Introduction: Complications such as thrombus and restenosis continue to be the major limitations for clinical application of cardiovascular devices[1]. Surface modification to provide specific bioactivities and enable the biomaterials to long-term directing intravascular response plays important role in improving the performance and function of cardiovascular devices[2]. Cu(II) as the key enzymes co-factor was found recently to stimulate endothelium regeneration effectively[3]. Moreover,Cu(II) plays key role in scavenging free radical in blood and enhancing hemangiectasis by catalytic reduction of NO[4],[5]. Take the advantage of specific intermolecular reaction, in this study, a novel Cu(II)-loaded heparin/poly-l-lysine nanoparticle was prepared and immobilized to cardiovascular material surface to improve the biocompatibility.
Materials and Methods: As shown in Fig.1, poly-dopamine coating was firstly deposited to 316L SS surface; after that, copper chloride solution was mixed with poly-l-lysine (PLL) to form Cu(II)/PLL complex; then the complex was mixed with heparin solution and Cu(II)-loaded nanoparticles were prepared via intermolecular electrostatic interaction. Finally, the nanoparticles were immobilized to dopamine-coated surface. The physicochemical properties of modified surface were characterized, the anticoagulation property and cellular compatibility were evaluated.
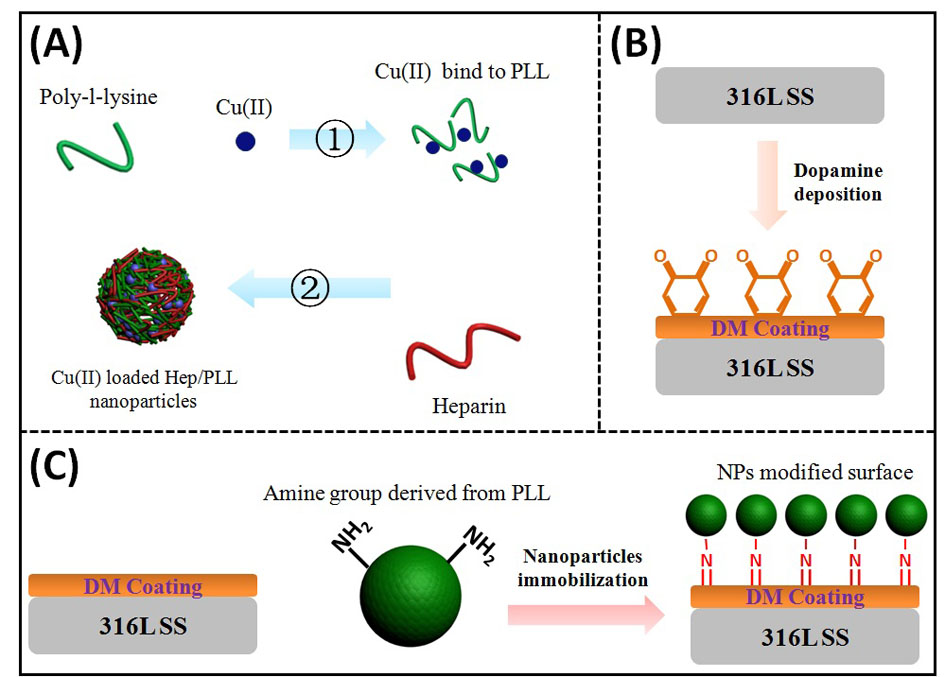
Fig. 1.(A) Preparation of Cu(II)-loaded nanoparticles, (B) dopamine coating deposition and (C) nanoparticles immobilized to material surface.
Results and Discussion: According to Fig.2, Cu(II)-loaded nanoparticles were immobilized to dopamine-coated surface successfully (Fig.2A). The Cu(II) incorporated into the nanoparticle mainly by interacting with the amino group and carbonyl group derived from PLL (Fig.2B). In this study, with the increasing of Cu(II) concentration, the absolute value of nanoparticle zeta potential was gradually decreased, which indicated the stability was decreased and particle agglomeration may occur. Besides, the increasing of Cu(II) concentration may reduce the amino group exposing density and thereby bring down the particle binding amount (Fig.2C). The nanoparticles modified surface displayed favorable Cu(II) sustained release property, as well as NO catalytic ability (Fig.2D and E). Biocompatibility evaluation result indicated that the nanoparticle modified surface displayed favorable anti-coagulation and anti-restenosis effect (Fig.2F and H). However, when the Cu(II) concentration was less than 0.5 mM, the modified surface may inhibit endothelial cells (ECs) growthing due to the high nanoparticle binding density. When the Cu(II) concentration was greater than 2.5 mM, the particle binding density was too little to direct ECs behavior. The nanoparticle modified surface displayed favorable ECs compatibility when Cu(II) concentration in the range of 0.5~2.5 mM (Fig.2G).
