Introduction: The earliest steps of development are characterized by cellular reorganization and differentiation within a three-dimensional (3D) microenvironment. This 3D context allows for a complex spatial interplay between biochemical and physical signals, and governs important cellular rearrangements leading to morphogenesis. In-vitro approaches have attempted to recapitulate key features of these processes, and it has now become possible to generate an increasing variety of self-organizing multicellular tissue constructs termed organoids. While important aspects of the 3D in-vivo organization have been recreated in these organoid systems, such studies have been exclusively performed in MatrigelTM, a poorly defined proteinaceous mixture whose properties cannot be readily modulated[1]-[3] . As such, the uncharacterized interactions between cells and this extracellular matrix (ECM) have proven to be a major challenge to understanding the underlying regulatory mechanisms governing morphogenesis.
In this work, we employ tunable synthetic ECM hydrogels in a high throughput screening approach[4] in order to disentangle the contributions of biochemical and physical components of the microenvironment in the specification of stem cell fate and morphogenesis.
Materials and Methods: A library of molecular building blocks was independently mixed and cross-linked in-situ to form cell-containing 3D scaffolds with independently controllable mechanical and biochemical properties. Differentiation, proliferation and apico-basal (AB) polarity were evaluated, and an optimized matrix was derived. A “signature of differentiation” was thus identified.
Further differentiation including treatment with retinoic acid was performed in order to pattern the generated cysts in the identified optimal matrix.
Results and Discussion: We develop a high-throughput approach to systematically generate combinatorial interactions between various components of the cellular microenvironment in 3D and deploy it to elucidate the mechanisms controlling early neuroepithelial development from single mouse embryonic stem cells. We show the synergistic roles of matrix elasticity, degradability and ECM protein composition in specifying neural fate and initiating apico-basal polarity. We explore how matrix characteristics relate to dynamic symmetry-breaking events in such multicellular constructs, and show how apico-basal polarity is required for initiating subsequent dorso-ventral patterning.
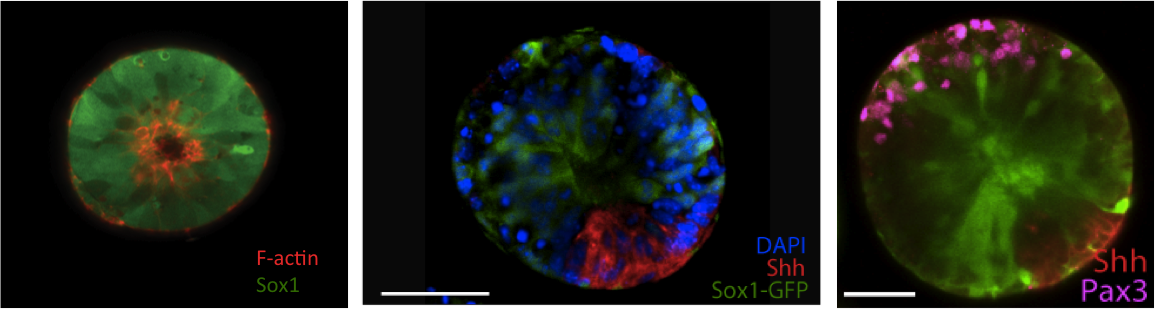
We demonstrate that these morphogenetic processes are tightly coupled to the physical characteristics of the matrix, and demonstrate for the first time that a patterned neural tube-like organoid can be generated within an optimized, fully synthetic matrix.
Conclusion: Using a synthetic 3D microenvironemental screening platform, we probe the combined effects of matrix properties and signaling proteins on early neural fate. This in-vitro model system allows us to precisely and controllably explore the role of the extrinsic microenvironment in directing self-organization and patterning. Ongoing work focuses on further utilizing this synthetic in-vitro model system to explore the emergence and migration of neural crest cells from the dorsal aspect of these neural tube-like organoids.
References:
[1] Takebe, T. et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 499, 481-484 (2013).
[2] Lancaster, M. A. et al. Cerebral organoids model human brain development and microcephaly. Nature 501, 373-379 (2013).
[3] Meinhardt, A. et al. 3D reconstitution of the patterned neural tube from embryonic stem cells. Stem Cell Reports 3, 987-999 (2014).
[4] Ranga, A. et al. 3D niche microarrays for systems-level analyses of cell fate. Nature communications 5 (2014).