Introduction: The Innovabone project aims to develop a biomimetic product that consists of a bespoke scaffold and a bioactive self-setting gel, which will provide a microenvironment that contains active elements such as growth factors and CaP nanoparticles to promote bone repair. Scaffolds composed of different lactic acid (LA) and caprolactone (CL) ratios were produced using a two photon polymerisation (2PP). In this study, in vitro degradation and compressive properties were conducted for the produced scaffold in phosphate buffered saline (PBS) at 37oC. Cytocompatibility of the scaffolds was assessed using human mesenchymal stem cells (MSCs)[1].
Experimental Methods: Scaffold Production – Scaffolds were provided by IBA and TETRA and manufactured by a 2PP polymerisation technology[2] in which the 3D structure was based on a Schwarz Primitive minimal surface derived unit cells. Three different LA:CL ratios (16:4, 18:2 and 9:1) were investigated and samples were coded LC16:4, 18:2 and 9:1.
In vitro degradation and Mechanical testing -Degradation study of the scaffolds was performed according to the standard BS EN ISO 10993-13:2010 at 37°C using PBS buffer (pH =7.4 ± 0.2) and accelerated testing was also applied at 50 and 65°C. Compression testing was conducted using Hounsfield tester according to the standard ASTM 1621-10: 2010 at 25±1oC.
Cell Culture – Human MSCs were seeded at a concentration of 1x106 cells per scaffold and cultured in DMEM supplemented with 10% foetal calf serum, 1% L-Glutamine, 1% non-essential amino acids, 1% penicillin/streptomycin [standard medium], 0.1 μM dexamethasone, 50 μM ascorbic acid phosphate, and 10 mM β-glycerophosphate [Osteogenic (OS) medium)] at 37°C and 5% CO2.
Cell viability, metabolic activity, and markers of osteo-blastic differentiation were measured using Neutral Red, PrestoBlue, SigmaFAST & Alizarin Red assays respectively. RESULTS AND DISCUSSION
Percentage of mass loss for LC16:4, 18:2 and 9:1 scaffolds showed a gradual increase versus degradation time as can be seen from Figure 1.
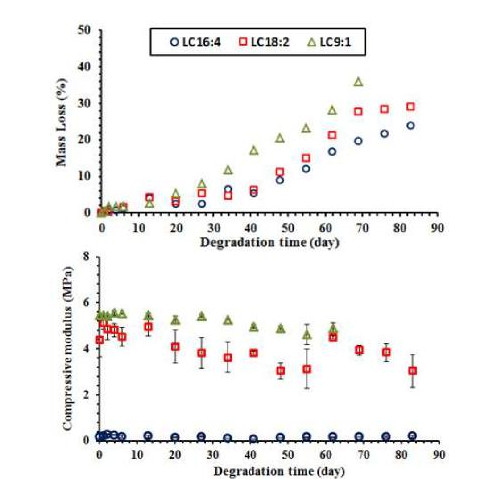
Figure 1: Change in mass loss and compressive modulus of LC scaffolds against degradation time at 37oC.
LC16:4 showed lower mass loss (ca. 20%) in comparison with LC18:2 and 9:1. This could be ascribed to the variation in ɛ -caprolactone to D,L lactide ratio (CL/LA) ratio between the scaffold materials. Compressive properties for LC18:2 and 9:1 scaffolds were also significantly higher (P<0.001) than LC16:4. Mechanical properties of these scaffolds are mainly dependent on their materials composition (i.e. CL/LA ratio) as all scaffolds have similar architecture. Accelerated degradation results show prediction of degradation rates and the activation energies from half mass loss results were found to be 87.9, 82.7 and 94.9 kJ mol-1 for LC16:4, LC18:2 and LC9:1 respectively.
Metabolic activity and ALP of cells within the scaffolds is shown in Figure 2. LC18:2 promoted higher cell metabolic activity but earlier time points showed no significant difference (p>0.05) between the three compositions. LC18:2 had also the highest ALP activity in comparison with LC16:4 and 9:1 scaffolds.
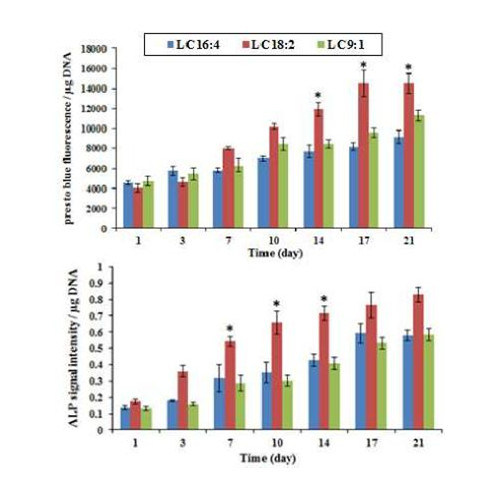
Figure 2: Metabolic activity and ALP for LCM scaffolds across 21 days of MSCs culturing.
LC18:2 scaffolds had significantly more live cells attached (ca. 20%) and mineralisation (ca. 30%) in comparison with LC16:4 and 9:1.
Conclusion: Variation of degradation and mechanical properties of LC scaffolds are related to their chemical composition and accelerated degradation was predictable. All three types of scaffold were capable of supporting cell proliferation and osteogenic differentiation of human MSCs over 21 days and LC16:4 showed the best bone cytocompatibility response. These scaffolds have a potential for use in bone repair applications.
References:
[1] Rashidi, H., et al., Cells Tissues Organs, 2012. 195(6): 484-94.
[2] Davis, K.A.et al., Biomaterials, 2003. 24(14): 2485-95.