
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 10 January 2018
Sec. Experimental Pharmacology and Drug Discovery
Volume 8 - 2017 | https://doi.org/10.3389/fphar.2017.00985
This article is part of the Research Topic Purinergic Pharmacology, Volume I View all 62 articles
Adenosine receptors (AR) are a family of G-protein coupled receptors, comprised of four members, named A1, A2A, A2B, and A3 receptors, found widely distributed in almost all human body tissues and organs. To date, they are known to participate in a large variety of physiopathological responses, which include vasodilation, pain, and inflammation. In particular, in the central nervous system (CNS), adenosine acts as a neuromodulator, exerting different functions depending on the type of AR and consequent cellular signaling involved. In terms of molecular pathways and second messengers involved, A1 and A3 receptors inhibit adenylyl cyclase (AC), through Gi/o proteins, while A2A and A2B receptors stimulate it through Gs proteins. In the CNS, A1 receptors are widely distributed in the cortex, hippocampus, and cerebellum, A2A receptors are localized mainly in the striatum and olfactory bulb, while A2B and A3 receptors are found at low levels of expression. In addition, AR are able to form heteromers, both among themselves (e.g., A1/A2A), as well as with other subtypes (e.g., A2A/D2), opening a whole range of possibilities in the field of the pharmacology of AR. Nowadays, we know that adenosine, by acting on adenosine A1 and A2A receptors, is known to antagonistically modulate dopaminergic neurotransmission and therefore reward systems, being A1 receptors colocalized in heteromeric complexes with D1 receptors, and A2A receptors with D2 receptors. This review documents the present state of knowledge of the contribution of AR, particularly A1 and A2A, to psychostimulants-mediated effects, including locomotor activity, discrimination, seeking and reward, and discuss their therapeutic relevance to psychostimulant addiction. Studies presented in this review reinforce the potential of A1 agonists as an effective strategy to counteract psychostimulant-induced effects. Furthermore, different experimental data support the hypothesis that A2A/D2 heterodimers are partly responsible for the psychomotor and reinforcing effects of psychostimulant drugs, such as cocaine and amphetamine, and the stimulation of A2A receptor is proposed as a potential therapeutic target for the treatment of drug addiction. The overall analysis of presented data provide evidence that excitatory modulation of A1 and A2A receptors constitute promising tools to counteract psychostimulants addiction.
Drug addiction is a complex chronic cognitive disorder characterized by drug seeking and compulsive use, which is difficult to control despite its harmful consequences. According to DSM-5 (American Psychiatric Association, 2013), which is used to define mental disorders in epidemiologic studies, it is now accepted that the criteria used to clinically define the terms abuse and dependence should be combined to form a new category known as Substance use disorders, including craving as a new criterion to increase diagnostic accuracy (Hasin et al., 2013). In terms of epidemiology, drug addiction is currently a global health problem, as can be deduced from comparison of data obtained from the Global Burden of Diseases Study between 1990 and 2015. For the period 1990–2015, global exposure to drug use increased by 30.2% for both sexes. In addition, by 2015 drug use was a major risk factor for early death and disability in developed countries like the United States, Canada, Australia, and the United Kingdom, being the 5th leading global risk factor for men and the 12th for women (GBD 2015 Risk Factors Collaborators, 2016).
Psychostimulants are a broad class of drugs whose effects include increases in arousal, wakefulness, cardiovascular stimulation, vigilance, and attention, and which constitute one of the most abused classes of prohibited drugs in the world, including as representative examples cocaine and amphetamine-like molecules (Chesworth et al., 2016). According to the 2017 report of the European Monitoring Centre for Drugs and Drug Addiction (European Monitoring Centre for Drugs and Drug Addiction, 2017), it was estimated that in the year 2016, the global annual prevalence among Europeans aged 15 or over was 3.5 million users of cocaine, 2.7 million users of MDMA and 1.8 million users of amphetamines which corresponds to 1.0, 0.8, and 0.5% of the European adults, respectively, which occasional consumed mentioned psychostimulants during 2016.
To date, the therapies developed to manage drug addiction are inadequate and unsatisfactory, and many scientists around the world are focusing on new strategies to improve them. Even though numerous aspects of this phenomenon are not well understood, the neurochemical mechanism common to all drugs causing abuse in humans is the increase of the neurotransmitter dopamine (DA) released from the ventral tegmental area (VTA), to a region in the mesocorticolimbic part of the brain, like the nucleus accumbens (NAc) and the prefrontal cortex (Filip et al., 2012; Morales and Margolis, 2017). This, in turn, increases the physiological reward and reinforcement mechanisms (Nestler and Landsman, 2001). This point is particularly important because DA not only mediates the effects of acute rewarding, but is also thought to be involved in the increased motivation to consume psychostimulants in psychostimulant abusers (Volkow et al., 2012). Furthermore, abuse of psychostimulants may induce changes in brain regions not only with relevance for addictive behavior, but may also promote long-term adverse consequences in areas related to memory and cognition (Nyberg, 2014). In addition, relapse into drug use after abstinence has been attributed to exposure to cues, stress or re-exposure to the drug itself that induce drug craving; the incubation of craving being a common phenomenon reported for most drugs of abuse, including psychostimulants, that may last from the beginning of abstinence for extended periods of time. Although little is known about the molecular mechanisms that lead to the incubation of craving during drug abstinence, vulnerability to relapse correlates with changes in the activity and structure of neurons from the limbic and frontal cortical circuitry, induced by the drug use (Pickens et al., 2011; Wolf, 2016).
The repeated ingestion of psychostimulants, as for most substances with marked abuse potential, shares one of the following two common features consistently reported in the literature: on the one hand, psychostimulants, by blocking molecular reuptake, enhance the extracellular neurotransmitter concentration in the synapses of monoaminergic neurons (Cooper et al., 1996; Rothman and Baumann, 2003; Wood et al., 2014); on the other hand, psychostimulants increase DA release in the NAc, a critical area for the reward circuit (Preedy, 2016). There is a growing body of scientific evidence demonstrating that psychostimulants affect dopaminergic neurons in the limbic reward system, and that this effect underlies addiction to stimulants (Siciliano et al., 2015).
Adenosine, an ubiquitous endogenous nucleoside, has been implicated in the reward-related behavior, and represents a novel and interesting target to interfere with it, as a consequence of its modulatory function on neurotransmission exerted by DA, glutamate and acetylcholine (Linden, 2001; Cunha, 2005; Gomes et al., 2011; Lopes et al., 2011; Borea et al., 2016; Burnstock, 2017; Jacobson et al., 2017). Interestingly, adenosine levels are modified following acute or chronic consumption of drugs of abuse and psychostimulants (Hack and Christie, 2003; Brown and Short, 2008; Filip et al., 2012), suggesting that a better comprehension of adenosine signaling in the brain during addiction may open new pharmacological frontiers to explore potential treatments in preclinical studies and clinical trials over the next years (Stone, 1981; Clark and Dar, 1989; Krauss et al., 1993; Bonci and Williams, 1996; Salem and Hope, 1999). The question of whether adenosine signaling can be used as a potential therapy in abuse disorders remains to be answered. Therefore, the goal of this review is to discuss current scientific evidence based on animal models of psychostimulant addiction, and to suggest promising candidates in the search for pharmacological interventions. We will include, when available, the effects of adenosine receptor (AR) ligands on the complex process of behavior related to psychostimulant consumption, seeking, withdrawal, craving and relapse, and we will restrict our discussion to what we can consider psychostimulant drugs, namely cocaine and amphetamine-like molecules.
The main brain circuitry associated with addiction is distributed across multiple areas. This circuitry is associated with the three stages of the addiction cycle (Koob and Volkow, 2010). The reinforcing effects in an initial binge/intoxication stage are mediated by DA and opioid neurotransmission, and depend on modifications in the VTA and striatum (particularly NAc). The negative emotional stage of withdrawal may be due to activation of the amygdala with norepinephrine, dynorphin and corticotropin-releasing factor. The third stage, craving, depends on the prefrontal cortex, amygdala and hippocampus, and glutamate is the major neurotransmitter involved (reviewed in Kelley and Berridge, 2002; Koob and Volkow, 2010; Kim et al., 2017; Figure 1). The NAc acts as a hub of convergence from the different regions, and it has been considered a key element in the neuronal circuitry of drug addiction. This nucleus is composed of two distinct populations of medium spiny neurons (MSN) with different levels of dopamine D1 and D2 receptors and projections (direct and indirect basal ganglia pathways), which are positively and negatively coupled to cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA) signaling, respectively. Striatonigral MSN (direct pathway) send substantia P and dynorphin projections to substantia nigra/VTA and globus pallidus interna, and are enriched with dopamine D1 receptors. Striatopallidal MSN (indirect pathway) send enkephalin projections to globus pallidus externa and are enriched with dopamine D2 receptors (Lobo, 2009). Taking together several studies using different approaches such as analysis of psychiatric disorders, transgenic mice, neuropharmacology and optogenetic techniques, it has been possible to postulate an integrative representation of the synaptic connections in NAc and the neurotransmitter systems (Silberberg and Bolam, 2015; Figure 2).
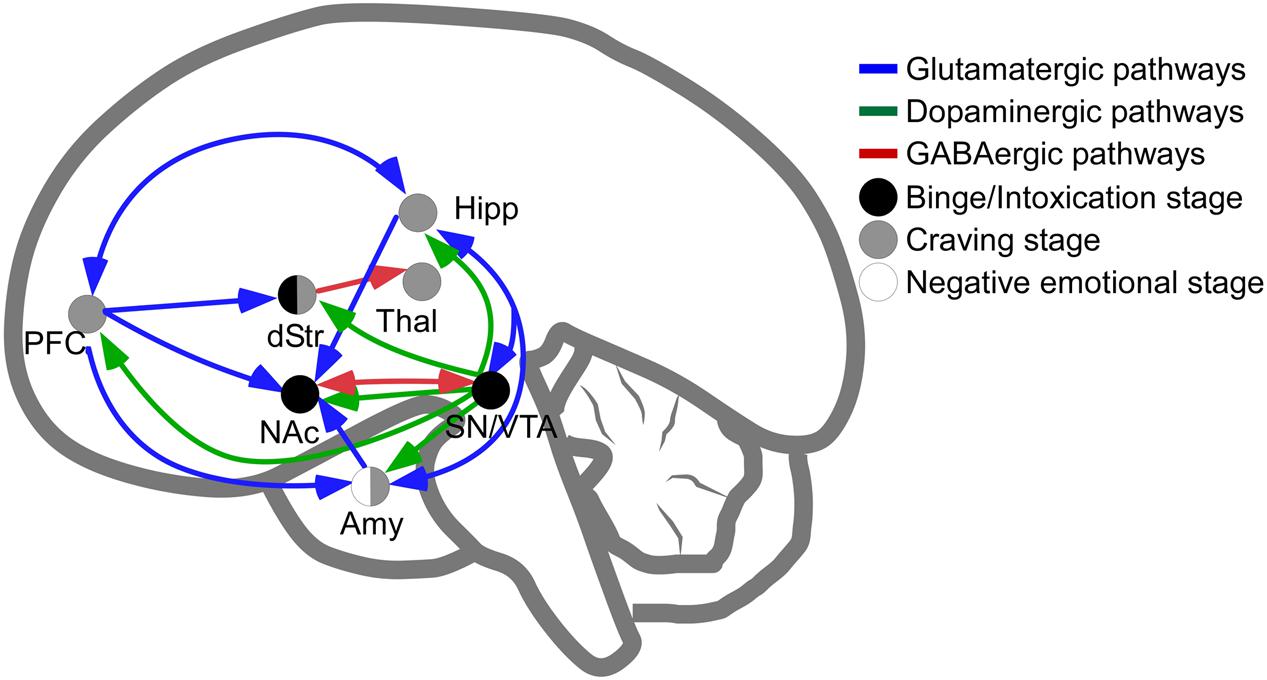
FIGURE 1. Schematic representation of pathways involved in intoxication, withdrawal and craving stages of addiction. Release of dopamine in the nucleus accumbens is a common feature of psychostimulants reinforcement at initial stages of psychostimulants intake (dots in black are regions directly involved in binge and intoxication). The negative emotional stage of withdrawal appears to be related to the activation of amygdala (dots in white are regions directly involved in negative emotional stage) and, finally, the latter stage of psychostimulant addiction, craving, depends on prefrontal cortex, amygdala and hippocampal activities (dots in gray). In blue, glutamatergic pathways; in green, dopaminergic pathways; in red, GABAergic connections. NAc, Nucleus Accumbens; Amy, Amygdala; dStr, dorsal Striatum; Hipp, Hippocampus; PFC, Prefrontal Cortex; SN/VTA, Substantia Nigra and Ventral Tegmental Area; Thal, Thalamus.
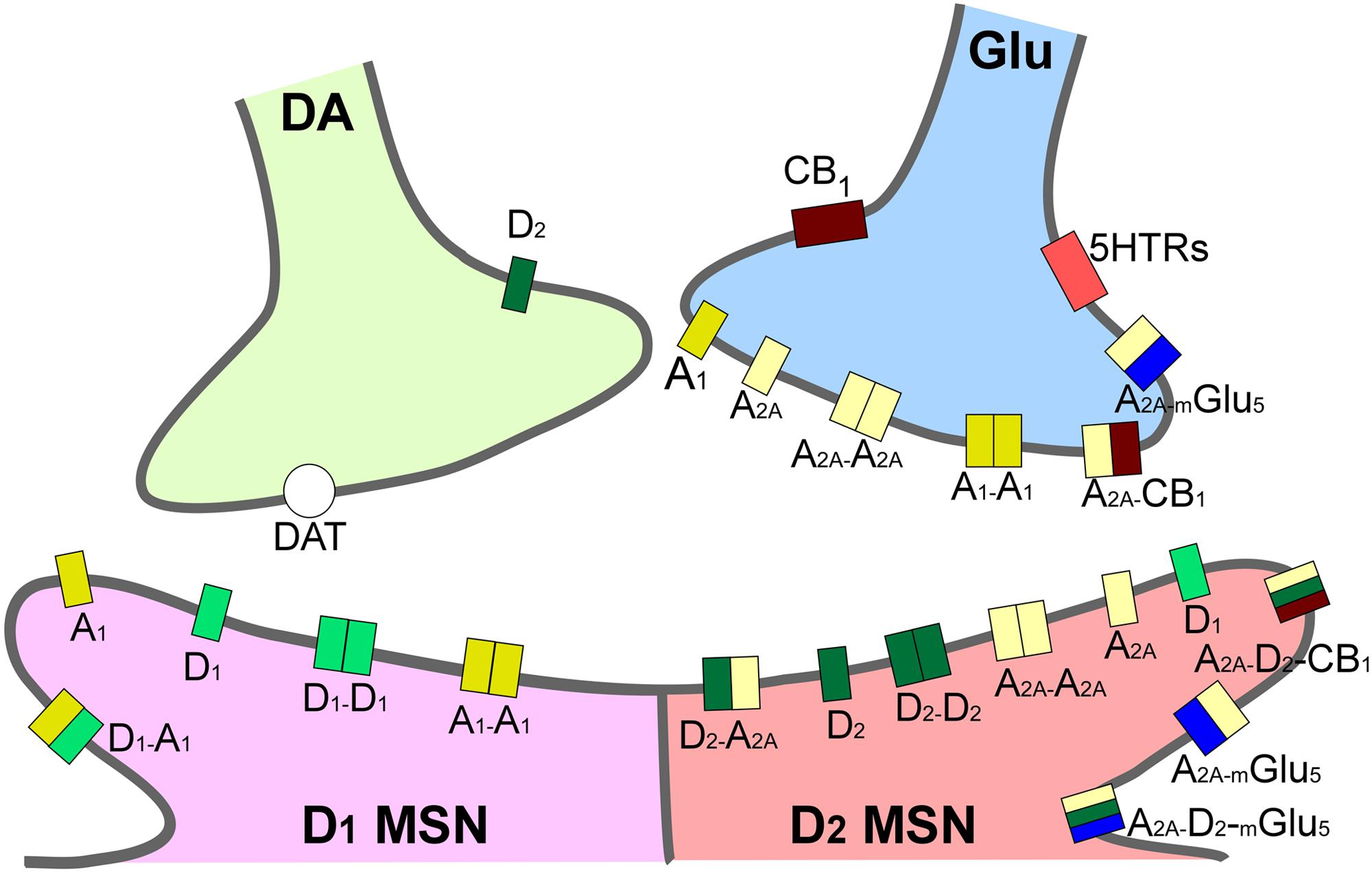
FIGURE 2. Integrative scheme of reward circuit in striatum with focus on adenosine and dopamine receptors and their interactions. A1 and A2A receptors are located pre- and post-synaptically forming homo-, heterodimers and oligomers in the dendritic spines of medium spiny neurons in striatum (MSN). Glutamatergic input from cortex and dopaminergic input from ventral tegmental area project to both MSNs expressing D1-like and MSNs expressing D2-like dopamine receptors. A2A, adenosine 2A receptors; CB1, endocannabinoid CB1 receptors; DAT, dopamine active transporters; D1, dopamine D1 receptors; D2, dopamine D2 receptors; mGlu5, metabotropic glutamate subtype 5 receptors; 5HTRs, serotonin receptors.
