Core cross-linked star polymers are of great interest for their potential application in gene and drug delivery[1] or biomaterial building blocks[2]. Devising functional polymeric building blocks for a given biomaterial or biomedical application often requires iterative optimization steps, which is both labour intensive and time consuming. Here we introduce a novel rapid aqueous one-pot arm-first star polymer biomaterial synthesis scheme that yields narrow dispersity stars within only 10 minutes of reaction and requires a single purification step. This novel methodology has been extensively characterized and shown to enable the high throughput generation of complex star polymer biomaterial libraries.
The general two-step reaction scheme is shown in Figure 1. Briefly, a solution of acrylamide monomer (Fig. 1b), raft agent (PABTC) and initiator (VA-044) was prepared in DI water/20% dioxane ([M]:[CTA]:[I] stoichiometry is detailed in Fig. 1a). Using a heat-block, the solution was heated to 100°C for 3-5 minutes to polymerize the macroCTA. To yield star polymers, a solution of cross-linker (di(ethylene glycol)diacrylate) and initiator was directly added to the macroCTA solution and further reacted at 100°C for 3-5 minutes. MacroCTA and star polymers were characterized by NMR, GPC and DLS and could readily be purified by dialysis.
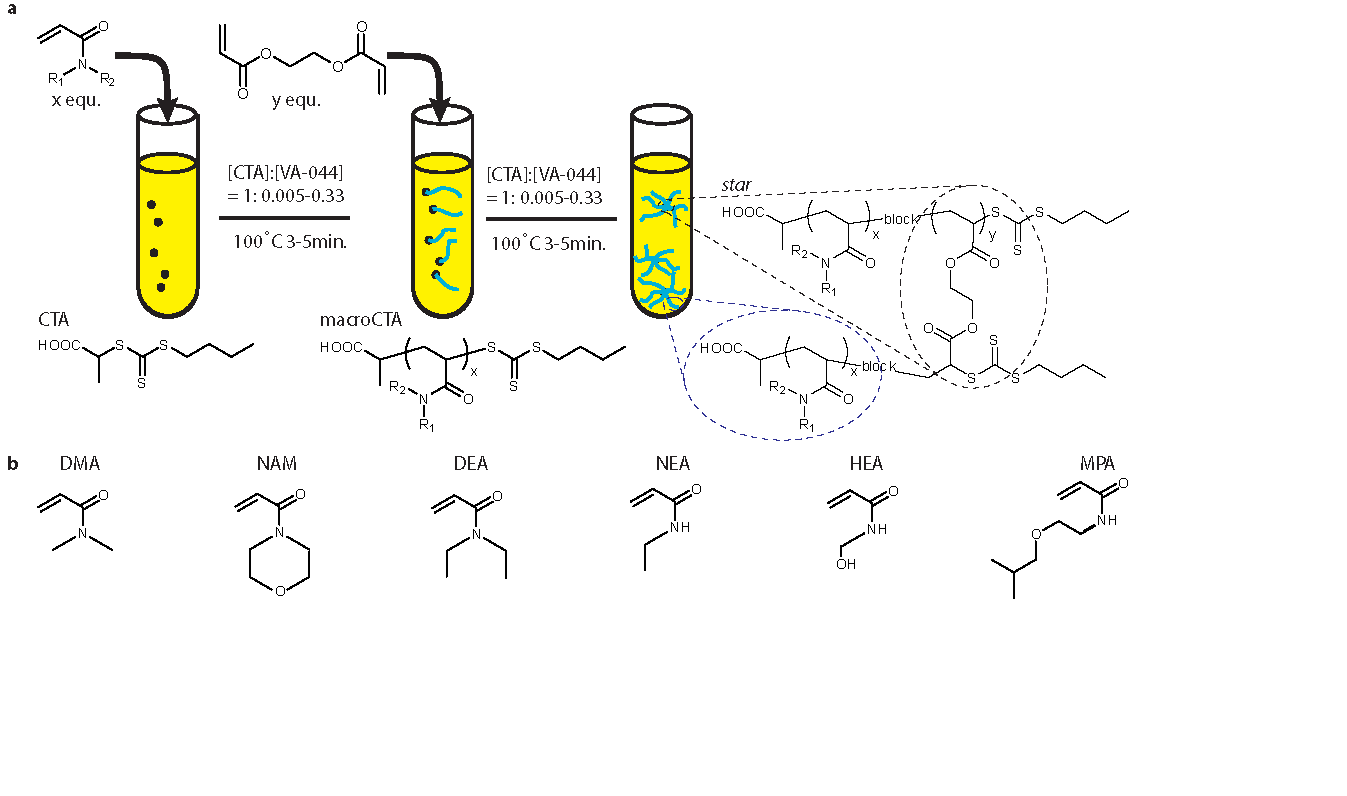
Figure 1. Star synthesis. a. Schematic representation of the aqueous one-pot star formation. b. List of acrylamide monomer used for star polymer synthesis.
