Introduction: Although chitosan-based hydrogels have been extensively investigated for various soft tissues, their potential use for vocal fold engineering has been overlooked. The present study was to investigate cross-linked glycol-chitosan/collagen (type I or type III)/glyoxal hydrogels for targeted vocal fold engineering. The effects of glyoxal, collagen type I, and collagen type III concentrations on the cell viability, cell adhesion, and cell migration as well as hydrogel viscoelastic properties, biochemical stability, and mechanical stability were studied.
Materials and Methods: Hydrogel Preparation: Cell-seeded hydrogels containing immortalized human vocal fold fibroblasts were prepared to obtain target concentrations of 2×106 cells/mL, glycol-chitosan 2%, and glyoxal 0.0075% (Group#1), glyoxal 0.005% (Group#2), or glyoxal 0.0025% (Group#3)[1]. Solutions of 0.3% collagen type I and 0.1% collagen type III were mixed with the Group#2 constituents to obtain the target concentrations shown in Table 1.
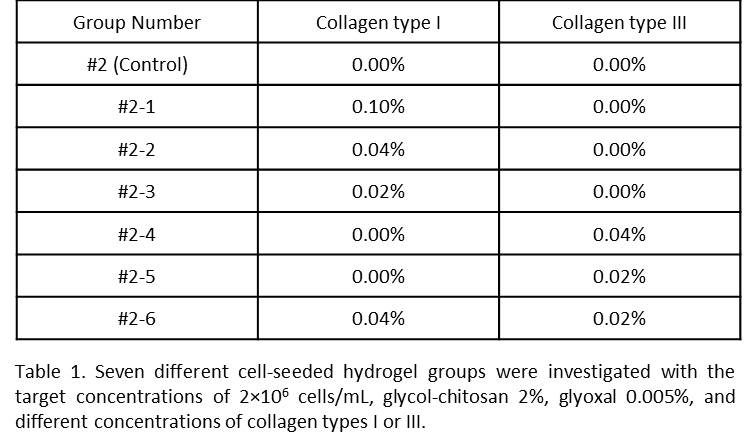
Cell Viability: The LIVE/DEAD Viability/Cytotoxicity kit was used to assess the viability of cells encapsulated in the hydrogels one hour, 3 and 7 days after preparation. The viability rate was obtained by dividing the number of live cells to the total number of cells. Cell Adhesion: Cells were cultured on the surface of hydrogel films. Cells were imaged before and after being washed with PBS 1X at determined time points. Cell Migration: Vybrant Dil was used to stain the cell membrane. The labelled cells with the target concentration of 5×105 cells/mL were used to prepare cell-seeded hydrogels. A confocal fluorescence microscope was used to image each sample for four consecutive hours. Rheometry: A TA Instrument rheometer was used to measure the shear elastic and loss moduli of the hydrogels at room temperature. A controlled stress frequency sweep test was performed over the frequency range between 0.5 Hz and 10 Hz. Biochemical Stability: Hydrogels were incubated in the solution of 13.0 µg/mL lysozyme in PBS 1X, with gentle mechanical agitation at 75 rpm over the period of study. After 3, 7, 14, 21, and 28 days, samples were lyophilized, and the dry weights were measured. Mechanical Stability (Fatigue Test): The scaffold was injected into the cavity of a custom-built phono-mimetic bioreactor, as shown in Figure 1, and phonated non-stop for 72 hours[2]. Subsequently, the gel was harvested and its mechanical integrity was evaluated.
