Introduction: Titanium (Ti), zirconium (Zr), and gold (Au) are highly corrosion-resistant materials. Ti and Zr are covered by surface passive oxide films, while Au is not. However, the bioactivity of metals is caused not only by good corrosion resistance but also by other properties. Thus, the aim of this study was to investigation of this difference in cellular and gene expression responses to those metals, which makes it possible to deeper understanding the mechanism of bone formation on metallic biomaterials. In this study, a mouse osteoblast-like cell (MC3T3-E1) was incubated on Ti, Zr and Au. After osteogenic differentiation induction, calcified extracellular matrix, osteoinduction to metals were investigated. Our study is important for understanding of the potential mechanisms of bone formation of preosteoblest at metal-cell interface.
Materials and Methods: Ti, Zr and Au specimens were prepared by sputter-deposition onto cover glasses. The surface of specimens was characterized by atomic force microscopy, X-ray diffraction, and X-ray photoelectron spectroscopy. A MC3T3-E1 (RIKEN cell bank, Japan) were cultured in a culture medium. The calcification of MC3T3-E1 cells differentiation-induced for 5, 7, 10, 14, 21, and 28 d on each specimen was evaluated by alizarin red S staining. Meanwhile, gene expression in cells was determined by miRNA TaqMan® qRT-PCR assays (Applied Biosystems) on a LightCycler® 480 (Roche) according to suppliers protocol. Primers for real time RT-PCR were Runx2, Col1a1, Akp2, Ibsp, Spp1, Bglap1, and Ifitm5.
Results and Discussion: A quicker calcification (after 5-day incubation) can be obtained (Fig. 1). MC3T3-E1 cultured on Ti and Zr surfaces showed a better bioactivity at 7 d, 10 d, and 14 d, compared with Au. On the other hand, gene expression analysis results showed that different metals presented different expression levels of genes involved in bone formation during the incubation. In the initial stage (1 to 7 days after inducing the differentiation), Au showed a higher gene expression in Ibsp and Spp1. After 7-day incubation, Ti reached to the highest gene expression level in Ibsp, Spp1, and Runx2. Ti also showed an advanced gene expression level in Bglap1. At the same time, the gene expression responses inMC3T3-E1 to Zr were not prominent compared with Ti and Au. Moreover, the premier calcification phenomenon obtained by alizarin red S staining was also confirmed by genic diagnosis with target genes of Akp2 and Ifitm5. Based on this finding, the initial osteoblast-like cells may form a premier calcification during the junior cellular proliferation and differentiation, which would be important for promoting the secondary cellular adhesion and differentiation (Fig. 1). The conformation of proteins adsorbed on the metallic surfaces was supposed to be one of important factors for the cellular osteoinduction and calcification at metal-cell interface.
Conclusion: Although similar visualized calcificaiton of Ti and Zr was obtained, the genic diagnosis showed that the osteoinduction and calcification behaviors of cells were different to metals. This work possibly contribute to promote the novel metallic biomaterials design and use of those biomaterials for orthopedic/dental applications.
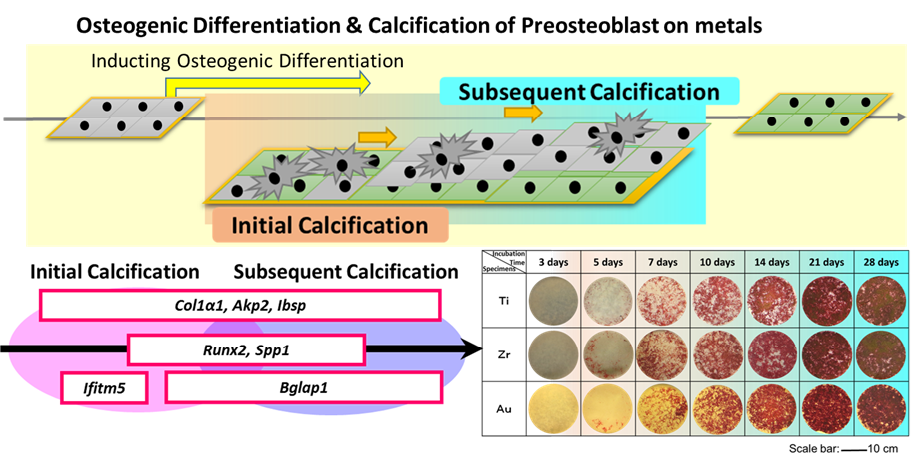
Fig. 1. Model of the role of target genes in promoting calcification after the induction of osteogenic differentiation and images of specimens after alizarin red S staining.