Low temperature 3D printing (3DP) has been employed to fabricate scaffolds, where the powder is bound by aqueous (often acidic) binder solutions delivered from the inkjets [1],[2]. However, a key requirement for 3DP of the scaffolds is the availability of printable and biocompatible powder-binder systems. This study focus on optimizing the formulation of the citric acid solution used in low temperature 3DP of the scaffolds for maximize compatibility and mechanical strength, with a supplementation of Pluronic F-127(0.1wt%) to reduce the surface tension of the binder solution. Dilutions of citric acid (20- 40wt %) are used as the base binder solution for 3DP the scaffolds. In order to further enhance the biocompatibility, collagen solution (2wt% ) is used to modify the scaffolds.
Hydroxyapatite(HA) scaffolds, Hydroxyapatite/Chitosan(HA/CS:70/30) scaffolds and collagen -HA/CS scaffolds are fabricated.The cytocompatibility of the scaffold materials, micro morphology observation, hydrophily, compress strength and alkaline phosphatase (ALP) activity of MC3T3-E1 cells cultured on 3D printed scaffolds are assessed. MC3T3-E1 cells are cultured on scaffolds at an initial density of 2 × 104 cells per scaffold for 3,7 and 14 days. Subsequently, 360 μl of α-MEM medium and 40 μl Cell Counting Kit-8 solution are added to each well at each time point. The absorbance is measured at 450 nm using a VeritasTM microplate reader.
Reducing the acid concentration from 40wt% to 28.3wt% significantly improves cell viability (from10%±1% viability to 63.8% ±2.1% viability); and no further improvements are observed by reduction to 20wt% (70.1%±4.3% viability).
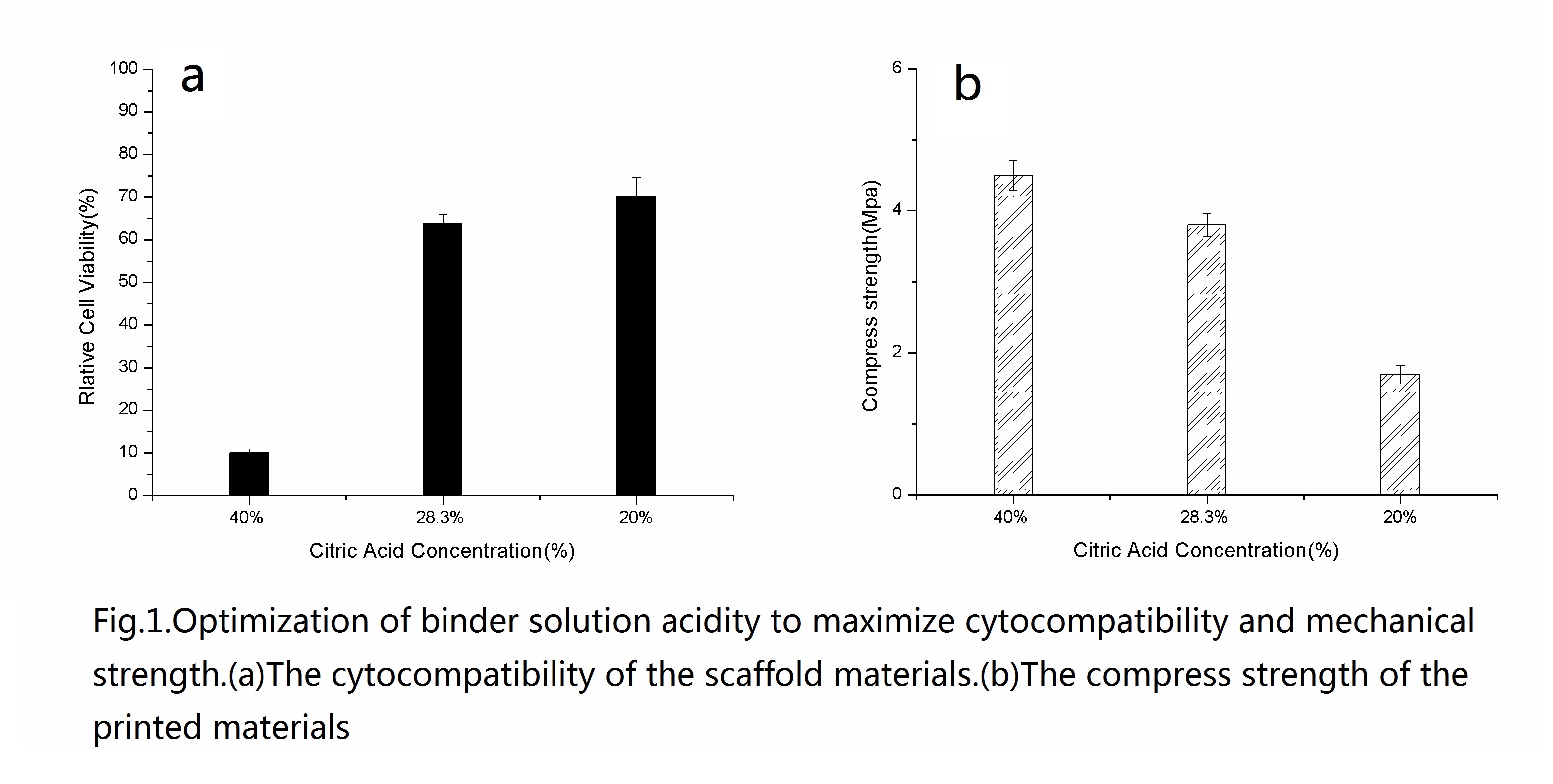
Using 28.3wt% citric acid produces significantly stronger scaffold materials over 20wt%. Therefore, 28.3wt% citric acid is selected for all subsequent experiments.
