Introduction: In vivo cyclic stretch strongly regulates vascular smooth muscle cells (vSMCs) phenotype and behavior[1]. Thus, the development of reliable models to study the mechanisms by which mechanical forces influence vSMCs would be essential for a better understanding of the vascular biology in physio-pathological conditions. In this context, the main objective of this study was to compare 2D and 3D culture-based models of stretched vSMCs with the aim to elucidate how the environment surrounding the cells influences cellular response.
Materials and Methods: For 2D cultures, vSMCs were seeded onto UniFlex® plates as monolayers at a density of 300x103 cells/mL (Fig.1, A). For 3D cultures, cellularized collagen gels were prepared by mixing sterile collagen solution with cell suspension[2], to a final cell density of 50x105 cells/mL, and poured onto the central region experiencing uniaxial strain of UniFlex® plates (Fig.1, B). During the experiments, a regimen of 7% cyclic strain at 1 Hz was chosen to stretch the system for 2 and 5 days.
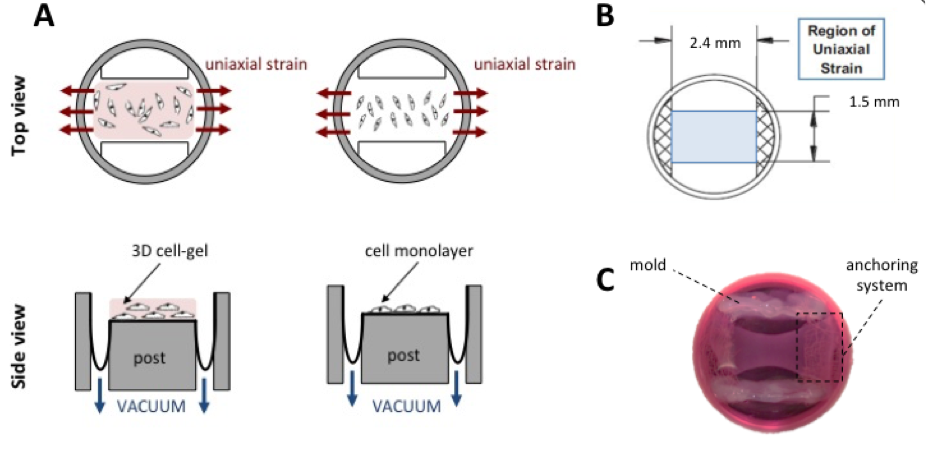
After culture, to compare the effect of mechanical stimulation on vSMCs in both 2D and 3D models, we performed: i) western Blot analyses (WB) to detect the expression of contractile-phenotype markers (i.e. α-actin and calponin); ii) immunofluorescence analysis for staining α-actin and calponin; iii) measurements of cell alignment.
Results and Discussion: Independently of the model dimensionality (2D or 3D), vSMCs showed strain-dependent alignment: in 2D cultures, cells aligned nearly perpendicular (80°-90°) to strain direction; in 3D cultures, cells aligned parallel to strain (at an angle of ca 0°), similarly to in vivo-like conditions. No-stretched 2D and 3D cultured cells arranged in a random orientation.
In the 2D experiments, WB analyses indicated a slight downregulation of α-SM actin (Fig. 2, A) and calponin in strained vSMCs compared to static controls (Fig. 2, B). In 3D cell-gels, instead, no differences in α-SM actin expression were detected between strained samples and controls for all time points (Fig. 2, C), while calponin expression was strongly upregulated by mechanical strain after 5 days of culture (Fig. 2, D).
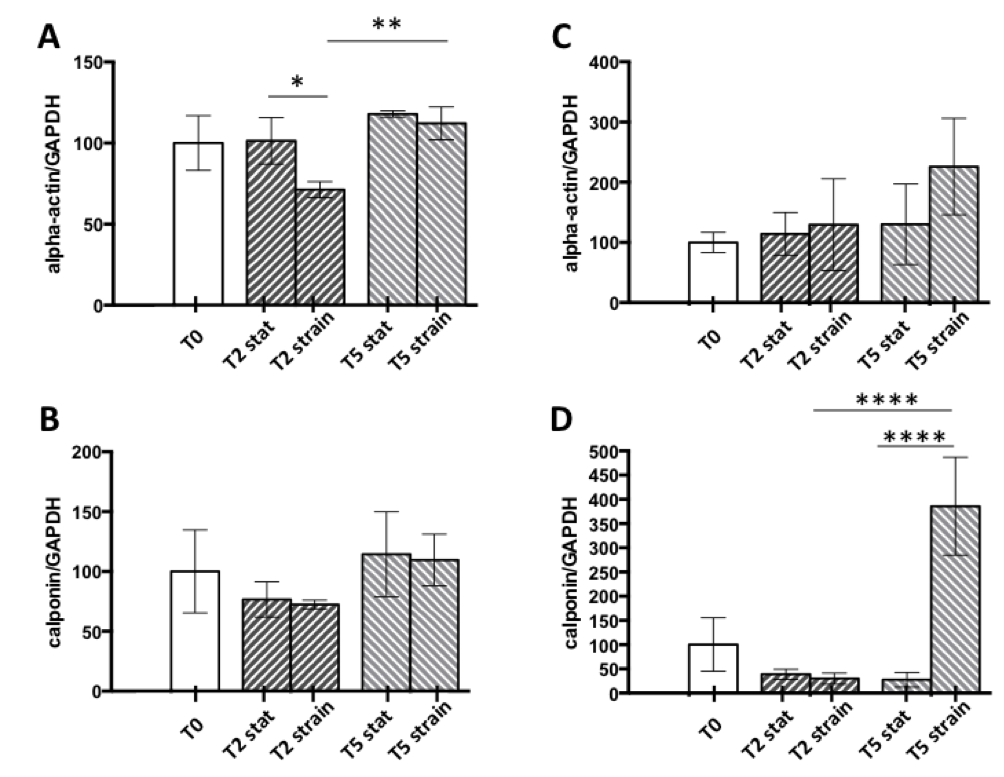
Immunostaining performed on both 2D and 3D models corroborated our results. These preliminary results suggest that dynamic stimulation in 2D cultures had minimal influence on the vSMC contractile phenotype, while a significant effect could be observed for 3D cultures.
Conclusions: In this study, we confirmed the important roleof mechanical stimuli on vSMCs behavior, highlighting 3D culture support can strongly affect the final response of cells. Future works will be focused on identifying the mechanisms involved in the response of vSMCs to dynamic stimulation.
NB was awarded of a PhD Scholarship from the Italian Ministry of Education, completed with a mobility scholarship from Scuola Interpolitecnica di Dottorato, Italy. DP was awarded of a Scholarship from NSERC CREATE Program in Regenerative Medicine (www.ncprm.ulaval.ca). This work was partially supported by NSERC-Canada, CIHR-Canada, CFI-Canada, FRQ-NT-Quebec, MRI-Quebec, and MURST.
References:
[1] Chen, L.-J., Wei, S.-Y. & Chiu, J.-J. Mechanical regulation of epigenetics in vascular biology and pathobiology. J. Cell. Mol. Med. 17, 437–448 (2013).
[2] Meghezi, S. et al. Engineering 3D Cellularized Collagen Gels for Vascular Tissue Regeneration. J. of Vis. Exp. (2015). doi:10.3791/52812.