM micrographs showing canals formed in phloem layers of KEDS symptomatic plants with raphides in the inner lumen without (A) or with mucilage (B). Results of the EDS analysis of both raphides and mucilage are presented in (B).
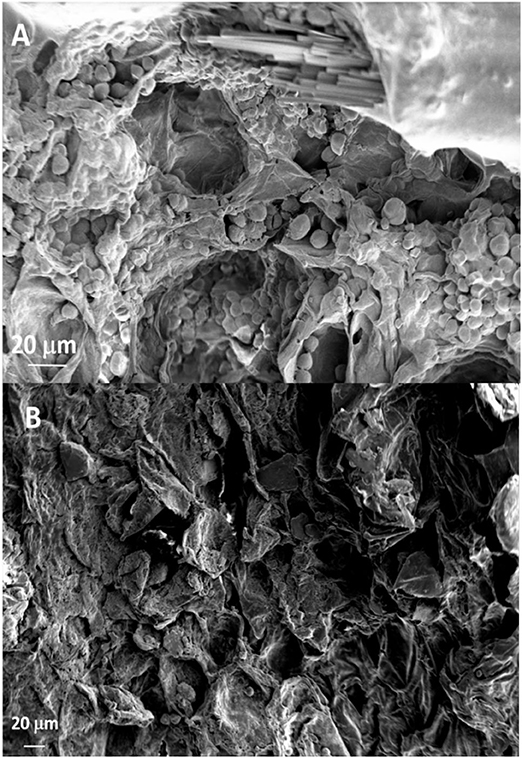
Figure 6. SEM micrographs showing the phloem layer with (A) or without (B) starch granules of roots from Ridged + Compost (RC) or Control (C) treatments, respectively.
None of the described anatomical oddities were observed in roots collected from a healthy plant grown in a private garden, showing a vigorous growth and fruitset and lacking any visible symptom of KEDS; these roots evidenced larger and more numerous xylem vessels than all the other samples analyzed (Figure 4E).
From the analysis of air temperature data from 1 to 168 DAMB it appeared evident that the average daily temperature remained almost continuatively above 20°C until about 90 DAMB during all three growing seasons, and the maximum daily temperature largely continuously exceeded 25°C, reaching 30–35°C, particularly in 2019 (Figure 7). This meteorological condition was reflected on soil temperature: in 2019 it was exceeding 20°C for more than 3 months after bloom (Figure 8A).
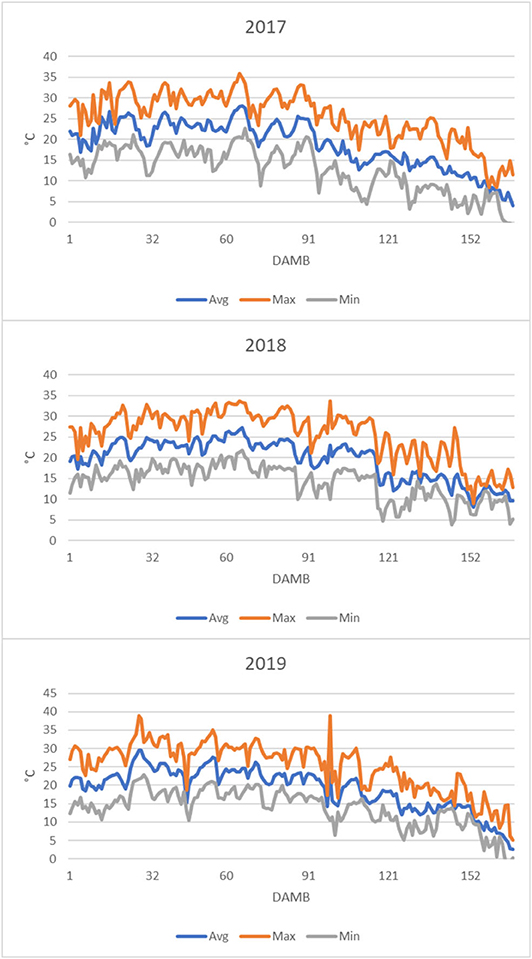
Figure 7. Air temperatures profiles recorded in the kiwifruit orchard after mid bloom during the growth seasons concerned by the trial (Min, minimum; Max, maximum; Avg, average).
