Redox injectable gel for anti-adhesion agent
-
1
University of Tsukuba, Department of Materials Science, Master’s School of Medical Sciences and WPI-MANA Satellite, Japan
-
2
The University of Tsukuba, Department of Medicine, Japan
-
3
University of Tsukuba, Laboratory Animal Resource Center, Japan
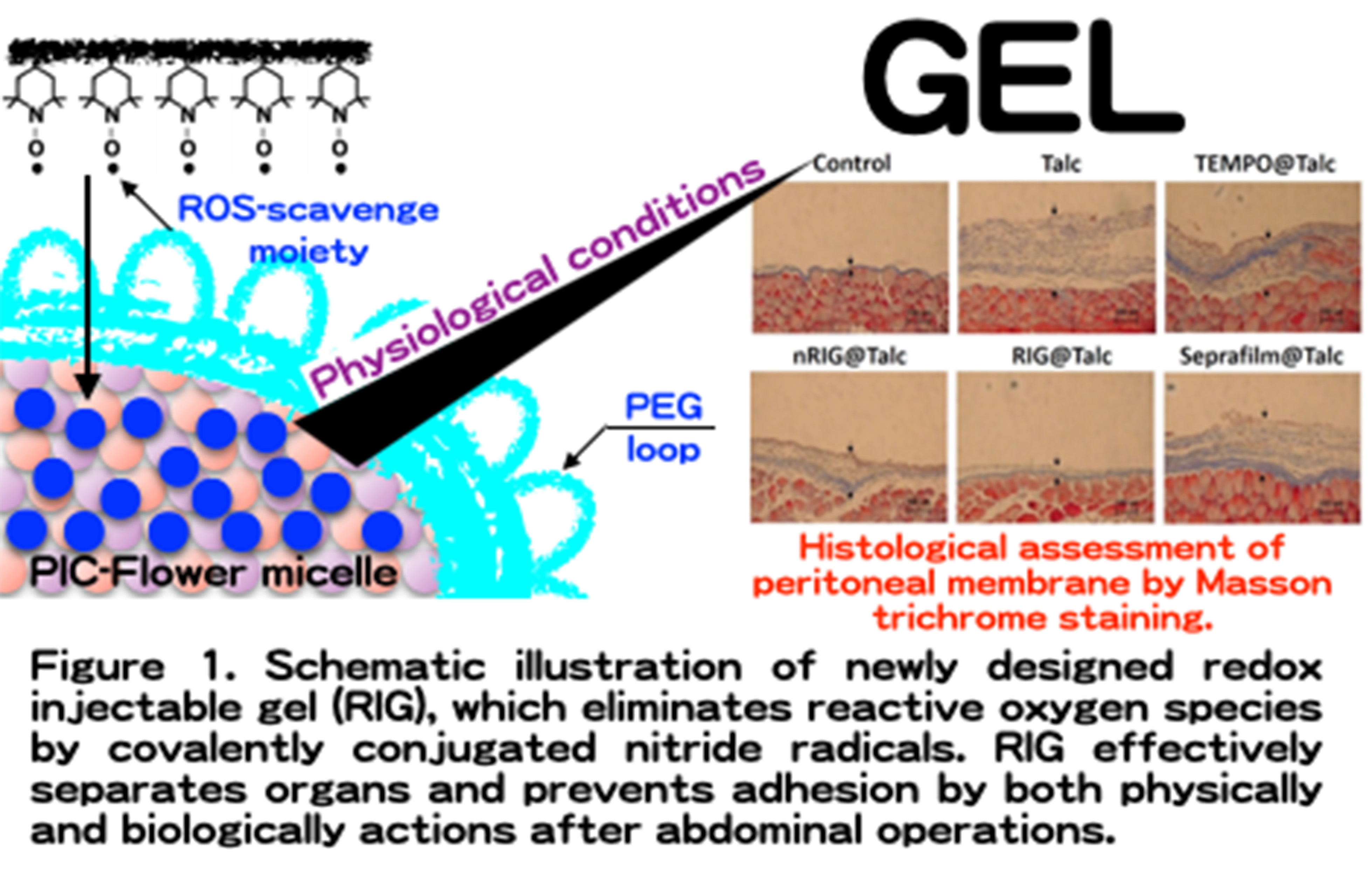
Introduction: Postsurgical tissue adhesion formation caused by inflammation and oxidative stress is one of serious issues because it induces severe clinical disorders. Currently, the most useful approach for reducing adhesions is the use of anti-adhesions by physical barrier using liquid and solid materials to isolate the traumatized tissue from surrounding other tissues. However, these materials function merely as physical barrier but has no effect to the inflammation caused by stimulation of cells and organ surfaces. In this study, we newly designed redox injectable gel (RIG), which possesses nitroxide radicals as a reactive oxygen species (ROS) scavenger for high performance anti-adhesion agent.
Materials and methods: The redox flower micelle was prepared by a polyion complex between polyamine-PEG-polyamine triblock copolymer possessing nitroxide radicals as a side chain of polyamine segments and poly(acrylic acid). Retention of the RIG in abdominal cavity was monitored by IVIS measurements. Talc-induced adhesion model mice were prepared and applied or evaluation of organ adhesions. Hematological analysis was performed using an automatic hematology analyzer for evaluation of the number of white blood cells. Histological assays were performed using the Masson trichrome assays.
Results and Discussions: Because polyion complex flower type micelles was used as redox-injectable gel, which forms a gel matrix in response to temperature under physiological ionic strength, its prolonged retention in the abdominal cavity increased the effect of RIG as a physical barrier, preventing the adhesion between tissues. RIG possessing nitroxide radicals was confirmed to scavenge excessively generated ROS and effectively suppressed inflammation in talc-induced adhesion model mice. It is also confirmed that white blood level of the talc-induced organ adhesion increased significantly, while RIG treatment suppressed it, indicating that the present RIG treatment effectively prevents diffusion of local inflammation to entirely body. Due to the combination of physically separation and biologically elimination of generated ROS, RIG dramatically inhibited the formation of tissue adhesions compared with commercial anti-adhesion agent (Seprafilm®). Another advantage of RIG is that it can be applied into the body as a liquid form, and transform into gel under physiological conditions. Therefore, RIG is recommended as a suitable anti-adhesion agent on the occasion of delicate surgical operations using endoscopic, catheter, and robotic surgeries.
Conclusion: We developed a high performance anti-adhesion agent using RIG with anti-oxidative function. RIG showed prolonged local retention in peritoneal cavity and effectively suppressed inflammation by sustained ROS scavenging activity in talc-induced adhesion model mice, which resulted in the dramatic suppression of adhesion formation. This gelation system under physiological conditions can be applied to both the open surgery and the occasion of delicate surgical operation using endoscopic, catheter and robotic surgeries. These findings indicate RIG has great potential for high performance anti-adhesion agent.
References:
[1] H.Nakagawa, et al., Biomaterials, Vol. 69,165-173 (2015)
Keywords:
in vivo,
nanoparticle,
gel,
Oxidation resistance
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
New Frontier Oral
Topic:
Nano-structured materials for unique functions
Citation:
Nagasaki
Y,
Nakagawa
H,
Matsumoto
Y,
Matsumoto
Y and
Miwa
Y
(2016). Redox injectable gel for anti-adhesion agent.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.01139
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.
*
Correspondence:
Dr. Yoshihiro Miwa, University of Tsukuba, Laboratory Animal Resource Center, Tsukuba, Japan, Email1