Adenosine acts in the central nervous system (CNS) as a neuromodulator, with DA neurotransmission being one of its targets. The modulation of dopaminergic activity is mediated by two main subtypes of AR, being the antagonist of DA receptors. Specifically, A1 receptors colocalize with D1 receptors, and A2A receptors with D2 receptors in heteromeric complexes (Fuxe et al., 2010). Building on this rationale, the case of A2A receptors is particularly important when we are studying the effects of drugs of abuse, for reasons that have been extensively reviewed previously (Hack and Christie, 2003; Brown and Short, 2008; Filip et al., 2012), and that we only briefly enumerate here. First, A2A receptors are highly expressed in the striatum, a key brain nucleus for the reward circuitry, and A2A are crucial receptors, modulating behavioral responses induced by drugs of abuse (Ferré et al., 2007); indeed, their genetic deletion in mice results in a selective decrease of locomotor responses to cocaine and amphetamine (Chen et al., 2000). Furthermore, A2A receptors are able to form heteromers with other adenosine subtypes, resulting in, e.g., the A1/A2A heteromer (Ferré et al., 2008a), and also with families of receptors relevant for the treatment of some neuropsychiatric disorders and drug addiction, producing, e.g., A2A/mGlu5 (Ferré et al., 2002), A2A/D2 (Ferré et al., 2008b; Bonaventura et al., 2015), and A2A/CB1 receptor heteromers (Tebano et al., 2012). This spectrum of interaction opens a wide range of possibilities in the field of AR pharmacology (Casadó et al., 2009). Interestingly, the oligomer formed by A2A/mGlu5/D2 receptors confers to the A2A subtype the ability to modulate the effects of psychostimulants in striatal neurons, through the balance of GABAergic, dopaminergic and glutamatergic signaling (Cabello et al., 2009; Kniazeff et al., 2011). The modulation of dopaminergic and glutamatergic signaling by A2A receptors has been particularly important in the field of psychiatric disorders, as DA and glutamate are two key players in the processing of moods, which could also be very relevant in drug addiction-related disorders (Cunha et al., 2008).
In the CNS, extracellular adenosine exists in basal conditions, and its concentration may increase under pathological situations, including hypoxia, ischemia or cell injury. Adenosine is produced by different mechanisms, including metabolism of ATP released from neurons or glial cells. Specifically, ATP undergoes dephosphorylation to ADP and AMP by the activity of particular enzymes named ectonucleoside triphosphate diphosphohydrolase (CD39), and to adenosine through a specific ecto-5′-nucleotidase (CD73) enzyme (Drury and Szent-Györgyi, 1929). Alternatively, adenosine may derive from hydrolysis of intracellular AMP through a cytoplasmic 5′-nucleotidase, or S-adenosyl-homocysteine (SAH) by SAH hydrolase, and may be released through facilitated diffusion, using bi-directional equilibrative nucleoside transporters (ENT) (Dunwiddie, 1985; Ledent et al., 1997; Ribeiro, 1999; Hack and Christie, 2003). Under resting conditions, extra- and intra-cellular levels of adenosine are very similar, but in pathophysiological states (inflammation, ischemia, and hypoxia), characterized by high levels of this nucleoside, transport through ENTs is the main mechanism responsible for extracellular adenosine removal. Inside the cell, adenosine is deaminated to inosine through adenosine deaminase (ADA), or phosphorylated to AMP by adenosine kinase (AK). These enzymes display different affinities for adenosine, with AK more affine than ADA; thus in physiologic conditions adenosine is preferentially transformed to AMP, whilst in pathological states predominantly to inosine, a process which occurs also in the extracellular milieu (Godinho et al., 2015; Borea et al., 2016).
Adenosine affects several functions in the body, exerting its physiological effects through regulation of four G-protein coupled receptors (GPCRs) named A1, A2A, A2B, and A3, characterized by different affinities for adenosine, tissue distribution, and coupling with effector systems. They have been cloned and pharmacologically characterized in different species, presenting a sequence homology of around 80–95%, except for A3 receptors which vary depending on species and show a variance of 30% in amino-acid composition between human and rat. The A3 receptor, in contrast to other AR, was the first to be isolated and then pharmacologically characterized (Meyerhof et al., 1991). All AR show a common structure, characterized by seven transmembrane domains connected by three intracellular and extracellular domains (Fredholm et al., 2000). At the extracellular level, the N-terminus presents specific glycosylation sites, while at the intracellular side, the C-terminus contains phosphorylation and palmitoylation sites, important for receptor desensitization. The A2A receptor has a longer C-terminus tail constituted by 122 amino acids, whereas A1, A2B, and A3 receptors’ C-terminal domains comprise 30–40 amino acids (Fredholm et al., 2001). Recently, important crystallization results have determined the structures of human A1 and A2A receptors, thus allowing better drug design for A1 and A2A receptor-selective ligands (Jaakola et al., 2008; Lebon et al., 2011; Xu et al., 2011; Carpenter et al., 2016; Glukhova et al., 2017; Sun et al., 2017). In the case of A3 receptors, structure-based molecular modeling techniques have led to the rational design of potent A3 receptor-selective ligands (Ciancetta and Jacobson, 2017). Furthermore, AR may be present in the cell membrane in homomer isolated forms or in heteromers and oligomers, providing another possibility of intervention for drug development (Cabello et al., 2009). Specifically, the ability of AR to interact with many other receptors, such as A2A/D2 receptor heterodimers located in the striatum (represented in Figure 2), means they play a pivotal role in the modulation and integration of neurotransmission, and may be targeted by drugs for the treatment of neurological diseases, including drug addiction (Chen et al., 2013).
AR are widely distributed in almost all organ and tissues, spanning brain, heart, lung, liver, kidney, bone, eye, skin, joints, and blood cells, suggesting that these proteins are potentially able to affect almost every physiological function (Peleli et al., 2017).
Specifically, as for the presence of each AR subtype in CNS, the A1 receptor is mainly present in the cortex, hippocampus, cerebellum, nerve terminals, spinal cord, and glia (Chen et al., 2013). This wide range of locations reflects the multitude of physiological effects orchestrated by it, including inhibition of neurotransmitter release, reduction of neuronal excitability, sedation, anticonvulsant and anxiolytic effects, analgesia and regulation of sleep (Stenberg et al., 2003; Gessi et al., 2011; Sawynok, 2016; Vincenzi et al., 2016a,b; Varani et al., 2017). These effects are mediated through A1 receptors coupling to Gi/Go proteins, inhibition of AC, activation of phospholipase-C (PLC)β and, particularly in neurons, activation of potassium channels and deactivation of Q-, P-, and N-type Ca2+ channels. They also modulate mitogen-activated protein kinases (MAPK) with important functional effects (Schulte and Fredholm, 2003).
The A2A receptor is highly expressed in the striatum, mainly present in GABAergic striatopallidal neurons, corticostriatal glutamatergic terminals, and cholinergic interneurons, but it is also detectable in the olfactory tubercle, cerebral cortex, hippocampus, neurons, and glial cells, where it induces excitotoxicity by affecting release of glutamate, activation of glia and infiltration of immune cells from the periphery, through blood brain barrier passage (Fuxe et al., 2003, 2007b; de Lera Ruiz et al., 2014). The A2A receptor generally couples to Gs proteins to increase cAMP levels, but in the brain it stimulates Golf, a specific Gs protein in neurons also associated with AC (Kull et al., 2000), which is supposed to have a prominent role as a mediator of the locomotor effects of some psychostimulant drugs (Hervé et al., 2001). The signaling cascade starting from cAMP and PKA regulates different proteins such as cAMP responsive element binding protein (CREB) and DA- and cAMP-regulated phosphoprotein (DARPP-32) (Preti et al., 2015), which is also involved in the responses to psychostimulant drugs (Engmann et al., 2015). CREB phosphorylation then increases transcription of immediate early genes such as c-fos and other genes like preproenkephalin (Ferré, 2008). In addition, the A2A receptor, with its long C-terminus, could also bind to different accessory proteins like D2 receptors, ADP-ribosylation factor nucleotide site opener (ARNO), α-actinin, translin-associated protein X (TRAX) and ubiquitin-specific protease (USP4). A2A receptor activation may also trigger the Ras/Raf-1/MEK/ERK pathway through PKA-dependent or independent mechanisms (Schulte and Fredholm, 2003).
A2B receptors are present in astrocytes, neurons, and microglia, but their role in the CNS is less well characterized in comparison to the other AR subtypes. As for the effector systems, it activates Gs proteins/cAMP/PKA phosphorylation. Furthermore, the A2B receptor stimulates an increase in Gq protein/PLC/Ca2+, while modulating ion channels through βγ subunits. Coupling to MAPK has been also reported (Merighi et al., 2017).
Finally, for A3 receptors, a low level of expression has been detected in the brain, in which it was detected in the cortex, thalamus and hypothalamus, hippocampus, motor nerve terminals, retinal ganglion cells, pial and intracerebral arteries and glia (Borea et al., 2015; Jacobson et al., 2017). The A3 receptor couples to Gi proteins and decreases cAMP levels, whilst through Gq proteins or Gβγ subunits, it stimulates PLC and increase Ca2+ concentration. In addition, a pathway presenting RhoA, a monomeric G-protein and PLD, is relevant for the neuroprotection effects of A3 receptors. Inhibition of the transcription hypoxia-inducible factor (HIF-1) has been reported in astrocytes with neuromodulatory effects through MAPK and Akt modulation (Gessi et al., 2013). Interestingly, the reduction of neuroinflammation has been related to analgesia (Janes et al., 2014).
In this section, we will describe the current state of knowledge about how adenosine signaling can interfere with common addictive psychostimulant consumption, focusing on data obtained from animal, particularly murine, models and from human studies. Animal models not only give us useful information on the pathophysiological mechanisms of psychostimulant drugs intake that are not accessible to study in human subjects but also provide an useful tool to assay pharmacological approaches to explore potential treatments before develop clinical trials. Certainly, although animal models may produce similar responses to those observed in humans, all the paradigms used in them for drug addiction research imply a lower degree of complexity than that observed in human drug addiction. Nowadays, more complex animal models have been developed to include some behavioral responses observed in human addictions, such as social peer influences in drug intake (Moser et al., 2011; Ross et al., 2015; Strickland and Smith, 2015). Nevertheless, in the last part of this section, information obtained from human studies is also provided and the actual information regarding this issue is discussed.
Psychostimulants can be classified into two broad categories depending on the mechanism by which DA levels are increased; namely, amphetamines (AMPH) behave as DA releasers, while cocaine acts to inhibit DA reuptake trough the inhibition of the DA active transporter (DAT) (Siciliano et al., 2015). In addition to the increase in levels of DA in the striatum, AMPH and cocaine are able to induce an increase in norepinephrine (NE), by blocking NE transporter (NET), while only cocaine also increases serotonin, by inhibiting serotonin transporter (SERT) (Phillips et al., 2014; Zwartsen et al., 2017).
Cocaine is extracted from coca leaves, mainly in South America where the coca plant is commonly grown. Despite being the most widely used illicit narcotic drug, cocaine has been used for centuries (if not millennia) for medical and cultural purposes (Johanson and Fischman, 1989). Although cocaine may be illegally distributed in several forms, mainly cocaine hydrochloride but also cocaine sulfate or crystalized as “crack,” the physiological and psychoactive effects of cocaine in different forms are similar (Hatsukami and Fischman, 1996).
The family of “amphetamines” or amphetamine-like psychostimulants includes a wide range of compounds which can be synthetized based on chemical substitutions of the original structure of alpha-methylphenethylamine. AMPH and methamphetamine (S(+)-methylamphetamine, METH) are the most studied compounds of the family, but other well-known psychostimulants in this family include methylphenidate, MDMA (3,4-methylenedioxymethamphetamine, “ecstasy”), ephedrine and cathinone (synthetic derivatives of which are known as “bath salts”) (Sulzer et al., 2005). Specifically, although cathinones have a synthetic profile similar to AMPH, their mechanism of action is rather similar to that of cocaine, being potent DAT inhibitors (López-Arnau et al., 2017). On the other hand, MDMA, in addition to inhibiting NET and DAT, is both a substrate for SERTs and an inhibitor of them, with an IC50 in the micromolar range, thus differentiating its behavior from that of AMPH, which is unable to affect SERTs (Rudnick and Wall, 1992; Baumann et al., 2005; Zwartsen et al., 2017). Although AMPH and METH have similar pharmacokinetics, they differ in their pharmacodynamic properties, with METH inducing DA release in the NAc more efficiently than AMPH (Goodwin et al., 2009). AMPH and METH have been studied for a long time, but their neurobiology remains largely unknown with discrepancies in the literature between pharmacological and genetic-based experiments.
As for psychostimulants-induced changes in AR expression in brain areas only few scientific reports have been reported. Specifically, during a study of cocaine withdrawal and its relationship with sleep architecture, Yang et al. (2011) reported that A1 receptor expression in the hippocampus of rats was reduced after 14 days of withdrawal, A2A receptor density was increased on withdrawal-day 8 and 14, while the A2B receptors remained unchanged. Other findings provide neurochemical evidence that after 10 days of cocaine self-administration, an up-regulation of functional A2A receptors in the NAc of rats was induced that returned to baseline expression levels after 7 days of drug withdrawal (Marcellino et al., 2007). In a study of the motivational mechanisms after cocaine self-administration and extinction, it was reported that, in the dorsal striatum of Wistar rats, there was an increase in the affinity of A2A receptors during maintenance and an increase in A2A receptor density after extinction from cocaine self-administration (Frankowska et al., 2013). Nevertheless, using a paradigm of escalating administration of cocaine dose (“binge”) and subsequent withdrawal, the density of adenosine A1 and A2A receptors in various brain nuclei of Fischer rats was not different nor in chronic cocaine-treated rats or in the long-term withdrawn rats group (Bailey et al., 2005). Finally, rats trained to self-administer METH for 14 days showed selective altered expression of AR, with A1 receptor levels increased in the NAc shell, caudate-putamen and prefrontal cortex, and A2A receptors decreased in the NAc shell and raised in the amygdala (Kavanagh et al., 2015). Interestingly, it is well known that GPCR act as an oligomer, and indeed homodimers of A2A and 5-HT1A receptors occur constitutively, and are further increased by agonists such as CGS 21680 and 8-OH-DPAT, or reduced by antagonists including SCH 58216 and methysergide, which could also contribute to psychostimulant addiction (Łukasiewicz, 2007).
Even though there are no consistent and complete studies about amphetamines- and cocaine-induced changes in AR expression in brain areas, a prominent role in the modulation of psychostimulant addiction attributed to adenosine is mediated through the activation of AR by complex mechanisms, affecting various aspects of this phenomenon including locomotor activity, discrimination, seeking behavior and reward.