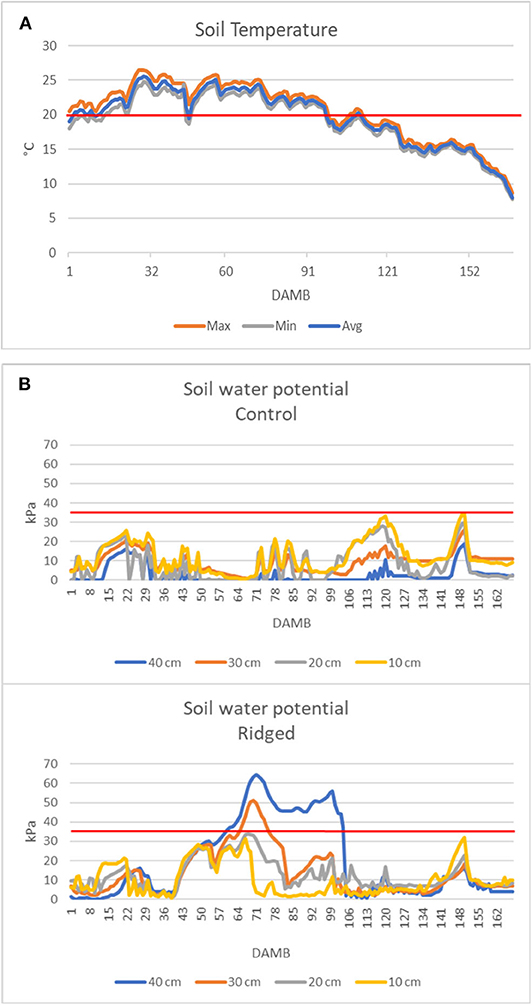
Figure 8. Soil temperature monitored at 20cm depth (A) and soil water potential measured at 10, 20, 30, and 40 cm depth in the Control (C) and Ridged (R) plots (B). The red line indicates the temperature at which root growth stops (Smith et al., 1989) (A) or the soil water potential at the field capacity (B).
Soil water potential in control plots remained at low levels, below the limit corresponding to soil field capacity, indicating an excessive soil moisture. In contrast, in ridged plots it exceeded the field capacity limit during the summer, indicating a good drainage (Figure 8B). However, unexpectedly, in ridged soils the soil water potential was highest at 40 cm depth and progressively lowered toward the surface layer.
The soil management played a major role in improving the kiwifruit plant resilience against the stresses causing the onset of KEDS symptoms: the incidence and the gravity of symptoms was significantly lower in ridged than in flatted treatments (Figure 1). Ridging is useful to improve the soil aeration and to prevent flooding conditions, as it was evidenced by the soil water potential, that was higher in ridged than in flatted soil (Figure 8B). Thus, it can be stated that ridging created most favorable conditions for kiwifruit roots growth and activity. However, it was not sufficient to prevent the onset of KEDS, not even after the addition of a massive amount of compost or of a consortium of selected beneficial rhizospheric microorganisms.
When the KEDS symptoms appeared on the above-ground part of the plant, a parallel deterioration of the root tissues was always visually observed and confirmed by microscopic and micrographic evidences of a roots anatomical disorder. In control plants (C), showing the worst KEDS symptoms, the anomalous root anatomy observed by SEM was particularly evident, indicating a stress to meristems as well as to xylematic and phloematic tissues (Figure 4A). Kiwifruit is characterized by a very high root oxygen demand (Smith et al., 1989). Meristems are the tissues most sensitive to oxygen depletion, as they are characterized by high cell density and metabolic activity which expose them to hypoxia, when cell metabolism shift from respiration to fermentation (Bailey-Serres and Voesenek, 2008). As a consequence, the high sugar demand, typical of these tissues, is further increased because of the lower energy yield due to the fermentative metabolism. Under such conditions, carbohydrates from photosynthesis are rapidly exhausted, followed by storage carbohydrates such as starch. As a consequence, if the hypoxic condition continues, cell death follows (Bailey-Serres and Voesenek, 2008). The anomalous xylem and phloem development and the damage to tissues contiguous to cambium observed in KEDS symptomatic plants (Figure 4) could be explained by insufficient sugars availability to secondary cambium and phellogen. Therefore, the stunted roots growth and progressive loss of functions in KEDS-symptomatic plants could be explained by the shortage of starch reserves evidenced by SEM analysis (Figure 6). In fact, the damage to root tips is particularly harming to kiwifruit, because it prevents the reactivation of suberized root tips that, typical in this species, ensures the persistence for more than 1 year of fibrous roots (Smith et al., 1990; Lemon and Considine, 1993; Bailey-Serres and Voesenek, 2008). Moreover, in kiwifruit the failure in the activation of secondary cambium, initiation of phellogen and reactivation of cell division in endodermis lead to rootlets death and progressive roots weakening over the years until plant death (Lemon and Considine, 1993).
