Introduction: Majority of corneal transplants are performed exclusively to replace a dysfunctional corneal endothelium (CE) due to Fuchs’ endothelial dystrophy (FED)[1]. Due to shortage of donor corneas, corneal cell injection therapy has been proposed as a minimally invasive alternative treatment for FED patients[2]-[4]. A significant clinical sign of FED is the presence of excrescences of membrane (DM) which are called corneal guttata (CG)[5]-[7]. Current studies on cell injection therapy are performed in animal model corneas which have unaltered DM and hence, do not recapitulate the topographical micro-environment of FED[2][4],[8],[9]. Studying the monolayer formation on guttata-laden surface will be key to the development of cell therapy as a viable clinical procedure for corneal patients. The aim of this study was to fabricate the synthetic guttata-like micro-structures based on patient guttata characterization and evaluate the feasibility of cell monolayer formation on a synthetic guttata-laden surface for cell therapy applications.
Material and Methods: Posterior corneal surfaces of FED patients were analyzed by using specular and transmission electron microscopy to characterize the CG dimensions. Soft lithography and hot embossing were used to fabricate similar dimensions guttata on polystyrene (synthetic guttata). The cell migration and monolayer formation on synthetic guttata was studied by first using B4G12 corneal cell line on various dimensions of guttata followed by primary human corneal endothelial cells (HCECs) on selected guttata. Cell interaction with synthetic guttata was studied with SEM imaging and monolayer formation was studied by using confocal microscopy in terms of ZO1 expression. Cell migration tracks were plotted by using manual tracking plugin in ImageJ and analyzed with Ibidi chemotaxis tool.
Results and Discussion: B4G12 cell monolayer formation on top of synthetic guttata pillars gradually increased as the pillar spacing and diameter was increased (Fig. 1). The cell morphology was elongated instead of polygonal on highest density pillars.
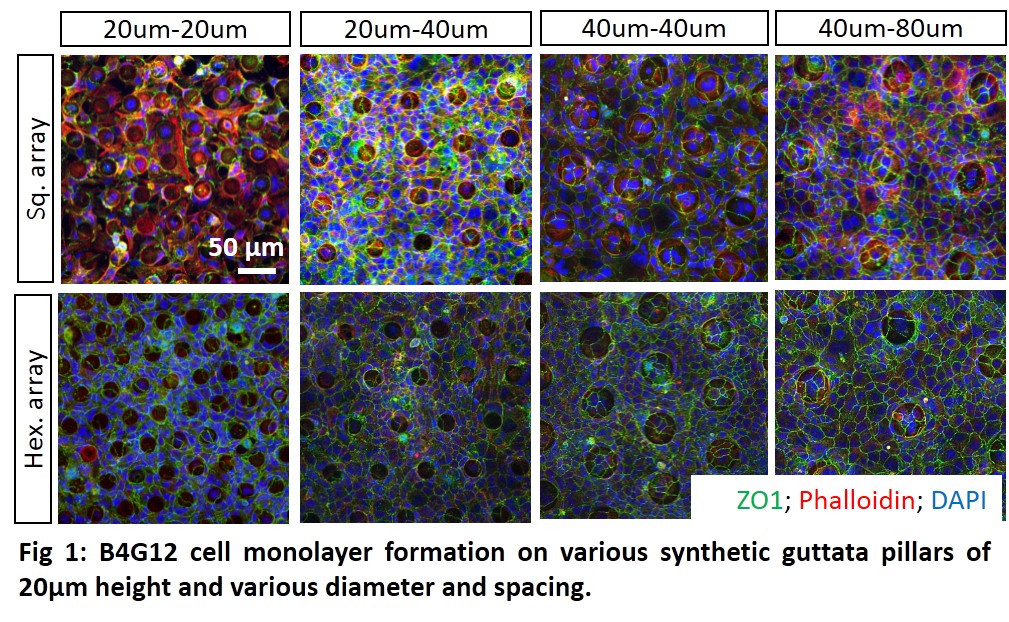
B4G12 cell migration distance, speed and directness analysis on synthetic guttata pillars showed that the highest density pillars presented the most challenging surface for cell monolayer formation, ZO1 expression and for cell migration.