Our reaction scheme is very rapid and star polymers with controlled composition and architecture are yielded within less than 10 minutes with just a few pipetting steps (Fig. 1a). The first step of our reaction scheme typically yields macroCTA with high conversion rate (>90%, NMR). Further reaction at 100°C for 3-5 minutes after the direct addition of cross-linker and initiator readily yielded the controlled formation (Đ=1.16±0.15) of star polymers as seen in Figure 2. Increased control over the reaction product was achieved with lower initiator concentrations (Fig. 2a). Interestingly, star polymers made of DMA macroCTA displayed biphasic behaviour when the amount of cross-linker per macroCTA was increased while stars from the other monomers display a strong linear correlation (R2>0.8), as shown by GPC analysis (Fig. 2a). Further characterization showed that the degree of polymerization of the macroCTA strongly affected the average number of arms per star, with stars made from short linear polymers having more arms when compared to those made from longer macroCTAs (Fig. 2b). Next we demonstrated the ability of this scheme to rapidly generate libraries of star polymers; 48 stars were made simultaneously (~10 minutes), as detailed in Figure 2c,d. We extended this scheme to the generation of more complex, combinatorial libraries of mikto-arm stars, as well as stars made from various block copolymers. Finally, cross-linking moieties can be directly substituted post-polymerization to the stars pendant ends to permit cross-linking and hydrogel formation through a well-known enzymatic scheme[3].
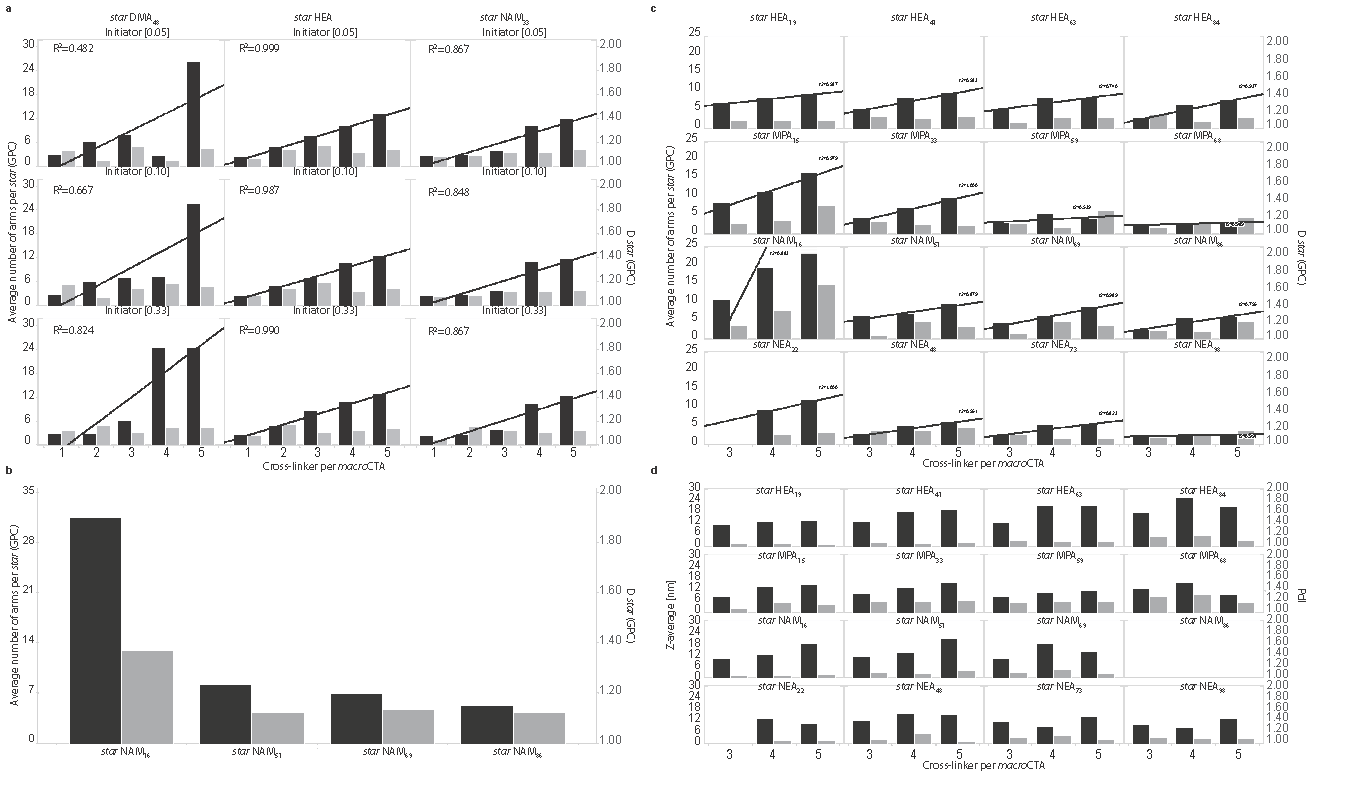
Figure 2. Characterization of star polymer formation. a. Graphical representation of the GPC characterization of star formation and the effect of initiator concentration. b. Graphical representation of the GPC characterization of star formation and the effect of macroCTA degree of polymerization. c. Graphical representation of the GPC characterization of star polymer library synthesis. d. Graphical representation of the DLS characterization of star polymer library synthesis.
In conclusion, the novel methodology presented drastically reduces star polymer reaction time whilst maintaining the typical level of control provided by RAFT polymerization. This process is readily amenable to high throughput discovery and enables rapid complex, combinatorial star polymer library generation to facilitate tailoring biomaterial building blocks for any desired application.
References:
[1] A. Gregory and M.H. Stenzel, Expert Opin. Drug Deliv., 8, 237-269, 2011
[2] Y. Dong, Y. Qin, M. Dubaa, J. Killion, Y. Gao, T. Zhao, D. Zhou, D. Duscher, L. Geever, G.C. Gurtner and W. Wang, Polymer Chem., 6, 6182-6192, 2015
[3] D.J. Menzies, A. Cameron, T. Munro, E. Wolvetang, L. Grondahl and J.J. Cooper-White, Biomacromolecules, 14, 413-423, 2013