In kiwifruit it is known that roots are the weakest sink in the competition within the vine for available photosynthates (Smith et al., 1989). The disproportionate allocation of photosynthetates to the developing fruit has a dominating influence over root activity (Buwalda and Smith, 1987), and root growth peaks only after the flush of fruit growth has been completed: the peak of growth of fibrous roots occurs at mid-summer, when the relative growth rate of the shoots and fruits have fallen to low levels (Buwalda and Hutton, 1988). However, it has been postulated that shoots have a stronger sink strength (ability to attract carbohydrate when supply is not limited) and sink priority (ability to compete when carbohydrate supply is limited) over fruits during the first 120 DAMB (Lacointe and Minchin, 2008). Afterwards, in case of competition for limited resources, vegetative growth continues at the expenses of fruit growth (Snelgar et al., 2012). Moreover, high temperatures strongly influence the photosynthates allocation between fruits and shoots, with increased vegetative vigor stimulated by high temperatures at the expenses of sugars accumulation in fruits, whose dry matter at harvest is lowered (Hall and Snelgar, 2008). Even though the effect of temperature on sink strength of roots with respect to fruits or shoots has not been thoroughly studied, it was reported that soil temperature above 20°C strongly reduced root growth in kiwifruit and increased shoot/root ratio (Smith et al., 1989). Therefore, considering the lower sink priority of roots over shoot growth (Smith et al., 1989), it could be speculated that growth and starch accumulation (Figure 6) in roots were dramatically hindered due to high temperatures occurred during summer over the 3 years of the experiment. Indeed, during all 3 years the temperature was very high from 1 to 90 DAMB, with the average daily temperature above 20°C and the maximum daily temperature largely exceeding 25°C, sometimes reaching 30–35°C, particularly in 2019 (Figure 7). Also soil temperature exceeded 20°C for more than 3 months after bloom (Figure 8A). High temperatures during summer also reduce accumulation of reserve carbohydrates in vines, causing a severe reduction of growth and flowering in the following season (Richardson et al., 2004). Based on these observations, we hypothesize that high summer temperatures could be considered the main triggering factor of the observed reduced root growth and functionality and general plant weakening, leading to KEDS onset.
The trunk growth peaked in summer (Figure 2), when the highest seasonal temperatures occurred (Figure 7), as previously observed by other authors (Smith et al., 1989; Hall and Snelgar, 2008) that reported a consequent unbalanced shoot/root development. In our trial, plants on ridged rows showed a significantly higher growth in comparison to those grown on flatted soil; however, the addition of the compost in the ridged treatment (RC) strongly reduced the trunk growth at mid July and delayed the growth rate curve of about a month with respect to both the not amended (R) or inoculated (RI) plants. The compost addition induced a slight decrease of trunk growth rate during hot summer also in flatted treatments, where growth was very poor, but allowed to induce a slight increase of growth rate when temperatures were milder.