Although the interactions between adenosine and DA in the striatum were previously known, the role of AR in AMPH-induced locomotor responses was first characterized only at the end of the last century. Turgeon et al. (1996) demonstrated that, in Sprague-Dawley rats, AMPH-induced behavior could be pharmacologically modulated by pretreatment with CHA, an A1 receptor agonist, or APEC, an A2A receptor agonist, which reduced locomotor responses induced by acute AMPH exposure. However, only the A2A receptor agonist inhibited c-Fos immunoreactivity, induced by AMPH, in striatum and NAc. In the case of METH, the experimental paradigm used to determine the role of AR in METH-mediated effects was METH-induced toxicity. In these studies, administration of the A1 agonist CPA attenuated the METH-provoked neurochemical tyrosine hydroxylase changes in Swiss-Webster mice (Delle Donne and Sonsalla, 1994) while, in other experimental models, represented by Wistar rats, both CPA and CGS 21680, an A2A receptor agonist, were able to attenuate METH-mediated DA release in the striatum (Gołembiowska and Zylewska, 1998a). In terms of METH-induced locomotor responses, it was also reported that administration of CHA and CGS 21680 before acute METH exposure in Wistar rats was able to inhibit METH-induced hyperlocomotion (Shimazoe et al., 2000) but, interestingly, when those same agonists were tested to study METH-induced sensitization (which occurs after repeated intermittent drug administration), only CGS 21680 was able to inhibit METH-induced increase of locomotion while CHA had no effect (Shimazoe et al., 2000). In addition, the activation of A2A receptors could also be the mechanism by which some herbal compounds, PAP9704 and ginsenoside herbal compounds, attenuate METH-induced hyperlocomotion and conditioned place preference in BALB/C AnNcrj mice as well as in C57BL/6 mice, respectively (Kwon et al., 2004; Shin et al., 2005). Furthermore, AMPH-induced stereotyped head movements in Wistar rats were attenuated in a dose-dependent manner with CGS 21680, poorly reduced when CPA was used and even potentiated when DMPX, an A2 receptor antagonist, was used (Poleszak and Malec, 2000). Finally, it seemed that the inhibition of AMPH-induced stereotyped head movements, through activation of A1 receptor, could depend on agonist properties, as Ribavirin, an A1 receptor agonist, reduced AMPH-induced total locomotor activity but had no effects on stereotypic activity in Wistar rats (Janać et al., 2005). Relevant literature concerning the functional effects of AR ligands in psychostimulant-induced phenomena, with a focus on rodent models, are presented in Supplementary Table S1.
A complete study of AR and their relationship with cocaine-induced locomotion was carried out by Poleszak and Malec (2002b) at the beginning of this century. They reported that CPA and CGS 21680, decreased both cocaine- and AMPH-induced locomotor activity. The agonist doses required to inhibit the effect of AMPH were higher than those which were active in cocaine-induced hyperactivity, while the A2 antagonist DMPX enhanced the effects of AMPH in Swiss mice (Poleszak and Malec, 2002b). Accordingly, the selective stimulation of A2A receptors in Wistar rats using CGS 2160 reduced the cocaine-induced locomotor response, the locomotor response during the development of sensitization, and the expression of sensitization in a cocaine challenge dose, while blocking A2A receptors with the antagonist MSX-3 induced the opposite effects in the three studied paradigms (Filip et al., 2006).
Genetic deletion of A2A receptors (A2A KO) in animal models of drug addiction provides a tool to understand the role of these receptors under certain circumstances by comparison to wild-type animals. Nevertheless, the results obtained using A2A KO animals on cocaine-, AMPH-, and METH-induced behavioral responses seem contradictory. In this sense, it was reported that in 129-Steel and hybrid C57BL/6 × 129-Steel mice, A2A KO attenuated cocaine-induced locomotor stimulation (Chen et al., 2000). Similar experiments, performed to demonstrate the effects of A2A genetic deletion with independence of the genetic background, were reproduced later in pure 129-Steel mice resulting in missed AMPH-induced locomotor sensitization (Chen et al., 2003). Accordingly, similar results were obtained when hybrid C57BL/6 × 129-Steel animals were used to generate tissue-specific A2A KO animals, where deletion of forebrain A2A receptors was carried out, which showed a loss of AMPH-mediated locomotor response (Bastia et al., 2005). In contrast, in CD1 background mice, it was reported that there were no differences in the cocaine-induced locomotor activity, sensitization and conditioned place preference between A2A KO animals and their littermates (Soria et al., 2006). Authors only found a lower rate of cocaine self-administration and motivation as well as lower efficacy of cocaine reinforcing effects in A2A KO mice (Soria et al., 2006). Interestingly, Soria et al. (2006) concluded with the hypothesis that separate neuronal substrates could mediate cocaine-induced locomotor effects and self-administration in an operant behavior paradigm. In order to corroborate this hypothesis, Shen et al. (2008) designed two different KO animals to distinguish between striatal versus non-striatal cocaine-mediated effects. In these experiments, cocaine-induced locomotor activity was enhanced in striatum-specific A2A KO mice (A2A receptors were deleted in striatal neurons) but attenuated in forebrain-specific A2A KO mice (A2A receptors were deleted in the neurons of striatum, cerebral cortex, and hippocampus). In addition, pharmacological inactivation (using KW6002, an A2A receptor antagonist with preferential affinity for post-synaptic A2A binding sites) of extra-striatal A2A receptors in striatum-specific A2A KO mice attenuated cocaine-induced hyperlocomotion, while the same antagonist enhanced cocaine-induced hyperlocomotion in the wild-type mice, reflecting the antagonism between striatal A2A receptors and extra-striatal A2A receptors (Shen et al., 2008). Finally, in CD1 A2A KO mice, a lesser increase in DA levels after acute cocaine exposure was reported while locomotor activity was further increased in A2A KO mice in comparison to wild-type littermates (Wells et al., 2012). The genetic models derived from the manipulation of AR, and their effect on the interaction of AR with psychostimulants, are presented in Supplementary Table S2.
Interestingly, there is some research available about the role of A3 receptors in modulation of AMPH- and METH-mediated actions. METH-induced DA release was measured in the rat striatum using APNEA, a putative A3 receptor agonist, which has a biphasic effect when perfused locally to the striatum via microdialysis. At the lower concentration studied, APNEA induced a decrease in DA outflow, but at the higher concentration studied, a clear increase in DA outflow was reported, which led researchers to conclude that the activation of A3 receptors exerts a rather toxic effect on DA neurons (Gołembiowska and Zylewska, 1998b). Nevertheless, when the A3 receptor was genetically deleted, it was reported that the resultant mice were much more sensitive to the toxic actions of METH, including Iba-1, caspase 3, TNF-α, and vesicular monoamine transport 2 (VMAT) increased expression (Shen et al., 2011), and also presented reduced AMPH-induced locomotor response (Björklund et al., 2008).
Adenosine receptors modulate psychostimulant-induced discriminative-stimulus effects, as A1 and A2A receptors antagonists (CPT and MSX-3 or DMPX, respectively) partially mimicked the discriminative-stimulus effects of METH, by increasing the levels of drug-lever selection, and potentiating the discriminative-stimulus actions of METH, as shown by significant leftward shifts of the METH dose-response curve, behaving like psychostimulant drugs (Munzar et al., 2002; Justinova et al., 2003). Surprisingly, CPA and CGS 21680 also shifted the dose-response curve to the left for cocaine, but not for METH, suggesting that A1 and A2A receptors have different influences on the discriminative-stimulus effects of METH and cocaine in Sprague-Dawley rats (Justinova et al., 2003).
Another relevant aspect in drug addiction which is strongly influenced by AR is the seeking behavior. In terms of the effect of A1 agonists, CPA microinfusions in the NAc of Sprague-Dawley rats inhibited cocaine seeking behavior (Hobson et al., 2013). On the other hand, treatment with A2A agonists such as NECA or CGS 21680 reduced the number of cocaine infusions self-administrated by rats, mainly due to an increase in the latency for the first cocaine infusion (Knapp et al., 2001). Accordingly, the activation of A2A receptors, using CGS 21680, antagonized the reinstatement of cocaine seeking in Sprague-Dawley rats (Bachtell and Self, 2009). In addition, it was reported that A2A receptor blockade, using CGS15943, increased cocaine-seeking in a dose-dependent manner and also reinstated cocaine-seeking, functioning as an intravenous reinforcer, in baboons (Weerts and Griffiths, 2003). The effects of activation and blockade of A2A receptors, using CGS 21680 and MSX-3, respectively, were also tested in Sprague-Dawley rats trained to press a lever for cocaine. Pretreatment with intra-NAc core microinjections of CGS 21680 reduced cocaine-induced reinstatement, while MSX-3 exacerbated it (O’Neill et al., 2012). Similar results were obtained in Sprague-Dawley rats, where intra-NAc microinjections of CPA and CGS 21680 inhibited the expression of cocaine sensitization, and microinjections of ABT-702 and DCF (AK and ADA inhibitors, respectively) blocked cocaine sensitization (Hobson et al., 2012). Interestingly, A2A receptor activation (with CGS 21680) in Wistar rats was able to affect food seeking with a similar potency to that observed for cocaine seeking, whilst A2A receptor antagonists increased cocaine-, but not food-, seeking behavior, suggesting that possibly a differential expression of A2A receptors occurs in striatopallidal GABAergic neurons involved in cocaine and food seeking (Wydra et al., 2015). In contrast, it was reported that although the activation of A1 (using CPA) and A2A (using CGS 21680) receptors impaired initial extinction responding, the blockade of presynaptic A2A receptors using SCH 442416 produced persistent impairment of cocaine-induced seeking in Sprague-Dawley rats (O’Neill et al., 2014).
As reported in Supplementary Table S1, the vast majority of studies have focused exclusively on males. Nevertheless, there is a large amount of literature concerning the differences in drug-mediated effects between males and females (for a recent review see Lynch, 2017). Although the focus of the present review is on the relationship between AR and psychostimulants, sex differences will be briefly commented upon to provide a fuller picture of the problem of addiction. In this regard, it has been reported that female rats self-administer higher levels of cocaine or METH, and escalate intake faster than males during extended daily psychostimulant access (Roth and Carroll, 2004; Reichel et al., 2012). Females also require shorter periods of time to show increased motivation to obtain cocaine (Lynch and Taylor, 2004). In addition, following short access self-administration, females show markedly higher levels of METH seeking (Ruda-Kucerova et al., 2015), and females present increased cocaine-seeking behavior during cocaine withdrawal (up to 6 months) compared to males (Kerstetter et al., 2008). Sex-dependent responses to AR’s ligands have also been reported in Sprague-Dawley rats using ATL444, a novel A2A/A1 receptor antagonist, in studies of motivation for cocaine. In these experiments, it was reported that ATL444 treatment acutely increased motivation for cocaine in females but, in males, induced a long-term decrease in motivation for cocaine (Doyle et al., 2012). Finally, one interesting more recent study, evaluating the effects of A2A receptor deletion on schizophrenia, found that AMPH induced a lower hyperlocomotion response in male CD1 A2A KO mice at 120–170 min, in comparison to wild type AMPH-treated mice; this effect was observed to a major extent in female CD1 A2A KO mice at 70–180 min, in comparison to wild type AMPH-treated mice (Moscoso-Castro et al., 2016).
Rewarding effects induced by AMPH and METH have mainly been evaluated using conditioned place preference procedures. In this way, Poleszak and Malec (2003) proved that CPA and, under certain conditions, CGS 21680, reduced the development of AMPH-induced conditioned place preference in Wistar rats; however, only CGS 21680 was able to decrease the expression of METH-induced conditioned place preference. In addition, ginseng saponins reduced the METH-induced circling behavior and conditioned place preference in C57BL/6 mice, via activation of the A2A receptor, as this reduction was reversed in a dose-dependent manner using the A2A receptor antagonist CSC. Interestingly, reduction of AP-1 DNA binding activity and proenkephalin gene expression induced by METH exposure were reduced by CSC (Shin et al., 2005). Furthermore, C57BL/6J mice with D2 receptors knocked down in the NAc core have been reported to exhibit a reduction in METH-induced locomotion, as in other paradigms (locomotor sensitization and conditioned place preference) after repeated METH-treatment, suggesting that D2 receptors are necessary mediators for the development of METH-induced rewarding effects (Miyamoto et al., 2014). Thus, the antagonism between A2A and D2 receptors further supports the conclusion that the activation of A2A receptors could be a promising way to counteract AMPH- and METH-induced rewarding effects.
A series of experiments to increase our understanding of the role of A1 and A2A receptors in METH-induced behavior were designed by Kavanagh et al. (2015), reporting that the initial METH-mediated rewarding effects may be tempered by A1 or A2A receptor activation in a model of rat self-administration. Therefore, they found that in Sprague-Dawley rats, the stimulation of A1 receptors using CPA reduced METH self-administration, and that the stimulation of both A1 and A2A receptors (using CPA and CGS 21680, respectively) reduced METH-induced place preference (Kavanagh et al., 2015). These results suggest that, taking into account the antagonism of A1/D1 and A2A/D2 heteromers, both A1 and A2A agonists will be useful to reduce METH-induced behaviors during the initial exposures to METH but, when METH exposures are more prolonged, the modulation of AR renders only the A1 agonist powerful enough to counteract the rewarding properties of METH. Accordingly, A2A KO animals (CD1 background) were less sensitive to METH rewarding properties, as METH exposure did not induce conditioned place preference in those animals and, although METH-self administration was not altered, the motivation to self-administer METH was reduced when compared with wild-type (Chesworth et al., 2016).
Finally, the important role of AR as possible pharmacological tools to treat psychostimulant addiction has also been tested in animal models using other members of the amphetamine family, albeit to a lesser extent. Specifically, it was demonstrated that SCH 58261, but not DPCPX, increased MDMA-induced hyperthermia (Vanattou-Saïfoudine et al., 2010) but, conversely, DPCPX, but not SCH 58261, enhanced MDMA-induced DA release from striatal slices (Vanattou-Saïfoudine et al., 2011). Accordingly, the blockade of A1 or A2A receptors using DPCPX or KW 6002, respectively, in mouse striatum increased the MDMA-mediated release of DA and 5-HT (Górska and Gołembiowska, 2015). In contrast to these pharmacological experiments, when A2A receptors were knocked down in a CD1 background model, MDMA-mediated reinforcement was dramatically decreased (although locomotor response was not altered) compared to wild-type littermates (Ruiz-Medina et al., 2011), suggesting that the lack of A2A receptors will increase resistance to psychostimulant rewarding properties.
The genomic era has provided the opportunity to study human polymorphisms and so to provide a tool to design personalized treatments according to observed mutations. Recent meta-analysis of case-control studies of psychostimulant users (cocaine, AMPH, and METH) have revealed that there is a general down-regulation of the dopaminergic system, as in psychostimulant users there is a decrease in DA release, in DA transporter availability, and also in the levels of D2 and D3 receptors, concluding that DA function is down-regulated both pre- and post-synaptically. This suggests that restoring DA function must be an important goal in the treatment of psychostimulant abusers (Ashok et al., 2017). Due to the antagonistic relationship between dopaminergic function and AR, some authors have studied the relationship between A1 and A2A gene polymorphisms and susceptibility to psychostimulant consumption/addiction. Most relevant human studies are presented in Supplementary Table S3.
The first study designed to discern the influence of A1 and A2A gene (ADORA1 and ADORA2A, respectively) polymorphisms on inter-individual variability in AMPH response was carried out by Hohoff et al. (2005). Using a sample of 99 healthy volunteers (50 men and 49 women), who received AMPH or a placebo, the authors reported that two ADORA2A polymorphisms (1976C/T and 2592C/Tins) were associated with increases in reported anxiety by participants after AMPH consumption (Hohoff et al., 2005). Nevertheless, these results should be regarded with caution, as the same research group could not reproduce them using the same methodology for a larger sample of individuals (Hart et al., 2013). In addition, a study by Kobayashi et al. (2010) searching for the relationship between ADORA2A variations and susceptibility to METH dependence/psychosis reported that, in a population of 171 Japanese METH dependent/psychotic patients (compared to 229 control subjects), six ADORA2A polymorphisms were found. The authors reported that only one single nucleotide polymorphism (SNP) of the A2A receptor gene was significantly associated with a subgroup of female patients (METH dependent/psychotic) that consumed only METH and no other psychostimulants or drugs (Kobayashi et al., 2010). Interestingly, that SNP was 1976C/T (rs5751876), the same that Hohoff et al. (2005) associated with anxiety after AMPH consumption, and this SNP is a synonymous variant, meaning that it cannot include amino acid substitutions. Finally, in the same Japanese population as the previous study, seven ADORA1 SNPs were identified but none was specific to any subgroup of METH dependent/psychotic patients, which would suggest that ADORA1 polymorphisms would make little or no contribution to METH vulnerability (Kobayashi et al., 2011); however, further research is needed to confirm this supposition. In addition, caffeine-induced anxiety has also been associated with ADORA2A 1976C/T polymorphism in a sample of 102 individuals (Childs et al., 2008), although other ADORA2A polymorphisms (such as 1976TT) also seem to be related to caffeine-induced anxiety, and could also influence predominantly women vulnerable to anxiety (Domschke et al., 2012; Gajewska et al., 2013).