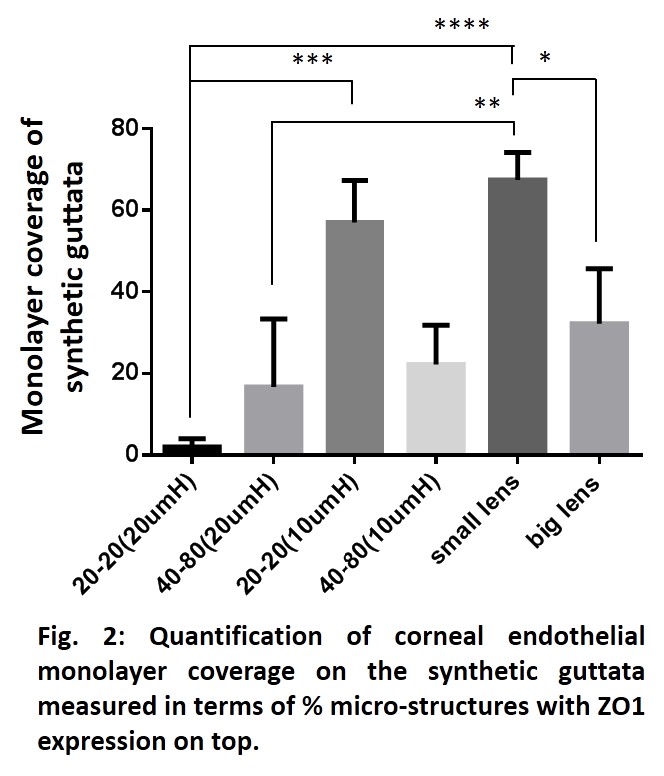
With primary HCECs, the cells were unable to form monolayer on highest density small pillars with migrated cells or with cell seeded on top of pillars. Decreasing the height and the diameter and making the edges round (lenses) led to an increased ZO1 expression on top of the micro-structures which indicated that the cells could form tight junction on synthetic guttata (Fig. 2).
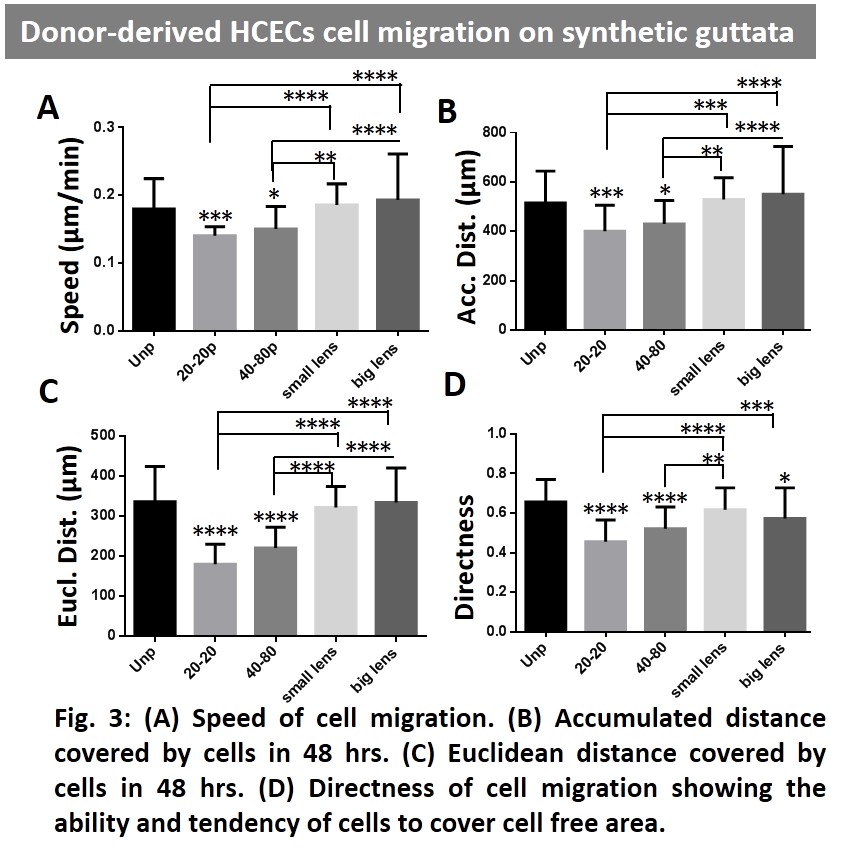
HCECs migration analysis showed that the synthetic guttata with round edges and less height (5µm) were relatively more favorable for cell migration and corneal endothelial monolayer formation (Fig. 3). The results suggest that the diseased guttata-laden corneal DM surface might potentially hinder the formation of corneal endothelium.
