Introduction: Biomaterials, dentistry and orthopaedics fields widely recognize that commercially pure Titanium (cpTi) is one the better materials for bone replacement. Despite its well-known clinical success, this biomaterial exhibits some drawbacks: 1) Ti implants present higher stiffness than bone (100-110 GPa against 20 GPa), which always implies a risk of failure due to bone resorption[1]; 2) Interfacial issues associated to presence of a fibrous tissue, which compromise implants osseointegration. Manufacturing of porous materials is one of the most plausible routes to address the first above issue of stress shielding risks[2]. The aim of this work is to develop, process and characterize porous biomaterials with enhanced surface properties by implementing both inhibited chemical etching and anodizing treatment.
Materials and Methods: Fabrication and characterization of porous cpTi samples. Commercially pure Titanium was used. The chemical composition was equivalent to cpTi Grade IV according to the ASTM F67-13 Standard[4]. Particles of ammonium bicarbonate (NH4HCO3, Alfa Aesar) were employed as space holder, 99.9 % of purity. The NH4HCO3 particles were sieved in a range between 100 and 200 μm. The mixture of cpTi and NH4HCO3 was fixed to a concentration of 50 vol.%. Surface modifications of cpTi porous samples: controlled etching and anodizing. Controlled etching of cpTi porous samples and anodizing were performed from previous studies of the authors on bulk Ti[5]: etching solution is a water based one with 2.2 vol.% of HF and a 0.5 ml/L % of an organic inhibitor (propargyl alcohol ethoxilated); temperature was 50°C and immersion times were 0, 5, 25, 125, 625 y 3125 s. Anodizing conditions were: 2 to 10 min, 12, 18 y 40 VDC, 60g/L H2SO4 and 150g/L H3PO4.
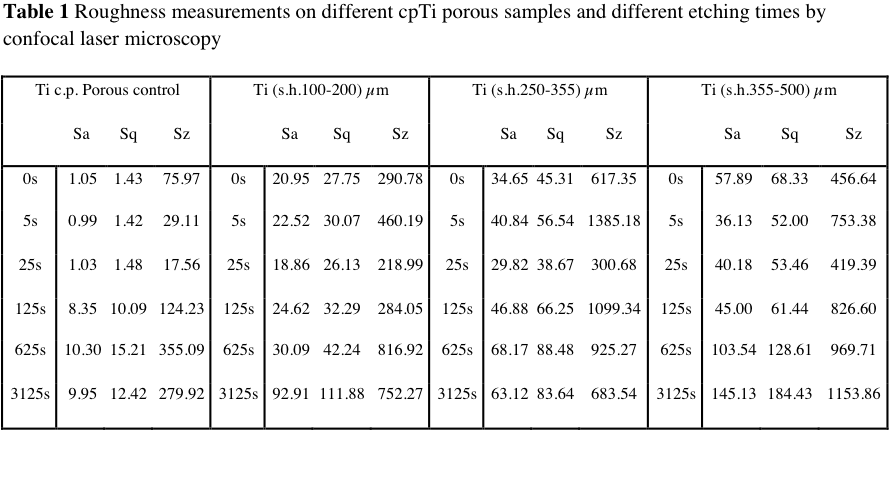
Results and Discussion: In the context of etching influence on samples with space holder, initial roughness is important (Fig.1). Controlled etching not only has allowed to increase the total and interconnected porosity, but also has implied the appearance of some new interesting features, which are suitable for bone repair[3]. Firstly, the increasing of interconnected porosity makes these materials attractive for both tissue engineering and regenerative medicine. Secondly, controlled etching has allowed the appearance of a micro and nano-porosity in the residual Ti matrix; this is an additional characteristic to be potentially explored to improve cells adhesion inside the pores, and to take advantage of those multi-scale pores (Fig.2). Roughness parameters appear reasonably low, which is a consequence of both starting size and geometry of cpTi particles. Rest of roughness parameters of cpTi-porous control samples presented in Table 1, for longer etching times, show higher values. Indeed, it would indicate that optimal etching time should be between 25s and 125s.
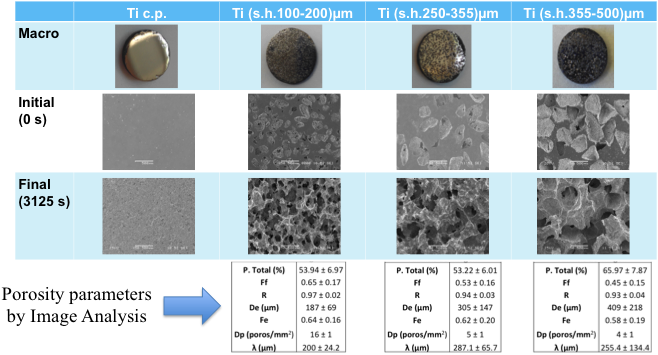

Conclusions: The aim of this new development was to address alternative routes to improve the balance between low stiffness, mechanical strength and surface properties of porous Ti implants. In this context, this new etching treatment have shown the ability to produce not only a controlled high porosity, but also new structural features in terms of multi-scale pores and roughness patterns.
Junta de Andalucía (Spain) / FEDER (EU), through the project Ref. P12-TEP-1401
References:
[1] J. Currey, in Handbook of Biomaterials Properties, ed. by J. H. Black, G. Hastings (Springer– Verlag, London 1998), p. 3
[2] J. Banhart, Prog. Mater. Sci., 46(6), 559-632 (2001)
[3] A. Bádenas C, Gil FX. Universitat Politècnica de Catalunya. Departament de Ciéncia dels Materials i Enginyeria Metallùrgica. http://hdl.handle.net/10803/6044. Accessed 15 (April 2005)
[4] Y. Torres, J.A. Rodríguez, S. Arias, M. Echeverry, S. Robledo, V. Amigó, Pavón, J.J., J. Mater. Sci. 47, 6565 a 6576 (2012)
[5] J. Pavón and R. Montes, Patent in process (University of Antioquia, 2015).