Despite the huge amount of evidence that connects AR with psychostimulant-mediated actions, the translation of this knowledge to the clinic has been quite slow in comparison with other areas. A few reasons related to particular characteristics of AR could be that AR receptors are widely distributed, not only in the CNS but throughout the human body, with adenosine signaling responsible for the regulation of a broad spectrum of physiologic and pathologic actions (for a more detailed discussion see Müller and Jacobson, 2011; Chen et al., 2013). For this reason, it is experimentally difficult to demonstrate the clinical effectiveness and safety of an AR ligand. Therefore, only two clinical studies (registered in website1) have studied psychostimulant dependence and its link with AR (Supplementary Table S3). One of these compared the responses of volunteers to acute caffeine (150 and 300 mg), AMPH (20 mg) and placebo between 13 cocaine users and 10 healthy control subjects (NCT00733993). The main target of the trial was to study caffeine-mediated effects in cocaine users. Nevertheless, although caffeine and AMPH produced a series of differential results across the cocaine and control groups, these outcomes were not systematic, perhaps due to limitations of the study itself (Lane et al., 2014). On the other hand, the effect of an acute dose (100 mg) of the A2A antagonist SYN115 was studied to elucidate the effects of this antagonist on brain function and behavior in a group of cocaine-dependent volunteers (NCT00783276). Some subjective effects (consistent with stimulation) were induced by SYN115 administration in cocaine users (Lane et al., 2012). Furthermore, the administration of SYN115 to cocaine-dependent volunteers increased brain activation in the orbitofrontal cortex, insula, and superior and middle temporal pole, as measured by fMRI while the participants were performing working memory tasks; this suggests that the blockade of A2A receptors could mitigate cocaine-associated neurobehavioral deficits (Moeller et al., 2012). In addition, no clinically significant adverse cardiovascular events were reported by the volunteers in either study (Lane et al., 2012; Moeller et al., 2012).
Finally, epidemiological and preclinical data demonstrate that gender differences exist for the three phases of drug abuse (represented in Figure 1). The pattern of gender differences establishes that women have lower prevalence of drug use disorders involving both licit and illicit drugs (including alcohol, sedatives, cannabis, tranquilizers, opioids, hallucinogens, and cocaine use disorders). Nevertheless, women that begin to self-administer drugs, even at lower doses than men do, escalate faster to addiction and present higher rates of relapse compared to men. These gender differences can be interpreted in terms of sociocultural factors as well as biological/physiological factors (reviewed in Lynch, 2006; Lev-Ran et al., 2013; Bobzean et al., 2014; Becker and Koob, 2016). Men and women also differ markedly in terms of psychostimulant use/abuse. For example for METH consumption, women tend to begin METH use at earlier ages and seem more dependent on METH consumption than men, although women do suffer a decreased degree of toxicity and respond better to treatment (Dluzen and Liu, 2008). In addition, women present more severe problems related to cocaine intake, beginning to use cocaine at earlier ages, and some pharmacological treatments for drug addiction have poor outcomes among women compared to men (Kennedy et al., 2013; DeVito et al., 2014). Several pathophysiological studies have demonstrated that the reinforcing effect of cocaine is strongly influenced by the female hormonal cycle; in fact, some authors suggest that gender differences in addiction are due to differences in the reinforcement pathways of neural systems induced by ovarian hormones (Anker and Carroll, 2011; Bobzean et al., 2014). These findings highlight the importance of taking gender into account when analyzing psychostimulant use, and designing prevention programs and personalized treatment programs.
As noted in previous sections, it has been reported that AR interact in an antagonistic way with DA receptors, A1 receptors being colocalized in heteromeric complexes with D1 receptors, and A2A receptors with D2 receptors, counteracting DA-induced behavioral effects (Ferré et al., 1997; Ginés et al., 2000; Hillion et al., 2002; Fuxe et al., 2007a). Specifically, the stimulation of striatal D2 receptors is responsible for the locomotor, sensitizing and rewarding effects of drugs of abuse such as cocaine and amphetamines, and A2A receptor stimulation counteracts them (Heffner et al., 1989; Popoli et al., 1994; Rimondini et al., 1997; Poleszak and Malec, 2000, 2002a; Shimazoe et al., 2000; Knapp et al., 2001; Bachtell and Self, 2009; Jastrzêbska et al., 2014; Johnson and Lovinger, 2016). In general, the process of addiction depends on an increase in DA neurotransmission in the striatum and an activation of its receptors. Specifically, cocaine induces its effects by indirectly increasing DA levels and directly activating D2 receptors (Fuxe et al., 2007a; Ferraro et al., 2012), thus enhancing dopaminergic signaling. The DA receptors most involved are of the D2 subtype, as demonstrated by their persistent striatal decrease following drug detoxification, and their induction of relapse as a consequence of chronic drug administration.
Interestingly, A2A and D2 receptors are co-expressed in the striatum, forming heteroceptors, especially in the GABAergic striatopallidal neurons, where A2A receptor activation increases GABA release and counteracts the effects induced by D2 receptors. These receptors may be linked to each other in two opposite ways. On the one hand, these receptor subtypes may form heteromers, causing antagonistic interactions between A2A receptors and D2 receptors at the AC level, related to Gs/olf and Gi type V AC signaling. On the other hand, at the membrane level, A2A receptor activation exerts a counterbalancing effect to D2 receptor stimulation, by reducing its affinity for DA and decreasing functional effects induced by D2 receptor stimulation (Ferré et al., 1991, 1994). In support of this relationship, transgenic animal models overexpressing A2A receptors in the brain showed reduced D2 receptors in the striatum. Accordingly, A2A receptor activation decreases behavioral responses to psychostimulants, indicating that the A2A receptor may represent a novel drug target for the treatment of drug addiction. In particular, A2A receptor stimulation decreases cocaine reward and seeking behavior, by reducing D2 agonist affinity (Pintsuk et al., 2016). In the context of the antagonistic interaction between A2A/D2 receptors, it has also been reported that the D2 receptor, through coupling to Gi, inhibits A2A receptor-mediated cAMP/PKA signaling, and thus CREB phosphorylation and c-fos expression (Pinna et al., 1997; Kull et al., 2000; Hillion et al., 2002). However, synergistic A2A and D2 receptor interaction has been revealed, again at the AC level in the striatum, linked to the overexpression of activator of G protein (AGS3) and Gs/olf and Gi type II/IV AC pathway. This relationship becomes important when AGS3 is upregulated, such as during ethanol consumption, and withdrawal from cocaine, ethanol or morphine, because its activity stabilizes and inhibits the GDP-bound form of Gi, at the same time increasing the βγ-dependent effect of Gs/olf protein, producing a strong increase in cAMP-PKA signaling. Even though in the striatum the first A2A/D2 antagonistic relationship is predominant, due to the higher distribution of AC V, when AGS3 is upregulated, such as during chronic exposure to addictive drugs, the synergistic interaction between A2A and D2 receptors becomes relevant, suggesting that A2A receptor antagonists may represent a class of drug to combat addiction and relapse (Ferré et al., 2008b). In addition, neuroprotection exerted by A2A receptor agonists seemed to be mediated by an increase in nuclear factor-κB (Kermanian et al., 2012, 2013; Soleimani et al., 2012).
Furthermore, neuromodulation of neuronal networks by systemic A2A receptor activation inhibits the reward and motivational properties of cocaine targeting A2A/D2 heteroreceptors in the striatopallidal GABA pathway. Microdialysis studies have related this effect to their increase and reduction of GABAergic and dopaminergic transmissions, respectively, in the NAc, as a consequence of an antagonistic A2A/D2 interaction, both at the membrane cell surface and at the intra-cellular level (Fuxe et al., 2007a; Trifilieff et al., 2011; Franco et al., 2013; Wydra et al., 2015; Borroto-Escuela et al., 2017). A2A/D2 heteromers involved in reward mechanisms reside in GABAergic neurons of the ventral striatopallidal area that are responsible for rewarding, motivational and seeking effects induced by cocaine, as well as by food (Wydra et al., 2013). However, both systemic treatment with an A2A receptor antagonist and its direct injection into the NAc reduced relapse in heroin-addicted rats and prevented DA increases in the NAc shell induced by tetrahydrocannabinol (THC), but not those mediated by cocaine (Yao et al., 2006; Justinova et al., 2011). Furthermore, A2A receptor antagonists alone may behave like psychostimulants by triggering cocaine-seeking behavior, thus decreasing their utility in the treatment of drug-dependence. Indeed, some findings on the addictive properties of A2A receptor antagonists have reported that they substituted for cocaine in baboons (Weerts and Griffiths, 2003), also inducing conditioned place preference (Harper et al., 2006) and restored cocaine-seeking behaviors in rats (O’Neill et al., 2014). In addition, the blockade of A2A receptors increased DA in the striatal network in cocaine-dependent subjects, which resulted in major prefrontal cortex stimulation (Moeller et al., 2012). However, in rats trained to self-administer heroin, the administration of A2A antagonists eliminated reinstatement (Yao et al., 2006), opening the possibility of using A2A antagonists as therapeutic ligands in the management of abstinence in the addiction of some drugs. The effect of cocaine exposure in fetal brains and the modulation of DA and adenosine effects have also been addressed; specifically, from E8 to E14 embryonic days, cocaine treatment induced changes in DA and adenosine signaling which increased basal cAMP levels in the striatum and cerebral cortex. This effect could be reverted by blocking A2A receptors (using SCH58261), suggesting that A2A receptors could be considered good candidates as targets to treat prenatal cocaine exposure-related syndromes. Indeed, D2 and A2A receptors counterbalance each other’s effects in the embryonic brain in a similar manner to what happens in the mature brain (Kubrusly and Bhide, 2010).
A2A receptors, independent of their interaction with D2 receptors in A2A/D2 heteromers, are also present in other complexes with mGlu5 and CB1 in striatal GABAergic neurons, as well as with A1, mGlu5, and CB1 in striatal glutamatergic terminals (Figure 2), that may be involved in the modulation of reward, exerting an important role in the regulation of dopaminergic and glutamatergic effects in addiction (Kalivas and Volkow, 2011; Cahill et al., 2014; Zhang et al., 2014; Johnson and Lovinger, 2016). In this sense, the blockade of A2A receptors increased cocaine-mediated locomotor effects through the activation of CB1 receptors in rat striatum (Tozzi et al., 2012). It has also been demonstrated that an interaction between A2A receptors and metabotropic glutamate 5 receptors (mGlu5) in the striatum avoided METH-, but not cocaine-, induced hyperactivity and rewarding behavior, making a combined antagonism of A2A and mGlu5 receptors in the therapy of METH addiction possible (Wright et al., 2016). Accordingly, the influence of adenosine on glutamatergic transmission in the striatal region has been reported (Fuxe et al., 2008). Finally, it was reported that the functions and pharmacology of extra-striatal A2A receptors must also be taken into account (Shen et al., 2008), although their function could not be totally extrapolated from the available data for striatal A2A receptors. In this sense, the apparent controversial data obtained from pharmacological studies and genetic approaches using KO animals (presented in Supplementary Tables S1, S2) must be carefully exposed as the genetic background effects in A2A KO animals may invalidate them as a model to study A2A receptors (Filip et al., 2012). To conclude, the exploitation of the full potential of AR as drug targets will not only necessitate a full comprehension of AR-mediated mechanisms, but will also require the availability of ligands which let us distinguish among the different receptor populations discussed in this paper (Popoli and Pepponi, 2012).
The consumption of legal psychostimulants has increased over recent years. For example, the misuse of prescribed psychostimulants, which are approved for the treatment of attention deficit hyperactivity disorder, for weight control or for the treatment of narcolepsy (Phillips et al., 2014), both by the patients themselves, and by non-affected individuals, based on misconceptions or simple lack of knowledge of the associated risks, is becoming more and more common nowadays (Lakhan and Kirchgessner, 2012; McHugh et al., 2015). This has been the case for methylphenidate consumption among college students as a study aid to enhance their academic performance (Maier et al., 2013; Webb et al., 2013; Vrecko, 2015). Although their use as a study aid is not the only reason why these substances are consumed (Drazdowski, 2016), this is one historically significant example, with reports of the use of amphetamines as study aids dating back to 1937 (Strohl, 2011).
In addition, caffeine, which is the most consumed psychoactive drug in the world, could also be of particular importance when addressing psychostimulant or drug addiction-related problems. Indeed, caffeine is commonly found as an adulterant in the preparation of illicit drugs (Prieto et al., 2016) and in energy drinks consumed in combination with alcohol or other psychostimulants (Reissig et al., 2009; Vanattou-Saïfoudine et al., 2012; Ferré, 2016). Interestingly, both acute and chronic adverse effects rise following concurrent consumption of caffeine and psychostimulant drugs. Specifically, caffeine worsens the psychostimulant’s toxicity by increasing hyperthermia, cardiotoxicity, and seizures, as well as influencing the stimulatory, discriminative, and reinforcing effects of psychostimulant drugs. These effects have been investigated for the cases of MDMA and cocaine ingested with caffeine (Comer and Carroll, 1996; Kuzmin et al., 1999; Vanattou-Saïfoudine et al., 2012; Górska et al., 2017). The molecular mechanism underlying the action of caffeine is the antagonism of AR, with A1 and A2A subtypes the most involved. Indeed, it has been reported that caffeine induces increased DA release through A1 receptor blockade (Okada et al., 1997). More recently, findings by Ferré (2016) attribute caffeine potentiation of the psychomotor activating and reinforcing effects of psychostimulants to the existence of A2A/D2 heteromers, where the antagonism of A2A receptors by caffeine reverts the inhibitory brake exerted by adenosine on D2 receptor signaling. Therefore, as ingestion of caffeine with cocaine and MDMA can significantly alter the drug-induced effects, understanding the molecular mechanisms underpinning this interaction will help to define correct approaches for the management of these side effects and toxicity.
Although the A2A receptor has been far more extensively studied (refer to Supplementary Table S1 for summary), some papers considered in this review do highlight the role of A1 receptor activation to modulate psychostimulant-mediated effects. The properties of A1 agonists, mainly CPA, as anxiolytics have been previously reported in mice lacking A1 receptors (Giménez-Llort et al., 2002), in animal models of cocaine or alcohol consumption (Prediger et al., 2006; Hobson et al., 2013; O’Neill et al., 2014), and in classical behavioral studies (Jain et al., 1995). In this sense, electrophysiological studies in basolateral amygdala reported that application of CPA inhibits excitatory postsynaptic currents and glutamate release (Rau et al., 2014). This evidence, and the experiments reported in this review, will make A1 receptor signaling an important target for the development of novel pharmacological treatments for the common anxiety-like disorders reported during the process of drug-seeking and withdrawal, and as such will produce lasting changes in relapse susceptibility.
Evidence for the role of A2A receptors in psychostimulant-mediated effects seems to be somewhat contradictory between different pharmacological studies, experimental models tested (particularly self-administration vs. experimenter-administration) and genetic approaches using A2A KO animals (refer to Supplementary Table S2 for more detail). However, the overall analysis of the presented data indicates that excitatory modulation of GPCR heteroreceptor complexes, in this case A2A/D2 heteroreceptors using A2A agonists, is a promising tool to counteract psychostimulant-induced effects. Indeed, a prominent role in the modulation of psychostimulant addiction attributed to adenosine is mediated through A2A activation by complex mechanisms, affecting various aspects of this phenomenon including locomotor activity, discrimination, seeking behavior and reward.