Conclusion: The results of this study shows that the pre-existing DM guttata could interfere with injected cells and corneal endothelial monolayer formation within eye. The surgical removal of preexisting guttata before cell therapy could potentially increase the chance of cell therapy success. Additionally, the characterization of the types of guttata on corneal DM surface of FED patients could also be important for patient-specific cell therapy procedure.
MOE AcRF Tier 1 – FRC R-397-000-217-112; National Research Foundation Translational and Clinical Research (TCR) Programme Grant (NMRC/TCR/008-SERI/2013); National Medical Research Council NMRC/NIG/0037/2008; Mechanobiology Institute (MBI) R-714-007-006-271; Biomedical Research Council Translation Clinical Research Partnership (TCRP) Grant (TCR0101673); Singapore International Graduate Award (SINGA) Scholarship by A*STAR Graduate Academy
References:
[1] 2012 Eye banking statistical report. Eye Bank Association of America. 2012
[2] Moysidis SN, Alvarez-Delfin K, Peschansky VJ, Salero E, Weisman AD, Bartakova A, et al. Magnetic field-guided cell delivery with nanoparticle-loaded human corneal endothelial cells. Nanomedicine-Uk. 2015;11:499-509
[3] Bartakova A, Kunzevitzky N, Goldberg J. Regenerative Cell Therapy for Corneal Endothelium. Curr Ophthalmol Rep. 2014;2:81-90
[4] Okumura N, Koizumi N, Ueno M, Sakamoto Y, Takahashi H, Tsuchiya H, et al. ROCK Inhibitor Converts Corneal Endothelial Cells into a Phenotype Capable of Regenerating In Vivo Endothelial Tissue. Am J Pathol. 2012;181:268-77
[5] Eghrari AO, Gottsch JD. Fuchs’ corneal dystrophy. Expert Review of Ophthalmology. 2010;5:147-59
[6] Chiou AG, Kaufman SC, Beuerman RW, Ohta T, Soliman H, Kaufman HE. Confocal microscopy in cornea guttata and Fuchs' endothelial dystrophy. The British journal of ophthalmology. 1999;83:185-9
[7] Borderie VM, Baudrimont M, Vallée A, Ereau TL, Gray Fo, Laroche L. Corneal Endothelial Cell Apoptosis in Patients with Fuchs’ Dystrophy. Invest Ophth Vis Sci. 2000;41:2501-5
[8] Koizumi N, Okumura N, Kinoshita S. Development of new therapeutic modalities for corneal endothelial disease focused on the proliferation of corneal endothelial cells using animal models. Exp Eye Res. 2012;95:60-7
[9] Patel SV, Bachman LA, Hann CR, Bahler CK, Fautsch MP. Human corneal endothelial cell transplantation in a human ex vivo model. Invest Ophthalmol Vis Sci. 2009;50:2123-31