The root development observed on a representative plant at the end of the season (Supplementary Picture 1) was higher in the ridged treatments, and the RC plants showed the largest root biomass and the most balanced shoot/root growth (Table 1). The root anatomy observed by SEM can be considered a further indication of a better root functionality in plants from ridged treatments, sufficient to sustain the more active vegetative growth induced by high temperatures. It can be deduced that ridging is strongly favorable to growth of both shoot and root, and that the improvement of biological fertility, in particular by compost addition, can favor a more balanced growth in response to high temperature stress. A possible explanations of this phenomenon could be the modification of the pattern of nutrient availability (Griffin and Hutchinson, 2007).
High temperatures also reduce oxygen diffusion in soil (Ponnamperuma, 1984; Smith et al., 1989). The presence of numerous canals in the phloem layer of roots from plants showing strong KEDS symptoms (Figures 4, 5) could represent the plant response to hypoxic conditions through aerenchyma formation to facilitate oxygen diffusion from leaves to root tips (Bailey-Serres and Voesenek, 2008). Lysogenic aerenchyma formation occurs through programmed cell death, mediated by ethylene-induced increase of cytoplasmic Ca2+ (Bouranis et al., 2006; Bailey-Serres and Voesenek, 2008; Pegg et al., 2020). This involves the death, and often complete lysis, of cells with the disappearance of all cell components, including the cell walls (He et al., 1994). Numerous canals in the root phloem layer (Figures 4, 5) and abundant bundles of raphides in the inner lumen of these canals (Figure 5A) were observed in SEM micrographs of symptomatic plants. Kiwifruit is accumulating calcium oxalate (Ferguson, 1984) in specialized cells (idioblasts), which act as high capacity sinks for removal of excess calcium from nearby tissues (Kostman et al., 2001). As lysogenic aerenchyma formation follows a calcium-mediated cell death, it can be speculated that raphides form as a consequence of increased calcium concentration in lysogenic tissues, where lysed cells collapse, forming empty canals. This hypothesis is supported by the presence of mucilages associated to raphides in the inner lumen of phloem canals observed in SEM micrographs (Figure 5B). Viscous or jelly-like mucilage is typically found in kiwifruit vines and roots, and they have been characterized as composed by oligosaccharides (glucuronosylmannose, arabinose, fucose, galactose, and traces of xylose) (Ferguson, 1984), which are products of the cell walls degradation. Interestingly, the activity of cellulase, pectinase and xylanase increased in roots upon exposure to hypoxia or ethylene (Smith et al., 1990; He et al., 1994; Bragina et al., 2003; Bailey-Serres and Voesenek, 2008). Fine roots “coated” with a mucilaginous layer in the root-soil interface, which maintained a humid and reduced (bluish/grayish) micro-environment, were observed in symptomatic plants (Supplementary Picture 2). This can be considered a consequence of abundant cell lysis that follows the induction of the pseudo-aerenchyma formation. Since this was observed also in treatments where the soil conditions were inducive of good aeration (ridging, high water potential, high organic matter content), the high soil temperature could be hypothesized as the only possible cause of roots hypoxic stress. It has been observed in the uprooted plants, as well as in several kiwifruit orchards showing KEDS symptoms, that the soil near the roots appears wet, even when weather conditions are hot and dry and bulk soil is dry (Nari, personal observation). The presence of mucilages in the soil could also account for the unexpected difference in water potential between the surface and deep soil layers observed in ridged soil treatment: indeed, it was higher in deep layers than at surface during the hot summer period (Figure 8B). The surface tension and viscosity of mucilage can play a role in maintenance of root-soil contact in drying soils (Read and Gregory, 1997). The possible effects of these soil modifications on the roots water uptake capacity requires further studies.
The application of the microbial inoculum affected root growth synergistically with ridged tillage as well, but to a lesser extent in comparison to compost. The positive effect of microbial inocula on root development has been reported for several fruit crops, including kiwifruit (Liu et al., 2020). The inoculation of soil with the microbial consortium promoted the root growth, and the xylem and phloem layers appeared less destructured than control roots, even though mucilage and raphides were still present. The positive effect of the application of the microbial inoculum, as well as of compost, on the plant physiology was confirmed by the chlorophyll index, an indicator of the nutritional status of the plants (Porro et al., 2001), that significantly increased in CC, RC an RI treatments (Figure 3). The better physiological and growth conditions were reflected in fruit production, which was observed only on plants receiving compost or the microbial inoculum. It can be deduced that rhizospheric microorganisms can play a significant positive role in preventing or alleviating KEDS by increasing the resilience of plants to abiotic stresses, as previously reported for several plant growth promoting microorganisms (Bardi and Malusà, 2012).