In this review, we have discussed current scientific evidence mainly based on animal models of psychostimulant addiction, and suggested promising candidates in the search for pharmacological interventions. This is particularly important because, nowadays, the main treatments against psychostimulant addiction are focused on behavioral interventions (Phillips et al., 2014), while AR pharmacology could be a powerful weapon to modify the neurochemical alterations that occur during psychostimulant addiction. AR are widely distributed in the CNS where they mediate a myriad of functions and interact with other neurotransmitter systems, which provides an opportunity to modulate specific complex brain functions. In addition, selective ligands are available for the different AR subtypes, which increase the chances to achieve nuclei-specific modulation, representing a pharmacological opportunity to control addictive psychostimulant consumption and health-related problems. Certainly, identifying strategies to fully understand AR signaling in drug addiction may provide insight into the factors contributing to consumption/craving/relapse of abused psychostimulants, thus revealing novel therapeutic approaches. We suggest that efforts could be made in three main aspects of adenosine pharmacology affecting psychostimulant addiction. Firstly, we have stated in this review that there is broad experimental evidence that pharmacological stimulation of A1 and A2A receptors may counteract the effects induced by psychostimulants of abuse, but it is also important to highlight that approaches including a combination of AR drugs, like A1/A2A ligands, may help form a more robust strategy when AR are the basis of pharmacological interventions. Secondly, as stated in Section “Mechanisms of Adenosine Receptor-Mediated Pathways in Drug Addiction,” due to the ability of AR to form homomers, heteromers and oligomers, it is mandatory to obtain specific ligands capable of discriminating among those different receptor populations. Thirdly, due to the lack of information concerning the effects consequent to alcohol and psychostimulant co-abuse, which is very common in drug addiction (Althobaiti and Sari, 2016; Barrett et al., 2016; Sánchez-López et al., 2017), it would be of particular interest to investigate the role of AR in those interactions.
CC developed the original idea. SG and CC designed the review. IB-Y and SG prepared the images. SG and CC prepared the tables. CC and SM edited and reviewed the final version of the article. All listed authors contributed to writing the article.
This work was supported by Universidad de Castilla-La Mancha (GI20174050).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
IB-Y and CC would like to thank Prof. Mario Durán (UCLM) and Prof. Emilio Ambrosio (UNED) for their generosity and kind advice.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2017.00985/full#supplementary-material
TABLE S1 | The role of adenosine receptor (AR)’s ligands in psychostimulant-induced effects upon behavior and function. The most relevant animal studies on the interaction between adenosine and psychostimulants are shown. Arrows represent decrease (↓) and increase (↑). When adenosine ligands induced no effect, the symbol “≈” is used. Although PAP9704 and ginsenosides are not ligands of AR, the effects of those ligands are reported in this table as authors suggests that their biological effects are mediated by AR.
TABLE S2 | Genetic manipulation of AR in murine models and psychostimulant-induced effects. Genetic models derived from the manipulation of AR used to explore the interaction between adenosine and psychostimulants are shown. Arrows represent decrease (↓) and increase (↑). The symbol “≈” is used to indicate no difference between the genetic model and the wild-type animal (∗Unless other comparison is stated).
TABLE S3 | Most relevant human clinical studies targeting AR. Ordered by publication date, most relevant clinical studies carried out in humans targeting the relationship between AR and psychostimulant addiction are shown. For each reference it is described the type of study, the number of subjects enrolled and its main objective and results. The number of the clinical trial is provided for the studies registered at ClinicalTrials.gov.
Althobaiti, Y. S., and Sari, Y. (2016). Alcohol Interactions with psychostimulants: an overview of animal and human studies. J. Addict. Res. Ther. 7:281. doi: 10.4172/2155-6105.1000281
American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders, DSM-5, 5th Edn. Delhi: CBS Publishers & Distributors. doi: 10.1176/appi.books.9780890425596
Anker, J. J., and Carroll, M. E. (2011). Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Curr. Top. Behav. Neurosci. 8, 73–96. doi: 10.1007/7854_2010_93
Ashok, A. H., Mizuno, Y., Volkow, N. D., and Howes, O. D. (2017). Association of stimulant use with dopaminergic alterations in users of cocaine, amphetamine, or methamphetamine: a systematic review and meta-analysis. JAMA Psychiatry 74, 511–519. doi: 10.1001/jamapsychiatry.2017.0135
Bachtell, R. K., and Self, D. W. (2009). Effects of adenosine A2A receptor stimulation on cocaine-seeking behavior in rats. Psychopharmacology 206, 469–478. doi: 10.1007/s00213-009-1624-2
Bailey, A., Gianotti, R., Ho, A., and Kreek, M. J. (2005). Persistent upregulation of mu-opioid, but not adenosine, receptors in brains of long-term withdrawn escalating dose “binge” cocaine-treated rats. Synapse 57, 160–166. doi: 10.1002/syn.20168
Barrett, S. P., Jemcov, A., and Darredeau, C. (2016). Patterns and effects of alcohol and psychostimulant co-administration: a brief review of pharmacological considerations and subjective responses. Curr. Addict. Rep. 3, 138–143. doi: 10.1007/s40429-016-0080-4
Bastia, E., Xu, Y.-H., Scibelli, A. C., Day, Y.-J., Linden, J., Chen, J.-F., et al. (2005). A crucial role for forebrain adenosine A(2A) receptors in amphetamine sensitization. Neuropsychopharmacology 30, 891–900. doi: 10.1038/sj.npp.1300630
Baumann, M. H., Clark, R. D., Budzynski, A. G., Partilla, J. S., Blough, B. E., and Rothman, R. B. (2005). N-substituted piperazines abused by humans mimic the molecular mechanism of 3,4-methylenedioxymethamphetamine (MDMA, or ‘Ecstasy’). Neuropsychopharmacology 30, 550–560. doi: 10.1038/sj.npp.1300585
Becker, J. B., and Koob, G. F. (2016). Sex differences in animal models: focus on addiction. Pharmacol. Rev. 68, 242–263. doi: 10.1124/pr.115.011163
Björklund, O., Halldner-Henriksson, L., Yang, J., Eriksson, T. M., Jacobson, M. A., Daré, E., et al. (2008). Decreased behavioral activation following caffeine, amphetamine and darkness in A3 adenosine receptor knock-out mice. Physiol. Behav. 95, 668–676. doi: 10.1016/j.physbeh.2008.09.018
Bobzean, S. A. M., DeNobrega, A. K., and Perrotti, L. I. (2014). Sex differences in the neurobiology of drug addiction. Exp. Neurol. 259, 64–74. doi: 10.1016/j.expneurol.2014.01.022
Bonaventura, J., Navarro, G., Casadó-Anguera, V., Azdad, K., Rea, W., Moreno, E., et al. (2015). Allosteric interactions between agonists and antagonists within the adenosine A2A receptor-dopamine D2 receptor heterotetramer. Proc. Natl. Acad. Sci. U.S.A. 112, E3609–E3618. doi: 10.1073/pnas.1507704112
Bonci, A., and Williams, J. T. (1996). A common mechanism mediates long-term changes in synaptic transmission after chronic cocaine and morphine. Neuron 16, 631–639. doi: 10.1016/S0896-6273(00)80082-3
Borea, P. A., Gessi, S., Merighi, S., and Varani, K. (2016). Adenosine as a multi-signalling guardian angel in human diseases: when, where and how does it exert its protective effects? Trends Pharmacol. Sci. 37, 419–434. doi: 10.1016/j.tips.2016.02.006
Borea, P. A., Varani, K., Vincenzi, F., Baraldi, P. G., Tabrizi, M. A., Merighi, S., et al. (2015). The A3 adenosine receptor: history and perspectives. Pharmacol. Rev. 67, 74–102. doi: 10.1124/pr.113.008540
Borroto-Escuela, D. O., Carlsson, J., Ambrogini, P., Narváez, M., Wydra, K., Tarakanov, A. O., et al. (2017). Understanding the role of GPCR heteroreceptor complexes in modulating the brain networks in health and disease. Front. Cell. Neurosci. 11:37. doi: 10.3389/fncel.2017.00037
Brown, R. M., and Short, J. L. (2008). Adenosine A(2A) receptors and their role in drug addiction. J. Pharm. Pharmacol. 60, 1409–1430. doi: 10.1211/jpp/60.11.0001
Burnstock, G. (2017). Purinergic signalling: therapeutic developments. Front. Pharmacol. 8:661. doi: 10.3389/fphar.2017.00661
Cabello, N., Gandía, J., Bertarelli, D. C. G., Watanabe, M., Lluís, C., Franco, R., et al. (2009). Metabotropic glutamate type 5, dopamine D2 and adenosine A2a receptors form higher-order oligomers in living cells. J. Neurochem. 109, 1497–1507. doi: 10.1111/j.1471-4159.2009.06078.x
Cahill, E., Salery, M., Vanhoutte, P., and Caboche, J. (2014). Convergence of dopamine and glutamate signaling onto striatal ERK activation in response to drugs of abuse. Front. Pharmacol. 4:172. doi: 10.3389/fphar.2013.00172
Carpenter, B., Nehmé, R., Warne, T., Leslie, A. G. W., and Tate, C. G. (2016). Structure of the adenosine A(2A) receptor bound to an engineered G protein. Nature 536, 104–107. doi: 10.1038/nature18966
Casadó, V., Cortés, A., Mallol, J., Pérez-Capote, K., Ferré, S., Lluis, C., et al. (2009). GPCR homomers and heteromers: a better choice as targets for drug development than GPCR monomers? Pharmacol. Ther. 124, 248–257. doi: 10.1016/j.pharmthera.2009.07.005
Chen, J. F., Beilstein, M., Xu, Y. H., Turner, T. J., Moratalla, R., Standaert, D. G., et al. (2000). Selective attenuation of psychostimulant-induced behavioral responses in mice lacking A(2A) adenosine receptors. Neuroscience 97, 195–204. doi: 10.1016/S0306-4522(99)00604-1
Chen, J.-F., Eltzschig, H. K., and Fredholm, B. B. (2013). Adenosine receptors as drug targets–what are the challenges? Nat. Rev. Drug Discov. 12, 265–286. doi: 10.1038/nrd3955
Chen, J.-F., Moratalla, R., Yu, L., Martin, A. B., Xu, K., Bastia, E., et al. (2003). Inactivation of adenosine A2A receptors selectively attenuates amphetamine-induced behavioral sensitization. Neuropsychopharmacology 28, 1086–1095. doi: 10.1038/sj.npp.1300152
Chesworth, R., Brown, R. M., Kim, J. H., Ledent, C., and Lawrence, A. J. (2016). Adenosine 2A receptors modulate reward behaviours for methamphetamine. Addict. Biol. 21, 407–421. doi: 10.1111/adb.12225
Childs, E., Hohoff, C., Deckert, J., Xu, K., Badner, J., and de Wit, H. (2008). Association between ADORA2A and DRD2 polymorphisms and caffeine-induced anxiety. Neuropsychopharmacology 33, 2791–2800. doi: 10.1038/npp.2008.17
Ciancetta, A., and Jacobson, K. A. (2017). Structural probing and molecular modeling of the A3 adenosine receptor: a focus on agonist binding. Molecules 22:E449. doi: 10.3390/molecules22030449
Clark, M., and Dar, M. S. (1989). Effect of acute ethanol on release of endogenous adenosine from rat cerebellar synaptosomes. J. Neurochem. 52, 1859–1865. doi: 10.1111/j.1471-4159.1989.tb07268.x
Comer, S. D., and Carroll, M. E. (1996). Oral caffeine pretreatment produced modest increases in smoked cocaine self-administration in rhesus monkeys. Psychopharmacology 126, 281–285. doi: 10.1007/BF02247378
Cooper, J. R., Bloom, F. E., and Roth, R. H. (1996). The Biochemical Basis of Neuropharmacology, 7th Edn. New York, NY: Oxford University Press.
Cunha, R. A. (2005). Neuroprotection by adenosine in the brain: from A(1) receptor activation to A (2A) receptor blockade. Purinergic Signal. 1, 111–134. doi: 10.1007/s11302-005-0649-1
Cunha, R. A., Ferré, S., Vaugeois, J.-M., and Chen, J.-F. (2008). Potential therapeutic interest of adenosine A2A receptors in psychiatric disorders. Curr. Pharm. Des. 14, 1512–1524. doi: 10.2174/138161208784480090
de Lera Ruiz, M., Lim, Y.-H., and Zheng, J. (2014). Adenosine A2A receptor as a drug discovery target. J. Med. Chem. 57, 3623–3650. doi: 10.1021/jm4011669
Delle Donne, K. T., and Sonsalla, P. K. (1994). Protection against methamphetamine-induced neurotoxicity to neostriatal dopaminergic neurons by adenosine receptor activation. J. Pharmacol. Exp. Ther. 271, 1320–1326.
DeVito, E. E., Babuscio, T. A., Nich, C., Ball, S. A., and Carroll, K. M. (2014). Gender differences in clinical outcomes for cocaine dependence: randomized clinical trials of behavioral therapy and disulfiram. Drug Alcohol Depend. 145, 156–167. doi: 10.1016/j.drugalcdep.2014.10.007
Dluzen, D. E., and Liu, B. (2008). Gender differences in methamphetamine use and responses: a review. Gend. Med. 5, 24–35. doi: 10.1016/S1550-8579(08)80005-8
Domschke, K., Gajewska, A., Winter, B., Herrmann, M. J., Warrings, B., Mühlberger, A., et al. (2012). ADORA2A Gene variation, caffeine, and emotional processing: a multi-level interaction on startle reflex. Neuropsychopharmacology 37, 759–769. doi: 10.1038/npp.2011.253
Doyle, S. E., Breslin, F. J., Rieger, J. M., Beauglehole, A., and Lynch, W. J. (2012). Time and sex-dependent effects of an adenosine A2A/A1 receptor antagonist on motivation to self-administer cocaine in rats. Pharmacol. Biochem. Behav. 102, 257–263. doi: 10.1016/j.pbb.2012.05.001
Drazdowski, T. K. (2016). A systematic review of the motivations for the non-medical use of prescription drugs in young adults. Drug Alcohol Depend. 162, 3–25. doi: 10.1016/j.drugalcdep.2016.01.011
Drury, A. N., and Szent-Györgyi, A. (1929). The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J. Physiol. 68, 213–237. doi: 10.1113/jphysiol.1929.sp002608
Dunwiddie, T. V. (1985). The physiological role of adenosine in the central nervous system. Int. Rev. Neurobiol. 27, 63–139. doi: 10.1016/S0074-7742(08)60556-5
Engmann, O., Giralt, A., Gervasi, N., Marion-Poll, L., Gasmi, L., Filhol, O., et al. (2015). DARPP-32 interaction with adducin may mediate rapid environmental effects on striatal neurons. Nat. Commun. 6:10099. doi: 10.1038/ncomms10099
European Monitoring Centre for Drugs and Drug Addiction (2017). European Drug Report 2017: Trends and Developments. Luxembourg: Publications Office of the European Union.
Ferraro, L., Frankowska, M., Marcellino, D., Zaniewska, M., Beggiato, S., Filip, M., et al. (2012). A novel mechanism of cocaine to enhance dopamine d2-like receptor mediated neurochemical and behavioral effects. An in vivo and in vitro study. Neuropsychopharmacology 37, 1856–1866. doi: 10.1038/npp.2012.33
Ferré, S. (2008). An update on the mechanisms of the psychostimulant effects of caffeine. J. Neurochem. 105, 1067–1079. doi: 10.1111/j.1471-4159.2007.05196.x
Ferré, S. (2016). Mechanisms of the psychostimulant effects of caffeine: implications for substance use disorders. Psychopharmacology 233, 1963–1979. doi: 10.1007/s00213-016-4212-2
Ferré, S., Ciruela, F., Borycz, J., Solinas, M., Quarta, D., Antoniou, K., et al. (2008a). Adenosine A1-A2A receptor heteromers: new targets for caffeine in the brain. Front. Biosci. 13, 2391–2399.
