Introduction: Infections associated with indwelling medical implants and devices costs $5-10 billion per year to treat[1]. Over 90% of patients with an indwelling urinary catheter for more than 4 weeks will develop infections[2]. MgO nanoparticles (nMgO) have become of interest for use in biomedical applications due to their antimicrobial properties[4]. MgO powder has been shown to exhibit antimicrobial activity towards Escherichia coli and Staphylococcus aureus[3],[4]. MgO may also be a useful additive to polymer nanocomposites (NC) to reduce bacterial adhesion to medical devices, thus we studied bacterial adhesion on nMgO based nanocomposites.
Materials and Methods: To determine minimal inhibition concentration(MIC) and minimal bactericidal concentration (MBC) , nMgO were incubated with Escherichia coli (Ec) and Staphylococcus epidermidis (Se) with initial seeding density of 6-8x10^6 microorganism/ml. Ec and Se were also cultured in broth with pH of 7-10 or in broth with doping amounts of Mg2+.
The NC was made with PLGA 50:50 and 30% nMgO (nMgO/PLGA) by weight. The scaffold was incubated with Ec or Se for 24h in LB, TSB or artificial urine (AU).
Post culture solutions were analyzed by determining the pH, Mg2+ and colony forming units (CFU). Bacteria adherence on the NC, polymer control, and in the presence of nMgO was evaluated using SEM.
Results and Discussion: The MIC and MBC forEc is the same at 1.0 mg/ml nMgO. For Se, the MIC started at a lower concentration of 0.5 mg/ml and the MBC 0.7 mg/ml nMgO. As the nMgO concentration increased, there was higher Mg2+ in solution. In addition, there was no Ec or Se inhibition or death observed when the bacteria were cultured in higher pH of 8-10 or higher amounts of Mg2+. The nMgO/PLGA NC was able to inhibit bacteria adhesions compared to PLGA (Figure 1A-F), PU and glass in LBB and TSB. At magnification 500X, there were patches of bacteria on PLGA (Figure 1C’) and PU, showing signs of biofilm formation while the NC showed no bacterial patches. In the AU solution, there was minimal or undetectable bacteria adherence to all the different experimental groups. The pH and Mg2+concentrationswere significantly higher for the NC and nMgO groups compared to PLGA, PU and glass control in TSB and AU.
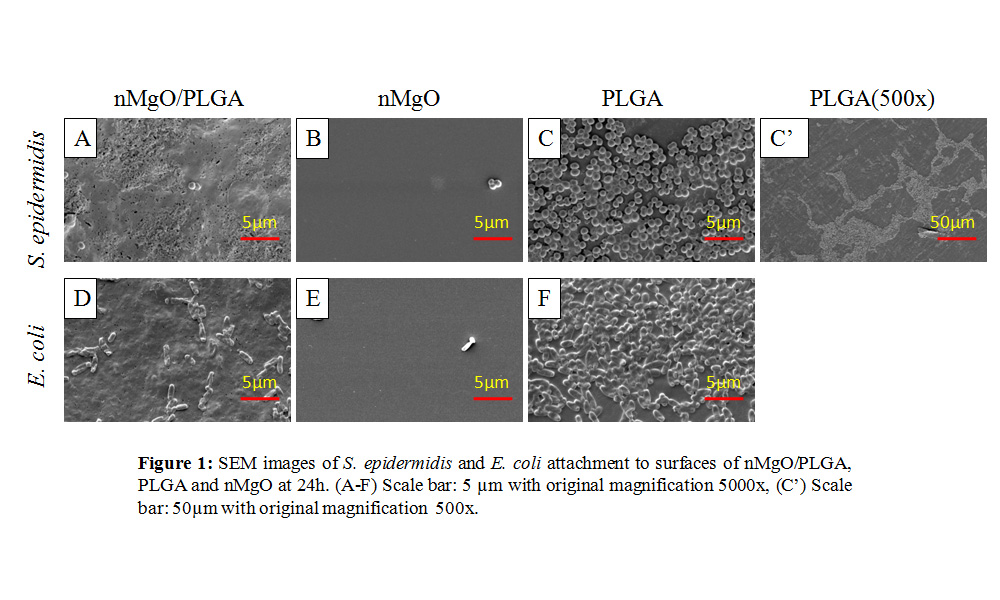
Conclusion: MgO nanoparticles were able to eradicate bacteria in solution as bare particles and inhibit bacteria adherence in the NC form. Use of nMgO in a variety of polymers could be useful in preventing bacteria adherence for biomedical applications.
NSF: Funding Sourse; CFAMM for SEM
References:
[1] Perl, T.M., et al., Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. New England Journal of Medicine, 2002. 346(24): p. 1871-1877.
[2] Choong, S., et al., Catheter associated urinary tract infection and encrustation. International Journal of Antimicrobial Agents, 2001. 17(4): p. 305-310.
[3] Sawai, J., et al., Antibacterial characteristics of magnesium oxide powder. World Journal of Microbiology and Biotechnology, 2000. 16(2): p. 187-194.
[4] Krishnamoorthy, K., et al., Antibacterial activity of MgO nanoparticles based on lipid peroxidation by oxygen vacancy. Journal of Nanoparticle Research, 2012. 14(9): p. 1-10.