All the evidences described support the hypothesis of KEDS as a climate change-derived phenomenon, through high temperature stress, directly affecting the kiwifruit plant growth and physiology and photoassimilates allocation, and indirectly inducing hypoxic soil conditions. Agronomic interventions, aimed at improving soil biological fertility and physical characteristics, can help to protect kiwifruit plant from this stress, but might be insufficient under prolonged and consecutive hot summer seasons, which are triggering KEDS in the following years. Therefore, other agronomic interventions would be necessary to counteract or avoid KEDS development. Reducing excessive warming of the plant/orchard microenvironment could be a strategy to be adopted through overtree microsprinkler irrigation, eventually associated to common soil microsprinkler irrigation; this would not negatively impact on the risk of PSA infection, being the bacterium strongly inhibited during summer (Scortichini et al., 2012). The positive effect of such strategy has been empirically observed in few commercial orchards, present in high risk areas, where any KEDS symptoms could be spotted. Alternatively, a more agro-ecological approach to orchard management (Malézieux, 2012) could also represent a technically and economically feasible solution to halt the spread of KEDS in kiwifruit orchards, by recreating the natural environmental conditions where Actinidia sp. thrives. All these options are currently under evaluation.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
LB, LN, and CM designed the study. LB, LN, and EM participated in its execution and data collection. LB elaborated the results and discussed them with LN, CM, and EM. MF did SEM and EDS analyses. LB wrote the first draft. EM and MF edited it. All authors contributed to the article and approved the submitted version.
This study was funded by Regione Piemonte—Direzione Agricoltura, Programma regionale di ricerca, sperimentazione e dimostrazione, DD no. 1066/2017.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We acknowledge the Azienda Agricola Giuliano Sacchetto di Sacchetto Bernardo (Saluzzo, CN) for the kind collaboration in making available and managing the experimental orchard. We also acknowledge the technicians of the Agrion Technical Services Coordination for their helpfulness in sharing their precious and wide observations and experiences gained in filed.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fagro.2020.580659/full#supplementary-material
Supplementary Picture 1. Root system development of a representative plant for each treatment applied to the kiwifruit orchard after 3 years of growth. The white sheet on the background measured 0.5 m2.
Supplementary Picture 2. Root surface from KEDS symptomatic plants appearing clotted due to mucilage secreted by the root (yellow arrows) and showing a reduced (blue/grayish) rhizospheric soil (white arrows) (A). Clumps of mucilage mixed to rod-shape crystals (yellow arrows) on the root surface and rhizospheric soil (B).
Bailey-Serres, J., and Voesenek, L. A. C. J. (2008). Flooding stress: acclimations and genetic diversity. Ann. Rev. Plant Biol. 59, 313–339. doi: 10.1146/annurev.arplant.59.032607.092752
Bardi, L. (2020). Early kiwifruit decline: a soil-borne disease syndrome or a climate change effect on plant–soil relations? Front. Agron. 2:3. doi: 10.3389/fagro.2020.00003
Bardi, L., and Malusà, E. (2012). “Drought and nutritional stresses in plants: alleviating role of rhizospheric microorganisms,” in Abiotic Stress: New Research, eds N. Haryana and S. Punj (Hauppauge, NY: Nova Science Publishers, Inc.), 1–56.
