Introduction: Many approaches have been employed to incorporate antimicrobial agents (AmAs) into biomaterials, including AmA surface adsorption or grafting as well as delivery based on elution. We are exploring an alternate approach (Fig. 1A-C) based on the hierarchical self-assembly of sub-micron-sized anionic microgels and cationic antimicrobials[1],[2]. When the average inter-gel spacing is ~1 μm, microgel coatings significantly reduce bacterial adhesion while maintaining desirable cell (e.g. osteoblast) interactions[3],[4]. However, active killing must be triggered to free surfaces from bacteria. Here we focus on AmA incorporation onto microgel-modified surfaces to inhibit gram-positive/negative bacteria colonization.
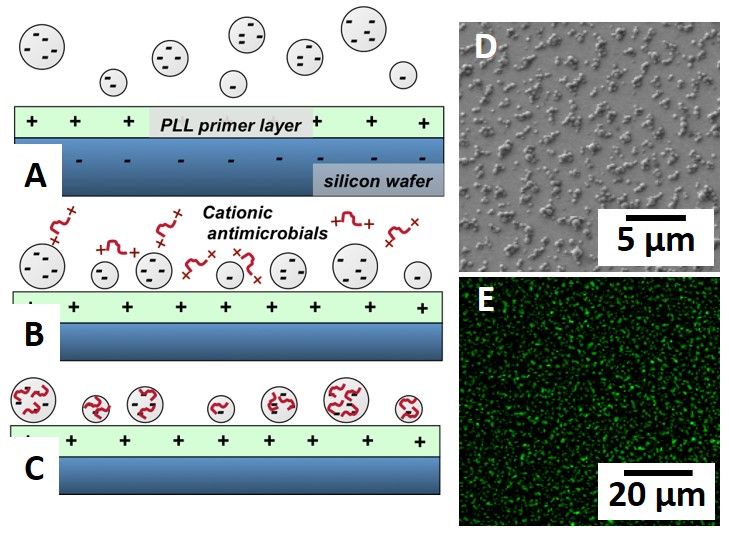
Fig.1 (A-C) Deposition of anionic microgels on a positively charged substrate followed by electrostaticantimicrobial loading; (D) SEM image of a microgel-modified surface; (E) Confocal image after FITC staing confirms AmA loading.
Materials and Methods: PEG-co-AA microgels were synthesized via suspension photopolymerization using PEG diacrylate and acrylic acid dissolved in dichloromethane and then dispersed in water. The microgels were filtered, cleaned, resuspended in 10 mM phosphate buffer, and electrostatically deposited onto poly(L-lysine)-primed Si. After deposition, different cationic antimicrobials (FDA-approved amikacin, colistion; Antimicrobial peptides (AMPs): L5, sub 5, KR-12, Bac 2A) were electrostatically loaded from AmA solutions into microgels on microgel-modified surfaces (Fig. 1 B,C). Antibacterial assays used a 107 CFU/mL inoculum followed by 48 h petrifilm culture.
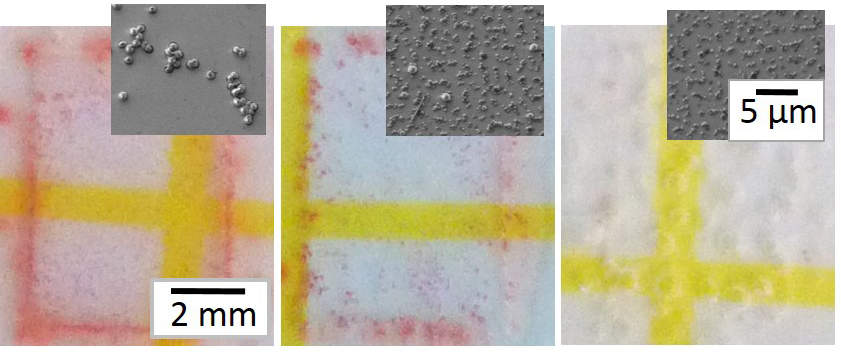
Fig.2 Petrifilm assays: of (A) PLL-primed Si; (B) AmA-free microgel-modified Si; (C)) AmA-loaded microgel-modified Si. Insets: SEM images of surfaces after inoculation.
Results and Discussion: Fig. 1D shows a typical surface morphology of (dry) PEG-co-AA microgels on Si. Cationic AmAs can be electrostatically loaded after microgel deposition and later visualized by FITC staining (Fig. 1E). The AmAs we studied range in size, charge, and hydrophobicity, all of which control their interactions with both microgels and bacteria. FITC staining shows that several AmAs remain sequestered within the microgels even after 72 h of soaking in PBS suggesting a non-traditional AmA release mechanism that is not diffusion controlled. Fig. 2 shows the results of petrifilm assays (red spots/pink area represent colonies) with the insets showing SEM images of innoculated surfaces. Bacteria adhere to and proliferate extensively on the PLL-modified positive control (Fig. 2A) and substantially less on the AmA-free microgel-modified surface (Fig. 2B). Significantly, AmA-loaded microgel-modified surfaces (Fig. 2C) very effectively resist colonization. These results suggest that AmAs are sequestered within microgels with release triggered by a bacterial challenge that brings a highly anionic/hydrophobic bacterial cell wall in close proximity to AmA-loaded microgels.
Conclusion: AmA-loaded microgel-modified surfaces both passively and actively resist bacterial colonization and unveil a new mechanism to inhibit biomaterials-associated infection.
This research project has been supported by the Army Research Office through grant #W911NF-12-1-0331.
References:
[1] Wu, Libera, et al. PEG-Based Microgels to Modify Biomaterials Surfaces. Macromol Symp, 2013. 329: 35.
[2] Wang, Libera, et al. Self-Assembled Poly(Ethylene Glycol) -Co- Acrylic Acid Microgels to Inhibit Bacterial Colonization of Synthetic Surfaces. Appl Matls Int, 2012. 4: 2498.
[3] Wang, Libera, Microgel-Modified Surfaces Enhance Short-Term Osteoblast Response. Colloids & Surf B, 2014. 118: 202.
[4] Wang, Libera, Busscher, et al. Length-Scale Mediated Differential Adhesion of Mammalian Cells and Microbes. Adv Func Matls, 2011. 21: 3916.