Ferré, S., Diamond, I., Goldberg, S. R., Yao, L., Hourani, S. M. O., Huang, Z. L., et al. (2007). Adenosine A2A receptors in ventral striatum, hypothalamus and nociceptive circuitry. Implications for drug addiction, sleep and pain. Prog. Neurobiol. 83, 332–347. doi: 10.1016/j.pneurobio.2007.04.002
Ferré, S., Fredholm, B. B., Morelli, M., Popoli, P., and Fuxe, K. (1997). Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci. 20, 482–487. doi: 10.1016/S0166-2236(97)01096-5
Ferré, S., Karcz-Kubicha, M., Hope, B. T., Popoli, P., Burgueño, J., Gutiérrez, M. A., et al. (2002). Synergistic interaction between adenosine A2A and glutamate mGlu5 receptors: implications for striatal neuronal function. Proc. Natl. Acad. Sci. U.S.A. 99, 11940–11945. doi: 10.1073/pnas.172393799
Ferré, S., O’Connor, W. T., Snaprud, P., Ungerstedt, U., and Fuxe, K. (1994). Antagonistic interaction between adenosine A2A receptors and dopamine D2 receptors in the ventral striopallidal system. Implications for the treatment of schizophrenia. Neuroscience 63, 765–773. doi: 10.1016/0306-4522(94)90521-5
Ferré, S., Quiroz, C., Woods, A. S., Cunha, R., Popoli, P., Ciruela, F., et al. (2008b). An update on adenosine A2A-dopamine D2 receptor interactions: implications for the function of G protein-coupled receptors. Curr. Pharm. Des. 14, 1468–1474.
Ferré, S., von Euler, G., Johansson, B., Fredholm, B. B., and Fuxe, K. (1991). Stimulation of high-affinity adenosine A2 receptors decreases the affinity of dopamine D2 receptors in rat striatal membranes. Proc. Natl. Acad. Sci. U.S.A. 88, 7238–7241. doi: 10.1073/pnas.88.16.7238
Filip, M., Frankowska, M., Zaniewska, M., Przegaliński, E., Müller, C. E., Agnati, L., et al. (2006). Involvement of adenosine A2A and dopamine receptors in the locomotor and sensitizing effects of cocaine. Brain Res. 1077, 67–80. doi: 10.1016/j.brainres.2006.01.038
Filip, M., Zaniewska, M., Frankowska, M., Wydra, K., and Fuxe, K. (2012). The importance of the adenosine A2A receptor-dopamine D(2) receptor interaction in drug addiction. Curr. Med. Chem. 19, 317–355. doi: 10.2174/092986712803414231
Franco, R., Martínez-Pinilla, E., Ricobaraza, A., and McCormick, P. J. (2013). Challenges in the development of heteromer-GPCR-based drugs. Prog. Mol. Biol. Transl. Sci. 117, 143–162. doi: 10.1016/B978-0-12-386931-9.00006-4
Frankowska, M., Marcellino, D., Adamczyk, P., Filip, M., and Fuxe, K. (2013). Effects of cocaine self-administration and extinction on D2 -like and A2A receptor recognition and D2 -like/Gi protein coupling in rat striatum. Addict. Biol. 18, 455–466. doi: 10.1111/j.1369-1600.2012.00452.x
Fredholm, B. B., Arslan, G., Halldner, L., Kull, B., Schulte, G., and Wasserman, W. (2000). Structure and function of adenosine receptors and their genes. Naunyn Schmiedebergs Arch. Pharmacol. 362, 364–374.
Fredholm, B. B., IJzerman, A. P., Jacobson, K. A., Klotz, K. N., and Linden, J. (2001). International union of pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 53, 527–552.
Fuxe, K., Agnati, L. F., Jacobsen, K., Hillion, J., Canals, M., Torvinen, M., et al. (2003). Receptor heteromerization in adenosine A2A receptor signaling: relevance for striatal function and Parkinson’s disease. Neurology 61, S19–S23. doi: 10.1212/01.WNL.0000095206.44418.5C
Fuxe, K., Ferré, S., Genedani, S., Franco, R., and Agnati, L. F. (2007a). Adenosine receptor-dopamine receptor interactions in the basal ganglia and their relevance for brain function. Physiol. Behav. 92, 210–217. doi: 10.1016/j.physbeh.2007.05.034
Fuxe, K., Marcellino, D., Borroto-Escuela, D. O., Guescini, M., Fernández-Dueñas, V., Tanganelli, S., et al. (2010). Adenosine-dopamine interactions in the pathophysiology and treatment of CNS disorders. CNS Neurosci. Ther. 16, e18–e42. doi: 10.1111/j.1755-5949.2009.00126.x
Fuxe, K., Marcellino, D., Genedani, S., and Agnati, L. (2007b). Adenosine A(2A) receptors, dopamine D(2) receptors and their interactions in Parkinson’s disease. Mov. Disord. 22, 1990–2017. doi: 10.1002/mds.21440
Fuxe, K., Marcellino, D., Rivera, A., Diaz-Cabiale, Z., Filip, M., Gago, B., et al. (2008). Receptor-receptor interactions within receptor mosaics. Impact on neuropsychopharmacology. Brain Res. Rev. 58, 415–452. doi: 10.1016/j.brainresrev.2007.11.007
Gajewska, A., Blumenthal, T. D., Winter, B., Herrmann, M. J., Conzelmann, A., Mühlberger, A., et al. (2013). Effects of ADORA2A gene variation and caffeine on prepulse inhibition: a multi-level risk model of anxiety. Prog. Neuropsychopharmacol. Biol. Psychiatry 40, 115–121. doi: 10.1016/j.pnpbp.2012.08.008
GBD 2015 Risk Factors Collaborators (2016). Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388, 1659–1724. doi: 10.1016/S0140-6736(16)31679-8
Gessi, S., Merighi, S., Fazzi, D., Stefanelli, A., Varani, K., and Borea, P. A. (2011). Adenosine receptor targeting in health and disease. Expert Opin. Investig. Drugs 20, 1591–1609. doi: 10.1517/13543784.2011.627853
Gessi, S., Merighi, S., Stefanelli, A., Fazzi, D., Varani, K., and Borea, P. A. (2013). A(1) and A(3) adenosine receptors inhibit LPS-induced hypoxia-inducible factor-1 accumulation in murine astrocytes. Pharmacol. Res. 76, 157–170. doi: 10.1016/j.phrs.2013.08.002
Giménez-Llort, L., Fernández-Teruel, A., Escorihuela, R. M., Fredholm, B. B., Tobeña, A., Pekny, M., et al. (2002). Mice lacking the adenosine A1 receptor are anxious and aggressive, but are normal learners with reduced muscle strength and survival rate. Eur. J. Neurosci. 16, 547–550. doi: 10.1046/j.1460-9568.2002.02122.x
Ginés, S., Hillion, J., Torvinen, M., Le Crom, S., Casadó, V., Canela, E. I., et al. (2000). Dopamine D1 and adenosine A1 receptors form functionally interacting heteromeric complexes. Proc. Natl. Acad. Sci. U.S.A. 97, 8606–8611. doi: 10.1073/pnas.150241097
Glukhova, A., Thal, D. M., Nguyen, A. T., Vecchio, E. A., Jörg, M., Scammells, P. J., et al. (2017). Structure of the adenosine A1 receptor reveals the basis for subtype selectivity. Cell 168, 867.e13–877.e13. doi: 10.1016/j.cell.2017.01.042
Godinho, R. O., Duarte, T., and Pacini, E. S. A. (2015). New perspectives in signaling mediated by receptors coupled to stimulatory G protein: the emerging significance of cAMP efflux and extracellular cAMP-adenosine pathway. Front. Pharmacol. 6:58. doi: 10.3389/fphar.2015.00058
Gołembiowska, K., and Zylewska, A. (1998a). Agonists of A1 and A2A adenosine receptors attenuate methamphetamine-induced overflow of dopamine in rat striatum. Brain Res. 806, 202–209.
Gołembiowska, K., and Zylewska, A. (1998b). N6-2-(4-aminophenyl)ethyladenosine (APNEA), a putative adenosine A3 receptor agonist, enhances methamphetamine-induced dopamine outflow in rat striatum. Pol. J. Pharmacol. 50, 299–305.
Gomes, C. V., Kaster, M. P., Tomé, A. R., Agostinho, P. M., and Cunha, R. A. (2011). Adenosine receptors and brain diseases: neuroprotection and neurodegeneration. Biochim. Biophys. Acta 1808, 1380–1399. doi: 10.1016/j.bbamem.2010.12.001
Goodwin, J. S., Larson, G. A., Swant, J., Sen, N., Javitch, J. A., Zahniser, N. R., et al. (2009). Amphetamine and methamphetamine differentially affect dopamine transporters in vitro and in vivo. J. Biol. Chem. 284, 2978–2989. doi: 10.1074/jbc.M805298200
Górska, A. M., and Gołembiowska, K. (2015). The role of adenosine A1 and A2A receptors in the caffeine effect on MDMA-induced DA and 5-HT release in the mouse striatum. Neurotox. Res. 27, 229–245. doi: 10.1007/s12640-014-9501-0
Górska, A. M., Kamińska, K., Wawrzczak-Bargieła, A., Costa, G., Morelli, M., Przewłocki, R., et al. (2017). Neurochemical and neurotoxic effects of MDMA (ecstasy) and caffeine after chronic combined administration in mice. Neurotox. Res. doi: 10.1007/s12640-017-9831-9 [Epub ahead of print].
Hack, S. P., and Christie, M. J. (2003). Adaptations in adenosine signaling in drug dependence: therapeutic implications. Crit. Rev. Neurobiol. 15, 235–274. doi: 10.1615/CritRevNeurobiol.v15.i34.30
Harper, L. K., Beckett, S. R., Marsden, C. A., McCreary, A. C., and Alexander, S. P. H. (2006). Effects of the A 2A adenosine receptor antagonist KW6002 in the nucleus accumbens in vitro and in vivo. Pharmacol. Biochem. Behav. 83, 114–121. doi: 10.1016/j.pbb.2005.12.014
Hart, A. B., de Wit, H., and Palmer, A. A. (2013). Candidate gene studies of a promising intermediate phenotype: failure to replicate. Neuropsychopharmacology 38, 802–816. doi: 10.1038/npp.2012.245
Hasin, D. S., O’Brien, C. P., Auriacombe, M., Borges, G., Bucholz, K., Budney, A., et al. (2013). DSM-5 criteria for substance use disorders: recommendations and rationale. Am. J. Psychiatry 170, 834–851. doi: 10.1176/appi.ajp.2013.12060782
Hatsukami, D. K., and Fischman, M. W. (1996). Crack cocaine and cocaine hydrochloride: are the differences myth or reality? JAMA 276, 1580–1588. doi: 10.1001/jama.1996.03540190052029
Heffner, T. G., Wiley, J. N., Williams, A. E., Bruns, R. F., Coughenour, L. L., and Downs, D. A. (1989). Comparison of the behavioral effects of adenosine agonists and dopamine antagonists in mice. Psychopharmacology 98, 31–37. doi: 10.1007/BF00442002
Hervé, D., Le Moine, C., Corvol, J. C., Belluscio, L., Ledent, C., Fienberg, A. A., et al. (2001). Galpha(olf) levels are regulated by receptor usage and control dopamine and adenosine action in the striatum. J. Neurosci. 21, 4390–4399.
Hillion, J., Canals, M., Torvinen, M., Casado, V., Scott, R., Terasmaa, A., et al. (2002). Coaggregation, cointernalization, and codesensitization of adenosine A2A receptors and dopamine D2 receptors. J. Biol. Chem. 277, 18091–18097. doi: 10.1074/jbc.M107731200
Hobson, B. D., Merritt, K. E., and Bachtell, R. K. (2012). Stimulation of adenosine receptors in the nucleus accumbens reverses the expression of cocaine sensitization and cross-sensitization to dopamine D2 receptors in rats. Neuropharmacology 63, 1172–1181. doi: 10.1016/j.neuropharm.2012.06.038
Hobson, B. D., O’Neill, C. E., Levis, S. C., Monteggia, L. M., Neve, R. L., Self, D. W., et al. (2013). Adenosine A1 and dopamine d1 receptor regulation of AMPA receptor phosphorylation and cocaine-seeking behavior. Neuropsychopharmacology 38, 1974–1983. doi: 10.1038/npp.2013.96
Hohoff, C., McDonald, J. M., Baune, B. T., Cook, E. H., Deckert, J., and de Wit, H. (2005). Interindividual variation in anxiety response to amphetamine: possible role for adenosine A2A receptor gene variants. Am. J. Med. Genet. B Neuropsychiatr. Genet. 139B, 42–44. doi: 10.1002/ajmg.b.30228
Jaakola, V.-P., Griffith, M. T., Hanson, M. A., Cherezov, V., Chien, E. Y. T., Lane, J. R., et al. (2008). The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science 322, 1211–1217. doi: 10.1126/science.1164772
Jacobson, K. A., Merighi, S., Varani, K., Borea, P. A., Baraldi, S., Aghazadeh Tabrizi, M., et al. (2017). A3 adenosine receptors as modulators of inflammation: from medicinal chemistry to therapy. Med. Res. Rev. doi: 10.1002/med.21456 [Epub ahead of print].
Jain, N., Kemp, N., Adeyemo, O., Buchanan, P., and Stone, T. W. (1995). Anxiolytic activity of adenosine receptor activation in mice. Br. J. Pharmacol. 116, 2127–2133. doi: 10.1111/j.1476-5381.1995.tb16421.x
Janać, B., Pesić, V., Peković, S., Rakić, L., and Stojiljković, M. (2005). Different effects of adenosine A1 agonist ribavirin on amphetamine-induced total locomotor and stereotypic activities in rats. Ann. N. Y. Acad. Sci. 1048, 396–399. doi: 10.1196/annals.1342.048
Janes, K., Esposito, E., Doyle, T., Cuzzocrea, S., Tosh, D. K., Jacobson, K. A., et al. (2014). A3 adenosine receptor agonist prevents the development of paclitaxel-induced neuropathic pain by modulating spinal glial-restricted redox-dependent signaling pathways. Pain 155, 2560–2567. doi: 10.1016/j.pain.2014.09.016
Jastrzêbska, J., Nowak, E., Smaga, I., Bystrowska, B., Frankowska, M., Bader, M., et al. (2014). Adenosine (A)(2A)receptor modulation of nicotine-induced locomotor sensitization. A pharmacological and transgenic approach. Neuropharmacology 81, 318–326. doi: 10.1016/j.neuropharm.2014.03.002
Johanson, C. E., and Fischman, M. W. (1989). The pharmacology of cocaine related to its abuse. Pharmacol. Rev. 41, 3–52.
Johnson, K. A., and Lovinger, D. M. (2016). Presynaptic G protein-coupled receptors: gatekeepers of addiction? Front. Cell. Neurosci. 10:264. doi: 10.3389/fncel.2016.00264
Justinova, Z., Ferré, S., Redhi, G. H., Mascia, P., Stroik, J., Quarta, D., et al. (2011). Reinforcing and neurochemical effects of cannabinoid CB1 receptor agonists, but not cocaine, are altered by an adenosine A2A receptor antagonist. Addict. Biol. 16, 405–415. doi: 10.1111/j.1369-1600.2010.00258.x
Justinova, Z., Ferré, S., Segal, P. N., Antoniou, K., Solinas, M., Pappas, L. A., et al. (2003). Involvement of adenosine A1 and A2A receptors in the adenosinergic modulation of the discriminative-stimulus effects of cocaine and methamphetamine in rats. J. Pharmacol. Exp. Ther. 307, 977–986. doi: 10.1124/jpet.103.056762
Kalivas, P. W., and Volkow, N. D. (2011). New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol. Psychiatry 16, 974–986. doi: 10.1038/mp.2011.46
Kavanagh, K. A., Schreiner, D. C., Levis, S. C., O’Neill, C. E., and Bachtell, R. K. (2015). Role of adenosine receptor subtypes in methamphetamine reward and reinforcement. Neuropharmacology 89, 265–273. doi: 10.1016/j.neuropharm.2014.09.030
Kelley, A. E., and Berridge, K. C. (2002). The neuroscience of natural rewards: relevance to addictive drugs. J. Neurosci. 22, 3306–3311.