Black, M., Patterson, K. J., Minchin, P. E. H., Gould, K. S., and Clearwater, M. J. (2012). Hydraulic responses of whole vines and individual roots of kiwifruit (Actinidia chinensis) following root severance. Tree Physiol. 31, 508–518. doi: 10.1093/treephys/tpr045
Bouranis, D. L., Chorianopoulou, S. N., Kollias, C., Maniou, P., Protonotarios, V. E., Siyiannis, V. F., et al. (2006). Dynamics of aerenchyma distribution in the cortex of sulfate-deprived adventitious roots of maize. Ann. Bot. 97, 695–704. doi: 10.1093/aob/mcl024
Bragina, T. V., Rodionova, N. A., and Grinieva, G. M. (2003). Ethylene production and activation of hydrolytic enzymes during acclimation of maize seedlings to partial flooding. Russ. J. Plant Physiol. 50, 794–98. doi: 10.1023/B:RUPP.0000003277.22914.6c
Buwalda, J. G., and Hutton, R. C. (1988). Seasonal changes in root growth of kiwifruit. Sci. Horticolt. 36, 251–260. doi: 10.1016/0304-4238(88)90059-3
Buwalda, J. G., and Smith, G. S. (1987). Accumulation and partitioning of dry matter and mineral nutrients in developing kiwifruit vines. Tree Physiol. 3, 295–307. doi: 10.1093/treephys/3.3.295
Chaparro, J. M., Sheflin, A. M., Manter, D. K., and Vivanco, J. M. (2012). Manipulating the soil microbiome to increase soil health and plant fertility. Biol. Fertil. Soils 48, 489–499. doi: 10.1007/s00374-012-0691-4
Diacono, M., and Montemurro, F. (2011). “Long-term effects of organic amendments on soil fertility,” in Sustainable Agriculture, Vol. 2, eds V. Lichtfouse, E. Hamelin, M. Navarrete, and M. Debaeke (Dordrecht: Springer), 761–786. doi: 10.1007/978-94-007-0394-0_34
Donati, I., Mauri, S., Cellini, A., Buriani, G., Fiorentini, L., Costa, G., et al. (2019). Influence of cultural practices on the incidence and severity of kiwifruit bacterial canker. Acta Horticult. 1243, 59–64. doi: 10.17660/ActaHortic.2019.1243.10
FAO (2018). Available online at: http://www.fao.org/faostat/en/#data/QC.
Ferguson, A. R. (1984). Kiwifruit: a botanical review. Horticult. Rev. 6, 1–64. doi: 10.1002/9781118060797.ch1
Ferguson, A. R. (2016). “World economic importance,” in The Kiwifruit Genome, Compendium of Plant Genomes, eds R. Testolin, H. W. Huang and A. R. Ferguson (Heidelberg: Springer International Publishing Switzerland), 37–42. doi: 10.1007/978-3-319-32274-2_3
Griffin, T. S., and Hutchinson, M. (2007). Compost maturity effects on nitrogen and carbon mineralization and plant growth. Compost Sci. Util. 15, 228–236. doi: 10.1080/1065657X.2007.10702338
Hall, A., and Snelgar, B. (2008). Temperature effects on kiwifruit dry matter at harvest. Acta Hortic. 803, 155–162. doi: 10.17660/ActaHortic.2008.803.19
Haye, T., and Weber, D. C. (2017). Special issue on the brown marmorated stink bug, Halyomorpha halys: an emerging pest of global concern. J. Pest Sci. 90, 987–988. doi: 10.1007/s10340-017-0897-1
He, C. J., Drew, M. C., and Morgan, P. W. (1994). Induction of enzymes associated with lysigenous aerenchyma formation in roots of Zea mays during hypoxia or nitrogen-starvation. Plant Physiol. 105, 861–865. doi: 10.1104/pp.105.3.861
Kostman, T. A., Tarlyn, N. M., Loewus, F. A., and Franceschi, V. R. (2001). Biosynthesis of L-ascorbic acid and conversion of carbons 1 and 2 of L-ascorbic acid to oxalic acid occurs within individual calcium oxalate crystal idioblasts. Plant Physiol. 125, 634–640. doi: 10.1104/pp.125.2.634