The compressive strength of HA/CS scaffold and collagen–HA/CS scaffold is more than that of HA scaffold with an additional 42.3% and 44.7% respectively.
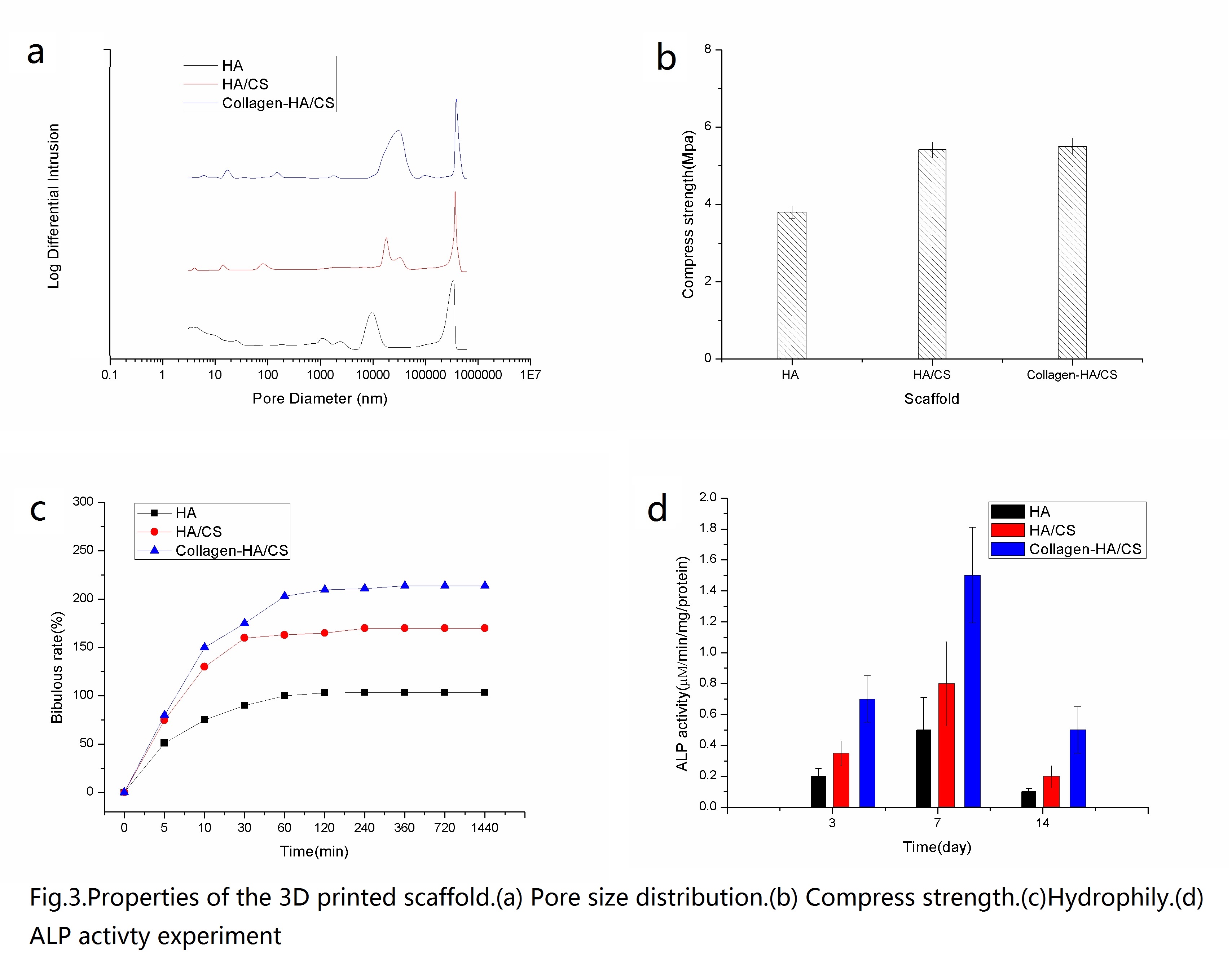
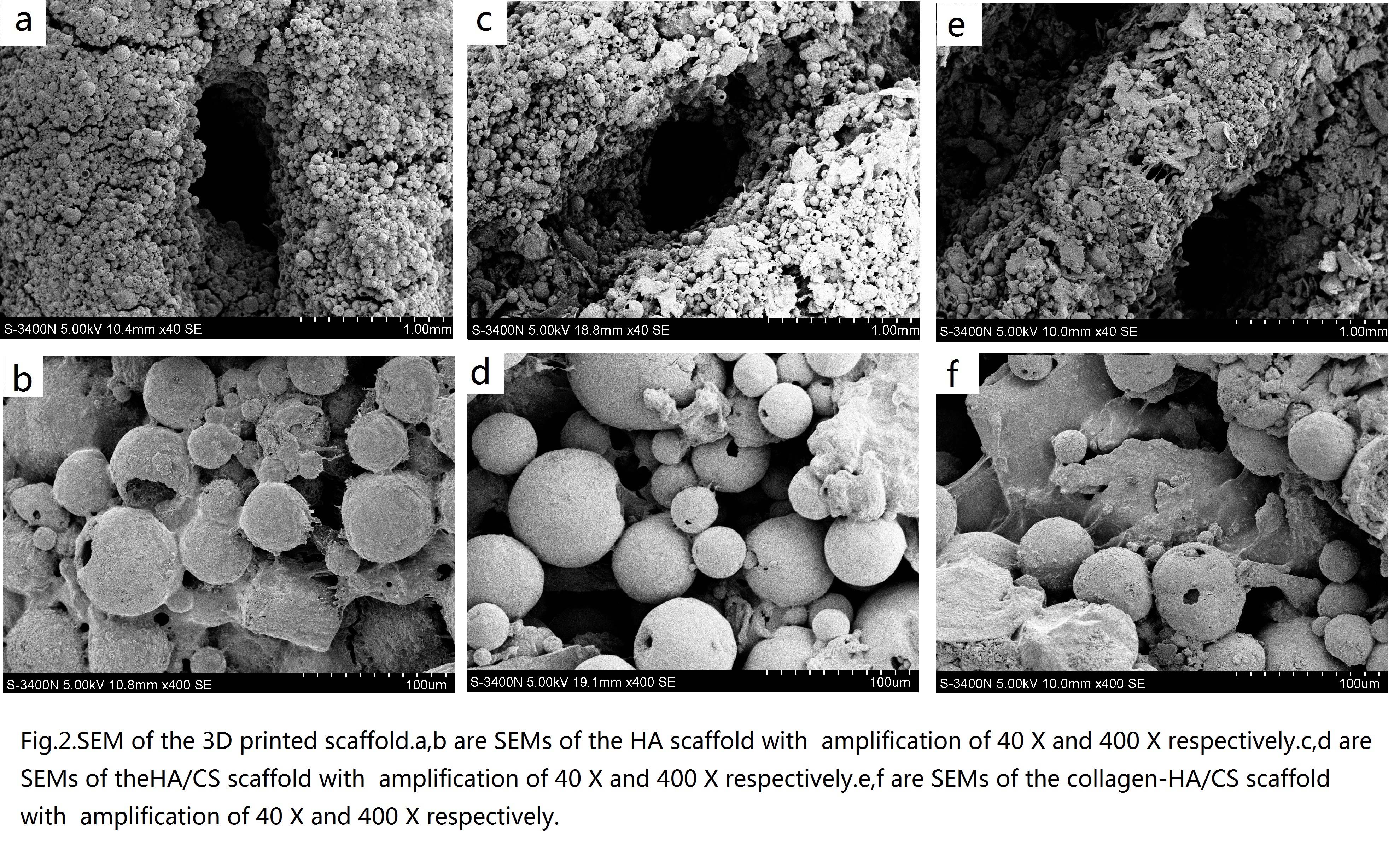
Moreover, the structure of collagen -HA/CS scaffold is complete without damage and stoppage of pores. The sharp edge of CS sheets is smoothed by the collagen.
Furthermore, the ALP activity of collagen -HA/CS scaffold is more 190.8% and 92.6% than those of HA scaffold and HA/CS composite material scaffold, respectively.
This study demonstrates that binder’s liquid properties (surface tension and viscosity) are important for 3D fabrication scaffolds mechanical properties and formulation quality. Moreover, the material integrate has impact on the scaffolds mechanical properties and biocompatibility. For instance, incorporating chitosan microparticle into the HA powder can improve the mechanical property of HA scaffold and the microstructure of the scaffolds. In addition, spraying binder solution with 2wt% collagen onto the every layer surface of HA/CS powder can further enhance the mechanical property of HA/CS scaffolds and improve the viability of MC3T3-E1cells cultured on the scaffold.
References:
[1] Susmita Bose,et al. Bone tissue engineering using 3D printing. Materials Today 16( 2013) 1369-7021
[2] Butscher A, Bohner M, Roth C, Ernstberger A, Heuberger R, Doebelin N, et al.Printability of calcium phosphate powders for three-dimensional printing of tissue engineering scaffolds. Acta Biomater 8 (2012) 373-85