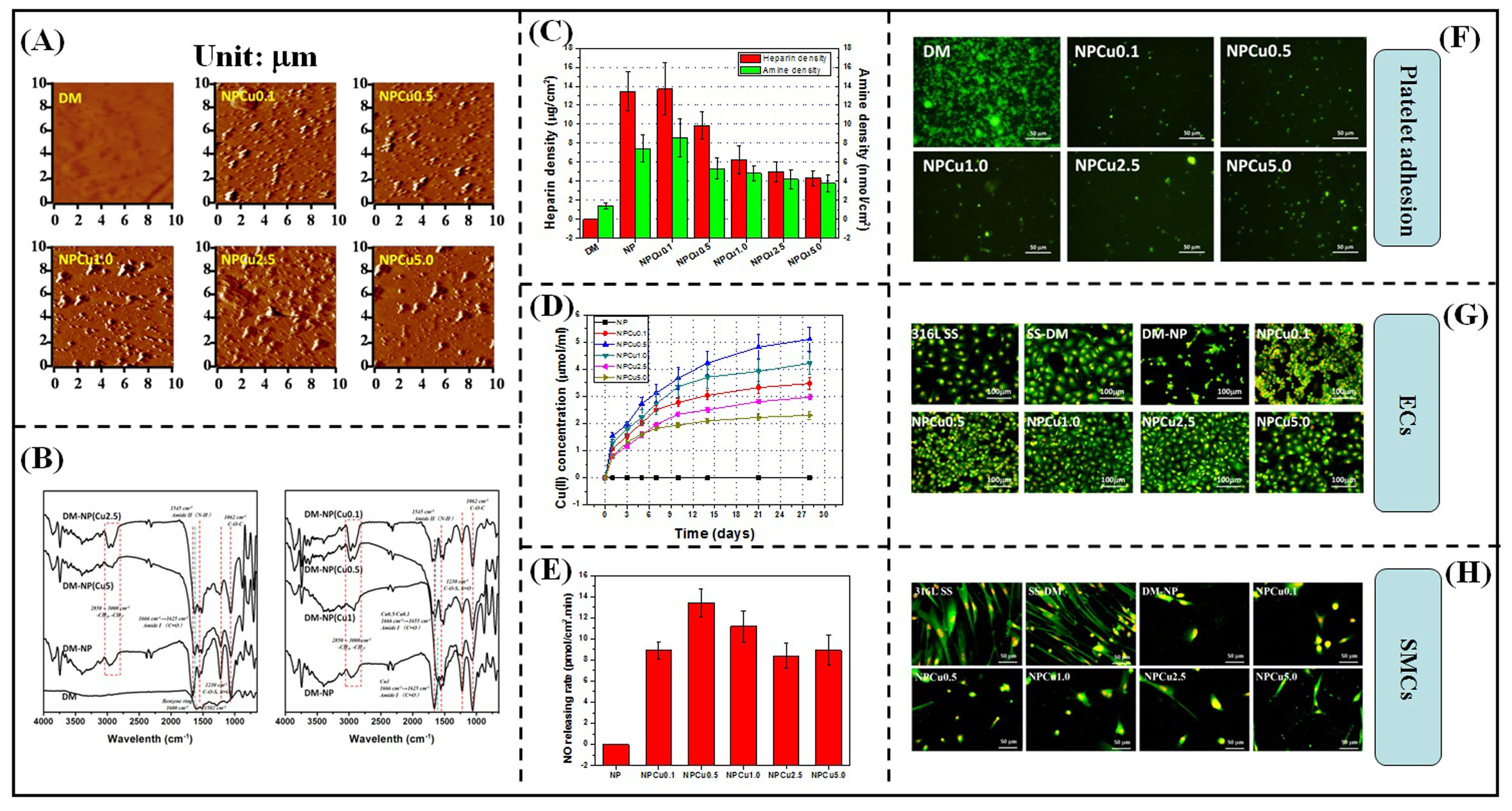
Fig. 2.(A) AFM images of nanoparticles modified surface, (B) FTIR spectra of nanoparticles modified surface, (C) quantitative characterization of heparin and amine exposing density, (D) Cu(II) release assay, (E) NO catalytic property, (F) platelet adhesion result, (G) ECs and (H) SMCs growthing profile on different sample surfaces.
Conclusions: This study provide a novel construction method of Cu(II)-loaded nano-coating. The size, stability, and binding density of nanoparticles was closely related to Cu(II) concentration. In a certain Cu(II) concentration range, the nano-coating displayed adequate stability and was found to selectively prevent thrombus and restenosis, while promote ECs growthing. This work may provide a promising approach for cardiovascular materials surface modification.
This work was supported in part by National Natural Science Foundation (No. 31470921 and No.31500778) and Foundation of Huaiyin Institute of Technology (No.491714296).
References:
[1] Garg S, Bourantas C, Serruys PW. New concepts in the design of drug-eluting coronary stents.Nat Rev Cardiol. 2013; 10(5): 248-260.
[2] Tao Liu, Kun Zhang, Shihui Liu, Junying Chen, Nan Huang. Endothelialization of implanted cardiovascular biomaterial surfaces: the development from in vitro to in vivo. J Biomed Mater Res A. 2014; 102(10): 3754-3772.
[3] Sen CK, Khanna S, Venojarvi M, Trikha P, Ellison EC, Hunt TK, et al. Copper induced vascular endothelial growth factor expression and wound healing. Am J Physiol Heart Circ Physiol. 2002;282:H1821–1827.
[4] Hwang S, Meyerhoff ME. Polyurethane with tethered copper(II)-cyclen complex: preparation, characterization and catalytic generation of nitric oxide from S-nitrosothiols. Biomaterials. 2008;29(16):2443-2452.
[5] Ren H, Wu J, Xi C, Lehnert N, Major T, Bartlett RH, Meyerhoff ME.Electrochemically Modulated Nitric Oxide (NO) Releasing Biomedical Devices via Copper (II)-Tri (2-pyridylmethyl) amine Mediated Reduction of Nitrite. ACS Appl Mater Interfaces. 2014;6(6):3779-3783.