Kennedy, A. P., Epstein, D. H., Phillips, K. A., and Preston, K. L. (2013). Sex differences in cocaine/heroin users: drug-use triggers and craving in daily life. Drug Alcohol Depend. 132, 29–37. doi: 10.1016/j.drugalcdep.2012.12.025
Kermanian, F., Mehdizadeh, M., Soleimani, M., Ebrahimzadeh Bideskan, A. R., Asadi-Shekaari, M., Kheradmand, H., et al. (2012). The role of adenosine receptor agonist and antagonist on Hippocampal MDMA detrimental effects; a structural and behavioral study. Metab. Brain Dis. 27, 459–469. doi: 10.1007/s11011-012-9334-6
Kermanian, F., Soleimani, M., Ebrahimzadeh, A., Haghir, H., and Mehdizadeh, M. (2013). Effects of adenosine A2a receptor agonist and antagonist on hippocampal nuclear factor-kB expression preceded by MDMA toxicity. Metab. Brain Dis. 28, 45–52. doi: 10.1007/s11011-012-9366-y
Kerstetter, K. A., Aguilar, V. R., Parrish, A. B., and Kippin, T. E. (2008). Protracted time-dependent increases in cocaine-seeking behavior during cocaine withdrawal in female relative to male rats. Psychopharmacology 198, 63–75. doi: 10.1007/s00213-008-1089-8
Kim, H. J., Lee, J. H., Yun, K., and Kim, J.-H. (2017). Alterations in striatal circuits underlying addiction-like behaviors. Mol. Cells 40, 379–385. doi: 10.14348/molcells.2017.0088
Knapp, C. M., Foye, M. M., Cottam, N., Ciraulo, D. A., and Kornetsky, C. (2001). Adenosine agonists CGS 21680 and NECA inhibit the initiation of cocaine self-administration. Pharmacol. Biochem. Behav. 68, 797–803. doi: 10.1016/S0091-3057(01)00486-5
Kniazeff, J., Prézeau, L., Rondard, P., Pin, J.-P., and Goudet, C. (2011). Dimers and beyond: the functional puzzles of class C GPCRs. Pharmacol. Ther. 130, 9–25. doi: 10.1016/j.pharmthera.2011.01.006
Kobayashi, H., Ujike, H., Iwata, N., Inada, T., Yamada, M., Sekine, Y., et al. (2010). The adenosine A2A receptor is associated with methamphetamine dependence/psychosis in the Japanese population. Behav. Brain Funct. 6:50. doi: 10.1186/1744-9081-6-50
Kobayashi, H., Ujike, H., Iwata, N., Inada, T., Yamada, M., Sekine, Y., et al. (2011). Association analysis of the adenosine A1 receptor gene polymorphisms in patients with methamphetamine dependence/psychosis. Curr. Neuropharmacol. 9, 137–142. doi: 10.2174/157015911795016958
Koob, G. F., and Volkow, N. D. (2010). Neurocircuitry of addiction. Neuropsychopharmacology 35, 217–238. doi: 10.1038/npp.2009.110
Krauss, S. W., Ghirnikar, R. B., Diamond, I., and Gordon, A. S. (1993). Inhibition of adenosine uptake by ethanol is specific for one class of nucleoside transporters. Mol. Pharmacol. 44, 1021–1026.
Kubrusly, R. C. C., and Bhide, P. G. (2010). Cocaine exposure modulates dopamine and adenosine signaling in the fetal brain. Neuropharmacology 58, 436–443. doi: 10.1016/j.neuropharm.2009.09.007
Kull, B., Svenningsson, P., and Fredholm, B. B. (2000). Adenosine A(2A) receptors are colocalized with and activate g(olf) in rat striatum. Mol. Pharmacol. 58, 771–777.
Kuzmin, A., Johansson, B., Zvartau, E. E., and Fredholm, B. B. (1999). Caffeine, acting on adenosine A(1) receptors, prevents the extinction of cocaine-seeking behavior in mice. J. Pharmacol. Exp. Ther. 290, 535–542.
Kwon, Y. S., Nabeshima, T., Shin, E.-J., Chun, W., Jhoo, J. H., Jhoo, W.-K., et al. (2004). PAP 9704, a Korean herbal medicine attenuates methamphetamine-induced hyperlocomotion via adenosine A2A receptor stimulation in mice. Biol. Pharm. Bull. 27, 906–909. doi: 10.1248/bpb.27.906
Lakhan, S. E., and Kirchgessner, A. (2012). Prescription stimulants in individuals with and without attention deficit hyperactivity disorder: misuse, cognitive impact, and adverse effects. Brain Behav. 2, 661–677. doi: 10.1002/brb3.78
Lane, S., Green, C., Steinberg, J., Ma, L., Schmitz, J., Rathnayaka, N., et al. (2012). Cardiovascular and subjective effects of the novel adenosine A(2A) receptor antagonist SYN115 in cocaine dependent individuals. J. Addict. Res. Ther. S1:009. doi: 10.4172/2155-6105.S1-009
Lane, S. D., Green, C. E., Schmitz, J. M., Rathnayaka, N., Fang, W. B., Ferré, S., et al. (2014). Comparison of caffeine and d-amphetamine in cocaine-dependent subjects: differential outcomes on subjective and cardiovascular effects, reward learning, and salivary paraxanthine. J. Addict. Res. Ther. 5:176. doi: 10.4172/2155-6105.1000176
Lebon, G., Warne, T., Edwards, P. C., Bennett, K., Langmead, C. J., Leslie, A. G. W., et al. (2011). Agonist-bound adenosine A2A receptor structures reveal common features of GPCR activation. Nature 474, 521–525. doi: 10.1038/nature10136
Ledent, C., Vaugeois, J. M., Schiffmann, S. N., Pedrazzini, T., El Yacoubi, M., Vanderhaeghen, J. J., et al. (1997). Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature 388, 674–678. doi: 10.1038/41771
Lev-Ran, S., Le Strat, Y., Imtiaz, S., Rehm, J., and Le Foll, B. (2013). Gender differences in prevalence of substance use disorders among individuals with lifetime exposure to substances: results from a large representative sample. Am. J. Addict. 22, 7–13. doi: 10.1111/j.1521-0391.2013.00321.x
Linden, J. (2001). Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu. Rev. Pharmacol. Toxicol. 41, 775–787. doi: 10.1146/annurev.pharmtox.41.1.775
Lobo, M. (2009). Molecular profiling of striatonigral and striatopallidal medium spiny neurons past, present, and future. Int. Rev. Neurobiol. 89, 1–35. doi: 10.1016/S0074-7742(09)89001-6
Lopes, L. V., Sebastião, A. M., and Ribeiro, J. A. (2011). Adenosine and related drugs in brain diseases: present and future in clinical trials. Curr. Top. Med. Chem. 11, 1087–1101. doi: 10.2174/156802611795347591
López-Arnau, R., Luján, M. A., Duart-Castells, L., Pubill, D., Camarasa, J., Valverde, O., et al. (2017). Exposure of adolescent mice to 3,4-methylenedioxypyrovalerone increases the psychostimulant, rewarding and reinforcing effects of cocaine in adulthood. Br. J. Pharmacol. 174, 1161–1173. doi: 10.1111/bph.13771
Łukasiewicz, S., Błasiak, E., Faron-Górecka, A., Polit, A., Tworzydło, M., Górecki, A., et al. (2007). Fluorescence studies of homooligomerization of adenosine A2A and serotonin 5-HT1A receptors reveal the specificity of receptor interactions in the plasma membrane. Pharmacol. Rep. 59, 379–392.
Lynch, W. J. (2006). Sex differences in vulnerability to drug self-administration. Exp. Clin. Psychopharmacol. 14, 34–41. doi: 10.1037/1064-1297.14.1.34
Lynch, W. J. (2017). Modeling the development of drug addiction in male and female animals. Pharmacol. Biochem. Behav. doi: 10.1016/j.pbb.2017.06.006 [Epub ahead of print].
Lynch, W. J., and Taylor, J. R. (2004). Sex differences in the behavioral effects of 24-h/day access to cocaine under a discrete trial procedure. Neuropsychopharmacology 29, 943–951. doi: 10.1038/sj.npp.1300389
Maier, L. J., Liechti, M. E., Herzig, F., and Schaub, M. P. (2013). To dope or not to dope: neuroenhancement with prescription drugs and drugs of abuse among Swiss University students. PLOS ONE 8:e77967. doi: 10.1371/journal.pone.0077967
Marcellino, D., Roberts, D. C. S., Navarro, G., Filip, M., Agnati, L., Lluís, C., et al. (2007). Increase in A2A receptors in the nucleus accumbens after extended cocaine self-administration and its disappearance after cocaine withdrawal. Brain Res. 1143, 208–220. doi: 10.1016/j.brainres.2007.01.079
McHugh, R. K., Nielsen, S., and Weiss, R. D. (2015). Prescription drug abuse: from epidemiology to public policy. J. Subst. Abuse Treat. 48, 1–7. doi: 10.1016/j.jsat.2014.08.004
Merighi, S., Bencivenni, S., Vincenzi, F., Varani, K., Borea, P. A., and Gessi, S. (2017). A2B adenosine receptors stimulate IL-6 production in primary murine microglia through p38 MAPK kinase pathway. Pharmacol. Res. 117, 9–19. doi: 10.1016/j.phrs.2016.11.024
Meyerhof, W., Müller-Brechlin, R., and Richter, D. (1991). Molecular cloning of a novel putative G-protein coupled receptor expressed during rat spermiogenesis. FEBS Lett. 284, 155–160. doi: 10.1016/0014-5793(91)80674-R
Miyamoto, Y., Iida, A., Sato, K., Muramatsu, S., and Nitta, A. (2014). Knockdown of dopamine D2 receptors in the nucleus accumbens core suppresses methamphetamine-induced behaviors and signal transduction in mice. Int. J. Neuropsychopharmacol. 18:yu038. doi: 10.1093/ijnp/pyu038
Moeller, F. G., Steinberg, J. L., Lane, S. D., Kjome, K. L., Ma, L., Ferré, S., et al. (2012). Increased orbitofrontal brain activation after administration of a selective adenosine A(2A) antagonist in cocaine dependent subjects. Front. Psychiatry 3:44. doi: 10.3389/fpsyt.2012.00044
Morales, M., and Margolis, E. B. (2017). Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat. Rev. Neurosci. 18, 73–85. doi: 10.1038/nrn.2016.165
Moscoso-Castro, M., Gracia-Rubio, I., Ciruela, F., and Valverde, O. (2016). Genetic blockade of adenosine A2A receptors induces cognitive impairments and anatomical changes related to psychotic symptoms in mice. Eur. Neuropsychopharmacol. 26, 1227–1240. doi: 10.1016/j.euroneuro.2016.04.003
Moser, P., Wolinsky, T., Duxon, M., and Porsolt, R. D. (2011). How good are current approaches to nonclinical evaluation of abuse and dependence? J. Pharmacol. Exp. Ther. 336, 588–595. doi: 10.1124/jpet.110.169979
Müller, C. E., and Jacobson, K. A. (2011). Recent developments in adenosine receptor ligands and their potential as novel drugs. Biochim. Biophys. Acta 1808, 1290–1308. doi: 10.1016/j.bbamem.2010.12.017
Munzar, P., Justinova, Z., Kutkat, S. W., Ferré, S., and Goldberg, S. R. (2002). Adenosinergic modulation of the discriminative-stimulus effects of methamphetamine in rats. Psychopharmacology 161, 348–355. doi: 10.1007/s00213-002-1075-5
Nestler, E. J., and Landsman, D. (2001). Learning about addiction from the genome. Nature 409, 834–835. doi: 10.1038/35057015
Nyberg, F. (2014). Structural plasticity of the brain to psychostimulant use. Neuropharmacology 87, 115–124. doi: 10.1016/j.neuropharm.2014.07.004
Okada, M., Kiryu, K., Kawata, Y., Mizuno, K., Wada, K., Tasaki, H., et al. (1997). Determination of the effects of caffeine and carbamazepine on striatal dopamine release by in vivo microdialysis. Eur. J. Pharmacol. 321, 181–188. doi: 10.1016/S0014-2999(96)00938-7
O’Neill, C. E., Hobson, B. D., Levis, S. C., and Bachtell, R. K. (2014). Persistent reduction of cocaine seeking by pharmacological manipulation of adenosine A1 and A 2A receptors during extinction training in rats. Psychopharmacology 231, 3179–3188. doi: 10.1007/s00213-014-3489-2
O’Neill, C. E., LeTendre, M. L., and Bachtell, R. K. (2012). Adenosine A2A receptors in the nucleus accumbens bi-directionally alter cocaine seeking in rats. Neuropsychopharmacology 37, 1245–1256. doi: 10.1038/npp.2011.312
Peleli, M., Fredholm, B. B., Sobrevia, L., and Carlström, M. (2017). Pharmacological targeting of adenosine receptor signaling. Mol. Aspects Med. 55, 4–8. doi: 10.1016/j.mam.2016.12.002
Phillips, K. A., Epstein, D. H., and Preston, K. L. (2014). Psychostimulant addiction treatment. Neuropharmacology 87, 150–160. doi: 10.1016/j.neuropharm.2014.04.002
Pickens, C. L., Airavaara, M., Theberge, F., Fanous, S., Hope, B. T., and Shaham, Y. (2011). Neurobiology of the incubation of drug craving. Trends Neurosci. 34, 411–420. doi: 10.1016/j.tins.2011.06.001
Pinna, A., Wardas, J., Cristalli, G., and Morelli, M. (1997). Adenosine A2A receptor agonists increase Fos-like immunoreactivity in mesolimbic areas. Brain Res. 759, 41–49. doi: 10.1016/S0006-8993(97)00214-X
Pintsuk, J., Borroto-Escuela, D. O., Pomierny, B., Wydra, K., Zaniewska, M., Filip, M., et al. (2016). Cocaine self-administration differentially affects allosteric A2A-D2 receptor-receptor interactions in the striatum. Relevance for cocaine use disorder. Pharmacol. Biochem. Behav. 144, 85–91. doi: 10.1016/j.pbb.2016.03.004
Poleszak, E., and Malec, D. (2000). Influence of adenosine receptor agonists and antagonists on amphetamine-induced stereotypy in rats. Pol. J. Pharmacol. 52, 423–429.
Poleszak, E., and Malec, D. (2002a). Adenosine receptor ligands and cocaine in conditioned place preference (CPP) test in rats. Pol. J. Pharmacol. 54, 119–126.
Poleszak, E., and Malec, D. (2002b). Cocaine-induced hyperactivity is more influenced by adenosine receptor agonists than amphetamine-induced hyperactivity. Pol. J. Pharmacol. 54, 359–366.
Poleszak, E., and Malec, D. (2003). Effects of adenosine receptor agonists and antagonists in amphetamine-induced conditioned place preference test in rats. Pol. J. Pharmacol. 55, 319–326.
Popoli, P., and Pepponi, R. (2012). Potential therapeutic relevance of adenosine A2B and A2A receptors in the central nervous system. CNS Neurol. Disord. Drug Targets 11, 664–674. doi: 10.2174/187152712803581100
Popoli, P., Pèzzola, A., and de Carolis, A. S. (1994). Modulation of striatal adenosine A1 and A2 receptors induces rotational behaviour in response to dopaminergic stimulation in intact rats. Eur. J. Pharmacol. 257, 21–25. doi: 10.1016/0014-2999(94)90689-0
Prediger, R. D. S., da Silva, G. E., Batista, L. C., Bittencourt, A. L., and Takahashi, R. N. (2006). Activation of adenosine A1 receptors reduces anxiety-like behavior during acute ethanol withdrawal (hangover) in mice. Neuropsychopharmacology 31, 2210–2220. doi: 10.1038/sj.npp.1301001
Preedy, V. R. (2016). Neuropathology of Drug Addictions and Substance Misuse: General Processes and Mechanisms, Prescription Medications, Caffeine and Areca, Polydrug Misuse, Emerging Addictions and Non-Drug Addictions, Vol. 3. Cambridge, MA: Academic Press.