Lacointe, A., and Minchin, P. E. H. (2008). Modelling phloem and xylem transport within a complex architecture. Func. Plant Biol. 35:772780. doi: 10.1071/FP08085
Lemon, C. W., and Considine, J. A. (1993). Anatomy and histochemistry of the root system of the kiwifruit vVine, Actinidia deliciosa var. deliciosa. Ann. Bot. 71, 117–129. doi: 10.1006/anbo.1993.1015
Ling, Q., Huang, W., and Jarvis, P. (2011). Use of a SPAD-502 meter to measure leaf chlorophyll concentration in Arabidopsis thaliana. Photosyn. Res. 107, 209–214. doi: 10.1007/s11120-010-9606-0
Liu, Z., Guo, Q., Feng, Z., Liu, Z., Li, H., Sun, Y., et al. (2020). Long-term organic fertilization improves the productivity of kiwifruit (Actinidia chinensis Planch.) through increasing rhizosphere microbial diversity and network complexity. Appl. Soil Ecol. 147:103426. doi: 10.1016/j.apsoil.2019.103426
Malézieux, E. (2012). Designing cropping systems from nature. Agron. Sustain. Develop. 32, 15–29. doi: 10.1007/s13593-011-0027-z
Montanaro, G., Dichio, B., Mininni, A. N., and Xiloyannis, C. (2014). Actinidia irrigata a scorrimento: pratica da abbandonare. Inform. Agr. 2, 73–76. Available online at: http://hdl.handle.net/11563/67691
Nari, L., and Vittone, G. (2017). La moria dell'actinidia: sintomi, cause e possibili rimedi. Inform. Agr. 16, 2–5. Available online at: http://www.ediagroup.it/BDO/BDO_popupAbstract.asp?D=129632
Pegg, T., Edelmann, R. R., and Gladish, D. K. (2020). Immunoprofiling of cell wall carbohydrate modifications during flooding-induced aerenchyma formation in fabaceae roots. Front. Plant. Sci. 10:1805. doi: 10.3389/fpls.2019.01805
Ponnamperuma, F. N. (1984). “Effects of flooding on soils,” in Flooding and Plant Growth ed T. T. Kozlowski (Orlando, FL: Academic Press), 9–45. doi: 10.1016/B978-0-12-424120-6.50007-9
Porro, D., Dorigatti, C., Stefanini, M., and Ceschini, A. (2001). Use of SPAD meter in diagnosis of nutritional status in apple and grapevine. Acta Hortic. 564, 243–252. doi: 10.17660/ActaHortic.2001.564.28
Prencipe, S., Savian, F., Nari, L., Ermacora, P., Spadaro, D., and Martini, M. (2020). First report of Phytopythium vexans causing decline syndrome of Actinidia deliciosa ‘Hayward’ in Italy. Plant Dis. 104:2032. doi: 10.1094/PDIS-10-19-2101-PDN
RAN (2010–2020). Rete Agrometeorologica Nazionale – MIPAAF. Availbale online at: https://www.politicheagricole.it/flex/FixedPages/Common/miepfy200_reteAgrometeorologica.php/L/IT.
Read, D. B., and Gregory, P. J. (1997). Surface tension and viscosity of axenic maize and lupin root mucilages. New Phytol. 137, 623–628. doi: 10.1046/j.1469-8137.1997.00859.x
Reid, J. B., Tate, K. G., Brown, N. S., and Cheah, L. H. (1991). Effects of flooding and alluvium deposition on kiwifruit (Actinidia deliciosa): 1. Early vine decline. J. Crop Horticult. Sci. 19, 247–257. doi: 10.1080/01140671.1991.10421808
Richardson, A. C., Marsh, K. B., Boldingh, H. L., Pickering, A. H., Bulley, S. M., Frearson, N. J., et al. (2004). High growing temperatures reduce fruit carbohydrate and vitamin C in kiwifruit. Plant, Cell and Environment 27, 423–435.