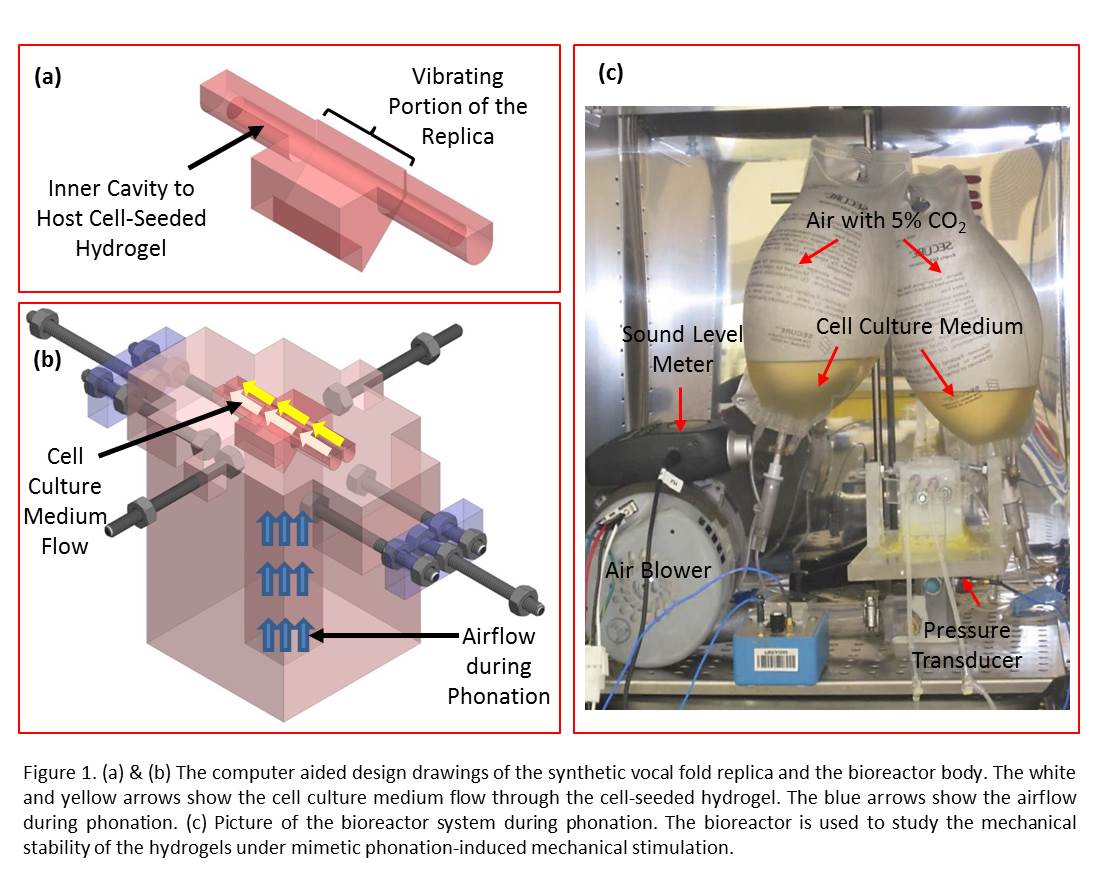
Results and Discussion: The viability rates were above 80.0% for all the groups one week after encapsulation inside the hydrogels. Representative images are shown in Figure 2.
Cell adhesion was improved in the groups containing either collagen type I or collagen type III. The average cell motility speed (µm/minute) was 0.09±0.03, 0.07±0.043, and 0.09±0.02 for groups 1, 2, and 3, respectively. The storage and loss moduli (in Pa) were 307±25 and 4±1, 149±31 and 3±1, 55±17 and 3±1, 785±23 and 46±3, 1288±15 and 37±3, 1170±11 and 43±5 for groups 1, 2, 3, 2-1, 2-4, and 2-6, respectively. Polymeric materials may fail under dynamic loading. Vocal folds undergo complex mechanical loading during phonation. The hydrogels were mechanically stable under mimetic loading in the bioreactor.
Conclusion: Our results suggest that glycol-chitosan/collagen/glyoxal hydrogels could be promising candidates for use in human vocal fold tissue repair and regeneration.
This work was supported by grants NIDCD R01-DC005788 (Mongeau, L.) and R03DC012112-01 (Li, N. Y. K.) from the National Institutes of Health. The authors would like to thank Professor Susan Thibeault (Department of Surgery, University of Wisconsin) for providing the I-HVFFs, Professor Hojatollah Vali for sharing his cell culture facility, Miss Annie Hélène Douillette (Department of Mechanical Engineering, McGill University) for her help with culturing the cells, and Dr. Eric Boucher (Department of Anatomy and Cell Biology, McGill University) for many fruitful discussions. The authors also thank the Advanced BioImaging Facility (ABIF, Life Sciences Complex, McGill University) for sharing their facility.
References:
[1] Heris, H. K., N. Latifi, H. Vali, N. Li and L. Mongeau (2015). "Investigation of Chitosan-glycol/glyoxal as an Injectable Biomaterial for Vocal Fold Tissue Engineering." Procedia Engineering 110: 143-150.
[2] Latifi, N., H. K. Heris, S. Kazemirad and L. Mongeau (2014). Development of a self-oscillating mechanical model to investigate the biological response of human vocal fold fibroblasts to phono-mimetic stimulation. ASME 2014 International Mechanical Engineering Congress and Exposition. Montreal, Quebec, Canada, American Society of Mechanical Engineers. 3: V003T003A002-V003T003A002.