Preti, D., Baraldi, P. G., Moorman, A. R., Borea, P. A., and Varani, K. (2015). History and perspectives of A2A adenosine receptor antagonists as potential therapeutic agents. Med. Res. Rev. 35, 790–848. doi: 10.1002/med.21344
Prieto, J. P., Scorza, C., Serra, G. P., Perra, V., Galvalisi, M., Abin-Carriquiry, J. A., et al. (2016). Caffeine, a common active adulterant of cocaine, enhances the reinforcing effect of cocaine and its motivational value. Psychopharmacology 233, 2879–2889. doi: 10.1007/s00213-016-4320-z
Rau, A. R., Ariwodola, O. J., and Weiner, J. L. (2014). Presynaptic adenosine A1 receptors modulate excitatory transmission in the rat basolateral amygdala. Neuropharmacology 77, 465–474. doi: 10.1016/j.neuropharm.2013.10.029
Reichel, C. M., Chan, C. H., Ghee, S. M., and See, R. E. (2012). Sex differences in escalation of methamphetamine self-administration: cognitive and motivational consequences in rats. Psychopharmacology 223, 371–380. doi: 10.1007/s00213-012-2727-8
Reissig, C. J., Strain, E. C., and Griffiths, R. R. (2009). Caffeinated energy drinks—A growing problem. Drug Alcohol Depend. 99, 1–10. doi: 10.1016/j.drugalcdep.2008.08.001
Ribeiro, J. A. (1999). Adenosine A2A receptor interactions with receptors for other neurotransmitters and neuromodulators. Eur. J. Pharmacol. 375, 101–113. doi: 10.1016/S0014-2999(99)00230-7
Rimondini, R., Ferré, S., Ogren, S. O., and Fuxe, K. (1997). Adenosine A2A agonists: a potential new type of atypical antipsychotic. Neuropsychopharmacology 17, 82–91. doi: 10.1016/S0893-133X(97)00033-X
Ross, E. J., Graham, D. L., Money, K. M., and Stanwood, G. D. (2015). Developmental consequences of fetal exposure to drugs: what we know and what we still must learn. Neuropsychopharmacology 40, 61–87. doi: 10.1038/npp.2014.147
Roth, M. E., and Carroll, M. E. (2004). Sex differences in the escalation of intravenous cocaine intake following long- or short-access to cocaine self-administration. Pharmacol. Biochem. Behav. 78, 199–207. doi: 10.1016/j.pbb.2004.03.018
Rothman, R. B., and Baumann, M. H. (2003). Monoamine transporters and psychostimulant drugs. Eur. J. Pharmacol. 479, 23–40. doi: 10.1016/j.ejphar.2003.08.054
Ruda-Kucerova, J., Amchova, P., Babinska, Z., Dusek, L., Micale, V., and Sulcova, A. (2015). Sex differences in the reinstatement of methamphetamine seeking after forced abstinence in sprague-dawley rats. Front. Psychiatry 6:91. doi: 10.3389/fpsyt.2015.00091
Rudnick, G., and Wall, S. C. (1992). The molecular mechanism of “ecstasy” [3,4-methylenedioxy-methamphetamine (MDMA)]: serotonin transporters are targets for MDMA-induced serotonin release. Proc. Natl. Acad. Sci. U.S.A. 89, 1817–1821. doi: 10.1073/pnas.89.5.1817
Ruiz-Medina, J., Ledent, C., Carretón, O., and Valverde, O. (2011). The A2a adenosine receptor modulates the reinforcement efficacy and neurotoxicity of MDMA. J. Psychopharmacol. 25, 550–564. doi: 10.1177/0269881110389210
Salem, A., and Hope, W. (1999). Role of endogenous adenosine in the expression of opiate withdrawal in rats. Eur. J. Pharmacol. 369, 39–42. doi: 10.1016/S0014-2999(99)00046-1
Sánchez-López, E., Marcos, A., Ambrosio, E., Mayboroda, O. A., Marina, M. L., and Crego, A. L. (2017). Investigation on the combined effect of cocaine and ethanol administration through a liquid chromatography-mass spectrometry metabolomics approach. J. Pharm. Biomed. Anal. 140, 313–321. doi: 10.1016/j.jpba.2017.03.061
Sawynok, J. (2016). Adenosine receptor targets for pain. Neuroscience 338, 1–18. doi: 10.1016/j.neuroscience.2015.10.031
Schulte, G., and Fredholm, B. B. (2003). Signalling from adenosine receptors to mitogen-activated protein kinases. Cell. Signal. 15, 813–827. doi: 10.1016/S0898-6568(03)00058-5
Shen, H., Luo, Y., Yu, S.-J., and Wang, Y. (2011). Enhanced neurodegeneration after a high dose of methamphetamine in adenosine A3 receptor null mutant mice. Neuroscience 194, 170–180. doi: 10.1016/j.neuroscience.2011.08.013
Shen, H.-Y., Coelho, J. E., Ohtsuka, N., Canas, P. M., Day, Y.-J., Huang, Q.-Y., et al. (2008). A critical role of the adenosine A2A receptor in extrastriatal neurons in modulating psychomotor activity as revealed by opposite phenotypes of striatum and forebrain A2A receptor knock-outs. J. Neurosci. 28, 2970–2975. doi: 10.1523/JNEUROSCI.5255-07.2008
Shimazoe, T., Yoshimatsu, A., Kawashimo, A., and Watanabe, S. (2000). Roles of adenosine A(1) and A(2A) receptors in the expression and development of methamphetamine-induced sensitization. Eur. J. Pharmacol. 388, 249–254. doi: 10.1016/S0014-2999(99)00899-7
Shin, E.-J., Nabeshima, T., Suh, H.-W., Jhoo, W.-K., Oh, K.-W., Lim, Y.-K., et al. (2005). Ginsenosides attenuate methamphetamine-induced behavioral side effects in mice via activation of adenosine A2A receptors: possible involvements of the striatal reduction in AP-1 DNA binding activity and proenkephalin gene expression. Behav. Brain Res. 158, 143–157. doi: 10.1016/j.bbr.2004.08.018
Siciliano, C. A., Calipari, E. S., Ferris, M. J., and Jones, S. R. (2015). Adaptations of presynaptic dopamine terminals induced by psychostimulant self-administration. ACS Chem. Neurosci. 6, 27–36. doi: 10.1021/cn5002705
Silberberg, G., and Bolam, J. P. (2015). Local and afferent synaptic pathways in the striatal microcircuitry. Curr. Opin. Neurobiol. 33, 182–187. doi: 10.1016/j.conb.2015.05.002
Soleimani, M., Katebi, M., Alizadeh, A., Mohammadzadeh, F., and Mehdizadeh, M. (2012). The role of the A2A receptor in cell apoptosis caused by MDMA. Cell J. 14, 231–236.
Soria, G., Castañé, A., Ledent, C., Parmentier, M., Maldonado, R., and Valverde, O. (2006). The lack of A2A adenosine receptors diminishes the reinforcing efficacy of cocaine. Neuropsychopharmacology 31, 978–987. doi: 10.1038/sj.npp.1300876
Stenberg, D., Litonius, E., Halldner, L., Johansson, B., Fredholm, B. B., and Porkka-Heiskanen, T. (2003). Sleep and its homeostatic regulation in mice lacking the adenosine A1 receptor. J. Sleep Res. 12, 283–290. doi: 10.1046/j.0962-1105.2003.00367.x
Stone, T. W. (1981). The effects of morphine and methionine-enkephalin on the release of purines from cerebral cortex slices of rats and mice. Br. J. Pharmacol. 74, 171–176. doi: 10.1111/j.1476-5381.1981.tb09970.x
Strickland, J. C., and Smith, M. A. (2015). Animal models of social contact and drug self-administration. Pharmacol. Biochem. Behav. 136, 47–54. doi: 10.1016/j.pbb.2015.06.013
Strohl, M. P. (2011). Bradley’s Benzedrine studies on children with behavioral disorders. Yale J. Biol. Med. 84, 27–33.
Sulzer, D., Sonders, M. S., Poulsen, N. W., and Galli, A. (2005). Mechanisms of neurotransmitter release by amphetamines: a review. Prog. Neurobiol. 75, 406–433. doi: 10.1016/j.pneurobio.2005.04.003
Sun, B., Bachhawat, P., Chu, M. L.-H., Wood, M., Ceska, T., Sands, Z. A., et al. (2017). Crystal structure of the adenosine A2A receptor bound to an antagonist reveals a potential allosteric pocket. Proc. Natl. Acad. Sci. U.S.A. 114, 2066–2071. doi: 10.1073/pnas.1621423114
Tebano, M. T., Martire, A., and Popoli, P. (2012). Adenosine A(2A)-cannabinoid CB(1) receptor interaction: an integrative mechanism in striatal glutamatergic neurotransmission. Brain Res. 1476, 108–118. doi: 10.1016/j.brainres.2012.04.051
Tozzi, A., de Iure, A., Marsili, V., Romano, R., Tantucci, M., Di Filippo, M., et al. (2012). A2A adenosine receptor antagonism enhances synaptic and motor effects of cocaine via CB1 cannabinoid receptor activation. PLOS ONE 7:e38312. doi: 10.1371/journal.pone.0038312
Trifilieff, P., Rives, M.-L., Urizar, E., Piskorowski, R. A., Vishwasrao, H. D., Castrillon, J., et al. (2011). Detection of antigen interactions ex vivo by proximity ligation assay: endogenous dopamine D2-adenosine A2A receptor complexes in the striatum. Biotechniques 51, 111–118. doi: 10.2144/000113719
Turgeon, S. M., Pollack, A. E., Schusheim, L., and Fink, J. S. (1996). Effects of selective adenosine A1 and A2a agonists on amphetamine-induced locomotion and c-Fos in striatum and nucleus accumbens. Brain Res. 707, 75–80. doi: 10.1016/0006-8993(95)01223-0
Vanattou-Saïfoudine, N., Gossen, A., Harkin, A., and Neuropsychopharmacology Research Group (2011). A role for adenosine A(1) receptor blockade in the ability of caffeine to promote MDMA “Ecstasy”-induced striatal dopamine release. Eur. J. Pharmacol. 650, 220–228. doi: 10.1016/j.ejphar.2010.10.012
Vanattou-Saïfoudine, N., McNamara, R., and Harkin, A. (2010). Mechanisms mediating the ability of caffeine to influence MDMA (‘Ecstasy’)-induced hyperthermia in rats. Br. J. Pharmacol. 160, 860–877. doi: 10.1111/j.1476-5381.2010.00660.x
Vanattou-Saïfoudine, N., McNamara, R., and Harkin, A. (2012). Caffeine provokes adverse interactions with 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) and related psychostimulants: mechanisms and mediators. Br. J. Pharmacol. 167, 946–959. doi: 10.1111/j.1476-5381.2012.02065.x
Varani, K., Vincenzi, F., Merighi, S., Gessi, S., and Borea, P. A. (2017). Biochemical and pharmacological role of A1 adenosine receptors and their modulation as novel therapeutic strategy. Adv. Exp. Med. Biol. doi: 10.1007/5584_2017_61 [Epub ahead of print].
Vincenzi, F., Borea, P. A., and Varani, K. (2016a). Anxiolytic properties of A1 adenosine receptor PAMs. Oncotarget 8, 7216–7217. doi: 10.18632/oncotarget.13802
Vincenzi, F., Ravani, A., Pasquini, S., Merighi, S., Gessi, S., Romagnoli, R., et al. (2016b). Positive allosteric modulation of A1 adenosine receptors as a novel and promising therapeutic strategy for anxiety. Neuropharmacology 111, 283–292. doi: 10.1016/j.neuropharm.2016.09.015
Volkow, N. D., Wang, G.-J., Fowler, J. S., and Tomasi, D. (2012). Addiction circuitry in the human brain. Annu. Rev. Pharmacol. Toxicol. 52, 321–336. doi: 10.1146/annurev-pharmtox-010611-134625
Vrecko, S. (2015). Everyday drug diversions: a qualitative study of the illicit exchange and non-medical use of prescription stimulants on a university campus. Soc. Sci. Med. 131, 297–304. doi: 10.1016/j.socscimed.2014.10.016
Webb, J. R., Valasek, M. A., and North, C. S. (2013). Prevalence of stimulant use in a sample of US medical students. Ann. Clin. Psychiatry 25, 27–32.
Weerts, E. M., and Griffiths, R. R. (2003). The adenosine receptor antagonist CGS15943 reinstates cocaine-seeking behavior and maintains self-administration in baboons. Psychopharmacology 168, 155–163. doi: 10.1007/s00213-003-1410-5
Wells, L., Opacka-Juffry, J., Fisher, D., Ledent, C., Hourani, S., and Kitchen, I. (2012). In vivo dopaminergic and behavioral responses to acute cocaine are altered in adenosine A2A receptor knockout mice. Synapse 66, 383–390. doi: 10.1002/syn.21527
Wolf, M. E. (2016). Synaptic mechanisms underlying persistent cocaine craving. Nat. Rev. Neurosci. 17, 351–365. doi: 10.1038/nrn.2016.39
Wood, S., Sage, J. R., Shuman, T., and Anagnostaras, S. G. (2014). Psychostimulants and cognition: a continuum of behavioral and cognitive activation. Pharmacol. Rev. 66, 193–221. doi: 10.1124/pr.112.007054
Wright, S. R., Zanos, P., Georgiou, P., Yoo, J.-H., Ledent, C., Hourani, S. M., et al. (2016). A critical role of striatal A2A R-mGlu5 R interactions in modulating the psychomotor and drug-seeking effects of methamphetamine. Addict. Biol. 21, 811–825. doi: 10.1111/adb.12259
Wydra, K., Gołembiowska, K., Suder, A., Kamińska, K., Fuxe, K., and Filip, M. (2015). On the role of adenosine (A)2A receptors in cocaine-induced reward: a pharmacological and neurochemical analysis in rats. Psychopharmacology 232, 421–435. doi: 10.1007/s00213-014-3675-2
Wydra, K., Golembiowska, K., Zaniewska, M., Kamińska, K., Ferraro, L., Fuxe, K., et al. (2013). Accumbal and pallidal dopamine, glutamate and GABA overflow during cocaine self-administration and its extinction in rats. Addict. Biol. 18, 307–324. doi: 10.1111/adb.12031
Xu, F., Wu, H., Katritch, V., Han, G. W., Jacobson, K. A., Gao, Z.-G., et al. (2011). Structure of an agonist-bound human A2A adenosine receptor. Science 332, 322–327. doi: 10.1126/science.1202793
Yang, S.-L., Han, J.-Y., Kim, Y.-B., Nam, S.-Y., Song, S., Hong, J. T., et al. (2011). Increased non-rapid eye movement sleep by cocaine withdrawal: possible involvement of A2A receptors. Arch. Pharm. Res. 34, 281–287. doi: 10.1007/s12272-011-0214-0
Yao, L., McFarland, K., Fan, P., Jiang, Z., Ueda, T., and Diamond, I. (2006). Adenosine A2a blockade prevents synergy between mu-opiate and cannabinoid CB1 receptors and eliminates heroin-seeking behavior in addicted rats. Proc. Natl. Acad. Sci. U.S.A. 103, 7877–7882. doi: 10.1073/pnas.0602661103
Zhang, S., Jin, Y., Liu, X., Yang, L., Ge, Z. J., Wang, H., et al. (2014). Methamphetamine modulates glutamatergic synaptic transmission in rat primary cultured hippocampal neurons. Brain Res. 1582, 1–11. doi: 10.1016/j.brainres.2014.07.040
Zwartsen, A., Verboven, A. H. A., van Kleef, R. G. D. M., Wijnolts, F. M. J., Westerink, R. H. S., and Hondebrink, L. (2017). Measuring inhibition of monoamine reuptake transporters by new psychoactive substances (NPS) in real-time using a high-throughput, fluorescence-based assay. Toxicol. In Vitro 45, (Pt 1), 60–71. doi: 10.1016/j.tiv.2017.05.010
Keywords: Adenosine A1 receptors, Adenosine A2A receptors, amphetamines, cocaine, dopamine, psychostimulants, behavioral effects, striatum
Citation: Ballesteros-Yáñez I, Castillo CA, Merighi S and Gessi S (2018) The Role of Adenosine Receptors in Psychostimulant Addiction. Front. Pharmacol. 8:985. doi: 10.3389/fphar.2017.00985
Received: 17 October 2017; Accepted: 22 December 2017;
Published: 10 January 2018.
Edited by:
Francisco Ciruela, University of Barcelona, SpainReviewed by:
Olga Valverde, Pompeu Fabra University, SpainCopyright © 2018 Ballesteros-Yáñez, Castillo, Merighi and Gessi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carlos A. Castillo, Y2FybG9zYS5jYXN0aWxsb0B1Y2xtLmVz Stefania Merighi, c3RlZmFuaWEubWVyaWdoaUB1bmlmZS5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.