Savé, R., and Serrano, L. (1986). Some physiological and growth responses of kiwi fruit (Actinidia chinensis) to flooding. Physiol. Plant 66, 75–78. doi: 10.1111/j.1399-3054.1986.tb01236.x
Scortichini, M., Marcelletti, S., Ferrante, P., Petriccione, M., and Firrao, G. (2012). Pseudomonas syringae pv. actinidiae: a re-emerging, multi-faceted, pandemic pathogen. Mol. Plant Pathol. 13, 631–640. doi: 10.1111/j.1364-3703.2012.00788.x
Singh, J., Silva, K. J. P., Fuchs, M., and Khan, A. (2019). Potential role of weather, soil and plant microbial communities in rapid decline of apple trees. PLoS ONE 14:e0213293. doi: 10.1371/journal.pone.0213293
Smith, G. S., Buwalda, J. G., Green, T. G. A., and Clark, C. J. (1989). Effect of oxygen supply and temperature at the root on the physiology of kiwifruit vines. New Phytol. 113, 431–437. doi: 10.1111/j.1469-8137.1989.tb00354.x
Smith, G. S., Judd, M. J., Miller, S. A., and Buwalda, J. G. (1990). Recovery of kiwifruit vines from transient waterlogging of the root system. New Phytol. 115, 325–333. doi: 10.1111/j.1469-8137.1990.tb00459.x
Snelgar, W. P., Minchin, P. E. H., Blatmann, P., and Hall, A. J. (2012). Sink priority on ‘Hayward’ kiwifruit vines. J. Crop Horticult. Sci. 40, 253–263. doi: 10.1080/01140671.2012.681386
Sorrenti, G., Toselli, M., Reggidori, G., Spinelli, F., Tosi, L., Giacopini, A., et al. (2016). Implicazioni della gestione idrica nella “moria del kiwi” del veronese. Rivista Frutticoltura 3, 2–7.
Spigaglia, P., Barbanti, F., Marocchi, F., Mastroleo, M., Baretta, M., Ferrante, P., et al. (2020). Clostridium bifermentans and C. subterminale are associated with kiwifruit vine decline, known as moria, in Italy. Plant Pathol. 69, 765–774. doi: 10.1111/ppa.13161
Tacconi, G., Giacopini, A., Vittone, G., Nari, L., Spadaro, D., Savian, F., et al. (2019a). Il punto sulla moria del kiwi a 8 anni dalla sua comparsa. Inform. Agr. 21, 34–36.
Tacconi, G., Giacopini, A., Vittone, G., Nari, L., Spadaro, D., Savian, F., et al. (2019b). Moria del kiwi: situazione disastrosa al nord, preoccupante nel resto d'Italia. Kiwi Informa 14, 32–37. Availbale online at: https://iris.unito.it/retrieve/handle/2318/1724385/568407/590.pdf
Tacconi, G., Paltrinieri, S., Mejia, J. F., Fuentealba, S. P., Bertaccini, A., Tosi, L., et al. (2015). Vine decline in kiwifruit: climate change and effect on waterlogging and Phytophthora in North Italy. Acta.Hortic. 1096, 93–97. doi: 10.17660/ActaHortic.2015.1096.7
Tacconi, G., Tosi, L., Giacopini, A., Bertaccini, A., Mazzucchi, U., Favaron, F., et al. (2014). “Vine decline in kiwifruit: climate change and effect on waterlogging and Phythopthora in North Italy,” in The 8th International Symposium on Kiwifruit-China (Dujiangyan).
Tosi, L., Giacopini, A., and Tacconi, G. (2015). La moria del kiwi, situazione e prospettive. Inform. Agr. 744, 67–70. Available online at: http://www.ediagroup.it/BDO/BDO_popupAbstract.asp?D=123393
USDA (1975). Soil Survey Staff 1975. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys. Washington, DC: Natural Resources Conservation Service; U.S. Department of Agriculture Handbook, 436.
Keywords: soil bioinocula, soil ridging, compost, abiotic stress, physiological disorder, root anatomy, raphides, climatic conditions
Citation: Bardi L, Nari L, Morone C, Faga MG and Malusà E (2020) Possible Role of High Temperature and Soil Biological Fertility on Kiwifruit Early Decline Syndrome. Front. Agron. 2:580659. doi: 10.3389/fagro.2020.580659
Received: 06 July 2020; Accepted: 02 September 2020;
Published: 06 October 2020.
Edited by:
Upendra Kumar, National Rice Research Institute (ICAR), IndiaReviewed by:
Avishek Banik, Presidency University, IndiaCopyright © 2020 Bardi, Nari, Morone, Faga and Malusà. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura Bardi, bGF1cmEuYmFyZGlAY3JlYS5nb3YuaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.