Introduction: The development and in vitro characterization of the Porcine Anorganic Bone Mineral (PABM) were presented in part I of this series. In part II we summarize the results from a canine intrabony defect model to validate the characteristics of PABM as an effective bone conductive matrix in supporting bone growth and regeneration. The use of PABM for maxillary bone augmentation in sinus lifting and socket bone augmentation in humans is presented.
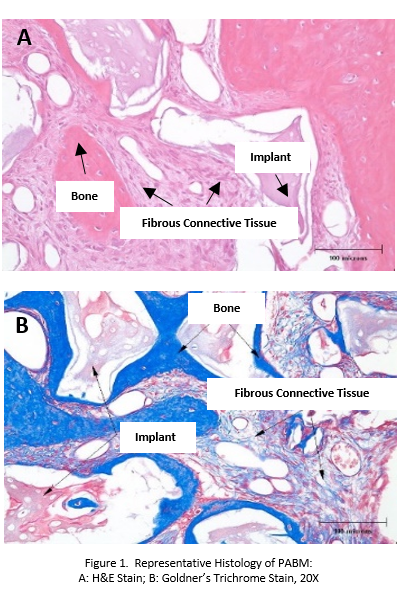
Methods: In Vivo Intrabony Defect Study in Canines: Five beagle dogs had the third pre-molars (P3) extracted from the mandible. After 14 weeks post-extraction healing, each animal had a bilateral defect created, filled with PABM and covered with a marketed collagen membrane (MatrixDerm®, Collagen Matrix, Inc.). At pre-determined time points (4, 8 and 12-weeks), explants were collected, fixed, and processed for histological analysis. Key parameters evaluated included cellular responses, bone healing activity and residual implant material.
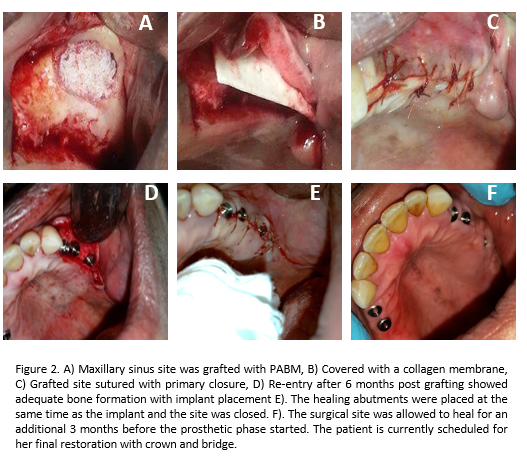
Human Case Studies: Case 1 was a maxillary sinus GBR procedure, using PABM and a collagen membrane (MatrixDerm®, Collagen Matrix, Inc.), performed on a 50-year-old female patient (See Fig. 2 for details of the procedure). Case 2 was a bone augmentation in a socket extraction site on a 65-year-old male. The defect site was filled with PABM, covered with a collagen membrane (MatrixFlex®, Collagen Matrix, Inc.) and sutured in place. Implant placement was performed 5 months post grafting and healing was uneventful. The tooth was fully restored (photos not shown).
Results and Discussion: In Vivo Intrabony Defect Study in Canines: Histologically, the total amount of bone and mature lamellar bone increased over time. Implantation of PABM supported bone regeneration (Fig. 1). The amount of implant material decreased slightly over the course of the study. PABM was nonirritant at all time intervals.
Human Case Study: Figure 2 showed an example of use of PABM and collagen membrane to augment maxillary bone defect. The quality and maturity of bone formation at 6 months were adequate for implant placement and final restoration. The clinical cases presented here demonstrate that the combination of PABM and collagen membrane can work in various dental implant surgeries requiring GBR procedures.
Conclusion: The use of a bone conductive matrix in conjunction with a membrane for GBR procedure has become a prerequisite step in the overall success of dental implantology. Our overall goal is to develop an ideal bone conductive matrix that will be accessible to clinicians. The in vitro characterization, animal study and human case studies presented here have demonstrated that PABM can fulfill the requirements for GBR applications.
The animal study was conducted at NAMSA. We thank Dr. John JH Choi and Dr. Cory Wanatick for performing the clinical case studies.